Abstract
The aim was to compare ovarian response and clinical outcome of potential high-responders after stimulation with highly purified menotropin (HP-hMG) or recombinant follicle-stimulating hormone (rFSH) for in vitro fertilisation/intracytoplasmic sperm injection. Retrospective analysis was performed on data collected in two randomized controlled trials, one conducted following a long GnRH agonist protocol and the other with an antagonist protocol. Potential high-responders (n = 155 and n = 188 in the agonist and antagonist protocol, respectively) were defined as having an initial anti-Müllerian hormone (AMH) value >75th percentile (5.2 ng/ml). In both protocols, HP-hMG stimulation in women in the high AMH category was associated with a significantly lower occurrence of high response (≥15 oocytes retrieved) than rFSH stimulation; 33% versus 51% (p = 0.025) and 31% versus 49% (p = 0.015) in the long agonist and antagonist protocol, respectively. In the potential high-responder women, trends for improved live birth rate were observed with HP-hMG compared with rFSH (long agonist protocol: 33% versus 20%, p = 0.074; antagonist protocol: 34% versus 23%, p = 0.075; overall population: 34% versus 22%, p = 0.012). In conclusion, the type of gonadotropin used for ovarian stimulation influences high-response rates and potentially clinical outcome in women identified as potential high-responders.
Keywords: GnRH agonist, GnRH antagonist, gonadotropins, live birth, ovarian response
Introduction
Individualization of the treatment strategy is currently one of the most relevant topics in reproductive medicine. The basis for individualization of treatment in patients undergoing their first in vitro fertilisation (IVF)/intracytoplasmic sperm injection (ICSI) cycle is prediction of the ovarian response to gonadotropin stimulation, forecasting poor, normal or high response [1]. Clinicians may then choose between various treatment strategies to maximize efficacy and safety in the different response categories. Albeit it has been suggested that a specific type of gonadotropin-releasing hormone (GnRH) protocol may be more suitable for either potential hyper-responders or potential poor-responders [2–5], no studies have explored whether a specific type of gonadotropin preparation may offer additional advantages in certain groups of patients, such as patients at risk of hyper-response.
Hyper-responders are usually defined as women with high numbers of oocytes retrieved following a standard protocol of controlled ovarian stimulation (COS). Although these patients are generally considered a good-prognosis group regarding reproductive success, it is currently debated whether a high ovarian response is associated with decreased chance of successful outcome as compared with a normal response. Two large retrospective studies suggest that pregnancy and live birth rates in fresh embryo transfer cycles are directly related to oocyte yield with an almost linear relationship between live birth and increasing number of oocytes retrieved, with a decline in live birth rates at high oocyte yields [6,7]. In contrast, other retrospective analyses have described that a high ovarian response does not compromise pregnancy rates [8,9]. Differences in patient populations or treatment protocols may explain the inconsistent results in the literature concerning outcome in high-responders.
It has been demonstrated that the relatively good chance of success in women with potential for being high-responders could be further increased by using a GnRH antagonist protocol with a starting gonadotropin dose of 150 IU daily [10,11], but it is not established if the type of gonadotropin preparation should be taken into consideration to further modulate the ovarian response. Indeed, highly purified menotropin (HP-hMG) and recombinant follicle-stimulating hormone (rFSH) are associated with differential follicular growth [12,13], which may be attributed to differences in FSH isoforms and overall profile of isoforms, as well as the luteinizing hormone (LH)-activity component in HP-hMG [14]. On this basis, it can be hypothesised that the effective number of high-responders may be different when women with high numbers of recruitable follicles are treated with either HP-hMG or rFSH.
The aim of the present study was to evaluate the impact of the type of gonadotropin preparation (HP-hMG versus rFSH) used for COS on ovarian response and clinical outcome in potential high-responders undergoing IVF/ICSI treatment. The women were classified as being at risk of a high response based on a high serum level of anti-Müllerian hormone (AMH) at start of stimulation. AMH has been demonstrated to be a reliable surrogate marker for the functional ovarian follicle reserve [15]. Further, a high basal concentration of AMH has been shown to be associated with excessive response to gonadotropin stimulation [16–22].
Materials and methods
This study was a retrospective analysis of data prospectively collected in two randomized controlled trials comparing treatment outcome in patients undergoing stimulation with HP-hMG (Menopur; Ferring Pharmaceuticals or rFSH (follitropin alfa, Gonal-F; Merck Serono and follitropin beta, Puregon; MSD) following a long GnRH agonist protocol or a GnRH antagonist protocol, as described elsewhere [12,13].
Study populations
The main inclusion criteria for the long agonist trial were women aged 21–37 years; primary infertility diagnosis being tubal factor, unexplained infertility, or mild male factor; FSH 1–12 IU/l. The main inclusion criteria for the antagonist trial were women aged 21–34 years; primary infertility diagnosis being unexplained infertility or mild male factor; FSH 1–12 IU/l. In both trials, women with polycystic ovaries were excluded.
Study protocols
In the long agonist protocol, down-regulation was performed with triptorelin (0.1 mg/d) (Decapeptyl; Ferring Pharmaceuticals). The gonadotropin dose was fixed at 225 IU/d for the first 5 d, followed by dose-adjustments according to ovarian response. In the antagonist protocol, the gonadotropin dose was fixed at 150 IU for the first 5 d and adjusted according to ovarian response from day 6 when GnRH antagonist (ganirelix, Orgalutran; MSD) was initiated (0.25 mg/d) and continued throughout gonadotropin-treatment. In both protocols, hCG (250 µg) (choriogonadotropin alpha, Ovitrelle; Merck Serono) was administered when three follicles of ≥17 mm were observed. Oocyte retrieval took place 36 ± 2 h later. Luteal support was provided by vaginal administration of progesterone (Crinone 90 mg/d, Merck Serono; Utrogestan 600 mg/d, Seid) starting the day after oocyte retrieval and for at least 13–15 d after embryo transfer. In the long agonist protocol, 1–2 embryos was transferred on day 3 and in the antagonist protocol 1 blastocyst was transferred on day 5. Delivery of (at least) one live-born neonate defined live birth.
Serum assays
AMH was analysed by enzyme-linked immunosorbent assay (long agonist trial: Immunotech Beckman Coulter AMH ELISA [A11893], Marseilles, France; antagonist trial: Beckman Coulter Gen 2 ELISA [A79765] Webster, TX, US; 1 ng/ml = 7.14 pmol/l). The AMH assays had a sensitivity of 0.35 and 0.08 ng/ml and total imprecision (% coefficient of variation) of <9.5 and <7.7 in Immunotech Beckman Coulter and Beckman Coulter Gen 2, respectively. FSH, estradiol and progesterone were analysed by electrochemiluminescence immunoassay (Roche-Diagnostics ECLIA).
Statistical analysis
In total, the two trials comprised 1372 women with an AMH value on stimulation day 1. Women were classified as potential high-responders if initial AMH was in the uppermost quartile of the observed AMH distribution. In both protocols, the 75th percentile was identical (5.2 ng/ml = 37.4 pmol/l). One hundred fifty-five women treated in the long GnRH agonist protocol (76 and 79 in the HP-hMG and rFSH groups, respectively) and 188 women in the GnRH antagonist protocol (87 and 101 in the HP-hMG and rFSH groups, respectively) were classified as potential high-responders.
In each protocol, baseline characteristics, end-of-stimulation data, ovarian response and embryo data were compared between the women grouped according to their AMH value on stimulation day 1 (>75th versus ≤75th percentile). Similar analyses were performed for the potential high-responders comparing gonadotropin treatments (HP-hMG versus rFSH) within each protocol. Continuous and categorical data were compared using the Wilcoxon test and the Chi-Square or Fisher’s exact test, respectively. For the potential high-responders, risk of high response (≥15 oocytes retrieved) and chance of live birth were compared between treatments using the Chi-square test. The observed differences in live birth rates between gonadotropin-treatment groups were further analysed in the pooled population of potential high-responders from both protocols to determine if they could be attributed to baseline characteristics or end-of-stimulation variables. For each variable, a logistic regression model was fitted including treatment group and the variable in question in the linear predictor. Only fresh treatment cycles were included in the present dataset.
Results
High AMH category versus non-high AMH category
In both the long agonist protocol and the antagonist protocol, the women in the high AMH category were characterized by younger age, longer menstrual cycle length, higher AFC, lower FSH and larger ovarian volume at start of stimulation than women in the non-high AMH category (p ≤ 0.003 for each variable) (Table 1).
Table 1.
Demographics and baseline, end-of-stimulation, oocyte and embryo data of the women grouped by the AMH concentration at start of stimulation (quartiles 1–3 versus quartile 4).
| Variable | Long GnRH agonist protocol |
GnRH antagonist protocol |
||||
|---|---|---|---|---|---|---|
| AMH Q1-Q3 ≤75th (≤5.2 ng/ml) (n = 468) |
AMH Q4 >75th (>5.2 ng/ml) (n = 155) |
p Value* | AMH Q1-Q3 ≤75th (≤5.2 ng/ml) (n = 561) |
AMH Q4 >75th (>5.2 ng/ml) (n = 188) |
p Value* | |
| Clinical characteristics | ||||||
| Age (years) | 31 (29, 34) | 30 (28, 32) | <0.001 | 31 (29, 33) | 30 (28, 32) | <0.001 |
| BMI (kg/m2) | 21.9 (20.3, 24.0) | 21.3 (20.1, 23.5) | 0.113 | 21.9 (20.3, 23.8) | 21.8 (20.5, 23.5) | 0.868 |
| Cycle length (days) | 28 (28, 29) | 29 (28, 30) | <0.001 | 28 (28, 29) | 29 (28, 30) | <0.001 |
| First treatment cycle, n (%) | 327 (70%) | 104 (67%) | 0.517 | 427 (76%) | 134 (71%) | 0.186 |
| Day 1 (before start of stimulation) | ||||||
| Ovarian volume (ml) | 8.5 (6.0, 11.8) | 10.4 (7.7, 14.3) | <0.001 | 10.6 (8.0, 14.2) | 13.2 (9.6, 16.8) | <0.001 |
| AFC (n) | 10 (7, 14) | 11 (8, 18) | <0.001 | 14 (11, 17) | 18 (15, 22) | <0.001 |
| AMH (ng/ml) | 3.0 (2.1, 4.0) | 7.0 (5.8, 8.5) | <0.001 | 2.4 (1.4, 3.6) | 6.9 (6.0, 8.7) | <0.001 |
| FSH (IU/l) | 3.8 (3.0, 4.9) | 3.4 (2.7, 4.4) | 0.003 | 7.2 (6.2, 8.5) | 6.5 (5.7, 7.6) | <0.001 |
| End-of-stimulation | ||||||
| Estradiol (nmol/l) | 5.5 (4.0, 7.3) | 8.5 (6.2, 13.0) | <0.001 | 5.7 (4.1, 8.2) | 8.7 (6.3, 13.3) | <0.001 |
| Progesterone (nmol/l) | 2.6 (2.0, 3.4) | 3.2 (2.4, 3.9) | <0.001 | 2.5 (1.9, 3.2) | 2.9 (2.1, 3.8) | <0.001 |
| Progesterone/estradiol ratio | 0.46 (0.35, 0.63) | 0.36 (0.24, 0.49) | <0.001 | 0.42 (0.30, 0.60) | 0.32 (0.21, 0.44) | <0.001 |
| Follicles ≥12 mm (n) | 10 (8, 13) | 15 (12, 19) | <0.001 | 10 (7, 13) | 15 (11, 18) | <0.001 |
| Endometrial thickness (mm) | 11 (9, 12) | 11 (10, 12) | 0.079 | 10 (9, 12) | 11 (10, 12) | 0.039 |
| Endometrial echogenicity pattern (hypo, iso, hyper) (%) | 39, 49, 13 | 34, 51, 15 | 0.544 | 40, 51, 9 | 36, 54, 10 | 0.668 |
| Cycle cancellation for ovarian hyper-response, n (%) | 2 (<1%) | 7 (5%) | 0.001 | 1 (<1%) | 1 (<1%) | 0.439 |
| Early OHSS (moderate/severe), n (%) | 1 (<1%) | 7 (5%) | <0.001 | 4 (<1%) | 8 (4%) | 0.003 |
| Intervention for ovarian hyper-response, n (%) | 2 (<1%) | 11 (7%) | <0.001 | 16 (3%) | 19 (10%) | <0.001 |
| Oocyte retrieval | ||||||
| Women with oocyte retrieval, n (%) | 446 (95%) | 145 (94%) | 0.392 | 537 (96%) | 185 (98%) | 0.088 |
| Oocytes retrieved (n) | 9 (6, 12) | 14 (10, 18) | <0.001 | 8 (5, 11) | 12 (9, 17) | <0.001 |
| Women with ≥15 oocytes retrieved, n (%) | 76 (16%) | 65 (42%) | <0.001 | 63 (11%) | 76 (40%) | <0.001 |
| Fertilisation and embryo data | ||||||
| Fertilisation rate (%) | 60 (33, 75) | 52 (29, 70) | 0.091 | 60 (43, 75) | 58 (42, 71) | 0.193 |
| Embryos on day 3 (n) | 2 (1, 5) | 3 (2, 6) | 0.029 | |||
| Women with top-quality embryo(s) on day 3, n (%)† | 199 (45%) | 73 (50%) | 0.229 | |||
| Blastocysts on day 5 (n) | 2 (1, 4) | 3 (1, 6) | <0.001 | |||
| Women with good-quality blastocyst(s) on day 5, n (%)‡ | 266 (50%) | 106 (57%) | 0.068 | |||
| Women with transfer, n (%)¶ | 397 (89%) | 122 (84%) | 0.119 | 462 (86%) | 159 (86%) | 0.976 |
Values are median (IQR) unless otherwise indicated.
Wilcoxon test (continous data); Chi-Square test or Fisher’s exact test (categorial data).
Top-quality embryos were defined as 4–5 cells on day 2, ≥7 cells on day 3, equally-sized blastomeres and ≤20% fragmentation on day 3 and no multinucleation.
Good-quality blastocysts were defined as blastocysts with expansion and hatching score ≥4 and with inner cell mass and trophectoderm grades of A or B, using the definitions described by Gardner & Schoolcraft [23].
Among women with oocytes retrieved.
Independent of the protocol used, women with high AMH exhibited significantly (p < 0.001 for each variable) higher serum levels of estradiol and progesterone as well as increased number of growing follicles ≥12 mm at end of stimulation than women with no-high AMH. Further, the women with high AMH had significantly (p ≤ 0.003 for each variable) more oocytes retrieved, increased occurrence of high response, higher frequency of early OHSS and interventions for hyper-response. In the long agonist protocol, cycle cancellation due to ovarian hyper-response occurred more frequently among women in the high AMH category (p = 0.001). At end of stimulation, no clinically relevant differences were noted in endometrial thickness or echogenicity patterns between the two AMH categories (Table 1).
Significantly more embryos on day 3 (long agonist protocol: p = 0.029) or blastocysts on day 5 (antagonist protocol: p < 0.001) were available in women with high AMH, but the proportion of women with top-quality embryo(s) or good-quality blastocyst(s) were similar in the two AMH categories (Table 1).
HP-hMG versus rFSH stimulation in high AMH category
Within each protocol, there were no clinically relevant differences between the two gonadotropin-treatment groups in the high AMH category regarding demographics, fertility history and markers of ovarian reserve (Table 2). BMI was significantly lower in rFSH-treated women, but was not of clinical relevance. At end of stimulation, higher estradiol levels (p = 0.012) and lower progesterone levels (p < 0.001) were observed with HP-hMG in the antagonist and long agonist protocol, respectively.
Table 2.
Comparison of baseline, end-of-stimulation, oocyte and embryo characteristics between HP-hMG- and rFSH-treated women with potential for being high-responders by a high AMH at start of stimulation.
| Variable | Long GnRH agonist protocol |
GnRH antagonist protocol |
||||
|---|---|---|---|---|---|---|
| AMH Q4: >75th (>5.2 ng/ml) |
AMH Q4: >75th (>5.2 ng/ml) |
|||||
| HP-hMG (n = 76) | rFSH (n = 79) | p Value* | HP-hMG (n = 87) | rFSH (n = 101) | p Value* | |
| Clinical characteristics | ||||||
| Age (years) | 30 (28, 32) | 30 (28, 32) | 0.743 | 30 (28, 33) | 30 (28, 31) | 0.039 |
| BMI (kg/m2) | 22.5 (20.7, 23.8) | 20.8 (19.8, 22.8) | 0.002 | 22.1 (21.0, 23.9) | 21.6 (20.1, 23.0) | 0.022 |
| Cycle length (days) | 29 (28, 30) | 29 (28, 30) | 0.682 | 29 (28, 30) | 29 (28, 31) | 0.382 |
| First treatment cycle, n (%) | 52 (68%) | 52 (66%) | 0.731 | 57(66%) | 77 (76%) | 0.105 |
| Day 1 (before start of stimulation) | ||||||
| Ovarian volume (ml) | 10.3 (7.9, 13.9) | 10.5 (7.7, 14.8) | 0.807 | 13.4 (9.1, 17.0) | 13.0 (9.9, 16.7) | 0.885 |
| AFC (n) | 12 (8, 20) | 11 (8, 16) | 0.486 | 18 (15, 22) | 18 (15, 22) | 0.934 |
| AMH (ng/ml) | 7.0 (5.9, 8.5) | 7.0 (5.7, 8.4) | 0.912 | 7.1 (6.2, 8.7) | 6.8 (6.0, 8.3) | 0.347 |
| FSH (IU/l) | 3.2 (2.6, 4.4) | 3.6 (2.8, 4.4) | 0.257 | 6.7 (5.6, 7.7) | 6.4 (5.7, 7.5) | 0.251 |
| End-of-stimulation | ||||||
| Estradiol (nmol/l) | 8.7 (6.4, 13.0) | 8.4 (6.1, 12.8) | 0.736 | 9.7 (6.8, 14.8) | 7.8 (5.5, 12.4) | 0.012 |
| Progesterone (nmol/l) | 2.7 (1.9, 3.6) | 3.6 (2.8, 4.5) | <0.001 | 2.8 (2.1, 3.9) | 3.0 (2.1, 3.8) | 0.857 |
| Progesterone/estradiol ratio | 0.31 (0.21, 0.42) | 0.43 (0.30, 0.52) | <0.001 | 0.26 (0.20, 0.41) | 0.34 (0.24, 0.47) | 0.011 |
| Follicles ≥12 mm (n) | 15 (12, 18) | 16 (13, 19) | 0.274 | 14 (11, 18) | 16 (12, 19) | 0.064 |
| Endometrial thickness (mm) | 11 (10, 12) | 11 (10, 12) | 0.522 | 11 (10, 12) | 11 (10, 12) | 0.478 |
| Endometrial echogenicity pattern (hypo, iso, hyper) (%) | 44, 43, 13 | 24, 60, 17 | 0.033 | 31, 58, 11 | 41, 50, 9 | 0.386 |
| Cycle cancellation for ovarian hyper-response, n (%) | 3 (4%) | 4 (5%) | 1.000 | 0 (0%) | 1 (<1%) | – |
| Early OHSS (moderate/severe), n (%) | 3 (4%) | 4 (5%) | 1.000 | 3 (3%) | 5 (5%) | 0.727 |
| Intervention for ovarian hyper-response, n (%) | 5 (7%) | 6 (8%) | 1.000 | 7 (8%) | 12 (12%) | 0.470 |
| Oocyte retrieval | ||||||
| Women with oocyte retrieval, n (%) | 72 (95%) | 73 (92%) | 0.555 | 85 (98%) | 100 (99%) | 0.475 |
| Oocytes retrieved (n) | 12 (9, 16) | 15 (11, 20) | 0.007 | 12 (8, 15) | 14 (10, 19) | 0.033 |
| Women with ≥15 oocytes retrieved, n (%) | 25 (33%) | 40 (51%) | 0.025 | 27 (31%) | 49 (49%) | 0.015 |
| Fertilisation and embryo data | ||||||
| Fertilisation rate (%) | 50 (27, 73) | 56 (35, 69) | 0.826 | 57 (43, 69) | 60 (41, 73) | 0.663 |
| Embryos on day 3 (n) | 3 (2, 6) | 4 (2, 6) | 0.806 | |||
| Women with top-quality embryo(s) on day 3, n (%)† | 38 (53%) | 35 (48%) | 0.561 | |||
| Blastocysts on day 5 (n) | 3 (1, 6) | 3 (1, 6) | 0.969 | |||
| Women with good-quality blastocyst(s) on day 5, n (%)‡ | 55 (65%) | 51 (51%) | 0.060 | |||
| Women with transfer, n (%)¶ | 61 (85%) | 61 (84%) | 0.848 | 72 (85%) | 87 (87%) | 0.655 |
Values are median (IQR) unless otherwise indicated.
Wilcoxon test (continous data); Chi-Square test or Fisher’s exact test (categorial data).
Top-quality embryos were defined as 4–5 cells on day 2, ≥7 cells on day 3, equally-sized blastomeres and ≤20% fragmentation on day 3 and no multinucleation.
Good-quality blastocysts were defined as blastocysts with expansion and hatching score ≥4 and with inner cell mass and trophectoderm grades of A or B, using the definitions described by Gardner & Schoolcraft [23].
Among women with oocytes retrieved.
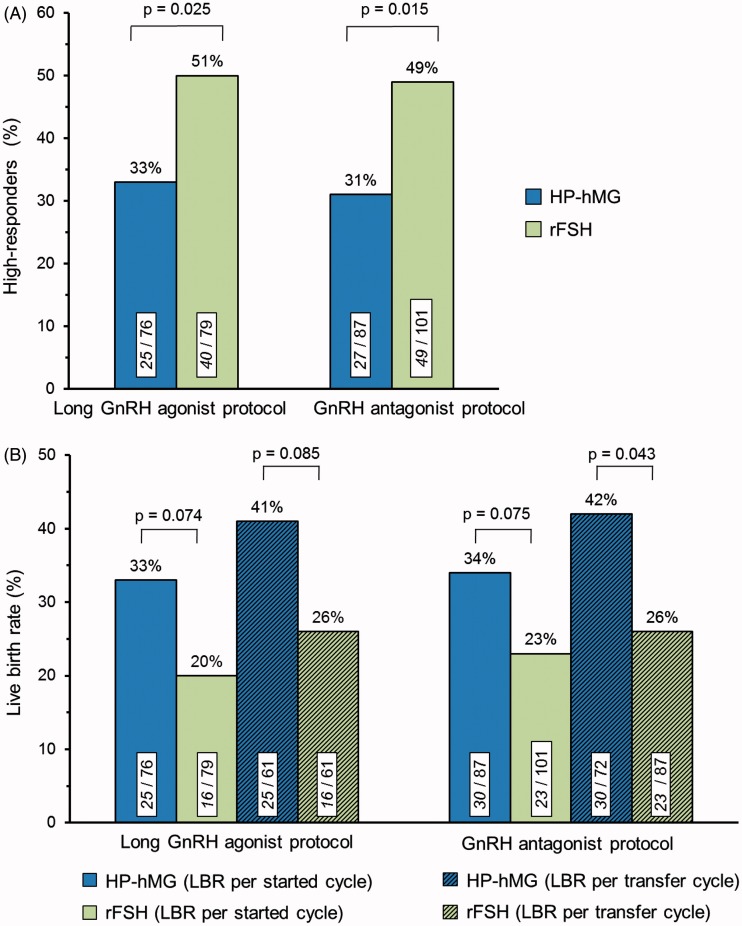
HP-hMG was associated with lower median number of oocytes retrieved in women with high AMH compared with rFSH (long agonist protocol: −3 oocytes, p = 0.007; antagonist protocol: −2 oocytes, p = 0.033) (Table 2). In both protocols, the percentage of women with a high ovarian response was significantly lower for HP-hMG compared with rFSH (long agonist protocol: 33% versus 51%, p = 0.025; antagonist protocol: 31% versus 49%, p = 0.015) (Figure 1A). Therefore, the risk of high response was consistently reduced with HP-hMG by 35 and 37%, respectively. There were no apparent differences between the two gonadotropin groups concerning cycle cancellations due to excessive response, early moderate/severe OHSS or interventions for excessive response in either protocol.
Figure 1.
(A) Occurrence of high ovarian response (≥15 oocytes retrieved) and (B) live birth rates (LBR) among the women classified as potential high responders by a high initial AMH level. Values within bars are n/total. p Values are based on the Chi-Square test.
Within each protocol, fertilisation rate, number of embryos/blastocysts available for transfer, women with top-quality embryo(s)/good-quality blastocyst(s) and percentages of women with transfer were similar between the HP-hMG and rFSH groups in the high AMH category (Table 2). However, in both protocols a statistical trend (p < 0.10) for improved live birth rate per started cycle was observed for HP-hMG compared with rFSH (Figure 1B). When restricted to women with embryo transfer, the difference in live birth rate between HP-hMG and rFSH was statistically significant (p = 0.043) in the antagonist protocol.
When the data of women with high AMH from both protocols were integrated, HP-hMG treatment was associated with significantly lower incidence of high response [32% (52/163) versus 49% (89/180), p < 0.001] and increased live birth rate per started cycle [34% (55/163) versus 22% (39/180), p = 0.012] as well as per embryo transfer cycle [41% (55/133) versus 26% (39/148), p = 0.008] compared with rFSH treatment. The logistic regression analysis (Supplementary Table 1) indicated that the type of GnRH protocol did not explain the difference in live birth rates between HP-hMG and rFSH, as it remained significant (p = 0.012) in the adjusted analysis. The probability of a live birth significantly increased with the availability of a top-quality embryo/good-quality blastocyst for transfer (p < 0.001), while an increased progesterone level (p = 0.042) and increased progesterone/estradiol ratio (p = 0.042) at end of stimulation significantly decreased the probability of live birth (Supplementary Table 1). However, in all adjusted analyses the difference between the two gonadotropin preparations remained significant (p < 0.05) indicating that the higher live birth rate in women with high AMH and stimulated with HP-hMG could not be attributed to differences in the baseline and end-of-stimulation variables examined.
Discussion
Several previous studies have shown that AMH can accurately identify women who are at risk of having an excessive ovarian response to COS [16–22]. In the present study, the prevalence of patients with a high ovarian response (i.e. ≥15 oocytes retrieved) was approximately three times higher in women with high AMH (>5.2 ng/ml) than in women in the non-high AMH category in both the long GnRH agonist and GnRH antagonist protocol. Recent meta-analyses comparing outcome of GnRH agonist versus antagonist indicate that the incidence of severe OHSS is significantly lower in antagonist protocols [24,25]. The use of GnRH antagonist has therefore been advocated in predicted high-responders, such as patients with high basal AMH [11,26,27] and PCOS patients [4,5]. In the present study in women with high AMH, similar incidences of high response, early moderate/severe OHSS as well as need of intervention because of ovarian hyper-response were observed in the antagonist and long agonist protocols. Additional adjustments of the treatment regimen, beyond the type of protocol, may therefore be required in patients with high AMH to reduce high-response rate and maximize safe use of gonadotropins. Furthermore, the present study suggests that consideration should be made to the actual gonadotropin preparation to choose the optimal stimulation strategy for each patient, as HP-hMG was associated with a substantially lower high-response rate than rFSH. Hence, the risk of developing a high response where an excessive response is predicted may be reduced by approximately one third with the elective use of HP-hMG, even in the GnRH antagonist protocol.
The rationale for a more moderate ovarian response with HP-hMG, and thereby a reduced risk of hyper-response with HP-hMG compared with rFSH, may be attributed to the FSH and/or LH components in the preparations. Like other glycoproteins, FSH displays a high degree of structural heterogeneity due to differences in the amount and/or composition of the carbohydrate structures, in particular sialic acid residues. Human-derived FSH shows more complex isoform heterogeneity than rFSH expressed by Chinese hamster ovary (CHO) cells. This is most likely because the CHO cells lack the enzymatic functions to construct the more complex oligosaccharide structures found in humans [28]. The composition of the carbohydrate moieties has a significant impact on the in vivo bioactivity of the various isoforms of FSH by affecting the metabolic clearance rate, the binding properties to the FSH receptors on the granulosa cells of the ovary and its ability to activate the receptors [29–33]. Hence, despite administration of similar amounts of bioactive FSH as measured by the Steelman-Pohley in vivo rat assay [34], the different FSH isoform profiles of HP-hMG and rFSH may influence the in vivo biopotency in humans and thereby the rate of high ovarian response among the potential high-responders. Another hypothesis to be considered is that exposure to the LH activity in HP-hMG early in the stimulation induces an initial higher level of androgens compared with stimulation with rFSH [35]. A higher androgen level has been suggested to increase the sensitivity of the follicle to FSH via up-regulation of the FSH receptors at an early stage of the follicle development leading to decrease in granulosa cell proliferation and, therefore, affecting the androgen-estrogen tonus [35,36]. The shift in favour of androgens early in the stimulation with HP-hMG may induce a more selective follicle recruitment process, thereby influencing the number of follicles/oocytes that will develop during the COS [35].
Interestingly, when using either type of GnRH analogue in women with high AMH there were consistent trends of increased success rates with HP-hMG compared with rFSH. The logistic regression analyses in the overall population did not identify any specific variable that explained the different live birth rates between HP-hMG and rFSH, but indicated that progesterone levels and progesterone/estradiol ratios at the end of stimulation as well as availability of top-quality embryos/good-quality blastocysts influenced live birth rates. Several studies have reported that elevated progesterone levels in the late follicular phase decrease pregnancy/live birth rates [37–40], which is considered to be due to advancement of the endometrium [41,42], without affecting oocyte/embryo quality [43–45]. Also, an elevated progesterone/estradiol ratio on the day of hCG has been suggested to better reflect “premature luteinisation” [46] and to be associated with lower pregnancy rates in both agonist [47] and antagonist protocols [48]. It should be noted that the progesterone/estradiol ratio was significantly lower after HP-hMG treatment in both protocols in the present study due to the more estrogenic microenvironment induced by the HP-hMG preparation [35]. Finally, it has been suggested that the type of stimulation protocol and the magnitude of the ovarian response may have direct effects on oocyte quality and aneuploidy rate [49–52], and incidences of embryo chromosome abnormalities has been reported to be higher for women with high response to stimulation [52,53]. The present study reinforces that progesterone, progesterone/estradiol ratio and embryo quality plays a role in treatment outcome in patients at risk of hyper-response based on high serum AMH levels. The presence of LH-activity in the menotropin preparation may explain the potential treatment outcome differences between the HP-hMG and rFSH groups by influencing some of the endocrine [12,13,35] or embryo-quality parameters [35,54,55].
In conclusion, the present study suggests that women prospectively identified as potential high-responders by a high initial AMH have a lower rate of high ovarian response with HP-hMG than with rFSH during COS for IVF/ICSI treatment. The potential impact on clinical outcome should instigate additional investigations for confirming prospectively this finding and elucidate further the mechanisms implicated.
Acknowledgements
The authors thank Göran Pettersson, PhD, Reproductive Health, Ferring Pharmaceuticals for assistance in writing the article.
Footnotes
Declaration of interest: A. L. M. has received consultancy fees or payment for lectures or speaker bureaus from Beckman Coulter, Roche Diagnostics, Ferring Pharmaceuticals, IBSA, Merck Serono and MSD. J.-C. A. and B. M. K. are employees of Ferring Pharmaceuticals.
References
- 1.La Marca A, Sunkara SK. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Hum Reprod Update. 2014;20:124–40. doi: 10.1093/humupd/dmt037. [DOI] [PubMed] [Google Scholar]
- 2.Devroey P, Polyzos NP, Blockeel C. An OHSS-free clinic by segmentation of IVF treatment. Hum Reprod. 2011;26:2593–7. doi: 10.1093/humrep/der251. [DOI] [PubMed] [Google Scholar]
- 3.Pu D, Wu J, Liu J. Comparisons of GnRH antagonist versus GnRH agonist protocol in poor ovarian responders undergoing IVF. Hum Reprod. 2011;26:2742–9. doi: 10.1093/humrep/der240. [DOI] [PubMed] [Google Scholar]
- 4.Pundir J, Sunkara SK, El-Toukhy T, Khalaf Y. Meta-analysis of GnRH antagonist protocols: do they reduce the risk of OHSS in PCOS? Reprod Biomed Online. 2012;24:6–22. doi: 10.1016/j.rbmo.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 5.Kol S, Homburg R, Alsbjerg B, Humaidan P. The gonadotropin-releasing hormone antagonist protocol - the protocol of choice for the polycystic ovary syndrome patient undergoing controlled ovarian stimulation. Acta Obstet Gynecol Scand. 2012;91:643–7. doi: 10.1111/j.1600-0412.2012.01399.x. [DOI] [PubMed] [Google Scholar]
- 6.van der Gaast MH, Eijkemans MJ, van der Net JB, et al. Optimum number of oocytes for a successful first IVF treatment cycle. Reprod Biomed Online. 2006;13:476–80. doi: 10.1016/s1472-6483(10)60633-5. [DOI] [PubMed] [Google Scholar]
- 7.Sunkara SK, Rittenberg V, Raine-Fenning N, et al. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod. 2011;26:1768–74. doi: 10.1093/humrep/der106. [DOI] [PubMed] [Google Scholar]
- 8.Kok JD, Looman CW, Weima SM, Te Velde ER. A high number of oocytes obtained after ovarian hyperstimulation for in vitro fertilization or intracytoplasmic sperm injection is not associated with decreased pregnancy outcome. Fertil Steril. 2006;85:918–24. doi: 10.1016/j.fertnstert.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 9.Fatemi HM, Doody K, Griesinger G, et al. High ovarian response does not jeopardize ongoing pregnancy rates and increases cumulative pregnancy rates in a GnRH-antagonist protocol. Hum Reprod. 2013;28:442–52. doi: 10.1093/humrep/des389. [DOI] [PubMed] [Google Scholar]
- 10.Nelson SM, Yates RW, Lyall H, et al. Anti-Müllerian hormone-based approach to controlled ovarian stimulation for assisted conception. Hum Reprod. 2009;24:867–75. doi: 10.1093/humrep/den480. [DOI] [PubMed] [Google Scholar]
- 11.Yates AP, Rustamov O, Roberts SA, et al. Anti-Mullerian hormone-tailored stimulation protocols improve outcomes whilst reducing adverse effects and costs of IVF. Hum Reprod. 2011;26:2353–62. doi: 10.1093/humrep/der182. [DOI] [PubMed] [Google Scholar]
- 12.Nyboe Andersen A, Devroey P, Arce J-C for the MERIT Group. Clinical outcome following stimulation with highly purified hMG or recombinant FSH in patients undergoing IVF: a randomized assessor-blind controlled trial. Hum Reprod. 2006;21:3217–27. doi: 10.1093/humrep/del284. [DOI] [PubMed] [Google Scholar]
- 13.Devroey P, Pellicer A, Nyboe Andersen A, Arce J-C on behalf of the Menopur in GnRH Antagonist Cycles with Single Embryo Transfer (MEGASET) Trial Group. A randomized assessor-blind trial comparing highly purified hMG and recombinant FSH in a GnRH antagonist cycle with compulsory single-blastocyst transfer. Fertil Steril. 2012;97:561–71. doi: 10.1016/j.fertnstert.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Wolfenson C, Groisman J, Couto AS, et al. Batch-to-batch consistency of human-derived gonadotrophin preparations compared with recombinant preparations. Reprod Biomed Online. 2005;10:442–54. doi: 10.1016/s1472-6483(10)60819-x. [DOI] [PubMed] [Google Scholar]
- 15.La Marca A, Sighinolfi G, Radi D, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART) Hum Reprod Update. 2010;16:113–30. doi: 10.1093/humupd/dmp036. [DOI] [PubMed] [Google Scholar]
- 16.La Marca A, Giulini S, Tirelli A, et al. Anti-Mullerian hormone measurement on any day of the menstrual cycle strongly predicts ovarian response in assisted reproductive technology. Hum Reprod. 2007;22:766–71. doi: 10.1093/humrep/del421. [DOI] [PubMed] [Google Scholar]
- 17.Nelson SM, Yates RW, Fleming R. Serum anti-Müllerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles - implications for individualization of therapy. Hum Reprod. 2007;22:2414–21. doi: 10.1093/humrep/dem204. [DOI] [PubMed] [Google Scholar]
- 18.Lee TH, Liu CH, Huang CC, et al. Serum anti-Müllerian hormone and estradiol levels as predictors of ovarian hyperstimulation syndrome in assisted reproduction technology cycles. Hum Reprod. 2008;23:160–7. doi: 10.1093/humrep/dem254. [DOI] [PubMed] [Google Scholar]
- 19.Nardo LG, Gelbaya TA, Wilkinson H, et al. Circulating basal anti-Müllerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril. 2009;92:1586–93. doi: 10.1016/j.fertnstert.2008.08.127. [DOI] [PubMed] [Google Scholar]
- 20.Broer SL, Dólleman M, Opmeer BC, et al. AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: a meta-analysis. Hum Reprod Update. 2011;17:46–54. doi: 10.1093/humupd/dmq034. [DOI] [PubMed] [Google Scholar]
- 21.Anckaert E, Smitz J, Schiettecatte J, et al. The value of anti-Müllerian hormone measurement in the long GnRH agonist protocol: association with ovarian response, dose adjustments, embryo quality and pregnancy. Hum Reprod. 2012;27:1829–39. doi: 10.1093/humrep/des101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arce J-C, La Marca A, Mirner Klein B, et al. Antimüllerian hormone in gonadotropin releasing-hormone antagonist cycles: prediction of ovarian response and cumulative treatment outcome in good-prognosis patients. Fertil Steril. 2013;99:1644–53. doi: 10.1016/j.fertnstert.2012.12.048. [DOI] [PubMed] [Google Scholar]
- 23.Gardner DK, Schoolcraft WB. In-vitro culture of human blastocysts. In: Jansen R, Mortimer D, editors. Towards reproductive certainty: fertility and genetics beyond 1999. New York: The Parthenon Publishing Group; 1999. pp. 378–88. [Google Scholar]
- 24.Kolibianakis EM, Collins J, Tarlatzis BC, et al. Among patients treated for IVF with gonadotrophins and GnRH analogues, is the probability of live birth dependent on the type of analogue used? A systematic review and meta-analysis. Hum Reprod Update. 2006;12:651–71. doi: 10.1093/humupd/dml038. [DOI] [PubMed] [Google Scholar]
- 25.Al-Inany HG, Youssef MA, Aboulghar M, et al. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev. 2011;5:CD001750. doi: 10.1002/14651858.CD001750.pub3. [DOI] [PubMed] [Google Scholar]
- 26.Fleming R, Broekmans F, Calhaz-Jorge C, et al. Can anti-Müllerian hormone concentrations be used to determine gonadotrophin dose and treatment protocol for ovarian stimulation? Reprod Biomed Online. 2013;26:431–9. doi: 10.1016/j.rbmo.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 27.Nelson SM. Biomarkers of ovarian response: current and future applications. Fertil Steril. 2013;99:963–9. doi: 10.1016/j.fertnstert.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 28.Horsman G, Talbot JA, McLoughlin JD, et al. A biological, immunological and physico-chemical comparison of the current clinical batches of the recombinant FSH preparations Gonal-F and Puregon. Hum Reprod. 2000;15:1898–902. doi: 10.1093/humrep/15.9.1898. [DOI] [PubMed] [Google Scholar]
- 29.Flack MR, Bennet AP, Froehlich J, et al. Increased biological activity due to basic isoforms in recombinant human follicle-stimulating hormone produced in a human cell line. J Clin Endocrinol Metab. 1994;79:756–60. doi: 10.1210/jcem.79.3.8077357. [DOI] [PubMed] [Google Scholar]
- 30.Barrios-de-Tomasi J, Timossi C, Merchant H, et al. Assessment of the in vitro biological activities of the human follicle stimulating hormones. Mol Cell Endocrinol. 2002;186:189–98. doi: 10.1016/s0303-7207(01)00657-8. [DOI] [PubMed] [Google Scholar]
- 31.Ulloa-Aguirre A, Timossi C, Barrios-de-Tomasi J, et al. Impact of carbohydrate heterogeneity in function of follicle-stimulating hormone: studies derived from in vitro and in vivo models. Biol Reprod. 2003;69:379–89. doi: 10.1095/biolreprod.103.016915. [DOI] [PubMed] [Google Scholar]
- 32.Lambert A, Rodgers M, Mitchell R, et al. In-vitro biopotency and glycoform distribution of recombinant human FSH (ORG32489), Metrodin and Metrodin-HP. Hum Reprod. 1995;10:1928–35. doi: 10.1093/oxfordjournals.humrep.a136208. [DOI] [PubMed] [Google Scholar]
- 33.de Leeuw R, Mulders J, Voortman G, et al. Structure-function relationship of recombinant follicle stimulating hormone (Puregon) Mol Hum Reprod. 1996;2:361–9. doi: 10.1093/molehr/2.5.361. [DOI] [PubMed] [Google Scholar]
- 34.Steelman SL, Pohley FM. Assay of the follicle stimulating hormone based on the augmentation with human chorionic gonadotropin. Endocrinology. 1953;53:604–16. doi: 10.1210/endo-53-6-604. [DOI] [PubMed] [Google Scholar]
- 35.Smitz J, Andersen AN, Devroey P, Arce J-C MERIT Group. Endocrine profile in serum and follicular fluid differs after ovarian stimulation with HP-hMG or recombinant FSH in IVF patients. Hum Reprod. 2007;22:676–87. doi: 10.1093/humrep/del445. [DOI] [PubMed] [Google Scholar]
- 36.Luo W, Wiltbank MC. Distinct regulation by steroids of messenger RNAs for FSHR and CYP19A1 in bovine granulosa cells. Biol Reprod. 2006;75:217–25. doi: 10.1095/biolreprod.105.047407. [DOI] [PubMed] [Google Scholar]
- 37.Bosch E, Labarta E, Crespo J, et al. Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: analysis of over 4000 cycles. Hum Reprod. 2010;25:2092–100. doi: 10.1093/humrep/deq125. [DOI] [PubMed] [Google Scholar]
- 38.Kolibianakis EM, Venetis CA, Bontis J, Tarlatzis BC. Significantly lower pregnancy rates in the presence of progesterone elevation in patients treated with GnRH antagonists and gonadotrophins: a systematic review and meta-analysis. Curr Pharm Biotechnol. 2012;13:464–70. doi: 10.2174/138920112799361927. [DOI] [PubMed] [Google Scholar]
- 39.Huang R, Fang C, Xu S, et al. Premature progesterone rise negatively correlated with live birth rate in IVF cycles with GnRH agonist: an analysis of 2,566 cycles. Fertil Steril. 2012;98:664–70. doi: 10.1016/j.fertnstert.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 40.Ochsenkühn R, Arzberger A, von Schönfeldt V, et al. Subtle progesterone rise on the day of human chorionic gonadotropin administration is associated with lower live birth rates in women undergoing assisted reproductive technology: a retrospective study with 2,555 fresh embryo transfers. Fertil Steril. 2012;98:347–54. doi: 10.1016/j.fertnstert.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 41.Labarta E, Martínez-Conejero JA, Alamá P, et al. Endometrial receptivity is affected in women with high circulating progesterone levels at the end of the follicular phase: a functional genomics analysis. Hum Reprod. 2011;26:1813–25. doi: 10.1093/humrep/der126. [DOI] [PubMed] [Google Scholar]
- 42.Van Vaerenbergh I, Fatemi HM, Blockeel C, et al. Progesterone rise on HCG day in GnRH antagonist/rFSH stimulated cycles affects endometrial gene expression. Reprod Biomed Online. 2011;22:263–71. doi: 10.1016/j.rbmo.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Melo MA, Meseguer M, Garrido N, et al. The significance of premature luteinization in an oocyte-donation programme. Hum Reprod. 2006;21:1503–07. doi: 10.1093/humrep/dei474. [DOI] [PubMed] [Google Scholar]
- 44.Lahoud R, Kwik M, Ryan J, et al. Elevated progesterone in GnRH agonist down regulated in vitro fertilisation (IVFICSI) cycles reduces live birth rates but not embryo quality. Arch Gynecol Obstet. 2012;285:535–40. doi: 10.1007/s00404-011-2045-0. [DOI] [PubMed] [Google Scholar]
- 45.Xu B, Li Z, Zhang H, et al. Serum progesterone level effects on the outcome of in vitro fertilization in patients with different ovarian response: an analysis of more than 10,000 cycles. Fertil Steril. 2012;97:1321–7. doi: 10.1016/j.fertnstert.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 46.Younis JS, Haddad S, Matilsky M, Ben-Ami M. Premature luteinization: could it be an early manifestation of low ovarian reserve? Fertil Steril. 1998;69:461–5. doi: 10.1016/s0015-0282(97)00561-x. [DOI] [PubMed] [Google Scholar]
- 47.Elgindy EA. Progesterone level and progesterone/estradiol ratio on the day of hCG administration: detrimental cutoff levels and new treatment strategy. Fertil Steril. 2011;95:1639–44. doi: 10.1016/j.fertnstert.2010.12.065. [DOI] [PubMed] [Google Scholar]
- 48.Cetinkaya ES, Berker B, Aytac R, et al. The value of the progesterone-to-estradiol ratio on the day of hCG administration in predicting ongoing pregnancy and live birth rates in normoresponders undergoing GnRH antagonist cycles. Eur J Obstet Gynecol Reprod Biol. 2013;170:452–7. doi: 10.1016/j.ejogrb.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 49.Munne S, Magli C, Adler A, et al. Treatment-related chromosome abnormalities in human embryos. Hum Reprod. 1997;12:780–4. doi: 10.1093/humrep/12.4.780. [DOI] [PubMed] [Google Scholar]
- 50.Munné S, Ary J, Zouves C, et al. Wide range of chromosome abnormalities in the embryos of young egg donors. Reprod Biomed Online. 2006;12:340–6. doi: 10.1016/s1472-6483(10)61007-3. [DOI] [PubMed] [Google Scholar]
- 51.Haaf T, Hahn A, Lambrecht A, et al. A high oocyte yield for intracytoplasmic sperm injection treatment is associated with an increased chromosome error rate. Fertil Steril. 2009;91:733–8. doi: 10.1016/j.fertnstert.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 52.Rubio C, Mercader A, Alamá P, et al. Prospective cohort study in high responder oocyte donors using two hormonal stimulation protocols: impact on embryo aneuploidy and development. Hum Reprod. 2010;25:2290–7. doi: 10.1093/humrep/deq174. [DOI] [PubMed] [Google Scholar]
- 53.Soares SR, Rubio C, Rodrigo L, et al. High frequency of chromosomal abnormalities in embryos obtained from oocyte donation cycles. Fertil Steril. 2003;80:656–7. doi: 10.1016/s0015-0282(03)00787-8. [DOI] [PubMed] [Google Scholar]
- 54.Ziebe S, Lundin K, Janssens R, Helmgaard L, Arce J-C MERIT (Menotrophin vs Recombinant FSH in vitro Fertilisation Trial) Group. Influence of ovarian stimulation with HP-hMG or recombinant FSH on embryo quality parameters in patients undergoing IVF. Hum Reprod. 2007;22:2404–13. doi: 10.1093/humrep/dem221. [DOI] [PubMed] [Google Scholar]
- 55.Weghofer A, Munné S, Brannath W, et al. The impact of LH-containing gonadotropins on diploidy rates in preimplantation embryos: long protocol stimulation. Hum Reprod. 2008;23:499–503. doi: 10.1093/humrep/dem412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.