Abstract
The chemical management of the black leaf streak disease in banana caused by Mycosphaerella fijiensis (Morelet) requires numerous applications of fungicides per year. However this has led to fungicide resistance in the field. The present study evaluated the activities of six fungicides against the mycelial growth by determination of EC50 values of strains collected from fields with different fungicide management programs: Rustic management (RM) without applications and Intensive management (IM) more than 25 fungicide application/year. Results showed a decreased sensitivity to all fungicides in isolates collected from IM. Means of EC50 values in mg L−1 for RM and IM were: 13.25 ± 18.24 and 51.58 ± 46.14 for azoxystrobin, 81.40 ± 56.50 and 1.8575 ± 2.11 for carbendazim, 1.225 ± 0.945 and 10.01 ± 8.55 for propiconazole, 220 ± 67.66 vs. 368 ± 62.76 for vinclozolin, 9.862 ± 3.24 and 54.5 ± 21.08 for fludioxonil, 49.2125 ± 34.11 and 112.25 ± 51.20 for mancozeb. A molecular analysis for β-tubulin revealed a mutation at codon 198 in these strains having an EC50 greater than 10 mg L−1 for carbendazim. Our data indicate a consistency between fungicide resistance and intensive chemical management in banana fields, however indicative values for resistance were also found in strains collected from rustic fields, suggesting that proximity among fields may be causing a fungus interchange, where rustic fields are breeding grounds for development of resistant strains. Urgent actions are required in order to avoid fungicide resistance in Mexican populations of M. fijiensis due to fungicide management practices.
Keywords: banana, fungicides, Mycosphaerella fijiensis, sensitivity, Sigatoka
Introduction
Banana and plantain are constantly exposed to adverse conditions that are affecting their production. Currently two fungal diseases have threatened the production of bananas in the world, the black Sigatoka, also known as black leaf streak disease (BLSD) caused by Mycosphaerella fijiensis Morelet (anamorph Pseudocercospora fijiensis Morelet) and the Panama disease caused by Fusarium oxysporum cubense race 4 (FOC)(Grimm, 2008). Detrimental crop losses have led to a debate about the future of banana production. In Mexico the banana industry is widely affected by BLSD. Since its emergence in the southern state of Chiapas, Mexico in 1980’s, the disease has spread rapidly in almost all banana-producing areas in the country in the last 15 years. These areas include the Gulf of Mexico within Veracruz, Tabasco and Oaxaca states, the central-Pacific area (Michoacán, Colima, Jalisco and Nayarit) and south pacific (Chiapas) (Beltrán-García et al., 2009). The disease reduces yield from 50 to 100%, besides inducing a premature ripening of the fruit, both phenomena impacting the economy of banana growers (Marín et al., 2003).
In the commercial banana plantations, BLSD is controlled by the use of synthetic fungicides. The application of fungicides in large banana plantations is carried out by aircraft and in small plantations by tractor mounted (goose neck) or back pack sprayers. It is estimated that during 2010, the Mexican banana producers spent more than 55 million dollars on the purchase of fungicides, based on an approximate cost of $1100 per hectare (Marín et al., 2003). In Mexico, the banana plantations with black Sigatoka are conducted under three types of chemical control programs: the intensive, semi-intensive and rustic. The intensive management involves applying a protectant fungicide (mancozeb) up to 35 times per year with an alternation of 14–20 applications of systemic fungicides using preferably triazoles, strobilurins, morpholines and in some cases benzimidazoles. The semi-intensive program applied protective fungicides (mancozeb or chlorothalonil) with up to 15 times and 5–10 applications of benzimidazoles, strobilurins or triazoles as systemic fungicides; and the rustic program without fungicides treatment (Orozco-Santos et al., 2001). It is clear that the application of a chemical management program depends on the economic capacity of the banana grower, therefore in the same producing region may be a combination of management programs, which have influenced the fungal populations that have developed resistance to fungicides.
There are several reports showing local aspects of fungicide resistance in isolates of M. fijiensis collected in America and other parts of the world (Romero and Sutton, 1997, 1998; Chin et al., 2001; Pérez et al., 2003; Vawdrey and Grice, 2005; Amil et al., 2007). However in Mexico, a few studies focussed on the current situation of fungicides resistance in M. fijiensis populations have been conducted (Manzo-Sánchez et al., 2012; Martínez-Bolaños et al., 2012). In order to ascertain the influence of the number of fungicide applications on banana fields, we evaluated the sensitivities to the fungicides fludioxonil and vinclozolin and those fungicides used for black Sigatoka treatment such as azoxystrobin, carbendazim, propiconazole and mancozeb in fourty M. fijiensis isolates collected from plantations with rustic and intensive fungicide application programs. Also we analysed whether a mutation at codon 198 of β-tubulin fragment is present in those strains growing in concentrations higher than 10 mg L−1 of carbendazim.
Materials and Methods
The fungicides used in this study, including Azoxystrobin (methyl (E)-2-{2-[6-(2-cyanophenoxy) pyrimidin-4-yloxy]phenyl}-3-methoxyacrylate), carbendazim [methyl benzimidazol-2-ylcarbamate], Fludioxonil (4-[2,2-difluoro-1,3-benzodioxol-4-il]-1H-pirrol-3-carbonitrile) and vinclozolin [3-(3,5-dichlorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione], were purchased from Sigma Chemical Co. (St. Louis, MO). Technical grade Mancozeb [manganese ethylenebis (dithocarbamate) (polymeric) complex with zinc salt] was generously provided by GBM Co. (Grupo Bioquímico Mexicano) and Propiconazol [1-2-(2,4-diclorophenyl)-4-propyl-1,3-dioxolan-2-yl-methyl)-1H-1,2,4 triazole] was purchased as technical grade (Tilt, 250 from Syngenta). The fungicide concentrations shown in the parenthesis are in ppm (mg L−1): Carbendazim (0–200), Mancozeb (0–200) and Propiconazole (0–20) were dissolved in sterile distilled water; Azoxystrobin (0–50) and Vinclozolin (0–400), both dissolved in acetone and Fludioxonil (0–100) was dissolved in ethanol. The control contained water, acetone or ethanol without the fungicides. The highest volume of acetone or ethanol added to the culture (10 mL) was 100 μL. No detrimental effects of solvents on growth of the fungus were detected (data not shown).
A total of 40 monosporic isolates were recovered from seven commercial banana plantations located in Colima, Chiapas, Guerrero, Michoacán, Veracruz, Tabasco and Yucatán. The isolates were collected from banana leaves (Musa acuminata Colla “Grand naine” subgroup Cavendish (AAA) showing symptoms of black Sigatoka. The plants were randomly selected from fields under rustic (not sprayed with chemicals) or intensive application of fungicides to control black Sigatoka (more than 25 applications of protective and systemic fungicides). The most used fungicides in those fields were benomyl, azoxystrobin, tridemorph, propiconazole, mancozeb and chlorothalonil. The strains were stored at the laboratorio de Biotecnología of Universidad de Colima, Tecomán, Colima, México and Centro de Investigación Científica de Yucatán, Merida, Yucatán, México for further studies.
The identity of single-ascospores strains was confirmed by: the morphology of the mycelium and PCR techniques using the specific primers (ACTR/MFactF) reported previously to amplify M. fijiensis β-tubulin (Arzanlou et al., 2007).
To estimate EC50, 100 mg of mycelial mat were transferred and placed in 12-multiwell plate with 2.5 mL of fresh PDB medium and incubated with different fungicide concentrations for 7 days at 27 °C and 150 r/min. After incubation, the mycelia were collected by filtration using a filter paper in vacuum, washed with distilled water and placed in aluminium foil keeping in an oven at 65 °C until a constant weight. To assess the change in biomass content, 100 mg dry mycelial biomass (equivalent to 4.8 ± 0.2 mg dried weight) from the beginning of the experiment was used as reference.
EC50 values were calculated for each isolate from dry weight of mycelium recorded for each fungicide concentration and were compared with control weight.
A statistical analysis was performed by comparing the concentrations of fungicides tested with the two groups of strains. Analysis of variance ANOVA was used to determine possible differences between strains with an intensive treatment and those with a rustic treatment. The data was analyzed using STATGRAPHICS Centurion XVI.I.
Fungal DNA was extracted from each isolate using Ultra Clean Plant DNA Isolation Kit (Mo Bio Laboratories Inc.). Mycelial pellets were collected and ground using liquid nitrogen, with mortar and pestle until a fine powder was obtained. The DNA was obtained following the manufacturer instructions.
The β-tubulin fragment of 1159 bp from M. fijiensis was amplified by PCR with primers Pini a 5′-CAG ACC ATC TCC GGC GAA CAT G-3′ and Pini b 5′TAG ACG ACA TCT TGA GAC CGC G-3′ (Cañas-Gutiérrez et al., 2006). The PCR reaction was prepared with P0982-100RXN, (Sigma-Aldrich). The amplification was carried out in a NYX Technik ATC 201 thermocycler with an initial temperature of 94 °C for 2 min, followed by 35 cycles of 94 °C for 1 min, 59 °C for 2 min and 72 °C for 1 min, with a final extension of 5 min at 72 °C. The amplification products (30 μL) were separated by electrophoresis on a 2% agarose gel (Ultra PURE, GibcoBRL); once electrophoresis was completed the gel was stained with ethidium bromide solution and visualized under a UV transilluminator (Apollo instrumentation). The PCR fragment was subsequently digested with a restriction enzyme (Bsh12361, Fermentas) as follows: 20 μL of PCR product, 3 μL of Buffer, 1 unit of restriction enzyme and distilled water up to 30 μL. The restriction site assay was incubated for a total of 40 min, (30 min 37 °C, 10 min 80 °C) and restriction products were separated on a 3% agarose gel (Ultra PURE, GibcoBRL) stained with ethidium bromide and visualized under a UV transilluminator.
Results and Discussion
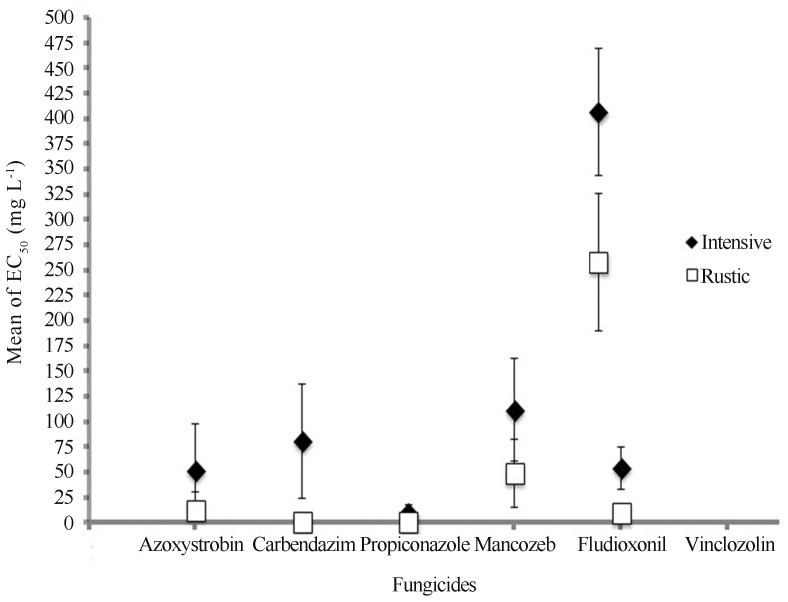
Throughout this study, 40 isolates were tested independently at least three times to evaluate the EC50 for six fungicides. Twenty isolates were selected from rustic management and twenty from fungicide-intensive treatment, to compare among the strains the sensitivity to fungicides in relation with the number of fungicide applications (Figure 1). A reduced sensitivity to all fungicides was observed in the intensive group compared to rustic isolates. We use the strain Mf-1 as the reference isolate for rustic and C1N for intensive, both strains were isolated in 1999 and showed a high sensitivity to fungicides and have been used in previous studies conducted in the laboratory (Beltrán-García et al., 2009). The statistical analysis revealed that the P-value of the F test is less than 0.05, suggesting that there is a statistically significant difference between the averages of the concentration of both treatments. Figure 1 shows the tolerance to fungicides concentration (average) of strains with an intensive treatment is higher than the concentration reached for those with a rustic treatment, since the bars never found at any point in the graph, it means that there is a difference between both behaviors to the concentration of fungicides. For azoxystrobin, a fungicide belonging to the strobilurins group or Q0I, mean EC50 values for populations collected from rustic and intensive plantations were 13.25 (± 18.24) and 51.58 (± 46.14) mg L−1 respectively. The EC50 values for the rustic group ranged from 0.05 to 50 and for intensive 0.25 to 125 mg L−1. Both groups have isolates that showed EC50 values greater than 10 mg L−1 (35% in rustic and 80% intensive). The Resistant factor (the average EC50 of less sensitive isolates/average EC50 of sensitive isolates) was 19.83 for rustic and 590.64 for intensive group. The Fungicide Resistance Action Committee (FRAC) in 2012 reported that the strains of M. fijiensis that are able to grow at concentrations above 10 mg L−1 should be considered resistant.
Figure 1.
Comparison of effective concentration of six fungicides for EC50 on mycelial growth in Mycophaerella fijiensis collected from banana fields with Rustic and Intensive managements of fungicides. All assays were done in triplicate.
Our analysis proved the presence of individual resistant strains in both groups, with bigger EC50 values in the intensive than in the rustic group. EC50 values in some strains could be compared to those obtained by Sierotzki et al. (2000) for an azoxystrobin-resistant strain isolated from Costa Rica in 2000, which tolerates 10 mg L−1 and has a mutation in the cytochrome b gene (G143A), responsible of this resistance. Recently Churchill (2011) reported azoxystrobin resistance in banana plantations of Colombia, Costa Rica, Guatemala and Panama.
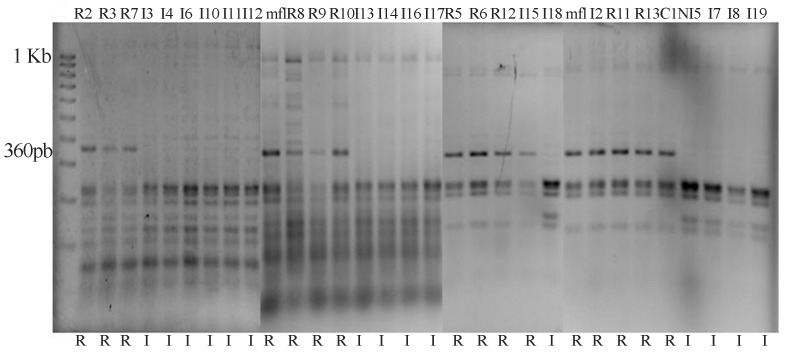
The benzimidazole fungicides are still widely used in Mexico to control BLSD, however their effectiveness is very low. The EC50 mean value for carbendazim in the rustic strains was 1.8575 (± 2.11) mg L−1 in comparison to 81.40 (± 56.50) mg L−1 for isolates from intensively managed plantations. The ranges for this fungicide were 0.5 to 150 mg L−1 for intensive and 0.10 to 7.5 mgL−1 for rustic. We found that almost all strains isolated from fields with intensive management are considered resistant to this fungicide. The Fungicide Resistance Action Committee-FRAC (2012), considers strains of M. fijiensis growing in benzimidazole fungicides at 10 mg L−1 as “resistant”. Figure 1 shows a difference of sensitivity of 43 times between groups. Resistance to benzimidazoles is caused by one or several single nucleotide polymorphisms (SNPs) in the β-tubulin gene, leading to a reduced binding affinity between the fungicide and the tubulin. For M. fijiensis, benzimidazole resistance is related with point mutations in the β-tubulin gene at codon 198 (Grimm, 2008). In this study, the change in codon 198 was examined and we found this mutation in those strains that have an EC50 greater than 10 mg L−1 (Figure 2).
Figure 2.
Molecular analysis of β-tubulin (at codon 198) in M. fijiensis strains from Intensive (I) and Rustic (R) banana plantations. The restriction pattern shows the loss of the 360 bp band in those strains collected from intensive management fields with fungicides. mf-1 and C1N are reference strains isolated in 1999 from rustic and intensive management fields respectively. Both strains are sensitivive to carbendazim with EC50 values of 0.2 and 5.0 mg L−1. Note: Figure composed of four runs of individual gels.
Resistant allele was identified by the loss of the band corresponding to 360 bp in the restriction analysis of the 1159 bp-PCR product for strains collected from rustic fields the band at 360 bp was observed, congruent with their sensitivity to carbendazim; it was found 100% correlation between sensitivity to the fungicide and the presence of the susceptible β-tubulin allele. We found that 80% of strains collected from intensive management had EC50 greater than 25 mg L−1, suggesting mutation at codon 198 of β-tubulin is not the only condition that confers resistance to high concentrations of carbendazim. In Saccharomyces cerevisiae attention has focussed on a gene named FLR1. This gene encodes a multidrug resistance (MDR) transporter of the major facilitator superfamily (MFS); the expression of this gene confers resistance to benomyl and mancozeb fungicides (Brôco et al., 1999; Teixeira et al., 2008). The FLR1 is up regulated by Yap1, a transcription factor (bZIP) that increases its activity in response to oxidative stress caused by fungicides. An analysis in silico of M. fijiensis genome shows a gene homologous to FLR1.
The FRAC (2012), considers strains of M. fijiensis growing in propiconazole at 3 mg L−1 as “resistant” However, Cañas-Gutierrez et al. (2009) considered as high tolerance strains that showed an EC50 higher than 1 mg L−1 and medium tolerance, those strains that grown on 0.20 to 0.40 mg L−1 of propiconazole. In our case intensive management group are highly resistant according to both criteria. The average value of EC50 for this fungicide was 1.225 mg L−1 (± 0.945) for rustic isolates and 10.01 (± 8.55) mg L−1 for intensive management. The higher standard deviation values for these strains are due to the broader range of EC50 (0.5 to 25 mgL−1). So, it is clear a notable loss of sensitivity to propiconazole occurs when the numbers of fungicide applications per year are increased. However this is the fungicide with smallest difference of EC50 mean values between groups (Figure 1). Propiconazole belongs to the DMI group, inhibitors of ergosterol biosynthesis (Luo and Schnabel, 2008; Wyand and Brown, 2005). DMI resistance has been reported in M. fijiensis and has been linked with one or more distinct point mutations at Y136F, A313G, Y461D, Y463D/H/N/S in the CYP51 gene, encoding eburicol 14α-demethylase (Cañas-Gutiérrez et al., 2009).
For vinclozolin, the mean EC50 values were higher in both groups, intensive management 368 ± 62.76 mg L−1 vs. 220 ± 67.66 mg L−1 in rustic management. Vinclozolin is a dicarboximide fungicide and has been used mainly for control of diseases caused by Botrytis cinerea and Monilia spp. The toxicity of this fungicide has been associated with reactive oxygen species (Lee et al., 1998; Cabral and Cabral, 2000), which induce lipid peroxidation and arrest growth through a secondary process of transformation of the fungicide. Until now reports of toxicity of vinclozolin on M. fijiensis have not been published. On the other hand, Lee et al. (1998) showed that the vinclozolin inhibitory concentration 50 (IC50) value for BC2 strain of B. cinerea was 2 μM (equivalent to 0.572 ng mL−1). All strains evaluated in this work tolerate more than 350 μM of this fungicide, so we can assume that M. fijiensis has a natural resistance to this class of fungicides. Therefore vinclozolin cannot be considered for control of BLSD.
The phenylpyrrole fludioxonil, is a derivative of pyrrolnitrin, a natural antifungal compound present in several Pseudomonas species. Fludioxonil inhibits both conidial and mycelial growth, but the latter process is more sensitive and has been commercially available in crop protection since the mid-1990s to control a broad spectrum of plant pathogenic fungi. The EC50 values for rustic isolates ranged from 2.5 to 12.5 mg L−1 with a mean EC50 value of 9.862 ± 3.24 mg L−1. The intensive group was less sensitive to fludioxonil with EC50 values between 10 and 75 mg L−1, with a mean of 54.5 ± 21.08 mg L−1. Similar to dicarboximides as vinclozin, fludioxonil has been reported as an inducer of swelling, abnormal branching and cell bursting in germ tubes, and appears to be 30–40 times more toxic than the dicarboximide in terms of in vitro hyphal growth for B. cinerea (Leroux, 2004). In our work the broad difference in toxicity between both fungicides is not observed, fludioxonil is only 7 times more effective than vinclozolin on the strains from the intensive group. As well as vinclozolin, fludioxonil toxicity has not been reported for M. fijiensis, but on function of current results it is not a good candidate to control BLSD in Mexico.
High cost and/or inefficiency of some systemic fungicides to control BLSD prompt banana producers in some regions of Mexico to apply mancozeb every week (Orozco-Santos et al., 2001; Martínez-Bolaños et al., 2012). Mancozeb belonging to dithiocarbamate fungicides is highly toxic to the conidia, inhibiting germination and germ tube elongation and epiphytic mycelial growth. Mancozeb induces oxidative stress indirectly by modifying thiol-rich proteins and inhibiting proteins with activity (Santos et al., 2009; Dias et al., 2010). There are no reports about in vitro EC50 value of mancozeb for M. fijiensis, despite being a widely used fungicide. In our work the EC50 for rustic isolates ranges from 3.5 to 100 mg L−1 with a mean EC50 of 49.21 ± 34.11 mg L−1. For the intensive group EC50 values ranges from 3.5 to 200 mg L−1 with a mean of 112.25 ± 51.20 mg L−1. Likely mancozeb application will select pathogen strains with less sensitivity, then these will become the strains that predominate in the field. Recently, M. fijiensis strains tolerant to 200 mg L−1 were found (manuscript in preparation), which could threaten the use of this fungicide to control BLSD in the future.
In summary this study shows a loss of sensitivity to azoxystrobin, carbendazim and propiconazole, and improved tolerance to mancozeb, fungicides widely used against black Sigatoka in Mexico. Fungicides like fludioxonil and vinclozolin are not functional for BLSD control. It is clear that continuous fungicide application modifies the behavior of individual isolates and exerts a selection pressure that correlates with high EC50 values in strains from fields with intensive management. Banana growers continue to apply intensively fungicides that promote resistant populations of M. fijiensis in their orchards and in the long run may trigger complete management failure and crop loss.
Acknowledgments
This research was supported in part by grants by CONACYT SEP-CB-79626 and FORDECYT-CONACYT No.116886. G.M-R and O. O-C thanks to CONACYT for fellowships 256660 and 275868 respectively. We thank Professor James F. White at RUTGERS University for comments and English revision of the manuscript.
References
- Amil AF, Heaney SP, Stanger C, Shaw MW. Dynamics of QoI sensitivity in Mycosphaerella fijiensis in Costa Rica during 2000 to 2003. Phytopathology. 2007;97:1451–1457. doi: 10.1094/PHYTO-97-11-1451. [DOI] [PubMed] [Google Scholar]
- Arzanlou M, Abeln ECA, Kema GHJ, Carlier J, Vried Id, Guzman M, Crous PW. Molecular diagnostic for the Sigatoka disease complex of banana. Phytopathology. 2007;97:1112–1118. doi: 10.1094/PHYTO-97-9-1112. [DOI] [PubMed] [Google Scholar]
- Beltrán-García MJ, Manzo-Sanchez G, Orozco-Santos M, Ogura T. Sigatoka negra: el cáncer de la producción de banano. Ciencia Desarrollo. 2009;35:60–63. [Google Scholar]
- Beltrán-García MJ, Manzo-Sánchez G, Guzman-González S, Arias-Castro C, Rodriguez-Mendiola M, Avila-Miranda M, Ogura T. Oxidative stress response of Mycosphaerella fijiensis, the causal agent of black leaf streak disease in banana plants, to hydrogen peroxide and paraquat. Can J Microbiol. 2009;55:887–894. doi: 10.1139/w09-023. [DOI] [PubMed] [Google Scholar]
- Brôco N, Tenreiro S, Viegas CA, Sa-Correia I. FLR1 gene (ORF YBR008c) is required for benomyl and methotrexate resistance in Saccharomyces cerevisiae and its benomyl-induced expression is dependent on pdr3 transcriptional regulator. Yeast. 1999;15:1595–1608. doi: 10.1002/(SICI)1097-0061(199911)15:15<1595::AID-YEA484>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Cabral SMJC, Cabral JPS. The primary mode of action of vinclozolin: are oxygen free radicals directly involved? Pest Biochem Physiol. 2000;66:145–152. [Google Scholar]
- Cañas-Gutiérrez GP, Patiño LF, Rodríguez-Arango E, Arango R. Molecular Characterization of benomyl-resistant isolates of Mycosphaerella fijiensis, collected in Colombia. J Phytopathol. 2006;154:403–409. [Google Scholar]
- Cañas-Gutiérrez GP, Angarita-Velásquez MJ, Restrepo-Flórez JM, Rodriguez P, Moreno CX, Arango R. Analysis of the CYP51 gene and encoded protein in propiconazole-resistant isolates of Mycosphaerella fijiensis. Pest Manag Sci. 2009;65:892–899. doi: 10.1002/ps.1770. [DOI] [PubMed] [Google Scholar]
- Chin KM, Wirz M, Laird D. Sensitivity of Mycosphaerella fijiensis from banana to trifloxystrobin. Plant Dis. 2001;85:1264–1270. doi: 10.1094/PDIS.2001.85.12.1264. [DOI] [PubMed] [Google Scholar]
- Churchill ACL. Mycosphaerella fijiensis, the black leaf streak pathogen of banana: progress towards understanding pathogen biology and detection, disease development, and the challenges of control. Mol Plant Pathol. 2011;12:307–328. doi: 10.1111/j.1364-3703.2010.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias PJ, Teixeira MC, Telo JP, Sa-Correia I. Insight into mechanism of toxicity and tolerance to the agricultural fungicide mancozeb in yeast, as suggested by a chemogenomics approach. OMICS. 2010;14:211–227. doi: 10.1089/omi.2009.0134. [DOI] [PubMed] [Google Scholar]
- FRAC (Fungicide Resistance Action Committee) Fungicide use guidelines. 10th Meeting Working Group Bananas; Orlando, FLA, USA. 2012. [Accessed 21 December 2012]. Avalaible at: http://www.frac.info/ [Google Scholar]
- Grimm D. A bunch of trouble. Science. 2008;322:1046–1047. doi: 10.1126/science.322.5904.1046. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Choi GJ, Cho KY. Correlation of lipid peroxidation in Botrytis cinerea caused by dicarboximide fungicides with their fungicidal activity. J Agric Food Chem. 1998;46:737–741. doi: 10.1021/jf970501c. [DOI] [PubMed] [Google Scholar]
- Leroux P. Chemical control of Botrytis and its resistance to chemical fungicides. In: Elad Y, Williamson P, Tudzinski P, Delen N, editors. Botrytis: Biology, pathology and control. Dordrecht: Kluwer Academic; 2004. pp. 195–222. [Google Scholar]
- Luo CX, Schnabel G. The cytochrome P450 lanosterol 14alpha-demethylase gene is a demethylation inhibitor fungicide resistance determinant in Monilinia fructicola field isolates from Georgia. Appl Environm Microbiol. 2008;74:359–366. doi: 10.1128/AEM.02159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzo-Sanchez G, Carrillo-Madrigal H, Guzman-Gonzalez S. Sensitivity in vitro of Mycosphaerella fijiensis, causal agent of black Sigatoka of banana to the fungicides benomyl, propiconazole and azoxystrobin. Rev Mex Fitopatol. 2012 http://www.redalyc.org/articulo.oa?id=61225129008.
- Marín DH, Romero RA, Guzman M, Sutton TB. Black sigatoka: an increasing threat to banana cultivation. Plant Dis. 2003;87:208–222. doi: 10.1094/PDIS.2003.87.3.208. [DOI] [PubMed] [Google Scholar]
- Martinez-Bolaños L, Teliz-Ortiz D, Rodriguez-Maciel C, Mora-Aguilera JA, Nieto-Angel D, Cortes-Flores I, Mejia-Sanchez D, Nava-Diaz C, Silva-Aguayo G. Fungicide resistance on Mycosphaerella fijiensis populations of southeastern Mexico. Agrociencia. 2012;46:707–717. [Google Scholar]
- Orozco-Santos M, Farias-Larios J, Manzo-Sanchez G, Guzmna-Gonzalez S. Black Sigatoka disease (Mycosphaerella fijiensis Morelet) in Mexico. Infomusa. 2001;10:33–37. [Google Scholar]
- Pérez L, Batlle A, Hernández M, Trujillo R, Alvarez C, Mendez A. Evolución de la sensibilidad a fungicidas de las poblaciones de Mycosphaerella fijiensis Morelet en banano en Cuba. Fitosanidad. 2003;7:49–54. [Google Scholar]
- Romero RA, Sutton TB. Sensitivity of Mycosphaerella fijiensis, causal agent of Black Sigatoka of banana, to propiconazole. Phytopathol. 1997;87:96–100. doi: 10.1094/PHYTO.1997.87.1.96. [DOI] [PubMed] [Google Scholar]
- Romero RA, Sutton TB. Characterization of benomyl resistance in Mycosphaerella fijiensis, cause of Black Sigatoka of banana, in Costa Rica. Plant Dis. 1998;82:931–934. doi: 10.1094/PDIS.1998.82.8.931. [DOI] [PubMed] [Google Scholar]
- Santos PM, Simoes T, Sá-Correia I. Insights into yeast adaptive response to the agricultural fungicide mancozeb: a toxicoproteomics approach. Proteomics. 2009;9:657–670. doi: 10.1002/pmic.200800452. [DOI] [PubMed] [Google Scholar]
- Sierotzki HS, Parisi U, Steinfeld I, Tenzer I, Poirey S, Gisi U. Mode of resistance to respiration inhibitors at the cytochrome bc1 enzyme complex of Mycosphaerella fijiensis field isolates. Pest Manag Sci. 2000;56:833–841. [Google Scholar]
- Teixeira MC, Dias PJ, Simões T, Sa-Correia I. Yeast adaptation to mancozeb involves the up-regulation of FLR1 under the coordinate control of Yap1, Rpn4, Pdr3, and Yrr1. Biochem Biophys Res Commun. 2008;367:249–255. doi: 10.1016/j.bbrc.2007.12.056. [DOI] [PubMed] [Google Scholar]
- Vawdrey LL, Grice K. Field evaluation of strobilurins, triazoles and acibenzolar to control Sigatoka disease in Australia. Infomusa. 2005;14:11–15. [Google Scholar]
- Wyand RA, Brown JK. Sequence variation in the CYP51 gene of Blumeria graminis associated with resistance to sterol demethylase inhibiting fungicides. Fungal Gen Biol. 2005;42:726–735. doi: 10.1016/j.fgb.2005.04.007. [DOI] [PubMed] [Google Scholar]