Abstract
The occurrence, resistance phenotype and molecular mechanisms of resistance of methicillin-resistant staphylococci from groin swabs of 109 clinically healthy dogs in Nsukka, Nigeria were investigated. The groin swab samples were cultured on mannitol salt agar supplemented with 10 μg of cloxacillin. Sixteen methicillin-resistant coagulase negative staphylococci (MRCoNS), all harbouring the mecA gene were isolated from 14 (12.8%) of the 109 dogs studied. The MRCoNS isolated were: S. sciuri subspecies rodentium, S. lentus, S. haemolyticus, and S. simulans with S. sciuri subspecies rodentium (62.5%) being the predominant species. Thirteen (81.3%) of the MRCoNS were resistant to tetracycline while 12 (75%) and 10 (62.5%) were resistant to kanamycin and trimthoprim-sulphamethoxazole respectively. None of the isolates was resistant to fusidic acid, linezolid and vancomycin. Thirteen (81.3%) of the MRCoNS were multi-drug resistance (MDR). Other antimicrobial genes detected were: blaZ, tet(K), tet(M), tet(L), erm(B), lnu(A), aacA-aphD, aphA3, str, dfr(G), catpC221, and catpC223. Methicillin-resistant staphylococci are common colonizers of healthy dogs in Nigeria with a major species detected being S. sciuri subsp. rodentium.
Keywords: methicillin-resistant, S. sciuri, resistant genes, dogs
Introduction
Staphylococci are Gram-positive cocci usually found as transient normal flora of the skin and mucous membranes of mammals and birds and can easily spread to humans by contact and through formites (Fusi Ngwa et al., 2007). According to Bergeron et al. (2011) the Staphylococcus genus groups together 45 species and 21 subspecies which are classified in two groups based on their ability to produce the enzyme coagulase: coagulase-positive staphylococci (CoPS) and coagulase-negative staphylococci (CoNS).
Thirty-eight different species are found within the CoNS group, including the S. sciuri subgroup, which consists of 3 species and 3 subspecies (S. sciuri subspecies rodentium, S. sciuri subspecies sciuri, S. sciuri subspecies carnaticus; S. lentus, and S. vitulinus) (Kloos and Bannerman, 1994). Staphylococcus sciuri, first described in 1976 by Kloos et al. (1976), is the most abundant member of the genus Staphylococcus with a wide habitat range (Kloos and Bannerman, 1994; Kloos et al., 1997). Although CoNS are saprophytic and rarely pathogenic (Kloos et al., 1997), multi-drug resistant strains have been associated with severe cases of difficult to treat infections, especially in immune compromised individuals (Zell et al., 2008).
Companion animals, particularly dogs and cats, are frequently implicated as potential reservoirs of methicillin-resistant staphylococci (Saleha and Zunita, 2010). Prevalence of staphylococcal species in companion animals is important because of the potential for zoonotic infections and possibility of resistance genes transfer (Rachal et al., 2009). Human colonization and infections with methicillin-resistant staphylococci (MRS) have been reported in several parts of Nigeria (Fusi Ngwa et al., 2007; Olowe et al., 2007; Ghebremedhin et al., 2009). However, no information exists on the occurrence and molecular characteristics of MRS from dogs in Nigeria. Thus, the objective of this study was to determine the occurrence, resistance phenotypes and molecular mechanisms of resistance of MRS in healthy dogs in Nsukka, Nigeria.
Material and Methods
Sample collection and processing
Dogs from the university town of Nsukka and four other surrounding communities (Ibagwa Aka, Obollo Afor, Orba, and Opi) were used for the study. A total of 109 clinically healthy dogs consisted of 20 individual household-dogs from the university town, 50 dogs displayed for sale in the local markets in the four communities, and 39 dogs presented to the University of Nigeria Veterinary Teaching Hospital, Nsukka for routine vaccination were sampled. Informed consent of the dog owners was obtained prior to sample collection. The cotton tip of each swab stick was moistened in sterile normal saline and gently rolled over the groin area for about 10 seconds. Each swab sample was streaked on mannitol salt agar (MSA; Oxoid, Basingstoke) supplemented with 10 μg/mL of cloxacillin. Inoculated plates were incubated at 37 °C for 24 to 48 h. On each plate that produced growth, three colonies were randomly selected and purified on nutrient agar. Purified colonies were subjected to Gram staining and catalase test and presumptive staphylococcal colonies (Gram-positive cocci in bunches with positive catalase test) were further evaluated for coagulase production (tube coagulase using rabbit plasma) and haemolysis on 5% sheep blood agar using standard procedures. Representative colony/colonies (if different morphology was shown) from each sample was/were selected for further analysis, including molecular identification.
Identification of staphylococci and methicillin-resistant staphylococci
Coagulase-negative staphylococci were identified by PCR amplification followed by sequencing of sodA gene (Poyart et al., 2001). The presence of mecA in all recovered isolates was investigated by PCR (Gómez-Sanz et al., 2011).
Antimicrobial resistance pheno- and genotype
Antimicrobial susceptibility of the methicilin-resistant staphylococcal isolates to 17 antimicrobial agents was determined by the agar disk-diffusion method. Antimicrobial agents tested were as follows: penicillin (10 U), oxacillin (1 μg), cefoxitin (30 μg) erythromycin (15 μg), clindamycin (2 μg), gentamicin (10 μg), kanamycin (30 μg), streptomycin (10 U), tobramycin (10 μg), tetracycline (30 μg), trimethoprim-sulphamethoxazole (25 μg), chloramphenicol (30 μg), ciprofloxacin (5 μg), mupirocin (200 μg), fusidic acid (10 μg), vancomycin (30 μg), and linezolid (30 μg). The disk-diffusion method and breakpoints recommended by Clinical and Laboratory Standards Institute (2010) were employed for all antimicrobials except for streptomycin and fusidic acid, where the methods and breakpoints recommended by the Société Française de Microbiologie (http://www.sfm.asso.fr) were used. The double-disk diffusion test (D-test) was performed on all isolates to detect inducible clindamycin resistance.
The presence of the following 30 antimicrobial resistance genes was investigated by PCR: mecA, blaZ, tet(K), tet(M), tet(L), erm(A), erm(B), erm(C), erm(T), mph(C), msr(A), msr(B), lnu(A), vga(A), vga(C), aacA-aphD, aphA-3, aadD, aadE, aadA, str, dfr(A), dfr(D), dfr(G), dfr(K), fexA, cfr, catpC194, catpC221, and catpC223 (Schwarz et al., 2001; Hanselman et al., 2008). Positive controls from the collection of the University of La Rioja were included in each PCR reaction.
Results
Occurrence of methicillin-resistant staphylococcal species
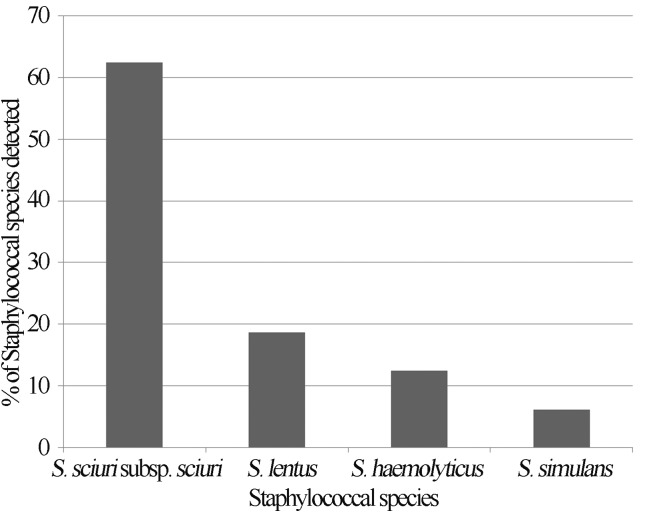
Sixteen methicillin-resistant coagulase negative staphylococci (MRCoNS), all harbouring the mecA gene were isolated from 14 (12.8%) of the 109 dogs sampled. The MRCoNS isolated belonged to four different species namely: S. sciuri subspecies rodentium, S. lentus, S. haemolyticus, and S. simulans, with S. sciuri subspecies rodentium (62.5%) being the predominant species detected (Figure 1).
Figure 1.
Occurrence (%) of MRCoNS species in healthy dogs in Nsukka, Nigeria.
Resistance pheno- and genotype of the MRS to other antimicrobials
Resistance phenotype of the staphylococcal isolates investigated as well as their corresponding molecular mechanisms of resistance are shown in Table 1. Thirteen (81.3%) of the MRCoNS were resistant to tetracycline while 12 (75%) and 10 (62.5%) were resistant to kanamycin and trimethoprim-sulphamethoxazole respectively. None of the isolates was resistant to fusidic acid, linezolid and vancomycin. High rate of multi-drug resistance (MDR) was observed among the MRCoNS as 13 (81.3%) of them were resistant to more than 3 classes of antimicrobial agents.
Table 1.
Species, antimicrobial resistance phenotype and resistance genes detected among MRCoNS isolated from dogs in Nuskka, Nigeria.
| Strain ID | Staphylococcal species | Resistance phenotypea | Resistance genes detected |
|---|---|---|---|
| C2854 | S. sciuri subsp. Rodentium | PEN-OXA-FOX-TET-ERY-CLI-GEN-KAN-TOB-SXT-CHL | mecA, tet(K), tet(M), erm(B), aacA-aphD, catpC223 |
| C2869 | S. sciuri subsp. Rodentium | PEN-OXA-FOX-TET-ERY-CLI-GEN-KAN-SXT-CHL-CIP | mecA, tet(K), erm(B), lnu(A), aacA-aphD, catpC221 |
| C2853 | S. sciuri subsp. Rodentium | PEN-OXA-FOX-TET-ERY-CLI-GEN-KAN-STR-SXT | mecA, tet(K), erm(B), aacA-aphD |
| C2865 | S. sciuri subsp. Rodentium | PEN-OXA-FOX-TET-ERY-CLI-GEN-KAN-STR-SXT | mecA, tet(K), tet(M), erm(B), lnu(A), aacA-aphD |
| C2855 | S. sciuri subsp. Rodentium | PEN-OXA-FOX-TET-ERY-CLI-KAN-SXT-CHL | mecA, tet(K), tet(M), erm(B), lnu(A), aacA-aphD, catpC221 |
| C2867 | S. sciuri subsp. Rodentium | PEN-OXA-FOX-TET-ERY-CLI-KAN-SXT-MUP | mecA, tet(K), tet(M), erm(B), lnu(A), aacA-aphD |
| C2864 | S. sciuri subsp. Rodentium | PEN-OXA-FOX-TET-ERY-CLI-GEN-KAN | mecA, tet(K), tet(M), erm(B), aacA-aphD |
| C2866 | S. sciuri subsp. Rodentium | PEN-OXA-FOX-TET-ERY-CLI-KAN | mecA, tet(K), tet(M), erm(B), lnu(A), aacA-aphD |
| C2852 | S. sciuri subsp. Rodentium | PEN-OXA-FOX-CHL | mecA, catpC223 |
| C2871 | S. sciuri subsp. Rodentium | PEN-OXA-FOX | mecA |
| C2861 | S. lentus | PEN-OXA-FOX-TET-ERY-CLI-KAN-SXT-CHL | mecA, tet(K), tet(M), tet(L), erm(B), aacA-aphD, dfr(G), catpC221 |
| C2860 | S. lentus | PEN-OXA-FOX-TET-STR-SXT | mecA, str, dfr(G) |
| C2871-2 | S. lentus | PEN-OXA-FOX-TET-KAN | mecA, tet(K), tet(M), aacA-aphD |
| C2873 | S. haemolyticus | PEN-OXA-FOX-TET-KAN-SXT-CHL | mecA, tet(K), aacA-aphD, aphA3, dfr(G), catpC221 |
| C2870 | S. haemolyticus | PEN-OXA-FOX-TET-KAN-SXT-CHL | blaZ, mecA, tet(K), aacA-aphD, dfr(G), catpC221 |
| C2857 | S. simulans | PEN-OXA-FOX | mecA |
PEN, penicillin; OXA, oxacillin; FOX, cefoxitin; TET, tetracycline; ERY, erythromycin; CLI, clindamycin; GEN, gentamicin; KAN, kanamycin; TOB, tobramycin; STR, streptomycin; SXT, trimethoprim-sulphamethoxazole; CHL, chloramphenicol; MUP, mupirocin; CIP, ciprofloxacin.
MRCoNS, Methicillin-resistant coagulase negative staphylococci.
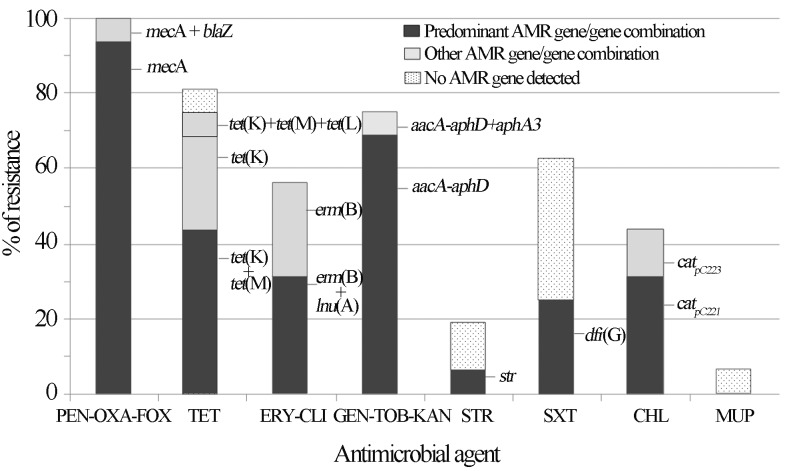
The percentage of each antimicrobial resistance gene and/or gene combination detected among the MRCoNS isolates is presented in Figure 2. The nine (56.3%) erythromycin resistant isolates were also resistant to clindamycin. Constitutive clindamycin resistance was observed in all but 3 MRCoNS isolates. These 3 isolates revealed the typical D-shaped halo around clindamycin disk, characteristic of inducible clindamycin resistance. Table 1 and Figure 2 show the different resistance genes detected among isolates. In some cases, the resistance mechanism implicated could not be detected even though a wide variety of resistance genes were tested. Six of the 10 MRCoNS that were also resistant to trimethoprim-sulphamethoxazole lacked any of the so far described genes encoding trimethoprim resistance [dfr(A), dfr(D), dfr(G), dfr(K)]. Based on these results, trimethoprim (5 μg/mL) and sulfonamide (20 μg/mL) antimicrobial agents were tested separately (CLSI 2010) and the results revealed that all trimethoprim-sulphamethoxazole resistant strains were also trimethoprim resistant. One methicillin-resistant S. sciuri subspecies rodentium (C2867) was resistant to mupirocin but mupA gene was not detected.
Figure 2.
Frequencies (%) of antimicrobial resistance (AMR) detected among the MRCoNS investigated in this study and resistance gene or gene combinations harboured by the resistant staphylococci. PEN, penicillin; OXA, oxacillin; FOX, cefoxitin; TET, tetracycline; ERY, erythromycin; CLI, clindamycin; GEN, gentamicin; KAN, kanamycin; TOB, tobramycin; STR, streptomycin; SXT, trimethoprim-sulphamethoxazole; CHL, chloramphenicol; MUP, mupirocin.
Discussion
Resistance of staphylococci to methicillin and other antimicrobials is a global problem in the chemotherapy of staphylococcal infections. As pointed out by Huebner and Goldmann (1999), this resistance has underscored the need for species identification which is an important step for monitoring the reservoir and distribution of these bacteria. In the present study, the major MR staphylococcal group present in the groin area of dogs in Nsukka was CoNS, with S. sciuri subspecies rodentium, being the predominant species and subspecies identified. Although CoNS are saprophytic and rarely pathogenic (Kloos and Bannerman, 1994), they have been associated with opportunistic infections, especially in immunocompromised individuals (Zell et al., 2008). Previous studies have shown S. sciuri to be present not only as part of the skin, nasal and oral microflora of healthy dogs, but also as a causative agent of infections, although at lower rates than S. pseudintermedius (Stepanovic et al., 2001; Hauschild and Wójcik, 2007). Staphylococcus sciuri was among the staphylococcal strains isolated from cases of canine pyoderma in Natal City, Brazil (Lima et al., 2012). A case of human wound infection by a multidrug resistant strain of S. sciuri has been reported (Coimbra et al., 2011). Thus, this staphylococcal species could constitute a health risk to both humans and animals especially when the natural mucocutaneous barrier is breached. Staphylococcus sciuri has been described as a natural reservoir of the methicillin resistance gene, mecA (Kloos et al., 1997; Couto et al., 2000), which may explain the high rate of mecA positive S. sciuri recorded in the present study. The other MRCoNS isolated in this study, although at low rates, have also been reported in dogs as commensal and/or pathogens (Hauschild and Wójcik, 2007).
The absence of MRCoPS colonization among the tested dog population is similar to the findings of Vengust et al. (2006) and Baptiste et al. (2005) who failed to detect the organism in healthy dogs in Slovenian and a community in United Kingdom, respectively. However, low MRCoPS carriage rates in healthy dogs have been reported by other authors (Hanselman et al., 2008; Loeffler et al., 2011), with S. pseudintermedius as the prominent MRCoPS species, even though their occurrence is still low (0–4.5%) (Gómez-Sanz et al., 2011; van Duijkeren et al., 2011). Failure to detect methicillin-resistant S. aureus and S. pseudintermedius among the sampled dogs suggests that these species are not predominant methicillin-resistant staphylococci in the study area. Alternatively, groin swabs from healthy dogs were used in the present study whereas most reports on S. pseudintermedius in dogs used nasal, skin, perineal or combined body-site samples (Rubin and Chirino-Trejo, 2011; van Duijkeren et al., 2011). It could therefore appear that the groin area of dogs is less colonized by methicillin-resistant S. pseudintermedius than the mucous or skin.
High rate of MDR was observed among the MRCoNS as 81.3% of them were resistant to more than 3 classes of antimicrobial agents. Cross resistance to other antimicrobials is most common in methicillin-resistant than in methicillin-sensitive staphylococcal isolates (Orrett and Land, 2006; John and Harvin, 2007). In our MRCoNS isolates, cross-resistance was due in most cases to tetracyclines, aminoglucosides, trimethoprim-sulphamethoxazole and/or macrolides-lincosamides. Interestingly, our isolates were recovered from healthy animals whereas nosocomial strains are more likely to be MDR than commensal (John and Harvin, 2007).
Only 2 of the 16 MRCoNS (both being S. sciuri subspecies rodentium) had the same resistance phenotype; indicating a high diversity of antimicrobial resistance profiles among the strains. Interestingly, no tetracycline resistance gene was detected in 1 tetracycline-resistant MR S. sciuri subspecies rodentium (C2860) (Table 1, Figure 2). Furthermore, 2 streptomycin-resistant MR S. sciuri subspecies rodentium did not present any streptomycin resistance genes, while 5 trimethoprim-sulphamethxazole resistant strains (again MR S. sciuri subsps. rodentium) equally did not harbour any of the so far described trimethoprim-sulphamethxazole resistant genes for staphylococci (Table 1, Figure 2). Chromosomal mutations conferring high-level resistance to streptomycin and over-expression of the enzyme dihydrofolate reductase (DHFR), target of trimethoprim, have been described (Schwarz and Chaslus-Dancla, 2001). It is also interesting to remark that one MR S. sciuri subsps. rodentium (C2867) was resistant to mupirocin but mupA gene could not be detected. Previous studies have shown an association between mupirocin usage and emergence of mupirocin resistance in staphylococci (Orrett 2008). However, mupirocin is not known to be used in veterinary practice in Nigeria, thus, mupirocin resistance in one of the Staphylococcus strain in the present study may not be attributed to mupirocin usage. These data altogether suggest the potential presence of novel characteristics and presents S. sciuri as a potential reservoir of novel antimicrobial resistant properties.
The findings in this study highlight the existence of MDR strains of MRCoNS, particularly S. sciuri subspecies rodentium, in healthy dogs in Nsukka, Nigeria. Since dogs are in close contact with their owners, the risk of transmission of MDR staphylococci between animals and humans as well as the possibility of transfer of mecA and other resistance genes from the CoNS to human pathogenic S. aureus are serious public health concerns.
Acknowledgments
This work was partially supported by Project SAF2009-08570 from the Ministry of Education and Science of Spain and FEDER. E. Gómez-Sanz has a fellowship from the Gobierno de La Rioja of Spain; and C. Lozano has a fellowship from the Ministerio de Ciencia e Innovación of Spain.
References
- Baptiste KE, Williams K, Williams NJ, Wattret A, Clegg PD, Dawson S, Corkill JE, O’Neill T, Hart CA. Methicillin-resistant staphylococci in companion animals. Emerg Infect Dis. 2005;11:1942–1944. doi: 10.3201/eid1112.050241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron M, Dauwalder O, Gouy M, Freydiere AM, Bes M, Meugnier H, Benito Y, Etienne J, Lina G, Vandenesch F, Boisset S. Species identification of staphylococci by amplification and sequencing of the tuf gene compared to the gap gene and by matrix-assisted laser desorption time-of-flight mass spectrometry. Eur J Clin Microbiol Infect Dis. 2011;30:343–354. doi: 10.1007/s10096-010-1091-z. [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI) CLSI Document M100-S20. Wayne, PA: 2010. Performance standards for antimicrobial susceptibility testing; Eighteenth informational supplement. [Google Scholar]
- Coimbra DG, Almeida AGCS, Junior JBO, da Silva LAF, Pimentel BJ, Gitai DLG, Moreina LS, Silva-Filho EA, de Andrade TG. Wound infection by multiresistant Staphylococcus sciuri identified by molecular methods. New Microbiologica. 2011;34:425–427. [PubMed] [Google Scholar]
- Couto I, Sanches IS, Sá-Leão R, de Lencastre H. Molecular characterization of Staphylococcus sciuri strains isolated from humans. J Clin Microbiol. 2000;38:1136–1143. doi: 10.1128/jcm.38.3.1136-1143.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol. 2005;3:948–958. doi: 10.1038/nrmicro1289. [DOI] [PubMed] [Google Scholar]
- Fusi Ngwa CN, Egri-Okwaji MT, Odugbemi T, Iroha E. A study on pediatric MRSA in Lagos, Nigeria. Int J Biol Chem Sci. 2007;1:54–60. [Google Scholar]
- Ghebremedhin B, Olugbosi MO, Raji AM, Layer F, Bakare RA, Konig B, Konig W. Emergence and community-associated MRSA strain with a unique resistance profile in Southwest Nigeria. J Clin Microbiol. 2009;47:2975–2980. doi: 10.1128/JCM.00648-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Sanz E, Torres C, Lozano C, Saenz Y, Zarazaga M. Detection and characterization of methicillin-resistant Staphylococcus pseudintermedius in healthy dogs in La Rioja, Spain. Comp Immun Microbiol Infect Dis. 2011;34:447–453. doi: 10.1016/j.cimid.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Hanselman BA, Kruth S, Weese JS. Methicillin-resistant staphylococcal colonization in dogs entering a veterinary teaching hospital. Vet Microbiol. 2008;126:277–281. doi: 10.1016/j.vetmic.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Hauschild T, Wójcik A. Species distribution and properties of staphylococci from canine dermatitis. Res Vet Sci. 2007;82:1–6. doi: 10.1016/j.rvsc.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Huebner J, Goldmann DA. Coagulase-negative staphylococci: role as pathogens. Annu Rev Med. 1999;50:223–236. doi: 10.1146/annurev.med.50.1.223. [DOI] [PubMed] [Google Scholar]
- John JF, Harvin AM. History and evolution of antibiotic resistance in coagulase-negative staphylococci: Susceptibility profiles of new anti-staphylococcal agents. Therap Clin Risk Manag. 2007;3:1143–1152. [PMC free article] [PubMed] [Google Scholar]
- Kloos WE, Schleifer KH, Smith RF. Characterization of Staphylococcus sciuri sp. nov. and its subspecies. Int J Syst Bacteriol. 1976;26:22–37. [Google Scholar]
- Kloos WE, Bannerman TL. Update on clinical significance of coagulase negative staphylococci. Clin Microbiol Rev. 1994;7:117–140. doi: 10.1128/cmr.7.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloos WE, Ballard BN, Webster JA, Hubner RJ, Tomasz A, Couto I, Sloan GL, Dehart HP, Fiedler F, Schubert K, de Lencastre H, Sanches IS, Heath HE, Leblanc PA, Ljungh A. Ribotype delineation and description of Staphylococcus sciuri subspecies and their potential as reservoirs of methicillin resistance and staphylolytic enzyme genes. Int J Syst Bacteriol. 1997;47:313–323. doi: 10.1099/00207713-47-2-313. [DOI] [PubMed] [Google Scholar]
- Lima LF, Lira AC, Coutinho HDM, Junior JP, Barreto HM. Antimicrobial resistance in staphylococci isolated from canine pyoderma. Comunicata Scientiae. 2012;3:181–185. [Google Scholar]
- Loeffler A, Pfeiffer DU, Lindsay JA, Magalhães RJ, Lloyd DH. Prevalence of and risk factors for MRSA carriage in companion animals: a survey of dogs, cats and horses. Epidemiol Infect. 2011;139:1019–1028. doi: 10.1017/S095026881000227X. [DOI] [PubMed] [Google Scholar]
- Olowe OA, Eniola KIT, Olowe RA, Olayemi AM. Antimicrobial susceptibility and betalactamase detection of MRSA in Osogbo, S.W. Nigeria. Nat Sci. 2007;5:44–48. [Google Scholar]
- Orrett FA. The emergence of mupirocin resistance among clinical isolates of methicillin-resistant Staphylococcus aureus in Trinidad: a first report. Jap J Infect Dis. 2008;61:107–110. [PubMed] [Google Scholar]
- Orrett FA, Land M. Methicillin-resistant Staphylococcus aureus prevalence: current susceptibility patterns in Trinidad. BMC Infect Dis. 2006;6 doi: 10.1186/1471-2334-6-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyart C, Quesne G, Boumaila C, Trieu-Cuot P. Rapid and accurate species-level identification of coagulase-negative staphylococci by using the sodA gene as a target. J Clin Microbiol. 2001;39:4296–4301. doi: 10.1128/JCM.39.12.4296-4301.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachal T, Leonard K, Martinez L, Breaux JG, Corbin A, Nathaniel R. Prevalence of SCCmec types in methicillin resistant Staphylococcus intermedius in healthy pets from Southeastern United States. J Infect Dis Immun. 2009;1:006–010. [Google Scholar]
- Rubin JE, Chirino-Trejo M. Prevalence, sites of colonization, and antimicrobial resistance among Staphylococcus pseudintermedius isolated from healthy dogs in Saskatoon, Canada. J Vet Diagn Invest. 2011;23:351–354. doi: 10.1177/104063871102300227. [DOI] [PubMed] [Google Scholar]
- Saleha A, Zunita Z. Methicillin resistant Staphylococcus aureus (MRSA): An emerging veterinary and zoonotic pathogen of public health concern and some studies in Malaysia. J Anim Vet Adv. 2010;9:1094–1098. [Google Scholar]
- Schnellmann C, Gerber V, Rossano A, Jaquier V, Panchaud Y, Doherr MG, Thomann A, Straub R, Perreten V. Presence of new mecA and mph(C) variants conferring antibiotic resistance in Staphylococcus spp. isolated from the skin of horses before and after clinic admission. J Clin Microbiol. 2006;44:4444–4454. doi: 10.1128/JCM.00868-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S, Chaslus-Dancla E. Use of antimicrobials in veterinary medicine and mechanisms of resistance. Vet Res. 2001;32:201–225. doi: 10.1051/vetres:2001120. [DOI] [PubMed] [Google Scholar]
- Stepanovic S, Dimitrijevic V, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M. Staphylococcus sciuri as a part of skin, nasal and oral flora in healthy dogs. Vet Microbiol. 2001;82:177–185. doi: 10.1016/s0378-1135(01)00377-7. [DOI] [PubMed] [Google Scholar]
- van Duijkeren E, Kamphuis M, van der Mije IC, Laarhoven LM, Duim B, Wagenaar JA, Houwers DJ. Transmission of methicillin-resistant Staphylococcus pseudintermedius between infected dogs and cats and contact pets, humans and the environment in households and veterinary clinics. Vet Microbiol. 2011;150:338–343. doi: 10.1016/j.vetmic.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Vengust M, Anderson MEC, Rousseau J, Weese JS. Methicillin-resistant staphylococcal colonization in clinically healthy dogs and horses in the community. Lett App Microbiol. 2006;43:602–606. doi: 10.1111/j.1472-765X.2006.02018.x. [DOI] [PubMed] [Google Scholar]
- Zell C, Resch M, Rosenstein R, Albrecht T, Hertel C, Gotz F. Characterization of toxin production of coagulase-negative staphylococci isolated from food and starter cultures. Int J Food Microbiol. 2008;127:246–251. doi: 10.1016/j.ijfoodmicro.2008.07.016. [DOI] [PubMed] [Google Scholar]