Abstract
Escherichia coli O157:H7 has been incriminated in food poisoning outbreaks and sporadic cases of hemorrhagic colitis and hemolytic uremic syndrome in many countries. Considering the high susceptibility of Minas Frescal cheese to contamination by E. coli O157:H7, the aim of this study was to determine the occurrence of this pathogen through PCR (Polymerase Chain Reaction) and ELISA (VIDAS ECO O157®, bioMérieux, Lyon, France) test. Thirty cheese samples manufactured by artisan farmhouse producers were collected from open-air markets in Goiânia and thirty from industries under Federal Inspection located in Goiás State which trade their products in supermarkets in Goiânia. E. coli O157:H7 was detected in 6.67% samples collected in open air markets using ELISA, and 23,33% with PCR. The pathogen was not detected in samples from industries under Federal Inspection.
Keywords: Minas Frescal cheese, STEC/VTEC, PCR, VIDAS ECO O157®
Introduction
Food-borne diseases are of major importance due to public health concerns and damages to consumers. Escherichia coli O157:H7 was first linked to food-borne diseases in the United States of America after an outbreak by ingestion of undercooked hamburgers at a fast food restaurant in 1982 (Riley, et al., 1983). Since then Escherichia coli O157:H7 has become a worldwide threat to public health and is one of today’s most troubling food-borne pathogens. The main E. coli O157:H7 reservoir is the gastrointestinal tract of cattle, being a source of potential contamination during milking procedures (Hussein and Sakuma, 2005). E. coli O157: H7 was detected in 6.8% of feces samples from cattle and 0.2% of dairy cows in Argentina (Padola, et al., 2004; Fernández, et al., 2010), while in the United States, LeJeune et al. (2006) isolated this serotype in 0.6% of dairy cows from Ohio State. Undercooked beef, unpasteurized milk and use of water contaminated by cattle manure have been associated to human outbreaks of hemorrhagic colitis and hemolytic uremic syndrome (Hussein and Sakuma, 2005; Sandrini, et al., 2007; Karmali, et al., 2009).
Verocytotoxin-producing E. coli O157 (VTEC/STEC) comprise over 400 serotypes (Scheutz, et al., 2005). Serotype O157:H7 is prevalent in many world regions (Armstrong, et al., 2006) and considered the most dangerous vegetative pathogen associated with raw milk and raw milk cheeses (Baylis, 2009). Murphy et al. (2007) detected five verocytotoxigenic E. coli O157 isolates in a surveillance of dairy production holdings supplying raw milk to farmhouse cheese makers in Ireland. Paneto et al. (2007) analyzed 50 samples of cheese produced from raw milk obtained from Araguaina (Tocantins State, Brazil) supermarkets and determined STEC occurrence in 6%. In a review conducted by Baylis (2009) numerous cases of food poisoning by E. coli O157:H7 due to unpasteurized milk products consumption were reported.
In Brazil, no E. coli O157:H7 food-borne outbreak was reported so far although this serotype has been isolated from sporadic cases of human diarrhea (Irino, et al., 2002) and cattle feces (Riley, et al., 1983; Cerqueira, et al., 1999; Sandrini, et al., 2007; Stella et al., 2008).
Raw milk cheese may be a source of E. coli as the pathogen may survive the manufacturing process. Brazilian government legislation provides for the use of pasteurized milk in cheese production but farm cheese craft manufacturers still use raw milk. The classic example in Brazil is Minas Frescal cheese where a production of 28,8 metric tons was reported in 2004 (Brasil, 2004). As a fresh product susceptible to microbiological changes it is frequently involved in outbreaks of food poisoning.
This study was developed in order to verify the occurrence of E. coli O157:H7 in cheese commercialized in Goiania, by PCR and ELISA (VIDAS ECO O157 commercial kit ®).
Materials and Methods
From January to March 2009 60 samples of Minas Frescal cheese were collected, 30 of which came from open-air street markets (farmers market) from Goiânia city, Goiás State and 30 from milk and dairy plants under Federal Inspection (IF), located in the State of Goias.
The occurrence of E. coli O157:H7 in Minas Frescal cheese was determined by the commercial kit VIDAS® ECO O157 (bioMérieux, Lyon, France) and a polymerase chain reaction (PCR) protocol. Analytical procedures were directed primarily to the presence of somatic antigen “O157” and then positive samples were processed for the detection of flagellar antigen “H7”.
The kit VIDAS ® ECO O157 (BioMérieux, Lyon, France) was used in accordance with manufacturer’s instructions. To confirm a positive result as E. coli O157:H7 isolation of the microorganism and specific serological tests were performed following the AFNOR’s protocol for general food products (Association Française de Normalisation - No. BIO-12/8-07/00).
Initially, 25 g of each individual sample were enriched in 225 mL of modified trypcase-soy broth (Difco, USA) supplemented with an aqueous solution of acriflavine-HCl (final concentration of 10 mg/L) and incubated at 41 °C for 6 to 7 h. Then, 1 mL of pre-enrichment broth was transferred to 9 mL of MacConkey broth (Difco, USA) with Cefixime (final concentration 0,05 mg/L) and potassium tellurite (final concentration 2.5 mg/L), incubating at 36 °C for 18 h. After incubation, 2 mL of broth were transferred into a new tube, and heated to 100°C for 15 min. Next, 0.5 mL of warmed broth were analyzed by the kit VIDAS® ECO O157 and the rest of the sample was kept refrigerated. In cases of positive results, the broth was plated on MacConkey sorbitol agar (BD, Heidelberg, Germany) and incubated at 35 °C ± 1 °C for 24–48 h. Subsequently, 5 to 10 colony forming units (CFU) suggestive of E. coli were selected, plated on CHROMagar O157 agar® (BD, Heidelberg, Germany) and incubated at 35 °C ± 1 °C for 24–48 h.
Typical CFUs were transferred to triple sugar iron agar tubes and after incubation yellow bezel gas production and no H2S tubes were submitted to biochemical characterization. Results were considered E. coli when positive for indole production, methyl red reaction, decarboxylation of lysine and ornithine, and negative for Voges Proskauer reaction, citrate and sorbitol utilization. Isolates with typical biochemical pattern underwent serology by using anti-O157 and H7 antisera (Difco, USA).
At all steps Escherichia coli (EHEC) O157:H7 INCQS 00171 was used as positive control strain. Reference strains were acquired from the collection of the Laboratory for Reference Materials, National Institute for Quality Control in Health (INCQS) Oswaldo Cruz Foundation.
For the detection of Escherichia coli O157:H7 by PCR two sets of primers were used. RFB (Hu, et al., 1999) primer pair are complementary to the gene region rfbE involved in the biosynthesis of O157 somatic antigen and Flic (Wang, et al., 2002) primer pair are complementary to region of fliC encoding the H7 flagellar antigen.
An aliquot of 1.5 mL of MacConkey broth with Cefixime and Potassium Tellurite was centrifuged at 10.000 rpm for 10 min. Then, the supernatant was discarded and the pellet was re-suspended in 500 μL of Tris-EDTA (TE, 10 mm Tris, 1 mM EDTA, pH 8.0). Genomic DNA extraction was performed following High Pure PCR Template Preparation Kit (Roche®, Mannheim, Germany) manual instructions. DNA quality and concentration were estimated in 0.8% agarose gel electrophoresis by visual comparison with standard molecular weight DNA Low Mass Ladder (Invitrogen®, Brazil) and adjusted to 20 ng/mL.
The PCR reactions and amplification conditions followed primer’s authors indications (Hu, et al., 1999; Wang, et al., 2002). As negative control for PCR reaction mixture, ultra-pure water was used replacing the DNA template and E. coli reference strain (EHEC) O157: H7 INCQS 00171 was used as positive control.
Results
Samples from free markets
Minas Frescal cheese from street fairs were positive for E. coli O157 in 83.33% samples (25/30) when analyzed by the kit VIDAS® ECO O157. However, when suspected microorganisms were submitted to isolation and serology, only 2/30 (6.67%) wereconfirmed as belonging to serotype O157:H7.
Concerning the detection of E. coli O157:H7 by PCR (Figures 1 and 2), 70% (21/30) samples from street markets were positive to RfbF/RfbR primers and 23.33% (7/30) to Flic-a/Flic-b primers.
Figure 1.
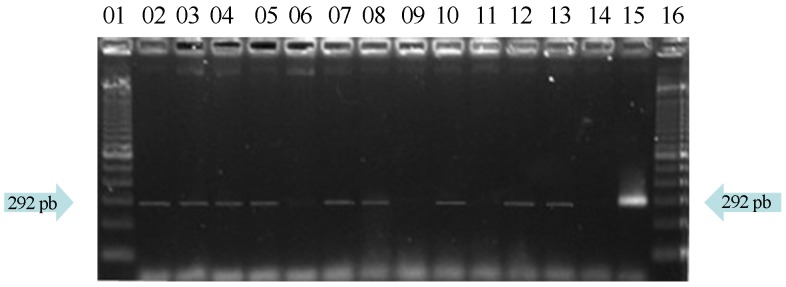
Agarose gel electrophoresis of “O” antigen detection by PCR with primers RfbR/RfbR (292pb). Lines 1 and 16 denotes molecular weight (DNA Ladder 100 bp); line 15 display positive control (INCQS 00171); lines 2–5, 7, 8, 10, 12 and 13 positive samples; line 14 negative control; lines 6, 9 and 11 negative samples.
Figure 2.
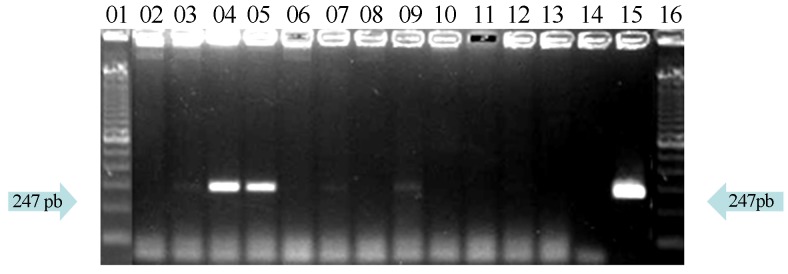
Agarose gel electrophoresis of “H” antigen detection by PCR with primers FliC-a/FliC-b (247pb). Lines 1 and 16 denotes molecular weight marker (DNA Ladder 100 bp), line 15 positive control (INCQS 00171); lines 3, 4, 5, 7 and 9 positive samples; line 14 negative control; lines 2, 6, 8 and 10–13 negative samples.
Samples obtained from plants with Federal Inspection (IF)
When the kit VIDAS® ECO O157 was used as a diagnostic technique on samples from plants under IF, 66.67% (20/30) were positive, but none was confirmed by conventional bacteriological culture of the microorganism.
When the PCR technique was employed, the primer pair RfbF/RfbR detected 50% (15/30) positive for the antigen “O157”, however, the pair of primers Flic-a/Flic-b did not detect any positive for the antigen “H7”.
Discussion
Detection of E. coli O157:H7 in Minas Frescal cheese confirms the presence of this pathogen in dairy products marketed in Brazil, and this result is in accordance with previous reports from Cerqueira et al. (1999), Irino et al. (2005) and Sandrini et al. (2007). In spite of these findings, the pathogen was only confirmed in artisan farmhouse cheeses, produced with unpasteurized milk, generally under inappropriate hygienic conditions. Toxigenic E. coli in raw milk and cheese made from unpasteurised milk was also reported by Quinto and Cepeda (1997) in Spain.
Toxigenic E. coli O157:H7 in raw milk and cheese made from unpasteurised milk was also reported by De Reu et al. (2004), in Belgium, who isolated the pathogen in 0.7% samples of raw milk from farms that directly trade their products, Altalhi and Hassan (2009) found STEC in three isolates (9.1%) from raw milk in Saudi Arabia and by Zweifel et al. (2010) in Switzerland, who found E. coli STEC in 5.7% samples of cheese made with raw milk.
The absence of this pathogen in processed Minas Frescal cheese from creameries under IF is in accordance with the results obtained in Italy by Zago et al. (2007), and strengthens the use of Good Manufacturing Practices and the application of specific normatives, such as IN 51-Ministry of Agriculture (2002), enhancing the importance of control procedures along the entire “farm-to-fork” chain.
In contrast, the occurrence of E. coli O157:H7 in Minas Frescal cheese samples from free markets may be partly explained by the low quality of farm-elaborated milk products associated to the absence or inadequate monitoring of artisan cheesemakers. Raw milk has already been signaled as a significant source of food borne pathogens, including E. coli O157:H7 and there have been numerous food-poisoning outbreaks associated with direct consumption of raw milk or milk that has been inadequately heat-treated (Hussein and Sakuma, 2005; Baylis, 2009).
The isolation of E. coli O157 and specifically the serotype O157:H7 have confirmed that Brazilian dairy cattle are a reservoir of the pathogen. Results from this study strongly suggest the need for more research concerning this and other E. coli serotypes related to food safety and food borne infection in humans.
These results also call the attention from veterinary inspectors and health authorities regarding production and marketing of Minas Frescal cheese. Preparation of cheese without appropriate controls and good hygiene practices and sanitary procedures constitutes a risk to public health.
Acknowledgments
We thank Sandra Queiroz Porto de Mesquita, Eurione Antonio Garcia da Veiga Jardim, Camila Silveira Melo, Wesdras Martins dos Santos, Jacqueline Santos Marques, Jaison Pereira de Oliveira and Marcele Louise Tadaieski for their exellent technical assistance. The authors are very grateful for the support of CAPES for funding Postdoctoral fellowship of Dr. Rolando Mazzoni.
References
- Altalhi AD, Hassan SA. Bacterial quality of raw milk investigated by Escherichia coli and isolates analysis for specific virulence-gene markers. Food Control. 2009;20:913–917. [Google Scholar]
- Armstrong GL, Hollingsworth J, Morris JG. Emerging foodborne pathogens Escherichia coli O157:H7 as a model of entry of a new pathogen into the food supply of the developed country. Epidemiol Rev. 1996;18:29. doi: 10.1093/oxfordjournals.epirev.a017914. [DOI] [PubMed] [Google Scholar]
- Baylis CL. Raw milk and raw milk cheeses as vehicles for infection by Verocytotoxin-producing Escherichia coli. Int J Dairy Tech. 2009;62:293–307. [Google Scholar]
- Beutin L. Prevalence and characteristics of Shiga toxin-producing Escherichia coli in swiss raw milk cheeses collected at producer level. J Dairy Sc. 2008;91:2561–2565. doi: 10.3168/jds.2008-1055. [DOI] [PubMed] [Google Scholar]
- Brasil. Ministério da Agricultura, Pecuária e Abastecimento. Regulamento Técnicos de Produção, Identidade, Qualidade, Coleta e Transporte de Leite. [Acessed July 13 2010];Instrução Normativa N° 51 de 18 de setembro de 2002. 2002 Available at: http://www.qualidadedoleite.com.br/hd/arquivos/IN51de2002_leitebnormas.pdf.
- Brasil. Ministério da Agricultura, Pecuária e Abastecimento. Embrapa Gado de Leite. Tabela 04.24. Produção brasileira de queijos sob inspeção federal. [Acessed July 13 2010];Estatísticas do Leite - Indústrias, 2004. 2004 Available at: http://www.cnpgl.embrapa.br/nova/informacoes/estatisticas/industria/tabela0404.php.
- Cerqueira AMF, Guth BEC, Joaquim RM, Andrade JRC. High occurrence of shiga toxin-producing Escherichia coli (STEC) in healthy cattle in Rio de Janeiro states, Brazil. Vet Microbiol. 1999;90:111–121. doi: 10.1016/s0378-1135(99)00123-6. [DOI] [PubMed] [Google Scholar]
- De Reu’ K, Gruspeerdt K, Herman L. A Belgian survey of hygiene indicator bacteria and pathogenic bacteria in raw milk and direct marketing of raw milk farm products. J Food Saf. 2004;24:17–36. [Google Scholar]
- Fernández D, Irino K, Sanz ME, Padola NL, Parma AE. Characterization of Shiga toxin-producing Escherichia coli isolated from dairy cows in Argentina. Lett Appl Microbiol. 2010;51:377–382. doi: 10.1111/j.1472-765X.2010.02904.x. [DOI] [PubMed] [Google Scholar]
- Hu Y, Zhang Q, Meitzler JC. Rapid and sensitive detection of Escherichia coli O157:H7 in bovine faeces by a multiplex PCR. J Appl Microbiol. 1999;87:867–876. doi: 10.1046/j.1365-2672.1999.00938.x. [DOI] [PubMed] [Google Scholar]
- Hussein HS, Sakuma T. Invited Review: Prevalence of shiga toxin-produncing Escherichia coli in dairy cattle and their products. J Dairy Sci. 2005;88:450–465. doi: 10.3168/jds.s0022-0302(05)72706-5. [DOI] [PubMed] [Google Scholar]
- Irino K, Vaz TMI, Kato MAMF, Naves ZVF. O157:H7 Shiga toxin-producing Escherichia coli strains associated with sporadic cases of diarrhea in São Paulo. Emerg Infect Dis. 2002;8:446–447. doi: 10.3201/eid0804.010490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irino K, Kato MAMF, Vaz TMI, Ramos I, Souza MAC, Cruz AS, Gomes TAT, Vieira MAM, Guth BEC. Serotypes and virulences markers of Shiga toxin-producing Escherichia coli (STEC) isolated from dairy cattle in São Paulo State, Brazil. Vet Microbiol. 2005;105:29–36. doi: 10.1016/j.vetmic.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Karmali MA, Gannon V, Sargeant JM. Verocytotoxin-producing Escherichia coli (VTEC) Vet Microbiol. 2009;140:360–370. doi: 10.1016/j.vetmic.2009.04.011. [DOI] [PubMed] [Google Scholar]
- LeJeune TJ, Hancock D, Wasteson Y, Skjerve E, Urdahl AM. Comparison of E. coli O157 and Shiga toxin-encoding genes (stx) prevalence between Ohio, Usa and Norwegian dairy cattle. Int J Food Microbiol. 2006;109:19–24. doi: 10.1016/j.ijfoodmicro.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Murphy M, Buckley JF, Whyte P, O’Mahony M, Anderson W, Wall PG, Fanning S. Surveillance of dairy production holdings supplying raw milk to the farmhouse cheese sector for Escherichia coli O157, O26 and O111. Zoonoses Public Health. 2007;54:358–365. doi: 10.1111/j.1863-2378.2007.01073.x. [DOI] [PubMed] [Google Scholar]
- Padola NL, Sanz ME, Blanco JE, Blanco M, Blanco J, Etcheverria AI, Arroyo GH, Usera MA, Parma AE. Serotypes and virulence genes of Shigatoxigenic Escherichia coli (STEC) isolates from a feedlot in Argentina. Vet Microbiol. 2004;100:3–9. doi: 10.1016/S0378-1135(03)00127-5. [DOI] [PubMed] [Google Scholar]
- Paneto BR, Schocken-Iturrino RP, Macedo C, Santo E, Marin JM. Occurrence of toxigenic Escherichia coli in raw milk cheese in Brazil. Arq Bras Med Vet Zootec. 2007;59:2508–512. [Google Scholar]
- Quinto EJ, Cepeda A. Incidence of toxigenic Escherichia coli in soft cheese made with raw or pasteurized milk. Lett Appl Microbiol. 1997;24:291–295. doi: 10.1046/j.1472-765x.1997.00072.x. [DOI] [PubMed] [Google Scholar]
- Riley LW, Remis RS, Helgerson SD, McGee HB, Wells JG, Davis BR, Hebert RJ, Olcott ES, Johnson LM, Hargrett T, Blake A, Cohen L. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983;308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- Sales SS, Costa FN, Alves LMC, Barrozo LM, Holanda-Viana AM, Mesquista ERL, Neto VM. Occurrence of Shiga toxin-producing Escherichia coli (STEC) in intestinal microbiota of bovine destined to the slaughter in the city of São Luís - MA/Brazil. Rev Port de Cienc Vet. 2006;101:559–560. 245–251. [Google Scholar]
- Sandrini CNM, Pereira MA, Brod CS, Carvalhal JB, Aleixo JAG. Escherichia coli verotoxigênica: isolamento e prevalência em 60 propriedades de bovinos de leite da região de Pelotas, RS, Brasil. Cienc Rural. 2007;37:175–182. [Google Scholar]
- Scheutz F, Strockbine NA. Escherichia . In: Garrity GM, Brenner DJ, Krieg NR, Staley JT, editors. Bergey’s Manual of Systematic Bacteriology. Springer; New York: 2005. pp. 607–624. [Google Scholar]
- Stella AE, Rigobelo EC, Oliveira AC, Maluta RP, Marin JM, Ávila FA. Ocorrência e sensibilidade microbiana de linhagens de Escherichia coli enteropatogências isoladas de propriedades leiteiras na região de Ribeirão Preto-SP, Brasil. Vet Zootec. 2008;15:66–74. [Google Scholar]
- Wang G, Clark CG, Rodgers FG. Detection in Escherichia coli of the genes encoding the major virulence factor, the genes defining the O157:H7 serotype, and components of the type 2 shiga toxin family by multiplex PCR. J Clin Microbiol. 2002;40:3613–3619. doi: 10.1128/JCM.40.10.3613-3619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zago M, Bonvini B, Platero AMM, Mucchetti G, Carminati D, Giraffa F. Characterisation of Escherichia coli isolated from raw milk cheeses. An Microbiol. 2007;57:49–54. [Google Scholar]
- Zweifel C, Giezendanner N, Corti S, Krause G, Beutin L, Danuser J, Stephan R. Characteristics of Shiga Toxin-Producing Escherichia coli Isolated from Swiss Raw Milk Cheese within a 3-Year Monitoring Program. J Food Prot. 2010;73:88–91. doi: 10.4315/0362-028x-73.1.88. [DOI] [PubMed] [Google Scholar]


