Abstract
Our understanding of kisspeptin and its actions depends, in part, on a detailed knowledge of the neuroanatomy of the kisspeptin signaling system in the brain. In this chapter, we will review our current knowledge of the distribution of kisspeptin cells, fibers, and receptors in the mammalian brain, including the development, phenotype, and projections of different kisspeptin sub-populations. A fairly consistent picture emerges from this analysis. There are two major groups of kisspeptin cell bodies: a large number in the arcuate nucleus (ARC) and a smaller collection in the rostral periventricular area of the third ventricle (RP3V) of rodents and preoptic area (POA) of non-rodents. Both sets of neurons project to GnRH cell bodies, which contain Kiss1r, and the ARC kisspeptin population also projects to GnRH axons in the median eminence. ARC kisspeptin neurons contain neurokinin B and dynorphin, while a variable percentage of those in the RP3Vof rodents contain galanin and/or dopamine. Neurokinin B and dynorphin have been postulated to contribute to control of GnRH pulses and steroid negative feedback, while the role of galanin and dopamine in rostral kisspeptin neurons is not entirely clear. Kisspeptin neurons, fibers, and Kiss1r are found in other areas, including widespread areas outside the hypothalamus, but their physiological role(s) in these regions remains to be determined.
Keywords: kisspeptin cells, kisspeptin fibers, Kiss1r, KNDy, kisspeptin efferents, arcuate nucleus, RP3V, preoptic area, puberty
3.1 Introduction
A clear understanding of the anatomy of the kisspeptin system is a necessary foundation for current and future work on the physiology and pathology of kisspeptin. Indeed, the early recognition of two distinct subpopulations of kisspeptin cell bodies in rodents was critical to the development of current models for the role of kisspeptin in mediating the feedback actions of steroids (1). As the physiological functions of kisspeptin expand, knowledge of the phenotype, and of the afferent and efferent connections, of different kisspeptin cell populations becomes even more critical. Thus the primary purpose of this chapter is to review our current understanding of this neural circuitry. The neuroanatomy of this system has been reviewed in the last few years (2–4) so this material should be considered an update of these reviews, with an emphasis on comparative aspects of kisspeptin neuroanatomy in mammals. It is important to recognize that there is also considerable information on this subject in non-mammalians species, particularly in fish, but this is beyond the scope of our chapter. Interested readers are referred to recent reviews of this topic (5;6).
This review will focus on distribution of kisspeptin cell bodies, fibers, and kisspeptin receptors with some discussion of the overlap of the latter two. We will also consider co-localization of neuropeptides, steroid receptors and other neurotransmitters in different kisspeptin subpopulations and evidence on where these subpopulations project. Finally, we think it is important to consider developmental neuroanatomy and sexual dimorphism of the kisspeptin system. Although this will be somewhat redundant with other chapters in this book, this information is required for a full understanding of the current neuroanatomical literature on kisspeptin expression in mammals.
3.2 Distribution of Kisspeptin and Kiss1 in the adult brain
Since the discovery of its central role in reproduction in 2003, there have been a number of studies documenting the localization of kisspeptin in the brain using either immunocytochemistry (ICC) for cells and fibers, or in situ hybridization (ISH) for cells. The original studies using ICC to identify kisspeptin-positive cells and fibers were confounded by cross-reactivity of the antibodies with related peptide members of the RFamide family (7), but more recently a number of antibodies have been generated which have been shown by the use of careful positive and negative controls to be specific to kisspeptin (8–10). Using these specific antibodies, and cDNA and RNA probes against kisspeptin sequences, the distribution of kisspeptin cells and fibers has now been mapped out in a variety of mammalian species. Not surprisingly, much of this work has been done in mice (8;11–17) and rats (14;18–25), but some data is also available in other rodents (hamster (26–29) and guinea pig (30)). Ruminants, particularly sheep (9;10;31–35) and goats (36–38), have also been studied extensively, while there is less information on expression in monkeys (39–43) and humans (41;44) (Table 3.1; Table 3.2).
Table 3.1.
Location of Kisspeptin/Kiss1 Cells in the Adult Brain
| Species | ARC | Preoptic Region POA RP3V | ME/Inf. stalk | DMH | VMH | Medial Amygdalaa | BNST | Other regions | ISH Reference | ICC Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Human | +++ | + | + | (1) | (2) | ||||||
| Rhesus Monkey | +++ | ++ | + | + | (1,3,4) | (5,6) | |||||
| Mouse | ++ | + | ++ | + | + | + | +b | (7–12) | (13,14) | ||
| Rat | +++ | ++ | + | + | + | +c | (12, 15–20) | (19–22) | |||
| Hamster | ++ | ++ | (23, 24) | (25, 26) | |||||||
| Guinea pig | +++ | + | + | +d | (27) | (27) | |||||
| Sheep | +++ | ++ | + | + | (28–31) | (30–34) | |||||
| Goat | +++ | + | (35, 36) (37) | ||||||||
| Horse | +++ | + | + | (38, 39) | |||||||
numbers based on diestrous females, ++ in proestrous females and males
neocortex, insular cortex, piriform cortex, lateral septum, nucl. solitary tract (based on (40), Kiss1 gene-driven Cre activity)
anterior parvocellular portion of the PVN
intermediate and caudal periventricular area
Rometo AM, Krajewski SJ, Lou Voytko M, Rance NE. Hypertrophy and Increased Kisspeptin Gene Expression in the Hypothalamic Infundibular Nucleus of Postmenopausal Women and Ovariectomized Monkeys. J Clin Endocrinol Metab. 2007 July 1, 2007;92(7):2744–50.
Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, et al. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. European Journal of Neuroscience. 2010;31(11):1984–98.
Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proceedings of the National Academy of Sciences of the United States of America. 2005 February 8, 2005;102(6):2129–34.
Smith JT, Shahab M, Pereira A, Pau KYF, Clarke IJ. Hypothalamic Expression of KISS1 and Gonadotropin Inhibitory Hormone Genes During the Menstrual Cycle of a Non-Human Primate. Biology of Reproduction. 2010 June 23, 2010:10.1095/biolreprod.110.085407.
Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural Interactions between Kisspeptin and GnRH Neurons in the Mediobasal Hypothalamus of the Male Rhesus Monkey (Macaca mulatta) as Revealed by Double Immunofluorescence and Confocal Microscopy. Endocrinology. 2008 September 1, 2008;149(9):4387–95.
Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B Stimulates GnRH Release in the Male Monkey (Macaca mulatta) and Is Colocalized with Kisspeptin in the Arcuate Nucleus. Endocrinology. 2010 June 23, 2010:en.2010–0223.
Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, et al. A Role for Kisspeptins in the Regulation of Gonadotropin Secretion in the Mouse. Endocrinology. 2004 September 1, 2004;145(9):4073–7.
Han S-K, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, et al. Activation of Gonadotropin-Releasing Hormone Neurons by Kisspeptin as a Neuroendocrine Switch for the Onset of Puberty. J Neurosci. 2005 December 7, 2005;25(49):11349–56.
Kauffman AS, Kim, J., Clifton, D.K., Steiner, R.A. Regulation of Kiss1 gene expression by sex steroids in the medial amygdala of mice. Society for Neuroscience 37th Annual Meeting, San Diego, CA. 2007:Society for Neuroscience, 2007.
Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 Gene Expression in the Brain of the Female Mouse. Endocrinology. 2005 September 1, 2005;146(9):3686–92.
Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, et al. Differential Regulation of KiSS-1 mRNA Expression by Sex Steroids in the Brain of the Male Mouse. Endocrinology. 2005 July 1, 2005;146(7):2976–84.
Kim J, Semaan SJ, Clifton DK, Steiner RA, Dhamija S, Kauffman AS. Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology. [Research Support, N.I.H., Extramural Research Support, U.S. Gov't, Non-P.H.S.]. 2011 May;152(5):2020–30.
Clarkson J, Herbison AE. Postnatal Development of Kisspeptin Neurons in Mouse Hypothalamus; Sexual Dimorphism and Projections to Gonadotropin-Releasing Hormone Neurons. Endocrinology. 2006 December 1, 2006;147(12):5817–25.
Clarkson J, Tassigny XdAd, Colledge WH, Caraty A, Herbison AE. Distribution of Kisspeptin Neurones in the Adult Female Mouse Brain. Journal of Neuroendocrinology. 2009;21(8):673–82.
Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, et al. Involvement of Anteroventricular Periventricular Matastin/Kisspeptin Neurons in Estrogen Positive Feedback Action on Leutentizing Hormone Release in Female Rats. Journal of Reproduction and Development. 2007;53(2):367–78.
Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, et al. Kisspeptin Activation of Gonadotropin Releasing Hormone Neurons and Regulation of KiSS-1 mRNA in the Male Rat. Neuroendocrinology. 2004;80(4):264–72.
Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, et al. Sexual Differentiation of Kiss1 Gene Expression in the Brain of the Rat. Endocrinology. 2007 April 1, 2007;148(4):1774–83.
Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 Neurons in the Forebrain as Central Processors for Generating the Preovulatory Luteinizing Hormone Surge. J Neurosci. 2006 June 21, 2006;26(25):6687–94.
Takase K, Uenoyama, Y., Inoue, N., Matsui, H., Yamada, S., Shimizu, M., Homma, T., Tomikawa, J., Kanda, S., Matsumoto, H., Oka, Y., Tsukamura, H., Maeda, K.I. Possible Role of Oestrogen in Pubertal Increase of Kiss1/Kisspeptin Expression in Discrete Hypothalamic Areas of Female Rats. Journal of Neuroendocrinology. 2009;21(6):527–37.
Xu Z, Kaga S, Mochiduki A, Tsubomizu J, Adachi S, Sakai T, et al. Immunocytochemical localization of kisspeptin neurons in the rat forebrain with special reference to sexual dimorphism and interaction with GnRH neurons. Endocr J. 2012 Feb 29;59(2):161–71.
Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, et al. Involvement of Central Metastin in the Regulation of Preovulatory Luteinizing Hormone Surge and Estrous Cyclicity in Female Rats. Endocrinology. 2005 October 1, 2005;146(10):4431–6.
Desroziers E, Mikkelsen J, Simonneaux V, Keller M, Tillet Y, Caraty A, et al. Mapping of kisspeptin fibres in the brain of the pro-oestrus rat. Journal of Neuroendocrinology. 2010:Accepted Article; 10.1111/j.365-2826.010.02053.x.
Ansel L, Bolborea M, Bentsen AH, Klosen P, Mikkelsen JD, Simonneaux V. Differential Regulation of Kiss1 Expression by Melatonin and Gonadal Hormones in Male and Female Syrian Hamsters. J Biol Rhythms. 2010 April 1, 2010;25(2):81–91.
Revel FG, Saboureau M, Masson-Pévet M, Pévet P, Mikkelsen Jens D, Simonneaux V. Kisspeptin Mediates the Photoperiodic Control of Reproduction in Hamsters. Current Biology. 2006;16(17):1730–5.
Greives TJ, Mason AO, Scotti M-AL, Levine J, Ketterson ED, Kriegsfeld LJ, et al. Environmental Control of Kisspeptin: Implications for Seasonal Reproduction. Endocrinology. 2007 March 1, 2007;148(3):1158–66.
Mason AO, Greives TJ, Scotti M-AL, Levine J, Frommeyer S, Ketterson ED, et al. Suppression of kisspeptin expression and gonadotropic axis sensitivity following exposure to inhibitory day lengths in female Siberian hamsters. Hormones and Behavior. 2007;52(4):492–8.
Bosch MA, Xue C, Ronnekleiv OK. Kisspeptin expression in guinea pig hypothalamus: effects of 17beta-estradiol. J Comp Neurol. [Research Support, N.I.H., Extramural]. 2012 Jul 1;520(10):2143–62.
Estrada KM, Clay, C.M., Pompolo, S., Smith, J.T., Clarke, I.J. Elevated KiSS-1 Expression in the Arcuate Nucleus Prior to the Cyclic Preovulatory Gonadotrophin-Releasing Hormone/Lutenising Hormone Surge in the Ewe Suggests a Stimulatory Role for Kisspeptin in Oestrogen-Positive Feedback. Journal of Neuroendocrinology. 2006;18(10):806–9.
Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 Messenger Ribonucleic Acid Expression in the Hypothalamus of the Ewe Is Regulated by Sex Steroids and Season. Endocrinology. 2007 March 1, 2007;148(3):1150–7.
Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, et al. Variation in Kisspeptin and RFamide-Related Peptide (RFRP) Expression and Terminal Connections to Gonadotropin-Releasing Hormone Neurons in the Brain: A Novel Medium for Seasonal Breeding in the Sheep. Endocrinology. 2008 November 1, 2008;149(11):5770–82.
Smith JT, Li Q, Pereira A, Clarke IJ. Kisspeptin Neurons in the Ovine Arcuate Nucleus and Preoptic Area Are Involved in the Preovulatory Luteinizing Hormone Surge. Endocrinology. 2009 December 1, 2009;150(12):5530–8.
Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neuroscience Letters. 2006;401(3):225–30.
Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CVR, Jafarzadehshirazi MR, et al. Kisspeptin Neurons in the Arcuate Nucleus of the Ewe Express Both Dynorphin A and Neurokinin B. Endocrinology. 2007 December 1, 2007;148(12):5752–60.
Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The Kisspeptin/Neurokinin B/Dynorphin (KNDy) Cell Population of the Arcuate Nucleus: Sex Differences and Effects of Prenatal Testosterone in Sheep. Endocrinology. 2010 January 1, 2010;151(1):301–11.
Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, et al. Gonadotrophin-Releasing Hormone Pulse Generator Activity in the Hypothalamus of the Goat. Journal of Neuroendocrinology. 2009;21(10):813–21.
Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, et al. Neurokinin B and Dynorphin A in Kisspeptin Neurons of the Arcuate Nucleus Participate in Generation of Periodic Oscillation of Neural Activity Driving Pulsatile Gonadotropin-Releasing Hormone Secretion in the Goat. J Neurosci. 2010 February 24, 2010;30(8):3124–32.
Matsuyama S, Ohkura S, Mogi K, Wakabayashi Y, Mori Y, Tsukamura H, et al. Morphological evidence for direct interaction between kisspeptin and gonadotropin-releasing hormone neurons at the median eminence of the male goat: an immunoelectron microscopic study. Neuroendocrinology. [Research Support, Non-U.S. Gov't]. 2011;94(4):323–32.
Decourt C, Tillet Y, Caraty A, Franceschini I, Briant C. Kisspeptin immunoreactive neurons in the equine hypothalamus: Interactions with GnRH neuronal system. Journal of Chemical Neuroanatomy. 2008;36(3–4):131–7.
Magee C, Foradori CD, Bruemmer JE, Arreguin-Arevalo JA, McCue PM, Handa RJ, et al. Biological and Anatomical Evidence for Kisspeptin Regulation of the Hypothalamic-Pituitary-Gonadal Axis of Estrous Horse Mares. Endocrinology. 2009 June 1, 2009;150(6):2813–21.
Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J, Jr., Atkin S, Bookout AL, et al. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. [Research Support, N.I.H., Extramural Research Support, U.S. Gov't, Non-P.H.S.]. 2011 Jan 26;173:37–56.
+++, many (50–150); ++, moderate (15–50); +, few (<15 or numbers not reported)
Table 3.2.
Distribution of Kisspeptin Fibers in the Adult Brain (identified KNDy projections, based on dual immunostaining for peptides and/or tract tracing, in yellow)
| Species | ARC | Preoptic region POA/RP3V |
ME Inter Exter |
PVN | VMM | DMH | LHA | SON | BNST | OVLT | Medial Septum |
Lateral Septum |
DBB | Other areas |
Referencesd | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human | ++ | ++ | ++ | ++ | + | + | + | + | + | + | 45 | ||||||
| Rhesus Monkey | ++ | + | ++ | + | 40,41 | ||||||||||||
| Mouse | ++ | + | + | ++ | + | + | + | + | + | + | + | + | +a | 8,11,17,107 | |||
| Rat | ++ | + | + | ++ | + | ++ | + | + | + | + | + | + | + | + | +b | 18,19,22,24,108,110 | |
| Hamster | ++ | + | 28 | ||||||||||||||
| Guinea pig | ++ | + | + | ++ | ++ | + | + | + | + | + | + | + | +c | 30 | |||
| Sheep | ++ | + | ++ | + | + | + | + | 9,10,35,36 | |||||||||
| Goat | ++ | + | ++ | + | 37 – 39 | ||||||||||||
| Horse | ++ | + | ++ | 48 | |||||||||||||
Other regions include: lateral preoptic area, contralateral ARC, posterior hypothalamic area, medial amygdala, paraventricular thalamic nucleus, periaqueductal gray, locus coeruleus.
Other regions include: septohypothalamic area, contralateral ARC, retrochiasmatic area, suprachiasmatic nucleus; medial tuberal nucleus; posterior hypothalamus.
Other regions include: ventral premammillary nucleus
Reference numbers are from citations at end of chapter
++, dense fibers; +, moderate or few fibers
Subsection 3.2.1 Kisspeptin cell bodies
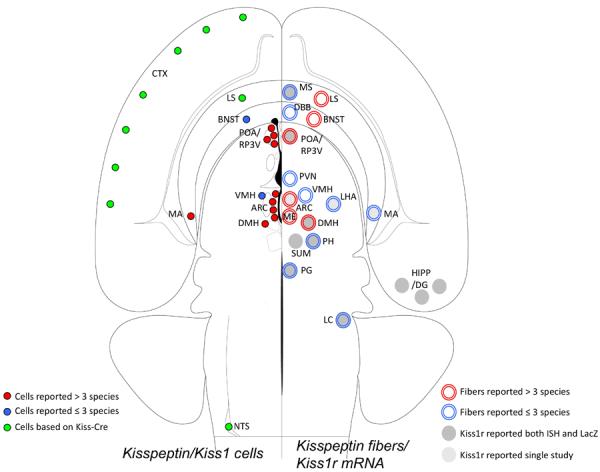
Across the species examined, there are two major populations of kisspeptin cells that have been identified in the diencephalon: a group in the arcuate nucleus (infundibular nucleus in humans) and the other in the preoptic region. The arcuate (ARC) population is the largest group of kisspeptin cells seen in the mammalian hypothalamus (2). In rodents, kisspeptin cells in this group are present at all rostral-caudal levels (12;15), while in monkeys and sheep, they are located mostly at middle and caudal levels (10;39). In addition to the ARC, a second prominent diencephalic group of kisspeptin cells is seen in the preoptic region. In rodents, the latter group is located in the rostral preoptic area of the third ventricle (RP3V), and consists of kisspeptin cells clustered in the anteroventral periventricular nucleus (AVPV) that extend caudally into the adjacent periventricular preoptic zone (PeN). This distribution in rodents is based largely on studies in females, since males have few if any kisspeptin cells in this region (see Section 3.5.2). In contrast to female rodents, female primates and ruminants (primates and ruminants) appear to lack a well-defined RP3V population, and instead kisspeptin cells are scattered slightly more laterally within the medial preoptic region. It seems likely that kisspeptin cells in the RP3V of rodents, and those in the preoptic region in sheep, goats and primates, are homologous, but the precise functional roles of each of the populations may differ between species (45). The only species in which a distinct preoptic population has yet to be demonstrated is the horse, despite the use of specific antibodies (46;47). Since these rostral kisspeptin populations have been implicated in the estrogen-induced preovulatory LH surge in many species (45), the absence of them in the horse correlates with evidence that the preovulatory LH increase in mares is due to withdrawal of steroid negative feedback, rather than the stimulatory actions of estradiol (48).
Identification of precise cell numbers in these populations is somewhat complicated by the fact that kisspeptin mRNA and peptide expression in the preoptic region and ARC is under opposite regulatory control by gonadal steroid hormones. Thus, estradiol in females in general stimulates kisspeptin expression in the RP3V and POA, while inhibiting it in the ARC (45). Nonetheless, comparison of cell numbers in the female brain under optimal hormonal conditions (estradiol treatment in the case of the preoptic population, and ovariectomy in the case of the ARC) suggests that the absolute number of kisspeptin cells in the ARC is generally two to four-fold greater than that in the RP3V or POA (2). Thus, the ARC kisspeptin cell population is most consistent among mammals in its presence and contains the greatest number of cells.
Another complication that raises both technical and interesting biological issues is the effect of endocrine status on location of kisspeptin-ir within cells. Specifically, estradiol (E2) may alter the location of kisspeptin within ARC neurons. Thus, in rats (18) and mice (49;50), intact and E2-treated OVX animals show a dense network of fibers with few, if any, visible cell bodies in the ARC; in contrast, tissue from OVX females contain kisspeptin-ir cell bodies, but few fibers. A similar effect of E2 has recently been reported in guinea pigs (30). It has been argued that the kisspeptin-ir fibers in the ARC arise from the AVPV because E2 stimulates kisspeptin expression in that area, but not the ARC (51). However, data using dual-ICC to identify efferents from ARC kisspeptin cells in rats, sheep, and monkeys indicate that many of the kisspeptin fibers in the ARC arise from cell bodies in this area (see below). Moreover, the dissociation of effects of E2 on kisspeptin expression in AVPV cell bodies and ARC fibers of adult hpg mice is not consistent with these fibers arising from the AVPV in this species (50). If these kisspeptin fibers do originate from ARC cell bodies, then the increase in immunoreactivity could be due to either: 1) increased, or no change, in transport out of the cells coupled with a decrease in kisspeptin synthesis, or 2) simply a build-up of kisspeptin protein because secretion has been inhibited more than synthesis and transport. Interestingly, this phenomenon is not seen in male rats (18) and is seen two days after colchicine administration in females (18) so it must reflect a chronic shift in location of kisspeptin proteins. Regardless of the mechanisms, the potential of changes in distribution of kisspeptin within a neuron argues for caution in interpreting changes based on ICC analysis.
In addition to the ARC and preoptic region there are a number of other hypothalamic areas that have been shown to contain populations of kisspeptin cells in the mammalian brain (Table 3.1). In the monkey and human, a small number of kisspeptin cells, likely an extension of the ARC group, are seen in the median eminence (monkey, (39)) and infundibular stalk (human, (44)). The dorsomedial nucleus of the hypothalamus (DMH) contains a small group of kisspeptin cells, demonstrated by ICC using specific antibodies, in mice, guinea pigs, sheep and horses, but these cells are not present in the rat or hamster (Table 3.1). Likewise, there is species variation in the presence of cells in the ventromedial hypothalamic nucleus (VMH), with cells being detected in the sheep (in some reports (32) but not others (33)) and rats (24), but not in other species; as in the case of kisspeptin cells in the median eminence (39), these cells may be an extension of the ARC population, in this case laterally. Regardless, evidence of kisspeptin cells in the DMH and VMH in a number of species rests primarily on ICC data, and additional ISH data would be worthwhile to verify their presence in these species.
There are also a number of kisspeptin populations that reside outside of the classical boundaries of the hypothalamus. These include a cluster of Kiss1 mRNA-expressing cells in the medial nucleus of the amygdala, seen in both rats and mice, and a small number of Kiss1 cells in the bed nucleus of the stria terminalis (BNST) of mice, rats and rhesus monkeys (Table 3.1). Like the RP3V population, Kiss1 expression in the medial amygdala is under the stimulatory influence of gonadal steroid hormones (14). The localization of these populations in circuitry that mediates pheromonal control of sexual behavior (52;53) and neuroendocrine function (54) suggests that they may play a role in these functions, but this remains to be explored.
Finally, recent findings suggest that kisspeptin cells may be present in widespread areas of the brain outside the hypothalamus and limbic system. This evidence is based on observations of transgenic mice in which Kiss1 drives the expression of Cre recombinase and other reporter genes (55). Using such mice, Cre expression has been detected in widespread cortical areas including layers 5 and 6 of neocortex, insular cortex, and piriform cortex, as well as in the lateral septum, and in the nucleus of the solitary tract in the brainstem. . A potential caveat to these observations is the possibility that Cre reporter expression in some of these regions represents kisspeptin that is transiently expressed during development but not during adulthood (56). However, at least in the neocortex, Kiss1-driven Cre activity is confirmed by the presence of light Kiss1 mRNA-labeling by ISH, although peptide is not detectable in these cells perhaps because of the low level of mRNA expression. The function(s) of kisspeptin in cortex and these other regions is at present a mystery but seems likely to portend functions of this peptide that extend far beyond its recognized roles in reproduction and neuroendocrine function.
Subsection 3.2.2 Kisspeptin fibers
The overall pattern and distribution of kisspeptin fibers has been analyzed in a number of ICC studies (Table 3.2), representing the same range of species in which cell bodies have been studied. In addition, in the case of the RP3V and ARC populations, the specific projections of each has been analyzed using either tract tracing combined with immunocytochemistry, or multiple-label immunocytochemistry, and these findings are summarized in Section 3.4. In all species examined to date, the densest accumulation of kisspeptin fibers and terminals appear to be within the regions that contain the two major hypothalamic populations, the ARC and RP3V, as well as within the internal zone of the median eminence (Table 3.2). Kisspeptin fibers, albeit fewer in number, have also been reported in the external zone of the median eminence in most species, the site of neurosecretory release of GnRH. As noted previously (2), the paucity and/or lack (e.g., mice) of kisspeptin fibers in the external zone, suggests that if kisspeptin is to effect release of GnRH at the level of the median eminence (57) it likely does so via diffusion and/or volume transmission from the internal to external layers.
Thus far, the species where kisspeptin fibers have been thoroughly mapped outside of the ARC, RP3V and median eminence include human, mouse, rat, guinea pig and sheep, and in general the studies have revealed a fairly consistent pattern with a majority of kisspeptin fibers being located predominantly within medially-located hypothalamic nuclei and preoptic regions (Table 3.2). For example, in each of these species, kisspeptin-immunoreactive fibers are found in the DMH; in addition, in all species except humans, kisspeptin fibers are seen in the BNST. There are also some species differences: for example, the PVN contains moderate to low numbers of kisspeptin fibers in humans, mice and rats, but not in guinea pigs or sheep; by contrast, the VMH which contains fibers in humans, guinea pigs and sheep is devoid of such fibers in rats and mice. Since immunoreactive fibers can represent either terminal boutons or axons of passage, these differences may reflect variation in postsynaptic targets of kisspeptin cells or simply in the route which fibers take to reach those targets.
In addition to medially located nuclei, in the mouse, rat and human hypothalamus, a few kisspeptin fibers are also seen consistently in the lateral preoptic and lateral hypothalamic areas (Table 3.2). Rostrally, kisspeptin-immunoreactive fibers in these species are present in a variety of forebrain structures, including the medial and lateral septum, the diagonal band of Broca, and organum vasculosum of the lamina terminalis (OVLT). Finally, in mice and rats, kisspeptin fibers have been reported in the caudal hypothalamus, and in mice also in more distant locations: the periaqueductal gray of the midbrain, the locus coeruleus, the paraventricular nucleus of the thalamus, as well as the medial amygdala (Table 3.2). Whether kisspeptin fibers in these more widespread areas are seen in other species is not known. Likewise, the precise origin of these fibers remains to be determined; while some are likely to arise from the ARC or RP3V kisspeptin cells (see Section 3.4), it is possible that others originate from populations located outside of the hypothalamus (see above).
Subsection 3.2.3 Co-localization of other peptide/transmitters
There is increasing evidence of anatomical heterogeneity among kisspeptin cell populations, specifically with respect to their co-expression of other neuropeptides and neurotransmitters. The most consistent example of this to date is in the ARC population where a majority of kisspeptin neurons express two other neuropeptides, neurokinin B and dynorphin, each of which has been strongly implicated in the physiological control of GnRH secretion (58;59). The high degree of co-localization of kisspeptin, NKB and dynorphin in the ARC population (Table 3.3A) and its conservation across species (Fig. 3.1) has led to the acronym of “KNDy” neurons (60). KNDy cells form reciprocal connections with each other, as well as project to GnRH neurons, and the combination of excitatory (kisspeptin, NKB) and inhibitory (dynorphin) actions within this circuitry has provided the foundation for models of how this kisspeptin population is involved in the generation and control of GnRH pulses (38;60–62). Although the percentage of KNDy peptide colocalization is uniformly high among female mammals and male rodents and ruminants (Table 3.3A), it is slightly lower in male monkeys (40) and humans (44), even in the absence of gonadal steroids that inhibit kisspeptin expression in the ARC. In addition, perturbations in the organizational effects of gonadal steroids during development can lead to an altered ratio of KNDy peptides within the adult ARC population (31).
Table 3.3A.
Percentage of ARC Kisspeptin cells co-localizing other neuropeptides/transmitters
Fig. 1.
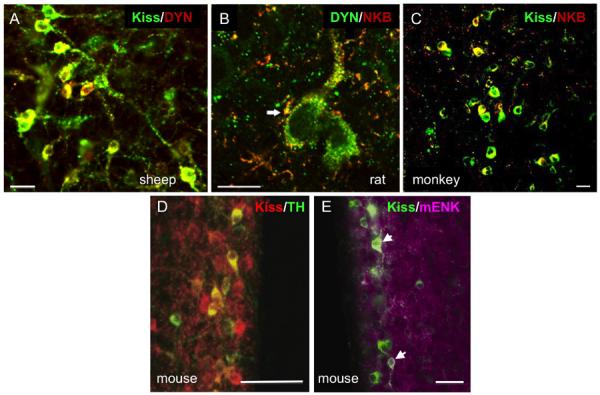
Preoptic kisspeptin cells in sheep and goats do not appear to colocalize either NKB or dynorphin, although a subset of RP3V neurons does appear to contain both peptides in the mouse (63) (Table 3.3B); other species have not as yet been examined for this possible colocalization. However, a sizeable percentage of RP3V cells in both the mouse (64;65) and rat (21) appear to colocalize tyrosine hydroxylase (TH) (Fig. 3.1D), the rate-limiting enzyme for dopamine biosynthesis (64), although the precise percentage in the rat appears to vary by steroidal status and by whether double-label ICC or ISH was used (Table 3.3B). By contrast, kisspeptin cells in the POA or ARC of the sheep do not contain TH (66) even though they are close to adjacent dopaminergic neurons in both regions. In addition, a sizable percentage of RP3V kisspeptin cells in the mouse co-localize met-enkephalin (Fig. 3.1E) and some colocalize galanin (67); KNDy neurons in the mouse also co-localize galanin but not met-enkephalin (Table 3.3A).
Table 3.3B.
Percentage of RP3V or POA Kisspeptin cells co-localizing other neuropeptides/transmitters
In addition to co-localization of other peptides, cells of both the ARC and RP3V kisspeptin populations contain the classical amino acid transmitters, glutamate and GABA (Tables 3.3A & B), as revealed by co-localization of the markers, vesicular glutamate transporter-2 (vGlut2) and gamma amino acid decarboxylase (GAD)-67, respectively. However, the ARC population is predominantly glutamatergic, whereas RP3V cells are mostly GABAergic (55). Preliminary observations in the sheep (68) suggest that this distinction between ARC and RP3V populations holds in other mammals as well. Finally, it should be noted that kisspeptin cells located in other regions (e.g., medial amygdala) have not yet been examined for possible co-expression of other peptides or transmitters. Given the likely functional heterogeneity of these anatomically distributed populations, it seems probable that their neurochemical phenotypes will be similarly diverse.
Subsection 3.2.4 Co-localization of steroid receptors
The key role that kisspeptin cells play in the steroid feedback control of GnRH neurons is underlined by the high degree of co-localization of nuclear receptors for gonadal sex steroids in these cells. Indeed, in all species examined, a majority of both ARC and RP3V/preoptic kisspeptin cells co-localize estrogen receptor (ER)-alpha (the isoform responsible for estradiol's feedback actions upon GnRH secretion), progesterone receptor (PR) and androgen receptor (AR) (2;15;69). In the ARC population of rats, mice and sheep, the percentages of co-localization of ER-alpha range from 70–99%, whereas in the RP3V and preoptic region they range from 50–99% (9;15;18;33;70). The percentage of both ARC and RP3V populations that co-localize the beta isoform of the estrogen receptor is considerably less, ranging from 11–25% in the ARC and 21–31% in the RP3V of rats and mice (15;23). Other kisspeptin populations have not been directly examined for steroid receptor co-localization, but given that sex steroids regulate Kiss1 expression in the medial amygdala in rats and mice (14), and the presence of ER and AR in this area (71), it seems likely that kisspeptin cells in the amygdala are also a direct target for gonadal steroids. Preliminary evidence suggests that other nuclear steroid receptors are also present in kisspeptin cells: approximately 50% of KNDy cells in the ovine ARC co-localize type II glucocorticoid receptors (72). Furthermore, there is recent evidence that other types of receptors for circulating hormones are present in ARC kisspeptin cells: receptors for prolactin (73) and insulin (74) have each been colocalized to a subset of KNDy neurons. Taken together, these findings point to a potential convergence of endogenous hormonal cues onto the ARC kisspeptin population, which may place them in a unique position to respond to multiple signals related to stress, nutrition, and the environment as well as reproductive endocrine status.
3.3 Distribution of Kiss1r
In contrast to the wealth of information on the neuroanatomical distribution of kisspeptin cells and fibers, there is very limited data available on the location of Kiss1r mRNA and no data on Kiss1r protein. The only data in humans is from early studies before the role of Kiss1r in reproductive neuroendocrinology was recognized, so they provide almost no information on hypothalamic expression of this receptor (75;76). Moreover, most of the studies since then in monkeys and rodents used RT-PCR of mRNA extracted from large tissues blocks or microdissected areas (Table 3.4). Consequently, these reports do not provide any information on the location of Kiss1r within these relatively large volumes of tissue. Quantitative comparisons between areas using this approach are also problematic because the ratio of Kiss1r mRNA to a housekeeping gene is partially dependent on the percentage of Kiss1r-containing cells within the block. Thus variations in the precision of microdissection contribute significantly to the values reported. There have been a number of studies using ISH, or related techniques, that can provide cellular resolution, but most of these have been focused on whether GnRH neurons contain Kiss1r and do not provide more general neuroanatomical information. Thus, detailed descriptions in this section rely largely on two studies. One of these used ISH in rats, but provided only a few low-power images (77). The other used transgenic mice in which IRES-LacZ cassettes had been inserted into the Kiss1r gene so that β-galactosidase could be identified with Xgal staining as a marker for Kiss1r-containing cells (78). Although this provides the only detailed description of Kiss1r expression in the murine brain, the results must be interpreted conservatively until confirmed with conventional ISH or ICC.
Table 3.4.
Distribution of Kiss1r mRNA based on RT-PCR, in situ hybridization using radioactive probes (ISH) or immunohistochemistry for β-galactosidase (β-gal)
| Species | MBH /ARC* |
Preoptic region POA/RP3V |
AHA | DMH | PH | LHA | Medial Septum |
Hippo /DG |
Medial Amgdala |
Locus Coer. |
GnRH neurons |
Other Regions |
eReferences | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human (PCR) | ++ | +++ | +++ | ++ | +a | 76,77 | ||||||||
| Rhesus Monkey (PCR) | + (M) ++(F) | ++(M) +(F) | 43,80,81 | |||||||||||
| Mouse (PCR) | ++ | ++ | ++ | +++ | + | 95% | +b | 86 | ||||||
| Mouse (β-gal) | − | ++ | − | − | − | ++ | − | ++ | +++ | − | + | 40–70% | +c | 79,90 |
| Rat (PCR) | + | ++ | ++ | + | 22,82–85 | |||||||||
| Rat (ISH) | +++ | ++ | ++ | +++ | ++ | +++ | P | +++ | +++ | +++ | 77% | +d | 20,78 | |
| Sheep (ISH) | P | P | + | 78–90% | 87 – 89 | |||||||||
PCR data is from MBH in humans and ARC in rodents and sheep
P: present but not quantified (from reports of Kiss1r in GnRH neurons)
Other regions include: Thalamus, substantial nigra, cingulated gyms;
Other regions include: cerebellum, striatum;.
Other regions include: supramammillary nucleus, anteroventralthalamus, hibenula; periaqueductal gray;
Other regions include: zona incerta, olfactory cortex, ventral premammillary, periaqueductal gray
Reference numbers are from citations at end of chapter
Subsection 3.3.1 Hypothalamic expression
Even with these caveats in mind, some clear general patterns emerge when Kiss1r expression in the hypothalamus is examined across species. In all species studied, there is clear Kiss1r expression in the POA (Table 3.4). In monkeys (42;79;80), rats (22;81–84) and mice (85) Kiss1r was identified using RT-PCR of tissue block extracts, and many of the latter studies used microdissected POA tissue (81;82;84;85). Moreover, this conclusion is supported by ISH data in sheep (35;86;87), rats (20), and mice (13;78;88) which also demonstrated co-localization of Kiss1r in GnRH neurons (Table 3.4). The second relatively consistent location of Kiss1r within the hypothalamus is in the ARC (Table 3.4). Kiss1r mRNA has been found in the ARC using both RT-PCR in rats (22;81–84) and mice (85), and ISH in sheep (86) and rats (77); it is also likely that the Kiss1r found in the MBH of monkeys (42;79;80) also reflects, in part, expression in the ARC. In contrast, no Kiss1r expression was found in mice using Xgal ICC (78), but this observation needs to be confirmed using other approaches since Kiss1r was readily detectable in micropunches of the murine ARC (85). There are two consistent reports of Kiss1r in the posterior hypothalamus of rats (77) and mice (78) and in the RP3V of rats (83;84), although it was not observed in the latter area in mice using Xgal ICC (78). Similarly, this receptor has been observed in the AHA, DMH, LHA, ventral premamillary nuclei, and zona incerta of rats (77), but not mice (78); whether this reflects species or technical differences awaits further work.
Subsection 3.3.2 Extra-hypothalamic expression
There is even less data on expression of Kiss1r in areas outside the hypothalamus, and the only species in which there is information on other neural areas are rats (77), mice (78), and humans (75;76). As noted above, the latter comes from two early reports using RT-PCR before the role of Kiss1r in reproduction had been discovered. The highest levels of Kiss1r are consistently found in the hippocampus, with relatively high expression also observed in the amygdala (humans and rats), periaqueductal gray (rats, mice), and locus coereulus (humans, rats). Relative high expression of Kiss1r was also reported in the supramammillary nuclei of mice, but data on this region is not available for other species. Similarly, there is evidence for Kiss1r expression in the primary olfactory cortex of rats, and the caudate nucleus of humans that is not available in other species. Finally, there are a number of reports that Kiss1r is found in the anterior pituitary of humans (75), baboons (89), sheep (90;91), pigs (92), and rats (93;94). One of these studies observed that co-localization in the rat pituitary was limited to gonadotropes using dual ICC (94), but there was no differential expression of Kiss1r in cellular fractions of ovine pituitary enriched for gonadotropes, compared to fractions enriched for somatotropes or lactotropes (91). The physiological role of Kiss1r in the pituitary remains unclear because effects of kisspeptin on LH secretion from pituitary cells in vitro are not consistently observed (95;96), and when kisspeptin stimulates LH secretion, its effects are generally modest compared to those of GnRH (91;93;97;98). Moreover, although kisspeptin was detected in hypophysial portal blood, the low concentrations and lack of correlation with LH concentrations in jugular samples led the authors to conclude that hypothalamic release of kisspeptin played no physiological role in regulation of LH in the ewe (91). It should be noted that kisspeptin mRNA and protein have been observed in the anterior pituitary in species ranging from rats (94) to primates (99), so Kiss1r could play a paracrine role in this tissue.
Subsection 3.3.3 Cellular expression
Not surprisingly most studies that have examined cellular Kiss1r localization have focused on GnRH neurons. Studies using dual ISH (Table 3.4) consistently report that a high percentage of GnRH cells from the POA contain Kiss1r; in female sheep this percentage ranged from 78 to 90% (35;86;87), in male rats it averaged 77% (20), and in adult male (13) and female (100) mice greater than 85% co-localization was observed. A slightly lower percentage ranging from 55% (88) to 68% (78) was observed in male mice using Xgal as a marker for Kiss1r and the lowest percentage of colocalization (3/8 cells) was obtained using single cell RT-PCR (101). The relative low level of expression in the latter study may have been due to the use of fetal cells (78). A high degree of expression of Kiss1r in GnRH neurons is also supported by the 90% of murine GnRH neurons that respond to kisspeptin in slice preparations (13), since this effect is independent of other neural input (13;102).
Only two other cell types have been examined for possible expression of Kiss1r. Based on dual ISH techniques, kisspeptin neurons in the POA and ARC of the sheep do not contain Kiss1r (86). On the other hand, ARC POMC neurons in mice most likely do contain Kiss1r because these cells consistently respond to direct application of kisspeptin in slice preparation, even when pharmacologically isolated from other neural inputs (85). It will be important, however to confirm this conclusion using anatomical approaches.
Subsection 3.3.4 Matches/mismatches of Kiss1r and kisspeptin fibers
Under most circumstances, Kiss1r are presumably only physiologically important if they are located in neurons innervated by kisspeptin-containing synapses. Thus the occurrence of receptor-ligand matches and mismatches in the kisspeptin system can provide potentially useful information about physiological significance. On the other hand, the presence of kisspeptin terminals in areas devoid of Kiss1r raises the possibility that kisspeptin exerts its effect in these areas via other, as yet unidentified, receptors. Alternatively, since kisspeptin is often colocalized with a number of other important neuropeptides (i.e., NKB and dynorphin) and transmitters (i.e., glutamate) (see section 3.2.3) such synapses may be providing input from other components within these terminals, as appears to be the case for kisspeptin input to KNDy cells (60). It should also be noted that because of the paucity of data on Kiss1r, only limited conclusions can be drawn on receptor-ligand matches/mismatches for the kisspeptin system at this time, and these are based largely on Kiss1r data in rats (77) and mice (78). For example, we cannot determine whether Kiss1r is matched with the kisspeptin-ir fibers that are observed in the median eminence of all species (Table 3.1), because there is no information on distribution of Kiss1r protein. In light of the expression of Kiss1r mRNA in GnRH cell bodies (see above), it is likely that the receptor protein is present in GnRH terminals in the median eminence, but this needs to be directly confirmed.
Within the hypothalamic-POA areas, the most obvious match between Kiss1r and kisspeptin-ir fibers is in the POA where both have been observed in mice, rats, sheep, and monkeys (Tables 3.1 & 3.2), which reflects, in part, kisspeptin innervation of GnRH neurons (Fig. 3.3). The second most consistent area of overlap is the ARC where kisspeptin fibers have been observed in all species and evidence for Kiss1r reported in mice, rats, and sheep, although data in mice are conflicting because Kiss1r was not observed in this area using Xgal ICC. The final hypothalamic areas with a consistent match are the medial/lateral septum and the posterior hypothalamus of rats and mice. For both the DMH and RP3V, matches have been observed in rats, while mice apparently have kisspeptin fibers, but not Kiss1r (based on Xgal ICC). Other areas of mismatch include the PVN and SON of rats and the LHA of mice which contains kisspeptin-ir but no detectable Kiss1r; conversely, the LHA of rats contain Kiss1r, but no corresponding fibers. At a cellular level, there is clear match of Kiss1r in GnRH cells and kisspeptin-positive synapses onto GnRH neurons in all species examined to date. However, since not all GnRH cells appear to be innervated by kisspeptin fibers (2), it is unclear whether this correspondence is seen at an individual cell by cell level. In contrast, a majority of ARC KNDy neurons are innervated by other KNDy neurons, but none of these contain Kiss1r, at least in the sheep.
Fig. 3.
Outside the hypothalamus, there is a major mismatch in the dentate gyrus and other areas of the hippocampus in which there is strong evidence for Kiss1r, in the absence of kisspeptin-positive fibers in both rats and mice. It should be noted, however, that there is evidence for Kiss1 mRNA (by PCR) in the dendate gyrus (103;104) so this possible mismatch should be further investigated. A similar mismatch is evident in the supramammillary nuclei of mice, but there are no data on this area in rats. Conversely, kisspeptin-ir fibers are present in the medial amygdala of mice (8), with no evidence for Kiss1r at this time. The clearest areas of matching localization are the periaquaductal gray and locus coeruleus of mice (8) ; these areas also contain Kiss1r in rats (77), but whether kisspeptin neurons project to these regions in this species remains to be determined.
Section 3.4 Anatomical connections of kisspeptin cells
Subsection 3.4.1 Efferent projections
To date, the projections of specific subsets of kisspeptin cells have been investigated using two approaches. The first takes advantage of the co-expression of other neuropeptides and transmitters that are co-expressed uniquely in one population: the most common example of this is the co-localization of KNDy peptides which can be used to define the projections arising from ARC kisspeptin cells (44;86;105–107). The second approach has been to combine stereotaxic injections of anterograde tract tracers (Fluoro-Gold, biotinylated dextran amine) with ICC for kisspeptin so that fibers dual-labelled for both the tracer and kisspeptin can be inferred to have arisen in the injected region (e.g., ARC) (108;109).
Dual-label immunostaining of kisspeptin fibers with either NKB and/or dynorphin has been the most common approach to selectively analyze the projections of the ARC kisspeptin population, and has been employed in sheep (60), rats (105), mice (106), and human tissue (44). In addition, dual-label ICC for NKB and dynorphin, which is unique to the KNDy population, has been used in rats (105), goats (36), and monkeys (40). The results suggest some common, shared projections of ARC kisspeptin cells across species, but some significant differences as well (Table 3.2). In all species examined, fibers arising from KNDy cells form the densest projections locally, within the ARC and to the internal zone of the median eminence. There are also consistent projections of KNDy fibers to the preoptic region, including the RP3V in rodents, although the density of these fibers is generally less than in the ARC. In the rat, sheep and goat, a few KNDy fibers also extend into the external zone of the median eminence. In sheep, rats and mice, KNDy cell efferents appear to be more widespread than in the monkey or human, and include projections in the DMH, BNST, and in rodents, the PVN, LHA and septal region (Table 3.2). Tracer injections into the ARC of mice combined with kisspeptin ICC revealed a similar distribution of KNDy efferents as did co-localization of the peptides, with projections to the PVN, DMH, and LHA, as well as revealing more distant targets including the posterior hypothalamic region and the periaqueductal gray of the midbrain (6). In both rats and mice, KNDy cells also project across the midline to the contralateral ARC, perhaps serving to coordinate activity between the KNDy population on both sides of the brain, and their ipsilateral projections to the preoptic area (and presumably GnRH neurons).
In contrast to the ARC population, much less is known about the projections of preoptic and RP3V kisspeptin cells. Dual-labeling of kisspeptin and TH in mice has revealed projections from RP3V kisspeptin cells to the POA where they contact GnRH neurons (64). Similarly, anterograde tracer injections into the mouse RP3V, combined with kisspeptin ICC, revealed a set of projections from this population that includes several nuclei of the POA, as well as other areas in the hypothalamus and septal region (108). The latter projection sites closely overlap those of the ARC population, and include the BNST, PVN, DMH, and periaqueductal gray, as well as projections to the ipsilateral ARC itself. However, there are a few areas that receive inputs from either the RP3V or ARC populations but not both: the lateral septum receives input from RP3V but not ARC kisspeptin cells, while the lateral preoptic region and lateral hypothalamic area contain projections from KNDy cells but not the RP3V (108).
There are two significant caveats to these observations on the origin of kisspeptin fibers. First, co-localization of kisspeptin and other neuropeptides in individual fibers and boutons does not exclude the possibility that these are fibers of passage en route to another target. Second, as noted above, caution must be used when evaluating projections based on co-localization of KNDy peptides, since the ability to detect these markers is clearly dependent on gonadal hormonal status as well as the dynamics of peptide storage/release at the axon terminal. The recent generation of several strains of Kiss1-Cre mice (110) may soon provide another approach that has the potential to circumvent these limitations. Specifically, Kiss1-Cre mice can be crossed with transgenic strains bearing Cre-inducible markers such as TdTomato or mCherry to provide complete anterograde filling of axons arising specifically from kisspeptin cells. To achieve selective anterograde labeling of individual Kiss1 cell populations (e.g., ARC, RP3V, amygdala), Cre-inducible virus lines expressing these markers could be injected into these regions in Kiss1-Cre mice. Alternatively, markers for other neuropeptides co-expressed in kisspeptin cells (see section 3.2 above), could be incorporated into this strategy (e.g., crossing NKB-FLP mice with FLP-inducible Kiss-Cre). In addition, markers could be linked to synaptophysin or other synaptic terminal proteins to allow for identification of boutons at a light microscopic level that represent bona fide synaptic terminals rather than fibers of passage. The use of transgenic/viral vector approaches for neuroanatomical studies of the kisspeptin system holds much promise, and should provide critical information on the anatomy and function of Kiss1 cells and their connections.
Subsection 3.4.2 Afferent inputs
The array of synaptic inputs received by kisspeptin cells has only recently begun to be systematically studied, so there is little data to compare among species or different kisspeptin cell populations. The best-studied kisspeptin cell population with respect to afferents is the ARC subset. One of the major conserved anatomical features of KNDy cells are the reciprocal connections that exist between them, and that have been demonstrated at a light microscopic level using confocal microscopy (105;111) as well as at an electron microscopic level (112). These so-called “KNDy-KNDy” connections have been hypothesized to serve as a structural basis for synchronization of activity among KNDy cells, underlying their proposed role as a component of the GnRH pulse generator (38;60;62;63). It should be noted that it is not known whether these reciprocal connections represent axon collaterals within a single neuron (e.g., autosynapses), connections from neighboring KNDy cells, or inputs from a segregated subset of KNDy cells. Finally, in addition to KNDy-KNDy connections, evidence from tracing studies indicate that there are bilateral connections between KNDy cells on either side of the hypothalamus (109), as well as reciprocal connection between the POA/RP3V and KNDy populations (108). Thus the anatomical substrate exists for communication, not just among the ARC KNDy cells, but also between the two major hypothalamic kisspeptin populations, as well as between kisspeptin cells on both sides of the brain.
As noted earlier, a majority of KNDy cells are glutamatergic (Table 3.3A), and not surprisingly, KNDy terminals in contact with other KNDy cells also contain glutamatergic markers (68); in addition, KNDy cells receive input from non-KNDy glutamatergic fibers presumably arising from other regions. Preliminary observations in the sheep suggest that KNDy cells also receive GABAergic inputs, although whether these derive in part from neighboring KNDy cells is not known.
Transgenic mice in which the leptin receptor (LepR) drives expression of the anterograde transneuronal tracer, wheat germ agglutinin (WGA), have been used to demonstrate that KNDy cells receive synaptic input from LepR-containing cells (113), consistent with evidence that KNDy cells play a functional role in conveying the influence of leptin on the reproductive axis (114). Dual-label ICC studies have also shown close contacts between NPY and POMC fibers and KNDy cells in the sheep ARC (115), suggesting that afferents from these metabolic control neurons may be part of the pathways by which leptin modulates the activity of kisspeptin neurons and their control of GnRH (114). Such input may be particularly important in sheep since KNDy neurons in this species appear to lack LepR (113).
Less is known about the specific afferents that contact RP3V or preoptic kisspeptin cells. In the mouse, there is clear evidence that RP3V cells of the AVPV receive direct input from vasopressin (VP) and vasoactive-intestinal polypeptide (VIP) cells of the suprachiasmatic nucleus (SCN), the site of a central circadian clock that synchronizes the phase of clock cells in other regions of the brain (116). This input is likely to function as part of the circadian gate regulating the timing of the GnRH/LH surge in rodents (117), and may differ in species in which the surge is not under circadian control (45). Finally, while there is little anatomical data on other inputs to RP3V neurons, recent electrophysiological studies of mouse AVPV kisspeptin neurons in slice preparations (118) have provided evidence that these cells like KNDy neurons are under the presynaptic influence of glutamate and GABA, as well as the inhibitory RFamide peptide, RFRP-3, the mammalian ortholog of avian gonadotropin-inhibiting hormone (119).
Subsection 3.4.3 Reciprocal Connections with GnRH neurons
There is now clear evidence that kisspeptin cells provide direct synaptic input to GnRH neurons (Fig. 3.2), from both light and electron microscopic (EM) studies in rodents, goats and sheep. Using confocal ICC, kisspeptin-positive close contacts have been observed upon GnRH cell bodies and dendrites in mice (11), sheep (34), horses (46), monkeys (39), and humans (44). In sheep, these contacts have been co-localized with synaptophysin providing further evidence as to their identity as bona fide synaptic terminals (120). More importantly, there is now direct EM evidence in mice of axo-dendritic and axo-somatic contacts between kisspeptin fibers and preoptic GnRH cells (106). This compelling observation needs to be confirmed in other species such as monkeys and sheep where GnRH neurons examined at an EM level are frequently surrounded and separated from nearby presynaptic terminals by astroglial processes (121;122).
Fig. 2.
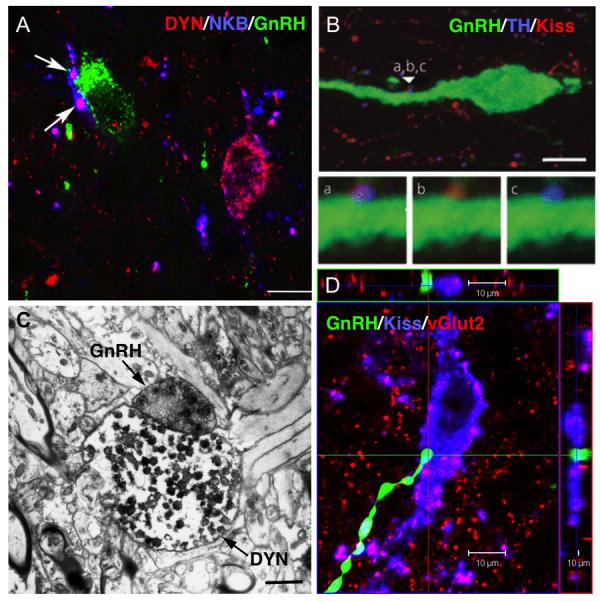
In addition to inputs at the level of GnRH cell bodies, there is also EM evidence that direct membrane appositions exist between kisspeptin and GnRH terminals within the median eminence. In rats, kisspeptin-positive terminals formed direct membrane contacts with GnRH terminals, although these contacts were seen in the internal zone of the median eminence (123). Similarly, in the goat (36), kisspeptin and GnRH terminals were seen to form direct axo-axonic contacts; however these contacts were seen in the external zone, unlike those observed in rats. In both species, axo-axonic contacts between kisspeptin and GnRH terminals lacked typical synaptic morphological specializations (e.g., synaptic densities, clefts) so that the mechanism of communication remains unclear. Consistent with the fact that in most species, kisspeptin fibers are sparse in the external zone (Table 3.2), kisspeptin cells in mice are not labeled by peripheral injections of tracers, indicating that they do not have access to fenestrated capillaries as GnRH cells do (108). Thus even given the presence of direct axo-axonic connections, it seems likely that for kisspeptin to regulate GnRH release within the median eminence, it must act via diffusion either to the external zone or through actions on other local intermediaries (e.g., glial cells). However, there is no evidence for the presence of Kiss1r in glial cells at this time.
While the precise origin of all kisspeptin inputs to GnRH cell bodies and terminals has yet to be defined, using the techniques described above (“Efferent projections”), there is evidence that at least some of this input arises from both RP3V and ARC populations. In mice, dual-labeled kisspeptin/TH terminals arising from the RP3V population innervate POA GnRH neurons (Fig. 3.2B), although they represent less than 20% of all kisspeptin contacts on those cells (64). Recent work using galanin as a co-marker for RP3V kisspeptin cells and NKB as a co-marker for ARC kisspeptin (KNDy) cells confirmed inputs to GnRH cell bodies from RP3V kisspeptin cells in mice, as well as showing direct inputs from the ARC population in this species (106). Once again, the identified inputs represented a small percentage of the total number of kisspeptin inputs to GnRH neurons. In the sheep, kisspeptin terminals arising from the ARC contact GnRH neurons in both the POA and MBH (where they are also located in this species) (Fig. 3.2A), and double-labeled KNDy terminals appear to account for the largest percentage of the total number of kisspeptin afferents. Finally, there is evidence in multiple species that KNDy cells provide input to the median eminence (Table 3.2), and that at least some of this input forms the close contacts observed with GnRH terminals, at least in the internal zone. In rats and sheep, dynorphin fibers of ARC origin (because they co-localize NKB) innervate the median eminence (105;111), and dynorphin terminals in the sheep make direct contacts with GnRH terminals at an EM level (Fig. 3.2C). Thus, the available evidence to date suggest that while both RP3V and ARC kisspeptin populations contribute direct inputs to GnRH cell bodies, inputs at the level of GnRH terminals in the median eminence arises from the ARC. We would note that these identified inputs represent a small percentage of the total kisspeptin input to GnRH neurons, but it is not clear whether this reflects limitations of the use of peptide co-markers (see above) or the presence of inputs from other kisspeptin cell populations. Again, the use of transgenic approaches where all axonal projections from a given kisspeptin population can be completely labeled should help to resolve this question.
Finally, in addition to kisspeptin inputs to GnRH neurons and terminals, there is also evidence that the reciprocal connection exists: that is, GnRH afferent input to kisspeptin cells. Specifically, confocal studies have shown close contacts between GnRH fibers in the MBH, and kisspeptin cells of the ARC in the rhesus monkey (30) and sheep (Fig. 3.2D). Thus, in these species, kisspeptin (KNDy) neurons, which are in themselves reciprocally interconnected, may comprise part of a larger reciprocal circuitry that includes GnRH neurons and the POA kisspeptin population (see above). One may speculate that if both GnRH and kisspeptin neurons are capable of displaying intrinsic pulsatile activity (37;124), a reciprocal network involving interconnections at multiple levels may be important in conferring synchronization of phase upon this rhythm in order to generate a coherent GnRH pulse.
3.5 Development and sex differences in the kisspeptin signaling system
In comparing kisspeptin and Kiss1 expression across mammalian species it is important to keep in mind developmental changes and sexual differences in expression. Sexually dimorphic expression of at least some kisspeptin populations has been found in all mammals in which it has been examined. Moreover, it is not surprising that the developmental trajectory of both kisspeptin and Kiss1 varies with the time course of maturation in each species. In this section, we will review the data available on these two issues for the limited number of species in which it is available.
Subsection 3.5.1 Development of kisspeptin and Kiss1 expression in females
Because kisspeptin was implicated in the onset of puberty at the time that its reproductive function was discovered, it is not surprising that most developmental studies have focused on the pubertal transition. Thus, although the role of kisspeptin in puberty is discussed elsewhere in this book, it will also be considered here as it represents an integral part of most reports on its developmental expression. Early studies in rats reported an increase in Kiss1 mRNA expression associated with pubertal development (125). However, those studies used RT-PCR to measure mRNA levels from whole hypothalami, so individual areas of the hypothalamus were not examined. As detailed previously, kisspeptin cells in the RP3V area and the ARC are differentially regulated. Thus, changes in each area must be considered separately.
One technical challenge to interpreting developmental changes in RP3V kisspeptin neurons, particularly in the rat, is that detection of kisspeptin-ir cells within this region seems to depend upon the use of colchicine pretreatment in order to enhance detection of immunoreactive peptide in cell bodies. In studies that have not used colchicine pretreatment, detection of kisspeptin-ir cells in the RP3V is difficult (19;22;126–128). However, kisspeptin-positive cells are readily apparent in studies that have used colchicine (18;25) or monitored Kiss1 mRNA (127;129), perhaps suggesting a very rapid secretion or turnover of the peptide in RP3V neurons of the rat. In sheep, much like non-colchicine treated rats, kisspeptin neurons in the POA (but not those in the ARC) were difficult to detect in young ewes and could not be quantified during the pubertal transition (130). In contrast, RP3V kisspeptin-ir neurons in the mouse are easily detectable without colchicine treatment. In mice and colchicine-treated rats, RP3V kisspeptin neurons are not usually detected before about PND 10 (11;49;50;65;127;129). A pubertal increase in RP3V kisspeptin immunoreactivity and Kiss1 mRNA expression has been reported in several studies for females of both species (25;49–51;127). In mice, kisspeptin-positive fibers become evident around GnRH neurons beginning at PND25, a time when changes in RP3V kisspeptin cell numbers and Kiss1 mRNA expression is increasing dramatically (11). In ovariectomized, estradiol-implanted ewes, POA Kiss1 mRNA-containing cell numbers increased around the time of puberty (131), but this change was independent of changes in LH pulse frequency associated with puberty in this species.
In the ARC, kisspeptin-positive cells or Kiss1 mRNA are detectable within the first few days postnatally in the female rat (126;127;129). Subsequently, kisspeptin immunoreactivity and Kiss1 mRNA levels increase in a puberty-associated manner (25;126;127). More recently, the number of kisspeptin-positive cells in the ARC were found to be higher in young, postpubertal ewes compared to prepubertal animals; these changes mirrored differences in LH pulse frequency (130). Changes in ARC kisspeptin-ir cell numbers in ewes from that study also paralleled an increase in the percentage of POA GnRH neurons that exhibited close appositions of kisspeptin-immunopositive varicosities, suggesting that potential changes in kisspeptin input to GnRH neurons during puberty in that species arises from the ARC kisspeptin cells. This is further supported by preliminary evidence that 50–70% of GnRH neurons receive close contacts from kisspeptin fibers that also contain dynorphin, indicating that they arise from KNDy neurons (Lehman and Goodman, unpublished data). A role for ovine ARC kisspeptin neurons in puberty is consistent with the positive correlation between the number of Kiss1 mRNA-containing cells in the middle ARC and an increase in LH pulse frequency during pubery in estradiol-treated ovariectomized ewes (131). These data fit well with those in primates where Kiss1 mRNA expression increased in female monkeys during the midpubertal phase of development (42). Interestingly, this increase in Kiss1 mRNA expression is paralleled by an increase in kisspeptin release in the primate median eminence (132) and in the amount of kisspeptin secretion induced by a GABA receptor antagonist (133). Thus, in these species, kisspeptin input from the ARC may play an important role in puberty onset.
In contrast to the rat, sheep and primate, the relative importance of ARC kisspeptin neurons in puberty onset of female mice is unclear. One limitation in studying this area in the mouse is that kisspeptin fiber density in the arcuate nucleus is usually so high that assessing numerical changes in kisspeptin-ir cell bodies has not been attempted. However, in one study that used RT-PCR, no changes in kisspeptin mRNA levels were observed in the ARC of female mice from PND10 to PND60 (50). In contrast, kisspeptin fiber density in the ARC was reported in that study to increase successively from PND10 to PND 30 and from PND30 to PND45. This raises the possibility of a mismatch between mRNA levels and protein production if kisspeptin was being produced locally (see above). Alternatively, these kisspeptin-ir fibers could come from the RP3V because changes in RP3V kisspeptin cell numbers often parallel changes in ARC kisspeptin fiber density (18;25;49;84). It was also reported that at least 40% of AVPV kisspeptin neurons project to the ARC in female mice (108). Based on these findings, it has been suggested that kisspeptin from the RP3V in the female mouse is more important than that from the ARC for puberty onset in this species (134). However, changes in kisspeptin expression in the ARC have been noted in the female hpg mouse, with a significant increase noted by PND30 (50). In addition, Kauffman et al. (135) reported increased ARC kisspeptin and NKB cell numbers following gonadectomy in juvenile female mice and suggested that disinhibition of ARC kisspeptin/NKB neurons in the ARC constitutes a critical element of the puberty triggering mechanism. This hypothesis is supported by the advancement of vaginal opening and increase in ARC Kiss1 mRNA levels in mice in which ERα was deleted from kisspeptin neurons (51). Clearly, more work is needed to determine the relative roles of the RP3V and ARC kisspeptin neurons in puberty onset for this species.
In addition to increased expression and release of kisspeptin during pubertal development, there may also be an increase in the ability of GnRH neurons to respond to kisspeptin as well. Very few studies have examined changes in Kiss1r over development and none have looked at protein expression of this receptor. Shahab et al. (42) reported a 3-fold increase in MBH Kiss1r mRNA levels during pubertal development in intact female monkeys. Takase et al. (25) observed an increase in Kiss1r mRNA around the time of puberty in the OVLT/POA of rats, while Kiss1r mRNA expression in the ARC did not change during this period. In mice, the percentage of GnRH neurons expressing Kiss1r was about 40% by PND 5 and rose to approximately 70% (or adult levels) by PND 20 (78). Given that both kisspeptin expression in the RP3V and input to GnRH neurons increase around PND25 in the female mouse (see above) and that Kiss1r expression is maximal by PND 20, the level of Kiss1r in GnRH neurons would appear not to be a limiting factor in the timing of puberty. This conclusion is consistent with evidence that administration of kisspeptin to pre- or midpubertal animals robustly stimulates GnRH/LH secretion in several species (42;125;131;132;136) and chronic administration has been shown to advance the timing of puberty in female rats (95;125). Interestingly, even though GnRH neurons in mice appear to express Kiss1r well before the normal timing of puberty onset, Han et al. (13) reported that the percentage of GnRH neurons in male mice that were activated by kisspeptin, as determined by gramicidin perforated patch clamping, increased during pubertal development. However, more recently, Dumalska et al. (137) reported that the response to kisspeptin in vGlut2-GFP-tagged cells in the medial septum that coexpressed GnRH did not change during pubertal development. More work will be necessary to confirm if increased coupling of Kiss1r to the electrical response of GnRH neurons plays a role in timing pubertal onset.
Subsection 3.5.2 Sex differences: developmental changes in males
Although a great deal of work has been done in females, there is evidence that changes in kisspeptin occur during development in males as well. While cell numbers in the RP3V of male rodents are much lower than that for females (discussed below), Han et al. (13) showed that the number of cells expressing kisspeptin mRNA was greater in adults than juveniles and that expression level per cell increased as well. Clarkson and Herbison (11) reported that kisspeptin-positive cell numbers began to increase at PND 25 and peaked at PND 45, but these changes may reflect increased circulating levels of gonadal steroids since they were abolished by gonadectomy at PND 20 and restored to normal with either testosterone or estradiol treatment (138). In contrast, others have reported very low cell numbers with no change (50;126) or only small changes (128) in the RP3V.
In the ARC, most changes reported in the male mouse have been fairly unremarkable. Kisspeptin fiber density increased from PND 10 to PND 45 in male mice (11), but no change in cell number or mRNA expression level has been shown to occur with development (13;50). It is important to note that kisspeptin-ir cell numbers increased in male hpg mice during development and that while kisspeptin cell numbers are not increased 4 days after castration at PND 14 in juvenile male mice, they are greater in adult male mice castrated at PND 14 (135). Thus steroid-independent changes occur in kisspeptin expression with development in male mice, although the relative importance of those changes remains to be determined. In male rats, cell numbers increase with pubertal development (126–128). In sheep, ARC kisspeptin-positive cell numbers decline with age in ram lambs between 6 and 12 months of age (130). Although these ages correspond to puberty onset in female lambs, males develop reproductively at a much earlier age (139); the significance of decline with age is unclear, but a similar pattern has been reported previously for cells in the ARC expressing Kiss1 mRNA in male rats (127;128).
Subsection 3.5.3 Hormonal control of sexual dimorphism in development
Because there are many more RP3V kisspeptin neurons in female than male rodents (11;21) and this area is the site where E2 acts to induce the preovulatory GnRH surge in these species (reviewed by (140)), work on the hormonal control of this sexual dimorphism focused on the well-established organizational effects of testosterone during the perinatal period. As described in detail elsewhere in this book (Chapter ?), studies in rats using neonatal gonadectomy and/or steroid treatments (21;141) support the hypothesis that testicular secretion of testosterone during this period is converted to E2, which then produces a permanent decrease in kisspeptin-expressing cells in the RP3V. This conclusion is also supported by the effects of genetic manipulations in mice that knocked out the aromatase enzyme (142) or alpha-fetoprotein (143). A similar sexual dimorphism in POA kisspeptin cell number has been observed in sheep (31) and humans (44), but the factors responsible remain unknown.
The effects of estradiol on kisspeptin cell numbers in the RP3V may not be limited to the perinatal period since OVX of mice at PND 15 reduced Kiss1 mRNA expression in the adult RP3V to male levels (49). On the other hand, in hypogonadal (hpg) mice (50) the absence of gonadal steroids did not alter the age-related increase in RP3V kisspeptin-ir cell numbers (although the maximal number was suppressed), but male hpg mice showed a similar increase in RP3V kisspeptin cells that was not evident in their WT littermates. It should be noted, however, that no age-related increases in Kiss1 mRNA (qRT-PCR) were observed in either male or female hpg mice (50) so there appears to be a mismatch between Kiss1 mRNA and protein expression in these mice.
In sheep (31) and humans (44), there are also significantly more kisspeptin neurons in the ARC of females than males, while in rodents ARC kisspeptin expression is similar in both sexes (21;135;141). There is no information on factors responsible for this sexual dimorphism in humans, but in sheep organizational effects of steroids likely play a role because this sex difference is seen in gonadectomized animals (130). However, prenatal masculinization of females with testosterone treatment during gestation did not influence kisspeptin cell numbers, but it did decrease the number of NKB-ir and dynorphin-ir cells to levels seen in males (31). Interestingly, the sexual differences in ARC kisspeptin expression develops between 6 and 12 months of ages as cell numbers in males declines, and is independent of gonadal steroids because it is evident in gonadectomized animals (130). In contrast, mice show a sexual dimorphism in ARC kisspeptin neurons that is lost during development. Specifically, OVX of prepubertal females results in an doubling of kisspeptin cell number, while castration of males prior to puberty has no effect (135), a sex difference not seen in adult mice (135). .
3.6 Conclusions
The mammalian kisspeptin system is remarkably consistent across all species studied to date, at least that portion of it that has a well-defined role in control of GnRH secretion. Major groups of cell bodies are found in two areas: a large number in the ARC and smaller set in the POA, which is concentrated in the RP3V of rodents (Fig 3.3). Most neurons in the ARC subpopulation also contain NKB and dynorphin, while a variable percentage of those in the RP3Vof rodents contain dopamine (as indicated by TH) and/or galanin (Tables 3.3A&B); whether these neurotransmitters are found in POA kisspeptin neurons in most other species remains to be determined. Moreover, the POA population is sexual dimorphic in a number of species, with more kisspeptin neurons in females than in males; a similar sexual dimorphism for the ARC population has been reported in sheep and human, but data on this in rodents is conflicting. Additional kisspeptin neurons are found in other hypothalamic and extra-hypothalamic areas in some species, but it is unclear whether or not this is a common distribution and the functional role of these neurons is unknown.
Based on the distribution of kisspeptin fibers, Kiss1r, and studies of afferent projections, both populations have functional projections to GnRH cell bodies, which represent an important site at which kisspeptin acts to stimulate GnRH secretion. Kisspeptin-ir fibers are also found in the external zone of the median eminence and there are direct membrane contacts between kisspeptin and GnRH axons in this region. These kisspeptin fibers appear to arise primarily from ARC KNDy neurons, although contacts from more rostral kisspeptin cells cannot be ruled out at this time. Although Kiss1r has yet to be identified in GnRH terminals, this is likely another site of kisspeptin action because this peptide stimulates GnRH release in vitro from the median eminence of rats (144) and sheep (86). It should be noted, however, that neither ARC nor RP3V kisspeptin cells in mice (108) or rats (123) have direct access to the portal vessels and as such are unlikely to act directly on GnRH terminals at the primary capillary bed of the median eminence.
There is also evidence for reciprocal connections within and between these two kisspeptin populations. The strongest data is for extensive reciprocal connections among KNDy neurons in a number of species, but there is also data that less abundant connections occur within the POA kisspeptin population in sheep. There is also clear evidence that KNDy neurons project to the RP3V of rodents and to some POA kisspeptin neurons in sheep. Conversely, almost half of the RP3V kisspeptin neurons project to the ARC in mice. It is important to note that the functional significance of kisspeptin release at these reciprocal connections is unclear because there is no evidence that kisspeptin neurons in the ARC or RP3V/POA contain Kiss1r. The other neurotransmitters within ARC KNDy neurons have been postulated to play a key role in synchronizing this population and driving episodic GnRH secretion. It is thus likely that reciprocal connections within the ARC play an important functional role, but whether that is true for the more rostral kisspeptin populations awaits further study.
Finally, although detailed discussion of the functional roles of these kisspeptin populations is beyond the scope of this chapter, as described elsewhere in this book there also appear to be common functional roles for these two kisspeptin populations. The rostral population is clearly involved in the preovulatory GnRH surge in many species, and the ARC population has been implicated in the negative feedback action of gonadal steroids and in possibly synchronizing GnRH neural activity during episodic secretion. The latter appears to play a key role in the onset of puberty in rodents, sheep, and primates, although the RP3V population likely contributes to this process in mice.
This review also identified several important gaps in our understanding of the neuroanatomy of kisspeptin signaling. The most obvious of these is the paucity of detailed information on the distribution of Kiss1r mRNA and the complete lack of any data on location of Kiss1r protein. Until more complete anatomical information is available on the location of these receptors, any conclusions as to physiological significance of most kisspeptin projections must be considered tentative at best. Information is also beginning to be developed on extra-hypothalamic kisspeptin cells and fibers, but this remains limited to just a few species. Thus these data need to be extended to a broader range of species and studies on their functional significance developed. Another anatomical issue of importance revolves around identifying inputs to kisspeptin cell bodies. While some data is available on afferents to ARC and POA/RP3V kisspeptin cell bodies, this information is fragmentary. A fuller understanding of the complete complement of presynaptic inputs to these, and other, kisspeptin cells will obviously be important for understanding their neural control. Finally, kisspeptin cells in the ARC, POA/RP3V and elsewhere are likely to play a significant role in the regulation of other physiological systems beyond reproduction. Identifying the full extent of kisspeptin efferents, and the distribution of their postsynaptic targets, is essential as a foundation for future functional dissection of these roles in the mammalian nervous system.
ACKNOWLEDGEMENTS
We thank Dr. Lique M. Coolen for her valuable assistance in the preparation of Fig. 3.3. This work supported by a grant from NIH (NIH RO1 HD033916)
Footnotes
The authors have nothing to declare
Reference List
- (1).Dungan HM, Clifton DK, Steiner RA. Minireview: Kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006;147(3):1154–1158. doi: 10.1210/en.2005-1282. [DOI] [PubMed] [Google Scholar]
- (2).Lehman MN, Merkley CM, Coolen LM, Goodman RL. Anatomy of the kisspeptin neural network in mammals. Brain Research. 2010;1364:90–102. doi: 10.1016/j.brainres.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Mikkelsen JD, Simonneaux V. The neuroanatomy of the kisspeptin system in the mammalian brain. Peptides. 2009;30(1):26–33. doi: 10.1016/j.peptides.2008.09.004. [DOI] [PubMed] [Google Scholar]
- (4).Oakley AE, Clifton DK, Steiner RA. Kisspeptin Signaling in the Brain. Endocrine Reviews. 2009;30(6):713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Akazome Y, Kanda S, Okubo K, Oka Y. Functional and evolutionary insights into vertebrate kisspeptin systems from studies of fish brain. Journal of Fish Biology. 2010;76(1):161–182. doi: 10.1111/j.1095-8649.2009.02496.x. [DOI] [PubMed] [Google Scholar]
- (6).Tena-Sempere M, Felip A, Gomez A, Zanuy S, Carrillo M. Comparative insights of the kisspeptin/kisspeptin receptor system: Lessons from non-mammalian vertebrates. General and Comparative Endocrinology. 2012;175(2):234–243. doi: 10.1016/j.ygcen.2011.11.015. [DOI] [PubMed] [Google Scholar]
- (7).Brailoiu GC, Dun SL, Ohsawa M, Yin DL, Yang J, Chang JK, et al. KiSS-1 expression and metastin-like immunoreactivity in the rat brain. Journal of Comparative Neurology. 2005;481(3):314–329. doi: 10.1002/cne.20350. [DOI] [PubMed] [Google Scholar]
- (8).Clarkson J, de Tassigny XD, Colledge WH, Caraty A, Herbison AE. Distribution of Kisspeptin Neurones in the Adult Female Mouse Brain. Journal of Neuroendocrinology. 2009;21(8):673–682. doi: 10.1111/j.1365-2826.2009.01892.x. [DOI] [PubMed] [Google Scholar]
- (9).Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neuroscience Letters. 2006;401(3):225–230. doi: 10.1016/j.neulet.2006.03.039. [DOI] [PubMed] [Google Scholar]
- (10).Goodman RL, Lehman MN, Smith JT, Coolen LM, De Oliveira CVR, Jafarzadehshirazi MR, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148(12):5752–5760. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- (11).Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; Sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147(12):5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- (13).Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. Journal of Neuroscience. 2005;25(49):11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Kim J, Semaan SJ, Clifton DK, Steiner RA, Dhamija S, Kauffman AS. Regulation of Kiss1 Expression by Sex Steroids in the Amygdala of the Rat and Mouse. Endocrinology. 2011;152(5):2020–2030. doi: 10.1210/en.2010-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146(9):3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- (16).Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, et al. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146(7):2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- (17).Hanchate NK, Parkash J, Bellefontaine N, Mazur D, Colledge WH, de Tassigny XD, et al. Kisspeptin-GPR54 Signaling in Mouse NO-Synthesizing Neurons Participates in the Hypothalamic Control of Ovulation. Journal of Neuroscience. 2012;32(3):932–945. doi: 10.1523/JNEUROSCI.4765-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. Journal of Reproduction and Development. 2007;53(2):367–378. doi: 10.1262/jrd.18146. [DOI] [PubMed] [Google Scholar]
- (19).Desroziers E, Mikkelsen J, Simonneaux V, Keller M, Tillet Y, Caraty A, et al. Mapping of kisspeptin fibres in the brain of the pro-oestrous rat. J Neuroendocrinol. 2010;22(10):1101–1112. doi: 10.1111/j.1365-2826.2010.02053.x. [DOI] [PubMed] [Google Scholar]
- (20).Irwig MS, Fraleyb GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80(4):264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- (21).Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, et al. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148(4):1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- (22).Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, et al. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146(10):4431–4436. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- (23).Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. Journal of Neuroscience. 2006;26(25):6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Xu ZF, Kaga S, Mochiduki A, Tsubomizu J, Adachi S, Sakai T, et al. Immunocytochemical localization of kisspeptin neurons in the rat forebrain with special reference to sexual dimorphism and interaction with GnRH neurons. Endocrine Journal. 2012;59(2):161–171. doi: 10.1507/endocrj.ej11-0193. [DOI] [PubMed] [Google Scholar]
- (25).Takase K, Uenoyama Y, Inoue N, Matsui H, Yamada S, Shimizu M, et al. Possible role of oestrogen in pubertal increase of Kiss1/kisspeptin expression in discrete hypothalamic areas of female rats. Journal of Neuroendocrinology. 2009;21(6):527–537. doi: 10.1111/j.1365-2826.2009.01868.x. [DOI] [PubMed] [Google Scholar]
- (26).Ansel L, Bolborea M, Bentsen AH, Klosen P, Mikkelsen JD, Simonneaux V. Differential Regulation of Kiss1 Expression by Melatonin and Gonadal Hormones in Male and Female Syrian Hamsters. Journal of Biological Rhythms. 2010;25(2):81–91. doi: 10.1177/0748730410361918. [DOI] [PubMed] [Google Scholar]
- (27).Greives TJ, Mason AO, Scotti MAL, Levine J, Ketterson ED, Kriegsfeld LJ, et al. Environmental control of kisspeptin: Implications for seasonal reproduction. Endocrinology. 2007;148(3):1158–1166. doi: 10.1210/en.2006-1249. [DOI] [PubMed] [Google Scholar]
- (28).Mason AO, Greives TJ, Scotti MAL, Levine J, Frommeyer S, Ketterson ED, et al. Suppression of kisspeptin expression and gonadotropic axis sensitivity following exposure to inhibitory day lengths in female Siberian hamsters. Hormones and Behavior. 2007;52(4):492–498. doi: 10.1016/j.yhbeh.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Revel FG, Saboureau M, Masson-Pevet M, Pevet P, Mikkelsen JD, Simonneaux V. Kisspeptin mediates the photoperiodic control of reproduction in hamsters. Current Biology. 2006;16(17):1730–1735. doi: 10.1016/j.cub.2006.07.025. [DOI] [PubMed] [Google Scholar]
- (30).Bosch MA, Xue CH, Ronnekleiv OK. Kisspeptin expression in guinea pig hypothalamus: Effects of 17 beta-estradiol. Journal of Comparative Neurology. 2012;520(10):2143–2162. doi: 10.1002/cne.23032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology. 2010;151(1):301–311. doi: 10.1210/en.2009-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Estrada KM, Clay CM, Pompolo S, Smith JT, Clarke IJ. Elevated KiSS-1 expression in the arcuate nucleus prior to the cyclic preovulatory gonadotrophin-releasing hormone/lutenising hormone surge in the ewe suggests a stimulatory role for kisspeptin in oestrogen-positive feedback. Journal of Neuroendocrinology. 2006;18(10):806–809. doi: 10.1111/j.1365-2826.2006.01485.x. [DOI] [PubMed] [Google Scholar]
- (33).Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148(3):1150–1157. doi: 10.1210/en.2006-1435. [DOI] [PubMed] [Google Scholar]
- (34).Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, et al. Variation in Kisspeptin and RFamide-Related Peptide ( RFRP) Expression and Terminal Connections to Gonadotropin-Releasing Hormone Neurons in the Brain: A Novel Medium for Seasonal Breeding in the Sheep. Endocrinology. 2008;149(11):5770–5782. doi: 10.1210/en.2008-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Smith JT, Li Q, Pereira A, Clarke IJ. Kisspeptin neurons in the ovine arcuate nucleus and preoptic area are involved in the preovulatory luteinizing hormone surge. Endocrinology. 2009;150(12):5530–5538. doi: 10.1210/en.2009-0712. [DOI] [PubMed] [Google Scholar]
- (36).Matsuyama S, Ohkura S, Mogi K, Wakabayashi Y, Mori Y, Tsukamura H, et al. Morphological Evidence for Direct Interaction between Kisspeptin and Gonadotropin-Releasing Hormone Neurons at the Median Eminence of the Male Goat: An Immunoelectron Microscopic Study. Neuroendocrinology. 2011;94(4):323–332. doi: 10.1159/000331576. [DOI] [PubMed] [Google Scholar]
- (37).Ohkura S, Wakabayashi Y, Uenoyama Y, Steiner RA, Tsukamura H, Maeda K, et al. Gonadotropin-releasing hormone pulse generator activity from the arcuate nucleus kisspeptin neurons in the goat hypothalamus. Endocrine Journal. 2010;57:S516. [Google Scholar]
- (38).Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, et al. Neurokinin B and Dynorphin A in Kisspeptin Neurons of the Arcuate Nucleus Participate in Generation of Periodic Oscillation of Neural Activity Driving Pulsatile Gonadotropin-Releasing Hormone Secretion in the Goat. Journal of Neuroscience. 2010;30(8):3124–3132. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology. 2008;149(9):4387–4395. doi: 10.1210/en.2008-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B Stimulates GnRH Release in the Male Monkey (Macaca mulatta) and Is Colocalized with Kisspeptin in the Arcuate Nucleus. Endocrinology. 2010;151(9):4494–4503. doi: 10.1210/en.2010-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. Journal of Clinical Endocrinology & Metabolism. 2007;92(7):2744–2750. doi: 10.1210/jc.2007-0553. [DOI] [PubMed] [Google Scholar]
- (42).Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(6):2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Smith JT, Periera A, Shahab M, Pau KYF, Clarke IJ. Hypothalamic expression of the kisspeptin gene (Kiss1) and the RFamide-related peptide (RFRP) gene during the menstrual cycle of a non-human primate. Endocrine Journal. 2010;57:S534. [Google Scholar]
- (44).Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, et al. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. European Journal of Neuroscience. 2010;31(11):1984–1998. doi: 10.1111/j.1460-9568.2010.07239.x. [DOI] [PubMed] [Google Scholar]
- (45).Goodman RL, Lehman MN. Minireview: Kisspeptin neurons from mice to men: similarities and differences. Endocrinology. 2012 doi: 10.1210/en.2012-1550. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Decourt C, Tillet Y, Caraty A, Franceschini I, Briant C. Kisspeptin immunoreactive neurons in the equine hypothalamus Interactions with GnRH neuronal system. Journal of Chemical Neuroanatomy. 2008;36(3–4):131–137. doi: 10.1016/j.jchemneu.2008.07.008. [DOI] [PubMed] [Google Scholar]
- (47).Magee C, Foradori CD, Bruemmer JE, Arreguin-Arevalo JA, Mccue PM, Handa RJ, et al. Biological and Anatomical Evidence for Kisspeptin Regulation of the Hypothalamic-Pituitary-Gonadal Axis of Estrous Horse Mares. Endocrinology. 2009;150(6):2813–2821. doi: 10.1210/en.2008-1698. [DOI] [PubMed] [Google Scholar]
- (48).Ginther OJ, Gastal EL, Gastal MO, Beg MA. Regulation of circulating gonadotropins by the negative effects of ovarian hormones in mares. Biology of Reproduction. 2005;73(2):315–323. doi: 10.1095/biolreprod.105.040253. [DOI] [PubMed] [Google Scholar]
- (49).Clarkson J, Boon WC, Simpson ER, Herbison AE. Postnatal Development of an Estradiol-Kisspeptin Positive Feedback Mechanism Implicated in Puberty Onset. Endocrinology. 2009;150(7):3214–3220. doi: 10.1210/en.2008-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Gill JC, Wang OL, Kakar S, Martinelli E, Carroll RS, Kaiser UB. Reproductive Hormone-Dependent and -Independent Contributions to Developmental Changes in Kisspeptin in GnRH-Deficient Hypogonadal Mice. Plos One. 2010;5(7) doi: 10.1371/journal.pone.0011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U, et al. Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(52):22693–22698. doi: 10.1073/pnas.1012406108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Newman SW. The medial extend amygdala in male reproductive behavior. A node in the mammalian socialbehavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- (53).Veening JG, Coolen LM. Neural activation following sexual behavior inthe male and female rat brain. Behav Neurosci. 1998;92:181–193. doi: 10.1016/s0166-4328(97)00190-3. [DOI] [PubMed] [Google Scholar]
- (54).Beltramino C, Taleusbik S. Facilitory and inhibitory effects of electrochemical stimulation of the amygdala onthe release of luteinizing hormone. Brain Res. 1978;144:95–107. doi: 10.1016/0006-8993(78)90437-7. [DOI] [PubMed] [Google Scholar]
- (55).Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J, Atkin S, Bookout AL, et al. Characterization of Kiss1 Neurons Using Transgenic Mouse Models. Neuroscience. 2011;173:37–56. doi: 10.1016/j.neuroscience.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Padilla SL, Carmody JS, Zeltser LM. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nature Medicine. 2010;16(4):403–405. doi: 10.1038/nm.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).de Tassigny XD, Fagg LA, Carlton MBL, Colledge WH. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology. 2008;149(8):3926–3932. doi: 10.1210/en.2007-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, et al. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145(6):2959–2967. doi: 10.1210/en.2003-1305. [DOI] [PubMed] [Google Scholar]
- (59).Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nature Genetics. 2009;41(3):354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Lehman MN, Coolen LM, Goodman RL. Minireview: Kisspeptin/Neurokinin B/Dynorphin (KNDy) Cells of the Arcuate Nucleus: A Central Node in the Control of Gonadotropin-Releasing Hormone Secretion. Endocrinology. 2010;151(8):3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Navarro VM, Gottsch ML, Wu M, Garca-Galiano D, Hobbs SJ, Bosch MA, et al. Regulation of NKB Pathways and Their Roles in the Control of Kiss1 Neurons in the Arcuate Nucleus of the Male Mouse. Endocrinology. 2011;152(11):4265–4275. doi: 10.1210/en.2011-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. Neurokinin B and the hypothalamic regulation of reproduction. Brain Research. 2010;1364:116–128. doi: 10.1016/j.brainres.2010.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. Journal of Neuroscience. 2009;29(38):11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Clarkson J, Herbison AE. Dual Phenotype Kisspeptin-Dopamine Neurones of the Rostral Periventricular Area of the Third Ventricle Project to Gonadotrophin-Releasing Hormone Neurones. Journal of Neuroendocrinology. 2011;23(4):293–301. doi: 10.1111/j.1365-2826.2011.02107.x. [DOI] [PubMed] [Google Scholar]
- (65).Semaan SJ, Murray EK, Poling MC, Dhamija S, Forger NG, Kauffman AS. BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice. Endocrinology. 2010;151(12):5807–5817. doi: 10.1210/en.2010-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Merkley CM, Coolen LM, Goodman RL, Lehman MN. Colocalization of met-enkephalin, but not galanin or tyrosine hydroxylase, within kisspeptin neurons in the sheep. Ann Meeting Endocrine Society. 2012 [Google Scholar]
- (67).Porteous R, Petersen SL, Yeo SH, Bhattarai JP, Ciofi P, de Tassigny XD, et al. Kisspeptin Neurons Co-Express Met-Enkephalin and Galanin in the Rostral Periventricular Region of the Female Mouse Hypothalamus. Journal of Comparative Neurology. 2011;519(17):3456–3469. doi: 10.1002/cne.22716. [DOI] [PubMed] [Google Scholar]
- (68).Merkley CM, Jackson L, Goodman RL, Lehman MN. Evidence for transcriptional activation of arcuate kisspeptin neurons, and glutamatergic input to kisspeptin during the preovulatory GnRH surge in sheep. Ann Meeting Endocrine Society. 2009:P3–220. [Google Scholar]
- (69).Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52(2):271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Clarkson J, d'Anglemont X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. Journal of Neuroscience. 2008;28(35):8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of Androgen and Estrogen-Receptor Messenger Rna-Containing Cells in the Rat-Brain - An Insitu Hybridization Study. Journal of Comparative Neurology. 1990;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- (72).Oakley AE, Coolen LM, Lehman MN, Wagenmaker ER, Karsch FJ. Are dynorphin neurons in the arcuate nucleus responsive to cortisol and influenced by the combined presence of cortisol and estradiol. Ann Meeting Endocrine Society. 2009 [Google Scholar]
- (73).Li Q, Rao A, Pereira A, Clarke IJ, Smith JT. Kisspeptin Cells in the Ovine Arcuate Nucleus Express Prolactin Receptor but not Melatonin Receptor. Journal of Neuroendocrinology. 2011;23(10):871–882. doi: 10.1111/j.1365-2826.2011.02195.x. [DOI] [PubMed] [Google Scholar]
- (74).Cernea M, Phillips R, Padmanabhan V, Coolen LM, Lehman MN. Colocalization of insulin receptors in KNDy but not GnRH neurons inthe ewe: effects of excess prenatal testosterone. Ann Meeting Soc for Neuroscience. 2012 [Google Scholar]
- (75).Kotani M, Detheux M, Vandenbbogaerde A, Communi D, Vanderwinden JM, Le Poul E, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. Journal of Biological Chemistry. 2001;276(37):34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- (76).Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. Journal of Biological Chemistry. 2001;276(31):28969–28975. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- (77).Lee DK, Nguyen T, O'Neill GP, Cheng R, Liu Y, Howard AD, et al. Discovery of a receptor related to the galanin receptors. Febs Letters. 1999;446(1):103–107. doi: 10.1016/s0014-5793(99)00009-5. [DOI] [PubMed] [Google Scholar]
- (78).Herbison AE, de Tassigny XD, Doran J, Colledge WH. Distribution and Postnatal Development of Gpr54 Gene Expression in Mouse Brain and Gonadotropin-Releasing Hormone Neurons. Endocrinology. 2010;151(1):312–321. doi: 10.1210/en.2009-0552. [DOI] [PubMed] [Google Scholar]
- (79).Kim W, Jessen HM, Auger AP, Terasawa E. Postmenopausal increase in KiSS-1, GPR54, and luteinizing hormone releasing hormone (LHRH-1) mRNA in the basal hypothalamus of female rhesus monkeys. Peptides. 2009;30(1):103–110. doi: 10.1016/j.peptides.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Shibata M, Friedman RL, Ramaswamy S, Plant TM. Evidence that down regulation of hypothalamic KiSS-1 expression is involved in the negative feedback action of testosterone to regulate luteinising hormone secretion in the adult male rhesus monkey (Macaca mulatta) Journal of Neuroendocrinology. 2007;19(6):432–438. doi: 10.1111/j.1365-2826.2007.01549.x. [DOI] [PubMed] [Google Scholar]
- (81).Kinsey-Jones JS, Li XF, Knox AMI, Wilkinson ES, Zhu XL, Chaudhary AA, et al. Down-Regulation of Hypothalamic Kisspeptin and its Receptor, Kiss1r, mRNA Expression is Associated with Stress-Induced Suppression of Luteinising Hormone Secretion in the Female Rat. Journal of Neuroendocrinology. 2009;21(1):20–29. doi: 10.1111/j.1365-2826.2008.01807.x. [DOI] [PubMed] [Google Scholar]
- (82).Knox AMI, Li XF, Kinsey-Jones JS, Wilkinson ES, Wu XQ, Cheng YS, et al. Neonatal Lipopolysaccharide Exposure Delays Puberty and Alters Hypothalamic Kiss1 and Kiss1r mRNA Expression in the Female Rat. Journal of Neuroendocrinology. 2009;21(8):683–689. doi: 10.1111/j.1365-2826.2009.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Quennell JH, Rizwan MZ, Relf HL, Anderson GM. Developmental and Steroidogenic Effects on the Gene Expression of RFamide Related Peptides and their Receptor in the Rat Brain and Pituitary Gland. Journal of Neuroendocrinology. 2010;22(4):309–316. doi: 10.1111/j.1365-2826.2010.01963.x. [DOI] [PubMed] [Google Scholar]
- (84).Yamada S, Uenoyama Y, Kinoshita M, Iwata K, Takase K, Matsui H, et al. Inhibition of metastin (kisspeptin-54)-GPR54 signaling in the arcuate nucleus-median eminence region during lactation in rats. Endocrinology. 2007;148(5):2226–2232. doi: 10.1210/en.2006-1529. [DOI] [PubMed] [Google Scholar]
- (85).Fu LY, van den Pol AN. Kisspeptin Directly Excites Anorexigenic Proopiomelanocortin Neurons but Inhibits Orexigenic Neuropeptide Y Cells by an Indirect Synaptic Mechanism. Journal of Neuroscience. 2010;30(30):10205–10219. doi: 10.1523/JNEUROSCI.2098-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Smith JT, Li Q, Yap KS, Shahab M, Roseweir AK, Millar RP, et al. Kisspeptin Is Essential for the Full Preovulatory LH Surge and Stimulates GnRH Release from the Isolated Ovine Median Eminence. Endocrinology. 2011;152(3):1001–1012. doi: 10.1210/en.2010-1225. [DOI] [PubMed] [Google Scholar]
- (87).Li Q, Roa J, Clarke IJ, Smith JT. Seasonal variation in the gonadotropin-releasing hormone response to kisspeptin in sheep: possible kisspeptin regulation of the kisspeptin receptor. Neuroendocrinology. 2012:93. doi: 10.1159/000335998. [DOI] [PubMed] [Google Scholar]
- (88).Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(5):1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Luque RM, Kineman RD, Tena-Sempere M. Regulation of hypothalamic expression of KiSS-1 and GPR54 genes by metabolic factors: Analyses using mouse models and a cell line. Endocrinology. 2007;148(10):4601–4611. doi: 10.1210/en.2007-0500. [DOI] [PubMed] [Google Scholar]
- (90).Bellingham M, Fowler PA, Amezaga MR, Rhind SM, Cotinot C, Mandon-Pepin B, et al. Exposure to a Complex Cocktail of Environmental Endocrine-Disrupting Compounds Disturbs the Kisspeptin/GPR54 System in Ovine Hypothalamus and Pituitary Gland. Environmental Health Perspectives. 2009;117(10):1556–1562. doi: 10.1289/ehp.0900699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Smith JT, Rao A, Pereira A, Caraty A, Millar RP, Clarke IJ. Kisspeptin is present in ovine hypophysial portal blood but does not increase during the preovulatory luteinizing hormone surge: Evidence that gonadotropes are not direct targets of kisspeptin in vivo. Endocrinology. 2008;149(4):1951–1959. doi: 10.1210/en.2007-1425. [DOI] [PubMed] [Google Scholar]
- (92).Li S, Ren J, Yang G, Guo Y, Huang L. Characterization of the porcine Kisspeptins receptor gene and evaluation as candidate for timing of puberty in sows. Journal of Animal Breeding and Genetics. 2008;125(4):219–227. doi: 10.1111/j.1439-0388.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- (93).Gutierrez-Pascual E, Martinez-Fuentes AJ, Pinilla L, Tena-Sempere M, Malagon MM, Castano JP. Direct pituitary effects of kisspeptin: Activation of gonadotrophs and somatotrophs and stimulation of luteinising hormone and growth hormone secretion. Journal of Neuroendocrinology. 2007;19(7):521–530. doi: 10.1111/j.1365-2826.2007.01558.x. [DOI] [PubMed] [Google Scholar]
- (94).Richard N, Galmiche G, Corvaisier S, Caraty A, Kottler ML. KiSS-1 and GPR54 genes are co-expressed in rat gonadotrophs and differentially regulated In vivo by oestradiol and gonadotrophin-releasing hormone. Journal of Neuroendocrinology. 2008;20(3):381–393. doi: 10.1111/j.1365-2826.2008.01653.x. [DOI] [PubMed] [Google Scholar]
- (95).Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochemical and Biophysical Research Communications. 2004;320(2):383–388. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- (96).Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, et al. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. Journal of Neuroendocrinology. 2004;16(10):850–858. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- (97).Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, et al. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology. 2005;146(1):156–163. doi: 10.1210/en.2004-0836. [DOI] [PubMed] [Google Scholar]
- (98).Suzuki S, Kadokawa H, Hashizume T. Direct kisspeptin-10 stimulation on luteinizing hormone secretion from bovine and porcine anterior pituitary cells. Animal Reproduction Science. 2008;103(3–4):360–365. doi: 10.1016/j.anireprosci.2007.05.016. [DOI] [PubMed] [Google Scholar]
- (99).Ramaswamy S, Gibbs RB, Plant TM. Studies of the Localisation of Kisspeptin Within the Pituitary of the Rhesus Monkey (Macaca mulatta) and the Effect of Kisspeptin on the Release of Non-Gonadotropic Pituitary Hormones. Journal of Neuroendocrinology. 2009;21(10):795–804. doi: 10.1111/j.1365-2826.2009.01905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Poling MC, Kim J, Dhamija S, Kauffman AS. Development, Sex Steroid Regulation, and Phenotypic Characterization of RFamide-Related Peptide (Rfrp) Gene Expression and RFamide Receptors in the Mouse Hypothalamus. Endocrinology. 2012;153(4):1827–1840. doi: 10.1210/en.2011-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Quaynor S, Hu L, Leung PK, Feng H, Mores N, Krsmanovic LZ, et al. Expression of a functional G protein-coupled receptor 54-kisspeptin autoregulatory system in hypothalamic gonadotropin-releasing hormone neurons. Molecular Endocrinology. 2007;21(12):3062–3070. doi: 10.1210/me.2007-0207. [DOI] [PubMed] [Google Scholar]
- (102).Pielecka-Fortuna JCZMSM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149:1979–1986. doi: 10.1210/en.2007-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Arai AC, Xia YF, Suzuki E, Kessler M, Civelli O, Nothacker HP. Cancer metastasis-suppressing peptide metastin upregulates excitatory synaptic transmission in hippocampal dentate granule cells. Journal of Neurophysiology. 2005;94(5):3648–3652. doi: 10.1152/jn.00590.2005. [DOI] [PubMed] [Google Scholar]
- (104).Arai AC, Orwig N. Factors that regulate KiSS1 gene expression in the hippocampus. Brain Research. 2008;1243:10–18. doi: 10.1016/j.brainres.2008.09.031. [DOI] [PubMed] [Google Scholar]
- (105).Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactlivity in the rat hypothalamus: Morphologic evidence of interrelated function within the arcuate nucleus. Journal of Comparative Neurology. 2006;498(5):712–726. doi: 10.1002/cne.21086. [DOI] [PubMed] [Google Scholar]
- (106).Kallo I, Vida B, Deli L, Molnar CS, Hrabovszky E, Caraty A, et al. Co-Localisation of Kisspeptin with Galanin or Neurokinin B in Afferents to Mouse GnRH Neurones. Journal of Neuroendocrinology. 2012;24(3):464–476. doi: 10.1111/j.1365-2826.2011.02262.x. [DOI] [PubMed] [Google Scholar]
- (107).True C, Kirigiti M, Ciofi P, Grove KL, Smith MS. Characterisation of Arcuate Nucleus Kisspeptin/Neurokinin B Neuronal Projections and Regulation during Lactation in the Rat. Journal of Neuroendocrinology. 2011;23(1):52–64. doi: 10.1111/j.1365-2826.2010.02076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Yeo SH, Herbison AE. Projections of Arcuate Nucleus and Rostral Periventricular Kisspeptin Neurons in the Adult Female Mouse Brain. Endocrinology. 2011;152(6):2387–2399. doi: 10.1210/en.2011-0164. [DOI] [PubMed] [Google Scholar]
- (109).Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain Projections of Arcuate Neurokinin B Neurons Demonstrated by Anterograde Tract-Tracing and Monosodium Glutamate Lesions in the Rat. Neuroscience. 2010;166(2):680–697. doi: 10.1016/j.neuroscience.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Dungan Lemko HM, Elias CF. Minreview: Kiss of the mutant mouse: how genetically altered mice advancedour understanding of kisspeptin's role in reproductive physiology. Endocrinology. 2012 doi: 10.1210/en.2012-1494. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Foradori CD, Amstalden M, Goodman RL, Lehman MN. Colocalisation of dynorphin A and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. Journal of Neuroendocrinology. 2006;18(7):534–541. doi: 10.1111/j.1365-2826.2006.01445.x. [DOI] [PubMed] [Google Scholar]
- (112).Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology. 2002;143(11):4366–4374. doi: 10.1210/en.2002-220586. [DOI] [PubMed] [Google Scholar]
- (113).Louis GW, Greenwald-Yarnell M, Phillips R, Coolen LM, Lehman MN, Myers MG. Molecular Mapping of the Neural Pathways Linking Leptin to the Neuroendocrine Reproductive Axis. Endocrinology. 2011;152(6):2302–2310. doi: 10.1210/en.2011-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Tena-Sempere M. KiSS-1 and reproduction: Focus on its role in the metabolic regulation of fertility. Neuroendocrinology. 2006;83(5–6):275–281. doi: 10.1159/000095549. [DOI] [PubMed] [Google Scholar]
- (115).Backholer K, Smith JT, Rao A, Pereira A, Iqbal J, Ogawa S, et al. Kisspeptin Cells in the Ewe Brain Respond to Leptin and Communicate with Neuropeptide Y and Proopiomelanocortin Cells. Endocrinology. 2010;151(5):2233–2243. doi: 10.1210/en.2009-1190. [DOI] [PubMed] [Google Scholar]
- (116).Mohawk JA, Green CB, Takahasi JS. Central and peripheral circadian clocks in mammals. Ann Rev Neurosci. 2012 doi: 10.1146/annurev-neuro-060909-153128. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian Regulation of Kiss1 Neurons: Implications for Timing the Preovulatory Gonadotropin-Releasing Hormone/Luteinizing Hormone Surge. Endocrinology. 2009;150(8):3664–3671. doi: 10.1210/en.2009-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Ducret E, Gaidamaka G, Herbison AE. Electrical and Morphological Characteristics of Anteroventral Periventricular Nucleus Kisspeptin and Other Neurons in the Female Mouse. Endocrinology. 2010;151(5):2223–2232. doi: 10.1210/en.2009-1480. [DOI] [PubMed] [Google Scholar]
- (119).Bentley GE, Ubuka T, McGuire NL, Calisi R, Perfito N, Kriegsfeld LJ, et al. Gonadotrophin-Inhibitory Hormone: A Multifunctional Neuropeptide. Journal of Neuroendocrinology. 2009;21(4):276–281. doi: 10.1111/j.1365-2826.2009.01851.x. [DOI] [PubMed] [Google Scholar]
- (120).Smith JT. Kisspeptin signalling in the brain: Steroid regulation in the rodent and ewe. Brain Research Reviews. 2008;57(2):288–298. doi: 10.1016/j.brainresrev.2007.04.002. [DOI] [PubMed] [Google Scholar]
- (121).Lehman MN, Karsch FJ, Robinson JE, Silverman AJ. Ultrastructure and Synaptic Organization of Luteinizing-Hormone-Releasing Hormone (Lhrh) Neurons in the Anestrous Ewe. Journal of Comparative Neurology. 1988;273(4):447–458. doi: 10.1002/cne.902730402. [DOI] [PubMed] [Google Scholar]
- (122).Witkin JW, O'Sullivan H, Miller R, Ferin M. GnRH perikarya in medial basal hypothalamus of pubertal female rhesus macaque are ensheathed with glia. Journal of Neuroendocrinology. 1997;9(12):881–885. doi: 10.1046/j.1365-2826.1997.00649.x. [DOI] [PubMed] [Google Scholar]
- (123).Uenoyama Y, Inoue N, Pheng V, Homma T, Takase K, Yamada S, et al. Ultrastructural Evidence of Kisspeptin-Gonadotrophin-Releasing Hormone (GnRH) Interaction in the Median Eminence of Female Rats: Implication of Axo-Axonal Regulation of GnRH Release. Journal of Neuroendocrinology. 2011;23(10):863–870. doi: 10.1111/j.1365-2826.2011.02199.x. [DOI] [PubMed] [Google Scholar]
- (124).Nunemaker CS, DeFazio RA, Geusz ME, Herzog ED, Pitts GR, Moenter SM. Long-term recordings of networks of immortalized GnRH neurons reveal episodic patterns of electrical activity. Journal of Neurophysiology. 2001;86(1):86–93. doi: 10.1152/jn.2001.86.1.86. [DOI] [PubMed] [Google Scholar]
- (125).Navarro VM, Fernandez-Fernandez R, Castellano JM, Roa J, Mayen A, Barreiro ML, et al. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004;561(Pt 2):379–386. doi: 10.1113/jphysiol.2004.072298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Desroziers E, Mikkelsen JD, Duittoz A, Franceschini I. Kisspeptin-immunoreactivity changes in a sex- and hypothalamic-region specific manner across rat postnatal development. J Neuroendocrinol. 2012 doi: 10.1111/j.1365-2826.2012.02317.x. [DOI] [PubMed] [Google Scholar]
- (127).Takumi K, Iijima N, Ozawa H. Developmental changes in the expression of kisspeptin mRNA in rat hypothalamus. J Mol Neurosci. 2011;43(2):138–145. doi: 10.1007/s12031-010-9430-1. [DOI] [PubMed] [Google Scholar]
- (128).Bentsen AH, Ansel L, Simonneaux V, Tena-Sempere M, Juul A, Mikkelsen JD. Maturation of kisspeptinergic neurons coincides with puberty onset in male rats. Peptides. 2010;31(2):275–283. doi: 10.1016/j.peptides.2009.11.017. [DOI] [PubMed] [Google Scholar]
- (129).Cao J, Patisaul HB. Sexually dimorphic expression of hypothalamic estrogen receptors alpha and beta and Kiss1 in neonatal male and female rats. J Comp Neurol. 2011;519(15):2954–2977. doi: 10.1002/cne.22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Nestor CC, Briscoe AM, Davis SM, Valent M, Goodman RL, Hileman SM. Evidence of a role for kisspeptin and neurokinin B in puberty of female sheep. Endocrinology. 2012;153(6):2756–2765. doi: 10.1210/en.2011-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Redmond JS, Baez-Sandoval GM, Spell KM, Spencer TE, Lents CA, Williams GL, et al. Developmental changes in hypothalamic Kiss1 expression during activation of the pulsatile release of luteinising hormone in maturing ewe lambs. J Neuroendocrinol. 2011;23(9):815–822. doi: 10.1111/j.1365-2826.2011.02177.x. [DOI] [PubMed] [Google Scholar]
- (132).Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008;149(8):4151–4157. doi: 10.1210/en.2008-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Kurian JR, Keen KL, Guerriero KA, Terasawa E. Tonic Control of Kisspeptin Release in Prepubertal Monkeys: Implications to the Mechanism of Puberty Onset. Endocrinology. 2012 doi: 10.1210/en.2012-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Clarkson J, Han SK, Liu X, Lee K, Herbison AE. Neurobiological mechanisms underlying kisspeptin activation of gonadotropin-releasing hormone (GnRH) neurons at puberty. Mol Cell Endocrinol. 2010;324(1–2):45–50. doi: 10.1016/j.mce.2010.01.026. [DOI] [PubMed] [Google Scholar]
- (135).Kauffman AS, Navarro VM, Kim J, Clifton DK, Steiner RA. Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am J Physiol Endocrinol Metab. 2009;297(5):E1212–E1221. doi: 10.1152/ajpendo.00461.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Plant TM, Ramaswamy S, Dipietro MJ. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology. 2006;147(2):1007–1013. doi: 10.1210/en.2005-1261. [DOI] [PubMed] [Google Scholar]
- (137).Dumalska I, Wu M, Morozova E, Liu R, van den PA, Alreja M. Excitatory effects of the puberty-initiating peptide kisspeptin and group I metabotropic glutamate receptor agonists differentiate two distinct subpopulations of gonadotropin-releasing hormone neurons. J Neurosci. 2008;28(32):8003–8013. doi: 10.1523/JNEUROSCI.1225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (138).Clarkson J, Shamas S, Mallinson S, Herbison AE. Gonadal steroid induction of kisspeptin Peptide expression in the rostral periventricular area of the third ventricle during postnatal development in the male mouse. J Neuroendocrinol. 2012;24(6):907–915. doi: 10.1111/j.1365-2826.2012.02294.x. [DOI] [PubMed] [Google Scholar]
- (139).Olster DH, Foster DL. Control of gonadotropin secretion in the male during puberty: a decrease in response to steroid inhibitory feedback in the absence of an increase in steroid-independent drive in the sheep. Endocrinology. 1986;118(6):2225–2234. doi: 10.1210/endo-118-6-2225. [DOI] [PubMed] [Google Scholar]
- (140).Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V) Brain Res Rev. 2008;57(2):277–287. doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (141).Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, Tomikawa J, et al. Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol Reprod. 2009;81(6):1216–1225. doi: 10.1095/biolreprod.109.078311. [DOI] [PubMed] [Google Scholar]
- (142).Bakker J, Pierman S, Gonzalez-Martinez D. Effects of aromatase mutation (ArKO) on the sexual differentiation of kisspeptin neuronal numbers and their activation by same versus opposite sex urinary pheromones. Horm Behav. 2010;57(4–5):390–395. doi: 10.1016/j.yhbeh.2009.11.005. [DOI] [PubMed] [Google Scholar]
- (143).Gonzalez-Martinez D, De Mees C, Douhard Q, Szpirer C, Bakker J. Absence of gonadotropin-releasing hormone 1 and Kiss1 activation in alpha-fetoprotein knockout mice: prenatal estrogens defeminize the potential to show preovulatory luteinizing hormone surges. Endocrinology. 2008;149(5):2333–2340. doi: 10.1210/en.2007-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (144).d'Anglemont de Tassigny X, Fagg LA, Dixon JPC, Day K, Leitch HG, Hendrick AG, et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(25):10714–10719. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]


