Abstract
Bacteriocins from lactic acid bacteria are ribosomal synthesized antibacterial proteins/peptides having wide range of applications. Lactobacillus pentosus SJ65, isolated from fermented Uttapam batter (used to prepare south Indian pan cake), produces bacteriocin having a broad spectrum of activity against pathogens. Optimization studies are of utmost important to understand the source of utilization and the conditions to enhance the production of metabolites. In the present study, an attempt was made to identify the parameters involved for maximal production of antimicrobial compounds especially bacteriocin from the isolate L. pentosus SJ65. Initially, optimal conditions, such as incubation period, pH, and temperature were evaluated. Initial screening was done using methodology one-variable-at-a-time (OVAT) for various carbon and nitrogen sources. Further evaluation was carried out statistically using Plackett-Burman design and the variables were analyzed using response surface methodology using central composite design. The optimum media using tryptone or soy peptone, yeast extract, glucose, triammonium citrate, MnSO4, dipotassium hydrogen phosphate and tween 80 produced maximum bacteriocin activity.
Keywords: bacteriocin, optimization, Lactobacillus pentosus, Plackett-Burman design, response surface methodology
Introduction
Lactic acid bacteria (LAB) are Gram positive bacteria that produce several antimicrobial compounds like lactic acid, acetic acid, and bacteriocin. Bacteriocins, cationic peptides exhibiting hydrophobic or amphiphilic properties, possess antibacterial activity against food spoilage and pathogenic organisms and are being used as food preservatives (Jeevaratnam et al., 2005). They also have high potential for biomedical applications. In addition, several low molecular weight compounds like benzoic acid, lactic acid, phenyl lactic acid, methylhydantoine, mevalonolactone produced by LAB also exhibits antifungal and antimicrobial properties. Earlier, we have identified many bacteriocinogenic lactobacilli isolates from fermented Uttapam batter (a cereal based fermented food source) to subspecies level showing broad spectrum of antibacterial activity (Saraniya and Jeevaratnam, 2012). Among the isolates, Lactobacillus pentosus SJ65 was found to be most potent and taken up for further study. Normally, LAB produce bacteriocins in very small quantities, requiring optimization of various factors for their maximal production.
The standard growth media like MRS (de Mann Rogosa and Sharpe) broth, Elliker broth, Brain Heart Infusion (BHI) broth, and Trypticase Soya digest Broth (TSB) are widely used for production of bacteriocin. Response surface methodology (RSM) is widely used in earlier studies for optimization of bacteriocin production using experimental designs, to analyse the linear and quadratic effects of variables and understand the interactions among the variables (Delgado et al., 2007; Kanmani et al., 2011; Myers and Montgomery, 2002; Wiese et al., 2010).
Current utility of these optimization techniques, especially for bacteriocins from lactic acid bacteria, have to be understood as they are extracellular released peptides with low yield. Previous studies have shown the crucial role of environmental factors in the production of bacteriocin (Kanmani et al., 2011; Kim et al., 2000). Hence, optimization of media components and non - nutritional parameters were done to increase the production of bacteriocin. The present study deals with the identification of optimal media and non-medium components for maximal production of antibacterial compounds, especially bacteriocin by L. pentosus SJ65 isolated from fermented Uttapam batter (Saraniya and Jeevaratnam, 2012).
Materials and Methods
Bacterial strains and media
L. pentosus strain, an isolate from fermented Uttapam batter was identified through 16S rRNA gene sequence and deposited in GenBank, NCBI (accession number JN573623). All media components were procured from HiMedia and Merck, India. The indicator strains (used in this study) were procured from Microbial Type culture collections (MTCC), Chandigarh, India. (unnecessary - All media were sterilized using autoclaved at 121 °C for 20 min).
Antibacterial assay
L. pentosus SJ65 was grown and the cell free supernatant (CFS) was collected by centrifugation at 8000 g for 10 min. The CFS was concentrated to 10 fold (CFSC) using rotavapor (Buchi, Swizterland) and the concentrate was adjusted to pH 6.0 using 1 M NaOH. The antibacterial activity was determined using agar well diffusion method (Tagg and McGiven, 1971) against Listeria monocytogenes and expressed as activity units (AU). One AU is defined as the reciprocal of the highest dilution exhibiting minimum inhibition zone against the indicator (Kanmani et al., 2011).
Evaluation of growth curve for L. pentosus SJ65
Initial evaluation was done to identify the optimal incubation period of growth for L. pentosus SJ65 using MRS broth/agar and represented as growth curve based on viable cell count using log CFU (colony forming units) and AU.mL−1.
Screening of carbon and nitrogen sources using OVAT methodology
The influence of various carbon and nitrogen sources was done using one-variable-at-a-time (OVAT). Carbon sources (glucose, fructose, sucrose, maltose, lactose, mannose, and galactose) at the concentration of 2% (w/v) were evaluated while other components were constant as MRS composition. The nitrogen sources (tryptone, soy peptone, peptone, skim milk, yeast extract, beef extract, malt extract, tri-ammonium citrate and ammonium nitrate) at a concentration of 2% (w/v) were analyzed with other constituents as that of MRS broth.
Analysis of minerals and non - nutritional conditions
Effect of various minerals like MnSO4 (0.005 to 0.05% (w/v) with 0.01% (w/v) increment), CaCl2, K2HPO4, and surfactant Tween 80 at various concentrations (0 to 0.5% (w/v) with 0.1% (w/v) increment) was studied. Non nutritional conditions like presence and absence of agitation (0 and 100 in orbital shaker) and initial inoculum (1, 2, 5, 7, and 10% (v/v) were also evaluated.
Designing of experiments and analysis of results
For experimental design and graphical analysis of data, Design - Expert trial version 8.0 (State-Ease Inc., Minneapolis, U.S.A.) was used. Eleven variables (tryptone, yeast extract, soy peptone, glucose, citrate, acetate, MgSO4, MnSO4, time, pH, and temperature) were chosen using Plackett-Burman design and significance of model was checked by F - test and goodness of fit by multiple correlations R. The most optimal variables from Placket-Burman were selected for further evaluation by response surface methodology using central composite design (CCD). A total of 26 experiments were performed using 4 essential variables (tryptone, soy peptone, glucose, and triammonium citrate). The relationships between experimental and predicted values were illustrated as contour plots. Values of p < 0.05 were considered significant. Further, the significant components were validated experimentally.
Antibacterial spectrum of L. pentosus SJ65 culture supernatant using optimised media
The antibacterial spectrum of L. pentosus SJ65 culture supernatant against various indicator organisms was done using agar well diffusion assay as described above and the activity was expressed as zone of inhibition in mm.
Results and Discussion
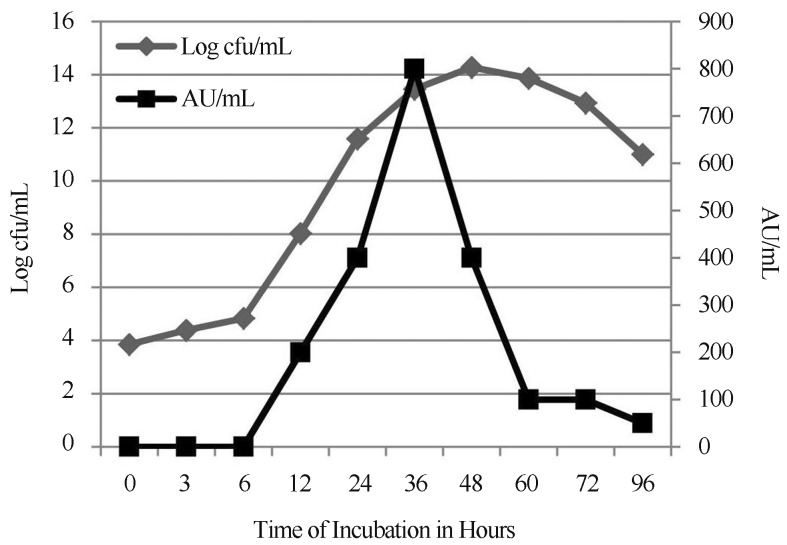
Antimicrobial compounds from lactic acid bacteria are well studied especially bacteriocins which are non-toxic, ribosomal synthesized antimicrobial peptides employed in food preservation (Ray, 1992). Presently, an attempt was made to understand the optimal factors and conditions involved for production of antimicrobial compounds from L. pentosus. Initial study was done using MRS agar/broth to identify the optimal incubation period for maximal production of bacteriocin and identified as 36 h of incubation with 800 AU.mL−1 (Figure 1). Li et al. (2002) suggested that composition of medium plays a significant role in production of bacteriocin. This led to the present study, which involved evaluation of various factors for maximal production of bacteriocin.
Figure 1.
Growth curve pattern obtained for L. pentosus J65.
Influence of C and N sources using OVAT
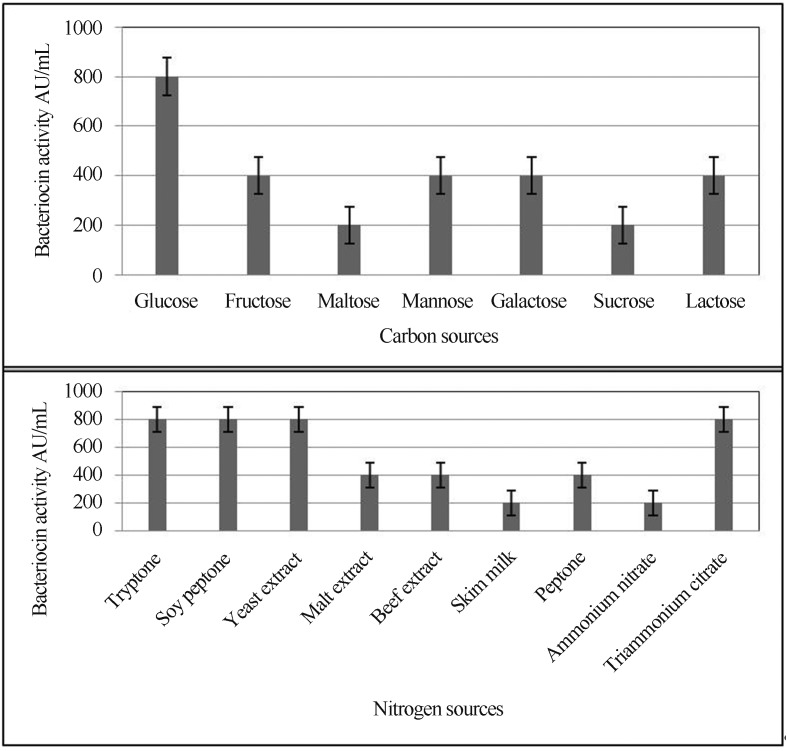
Several earlier studies have shown the precedence of OVAT before usage of Plackett - Burman and CCD (Kanmani et al., 2011; Preetha et al., 2007). Accordingly, initial screening was done using OVAT for selection of optimum carbon sources (Figure 2) wherein glucose was found to be the sole carbon source. Further evaluation of glucose at various concentrations indicated (20 g.L−1) was the optimally utilized by L. pentosus SJ65 having 1600 AU.mL−1 and at concentration of 30 g.L−1 bacteriocin activity was significantly reduced (p < 0.05). Todorov et al. (2004) showed a similar observation for L. pentosus while Aasen et al. (2000) observed it for L. sakei. Evaluation of various nitrogen sources (Figure 2) showed that tryptone, soy peptone, and yeast extract lead to a higher production. Similar observation was made regarding production of bacteriocin from L. pentosus ST712BZ (Todorov et al., 2004) with 3200 AU.mL−1. Essentially, tryptone and peptone are the major nitrogen sources while yeast extract is an essential source for growth factors as it is known to contain larger quantity of free amino acids, short peptides, and essential vitamins (Bridson, 1998).
Figure 2.
Effect of carbon and nitrogen sources using one variable at a time.
Influence of minerals and non-nutritional conditions
Non-ionic surfactant (Tween 80) is an important factor for bacteriocin secretion, which happens by altering the membrane fluidity. Earlier studies have shown that Tween 80 is an essential factor for bacteriocin production (Biswas et al., 1991; Rajaram et al., 2010) while Trinetta et al. (2008) reported Tween 80 as a non-essential factor. In this study, bacteriocin activity was observed maximum at concentration of 1 g.L−1 Tween 80. The concentration of minerals also plays an effective role in the production of bacteriocin. In the present study, MnSO4 has a significant effect on bacteriocin production at the concentration of 0.3 g.L−1. Di-potassium hydrogen phosphate, which acts as a buffering agent in regulating the pH of the medium, is also shown to have a positive effect at the concentration of 1 g.L−1, while increasing its concentration leads to a decreased activity. Presence of calcium chloride in the medium decreases the production of bacteriocin showing that lactic acid is an essential component for bacteriocin production. Calcium forms salts of lactic acid and decreases the availability of lactic acid. Studies involving agitation and initial inoculums were also evaluated. It has been reported that growth and bacteriocin production was improved with agitation at 100 rpm, compared with the static condition (Jozala et al., 2005; Liew et al., 2005). On the contrary, our results showed faster growth with agitation, but bacteriocin production was higher under static condition. In addition, initial inoculum of 5% showed maximal production of bacteriocin while production decreased at 10%, indicating a negative influence on bacteriocin production.
Analysis of variables using statistical tools
Lim et al. (2007) stated that OVAT is highly unreliable when there were large numbers of variables. Also, these methods are time consuming and do not involve mutual interaction among variables. Though the use of OVAT is time consuming, yet a clear understanding of usage of the particular carbon and nitrogen sources was achieved, which helped to confirm by statistical methodologies. Analysis with Plackett - Burman design involves 11 variables consisting of 16 experiments, including 4 mid - points. Table 1, show the experimental design and results of Plackett-Burman Design and the ANOVA were given in Table 2. The production of bacteriocin showed marked increase from 100 to 3200 AU.mL−1 at various levels of each component. Concentration of tryptone and glucose has strongly affected the production of bacteriocin, with a P value of < 0.05. The factorial model is augmented with coefficients to adjust the mean for curvature. This model separates problems due to curvature from those models, not fitting the factorial points, which is appropriate for calculating the diagnostics. If curvature is significant and lack of fit is insignificant, this model could be used for predicting only the factorial points, but not any other points. In the present study, the curvature and lack of fit were insignificant hence this model could be used for predicting all points. Thus, most influential variables from Plackett - Burman design, namely, tryptone (A), soy peptone (B), glucose (C), and triammonium citrate (D), were chosen for further evaluation at various levels of actual and coded values using CCD (Table 3). The final regression equation that was obtained in terms of actual factors that were mainly involved in bacteriocin production is depicted below,
Table 1.
Plackett-Burman experimental design and results.
| Run | Tryptone (g.L−1) | Yeast extract (g.L−1) | Glucose (g.L−1) | Citrate (g.L−1) | Acetate (g.L−1) | MgSO4 (g.L−1) | MnSO4 (g.L−1) | Soya peptone (g.L−1) | Time (h) | pH | Temp (°C) | Activ (AU.mL−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 20 | 5 | 5 | 0 | 0 | 0.3 | 0 | 20 | 48 | 5 | 45 | 800 |
| 2 | 5 | 5 | 5 | 2 | 5 | 0 | 0.05 | 20 | 48 | 5 | 25 | 800 |
| 3 | 12.5 | 3 | 12.5 | 1 | 2.5 | 0.15 | 0.03 | 12.5 | 36 | 6 | 35 | 1600 |
| 4 | 12.5 | 3 | 12.5 | 1 | 2.5 | 0.15 | 0.03 | 12.5 | 36 | 6 | 35 | 3200 |
| 5 | 5 | 1 | 20 | 0 | 5 | 0.3 | 0 | 20 | 48 | 7 | 25 | 1600 |
| 6 | 5 | 5 | 20 | 0 | 5 | 0.3 | 0.05 | 5 | 24 | 5 | 45 | 400 |
| 7 | 5 | 5 | 20 | 2 | 0 | 0 | 0 | 20 | 24 | 7 | 45 | 1600 |
| 8 | 20 | 1 | 5 | 0 | 5 | 0 | 0.05 | 20 | 24 | 7 | 45 | 800 |
| 9 | 12.5 | 3 | 12.5 | 1 | 2.5 | 0.15 | 0.03 | 12.5 | 36 | 6 | 35 | 1600 |
| 10 | 20 | 5 | 5 | 2 | 5 | 0.3 | 0 | 5 | 24 | 7 | 25 | 800 |
| 11 | 5 | 1 | 5 | 2 | 0 | 0.3 | 0.05 | 5 | 48 | 7 | 45 | 400 |
| 12 | 20 | 1 | 20 | 2 | 0 | 0.3 | 0.05 | 20 | 24 | 5 | 25 | 3200 |
| 13 | 12.5 | 3 | 12.5 | 1 | 2.5 | 0.15 | 0.03 | 12.5 | 36 | 6 | 35 | 1600 |
| 14 | 20 | 1 | 20 | 2 | 5 | 0 | 0 | 5 | 48 | 5 | 45 | 3200 |
| 15 | 20 | 5 | 20 | 0 | 0 | 0 | 0.05 | 5 | 48 | 7 | 25 | 1600 |
| 16 | 5 | 1 | 5 | 0 | 0 | 0 | 0 | 5 | 24 | 5 | 25 | 0 |
Table 2.
The ANOVA results for the Plackett-Burman design employed in the present study.
| Source | Sum of squares | df | Mean square | F value | p-value (Prob > F) |
|---|---|---|---|---|---|
| Model | 1.06E+07 | 5 | 2.11E+06 | 4.44 | 0.0217 |
| A-Tryptone | 2.61E+06 | 1 | 2.61E+06 | 5.49 | 0.0411 |
| C-Glucose | 5.33E+06 | 1 | 5.33E+06 | 11.2 | 0.0074 |
| D-CItrate | 1.92E+06 | 1 | 1.92E+06 | 4.03 | 0.0724 |
| H-Soya peptone | 4.80E+05 | 1 | 4.80E+05 | 1.01 | 0.339 |
| J-Time | 2.13E+05 | 1 | 2.13E+05 | 0.45 | 0.5184 |
| Lack of Fit | 2.84E+06 | 7 | 4.06E+05 | 0.63 | 0.7217 |
| Pure Error | 1.92E+06 | 3 | 6.40E+05 | ||
| Cor Total | 1.53E+07 | 15 |
Table 3.
Central composite experimental design using 4 variables obtained from Plackett-Burman and OVAT.
| Run | A: Tryptone (g.L−1) | B: Soy (g.L−1) peptone | C: Glucose (g.L−1) | D: Citrate (g.L−1) | Activity (AU.mL−1) |
|---|---|---|---|---|---|
|
|
|
||||
| Units | |||||
| 1 | 20 | 15 | 20 | 2 | 3200 |
| 2 | 20 | 20 | 15 | 2 | 800 |
| 3 | 15 | 20 | 20 | 5 | 1600 |
| 4 | 15 | 15 | 20 | 2 | 3200 |
| 5 | 20 | 15 | 20 | 5 | 6400 |
| 6 | 20 | 20 | 20 | 5 | 3200 |
| 7 | 15 | 20 | 20 | 2 | 1600 |
| 8 | 17.5 | 17.5 | 17.5 | 3.5 | 6400 |
| 9 | 15 | 15 | 15 | 2 | 100 |
| 10 | 15 | 15 | 20 | 5 | 1600 |
| 11 | 20 | 15 | 15 | 2 | 100 |
| 12 | 17.5 | 17.5 | 22.5 | 3.5 | 3200 |
| 13 | 17.5 | 17.5 | 17.5 | 3.5 | 6400 |
| 14 | 17.5 | 17.5 | 17.5 | 0.5 | 1600 |
| 15 | 22.5 | 17.5 | 17.5 | 3.5 | 3200 |
| 16 | 15 | 20 | 15 | 2 | 1600 |
| 17 | 17.5 | 17.5 | 17.5 | 6.5 | 3200 |
| 18 | 17.5 | 22.5 | 17.5 | 3.5 | 1600 |
| 19 | 15 | 15 | 15 | 5 | 400 |
| 20 | 20 | 15 | 15 | 5 | 3200 |
| 21 | 15 | 20 | 15 | 5 | 1600 |
| 22 | 17.5 | 17.5 | 17.5 | 3.5 | 3200 |
| 23 | 12.5 | 17.5 | 17.5 | 3.5 | 800 |
| 24 | 17.5 | 17.5 | 17.5 | 3.5 | 3200 |
| 25 | 20 | 20 | 15 | 5 | 3200 |
| 26 | 17.5 | 17.5 | 12.5 | 3.5 | 800 |
| 27 | 17.5 | 12.5 | 17.5 | 3.5 | 3200 |
| 28 | 20 | 20 | 20 | 2 | 1600 |
| (1) |
The quadratic regression model (Table 4) based on ANOVA indicates F-value of 5.27 from CCD, implying the significance of the model. The goodness of fit for the model was checked by regression coefficient (R2), which was equal to 0.8501 and this is in close agreement with the adjusted R2 value. “ Adeq Precision” measures the signal to noise ratio and ratio greater than 4 is considered to be desirable. This model indicated a ratio of 7.865, indicating an adequate signal. Overall, the model validates that each variable individually has a direct influence, wherein A, C, D, A2, B2, C2, and D2 lead to a p < 0.05, indicating these model terms as statistically significant variables. Similarly, the interaction variables of AD and BC were statistically significant.
Table 4.
Regression analysis of CCD using ANOVA.
| Source | Sum of squares | df | Mean square | F Value | p-value (Prob > F) |
|---|---|---|---|---|---|
| Model | 6.98E+07 | 14 | 4.99E+06 | 5.27 | 0.0024 |
| A-Tryptone | 9.13E+06 | 1 | 9.13E+06 | 9.64 | 0.0084 |
| B-Soy peptone | 1.60E+06 | 1 | 1.60E+06 | 1.69 | 0.2159 |
| C-Glucose | 1.09E+07 | 1 | 1.09E+07 | 11.55 | 0.0047 |
| D-Citrate | 6.20E+06 | 1 | 6.20E+06 | 6.55 | 0.0237 |
| AB | 1.69E+06 | 1 | 1.69E+06 | 1.79 | 0.2044 |
| AC | 4.90E+05 | 1 | 4.90E+05 | 0.52 | 0.4845 |
| AD | 8.41E+06 | 1 | 8.41E+06 | 8.89 | 0.0106 |
| BC | 6.00E+06 | 1 | 6.00E+06 | 6.34 | 0.0257 |
| BD | 62500 | 1 | 62500 | 0.066 | 0.8012 |
| CD | 4.23E+05 | 1 | 4.23E+05 | 0.45 | 0.5157 |
| A2 | 1.24E+07 | 1 | 1.24E+07 | 13.1 | 0.0031 |
| B2 | 9.19E+06 | 1 | 9.19E+06 | 9.71 | 0.0082 |
| C2 | 1.24E+07 | 1 | 1.24E+07 | 13.1 | 0.0031 |
| D2 | 9.19E+06 | 1 | 9.19E+06 | 9.71 | 0.0082 |
| Residual | 1.23E+07 | 13 | 9.46E+05 | ||
| Lack of Fit | 2.06E+06 | 10 | 2.06E+05 | 0.06 | 0.9997 |
| Pure Error | 1.02E+07 | 3 | 3.41E+06 | ||
| Cor Total | 8.21E+07 | 27 |
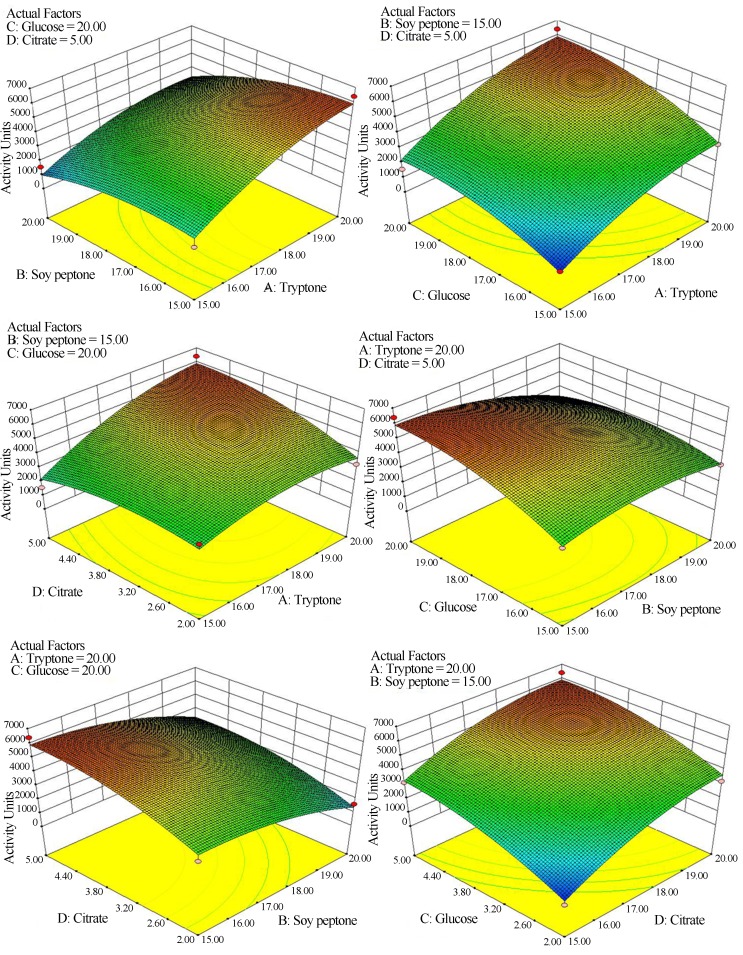
The three dimensional plot (Figure 3) based on the interaction between the variables showed an increase in bacteriocin production with increasing concentrations of tryptone, glucose, and citrate, while increasing concentration of soy peptone decreased production. The optimum values obtained from the 3D plot was also equal to the results obtained from the regression analysis (Eq. (1)). Thus, the optimized media components (per litre) were tryptone (15 g), yeast extract (1 g), tri-ammonium citrate (3.5 g), glucose (15 g), tween-80 (1 g), and di-potassium hydrogen phosphate (1 g). Validation of these optimized components in the form of a liquid medium gave a satisfactory result of 3200 to 6400 AU.mL−1 of bacteriocin activity for L. pentosus while the production in standard MRS broth was only 800 AU.mL−1. This medium is highly advantageous as a bacteriocin production of 4 to 8 fold increase was observed when compared to the routinely used MRS medium. The antibacterial activity assessed against several pathogenic indicator organisms and expressed in mm (Table 5) using the optimized media showed the effectiveness of the antibacterial compound. Further studies are essential to purify and identify the nature of this compound.
Figure 3.
Response surface methodology contour plots for production of bacteriocin using interaction of variables (A) tryptone (B) soy peptone (C) glucose and (D) Citrate.
Table 5.
Antibacterial spectrum analysis of culture supernatant using the optimized culture medium.
| Strains | L. pentosus SJ65 |
|---|---|
| Enterococcus faecalis MTCC 439 | 11 ± 1 |
| Leuconostoc mesenteroides MTCC 107 | 11 ± 1 |
| Lactobacillus fermentum MTCC 1745 | 12 ± 1 |
| Staphylocccus aureus MTCC 737 | 18 ± 3 |
| Bacillus subtilis MTCC 619 | 18 ± 2 |
| Listeria monocytogenes MTCC 657 | 20 ± 2 |
| Vibrio parahemolyticus MTCC 451 | 17 ± 3 |
Inhibition zone expressed in millimeters inclusive of well diameter 6 mm. Values are means of three independent experiments performed in duplicates with standard deviation.
In conclusion, optimization studies using a dominant tool response surface methodology have helped to identify the prime and most favourable nutritional and non-nutritional factors/conditions that are involved for maximal production of antibacterial compounds from L. pentosus SJ65.
Acknowledgments
Ms. A. SARANIYA, acknowledge the support of ICMR (Indian council of medical research), Delhi, India for financial support (3/1/3/JRF-2011/HRD-11-21148).
References
- Aasen IM, Moretro T, Katla T, Axelsson L, Storro I. Influence of complex nutrients, temperature and pH on bacteriocin production by Lactobacillus sakei CCUG 42687. Appl Microbiol Biotechnol. 2000;53:159–166. doi: 10.1007/s002530050003. [DOI] [PubMed] [Google Scholar]
- Biswas SR, Ray P, Johnson MC, Ray B. Influence of growth conditions on the production of a bacteriocin, pediocin AcH, by Pediococcus acidilactici H. Appl Environ Microbiol. 1991;57:1265–1267. doi: 10.1128/aem.57.4.1265-1267.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridson EY. The Oxoid Manual. Oxoid Ltd.; Hampshire, England: 1998. [Google Scholar]
- Delgado A, Arroyo Lopez FN, Brito D, Peres C, Fevereiro P, Garrido-Fernandez A. Optimum bacteriocin production by Lactobacillus plantarum 17.2b requires absence of NaCl and apparently follows a mixed metabolite kinetics. J Biotechnol. 2007;130:193–201. doi: 10.1016/j.jbiotec.2007.01.041. [DOI] [PubMed] [Google Scholar]
- Jeevaratnam K, Jamuna M, Bawa AS. Biological preservation of foods - bacteriocins of lactic acid bacteria. Indian J Biotechnol. 2005;4:446–454. [Google Scholar]
- Jozala AF, De Lencastre Novaes LC, Cholewa O, Moraes D, Vessoni Penna TC. Increase of nisin production by Lactococcus lactis in different media. Afr J Biotechnol. 2005;4:262–265. [Google Scholar]
- Kanmani P, Satish Kumar R, Yuvaraj N, Paari KA, Pattukumar V, Arul V. Optimization of media components for enhanced production of Streptococcus phocae PI80 and its bacteriocin using response surface methodology. Braz J Microbiol. 2011;42:716–720. doi: 10.1590/S1517-838220110002000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Ji GE, Ahn C. Purification and molecular characterization of a bacteriocin from Pediococcus sp. KCA 1202–10 isolated from fermented flastfish. Food Sci Biotechnol. 2000;9:270–276. [Google Scholar]
- Li C, Bai J, Cai Z, Ouyang F. Optimization of a cultural medium for bacteriocin production by Lactococcus lactis using response surface methodology. J Biotechnol. 2002;93:27–34. doi: 10.1016/s0168-1656(01)00377-7. [DOI] [PubMed] [Google Scholar]
- Liew SL, Ariff AB, Raha AR, Ho YW. Optimization of medium composition for the production of a probiotic microorganism, Lactobacillus rhamnosus, using response surface methodology. Int J Food Microbiol. 2005;102:137–142. doi: 10.1016/j.ijfoodmicro.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Lim C, Rahim RA, Ho YW, Arbakariya BA. Optimization of growth medium for efficient cultivation of Lactobacillus salivaris I24 using response surface method. Malaysian J Microbiol. 2007;3:41–47. [Google Scholar]
- Myers RH, Montgomery DC. Process and products optimization using designed experiments. John Wiley & Sons; New York: 2002. Response surface methodology. [Google Scholar]
- Preetha R, Jayaprakash NS, Philip R, Bright Singh IS. Optimization of medium for the production of a novel aquaculture probiotic, Micrococcus MCCB 104 using central composite design. Biotechnol Bioprocess Eng. 2007;12:548–555. [Google Scholar]
- Rajaram G, Manivasagan P, Thilagavathi B, Saravanakumar A. Purification and characterization of a bacteriocin produced by Lactobacillus lactis isolated from marine environment. Adv J Food Sci Technol. 2010;2:138–144. [Google Scholar]
- Ray B. Bacteriocins of starter culture bacteria as food biopreservative. In: Ray B, Daeschael M, editors. Food Biopreservatives of Microbial Origin. CRC Press; Florida: 1992. pp. 177–205. [Google Scholar]
- Saraniya A, Jeevaratnam K. Molecular characterization of bacteriocinogenic Lactobacillus species isolated from fermented Uttapam batter. Biosci Biotechnol Res Asia. 2012;9:417–421. [Google Scholar]
- Tagg JR, McGiven AR. Assay system for bacteriocins. Appl Microbiol. 1971;21:943. doi: 10.1128/am.21.5.943-943.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov SD, van Reenan CA, Dicks LMT. Optimization of bacteriocin production by Lactobacillus plantarum ST13BR, a strain isolated from barley beer. J Gen Appl Microbiol. 2004;50:149–157. doi: 10.2323/jgam.50.149. [DOI] [PubMed] [Google Scholar]
- Trinetta V, Rollini M, Manzoni M. Development of a low cost culture medium for sakacin A production by L. sakei. Process Biochem. 2008;43:1275–1280. [Google Scholar]
- Wiese B, Bru E, Juarez Tomas MS, Nader-Macias MEF. Optimization of low-cost culture media for the production of biomass and bacteriocin by a Urogenital Lactobacillus salivarius strain. Probiotics Antimicrob Proteins. 2010;2:2–11. doi: 10.1007/s12602-010-9037-4. [DOI] [PubMed] [Google Scholar]