Abstract
Membrane/lipid rafts (MLR) are plasmalemmal microdomains that are essential for neuronal signaling and synaptic development/stabilization. Statins inhibit HMG-CoA reductase, the rate-limiting enzyme in the biosynthesis of mevalonic, a precursor to cholesterol via the mevalonate pathway. Because there has been controversy over the effects of statins on neuronal and cognitive function, we investigated the impact of long-term atorvastatin treatment (5mg/kg/d for 7 months by oral gavage) on behavior, cognition, and brain biochemistry in mice. We hypothesized that long-term statin treatment would alter lipid rafts and cognitive function. Atorvastatin treatment resulted in behavioral deficits as measured in paradigms for basic exploration (open field activity) and cognitive function (Barnes maze, startle response) without impairment in global motor function (Rotor Rod). Furthermore, significant changes in MLR-associated proteins (syntaxin-1α and synaptophysin) and a global change of post-synaptic density protein-95 (PSD95) were observed. The observed decreases in the MLR-localized pre-synaptic vesicle proteins syntaxin-1α and synaptophysin suggest a molecular mechanism for the statin-associated impairment of cognitive function that was observed and that has been suggested by the clinical literature.
Keywords: brain, cholesterol, atorvastatin, lipid, hypercholesterolemia, pharmacology
Statins are used widely in the treatment of hypercholesterolemia. Statins exert their cholesterol lowering effects through inhibition HMG-CoA reductase (3-hydroxy-3-methyl-glutaryl-CoA reductase), the rate-limiting enzyme in cholesterol synthesis via the mevalonate pathway [1].
Prevention of myocardial infarction and stroke by statins have been demonstrated convincingly[2](meta-analysis including one investigation of simvastatin, three of pravastatin, and one of lovastatin). It has been estimated that more than 30 million Americans currently receive statins [3]. However, there has been increasing concern that statins may have adverse effects on cognitive function [4]. While long-term investigations in large populations suggest that statins reduce the incidence of Alzheimer’s disease (AD) and other dementias [5–7](meta-analyses: Swiger et al. including three investigations of simvastatin, five of pravastatin, and five of lovastatin; Jick et al. including investigations of simvastatin, pravastatin, atorvastatin, fluvastatin, and cerivastatin; Rockwood et al. including investigations of lipid-lowering agents not further specified), other work suggests that cognitive impairment may occur in some individuals. Specifically, simvastatin has been shown to cause deficits in attention and psychomotor speed, decline in neuropsychological performance [8], and case reports describe statin associated memory loss [9] (Case reports including 36 patients receiving simvastatin, 23 receiving atorvastatin, and one patient receiving pravastatin). Statin associated memory loss may be especially relevant to those with preexisting dementia or neurodegenerative diseases such as AD [10–12] (Evans et al. describes a survey of 168 patients including atorvastatin (n=118), simvastatin (n=69), pravastatin (n=42), lovastatin (n=20), fluvastatin (n=6), rosuvastatin (n=10), and cerivastatin (n=19); Feldman et al. is a randomized controlled trial investigating atorvastatin; Padala et al. describe a prospective pilot investigation including atorvastatin (n=8), simvastatin (n=5), fluvastatin (n=2), pravastatin (n=1), rosuvastatin (n=1), and lovastatin (n=1). The supporting information has been sufficient to prompt a warning by the U.S. Food and Drug Administration about the hazard of statin-associated cognitive deterioration [13].
To our knowledge there exists no pre-clinical model for the assessment of the effect of statins on cognition. It has been suggested that the adverse cognitive effects of statins are likely to be greatest with lipophilic statins, though this idea is the subject of debate [14,15] (King et al. investigating atorvastatin and simvastatin). In our study we evaluated the effect of long-term treatment with the lipophilic statin atorvastatin on behavior and cognition in mice. Pre-clinical findings from other laboratories and our group have demonstrated a protective role for cholesterol and MLR against neuronal toxicity and ischemic injury [15]. We therefore simultaneously examined the effect of atorvastatin on MLR-associated protein expression and on biochemical markers of synaptic function in the hippocampus.
All studies performed were approved by the Institutional Animal Care and Use Committee of the Veteran Affairs Medical Center, San Diego, and conform to relevant National Institutes of Health guidelines. Prior to behavioral testing and biochemical analysis inbred mice (C57BL/6J, 129/Sv and Black Swiss background) were treated daily with atorvastatin, as previously described [16] (Atorva, 5 mg/kg/day in 10% EtOH in tap water, 200 μl; n=9/group) or vehicle (Veh, 10% EtOH in tap water, 200 μl, n=10/group) for 7 months via oral gavage. All data were analyzed to determine parametric or non-parametric distribution and then analyzed by unpaired t tests or 2-way/3-way ANOVA followed by appropriate post hoc tests using Prism 6 (GraphPad Software, Inc.). Significance was set at p<0.05.
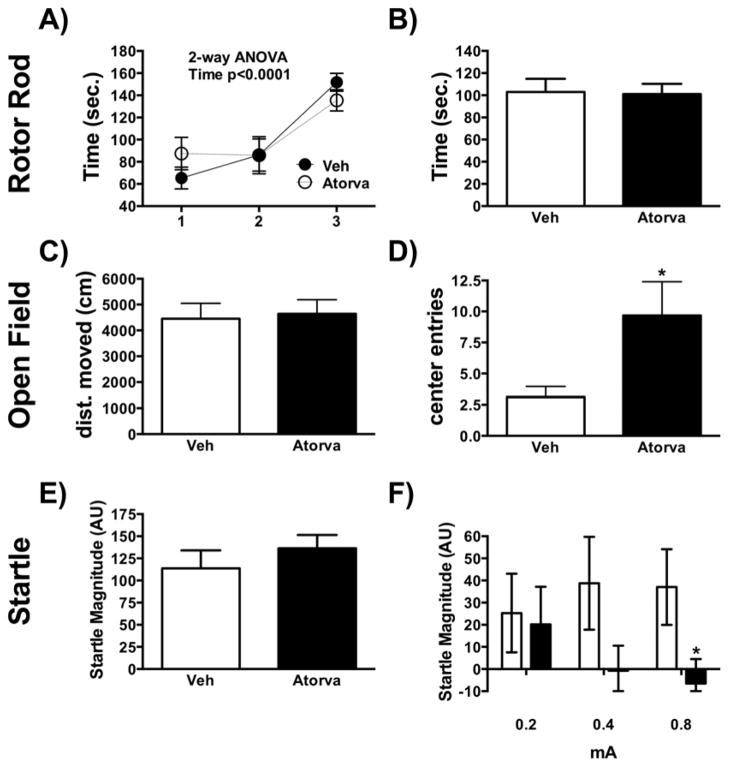
To determine whether statin treatment caused alterations in motor function and agility, the accelerating (0–40 rpm over a period of 300 sec) Rota-Rod (Med-Associates, VT) was used. It revealed no significant differences in task acquisition time and total average duration that both groups remained on the rod (Fig. 1A–B). In order to examine basic activity and general behavior, we assessed Open Field activity by computerized video tracking system (Polytracker, San Diego Instruments, San Diego, CA) software. We observed no difference between treatments in the total distance moved (Fig. 1C). However, atorvastatin administration was associated with a significant increase in center entries (t(16)=−2.288, p=0.036) (Fig. 1D). Startle chambers (San Diego Instruments, San Diego, CA) were used to assess baseline and context potentiated startle. No difference between groups was observed during baseline startle (Fig. 1E). As we have reported previously [17], startle potentiation is largest with the 90 dB intensity trials and that intensity was used in this study. A 3-way ANOVA of shock, statin treatment and startle intensity (Fig. 1E–F), revealed a shock x intensity effect [F(6,102)=6.15, p<0.001] and a statin x intensity interaction [F(2,34)=3.48, p<0.05] (Fig. 1F). Analysis of percentage change in startle reactivity across vehicle and statin treated animals revealed that statin treated animals had a trend toward reduced startle potentiation after shock [F(2,34)=2.34, p=0.087] in statin treated animals. In a post-hoc analysis, statin-treated animals showed significantly less context-potentiation after the 0.8 mA shock (t(15)=2.14, p=0.049, Welch’s test, Fig. 1F).
Figure 1.
(A, B) Rotor Rod; (C, D) Open Field; (E, F) Startle; Data are shown as mean±SEM; *p<0.05; Veh n=10; Atorva n=9.
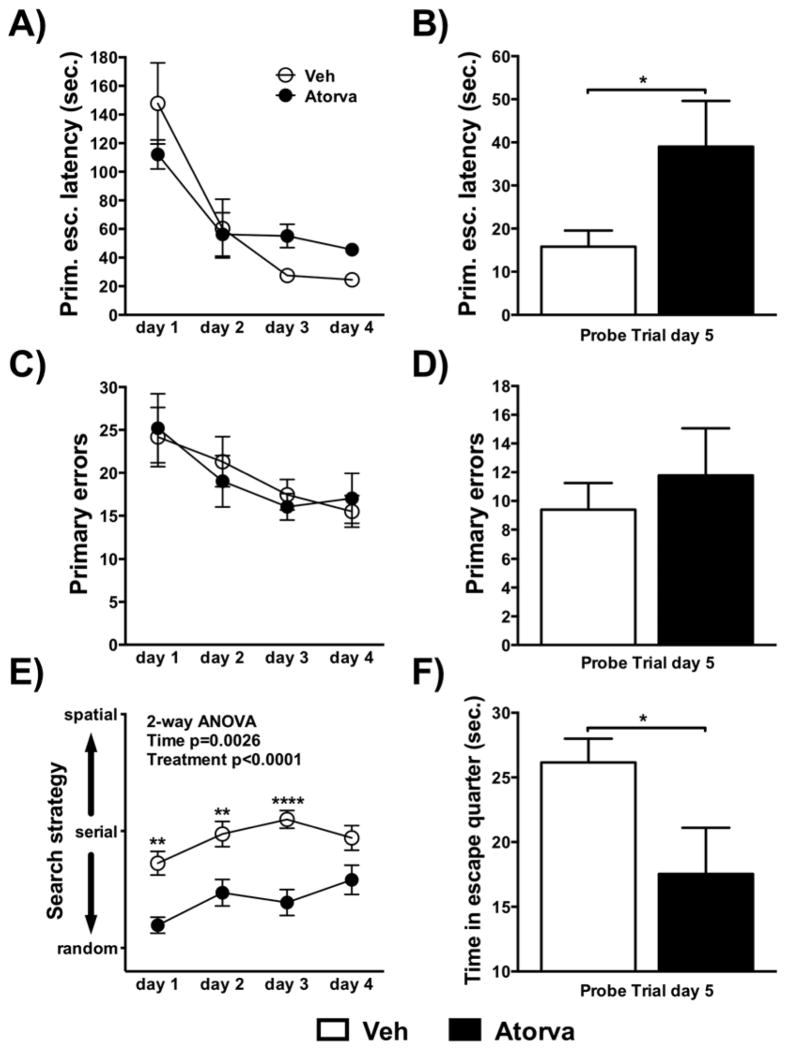
The Barnes maze was used to assess spatial learning and memory. Atorvastatin significantly increased primary escape latency (Fig. 2A–F, df(17)=2.156, p=0.046) and lowered time in the quarter of the escape tunnel on probe trial day 5 (t(17)=2.218, p=0.041). Atorvastatin treated mice were more likely to employ a random strategy to find the escape tunnel during the acquisition phase of the experiment [F(1, 17)=28.07, p<0.0001]. During the acquisition phase a significant effect of time was observed for the primary escape latency [F(3, 45)=21.22, p<0.0001], primary errors [F(3, 48)=4.043, p=0.012], and search strategy [F(3, 51)=5.402, p=0.003]. No difference between groups was observed for primary escape latency, primary errors during acquisition and primary errors during the probe trials.
Figure 2.
(A–H) Barnes maze: primary escape latency (A) acquisition and (B) probe trial; primary errors (C) acquisition and (D) probe trial; search strategy (E) acquisition; time spent in escape quarter (F) probe trial; Data are shown as mean±SEM; *p<0.05; Veh n=10; Atorva n=9.
In aggregate the behavioral data reveal that atorvastatin altered general behavior, as well as learning and memory without impacting motor function
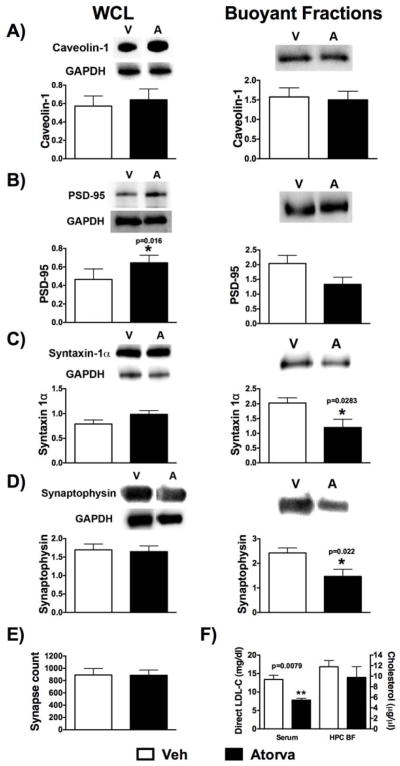
MLR, plasmalemmal cholesterol, and the cholesterol binding protein caveolin-1 (Cav-1) have previously been shown to play a critical role in the structural organization of receptors involved in post-synaptic neurotransmitter and neurotrophin signaling and in neurite growth [18]. We therefore assessed the effect of atorvastatin on the protein expression of Cav-1 and the post-synaptic density (PSD) marker PSD-95. There was no significant difference in Cav-1 protein expression in the whole cell lysate (WCL) or buoyant fractions (BF) following sucrose density fractionation between the groups [Fig 3A Cav-1: Veh (n=6) vs Atorva (n=5), t(9)=0.425, p=0.68 (mean±SEM 0.57±0.11 vs 0.64±0.12) for WCL; t(9)=0.221, p= 0.83 (mean±SEM 1.58±0.23 vs 1.50±0.22) for BF]. Although we observed only a trend toward a decreased PSD-95 in the BF for Atorva, there was a significant increase in WCL PSD-95 expression with Atorva, suggesting that this total cellular increase was localized to non-MLR regions [Fig 3B, Veh (n=6) vs Atorva (n=5), t(9)=1.888, p=0.09 (mean±SEM 2.04±0.28 vs 1.33±0.24) for BF; t(9)=2.960, p=0.016 (mean±SEM 0.47±0.05 vs 0.64±0.04) for WCL].
Figure 3.
Representative blots and quantification are shown for whole cell lysates (WCL) and pooled buoyant fractions for (A) Caveolin-1; (B) PSD-95; (C) Syntaxin-1α; (D) Synaptophysin; E) shows quantification of synapse counts (n=4/group); F) LDL-C from serum (n=6/group) and cholesterol of buoyant fractions from hippocampal tissue samples (n=4/group); Data are shown as mean±SEM; *p<0.05; Veh n=5; Atorva n=6 for biochemical analysis.
MLR and cholesterol have also been implicated in the regulation of the exocytotic machinery, specifically in the regulation of synaptic vesicle fusion with the plasma membrane and release of vesicle contents [19,20]. We therefore assessed protein expression of syntaxin-1α and synaptophysin, two established markers of pre-synaptic vesicles [20,21]. Although there was no significant change in protein expression in the WCL [Fig 3C - Syntaxin 1α: Veh (n=6) vs Atorva (n=5), t(9)=1.739, p=0.116 (mean±SEM 0.79±0.08 vs 0.99±0.075); Fig 3D - Synaptophysin: Veh (n=6) vs Atorva (n=5), t(9)=0.220, p=0.831 (mean±SEM 1.70±0.16 vs 1.65±0.154)], there was a significant decrease in BF expression of syntaxin-1α and synaptophysin [Fig 3C - Syntaxin 1α: Veh (n=6) vs Atorva (n=5), t(9)=2.608, p=0.028 (mean±SEM 2.03±0.18 vs 1.20±0.28); Fig 3D - Synaptophysin: Veh (n=6) vs Atorva (n=5), t(9)=2.764, p=0.022 (mean±SEM 2.42±0.21 vs 1.47±0.29)]. WCLs were all normalized to GAPDH and fractions were generated from equal protein loading (1 mg/ml) for all samples.
Our data show that atorvastatin resulted in a decrease in MLR-localized pre-synaptic vesicle proteins syntaxin-1α and synaptophysin
Electron micrographs (25 images/animal, n=4/group) were analyzed, as previously described [18], by three blinded independent observers. We observed no statistical difference in hippocampal (CA1 region) synapse counts between the two treatment groups [Fig 3E - total synapse counts: Veh (n=4) vs Atorva (n=4), t(6)=0.030, p=0.976 (mean±SEM 891.10±104.10 vs 886.90±84.65)].
After five weeks of Atorva or Veh treatment, non-fasting direct serum LDL cholesterol (LDL-C) was measured (IDEXX Laboratories, West Sacramento, CA, USA). Atorva treatment significantly reduced LDL-C [Fig 3F – LDL-C in serum: Veh (n=6) vs Atorva (n=6), U(10)=0, p=0.0079 (mean rank 8.00 vs 3.00)]. No significant difference was detected in the cholesterol content [Amplex® Red Cholesterol Assay Kit (Life Technologies™)] of the hippocampal BFs [Fig 3F - total cholesterol in BF: Veh (n=4) vs Atorva (n=4), t(6)=0.860, p=0.423 (mean±SEM 11.79±1.18 vs 9.78±2.03)].
This is the first preclinical investigation to demonstrate that long-term administration of the lipophilic statin atorvastatin causes cognitive deficits. Deficits in basic exploration (open field activity) and cognitive function (Barnes maze, Startle response) were observed, in the absence of impaired motor function (Rotor Rod). In addition, significant biochemical changes were observed in MLR-associated proteins necessary for synaptic vesicular membrane tethering and exocytotic release.
Currently there are seven statins available in the United States: atorvastatin, fluvastatin, and rosuvastatin are synthetically derived, while lovastatin, pravastatin, and simvastatin are derived by fermentation. Of these pravastatin and rosuvastatin are hydrophilic (acid form), while the others are lipophilic (lactone form) [22]. It has been demonstrated in human trials and in animal experiments that the lipophilic statins, lovastatin and simvastatin, are capable of crossing the blood brain barrier (BBB), but that the more hydrophilic pravastatin cannot [23]. Short-term statin treatment does not alter cholesterol levels in the brain. Experiments in brain slices from rats have demonstrated that high doses of statins affect brain cholesterol production but not brain cholesterol content [19]. However, the insignificant effect on brain cholesterol content in these investigations may be a function of the extremely long half-life of brain cholesterol [24].
As previously mentioned, several studies suggest cognitive impairments caused by statin treatment. Evans et al. reported statin-related cognitive adverse events [10] (see introduction for list of statins used). In another observational study, patients with existing dementia exhibited improved cognition only after discontinuation of statins and worsening with rechallenge [12] (see introduction for list of statins used). In addition, case reports also present evidence of decreased memory function during statin treatment [9,15,25] (Galatti et al. describe one case involving rosuvastatin; the statins used in the other studies are listed in the introduction). Because of these increasing reports, the US Food and Drug Administration (FDA) recently added safety warnings to statins concerning confusion and memory loss (FDA Drug Safety February 29th, 2012) [13].
It is well established that proteins involved in vesicular tethering and the cytoskeletal re-arrangement that leads to vesicular release from the plasmalemma are localized to cholesterol-enriched plasmalemmal regions, i.e., MLR. Our observations of changes in the pre-synaptic vesicle proteins syntaxin-1α and synaptophysin suggest that defects in vesicular tethering and therefore neurotransmitter release may be involved in the cognitive changes we observed. There are additional possible mechanisms. Statins also inhibit the synthesis of isoprenoids. Inhibition of isoprenylation impairs the function of small GTP-binding proteins, e.g., Rho, Ras and Rac [21] and may thereby also contribute in part to aberrant signal transduction and neurotransmitter or hormonal release. However, it is widely believed that the isoprenoid-mediated effects of statins are of more rapid onset and offset than the cholesterol mediated changes. This mechanism might account for cognitive changes that have been reported to resolve rapidly after statin discontinuation [10]. We used normocholesterolemic mice so that vascular inflammation due to high cholesterol levels did not interfer with behavior and cognition. It is common practice for statin therapy to be initiated in patients after myocardial infarction, regardless of serum cholesterol levels. Recently, new cardiovascular disease prevention guidelines were released by the American Heart Association and the American College of Cardiology [26,27]. These guidelines will increase the number of people who are not hypercholesterolemic for whom statins will be recommended.
The biochemical and behavioral changes observed in our study are consistent with earlier observations related to synaptophysin knock out (KO) mice [28]. The synaptophysin KO mouse showed increased exploratory behavior in a novel environment, i.e., behaviors consistent with our observation of increased center visits in the open field in our atorvastatin treated mice. In addition, compromised learning and memory ability in the Morris water maze, which resemble our findings in the Barnes maze were also observed in that investigation. Our present observations suggest impairment of vesicular neurotransmitter release but do not allow us to predict which neurotransmitters might be involved.
Our study is the first to suggest that alterations in the membrane lipid raft associated proteins syntaxin-1α and synaptophysin may account for adverse effects of statins on neurocognitive behavior. Moreover our study provides a model for subsequent pre-clinical studies. The current literature shows beneficial as well as detrimental statin effects on cognitive function [29]. As with all medications careful clinical evaluation of side effects should be performed. Our investigation entailed a lipophilic statin. It would seem ideal that subsequent studies compare lipophilic and hydrophilic statins to determine whether the adverse effects on behavior and hippocampal biochemistry are lessened by administration of the latter.
Highlights.
We present a mouse model of a 7 months treatment with atorvastatin
Atorvastatin treatment resulted in behavioral and cognitive alterations
Syntaxin-1α and synaptophysin were changed in hippocampal buoyant fractions
Suggestion for mechanism of statin-associated cognitive impairment
Acknowledgments
This work was supported by: American Heart Association BGIA 2260359 (AEZ-H), Veteran Affairs Merit Award from the Department of Veterans Affairs BX001225 (BPH), and National Institutes of Health, Bethesda, MD, U.S.A., NS073653 (BPH).
We would like to acknowledge April Voong, Sarah E. Kellerhals, and Igor Guryev for their technical assistance during the behavioral studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liscum L, Cummings RD, Anderson RG, DeMartino GN, Goldstein JL, Brown MS. 3-Hydroxy-3-methylglutaryl-CoA reductase: a transmembrane glycoprotein of the endoplasmic reticulum with N-linked “high-mannose” oligosaccharides. Proc Natl Acad Sci U S A. 1983;80:7165–9. doi: 10.1073/pnas.80.23.7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LaRosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease: a meta-analysis of randomized controlled trials. JAMA. 1999;282:2340–6. doi: 10.1001/jama.282.24.2340. [DOI] [PubMed] [Google Scholar]
- 3.Taylor F, Ward K, Moore TH, Burke M, Davey Smith G, Casas J-P, Ebrahim S. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2011;1:CD004816. doi: 10.1002/14651858.CD004816.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maningat P, Breslow JL. Needed: pragmatic clinical trials for statin-intolerant patients. N Engl J Med. 2011;365:2250–1. doi: 10.1056/NEJMp1112023. [DOI] [PubMed] [Google Scholar]
- 5.Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356:1627–31. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- 6.Rockwood K, Kirkland S, Hogan DB, MacKnight C, Merry H, Verreault R, et al. Use of lipid-lowering agents, indication bias, and the risk of dementia in community-dwelling elderly people. Arch Neurol. 2002;59:223–7. doi: 10.1001/archneur.59.2.223. [DOI] [PubMed] [Google Scholar]
- 7.Swiger KJ, Manalac RJ, Blumenthal RS, Blaha MJ, Martin SS. Statins and cognition: a systematic review and meta-analysis of short- and long-term cognitive effects. Mayo Clin Proc. 2013;88:1213–21. doi: 10.1016/j.mayocp.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Muldoon MF, Ryan CM, Sereika SM, Flory JD, Manuck SB. Randomized trial of the effects of simvastatin on cognitive functioning in hypercholesterolemic adults. Am J Med. 2004;117:823–9. doi: 10.1016/j.amjmed.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 9.Wagstaff LR, Mitton MW, Arvik BM, Doraiswamy PM. Statin-associated memory loss: analysis of 60 case reports and review of the literature. Pharmacotherapy. 2003;23:871–80. doi: 10.1592/phco.23.7.871.32720. [DOI] [PubMed] [Google Scholar]
- 10.Evans MA, Golomb BA. Statin-associated adverse cognitive effects: survey results from 171 patients. Pharmacotherapy. 2009;29:800–11. doi: 10.1592/phco.29.7.800. [DOI] [PubMed] [Google Scholar]
- 11.Feldman HH, Doody RS, Kivipelto M, Sparks DL, Waters DD, Jones RW, et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology. 2010;74:956–64. doi: 10.1212/WNL.0b013e3181d6476a. [DOI] [PubMed] [Google Scholar]
- 12.Padala KP, Padala PR, McNeilly DP, Geske JA, Sullivan DH, Potter JF. The Effect of HMG: CoA Reductase Inhibitors on Cognition in Patients With Alzheimer’s Dementia: A Prospective Withdrawal and Rechallenge Pilot Study. Am J Geriatr Pharmacother. 2012 doi: 10.1016/j.amjopharm.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Food, Drug Administration. Drug Administration. no date. FDA expands advice on statins risk [actualized 29 Feb 2012] [Google Scholar]
- 14.Bulloj A, Leal MC, Surace EI, Zhang X, Xu H, Ledesma MD, et al. Detergent resistant membrane-associated IDE in brain tissue and cultured cells: Relevance to Abeta and insulin degradation. Mol Neurodegener. 2008;3:22. doi: 10.1186/1750-1326-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King DS, Wilburn AJ, Wofford MR, Harrell TK, Lindley BJ, Jones DW. Cognitive impairment associated with atorvastatin and simvastatin. Pharmacotherapy. 2003;23:1663–7. doi: 10.1592/phco.23.15.1663.31953. [DOI] [PubMed] [Google Scholar]
- 16.Liao Y, Zhao H, Ogai A, Kato H, Asakura M, Kim J, et al. Atorvastatin slows the progression of cardiac remodeling in mice with pressure overload and inhibits epidermal growth factor receptor activation. Hypertens Res. 2008;31:335–44. doi: 10.1291/hypres.31.335. [DOI] [PubMed] [Google Scholar]
- 17.Risbrough VB, Geyer MA, Hauger RL, Coste S, Stenzel-Poore M, Wurst W, Holsboer F. CRF1 and CRF2 receptors are required for potentiated startle to contextual but not discrete cues. Neuropsychopharmacology. 2009;34:1494–503. doi: 10.1038/npp.2008.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Head BP, Peart JN, Panneerselvam M, Yokoyama T, Pearn ML, Niesman IR, et al. Loss of caveolin-1 accelerates neurodegeneration and aging. PLoS One. 2010;5:e15697. doi: 10.1371/journal.pone.0015697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linetti A, Fratangeli A, Taverna E, Valnegri P, Francolini M, Cappello V, et al. Cholesterol reduction impairs exocytosis of synaptic vesicles. J Cell Sci. 2010;123:595–605. doi: 10.1242/jcs.060681. [DOI] [PubMed] [Google Scholar]
- 20.Sebastião AM, Colino-Oliveira M, Assaife-Lopes N, Dias RB, Ribeiro JA. Lipid rafts, synaptic transmission and plasticity: impact in age-related neurodegenerative diseases. Neuropharmacology. 2013;64:97–107. doi: 10.1016/j.neuropharm.2012.06.053. [DOI] [PubMed] [Google Scholar]
- 21.Kwon SE, Chapman ER. Synaptophysin regulates the kinetics of synaptic vesicle endocytosis in central neurons. Neuron. 2011;70:847–54. doi: 10.1016/j.neuron.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biondi E. Prescription of lipophilic statins to Alzheimer’s disease patients: some controversies to consider. Neurol Sci. 2011;32:195–201. doi: 10.1007/s10072-010-0440-0. [DOI] [PubMed] [Google Scholar]
- 23.Botti RE, Triscari J, Pan HY, Zayat J. Concentrations of pravastatin and lovastatin in cerebrospinal fluid in healthy subjects. Clin Neuropharmacol. 1991;14:256–61. doi: 10.1097/00002826-199106000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Andersson M, Elmberger PG, Edlund C, Kristensson K, Dallner G. Rates of cholesterol, ubiquinone, dolichol and dolichyl-P biosynthesis in rat brain slices. FEBS Lett. 1990;269:15–8. doi: 10.1016/0014-5793(90)81107-y. [DOI] [PubMed] [Google Scholar]
- 25.Galatti L, Polimeni G, Salvo F, Romani M, Sessa A, Spina E. Short-term memory loss associated with rosuvastatin. Pharmacotherapy. 2006;26:1190–2. doi: 10.1592/phco.26.8.1190. [DOI] [PubMed] [Google Scholar]
- 26.Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 [Google Scholar]
- 27.Stone NJ, Robinson J, Lichtenstein AH, Merz CNB, Blum CB, Eckel RH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 [Google Scholar]
- 28.Schmitt U, Tanimoto N, Seeliger M, Schaeffel F, Leube RE. Detection of behavioral alterations and learning deficits in mice lacking synaptophysin. Neuroscience. 2009;162:234–43. doi: 10.1016/j.neuroscience.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 29.Kelley BJ, Glasser S. Cognitive Effects of Statin Medications. CNS Drugs. 2014 doi: 10.1007/s40263-014-0147-5. [DOI] [PubMed] [Google Scholar]