Abstract
STUDY QUESTION
Can administration of a prostaglandin (PG) E2 receptor 2 (PTGER2) antagonist prevent pregnancy in adult female monkeys by blocking periovulatory events in the follicle without altering menstrual cyclicity or general health?
SUMMARY ANSWER
This is the first study to demonstrate that a PTGER2 antagonist can serve as an effective non-hormonal contraceptive in primates.
WHAT IS KNOWN ALREADY
The requirement for PGE2 in ovulation and the release of an oocyte surrounded by expanded cumulus cells (cumulus–oocyte expansion; C-OE) was established through the generation of PTGS2 and PTGER2 null-mutant mice. A critical role for PGE2 in primate ovulation is supported by evidence that intrafollicular injection of indomethacin in rhesus monkeys suppressed follicle rupture, whereas co-injection of PGE2 with indomethacin resulted in ovulation.
STUDY DESIGN, SIZE, DURATION
First, controlled ovulation protocols were performed in adult, female rhesus monkeys to analyze the mRNA levels for genes encoding PGE2 synthesis and signaling components in the naturally selected pre-ovulatory follicle at different times after the ovulatory hCG stimulus (0, 12, 24, 36 h pre-ovulation; 36 h post-ovulation, n = 3–4/time point). Second, controlled ovarian stimulation cycles were utilized to obtain multiple cumulus–oocyte complexes (COCs) from rhesus monkeys to evaluate the role of PGE2 in C-OE in vitro (n = 3–4 animals/treatment; ≥3 COCs/animal/treatment). Third, adult cycling female cynomolgus macaques were randomly assigned (n = 10/group) to vehicle (control) or PTGER2 antagonist (BAY06) groups to perform a contraceptive trial. After the first treatment cycle, a male of proven fertility was introduced into each group and they remained housed together for the duration of the 5-month contraceptive trial that was followed by a post-treatment reversibility trial.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Quantitative real-time PCR, COC culture and expansion, immunofluorescence/confocal microscopy, enzyme immunoassay, contraceptive trial, ultrasonography, complete blood counts, serum biochemistry tests and blood lipid profiles.
MAIN RESULTS AND THE ROLE OF CHANCE
Several mRNAs encoding proteins involved in PGE2 synthesis, metabolism and signaling increase (P < 0.05) in the periovulatory follicle after administration of an ovulatory hCG bolus. PGE2 signaling through PTGER2 induces cumulus cell expansion and production of hyaluronic acid, which are critical events for fertilization. Moreover, chronic administration of a selective PTGER2 antagonist resulted in a significant (P < 0.05 versus vehicle-treated controls) contraceptive effect without altering steroid hormone patterns or menstrual cyclicity during a 5-months contraceptive trial. Fertility recovered as early as 1 month after ending treatment.
LIMITATIONS, REASONS FOR CAUTION
This is a proof-of-concept study in a non-human primate model. Further investigations are warranted to elucidate the mechanism(s) of PTGER2 antagonist action in the primate ovary. Although PTGER2 antagonist treatment did not produce any obvious undesirable effects, improvements in the mode of administration, as well as the efficacy of these compounds, are necessary to consider such a contraceptive for women.
WIDER IMPLICATIONS OF THE FINDINGS
Monitoring as well as improving the efficacy and safety of female contraceptives is an important public health activity. Even though hormonal contraceptives are effective for women, concerns remain regarding their side-effects and long-term use because of the widespread actions of such steroidal products in many tissues. Moreover, some women cannot take hormones for medical reasons. Thus, development of non-hormonal contraceptives for women is warranted.
STUDY FUNDING/COMPETING INTEREST(S)
Supported by Bayer HealthCare Pharmaceuticals, The Eunice Kennedy Shriver NICHD Contraceptive Development and Research Center (U54 HD055744), NIH Office of the Director (Oregon National Primate Research Center P51 OD011092), and a Lalor Foundation Postdoctoral Basic Research Fellowship (MCP). The use of the Leica confocal was supported by grant number S10RR024585. Some of the authors (N.B., A.R., K.-H.F., U.F., B.B. and B.L.) are employees of Bayer Healthcare Pharma.
Keywords: prostaglandin E2 receptor, cumulus oocyte expansion, contraceptive trial, primates
Introduction
Recent advances in contraception for women generally center on modifications (reduced doses, altered ratios/types of steroid-derivatives or mode of administration) of older methods, particularly the sex steroid analogs (e.g. estrogens, progestins) comprising the ‘pill’ (Harper, 2005). Even though these hormonal contraceptives are very effective for women, concerns remain regarding their side-effects and long-term use because of the widespread actions of such steroidal products in many non-reproductive tissues (Hall et al., 2001; Mulac-Jericevic and Conneely, 2004). Alternatively, a better understanding of the molecular and cellular mechanisms required for ovarian/follicular function may lead to the identification of novel, more specific, non-hormonal targets for contraception.
Ovulation is a complex process wherein a fully developed follicle ruptures in response to the actions of the midcycle LH surge, releasing the cumulus–oocyte complex (COC) for passage into the reproductive tract and possible fertilization. However, release of the oocyte from the follicle must be preceded by detachment of the COC from the inner granulosa cell layer of the follicle wall. Detachment involves loss of cell–cell contacts and formation of a hyaluronic acid (HA)-rich extracellular matrix (ECM) between cumulus cells surrounding the oocyte, resulting in a large increase or ‘expansion’ of the COC. Recent data from non-primate species indicate that this process, termed cumulus–oocyte expansion (C-OE), involves a complex interaction of oocyte-, granulosa/cumulus- and serum-derived factors (Richards, 2005). While some of the paracrine-acting factors important for C-OE in rodents have been identified (Eppig, 1981; Richards, 2005; Peng et al., 2013), the molecular mechanisms responsible for initiating such complex processes are not fully understood. Clearly, however, HA is the main matrix component of the expanded COC and the amount synthesized through the induction of HA synthase-2 is closely correlated with the degree of expansion (Chen et al., 1993; Fulop et al., 1997; Kimura et al., 2002). The HA-containing matrix is stabilized by several plasma- and follicle-derived components (Richards, 2005) that include inter-α trypsin inhibitor and associated heavy chains (HC1–3), proteoglycans such as versican, the tumor necrosis factor alpha-induced protein 6 (TNFAIP6), pentraxin 3, as well as certain members of the a disintegrin and metalloproteinase with thrombospondin-like repeats family of proteases (Chen et al., 1992; Mukhopadhyay et al., 2001; Fulop et al., 2003; Salustri et al., 2004; Richards, 2005).
A critical role for LH-induced prostaglandin (PG) synthesis and action in coordinating the events necessary for ovulation and C-OE originated from studies demonstrating that non-steroidal anti-inflammatory drugs (NSAIDs; e.g. indomethacin or meloxicam) inhibited ovulation in a wide range of species, independent of any actions on pituitary LH (Sirois et al., 2004; Jesam et al., 2010). It is now recognized that these drugs block the ability of prostaglandin synthase (PTGS) enzymes to convert arachidonic acid into PGH2, the precursor of all other PGs. The requirement for PGE2 specifically in ovulation and the release of an expanded COC was further established through the generation of PTGS2 and PGE2 receptor subtype 2 (PTGER2) null-mutant mice. Female mice deficient for either PTGS2 or PTGER2 are infertile or subfertile, likely as a result of abnormal C-OE and/or follicle rupture (Hizaki et al., 1999; Ochsner et al., 2003). A critical role for PGE2 in the ovulatory process in primates is supported by evidence that intrafollicular injection of indomethacin in rhesus monkeys also suppressed ovulation, whereas co-injection of PGE2 with indomethacin resulted in follicle rupture (Duffy and Stouffer, 2002). Moreover, the involvement of PGE2 in C-OE is likely dependent on its ability to directly promote expansion through the synthesis and secretion of HA from cumulus cells (Eppig, 1981), potentially through the action of the PGE2 receptors PTGER2 and PTGER3 as they are preferentially expressed in the cumulus cells relative to the mural cells prior to rupture (Harris et al., 2011).
While it has been suggested that blocking PG synthesis through inhibition of PTGS activity would limit fertility (Norman, 2001) and possibly serve as a means to achieve contraception (Bata et al., 2006; Massai et al., 2007; Jesam et al., 2010), such an approach would eliminate the synthesis of all PGs and be expected to have greater side-effects than a specific inhibitor of the PGE2 pathway considered vital for periovulatory events (e.g. via PTGER2). DNA array studies conducted by our laboratory (Xu et al., 2011) and individual gene/protein expression analyses performed by others (Harris et al., 2011) revealed that the macaque follicle possesses the capacity to synthesize and respond to PGE2, which in turn supports a similar role for PGE2 as in rodent C-OE (Eppig, 1981). Due to obvious differences between ovarian activities in rodents and domestic animals compared with primates (e.g. size of the follicle, duration of the interval from onset of the gonadotrophin surge to follicle rupture, presence and ovulation of one dominant follicle), contraceptive methods designed to selectively block ovulation should be tested for efficacy and specificity in a primate model prior to clinical trials. Macaques are ideally suited for such studies as they share the above-stated characteristics with women.
Therefore, studies using adult, female macaques were designed to (i) analyze the mRNA levels for the PGE2 synthesis and signaling components in the single, naturally selected pre-ovulatory follicle at different time points after an ovulatory stimulus in vivo; (ii) determine the involvement of PGE2 signaling in primate C-OE in vitro, as well as to compare its effectiveness with that of gonadotrophins; (iii) assess whether a specific PTGER2 antagonist inhibits CO-E in vitro; (iv) assess the ability of a chronically administered PTGER2 antagonist to prevent pregnancy in adult females during natural menstrual cycles; (v) determine if a PTGER2 antagonist affects menstrual cyclicity, steroid hormone patterns, body weights and general health (complete blood counts, serum biochemistry tests, blood lipids, etc.) and (vi) evaluate if cessation of PTGER2 antagonist treatment restores fertility.
Materials and Methods
Animals
The general care and housing of macaques at the Oregon National Primate Research Center (ONPRC) was previously described (Wolf et al., 1990). The studies involving rhesus and cynomolgus macaques were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. The ONPRC Institutional Animal Care and Use Committee approved all study protocols and experiments prior to initiation. Rhesus macaques were used to analyze the mRNA levels of the PGE2 signaling components in the single naturally selected pre-ovulatory follicle and to perform the C-OE in vitro studies. Cynomolgus macaques were used to perform the contraceptive trial since these macaques are not seasonally anovulatory (such as rhesus), and are more adaptable to group handling and training.
Menstrual cycles of adult, female rhesus monkeys were monitored, and blood samples collected by saphenous venipuncture daily starting 4 days after the onset of menses until the next menstrual period as previously described (Duffy et al., 2000). Adult, female cynomolgus monkeys exhibiting normal body weights (2.5–5.0 kg), regular menstrual cycles and proven fertility, were kept under controlled conditions of temperature (22°C) and a standard daily light–dark cycle (12 h light: 12 h dark). Females were housed together in small social groups. Prior to initiation of treatment, the females were socialized to groups, and trained to enter a tunnel where they could be individually identified and examined daily for evidence of menstruation or vaginal sperm (vaginal swab with a cotton-tipped applicator).
Collection of periovulatory follicles and assessment of gene expression
Controlled ovulation (COv) protocols were performed to obtain the single, naturally selected dominant follicle at different time points after administering an ovulatory bolus of hCG to rhesus monkeys, as previously published (Young et al., 2003). The mRNA levels for genes encoding PGE2 synthesis and signaling components in pre-ovulatory follicles at different times after the ovulatory hCG stimulus (0, 12, 24, 36 h pre-ovulation, post-ovulation, n = 3–4 per time point) were assessed using cDNA that was synthesized as previously described (Young et al., 2002; Bogan et al., 2008a,b). The expression levels of genes involved in PGE2 synthesis and signaling were determined through the analyses of a published DNA microarray database (Xu et al., 2011). Based on the criteria of a significant (P < 0.05) ≥2-fold mRNA increase in the follicle after animals received a bolus of hCG, individual mRNA levels were subsequently verified by quantitative real-time PCR (qPCR) analyses. Gene probe sets included on the Affymetrix™ Rhesus Macaque Total Genome Array were used to BLAST the rhesus macaque genome sequence to obtain corresponding annotated, full-length cDNA sequences, which were then used to design qPCR primer and Taqman Probes as previously described (Bogan et al., 2008a,b). Sequences of all primers and probes are listed in Supplementary data, Table SI. Relative levels of target gene expression were normalized to ribosomal protein 18S levels.
Recovery and in vitro analyses of COCs
Controlled ovarian stimulation cycles were utilized to obtain multiple unexpanded COCs from rhesus monkeys for the C-OE assays in vitro. Briefly, recombinant human (rh) gonadotrophins were administered to promote the development of multiple large antral follicles (Chaffin et al., 2000). Beginning at menses (day 1–2), monkeys received rhFSH (60 IU, divided equally between 0800 and 1600 h injections, i.m., Schering-Plough, Oss, Netherlands) for 7–9 days as previously described (Hibbert et al., 1996). A GnRH antagonist (acyline; 75 µg/kg i.m.; NICHD, Bethesda, MD, USA) was administered on days 4–6 of treatment to prevent an endogenous gonadotrophin surge. The day before surgery, animals also received a single low dose of rhLH (30 IU, i.m., Merck Serono, Randolph, MA, USA) to allow further maturation of the follicle without eliciting cumulus expansion or ovulation. No hCG bolus was given. COCs were gently aspirated by hand syringe from anesthetized monkeys via laparoscopy to avoid disruption of the cumulus cells surrounding the oocyte.
Individual COCs were cultured in 100 µl TALP (Tyrode's, albumin, lactate, pyruvate) for 30 or 48 h in the following treatment groups (n = 3–4 animals/treatment; at least three COCs/animal/treatment): (i) 5% monkey serum (MS); (ii) MS + FSH (rhFSH; 100 ng/ml) + LH (rhLH; 100 ng /ml); (iii) MS + FSH; (iv) MS + LH; (v) MS + PGE2 (500 ng/ml, Cayman, Ann Arbor, MI, USA) and (vi) MS + PGE2 + ZK888 (PTGER2 antagonist; 15 µM, Bayer Healthcare AG, Berlin, Germany). Cultures were performed in Universal GPS® dishes (IVFonline, LLC, Guilford, CT, USA) containing media in the outer wells and water in the inner wells. Dishes were incubated at 37°C in a sealed chamber (6% CO2, 5% O2 and 89% N2) to minimize evaporation of media as no oil overlay was used to prevent partitioning of the lipid-soluble PGE2 out of the aqueous media. The MS used to provide the serum factors necessary for C-OE in vitro was obtained by pooling and filtering serum samples obtained from female monkeys during the early follicular phase of the natural cycle (day 3 or 4), which was subsequently charcoal-stripped (1:1vol:vol). The serum was then divided into aliquots and stored at −80°C. Negative controls included dimethylsulfoxide and/or ethanol, with no effects being observed in comparison with the control with MS alone. The presence of serum in the media for COC culture was based on the fact that serum factors are required for C-OE to occur. Data from non-primate species indicate that C-OE involves a complex interaction of oocyte-, granulosa/cumulus-, and serum-derived factors (Richards, 2005). Images of the individual COCs were acquired 0, 30 or 48 h post-treatment, using a digital camera attached to an Olympus CK40 microscope.
A fluorescence-based technique using HA binding protein (Back et al., 2005) was developed to evaluate the formation and deposition of a HA-rich ECM in expanded rhesus macaque COCs in vitro. Briefly, at the end of the culture period (30 h), COCs were fixed in 4% paraformaldehyde for 15 min at 37°C and then stored in washing buffer [0.5% bovine serum albumin, 0.1% powder milk, 1% goat serum, 1% donkey serum, 0.1% triton X-100, 0.05 M glycine in phosphate-buffered saline (PBS)] at 4°C. Following a blocking step with 5% goat serum in PBS, the COCs were incubated with biotinylated HA binding protein (1:250; Associates of Cape Cod, Inc.) for 2 h at 37°C. The COCs were then washed and incubated with Cy3-labeled streptavidin (1:1000; Jackson Immunoresearch) to reveal the presence of HA in the samples. Nuclear staining was detected using Hoechst 33342 (3 µM, Invitrogen) and F-actin was probed with Alexa 488-phalloidin (1:50; Invitrogen). COCs were analyzed by confocal microscopy (Leica SP5 AOBS, Leica Microsystems, Heidelberg, GE) using different objectives (PL APO CS 40× GLY UV and PL APO CS 63× 1.3 GLY UV). Full Z-stack data sets were collected with a HCX PL Apo CS 40× or 63× glycerol objective for each COC, with images taken every 0.5 µm.
A commercially available enzyme immunoassay (EIA) kit was used according to the manufacturer's instructions (Sigma-Aldrich, Saint Louis, MO, USA) to quantify the level of cAMP in the culture media. At the end of the COC cultures, the media were centrifuged at 600 g for 10 min and the supernatants were stored at −80°C. After thawing, 25 µl of culture media from each sample was dried down and reconstituted in 100 µl of assay buffer, as recommended by the manufacturer. The assay was performed using the acetylated cAMP protocol. The experiment was repeated a total of three times with COCs from different monkeys. The EIA had an inter-assay coefficient of variation of 8%.
PTGER2 antagonist contraceptive trial
Cycling female cynomolgus monkeys (6- to 16-years old) were randomly assigned (n = 10 per group) to vehicle (Control) or PTGER2 antagonist (BAY06; Bayer Healthcare AG, Berlin) groups. Treatments were administered subcutaneously at a dose of 10 mg/kg in 0.5 ml castor oil (vehicle) twice a day (0830, 1830 h) for BAY06, or once a day (0830) for Control, for 6 months. The BAY06 dose was based on pharmacokinetic studies on cynomolgus monkeys, relating steady state levels to dose–response curves for antagonist activity (unpublished, Bayer Healthcare AG) During the first month of the protocol, i.e. the pretrial cycle, females in both groups received their injections but were housed without the males. During this period, the males had visual and olfactory exposure to the females. After the pretrial cycle, a single male of proven fertility was introduced into each group and they remained housed together during the 5-month contraceptive trial.
All females were checked daily for evidence of menses or mating. Sperm were identified by light microscopy after transfer of vaginal secretions to a glass slide; positive identification of mating was considered when >20 sperm were observed from the vaginal swab. This limit on sperm number was selected since males frequently mount females without ejaculating, but a few sperm can pass into the vagina. At the end of the treatment interval, the male in the BAY06 group remained housed with the non-pregnant females for 5 additional months to assess the reversibility of the drug's contraceptive action. One monkey in the BAY06 group was removed from the protocol prior to mating due circumstances (viral infection) unrelated to drug treatment. Data from this monkey were eliminated from subsequent analyses. The infection was not transmitted to other animals in the BAY06 group.
The beginning and progression of each menstrual cycle was monitored starting from the initiation of menses, as well as hormone patterns of estradiol (E2) and progesterone, respectively. Blood samples were obtained weekly from the femoral vein of unanesthetized females throughout the entire protocol (treatment and reversibility intervals). Serum E2 and progesterone levels were assayed by the Endocrine Technology Support Core at the ONPRC. Also, a single blood sample was obtained from each female immediately prior to the first treatment (pretreatment sample) and on the final day of treatment (post-treatment sample) for complete blood counts, serum biochemistry tests and blood lipid profiles. These analyses were performed by the Clinical Pathology Laboratory at ONPRC and Quest Diagnostics Incorporated (Portland, OR, USA). Each animal was weighed weekly, with the last weight value defined as either at the end of the treatment or the value obtained the week that pregnancy was confirmed.
If progesterone levels exceeded 1.0 ng/ml for three consecutive weeks, animals were sedated with ketamine (1 mg/kg) and examined by ultrasonography using a GE Healthcare (Seattle, WA, USA) Model SP10–16 ultrasound equipped with a 4.5 mHz linear array abdominal transducer. Pregnancy was confirmed based on the presence of an intrauterine gestational sac, with a yolk sac or fetus. A viable pregnancy was defined as the presence of fetal cardiac activity in addition to elevated progesterone levels, whereas a biochemical pregnancy was determined when macaque chorionic gonadotrophin (mCG), as well as serum progesterone levels (for >3 weeks), were >1.0 ng/ml in the absence of an ultrasound evidence of fetal activity. Circulating mCG was confirmed retrospectively in samples from animals suspected of having a biochemical pregnancy by the Endocrine Technology Support Core, ONPRC using an established enzyme-linked immunosorbent assay (Munro et al., 1997). All pregnancies were subsequently terminated as previously described (Micks et al., 2012).
Statistical analyses
Statistical calculations were performed using Sigma Stat software package (Systat Software, Inc., Richmond, CA, USA). Differences in mRNA expression, as well as cAMP levels in the culture media, among groups were analyzed using normal or transformed data and a one-way analysis of variance (ANOVA) followed by comparison among means using either Holm–Sidak or Student–Newman–Keuls method. For non-parametric data, a Kruskal–Wallis ANOVA on ranks was used. Differences were considered significant at P < 0.05.
The Fisher's exact test was used to compare the proportion of females that became pregnant between the control and BAY06 treatment group, and per appropriately timed mating in each group during the contraceptive trial. A paired t-test was used to compare first and last values of weight and cycle length in each group. Moreover, percentage changes in body weight were analyzed by one-way ANOVA with one repeated measure, followed by a Neuman–Keuls test for comparison among groups and t-test between control and treatment groups. ANOVA with repeated measures was performed to analyze cycle lengths throughout antagonist treatment. When normality failed, non-parametric tests (such as Mann–Whitney rank sum test or Wilcoxon signed rank test) were performed. Values were considered significant at P < 0.05.
Results
Levels of mRNAs encoding PGE2 synthesis and signaling components in the primate follicle through the periovulatory interval
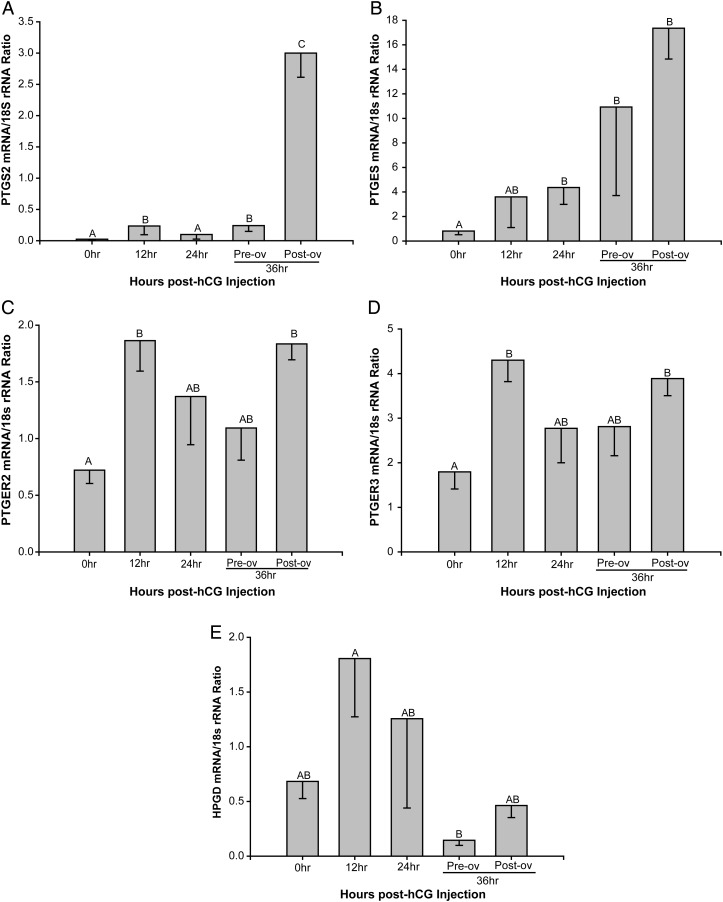
The level of mRNA corresponding to PTGS2; PGE2 synthase (PTGES), the enzyme responsible for converting PGH2 to PGE2; the PGE2 receptor subtypes PTGER2 and PTGER3; as well as hydroxy-PG dehydrogenase (HPDG), the enzyme responsible for metabolizing PGE2 to inactive metabolites were differentially regulated by an ovulatory bolus of hCG (Fig. 1). PTGS2 mRNA levels (Fig. 1A) increased 12-fold (P < 0.05) prior to ovulation at 12 and 36 h after hCG administration relative to levels before hCG administration (0 h). A secondary 150-fold increase in PTGS2 mRNA levels was noted after follicle rupture. PTGES mRNA levels (Fig. 1B) tended to increase at 12 h post-hCG, reaching significance (P < 0.05) at 24 and 36 h pre-ovulation, and then remained high after ovulation. The PGE2 receptors PTGER2 through four were expressed in the primate pre-ovulatory follicle, displaying different patterns of expression in the follicle during the periovulatory interval. Based on our previous microarray database (Xu et al., 2011), PTGER1 mRNA expression was below the threshold of detection (data not shown) and, therefore, not included for further analysis. Normalized qPCR analyses for PTGER2 and PTGER3 mRNAs (Fig. 1C and D) revealed a similar pattern of expression through the macaque periovulatory interval, with increased levels observed at 12 h post-hCG administration (2.6- and 2.4-fold, respectively; P < 0.05) that tended to decline at 24 and 36 h in the unruptured follicle. Moreover, both PTGER2 and PTGER3 mRNA levels exhibited a secondary increase after ovulation. Both microarray and qPCR analyses revealed appreciable PTGER4 mRNA, but levels did not vary during the periovulatory interval (data not shown). Lastly, the mRNA levels for hydroxy-PG Dehydrogenase (HPGD) were lower (P < 0.05) just before ovulation at 36 h compared with 12 h post-hCG (Fig. 1E). Collectively, these data are consistent with the concept that an ovulatory stimulus (i.e. hCG) results in increased PGE2 synthesis (PTGS2, PTGES) and responsiveness (PTGER2, PTGER3), as well as reduced PGE2 inactivation prior to rupture (HPGD), which in turn supports a role for PGE2 as a key regulator of processes necessary for ovulation in the naturally selected macaque follicle.
Figure 1.
Expression of PGE2 synthesis, degradation and signaling genes in the macaque periovulatory follicle. Levels of mRNAs that encode proteins involved in PGE2 synthesis (PTGS2; PGE2 synthase, PTGES; A and B, respectively), signaling (PTGER2; PTGER3; C and D, respectively) and metabolism (hydroxy-PG dehydrogenase, HPGD; E) were determined in single, naturally selected follicles obtained at the indicated times after administration of an ovulatory bolus of hCG. 18S rRNA served as the invariant control RNA for normalization. Different letters represent significant differences between groups (P < 0.05). Differences in mRNA expression among groups were analyzed using normal or transformed data and a one-way analysis of variance (ANOVA) followed by comparison among means using either the Holm–Sidak or Student–Newman–Keuls method. For non-parametric data, a Kruskal–Wallis ANOVA on ranks was used.
The role of PGE2 and gonadotrophins in regulating expansion of the macaque COC
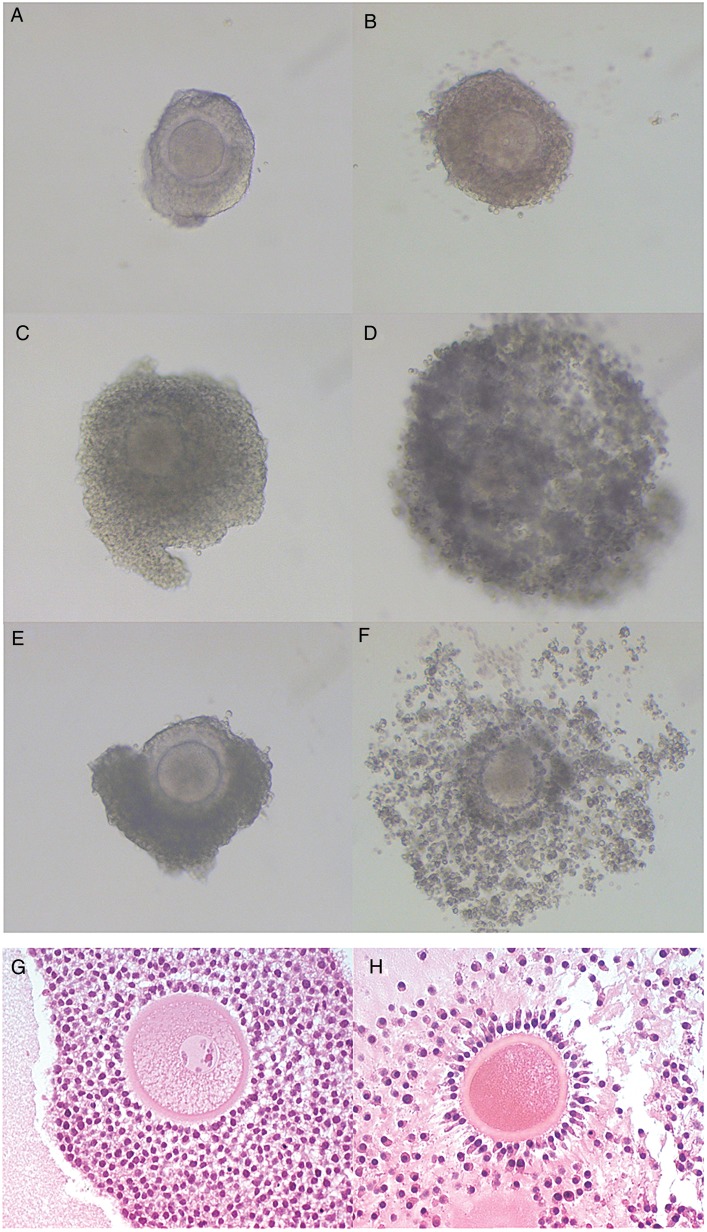
Following the optimization of the culture conditions for assessing cumulus cell expansion of primate COCs in vitro, studies were conducted to determine if PGE2 directly induces C-OE and the production of a HA-rich ECM. Because macaque COCs may exhibit morphological evidence of spontaneous expansion in vitro, optimal conditions that maintain COCs in an unexpanded state were determined and included culturing individual COCs without oil in TALP media containing 5% charcoal-stripped MS. Figure 2A–F provide representative images of COCs prior to (0 h) and 48 h after culture under different conditions. While rhesus macaque COCs cultured with 5% MS alone consistently did not undergo spontaneous C-OE (Fig. 2A and B), the addition of FSH and LH (Fig. 2C and D) or PGE2 (Fig. 2E and F) resulted in expansion of the surrounding cumulus cell layer. However, a difference in the degree of expansion was noted between the FSH/LH (Fig. 2D) and PGE2 treatment (Fig. 2F) treatment groups wherein the expansion elicited by PGE2 is typical of the C-OE that normally occurs in vivo (Fig. 2; compare G, pre-hCG with H, 24 h post-hCG).
Figure 2.
PGE2 induces macaque cumulus-oocyte expansion (C-OE). Images of COCs were taken prior to (A, C and E) and at the end of culture (48 h; B, D and F). Cumulus-oocyte complexes (COCs) were incubated with media + 5% monkey serum (MS; A and B), 5% MS + FSH + LH (100 ng each/ml; C and D), or 5% MS + PGE2 (500 ng/ml; E and F). All images were obtained at the same magnification (10×). Images of COCs residing within the ovulatory follicle were obtained from rhesus macaques undergoing controlled ovulation protocols as previously described (Young et al., 2003) prior to (0 h; unexpanded COC, G) and 24 h after hCG injection (expanded COC, H) to serve as a reference for in vivo C-OE. FSH, follicle stimulating hormone; LH, lutenising hormone; MS, monkey serum.
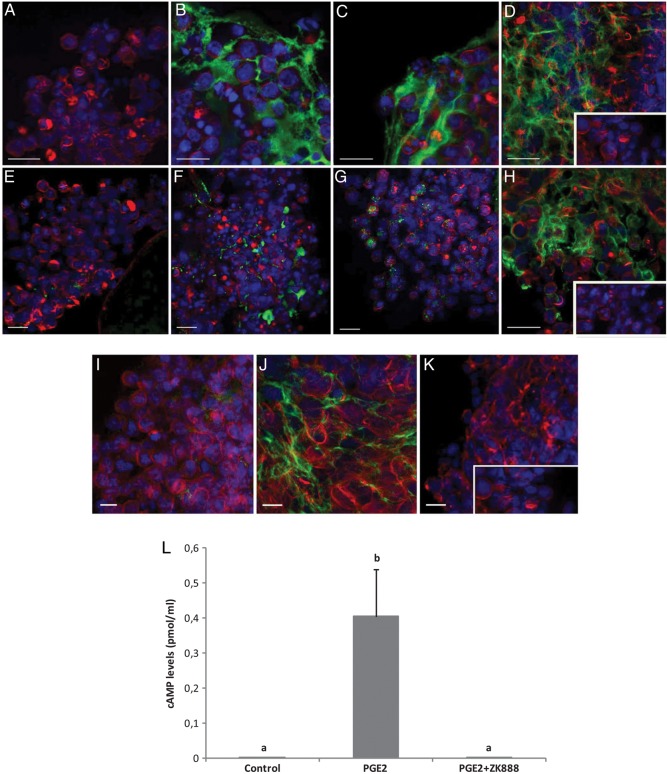
Because morphological assessment of expansion does not discriminate between simple loss of cell–cell contacts/death during two-dimensional culture and true expansion, a methodology was developed to assess a molecular indicator of C-OE: HA synthesis. A fluorescence-based technique using HA binding protein was employed to evaluate the formation and deposition of a HA-rich ECM in expanded rhesus macaque COCs in vitro. Figure 3 provides representative confocal microscopy images of HA immunofluorescence (green) in COCs from two different experiments (Fig. 3A–D and E–H, respectively). First, COCs incubated with medium containing only MS (Fig. 3A and E) did not stain positive for HA. However, exposure to FSH (Fig. 3B and F), LH (Fig. 3C and G), FSH plus LH (data not shown) or PGE2 (Fig. 3D and H) induced the synthesis and secretion of HA into the ECM between the cumulus cells. Gonadotrophin treatment of COCs (i.e. FSH, LH or a combination of the two) did not lead to a uniform HA staining pattern within the complex, nor did it consistently induce HA synthesis in different COCs. For example, Fig. 3B and C showed positive staining for HA after treatment of either FSH or LH; however, COCs from a different animal (Fig. 3F and G) exhibited low levels of HA staining. In contrast, treatment with PGE2 consistently induced synthesis and secretion of HA through the whole complex and among all COCs analyzed. The presence of HA was also observed within some oocytes regardless of treatment (not shown).
Figure 3.
Inhibition of PTGER2 signaling in macaque COCs blocks events critical for C-OE. COCs were obtained from three different monkeys undergoing controlled ovarian stimulation protocols (A–D; E–H; I–K). Extracellular hyaluronic acid (HA) was determined through the use of biotinylated HA binding protein. COCs were incubated (30 h) with control media (+5% MS; A and E); 5% MS + FSH (100 ng/ml; B and F); 5% MS + LH (100 ng/ml; C and G); 5% MS + PGE2 (500 ng/ml; D and H). COCs were also incubated with media + 5% MS (I), 5% MS + PGE2 (500 ng/ml; J), or with 5% MS + PGE2 + PTGER2 antagonist ZK888 (15 μm; K). (A–K) Nuclear staining is displayed in blue (Hoechst 33342) and F-actin (Phalloidin) in red. No staining was observed in the negative control (absence of the HA binding protein, inset). Scale bars = 10 μm. (L) Levels of cAMP in the culture media after incubation of COCs for 30 h in the different treatment groups (MS, PGE2, and ZK888 concentrations as above). Different letters represent significant differences between the treatment groups (P < 0.05). Differences in cAMP levels in the culture media among groups were analyzed using normal or transformed data and a one-way analysis of variance (ANOVA) followed by comparison among means using either the Holm–Sidak or Student–Newman–Keuls method.
The PTGER2 plays a critical role in regulating macaque C-OE
To further investigate the involvement of PGE2 signaling through the PTGER2 subtype in regulating C-OE, the first generation PTGER2-selective antagonist ZK888 was included in COC cultures containing PGE2. Addition of ZK888 prevented PGE2-mediated induction of HA synthesis in the surrounding cumulus cells (Fig. 3K) relative to macaque COCs cultured with PGE2 alone (Fig. 3J), suggesting that PTGER2 signaling stimulates cumulus cell HA synthesis. The control group that included COCs incubated with MS alone (Fig. 3I) displayed no HA staining. Moreover, background immunofluorescence was minimal, as assessed by omitting the biotinylated HA binding protein (Fig. 3D and H, K insets).
After 30 h of culture (Fig. 3L), cAMP levels increased in media obtained from COC cultures treated with PGE2 (P < 0.05) relative to the undetectable levels obtained from COCs cultured only in media containing serum (controls). The addition of the PTGER2 antagonist ZK888 blocked (P < 0.05) the ability of PGE2 to enhance cAMP levels. FSH or the combination of FSH and LH also increased cAMP levels in the media, whereas media from the LH group did not possess detectable levels of cAMP (data not shown).
A PTGER2 antagonist prevents pregnancy and is reversible in group-housed macaques
To determine the contraceptive effect of a second-generation PTGER2 antagonist, BAY06, a 5-month contraceptive trial was conducted using cynomolgus macaques. Table I summarizes the number of pregnancies and appropriately timed matings (i.e. around the midcycle E2 rise), plus pregnancy rates within the control and treatment groups. Through the span of the contraceptive trial, eight pregnancies from a total of 10 females were obtained in the vehicle-treated (control) group (80%). Of these pregnancies, one was a biochemical pregnancy. The percentage of pregnancies occurring per appropriately timed mating (based on the presence of vaginal sperm) was 38% (21 total timed matings), with four pregnancies achieved at the first-timed mating and the other four at the second. In contrast, from a total of nine monkeys in the BAY06 group, only two pregnancies occurred during treatment (22%; P < 0.05 relative to the 80% pregnancy rate in the control group). The first pregnancy occurred after two appropriately timed matings (third month of treatment) and the second after the third timed mating (fourth month of treatment). In the BAY06 group, the two pregnancies occurred as a result of 34 appropriately timed matings (6% pregnancy rate per timed mating; P < 0.05 versus the control group). The lower pregnancy rates (both per group and per timed mating) are not due to a lack of mating, as all of the BAY06 group experienced two or more matings during the period of optimal fertility based on identification of sperm on vaginal swabs.
Table I.
Number of timed matings and pregnancies in cynomolgus macaques during the 5-month contraceptive trial.
| Group | Animals | Timed matings | Pregnancies | % Pregnancies/Time mating | % Pregnancies/Group |
|---|---|---|---|---|---|
| Control | 10 | 21 | 8 | 38a | 80A |
| BAY06 | 9 | 34 | 2 | 6b | 22B |
a,bLetters represent significance differences (P < 0.05) in proportion of pregnancies in relation to timed mating among treatment groups.
A,BLetters represent significance differences (P < 0.05) in proportion of total females that became pregnant among treatment groups.
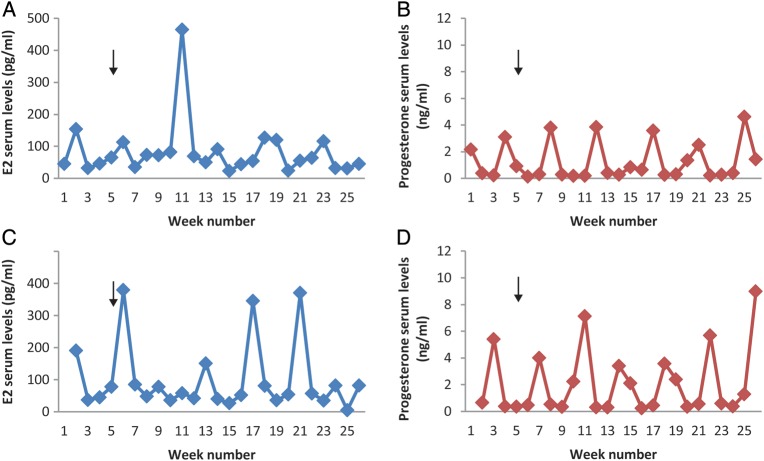
Levels of serum E2 and progesterone were measured to monitor ovarian activity and cyclicity (with progesterone an indirect index of luteinization of the dominant follicle) and to detect possible effects of the PTGER2 antagonist on these parameters. Remarkably, E2 and progesterone patterns were not affected by BAY06 treatment throughout the span of the contraceptive trial. Representative graphs of E2 and progesterone patterns in monkeys from each group are shown in Fig. 4. Since blood samples were collected weekly due to logistical issues and blood sampling guidelines, the values of these steroid hormones in each monkey do not reflect peak levels, nor do they define the precise interval of the follicular and luteal phases. However, the cyclic pattern of E2 and progesterone was easily observed and coincided with the menstrual cycles as delineated by the onset and presence of menses. Notably, menstrual cycle length was not significantly altered by BAY06 treatment relative to controls (Control: 31.0 ± 1.1 days and 30.9 ± 1.3 days versus BAY06: 28.4 ± 1.0 days and 30.2 ± 2.2 days; first and last treatment cycles, respectively) when analyzed by either comparing cycle length at the beginning and end of the contraceptive trial in all females or by comparing cycle lengths within an individual female within the drug group (Supplementary data, Figure S1).
Figure 4.
PTGER2 antagonist treatment does not alter menstrual cyclicity in cynomolgus macaques. Weekly E2 (A and C) and progesterone (B and D) serum levels through the contraceptive trial from representative animals in each group (Control, A and B; PTGER2 Antagonist BAY06, C and D) are shown. Between week 1 and 2, the Control and BAY06 treatment started and after Week 5 the breeder male was added to the groups (arrow, A–D). The control female was one of the 2 (out a total of 10 animals) that did not become pregnant, yet exhibited normal menstrual cyclicity. The representative female from BAY06 group was time-mated six times; with no pregnancy. In a previous trial, these females became pregnant.
Paired t-tests revealed that animal body weight declined (3.58 ± 0.24 versus 3.24 ± 0.18 kg; P < 0.05) in the PTGER2 antagonist treatment group. However, the percentage change in body weight in the PGTER2 antagonist group was modest (8 ± 3%), varied between animals, and did not differ significantly (P > 0.05) from controls. Typically, animals in both the control and BAY06 groups modestly increased their body weight by 5–6% (0.2 kg) during the 3 months after the contraceptive trial.
Blood samples were collected before and at the end of treatment to perform complete blood counts, blood lipids profiles as well as serum biochemistry tests. Values obtained in the treatment group did not differ from controls, and were within the expected range for cynomolgus monkeys during spontaneous menstrual cycles (data not shown).
During a 5 month reversibility trial, four out of seven monkeys became pregnant (60%) after the cessation of BAY06 treatment. These females had two to four appropriately timed matings during BAY06 treatment that did not lead to pregnancy. During the reversibility interval, two of the females became pregnant in the first-timed mating, whereas the other two became pregnant at the second- and fourth-timed mating. One of these pregnancies occurred very early within a month of ceasing BAY06 treatment. Although this event is included in the reversibility trial, it is possible that the timed mating spanned the last days of treatment, as well as the first days after treatment, leaving inconclusive results regarding whether this pregnancy occurred in the presence or absence of drug. The three females that did not get pregnant had five-, five- and three-timed matings during the reversibility trial; and six-, six- and two-timed matings through the course of BAY06 treatment, respectively.
Discussion
This study reveals that mRNA levels for the genes involved in PGE2 synthesis and signaling increase in the single, naturally selected pre-ovulatory follicle within the rhesus macaque ovary in response to an ovulatory stimulus. Also, it demonstrated that PGE2, in contrast to gonadotrophins, consistently induces C-OE and stimulates the secretion of HA in cumulus cells through PTGER2, suggesting a critical role for this PGE2 receptor in processes necessary for primate C-OE. Moreover, the fertility trial demonstrates a contraceptive effect of a PTGER2 antagonist in vivo without altering the hormone patterns or length of the natural menstrual cycle, or general health of the females. In addition, fertility recovers as early as 1 month after cessation of treatment. Thus, this study supports the idea that PGE2 receptor antagonists could serve as effective non-hormonal contraceptives for women.
The transcriptome of the pre-ovulatory follicle at selected times following an ovulatory stimulus was previously elucidated using Affymetrix Rhesus Macaque Total Genome Arrays™ (Xu et al., 2011), and the mRNAs involved in the PG/PGE2 synthesis and signaling pathway were validated by qPCR. The COv model offers the unique opportunity to analyze the naturally selected dominant follicle at defined intervals prior to and after exposure to an ovulatory gonadotrophin stimulus (i.e. a bolus of hCG) during the menstrual cycle in rhesus macaques (Young et al., 2003). Of these, mRNA encoding PTGS2 and PTGES (PGE2 synthesis), HPGD (PGE2 metabolism) as well as PTGER2 and PTGER3 (PGE2 signaling) were significantly regulated by an ovulatory bolus of hCG. PTGS2 mRNA levels increased before follicle rupture and again after ovulation, whereas PTGES peaked at 36 h in both unruptured and ruptured follicles. Isolated granulosa cells obtained from rhesus macaques undergoing an ovarian stimulation protocol comparable to that used in IVF clinics displayed a similar PTGS2 expression pattern post-hCG (Duffy and Stouffer, 2001). Moreover, our results are consistent with those of Duffy et al. (2005) who also noted that PTGES was the primary PGE2 synthase expressed in granulosa cells aspirated from cynomolgus macaque pre-ovulatory follicles after hCG administration. Although enzyme activity was not measured in these studies, the data correlate positively with the rise in PGE2 levels observed in the macaque follicle after an ovulatory stimulus (Duffy and Stouffer, 2001).
Our microarray database (Xu et al., 2011) also revealed significant levels of mRNAs encoding PTGER2, PTGER3, and PTGER4 receptor subtypes throughout the periovulatory interval in the macaque follicle, with PTGER2 and PTGER3 being significantly up-regulated by an ovulatory stimulus. The rapid (i.e. 12 h) increase in PTGER2 and PTGER3 mRNA occurs just prior to the initiation of events necessary for C-OE and coincides with the expression of key genes involved in this process (such as HAS2, TNFAIP6, AREG and EREG) in the rhesus macaque pre-ovulatory follicle (Xu et al., 2011). In mice, PGE2 actions mediated through PTGER2 were shown to be critical for C-OE and fertility (Ochsner et al., 2003). Supporting a possible involvement of PTGER2 and PTGER3 in primate C-OE, a recent study of COCs collected from cynomolgus macaques reported higher mRNA levels for these receptors in the cumulus cells relative to mural cells 36 h post-hCG treatment (Harris et al., 2011). In the present study, differential expression of PTGER2 and PTGER3 transcripts among the granulosa cell populations cannot be assessed since RNA extraction was performed from the whole pre-ovulatory follicle. After ovulation, a secondary rise in PTGS2, PTGER2 and PTGER3 mRNA was noted, which is likely related to corpus luteum formation and/or function in primates (Bogan et al., 2008a,b) and suggests a luteotropic role for PGE2.
A series of experiments were designed to define the involvement of PGE2 in primate C-OE, as well as to compare its effectiveness with that of gonadotrophins. The results showed that 5% MS alone did not allow for spontaneous C-OE, whereas the addition of either gonadotrophins (FSH and/or LH) or PGE2 induced expansion of the surrounding cumulus cell layer in vitro. In addition to a variable response, the expansion induced by gonadotrophins appeared morphologically distinct from the expansion induced by PGE2. The C-OE induced in response to PGE2 more closely resembles that which normally occurs in vivo, with the typical radial distribution of the cumulus cells within the matrix. Qualitative as well as quantitative analyses of C-OE historically focused on the change in COC area, which is widely considered the main manifestation of this process. However, C-OE is a complex process involving the formation of a HA-rich ECM with other components that stabilize the matrix. The cumulus cells secrete HA, which is the main component of the expanded matrix and the amount of HA synthesized is closely correlated with the degree of expansion (Chen et al., 1993). Thus, an immunofluorescence-based technique was developed to evaluate the presence or absence of HA within the COCs cultured under the different treatments. Using this molecular assessment of expansion, both gonadotrophins and PGE2 induced the synthesis and secretion of HA, leading to its localization in the ECM between cumulus cells. However, gonadotrophin stimulation of HA synthesis was not uniform throughout the entire COC, with the staining primarily localized to the periphery of the COCs. The presence of HA was also observed within some oocytes regardless of treatment, which is potentially involved in regulating perivitelline volume (Ueno et al., 2009). Moreover, the level of HA synthesis varied between COCs following gonadotrophin (FSH and/or LH) exposure. As FSH is able to induce C-OE in vitro in rodents (Eppig, 1979; Eppig, 1980a,b), it has been used for that purpose in different species, including pigs, cows, and women. However, LH is generally believed to be the primary gonadotrophin responsible for C-OE via its ability to trigger production of paracrine/autocrine factors that are ultimately responsible for C-OE (e.g. epidermal growth factor (EGF)-like ligand family members AREG and EREG, PGE2, GDF9/BMP15). It is possible that the variability in responsiveness of COCs treated with gonadotrophins in vitro is the consequence of COC desensitization due to the use of gonadotrophins in the ovarian stimulation protocol, or that the retrieved COCs are heterogeneous in terms of maturational state and, therefore, responded differently to gonadotrophins in vitro. In contrast to FSH and LH, COC treatment with PGE2 in vitro uniformly induced the synthesis and secretion of HA. The PGE2 response in macaque COCs may be due to activation of mitogen-activated protein kinase 3/1 through the induction of EGF-like ligands, as was reported in porcine COCs (Yamashita et al., 2011). EGF ligand synthesis and action is a requirement for C-OE as well as for meiotic maturation in mice (Park et al., 2004).
The importance of PGs in fertility has been widely reported in numerous mammalian species. In primates, signaling through at least one of the PTGER subtypes is critical for ovulation as PGE2 reverses the suppression of follicle rupture resulting from the intrafollicular injection of indomethacin (Duffy and Stouffer, 2002). In cynomolgus macaques, higher levels of PTGER2 and PTGER3 mRNA in the cumulus cells relative to mural granulosa cells were observed 36 h post-hCG treatment (Harris et al., 2011), further supporting a possible role for PGE2 signaling directly in the cumulus cells that is necessary for ovulation (i.e. C-OE). In rhesus macaques, PTGER2 appears to be critical for C-OE since the first generation PTGER2-selective antagonist ZK888 prevented PGE2 from inducing HA synthesis by COCs in vitro. Furthermore, oral administration of specific PTGS2 inhibitors can disrupt timely ovulation in women (Norman, 2001). In mice deficient for PTGER2, females are infertile and fail to undergo C-OE despite follicle rupture and COC release (Hizaki et al., 1999; Ochsner et al., 2003). Moreover, PGE2-PTGER2 signaling in mice plays a critical role in regulating the expression of cumulus cell genes critical for C-OE, including EGF ligands (e.g. AREG, EREG, betacellulin; Tamba et al., 2010). Interestingly, the selective PTGER2 antagonist ZK888 also blocked PGE2-mediated stimulation of cAMP by macaque COCs in this study, demonstrating that cAMP synthesis in the primate COC triggered by PGE2 is dependent on PTGER2. The PTGER2 subtype is a G protein-coupled receptor that when bound to ligand activates adenylate cyclase (Narumiya et al., 1999), which in turn leads to increased cAMP production that can be assessed by measuring cAMP levels in the media (Bodo et al., 2009). Increased cAMP levels were also observed in the media of macaque COCs treated with FSH or the combination of FSH and LH, but not in media from COCs treated with LH alone. Such results are consistent with the observation that LH receptor expression is low in murine cumulus cell (Peng et al., 1991).
Based on the purported critical roles that PGE2 plays in coordinating the molecular processes necessary for ovulatory events in the primate follicle, the contraceptive effect of chronic PTGER2 antagonist administration was assessed in female macaques. Only two of nine monkeys receiving the PTGER2 antagonist became pregnant during the 5-month trial despite 34 matings during the optimal window of fertility. In contrast, eight pregnancies from a total of 10 females were obtained after 21 appropriately timed mating in the vehicle-treated (control) group. As a proven-fertile male was added to each group, possible variability in pregnancy rates due to male fertility factors was not an issue. Importantly, pregnancy rates in total as well as per appropriately timed mating were significantly lower in the PTGER2 antagonist groups relative to controls, demonstrating that this non-hormonal intervention was able to prevent pregnancy. The 2 pregnancies in the PTGER2 antagonist group occurred only after 3 and 4 months of treatment. It is possible that chronic drug treatment increased metabolic pathways (e.g. CYP induction) to degrade this compound in macaques over time. The pregnancies observed in the PTGER2 antagonist group may be due, however, to a redundancy in action of local factors in controlling periovulatory events in the primate ovary. Indeed, it is possible that PGE2 action in the ovulatory follicle involves signaling through more than one PTGER receptor. As mentioned previously, PTGRE2 and PTGER3 subtypes were both proposed to be the key receptors mediating C-OE by PGE2 in cynomolgus macaques (Harris et al., 2011). Moreover, in the present study, both PTGER3 and PTGER2 mRNA levels increase significantly after hCG administration in rhesus macaques COCs. The major signaling pathway of PTGER3 is inhibition of adenylate cyclase via Gi, but it can also induce the elevation of intracellular calcium and cAMP (Sugimoto and Narumiya, 2007) depending on the splice variant that is being expressed. Thus, further studies addressing issues of PTGER selectivity, including dose and combinations of PTGER antagonists, are warranted.
During the contraceptive trial, the PTGER2 antagonist limited fertility without grossly affecting menstrual cyclicity despite chronic treatment. Steroid hormone (E2 and progesterone) patterns and cycle lengths were unaffected by PTGER2 antagonist treatment. These results are relevant to the goal of developing a non-hormonal contraceptive that preserves menstrual and hormonal cyclicity. Since menstrual cycle lengths were unaffected by PTGER2 antagonist treatment, either (i) a non-fertilizable oocyte was released (e.g. possessing an unexpanded cumulus cell layer), and/or (ii) ovulation did not occur and the COC remained trapped within the follicle without affecting the formation and function of the corpus luteum. Furthermore, these results also suggest that PTGER2 is not critical for luteinization/luteal development. Whether the reduced pregnancy rates in the current contraceptive trial are due to BAY06 inhibition of C-OE, follicle rupture or both remains to be determined. Further experiments are warranted to elucidate the mechanism(s) of action for the PTGER2 antagonist in the primate ovary. Also, since PGE2 reportedly plays a role in embryo implantation in mice (Ruan et al., 2012), interference within this process in primates by BAY06 cannot be discounted, but this was not evaluated in the current study.
Overall, the general health of the animals, as judged from complete blood counts, blood lipid profiles and serum biochemistry tests were not affected by PTGER2 antagonist treatment. The loss in body weight noted after treatment with a PTGER2 antagonist was modest. Weekly weight variation in these macaques is typically around 5–10% and our results suggest that the study protocol alone resulted in a slight reduction in weight. Moreover, after discontinuation of PTGER2 antagonist treatment, half of the females became pregnant, indicating no lasting effect on fertility. This is an important aspect of contraceptive development, since the inhibition of fertility must be temporary for those who subsequently desire to have children. While PTGER2 antagonist treatment did not produce any obvious undesirable effects, improvements in the mode of administration, as well as the efficacy of these compounds, are necessary to consider such a contraceptive for women.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Author's roles
M.C.P designed and performed research, analyzed data and wrote the paper; J.S., M.J.M. and T.A. performed research; N.B., A.R., K-H.F., U.F., B.B. and B.L. contributed new reagent; R.L.S., J.D.H. and M.B.Z. designed research and wrote/corrected the paper.
Funding
Supported by Bayer HealthCare Pharmaceuticals, The Eunice Kennedy Shriver NICHD Contraceptive Development and Research Center (U54 HD055744), NIH Office of the Director (Oregon National Primate Research Center P51 OD011092), and a Lalor Foundation Postdoctoral Basic Research Fellowship (MCP). The use of the Leica confocal was supported by grant number S10RR024585.
Conflict of interest
Some of the authors (N.B., A.R., K.-H.F., U.F., B.B., and B.L.) are employees of Bayer Healthcare Pharma, who provided the PTGER2 antagonists and sponsored the contraceptive trial by the researchers at ONPRC.
Supplementary Material
Acknowledgements
We are grateful for the expert contributions of the animal care staff and surgical unit of the Division of Comparative Medicine, as well as the Assisted Reproductive Technologies Core and the Endocrine Technology Support Core at ONPRC. Recombinant human FSH (Schering-Plough, Oss) and LH (Merck Serono) were generously donated for this project. A special thanks to Afua Asare for her technical assistance. Also, special thanks to Gernot Langer and Antonius ter Laak Global (Drug Discovery, Bayer Healthcare Pharma, Berlin, Germany) for their very valuable contributions to the development of PGE2 receptor antagonists.
References
- Back SA, Tuohy TM, Chen H, Wallingford N, Craig A, Struve J, Luo NL, Banine F, Liu Y, Chang A, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–972. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- Bata MS, Al-Ramahi M, Salhab AS, Gharaibeh MN, Schwartz J. Delay of ovulation by meloxicam in healthy cycling volunteers: a placebo-controlled, double-blind, crossover study. J Clin Pharmacol. 2006;46:925–932. doi: 10.1177/0091270006289483. [DOI] [PubMed] [Google Scholar]
- Bodo E, Kromminga A, Biro T, Borbiro I, Gaspar E, Zmijewski MA, van Beek N, Langbein L, Slominski AT, Paus R. Human female hair follicles are a direct, nonclassical target for thyroid-stimulating hormone. J Invest Dermatol. 2009;129:1126–1139. doi: 10.1038/jid.2008.361. [DOI] [PubMed] [Google Scholar]
- Bogan RL, Murphy MJ, Stouffer RL, Hennebold JD. Prostaglandin synthesis, metabolism, and signaling potential in the rhesus macaque corpus luteum throughout the luteal phase of the menstrual cycle. Endocrinology. 2008a;149:5861–5871. doi: 10.1210/en.2008-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogan RL, Murphy MJ, Stouffer RL, Hennebold JD. Systematic determination of differential gene expression in the primate corpus luteum during the luteal phase of the menstrual cycle. Mol Endocrinol. 2008b;22:1260–1273. doi: 10.1210/me.2007-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffin CL, Dissen GA, Stouffer RL. Hormonal regulation of steroidogenic enzyme expression in granulosa cells during the peri-ovulatory interval in monkeys. Mol Hum Reprod. 2000;6:11–18. doi: 10.1093/molehr/6.1.11. [DOI] [PubMed] [Google Scholar]
- Chen L, Mao SJ, Larsen WJ. Identification of a factor in fetal bovine serum that stabilizes the cumulus extracellular matrix. A role for a member of the inter-alpha-trypsin inhibitor family. J Biol Chem. 1992;267:12380–12386. [PubMed] [Google Scholar]
- Chen L, Russell PT, Larsen WJ. Functional significance of cumulus expansion in the mouse: roles for the preovulatory synthesis of hyaluronic acid within the cumulus mass. Mol Reprod Dev. 1993;34:87–93. doi: 10.1002/mrd.1080340114. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Stouffer RL. The ovulatory gonadotrophin surge stimulates cyclooxygenase expression and prostaglandin production by the monkey follicle. Mol Hum Reprod. 2001;7:731–739. doi: 10.1093/molehr/7.8.731. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Stouffer RL. Follicular administration of a cyclooxygenase inhibitor can prevent oocyte release without alteration of normal luteal function in rhesus monkeys. Hum Reprod. 2002;17:2825–2831. doi: 10.1093/humrep/17.11.2825. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Chaffin CL, Stouffer RL. Expression of estrogen receptor alpha and beta in the rhesus monkey corpus luteum during the menstrual cycle: regulation by luteinizing hormone and progesterone. Endocrinology. 2000;141:1711–1717. doi: 10.1210/endo.141.5.7477. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Seachord CL, Dozier BL. Microsomal prostaglandin E synthase-1 (mPGES-1) is the primary form of PGES expressed by the primate periovulatory follicle. Hum Reprod. 2005;20:1485–1492. doi: 10.1093/humrep/deh784. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Gonadotropin stimulation of the expansion of cumulus oophori isolated from mice: general conditions for expansion in vitro. J Exp Zool. 1979;208:111–120. doi: 10.1002/jez.1402080112. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Regulation of cumulus oophorus expansion by gonadotropins in vivo and in vitro. Biol Reprod. 1980a;23:545–552. doi: 10.1095/biolreprod23.3.545. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Role of serum in FSH stimulated cumulus expansion by mouse oocyte–cumulus cell complexes in vitro. Biol Reprod. 1980b;22:629–633. doi: 10.1093/biolreprod/22.3.629. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Prostaglandin E2 stimulates cumulus expansion and hyaluronic acid synthesis by cumuli oophori isolated from mice. Biol Reprod. 1981;25:191–195. doi: 10.1095/biolreprod25.1.191. [DOI] [PubMed] [Google Scholar]
- Fulop C, Salustri A, Hascall VC. Coding sequence of a hyaluronan synthase homologue expressed during expansion of the mouse cumulus–oocyte complex. Arch Biochem Biophys. 1997;337:261–266. doi: 10.1006/abbi.1996.9793. [DOI] [PubMed] [Google Scholar]
- Fulop C, Szanto S, Mukhopadhyay D, Bardos T, Kamath RV, Rugg MS, Day AJ, Salustri A, Hascall VC, Glant TT, et al. Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development. 2003;130:2253–2261. doi: 10.1242/dev.00422. [DOI] [PubMed] [Google Scholar]
- Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276:36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- Harper M. In search of a second contraceptive revolution. Sex Reprod Menopause. 2005;3:59–67. [Google Scholar]
- Harris SM, Aschenbach LC, Skinner SM, Dozier BL, Duffy DM. Prostaglandin E2 receptors are differentially expressed in subpopulations of granulosa cells from primate periovulatory follicles. Biol Reprod. 2011;85:916–923. doi: 10.1095/biolreprod.111.091306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbert ML, Stouffer RL, Wolf DP, Zelinski-Wooten MB. Midcycle administration of a progesterone synthesis inhibitor prevents ovulation in primates. Proc Natl Acad Sci USA. 1996;93:1897–1901. doi: 10.1073/pnas.93.5.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hizaki H, Segi E, Sugimoto Y, Hirose M, Saji T, Ushikubi F, Matsuoka T, Noda Y, Tanaka T, Yoshida N, et al. Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin E receptor subtype EP(2) Proc Natl Acad Sci USA. 1999;96:10501–10506. doi: 10.1073/pnas.96.18.10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesam C, Salvatierra AM, Schwartz JL, Croxatto HB. Suppression of follicular rupture with meloxicam, a cyclooxygenase-2 inhibitor: potential for emergency contraception. Hum Reprod. 2010;25:368–373. doi: 10.1093/humrep/dep392. [DOI] [PubMed] [Google Scholar]
- Kimura N, Konno Y, Miyoshi K, Matsumoto H, Sato E. Expression of hyaluronan synthases and CD44 messenger RNAs in porcine cumulus–oocyte complexes during in vitro maturation. Biol Reprod. 2002;66:707–717. doi: 10.1095/biolreprod66.3.707. [DOI] [PubMed] [Google Scholar]
- Massai MR, Forcelledo ML, Brache V, Tejada AS, Salvatierra AM, Reyes MV, Alvarez F, Faundes A, Croxatto HB. Does meloxicam increase the incidence of anovulation induced by single administration of levonorgestrel in emergency contraception? A pilot study. Hum Reprod. 2007;22:434–439. doi: 10.1093/humrep/del369. [DOI] [PubMed] [Google Scholar]
- Micks E, Shekell T, Stanley J, Zelinski M, Martin L, Riefenberg S, Adevai T, Jensen J. Medical termination of pregnancy in cynomolgus macaques. J Med Primatol. 2012;41:394–402. doi: 10.1111/jmp.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay D, Hascall VC, Day AJ, Salustri A, Fulop C. Two distinct populations of tumor necrosis factor-stimulated gene-6 protein in the extracellular matrix of expanded mouse cumulus cell-oocyte complexes. Arch Biochem Biophys. 2001;394:173–181. doi: 10.1006/abbi.2001.2552. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Conneely OM. Reproductive tissue selective actions of progesterone receptors. Reproduction. 2004;128:139–146. doi: 10.1530/rep.1.00189. [DOI] [PubMed] [Google Scholar]
- Munro CJ, Laughlin LS, Illera JC, Dieter J, Hendrickx AG, Lasley BL. ELISA for the measurement of serum and urinary chorionic gonadotropin concentrations in the laboratory macaque. Am J Primatol. 1997;41:307–322. doi: 10.1002/(SICI)1098-2345(1997)41:4<307::AID-AJP3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Norman RJ. Reproductive consequences of COX-2 inhibition. Lancet. 2001;358:1287–1288. doi: 10.1016/S0140-6736(01)06455-8. [DOI] [PubMed] [Google Scholar]
- Ochsner SA, Russell DL, Day AJ, Breyer RM, Richards JS. Decreased expression of tumor necrosis factor-alpha-stimulated gene 6 in cumulus cells of the cyclooxygenase-2 and EP2 null mice. Endocrinology. 2003;144:1008–1019. doi: 10.1210/en.2002-220435. [DOI] [PubMed] [Google Scholar]
- Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- Peng XR, Hsueh AJ, LaPolt PS, Bjersing L, Ny T. Localization of luteinizing hormone receptor messenger ribonucleic acid expression in ovarian cell types during follicle development and ovulation. Endocrinology. 1991;129:3200–3207. doi: 10.1210/endo-129-6-3200. [DOI] [PubMed] [Google Scholar]
- Peng J, Li Q, Wigglesworth K, Rangarajan A, Kattamuri C, Peterson RT, Eppig JJ, Thompson TB, Matzuk MM. Growth differentiation factor 9: bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci USA. 2013;110:E776–E785. doi: 10.1073/pnas.1218020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JS. Ovulation: new factors that prepare the oocyte for fertilization. Mol Cell Endocrinol. 2005;234:75–79. doi: 10.1016/j.mce.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Ruan YC, Guo JH, Liu X, Zhang R, Tsang LL, Dong JD, Chen H, Yu MK, Jiang X, Zhang XH, et al. Activation of the epithelial Na+ channel triggers prostaglandin E(2) release and production required for embryo implantation. Nat Med. 2012;18:1112–1117. doi: 10.1038/nm.2771. [DOI] [PubMed] [Google Scholar]
- Salustri A, Garlanda C, Hirsch E, De Acetis M, Maccagno A, Bottazzi B, Doni A, Bastone A, Mantovani G, Beck Peccoz P, et al. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development. 2004;131:1577–1586. doi: 10.1242/dev.01056. [DOI] [PubMed] [Google Scholar]
- Sirois J, Boerboom D, Sayasith K. Prostaglandin Biosynthesis and Action in the Ovary. In: Leung PCK, Adashi EY, editors. The Ovary. San Diego, CA: Elsevier Inc, Academic Press; 2004. pp. 233–247. [Google Scholar]
- Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- Tamba S, Yodoi R, Morimoto K, Inazumi T, Sukeno M, Segi-Nishida E, Okuno Y, Tsujimoto G, Narumiya S, Sugimoto Y. Expression profiling of cumulus cells reveals functional changes during ovulation and central roles of prostaglandin EP2 receptor in cAMP signaling. Biochimie. 2010;92:665–675. doi: 10.1016/j.biochi.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Ueno S, Yoshida N, Niimura S. Amount of hyaluronan produced by mouse oocytes and role of hyaluronan in enlargement of the perivitelline space. J Reprod Dev. 2009;55:496–501. doi: 10.1262/jrd.20226. [DOI] [PubMed] [Google Scholar]
- Wolf DP, Thomson JA, Zelinski-Wooten MB, Stouffer RL. In vitro fertilization-embryo transfer in nonhuman primates: the technique and its applications. Mol Reprod Dev. 1990;27:261–280. doi: 10.1002/mrd.1080270313. [DOI] [PubMed] [Google Scholar]
- Xu F, Stouffer RL, Muller J, Hennebold JD, Wright JW, Bahar A, Leder G, Peters M, Thorne M, Sims M, et al. Dynamics of the transcriptome in the primate ovulatory follicle. Mol Hum Reprod. 2011;17:152–165. doi: 10.1093/molehr/gaq089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y, Okamoto M, Kawashima I, Okazaki T, Nishimura R, Gunji Y, Hishinuma M, Shimada M. Positive feedback loop between prostaglandin E2 and Egf-like factors is essential for sustainable activation of MAPK3/1 in cumulus cells during in vitro maturation of porcine cumulus oocyte complexes. Biol Reprod. 2011;85:1073–1082. doi: 10.1095/biolreprod.110.090092. [DOI] [PubMed] [Google Scholar]
- Young KA, Hennebold JD, Stouffer RL. Dynamic expression of mRNAs and proteins for matrix metalloproteinases and their tissue inhibitors in the primate corpus luteum during the menstrual cycle. Mol Hum Reprod. 2002;8:833–840. doi: 10.1093/molehr/8.9.833. [DOI] [PubMed] [Google Scholar]
- Young KA, Chaffin CL, Molskness TA, Stouffer RL. Controlled ovulation of the dominant follicle: a critical role for LH in the late follicular phase of the menstrual cycle. Hum Reprod. 2003;18:2257–2263. doi: 10.1093/humrep/deg467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.