Abstract
We aimed to isolate and identify yeasts found in the tomato fruit in order to obtain isolates with biotechnological potential, such as in control of fungal diseases that damage postharvest fruits. We identified Candida orthopsilosis strains LT18 and LT24. This is the first report of this yeast on Lycopersicum esculentum fruits in Brazil.
Keywords: Lycopersicum esculentum Mill, Candida orthopsilosis, tomato fruits
The tomato fruit (Lycopersicum esculentum), which is used as a vegetable, is one of the most consumed food ingredients in the world (Mata et al., 2003), and Brazil is one of the major producers of tomato. In 2009, the national production reached 4.3 million tons and the world production was more than 152 million tons (FAOSTAT, 2011).
Yeasts are a large and diverse group of microorganisms consisting of more than 1000 species and can be found in soil, air, water, and food (Mislivec et al., 1992). Some species also co-exist with the natural microflora of fruits and vegetables and their colonization is influenced by environmental, harvest, or storage conditions (Skinner, 1980). Generally, many fruits and vegetables present nearly ideal conditions for the survival and growth of several types of microorganisms (Barth et al., 2009). Ferreira et al. (2010) studied the postharvest quality of the tomato fruit and revealed that the yeast and mold count decreases with the ripening of the fruit, but remains around the order of 103 CFU/g, indicating that the tomatoes preserved at room temperature need to be cleaned efficiently before consumption.
A diverse community of epiphytic microorganisms also colonizes the tomato fruit surface, effectively providing a further competitive barrier against the spoilage organisms (Barth et al., 2009). Therefore, the yeast microflora in the tomato fruit may present a biotechnological potential, such as in the fungal diseases control on postharvest fruit. The main mechanisms for the control of postharvest diseases by using microbial antagonists exploit microbial competition for nutrients and space, induced resistance, production of antibiotics, and direct parasitism (Sharma et al., 2009).
Pichia guilliermondii presents potential biocontrol activity against Botrytis cinerea in apples (Trofa et al., 2008) and against Rhizopus nigricans in tomato fruits (Zhao et al., 2008). Pichia anomala, isolated from the surface of coffee berries, is able to inhibit the spore production of Aspergillus ochraceus and Penicillium roqueforti (Ramos et al., 2010). Candida lambica is able to reduce up to 95.87% of A. ochraeus biomass in submerged culture (Beux, 2004). Therefore, we aimed to isolate and identify yeasts from the tomato fruit, which display characteristics similar to the Pichia genus in order to obtain isolates with biotechnological potential.
Ten tomato fruit samples were collected from different markets in Curitiba, Paraná State, Brazil, on October 2006. Tomato broth was prepared by disintegration and homogenization of tomatoes by using a sterile metal shredder. The broth was separated into sterile Erlenmeyer flasks and cultivated in a biochemical oxygen demand (BOD) incubator at 28°C for 5 days. Subsequently, the microorganisms were isolated on potato dextrose agar (PDA) (Merck, Darmstadt, Germany) by serial dilution in 0.1% peptone water.
The yeast groups were identified by the ability to ferment sugars such as glucose, galactose, sucrose, maltose, fructose, mannose, and raffinose according to the method described by Rocha (2006). The results were compared with those of a prior study (Back, 2006; Barnett and Pankahurst, 1974). Later, the yeasts were identified by growing them on CHROMAgar Candida (BD), by using the API 20C AUX system (bioMérieux) and by sequencing the ITS of their rDNA.
About 1 cm2 colonies of 5-day-old cultures were transferred to 2-mL Eppendorf tubes, each containing 300 μL cetyltrimethylammonium bromide (CTAB) buffer (2% CTAB [w/v], 1.4 M NaCl, 100 mM Tris-HCl, pH 8.0, 20 mM EDTA, 0.2% β-mercaptoethanol [v/v]) and approximately 80 mg of a silica mixture (silica gel H; Merck/Celite 545-Macherey Nagel & Co.; 2:1, w/w). The cells were disrupted manually using a sterile pestle for approximately 5 min. Subsequently, 200 μL CTAB buffer was added again, the mixture was vortexed, and then incubated for 10 min at 65 °C. After adding 500 μL chloroform, the solution was mixed and centrifuged for 5 min at 20,500 g force value and the supernatant was transferred to a new tube containing 2 volumes of ice-cold 96% ethanol. The DNA was allowed to precipitate for 30 min at −20 °C and then centrifuged again for 5 min at 20,500 g force value. Subsequently, the pellet was washed with cold 70% ethanol. After drying it at room temperature, the pellet was resuspended in 97.5 μL TE-buffer with 2.5 μL RNAse (20 U/mL) and incubated for 5 min at 37 °C; thereafter, it was stored at −20°C (Gerrits and Hoog, 1999).
The ITS of the ribosomal DNA (rDNA) was amplified using the primers V9G (5′-TTACGTCCCTGCCCTTTGTA-3′) and LS266 (5′-GCATTCCCAAACAACTCGACTC-3′) (9) and sequenced using ITS5 (5′-GGAAGTAAAAGTCGTAACAAGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) (White et al., 1990). Amplicons were cleaned using the GFX PCR DNA purification kit (GE Healthcare) and sequenced using an ABI 3130 automatic sequencer (Applied Biosystems). Sequences were edited and aligned using the Staden sequence analysis package v. 1.6.0 (Staden, 1996). Sequence analysis was performed using the sequence alignment software BLASTn run against the NCBI database. Phylogenetic analysis was performed using the software Mega 4.0.2 (Tamura et al., 2007) by applying the neighbor-joining method (Saitou and Nei, 1987) and the Jukes-Cantor correct distance model (Jukes and Cantor, 1969). The nucleotide sequence obtained in this study was submitted to GenBank.
Seventeen species of filamentous fungi were isolated from the tomato broth, and the analysis of macromorphology and reproductive structures revealed that they belonged to 4 genera: Penicillium, Aspergillus, Acremonium, and Trichoderma (Data not shown). Some species (e.g., Trichoderma harziamun) of these genera have previously shown the ability to control postharvest diseases in fruits (Batta, 2007), whereas others such as Penicillium expansum were agents of postharvest diseases (Yu et al., 2012). However, we chose to focus this work on yeast, so that the future implementation and bioprocess viability could be easily achieved.
We isolated 2 strains of yeast labeled LT18 and LT24, whose colonies were bright white, flat, and smooth, with regular, rounded borders and no pseudohyphae. Fermentation of the carbon sources was assessed in a previous identification of the yeast isolates, revealing that the strains could potentially belong to Pichia.
The morphological and physiological characters of LT18 and LT24 were similar to Pichia sp.; hence, a further identification was carried out. The strains LT18 and LT24 presented pink pigmentation and smooth texture on the chromogenic medium CHROMAgar, and they were identified as Candida parapsilosis by using the API 20C AUX system.
Sequences of ITS1-5.8S-ITS2 revealed a fragment of 422 bp for LT18 and 423 bp for LT24. Sequences of both strains presented with 98% (LT18) and 99% (LT24) similarity with the Candida orthopsilosis sequence EU557371. The sequences of the isolates LT18 and LT24 were deposited in GenBank with the respective accession numbers JN797502 and JN797503.
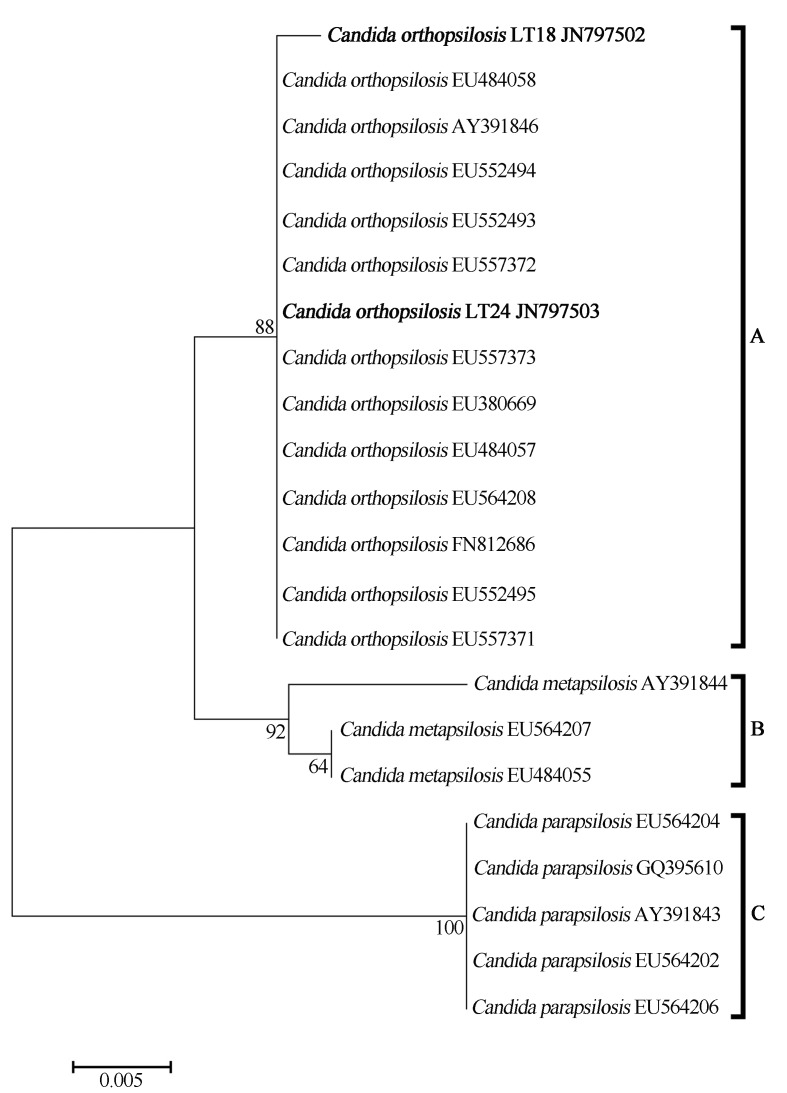
Twenty sequences previously deposited at GenBank (Table 1), which showed similarity in the range of 95–99% to the sequences of the isolates obtained in this study, were employed for constructing a phylogenetic tree (Figure 1). Three groups that showed high bootstrap values were obtained. Group A, 88% consistent, was composed of isolates of C. orthopsilosis, including the ones obtained in our study, LT18 and LT24; group B, with a bootstrap value of 92%, is represented by members of Candida metapsilosis; and finally group C, with a bootstrap value of 100%, contained isolates from C. parapsilosis.
Table 1.
Sequences from Candida spp. deposited in GenBank used in the phylogenetic analysis.
| Name | GenBank | Source | Origin | Strain Number |
|---|---|---|---|---|
| Candida orthopsilosis | JN797502 | Tomato fruit | Brazil | LT18 |
| Candida orthopsilosis | JN797503 | Tomato fruit | Brazil | LT24 |
| Candida orthopsilosis | EU557371 | Bloodstream infection | Brazil | L8201A |
| Candida orthopsilosis | EU564208 | Human blood culture | USA | ATCC 96141 |
| Candida orthopsilosis | EU484057 | Human Blood | Brazil | L7941 |
| Candida orthopsilosis | EU557372 | Bloodstream infection | Brazil | 840 |
| Candida orthopsilosis | FN812686 | Unknown | USA | 90–125 |
| Candida orthopsilosis | EU557373 | Bloodstream infection | Brazil | L7956 |
| Candida orthopsilosis | EU552495 | Human blood culture | Brazil | L7786 |
| Candida orthopsilosis | EU380669 | Human Blood | Brazil | L6786 |
| Candida orthopsilosis | EU552494 | Human blood culture | Brazil | L8106A |
| Candida orthopsilosis | EU484058 | Human Blood | Brazil | L6785 |
| Candida orthopsilosis | AY391846 | - | USA | MCO456 |
| Candida orthopsilosis | EU552493 | Human blood culture | Brazil | L8068A |
| Candida parapsilosis | EU564206 | Human Blood | USA | ATCC 90018 |
| Candida parapsilosis | CG395610 | Feeding production sample | China | A005 |
| Candida parapsilosis | EU564204 | Human blood culture | Brazil | L8367 |
| Candida parapsilosis | EU564202 | Human blood culture | Brazil | L8096 |
| Candida parapsilosis | AY391843 | Feces | Puerto Rico | CBS 604 |
| Candida metapsilosis | EU564207 | Unknown | USA | ATCC 96143 |
| Candida metapsilosis | AY391844 | Human sputum | Norway | CBS 2916 |
| Candida metapsilosis | EU484055 | Human Blood | Brazil | L8521 |
Figure 1.
Phylogenetic analysis of yeasts LT18 and LT24 belonging to the C. orthopsilosis specie. Neighbor-joining method. Numbers on the tree branches indicate the bootstrap value found from 10000 replicates. Mega Software 4.0.2 release. Strains available in GenBank accession Nos JN797502 and JN797503.
Identification via conventional biochemical systems corroborated with the data obtained by Tay et al. (2009), who also used the API 20C AUX system for preliminary identification of the isolates from the bloodstream of infected patients. All isolates were initially identified as C. parapsilosis, but were later differentiated into C. parapsilosis, C. orthopsilosis, C. metapsilosis, and Lodderomyces elongisporus through RAPD and ITS sequencing. Others studies (Silva et al., 2009; Toro et al., 2010) also differentiated a large set of C. parapsilosis isolates, previously identified by biochemical tests, into C. parapsilosis sensu stricto, C. orthopsilosis, and C. metapsilosis species by molecular identification (SADH gene restriction profile).
Further, Pitt and Hocking (1997) have isolated C. parapsilopsis from fruit juices, olives, meat, and seafood. This particular species is well known for causing nosocomial blood infections, especially among neonates and the immuno-compromised, and it is associated with Candidemia due to contaminated intravascular devices and parenteral nutrition (Almirante et al., 2006; Girmenia et al., 1996; Pfaller and Diekema, 2002, 2007; Safdar et al., 2002; Trofa et al., 2008). This yeast was also isolated from robusta coffee samples from the Congo Republic, akin to others species from this genera including Candida pelliculosa, Candida famata, and Candida tropicalis (Pee and Castelein, 1971).
The taxon C. parapsilosis was traditionally divided into 3 groups, I, II, and III. Recently, Tavanti et al. (2005) investigated the genetic heterogeneity of the taxon and proposed replacing groups II and III with C. orthopsilosis and C. metapsilosis, respectively.
The phylogenetic tree clusterings of all 3 species for the ITS region are consistent with those reported by Gomez-Lopez et al. (2008), Tavanti et al. (2005) and Tay et al. (2009), in which the differences in the sequence alignment validated the species separation. This result showed that C. orthopsilosis and C. metapsilosis are more closely related to each other than to C. parapsilosis, as shown in Figure 1.
In conclusion, our study is likely the first report of C. orthopsilosis found in L. esculentum Mill. fruits in Brazil. We recommend further studies on these strains in order to screen for biotechnologically important characteristics and exploit their use in postharvest fungal diseases control.
References
- Almirante BJ, Alonso-tarres L, Rodriguez-tudela JL, Pahissa A. Epidemiology, risk factors and prognosis of Candida parapsilosis bloodstream infections: case-control population-based surveillance study of patients in Barcelona, from 2002 to 2003. J Clin Microbiol. 2006;44(5):1681–1685. doi: 10.1128/JCM.44.5.1681-1685.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back W. Farbatlas und Handbuch der Getränkebiologie Teil 2. Verlag Hans Carl; Nürnburg: 2000. [Google Scholar]
- Barnett JA, Pankahurst RJ. A key for identifying yeasts based on physiological tests. Holland Publishing Company; Amsterdam: 1974. [Google Scholar]
- Barth M, Hankinson TR, Zhuang H, Breidt F. Microbiological Spoilage of Fruits and Vegetables. In: Sperber WH, Doyle MP, editors. Compendium of the Microbiological Spoilage of Foods and Beverages (Food Microbiology and Food Safety) Springer Science and Business Media; New York, USA: 2009. pp. 135–183. [Google Scholar]
- Batta YA. Control of postharvest diseases of fruit with an invert emulsion formulation of Trichoderma harzianum Rifai. Postharvest Biol Technol. 2007;43(1):143–150. [Google Scholar]
- Beux MR. PhD thesis. Curitiba, Brasil: Departamento de Biotecnologia, UFPR; 2004. Café - Estudo da Biodiversidade Microbiana de Frutos de Café do Brasil, Seleção de Cepas de Levedeuras e Bactérias Lácticas com Ação Fungistática Contra Aspergillus ochraeus Produtor de Ocratoxina A; p. 121. [Google Scholar]
- FAOSTAT. [Accessed 14 November 2011];Food and Agriculture Organization of United Nations Statistics Division. 2011 Available at: http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID=567#ancor.
- Ferreira SMR, Quadros DA, Karkle ENL, Lima JJ, Tullio LT, Freitas RJS. Qualidade pós-colheita do tomate de mesa convencional e orgânico. Ciênc Tecnol Aliment. 2010;30(4):858–869. [Google Scholar]
- Gerrits van den ende AHG, Hoog G. Variability and molecular diagnostics of the neurotropic species Cladophialophora bantiana. Studies in Mycology. 1999;43(1):151–162. [Google Scholar]
- Girmenia CP, Martino FDE, Bernardis F. Rising incidence of Candida parapsilosis fungemia in patients with hematologic malignancies: clinical aspects, predisposing factors and differential pathogenicity of the causative strains. Clin Infect Dis. 1996;23(3):506–514. doi: 10.1093/clinids/23.3.506. [DOI] [PubMed] [Google Scholar]
- Gomez-lopez A, Alastruey-izquierdo A, Rodriguez D, Almirante B, Pahissa A, Rodriguez-tudela JL, Cuenca-Estrella M. Prevalence and Susceptibility Profile of Candida metapsilosis and Candida orthopsilosis: Results from Population-Based Surveillance of Candidemia in Spain. Antimicrob. Agents Chemother. 2008;52(4):1506–1509. doi: 10.1128/AAC.01595-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukes TH, Cantor CR. Evolution of protein molecules. In: Munro HN, editor. Mammalian Protein Metabolism. Academic Press; New York, USA: 1969. pp. 21–132. [Google Scholar]
- Mata MERMC, Braga MED, Kross RK. Secagem Osmótica de Tomate: Efeito da epiderme. Rev Bras Prod Agroindust Especial. 2003;(1):77–84. [Google Scholar]
- Mislivec PB, Beuchat LR, Cousin MA. Yeasts and Molds. In: Vanderzant C, Splittstoesser DF, editors. Compendium of Methods for the Microbiological Examination of Foods. APHA; Washington, USA: 1992. pp. 239–243. [Google Scholar]
- Pee WV, Castelein J. The yeast flora of fermenting robusta coffee. East African Agricul Forest J. 1971;36:308–310. [Google Scholar]
- Pfaller MA, Diekema DJ. Role of sentinel surveillance of candidemia: trends in species distribution and antifungal susceptibility. J Clin Microbiol. 2002;40(10):3551–3557. doi: 10.1128/JCM.40.10.3551-3557.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20(1):133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt JI, Hocking AD. Fungi and food spoilage. Academic Press; London: 1997. [Google Scholar]
- Ramos DMB, SilvA CF, Batista LR, Schwan RF. Inibição in vitro de fungos toxigênicos por Pichia sp. e Debaryomyces sp. isoladas de frutos de café (Coffea arabica) Acta Sci Agron. 2010;32(3):397–402. [Google Scholar]
- Rocha CD. M.Sc. Dissertation. Curitiba, Brasil: Departamento de Patologia Básica, UFPR; 2006. Determinação dos pontos críticos de contaminação por leveduras em indústria de refrigerante; p. 42. [Google Scholar]
- Safdar A, Perling DS, Armstrong D. Hematogenous infections due to Candida parapsilosis: changing trends in fungemic patients at a comprehensive cancer center during the last four decades. Diagn Microbiol Infect Dis. 2002;44(1):11–16. doi: 10.1016/s0732-8893(02)00423-6. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sharma RR, Singh D, Singh R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biological Control. 2009;50(3):205–221. [Google Scholar]
- Silva AP, Miranda IM, Lisboa C, Pina-Vaz C, Rodrigues AG. Prevalence, Distribution, and Antifungal Susceptibility Profiles of Candida parapsilosis, C. orthopsilosis, and C. metapsilosis in a Tertiary Care Hospital. J Clin Microbiol. 2009;47(8):2392–2397. doi: 10.1128/JCM.02379-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner FA. Biology and activities of yeasts. Academic Press; London: 1980. [Google Scholar]
- Staden R. The Staden sequence analysis package. Mol Biotechnol. 1996;5(1):233–241. doi: 10.1007/BF02900361. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA 4: Molecular Evolutionary Genetics Analysis. Mol Biol Evol. 2007;24(8):1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tavanti A, Davidson AD, Gow NA, Maiden MC, Odds FC. Candida orthopsilosis and Candida metapsilosis spp. nov. to replace Candida parapsilosis groups II and III. J Clin Microbiol. 2005;43(1):284–292. doi: 10.1128/JCM.43.1.284-292.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay ST, Na SL, Chong J. Molecular differentiation and antifungal susceptibilities of Candida parapsilosis isolated from patients with bloodstream infections. J Med Microbiol. 2009;58(2):185–191. doi: 10.1099/jmm.0.004242-0. [DOI] [PubMed] [Google Scholar]
- Toro M, Torres MJ, Maite R, Aznar J. Characterization of Candida parapsilosis complex isolates. Clin Microbiol Infect. 2010;17(3):418–24. doi: 10.1111/j.1469-0691.2010.03302.x. [DOI] [PubMed] [Google Scholar]
- Trofa D, Gacser A, Nosanchuk JD. Candida parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev. 2008;21(4):606–25. doi: 10.1128/CMR.00013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. PCR Protocols: A guide to methods and applications. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. Academic Press; New York, USA: 1990. pp. 315–322. [Google Scholar]
- Yu Q, Chen Q, Chen Z, Xu H, Fu M, Li S, Wang H, Xu M. Activating defense responses and reducing postharvest blue mold decay caused by Penicillium expansum in peach fruit by yeast saccharide. Postharvest Biol Technol. 2012;74(1):100–107. [Google Scholar]
- Zhang D, Spadaro D, Garibaldi A, Gullino ML. Potential biocontrol activity of a strain of Pichia guilliermondii against grey mold of apples and its possible modes of action. Biological Control. 2011;57(3):193–201. [Google Scholar]
- Zhao Y, Tu K, Shao X, Jing W, Su Z. Effects of the yeast Pichia guilliermondii against Rhizopus nigricans on tomato fruit. Postharvest Biol Technol. 2008;49(1):113–120. [Google Scholar]