Abstract
An expeditious one-pot, ligand-free, Pd(OAc)2-catalyzed, three-component reaction for the synthesis of 2,3-diarylimidazo[1,2-a]pyridines was developed under microwave irradiation. With the high availability of commercial reagents and great efficiency in expanding molecule diversity, this methodology is superior to the existing procedures for the synthesis of 2,3-diarylimidazo[1,2-a]pyridines analogues.
Imidazo[1,2-a]pyridines are extremely popular among chemists and medicinal chemists due to their remarkable biological and pharmacological activities1 such as anticancer,2 anti-inflammatory,3 antibacterial,4 antiprotozonal,5 antiviral,6 antiulcer,7 and antifungal.8 Within the imidazo[1,2-a]pyridine family, several products have progressed to market, including alpidem (anxiolytic),9 necopidem (anxiolytic),10 saripidem (anxiolytic),11 zolpidem (insomnia),12 olprinone (cardiotonic),13 minodronic acid (bisphosphonate),14 divalpon (anxiolytic),15 and zolimidine (antiulcer).16
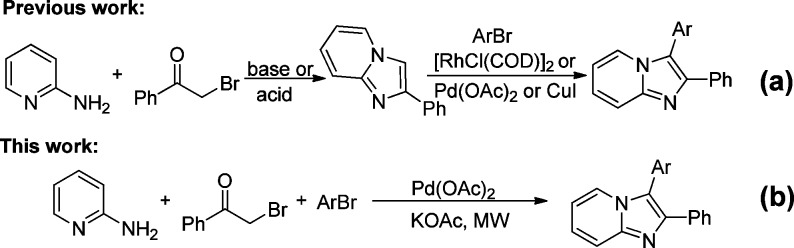
The imidazo[1,2-a]pyridine derivatives, 2,3-diarylimidazo[1,2-a]pyridines also exhibit a wide range of biological activities.17 In particular, medicinal chemists have focused on the core structure to produce inhibitors18 for PI3K, p38, and TGF-β kinases. Owing to the attractive biological properties of 2,3-diarylimidazo[1,2-a]pyridines, a variety of strategies to synthesize the core structure have been developed, focusing on (1) condensation between 2-aminopyridines and 2-bromo-1,2-diarylethanone;19 (2) Suzuki-type cross-coupling between 3-halo-2-arylimidazo[1,2-a]pyridines and arylboronic acid,20 and (3) copper-catalyzed conversion of pyridine to 2,3-diarylimidazo[1,2-a]pyridines.21 Very recently, Pd-, Rh-, and Cu- catalyzed direct C–H arylations of 2-arylimidazo[1,2-a]pyridines at C-3 have been extensively studied (Scheme 1a).22 The transformation is completed in stepwise sequences to afford 2,3-diarylimidazo[1,2-a]pyridines. Therefore, an efficient synthetic methodology involving commercially available and inexpensive starting materials is required to expeditiously synthesize diverse 2,3-diarylimidazo[1,2-a]pyridines. In order to develop a convenient synthetic route in conjunction with the possibility for rapid library diversification, we were interested in conducting the two-step cyclization/C–H arylation in one pot (Scheme 1b). To the best of our knowledge, there has never been a report of a one-pot, ligand-free, palladium-catalyzed, three-component reaction to afford 2,3-diarylimidazo[1,2-a]pyridines under microwave irradiation.
Scheme 1. Methods for the Synthesis of 2,3-Diarylimidazo[1,2-a]pyridines.
2-Aminopyridine (1a), 2-bromo-1-phenylethanone (2a), and 1-bromo-4-nitrobenzene (3a) were chosen as model substrates to optimize the reaction conditions. We initiated our studies by examining the influence of microwave irradiation on the conversion using KOAc as the base and 10 mol % of Pd(OAc)2 as the catalyst. First, we carried out a reaction in DMF at 100 °C under microwave irradiation for 30 min, but only trace amounts of product 4aaa were detected (19% yield, Table 1, entry 1). Increasing the temperature from 100 to 120 °C improved the yield to 36% (entry 2). A high yield of 66% was obtained with a temperature of 160 °C (entry 4). Unfortunately, yields decreased with higher temperatures (entries 5 and 6). The yield was optimized to 84% by increasing the reaction time to 1 h (entry 7). Then, the reaction was performed using different catalyst loads (entry 8–11). In the presence of 8 mol % of Pd(OAc)2, a yield of 78% was obtained and a significant yield was lost with lower catalytic loads.
Table 1. Optimization of Reaction Conditionsa.
| entry | Pd(OAc)2 (mol %) | base | solvent | temp (°C), time | yieldb (%) |
|---|---|---|---|---|---|
| 1 | 10 | KOAc | DMF | 100, 30 min | 19 |
| 2 | 10 | KOAc | DMF | 120, 30 min | 36 |
| 3 | 10 | KOAc | DMF | 140, 30 min | 42 |
| 4 | 10 | KOAc | DMF | 160, 30 min | 66 |
| 5 | 10 | KOAc | DMF | 170, 30 min | 51 |
| 6 | 10 | KOAc | DMF | 180, 30 min | 49 |
| 7 | 10 | KOAc | DMF | 160, 1 h | 84 |
| 8 | 2 | KOAc | DMF | 160, 1 h | 45 |
| 9 | 4 | KOAc | DMF | 160, 1 h | 56 |
| 10 | 6 | KOAc | DMF | 160, 1 h | 68 |
| 11 | 8 | KOAc | DMF | 160, 1 h | 78 |
| 12 | 10 | CsOAc | DMF | 160, 1 h | 67 |
| 13 | 10 | NaOAc | DMF | 160, 1 h | 54 |
| 14 | 10 | K2CO3 | DMF | 160, 1 h | 35 |
| 15 | 10 | Cs2CO3 | DMF | 160, 1 h | 23 |
| 16 | 10 | KOAc | DMAc | 160, 1 h | 77 |
| 17 | 10 | KOAc | NMP | 160, 1 h | 62 |
| 18 | 10 | KOAc | xylene | 160, 1 h | 35 |
| 19 | 10 | KOAc | dioxane | 160, 1 h | 24 |
| 20 | 10 | KOAc | butan-1-ol | 160, 1 h | 11 |
Reaction conditions: 1a (1 mmol), 2a (1 mmol), 3a (2 mmol), base (2 mmol), solvent (6 mL).
Isolated yield.
A variety of bases were investigated, such as KOAc, CsOAc, NaOAc, K2CO3, and Cs2CO3. KOAc was identified as the best base (Table 1). Finally, our study focused on testing various solvents, such as DMF, DMAc, NMP, xylene, dioxane, and 1-butanol, and among them, DMF was found to be superior (Table 1).
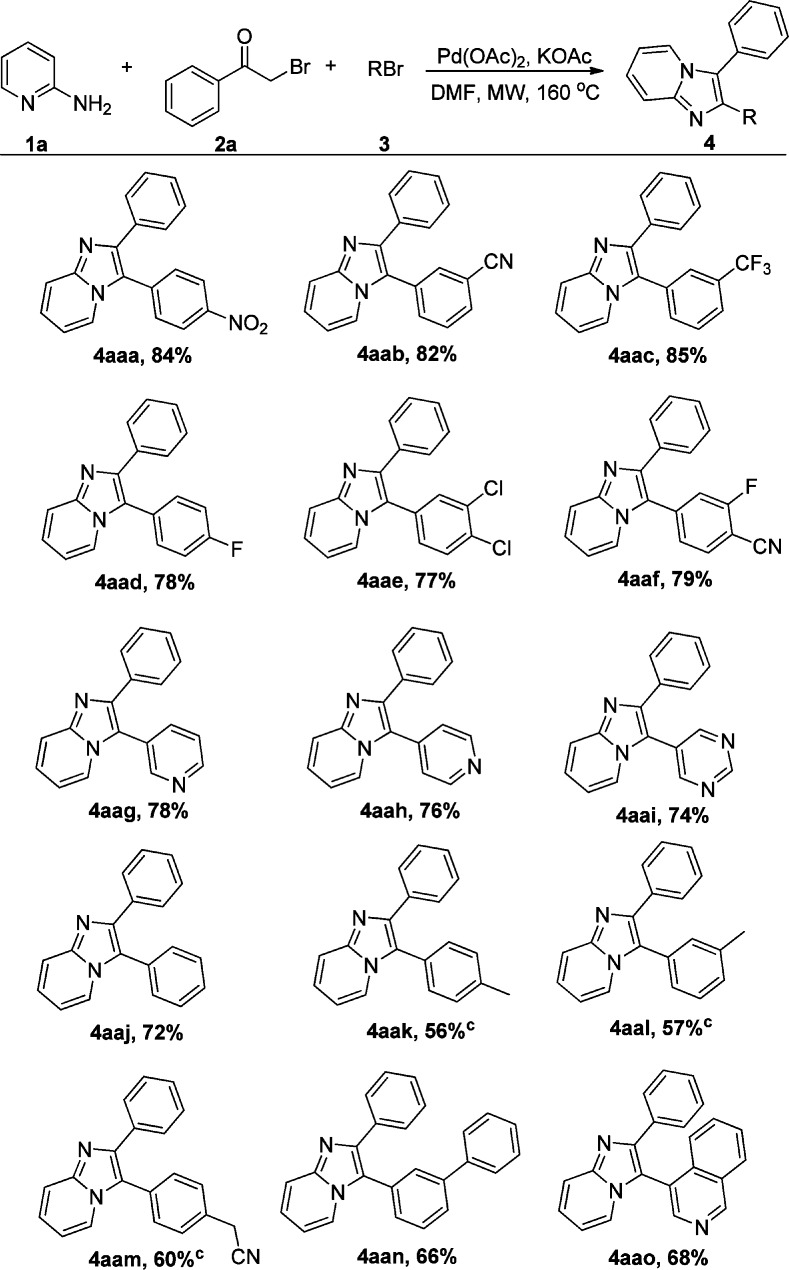
With the optimized reaction conditions in hand, we evaluated the scope and limitations of the transformation. We initially assessed the reactions of various aryl bromides with 1a and 2a (Scheme 2). First, a series of para- or meta-substituted electron-deficient aryl bromides were examined under the optimized conditions, and the corresponding products (4aaa–d) were produced in good to excellent yields. Subsequently, disubstituted aryl bromides were subjected to the reaction and transformation occurred smoothly. The one-pot three component reaction also worked well with bromopyridine or bromopyrimidine, leading to the products (4aag–i) in good yields. Bromobenzene was also successfully employed in the reaction to produce 4aaj in 72% yield. It should be noted that electron-rich aryl bromides gave slightly lower yields than electron-deficient systems, affording the corresponding 4aak–m in 56–60% yields. Biphenyl bromide and 4-bromoisoquinoline were also found to be suitable partners, giving 4aan and 4aao in yields of 66% and 68%, respectively.
Scheme 2. Scope of Phenyl Bromides,
Reaction conditions: 1a (1 mmol), 2a (1 mmol), 3 (2 mmol), Pd(OAc)2 (10 mol %), KOAc (2 mmol), DMF (6 mL), microwave, 160 °C, 1 h.
Isolated yield.
Reaction time is 2 h.
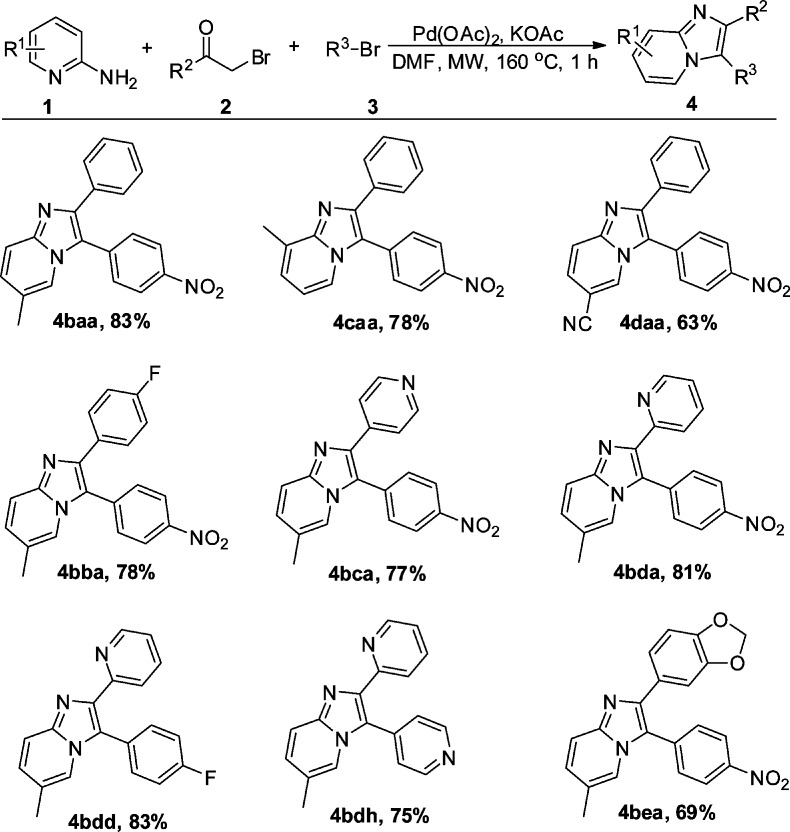
To further examine the efficiency of this one-pot reaction and to rapidly expand our unique compound collection, we extended the reaction scope to other 2-aminopyridines and 2-(bromophenyl)ethanones. As shown in Scheme 3, we were pleased to find that methyl substituted 2-aminopyridines could be smoothly transformed into the desired products (4baa and 4caa in 83% and 78% yield, respectively). The electron-withdrawing (−CN) substituent was well tolerated (4daa). To our delight, when 2-bromo-1-(4-fluorophenyl)ethanone, 4-(bromoacetyl)pyridine, and 2-(bromoacetyl)pyridine were subjected to the reaction, the corresponding products (4bba–bda) could be isolated in good to excellent yields (Scheme 3).
Scheme 3. Scope of Aminopyridines and 2-Bromophenylethanones,
Reaction conditions: 1a (1 mmol), 2a (1 mmol), 3 (2 mmol), Pd(OAc)2 (10 mol %), KOAc (2 mmol), DMF (6 mL), microwave, 160 °C, 1 h;
Isolated yield.
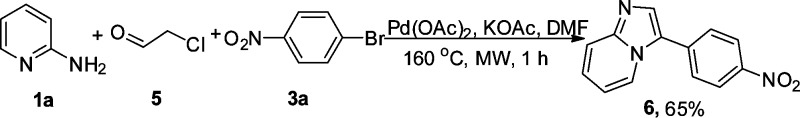
The reaction is not limited to synthesis of 2,3-diarylimidazo[1,2-a]pyridines. Under the same reaction conditions, 2-chloroacetaldehyde was successfully coupled with 1a and 3a to afford 2-arylimidazo[1,2-a]pyridine 6 in 65% yield (Scheme 4).
Scheme 4. Synthesis of 3-(4-Nitrophenyl)imidazo[1,2-a]pyridine via the One-Pot Procedure.
We also prepared compounds 4bca and 4bdh in yields of 77% and 75%, which have been found to be active on the TGFβ-R1 kinase through a high-throughput computational screen (Figure 1).23 Interestingly, both compounds are predicted to bind the kinase in a similar fashion to known TGFβ-R1 inhibitors (Figure 1)24 and have calculated properties with druglike space (Table 2).
Figure 1.
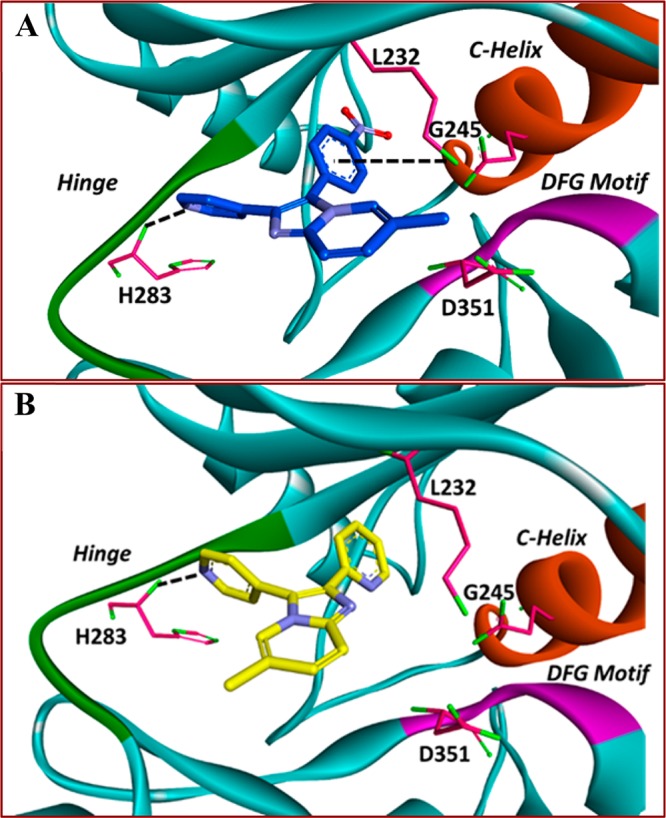
Computational modeling of 4bca (A) and 4bdh (B) in the TGFβ-R1 kinase (PDB: 3FAA(24)). In both cases, a pyridine moiety hydrogen bonds to the hinge with H283. 4bca creates a contact with the conserved lysine, L232. Both compounds access a lipophilic pocket to the left of L232. The hinge is shown in green, the DFG motif is shown in purple, and the C-Helix is shown in orange. Ligand/receptor interactions are denoted with black dotted lines.
Table 2. TGFβ-R1 Computational Affinity and Drug Properties.
| compd | ΔGa (kcal/mol) | Kdb (nM) | CLogPc | tPSAc (Å2) | MW (g/mol) |
|---|---|---|---|---|---|
| 4bca | –10.4 | 23.3 | 3.8 | 79.8 | 330 |
| 4bdh | –9.2 | 177.3 | 2.8 | 40.3 | 286 |
Calculated with Autodock Vina.
Calculated from ΔG using Kd = e–ΔG/RT at 298 K.
Calculated with ChemDraw.
In summary, we have developed an expeditious one-pot procedure to access 2,3-diarylimidazo[1,2-a]pyridines via a ligand-free, palladium-catalyzed, microwave-assisted three-component reaction. In addition, the availability of reagents and ease in expanding molecule diversity make the developed methodology operationally proficient and can facilitate rapid library construction. Further efforts to therapeutically evaluate the synthesized compounds are underway and will be reported in due course.
Acknowledgments
This work was supported by a training grant from The National Institutes of Health (T32 GM008804), University of Arizona startup funding, and The Caldwell Health Sciences Research Fellowship.
Supporting Information Available
Experimental procedure and characterization of all compounds and modeling procedures of compounds 4 and 6. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of all authors.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Enguehard-Gueiffier C.; Gueiffier A. Mini-Rev. Med. Chem. 2007, 7, 888. [DOI] [PubMed] [Google Scholar]
- a Byth K. F.; Geh C.; Forder C. L.; Oakes S. E.; Thomas A. P. Mol. Cancer Ther. 2006, 5, 655. [DOI] [PubMed] [Google Scholar]; b El-Sayed W. M.; Hussin W. A.; Al-Faiyz Y. S.; Ismail M. A. Eur. J. Pharmacol. 2013, 715, 212. [DOI] [PubMed] [Google Scholar]; c Kim O.; Jeong Y.; Lee H.; Hong S.-S.; Hong S. J. Med. Chem. 2011, 54, 2455. [DOI] [PubMed] [Google Scholar]; d Kamal A.; Reddy J.S.; Ramaiah M. J.; Dastagiri D.; Bharathi E. V.; Sagar M. V. P.; Pushpavalli S. N. C. V. L; Ray P.; Pal-Bhadra M. Med. Chem. Commun. 2010, 1, 355. [DOI] [PubMed] [Google Scholar]
- Lacerda R. B.; de Lima C. K.; da Silva L. L.; Romeiro N. C.; Miranda A. L.; Barreiro E. J.; Fraga C. A. Bioorg. Med. Chem. 2009, 17, 74. [DOI] [PubMed] [Google Scholar]
- a Shukla N. M.; Salunke D. B.; Yoo E.; Mutz C. A.; Balakrishna R.; David S. A. Bioorg. Med. Chem. 2012, 20, 5850. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Al-Tel T. H.; Al-Qawasmeh R. A.; Zaarour R. Eur. J. Med. Chem. 2011, 46, 1874. [DOI] [PubMed] [Google Scholar]
- Ismail M. A.; Arafa R. K.; Wenzler T.; Brun R.; Tanious F. A.; Wilson W. D.; Boykin D. W. Bioorg. Med. Chem. 2008, 16, 683. [DOI] [PubMed] [Google Scholar]
- a Véron J. B.; Allouchi H.; Enguehard-Gueiffier C.; Snoeck R.; Andrei G.; De Clercq E.; Gueiffier A. Bioorg. Med. Chem. 2008, 16, 9536. [DOI] [PubMed] [Google Scholar]; b Gudmundsson K. S.; Williams J. D.; Drach J. C.; Townsend L. B. J. Med. Chem. 2003, 46, 1449. [DOI] [PubMed] [Google Scholar]
- Kaminski J. J.; Doweyko A. M. J. Med. Chem. 1997, 40, 427. [DOI] [PubMed] [Google Scholar]
- Rival Y.; Grassy G.; Taudon A.; Ecalle R. Eur. J. Med. Chem. 1991, 26, 13. [Google Scholar]
- George P. G.; Rossey G.; Sevrin M.; Arbilla S.; Depoortere H.; Wick A. E.L. E. R. S. Monograph Ser. 1993, 8, 49. [Google Scholar]
- Depoortere H.; George P.. US 5064836, 1991.
- Sanger D. J. Behav. Pharmacol. 1995, 6, 116. [PubMed] [Google Scholar]
- Du B.; Shan A.; Zhang Y.; Zhong X.; Chen D.; Cai K. Am. J. Med. Sci. 2014, 347, 178. [DOI] [PubMed] [Google Scholar]
- Uemura Y.; Tanaka S.; Ida S.; Yuzuriha T. J. Pharm. Pharmacol. 1993, 45, 1077. [DOI] [PubMed] [Google Scholar]
- Sorbera L. A.; Castaner J.; Leeson P. A. Drugs Future 2002, 27, 935. [Google Scholar]
- Pellón R.; Ruíz A.; Lamas E.; Rodríguez C. Behav. Pharmacol. 2007, 18, 81. [DOI] [PubMed] [Google Scholar]
- Belohlavek D.; Malfertheiner P. Scand. J. Gastroenterol Suppl. 1979, 54, 44. [PubMed] [Google Scholar]
- a Scribner A.; Dennis R.; Lee S.; Ouvry G.; Perrey D.; Fisher M.; Wyvratt M.; Leavitt P.; Liberator P.; Gurnett A.; Brown C.; Mathew J.; Thompson D.; Schmatz D.; Biftu T. Eur. J. Med. Chem. 2008, 43, 1123. [DOI] [PubMed] [Google Scholar]; b Gudmundsson K. S.; Johns B. A. Org. Lett. 2003, 5, 1369. [DOI] [PubMed] [Google Scholar]; c Gudmundsson K. S.; Johns B. A. Bioorg. Med. Chem. Lett. 2007, 17, 2735. [DOI] [PubMed] [Google Scholar]; d Enguehard-Gueiffier C.; Fauvelle F.; Debouzy J.-C.; Peinnequin A.; Thery I.; Dabouis V.; Gueiffier A. Eur. J. Pharm. Sci. 2005, 24, 219. [DOI] [PubMed] [Google Scholar]; e Singhaus R. R.; Bernotas R. C.; Steffan R.; Matelan E.; Quinet E.; Nambi P.; Feingold I.; Huselton C.; Wilhelmsson A.; Goos-Nilsson A.; Wrobel J. Bioorg. Med. Chem. Lett. 2010, 20, 521. [DOI] [PubMed] [Google Scholar]
- a Colletti S. L.; Frie J. L.; Dixon E. C.; Singh S. B.; Choi B. K.; Scapin G.; Fitzgerald C. E.; Kumar S.; Nichols E. A.; O’Keefe S. J.; O’Neill E. A.; Porter G.; Samuel K.; Schmatz D. M.; Schwartz C. D.; Shoop W. L.; Thompson C. M.; Thompson J. E.; Wang R.; Woods A.; Zaller D. M.; Doherty J. B. J. Med. Chem. 2003, 46, 349. [DOI] [PubMed] [Google Scholar]; b Rupert K. C.; Henry J. R.; Dodd J. H.; Wadsworth S. A.; Cavender D. E.; Olini G. C.; Fahmy B.; Siekierka J. J. Bioorg. Med. Chem. Lett. 2003, 13, 347. [DOI] [PubMed] [Google Scholar]; c Follot S.; Debouzy J.-C.; Crouzier D.; Enguehard-Gueiffier C.; Gueiffier A.; Nachon F.; Lefebvre B.; Fauvelle F. Eur. J. Med. Chem. 2009, 44, 3509. [DOI] [PubMed] [Google Scholar]; d Enguehard C.; Renou J. L.; Allouchi H.; Leger J. M.; Gueiffier A. Chem. Pharm. Bull. 2000, 48, 935. [DOI] [PubMed] [Google Scholar]; e Kim O.; Jeong Y.; Lee H.; Hong S. S.; Hong S. J. Med. Chem. 2011, 54, 2455. [DOI] [PubMed] [Google Scholar]
- a Enguehard-Gueiffier C.; Musiu S.; Henry N.; Véron J. B.; Mavel S.; Neyts J.; Leyssen P.; Paeshuyse J.; Gueiffier A. Eur. J. Med. Chem. 2013, 64, 448. [DOI] [PubMed] [Google Scholar]; b Kettle J. G.; Brown S.; Crafter C.; Davies B. R.; Dudley P.; Fairley G.; Faulder P.; Fillery S.; Greenwood H.; Hawkins J.; James M.; Johnson K.; Lane C. D.; Pass M.; Pink J. H.; Plant H.; Cosulich S. C. J. Med. Chem. 2012, 55, 1261. [DOI] [PubMed] [Google Scholar]; c Yin L.; Erdmann F.; Liebscher J. J. Heterocycl. Chem. 2005, 42, 1369. [Google Scholar]; d Patel H. S.; Linn J. A.; Drewry D. H.; Hillesheim D. A.; Zuercher W. J.; Hoekstra W. J. Tetrahedron Lett. 2003, 44, 4077. [Google Scholar]
- a Enguehard C.; Renou J. L.; Collot V.; Hervet M.; Rault S.; Gueiffier A. J. Org. Chem. 2000, 65, 6572. [DOI] [PubMed] [Google Scholar]; b Bazin M. A.; Marchand P. Tetrahedron Lett. 2012, 53, 297. [Google Scholar]; c Akkaoui A. E.; Bassoude I.; Koubachi J.; Berteina-Raboin S.; Mouaddib A.; Guillaumet G. Tetrahedron 2011, 67, 7128. [Google Scholar]
- a Huang H.; Ji X.; Tang X.; Zhang M.; Li X.; Jiang H. Org. Lett. 2013, 15, 6254. [DOI] [PubMed] [Google Scholar]; b Yu J.; Jin Y.; Zhang H.; Yang X.; Fu H. Chem.—Eur. J. 2013, 19, 16804. [DOI] [PubMed] [Google Scholar]
- a Marhadour S.; Marchand P.; Pagniez F.; Bazin M. A.; Picot C.; Lozach O.; Ruchaud S.; Antoine M.; Meijer L.; Rachidi N.; Le Pape P. Eur. J. Med. Chem. 2012, 58, 543. [DOI] [PubMed] [Google Scholar]; b Fu H. Y.; Chen L.; Doucet H. J. Org. Chem. 2012, 77, 4473. [DOI] [PubMed] [Google Scholar]; c Liu Y.; He L.; Yin G.; Wu G.; Cui Y. Bull. Korean Chem. Soc. 2013, 34, 2340. [Google Scholar]; d Cao H.; Zhan H.; Lin Y.; Lin X.; Du Z.; Jiang H. Org. Lett. 2012, 14, 1688. [DOI] [PubMed] [Google Scholar]
- Trott O.; Olson A. J. J. Comput. Chem. 2010, 31, 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Bonafoux D.; Chuaqui C.; Boriack-Sjodin P. A.; Fitch C.; Hankins G.; Josiah S.; Black C.; Hetu G.; Ling L.; Lee W. C. Bioorg. Med. Chem. Lett. 2009, 19, 912. [DOI] [PubMed] [Google Scholar]; b Li H. Y.; Wang Y.; McMillen W. T.; Chatterjee A.; Toth J. E.; Mundla S. R.; Voss M.; Boyer R. D.; Sawyer J. S. Tetrahedron 2007, 63, 11763. [Google Scholar]; c Sawyer J. S.; Beight D. W.; Ciapetti P.; Decollo T. V.; Godfrey A. G.; Goodson T. Jr; Herron D. K.; Li H. Y.; Liao J.; McMillen W. T.; Miller S. C.; Mort N. A.; Yingling J. M.; Smith E. C. R.. PCT Int. Appl. WO 2002094833.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.