Abstract
STUDY QUESTION
Are differences in metabolic dysfunction between polycystic ovary syndrome (PCOS) and control women related to differences in their fat to lean mass (F/L) ratio?
SUMMARY ANSWER
Compared with controls of similar body mass index (BMI), women with PCOS demonstrate adverse body composition characterized by increased whole body fat relative to lean mass (i.e. a higher F/L ratio), which is associated with differences in metabolic dysfunction between the two groups.
WHAT IS KNOWN ALREADY
Previous studies examining body composition and insulin resistance (IR) in PCOS have yielded conflicting results. Excess total fat mass (i.e. fat mass index [fat BMI]) correlates with IR, whereas increased total lean mass (i.e. lean BMI) has been associated with higher insulin sensitivity. However, the role of the F/L ratio, which integrates the antagonistic effects of both fat and lean mass depots, on IR in PCOS, has not been investigated.
STUDY DESIGN, SIZE, DURATION
We conducted a prospective cross-sectional study of 120 women between the ages of 22–44 years to study the relation of the F/L ratio with measures of insulin action and secretion in both steady and dynamic states.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Sixty PCOS (by NIH, 1990 criteria) and 60 control (age, race and BMI-matched) women were prospectively studied for body composition (by bioelectrical impedance analysis [BIA]) and basal IR and insulin secretion by the homeostasis model assessment (HOMA-IR and HOMA-%β-cell function, respectively) in a tertiary care academic referral center. A subset of 12 PCOS and 12 matched control women also underwent a modified frequently sampled intravenous glucose tolerance test (FSIVGTT) to determine glucose uptake and insulin secretion in dynamic state.
MAIN RESULTS AND THE ROLE OF CHANCE
Our results indicate that women with PCOS demonstrated greater degrees of hyperandrogenism, and higher waist-to-hip ratio (WHR), %body fat, fat BMI, F/L, fasting insulin levels, and HOMA-IR and HOMA-%β-cell values, than controls. In models adjusted for WHR and free testosterone and diagnostic groups, fasting insulin levels, HOMA-IR, and HOMA-%beta cell function were positively related to the F/L ratio. A positive relationship was also found in both study groups between F/L and the FSIVGTT measures insulin sensitivity (Si) and acute insulin response to glucose (AIRg). The F/L tended to negatively correlate with glucose effectiveness or non-insulin-mediated glucose transport (Sg) only in PCOS women.
LIMITATIONS, REASONS FOR CAUTION
Regional tissue sub-compartments, which have been shown to have potential independent associations with metabolic variables, cannot be determined by bioelectrical impedance analysis (BIA).
WIDER IMPLICATIONS OF THE FINDINGS
The current results suggest that BIA could be used to assess F/L in place of dual energy X-ray absorptiometry (DXA) in research protocols, and that F/L could possibly be used as an alternative to WHR as a surrogate marker of metabolic dysfunction in clinical practice.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by grants R01-DK073632 and R01-HD29364 from the NIH and an endowment of the Helping Hand of Los Angeles, Inc. (to R.A.). The authors have no competing interests to declare.
TRIAL REGISTRATION NUMBER
Not applicable.
Keywords: PCOS, lean body mass, fat body mass, androgens, insulin resistance
Introduction
As the most common endocrine-metabolic disorder, polycystic ovary syndrome (PCOS) affects 7–10% of women even when the most conservative definition of PCOS is used (i.e. the NIH, 1990 definition) (Azziz et al., 2004a; Zawadzki and Dunaif, 1992) and is characterized by hyperandrogenism, chronic oligo-ovulation and polycystic ovaries. PCOS has been strongly associated with insulin resistance (IR) and obesity (Azziz et al., 2004b, 2009; Ezeh et al., 2013a), at least in patients seen in referral populations (Ezeh et al., 2013b). Approximately 70% of women with PCOS demonstrate IR and compensatory hyperinsulinemia, beyond that due to their degree of obesity (Dunaif et al., 1997; DeUgarte et al., 2005). While the prevalence of overall adiposity or obesity appears to be as common among women with PCOS as in the general population, >60% of such patients seen in the clinical setting are obese (Ezeh et al., 2013b).
Although increased body mass index (BMI) has been associated with increased IR and increased risk of developing metabolic syndrome, type 2 diabetes (T2DM), and cardiovascular disease (CVD) (Dunaif et al., 1997; Ezeh et al., 2013a), the relationships between BMI and IR in women with PCOS is heterogeneous. Some obese PCOS women demonstrate no measurable metabolic abnormalities and IR occurs in obese and lean PCOS women alike (Dunaif et al., 1997; Ezeh et al., 2013a). We have demonstrated adipose tissue dysfunction in these patients in the form of impaired glucose transport, exaggerated inflammatory markers, impaired adiponectin secretion and reduced GLUT-4 expression, independent of degree of obesity (Chazenbalk et al., 2010; Chen et al., 2013).
These data imply that factors other than exaggerated adiposity per se may account for the IR observed in PCOS. An over-reliance of previous studies on BMI as an assessment of adiposity may also be to blame for the heterogeneous relationship between obesity and metabolic dysfunction in PCOS, since it is limited by its inability to distinguish between fat and lean body mass. Although hyperinsulinemia and hyperandrogenemia have been shown to increase lean mass in post-menopausal women (Ding et al., 2006; Rariy et al., 2011), prior studies of body composition in PCOS have yielded conflicting results. Some investigators report increased lean mass (Douchi et al., 1999), decreased lean mass and higher fat mass (Kirchengaast and Huber, 2001), or normal whole body fat mass, with increased truncal fat mass and lean mass (Carmina et al., 2009), compared with BMI-matched controls. Some studies also indicate a strong association in PCOS between hyperandrogenism and preferential abdominal fat deposition with increased adiposity (Douchi et al., 1999) and IR (Coviello et al., 2006), although others have not consistently observed the reported improvements in insulin sensitivity with androgen suppression in PCOS (Bhattacharya and Jha, 2012). Furthermore, these reports of alterations in body composition associated with PCOS were not adjusted for confounders such as ethnic/racial composition, endogenous androgens and abdominal adiposity.
Excess total fat mass and its distribution have been strongly associated with IR, glucose intolerance and increased risks of T2DM and CVD (Svendsen et al., 2008; Neeland et al., 2012; Ezeh et al., 2013a), while increased total lean body mass, predominantly comprised of skeletal muscles, has been linked with increased whole body insulin sensitivity (Miller et al., 1994; Nam et al., 2001; Van Der Heijden et al., 2010; Srikanthan and Karlamangla, 2011). One possible mechanism determining the increased IR in females is an alteration in the lean to fat mass ratio. Prior studies of body composition in PCOS focused on lean or fat mass alone and did not explore how whole body fat deposition interacts with lean mass to affect IR. In this study we hypothesized that the F/L ratio, which integrates the antagonistic effects of both fat and lean mass depots, differs between PCOS patients and controls and accounts, at least in part, for the greater degrees of IR in the former.
Materials and Methods
Study population
Sixty women with PCOS and 60 (age, race and BMI-matched) healthy control women, aged 22–44 years, were prospectively studied at the Center for Androgen-Related Disorders (CARD) from 2008 to 2009. The presence of PCOS was defined by the 1990 National Institutes of Health (NIH) consensus criteria: namely the presence of oligo-ovulation and biochemical and/or clinical hyperandrogenism, excluding other known endocrinopathies as previously described (Zawadzki and Dunaif, 1992; Knochenhauer et al., 1998; Azziz et al., 2004b). Control women were recruited through advertisements and were healthy, without a history of endocrine disorders, non-hirsute, had long-term predictable eumenorrhea, and normal ovarian morphology on ultrasonography.
To ensure comparable groups, we prospectively recruited all PCOS subjects first and then a match among controls was sought, either from a previously recruited pool of controls or, if no control with the needed parameters (i.e. ±3 kg/m2 in BMI, ±5 years in age, and similar race to the PCOS subject) was so identified, then a new control was sought. Controls were then studied per the protocol of this study. This recruitment strategy yields cohorts of PCOS and controls similar in number, mean BMI, mean age and race distribution. The subset of patients who underwent a frequently sampled intravenous glucose tolerance test (FSIVGTT) was similarly recruited prospectively and consecutively as part of an ongoing study of adipose tissue dysfunction (Chazenbalk et al., 2010, 2012; Chen et al., 2013).
None of the women were taking any hormonal medication (including oral contraceptives or other medications that affect glucose metabolism such as metformin or the thiazolidinediones) and had not done so for at least 3 months before the study. The study was approved by the Institutional Review Board of Cedars-Sinai Medical Center in Los Angeles, CA, USA. All subjects provided written informed consent.
Subject evaluation
All subjects underwent a physical examination with blood sampling for hormone measurements, as previously described (Knochenhauer et al., 1998; Azziz et al., 2004b) and were normoglycemic by 2-h oral glucose tolerance testing (OGTT). In addition to height, weight and modified Ferriman–Gallwey (mFG) score, waist circumference (WC) was measured at the narrowest portion of the torso approximately midway between the lower costal margin and the iliac crest, and the hip circumference was measured over the widest portion of the gluteal and greater trochanteric region. The body mass index (BMI) and waist:hip ratio (WHR) were then calculated.
As previously reported (Knochenhauer et al., 1998), ovulatory dysfunction was defined by: (i) a history of eight or fewer menstrual cycles in a year, or (ii) menstrual cycles less than 26 d or >35 day in length or (iii) a d 22–24 (mid-luteal) progesterone (P4) level of <4 ng/ml in subjects with cycles 26–35 day in length. Clinical hyperandrogenism was defined by hirsutism with a modified Ferriman–Gallwey (mFG) score of ≥6. Hyperandrogenemia was defined by androgen values based on prior levels of a total testosterone (TT), free testosterone (FT) and DHEAS as previously described (Knochenhauer et al., 1998).
Blood samples were obtained within the first 7 days of a spontaneous vaginal bleed or, if anovulatory or amenorrheic, after a withdrawal bleed induced using oral micronized P4, as previously reported (Woods et al., 2002). We also screened all subjects with PCOS (including those with menstrual dysfunction) using a TSH, prolactin and 17-hydroxyprogesterone level, to exclude thyroid dysfunction, hyperprolactinemia and 21-hydroxylase deficient nonclassic adrenal hyperplasia, respectively.
Hormonal and biochemical analyses
Fasting blood samples for circulating total testosterone (total T), free testosterone (free T), dehydroepiandrosterone sulfate (DHEAS), sex hormone binding protein (SHBG), insulin and glucose concentrations were obtained on Day 3 through 8 following a vaginal bleed.
Total T was measured using high-turbulence liquid chromatography tandem mass spectrometry and free T determined by equilibrium dialysis (Quest Diagnostics, San Juan Capistrano, CA, USA). The serum levels of DHEAS were measured by a competitive immunoassay (Modular E170; Roche Diagnostics, Indianapolis, IN, USA). Insulin was assayed by chemiluminescence (ADVIA Centaur chemiluminescent immunoassay system; Siemens Healthcare, Deerfield, IN, USA). Serum glucose levels were measured using the hexokinase/glucose-6-phosphate dehydrogenase method (Roche Applied Sciences, Indianapolis, IN, USA).
Samples were batched at regular intervals for analysis to minimize the impact of inter-assay variability and provide study subjects with timely information. The intra- and inter-assay variations for total T, SHBG, DHEAS, A4, PRL, TSH, 17-HP and P4 have been previously reported and did not exceed 9% (Knochenhauer et al., 1998; DeUgarte et al., 2005).
Metabolic assessment
FSIVGTT was performed as previously described (Farah-Eways et al., 2004). Briefly, after an overnight fast, two intravenous catheters were placed in each forearm between 8:00 and 9:00 am. Intravenous administration of glucose (0.3 g/kg) was followed in 20 min by the administration of regular insulin (0.03 U/kg). Blood samples (2.0 ml) were collected 34 times from −20 min. (relative to glucose administration) to +180 min. Samples drawn into pre-chilled tubes containing EDTA (for insulin) or sodium fluoride potassium oxalate (for glucose), and plasma were frozen at −80°C until assayed.
Plasma glucose and insulin values were entered into the MINMOD computer program and data were analyzed in a single assay using minimal model of glucose kinetics to establish glucose-insulin interactions (Bergman, 1989). The calculated components of the modified FSIVGTT were: the acute insulin response to glucose (AIRg), which reflects the first phase endogenous insulin secretion in response to a bolus glucose injection; the insulin sensitivity index (Si), the insulin-mediated glucose uptake per unit of insulin evaluated by a bolus injection of known quantity of insulin; the glucose effectiveness (Sg), the ability of glucose per se, independent of changes in insulin, to increase glucose uptake and suppress endogenous output; and the disposition index (DI = Si x AIRg), representing the interaction of insulin sensitivity and the compensatory ability of the β-cell to secrete insulin.
Bioelectrical impedance
Body composition was assessed by bioelectrical impedance analysis (InBody 520, Biospace, Inc., Cerritos, CA, USA), a well-established and validated technique for assessment of body composition (National Institutes of Health, 1996). Measurements were performed according to the National Institutes of Health guidelines (National Institutes of Health, 1996) and recommendation of the manufacturer. Measurements obtained included total body fat mass (FM in kg), and total body lean mass (LM in kg). In addition, the fat BMI (fat mass in kg/ (height in m)2), lean BMI (lean mass in kg/(height in m)2 and the fat/lean mass (F/L) ratio were calculated, as previously described (Schutz et al., 2002).
Statistical analyses
Homeostasis model assessment for insulin resistance (HOMA-IR) and beta-cell secretion (HOMA-%β-cell) were calculated from fasting serum insulin concentration [in milliunits per liter] and fasting plasma glucose concentration [in millimoles] as previously described (Matthews et al., 1985). In addition to plots of each outcome measure, the Shapiro–Wilk-test was performed on the univariate distributions. If on examination of the univariate distributions normality assumptions were not met, we normalized such data using log transformation for subsequent analyses. Six variables (total T, free T, fasting glucose, fasting insulin, HOMA-IR and HOMA-%β-cell) which demonstrated a skewed distribution were log-transformed.
For each of the measures, we computed descriptive analyses and investigated bivariate relationships for potential use in multivariate analyses. Group differences were evaluated using unpaired t-tests for continuous variables. In the case of mFG score and DHEAS, which did not follow the parametric normal distribution on the original or log scale, and FSIVGTT, where our sample size was small for each group, we used the non-parametric Mann–Whitney test for bivariate comparisons. Categorical variables were analyzed with Chi-square tests or Fisher's exact test, as appropriate. Bivariate correlations between continuous variables were analyzed using Pearson correlations, except for mFG score, DHEAS and FSIVGTT where we used Spearman correlations. We conducted linear regression analyses to model the relationship between the F/L ratio and measures of metabolic dysfunction (fasting insulin, HOMA-IR and HOMA-%β-cell). To test our hypotheses of group differences (PCOS versus control), analysis of covariance models were used on our measures of metabolic dysfunction outcomes while adjusting for WHR, androgens and F/L ratio. Power analysis on R2 indicated that for the multiple linear regression test of R2 = 0 (alpha = 0.05), a sample size of 120 was sufficient for four covariates to detect a difference with an 80% power and an R2 of at least 9.4% (Chow et al., 2008).
A significance level of 0.05 was used for all statistical tests, with Bonferroni's method of adjustment for multiple analyses (Tables II and III). Data analyses were conducted using the Stats Direct statistics software package, version 2.7.8 2010 (Cheshire, England).
Table II.
Association of fat/lean mass ratio, abdominal adiposity and androgens with basal measures of metabolic dysfunction in polycystic ovary syndrome (PCOS) and Controls.
| Variables | Fasting Insulin (µIU/ml)a |
HOMA IRa |
HOMA-%β-cell functiona |
|||
|---|---|---|---|---|---|---|
| r | P-valueb | r | P-valueb | r | P-valueb | |
| PCOS (n = 60) | ||||||
| Fat/lean mass ratio | 0.49 | 0.0001 | 0.49 | 0.0001 | 0.48 | 0.0001 |
| Waist: hip ratio | 0.38 | 0.0077 | 0.41 | 0.0037 | 0.33 | 0.0144 |
| Free T (pg/ml)a | 0.53 | 0.0001 | 0.56 | 0.0001 | 0.49 | 0.0003 |
| Total T (ng/dl)a | −0.15 | 0.2869 | −0.15 | 0.3138 | −0.17 | 0.2369 |
| DHEAS (µg/dl) | 0.06 | 0.7077 | 0.09 | 0.5419 | 0.03 | 0.8521 |
| mFG score | 0.09 | 0.5545 | 0.08 | 0.5734 | 0.01 | 0.4997 |
| Controls (n = 60) | ||||||
| Fat/lean mass ratio | 0.55 | 0.0002 | 0.53 | 0.0003 | 0.54 | 0.0002 |
| Waist: hip ratio | 0.43 | 0.0068 | 0.4 | 0.0109 | 0.45 | 0.0037 |
| Free T (pg/ml)a | 0.31 | 0.0473 | 0.3 | 0.0516 | 0.3 | 0.0532 |
| Total T (ng/dl)a | 0.03 | 0.858 | −0.02 | 0.9047 | 0.08 | 0.6064 |
| DHEAS (µg/dl) | 0.09 | 0.5859 | 0.05 | 0.7517 | 0.11 | 0.5079 |
| mFG score | 0.11 | 0.5068 | −0.09 | 0.5898 | −0.13 | 0.4147 |
DHEAS is dehydroepiandrosterone sulfate; HOMA, homeostasis model assessment; mF-G is the modified Ferriman–Gallwey hirsutism score; T is testosterone.
Analysis by Pearson correlation.
aLog transformed prior to analysis.
bFor this analysis a significant P-value was considered after Bonferroni correction to be P < 0.0083. p values that are significant are denoted in bold.
Table III.
Results of multivariate models predicting basal metabolic measures in polycystic ovary syndrome patients and controls.
| Model | Independent variables | β-Coefficient (95% CI) | P-valuea | R2 | ΔR2 |
|---|---|---|---|---|---|
| Dependent variable = fasting insulin (µIU/ml)b | |||||
| 1 | Diagnostic group | 0.40 (0.19 to 0.60) | 0.0002 | 28.7 | |
| Free testosterone (pg/ml)b | 0.40 (0.13 to 0.67) | 0.0045 | |||
| Waist: hip ratio | 1.00 (0.25 to 1.76) | 0.0100 | |||
| 2 | Diagnostic group | 0.39 (0.20 to 0.57) | <0.0001 | 35.3 | 6.6 |
| Free testosterone (pg/ml)b | 0.40 (0.16 to 0.64) | 0.0012 | |||
| Fat/lean mass ratio | 0.56 (0.31 to 0.81) | <0.0001 | |||
| 3 | Diagnostic group | 0.36 (0.16 to 0.55) | 0.0005 | 37.5 | 8.8 |
| Free testosterone (pg/ml)b | 0.36 (0.12 to 0.62) | 0.0059 | |||
| Waist: hip ratio | 0.50 (−0.27 to 1.27) | 0.1972 | |||
| Fat/lean mass ratio | 0.50 (0.20 to 0.79) | 0.0011 | |||
| Dependent variable = HOMA-IRb | |||||
| 1 | Diagnostic group | 0.43 (0.22 to 0.65) | 0.0001 | 30.4 | |
| Free testosterone (pg/ml)b | 0.42 (0.14 to 0.70) | 0.0039 | |||
| Waist: hip ratio | 1.10 (0.31 to 1.90) | 0.0069 | |||
| 2 | Diagnostic group | 0.41 (0.21 to 0.61) | <0.0001 | 35.9 | 5.5 |
| Free testosterone (pg/ml)b | 0.42 (0.17 to 0.67) | 0.0013 | |||
| Fat/lean mass ratio | 0.61 (0.34 to 0.87) | <0.0001 | |||
| 3 | Diagnostic group | 0.39 (0.18 to 0.59) | 0.0003 | 40.0 | 9.6 |
| Free testosterone (pg/ml)b | 0.39 (0.12 to 0.65) | 0.0049 | |||
| Waist: hip ratio | 0.55 (−0.25 to 1.35) | 0.1733 | |||
| Fat/lean mass ratio | 0.55 (0.25 to 0.85) | 0.0006 | |||
| Dependent variable = HOMA-β cell% functionb | |||||
| 1 | Diagnostic group | 0.40 (0.17 to 0.62) | 0.0008 | 25.9 | |
| Free testosterone (pg/ml)b | 0.40 (0.10 to 0.70) | 0.0090 | |||
| Waist: hip ratio | 1.09 (0.26 to 1.93) | 0.0110 | |||
| 2 | Diagnostic group | 0.40 (0.19 to 0.60) | 0.0003 | 32.6 | 6.7 |
| Free testosterone (pg/ml)b | 0.42 (0.15 to 0.68) | 0.0023 | |||
| Fat/lean mass ratio | 0.60 (0.32 to 0.88) | <0.0001 | |||
| 3 | Diagnostic group | 0.35 (0.14 to 0.57) | 0.0018 | 33.6 | 7.7 |
| Free testosterone (pg/ml)b | 0.37 (0.08 to 0.65) | 0.0122 | |||
| Waist: hip ratio | 0.59 (−0.27 to 1.45) | 0.1785 | |||
| Fat/lean mass ratio | 0.50 (0.18 to 0.83) | 0.0029 | |||
Diagnostic group was coded as PCOS = 1 and Control = 0.
95% CI is the 95% confidence interval.
R2 is the regression coefficient of determination, estimating the percent of the total variation in the outcome that is accounted for by predicting variables.
ΔR2 is the percent change in R2 relative to R2 in model 1.
aFor this analysis a significant P-value was considered after Bonferroni correction to be P < 0.0042. p values that are significant are denoted in bold.
bLog transformed prior to analysis.
Results
Baseline features of study groups
The baseline characteristics of the subjects are described in Table I. PCOS and control groups did not differ significantly with respect to age, body mass index (BMI), height or racial/ethnic composition (P = 0.5134). As expected, women with PCOS had higher WHR, HOMA-IR and HOMA-%β-cell values, mF-G scores, and fasting insulin, baseline total T, free T and DHEAS levels than controls (Table I). All controls were euandrogenemic with the exception of one subject with isolated hyperandrogenemia with a free T of 10 pg/ml, who was included in the study nevertheless.
Table I.
Baseline anthropometric, endocrine and metabolic characteristics of study subjects.
| Variables | PCOS (n = 60) | Control (n = 60) | P-value |
|---|---|---|---|
| Age (years) | 30.37 ± 0.64 | 32.40 ± 0.91 | 0.0734 |
| Race (n and %) | |||
| Non-Hispanic White | 20 (33.3%) | 20 (33.3%) | 0.9999 |
| Hispanic White | 17 (28.3%) | 11 (18.3%) | 0.2805 |
| African-American | 10 (16.7%) | 15 (25%) | 0.3686 |
| Asian-American | 12 (20.0%) | 11 (18.3%) | 0.9999 |
| Mixed | 1 (1.7%) | 3 (5.0%) | |
| Anthropometric measures | |||
| BMI (kg/m2) | 29.5 ± 0.79 | 27.8 ± 0.92 | 0.1643 |
| Waist: hip ratio | 0.87 ± 0.01 | 0.84 ± 0.01 | 0.0357 |
| Lean and fat mass measures | |||
| % Body fat | 39.41 ± 0.98 | 35.27 ± 1.16 | 0.0075 |
| Fat BMI (kg/m2) | 11.96 ± 0.56 | 10.48 ± 0.72 | 0.0158 |
| Lean BMI (kg/m2) | 17.51 ± 0.26 | 17.64 ± 0.37 | 0.7676 |
| Fat/lean mass ratio | 0.67 ± 0.03 | 0.58 ± 0.03 | 0.0210 |
| Androgenism measures | |||
| mFG scorea | 7.72 ± 0.69 | 0.78 ± 0.18 | 0.0001 |
| Free T (pg/ml)b | 4.76 (0.80–15.20) | 1.77 (0.70–10) | 0.0001 |
| Total T (ng/dl)b | 45.32 (11–144) | 21.17 (8–79) | 0.0001 |
| DHEAS (µg/dl)a | 249.68 ± 17.34 | 194.92 ± 14.80 | 0.0089 |
| Metabolic function | |||
| Fasting insulin (µIU/ml)b | 10.07 (2–83) | 6.72 (2–20) | 0.0044 |
| HOMA IRb | 2.14 (0.40–17.42) | 1.39 (0.42–4.64) | 0.0043 |
| HOMA-%β-cell functionb | 37.54 (5.50–348.06) | 24.57 (4.97–73.10) | 0.0070 |
Analysis by unpaired t-test p-values reported for all variables, except for mFG score and DHEAS.
Values are means ± SE except for race and log-transformed data (indicated by b).
For this analysis P < 0.05 is considered significant. p values that are significant are denoted in bold.
DHEAS is dehydroepiandrosterone sulfate; HOMA, homeostasis model assessment; mF-G is the modified Ferriman–Gallwey hirsutism score; PCOS is polycystic ovary syndrome, T is testosterone.
aAnalysis by Mann–Whitney test.
bGeometric means, the antilog of the log scale mean, is reported for log-transformed data.
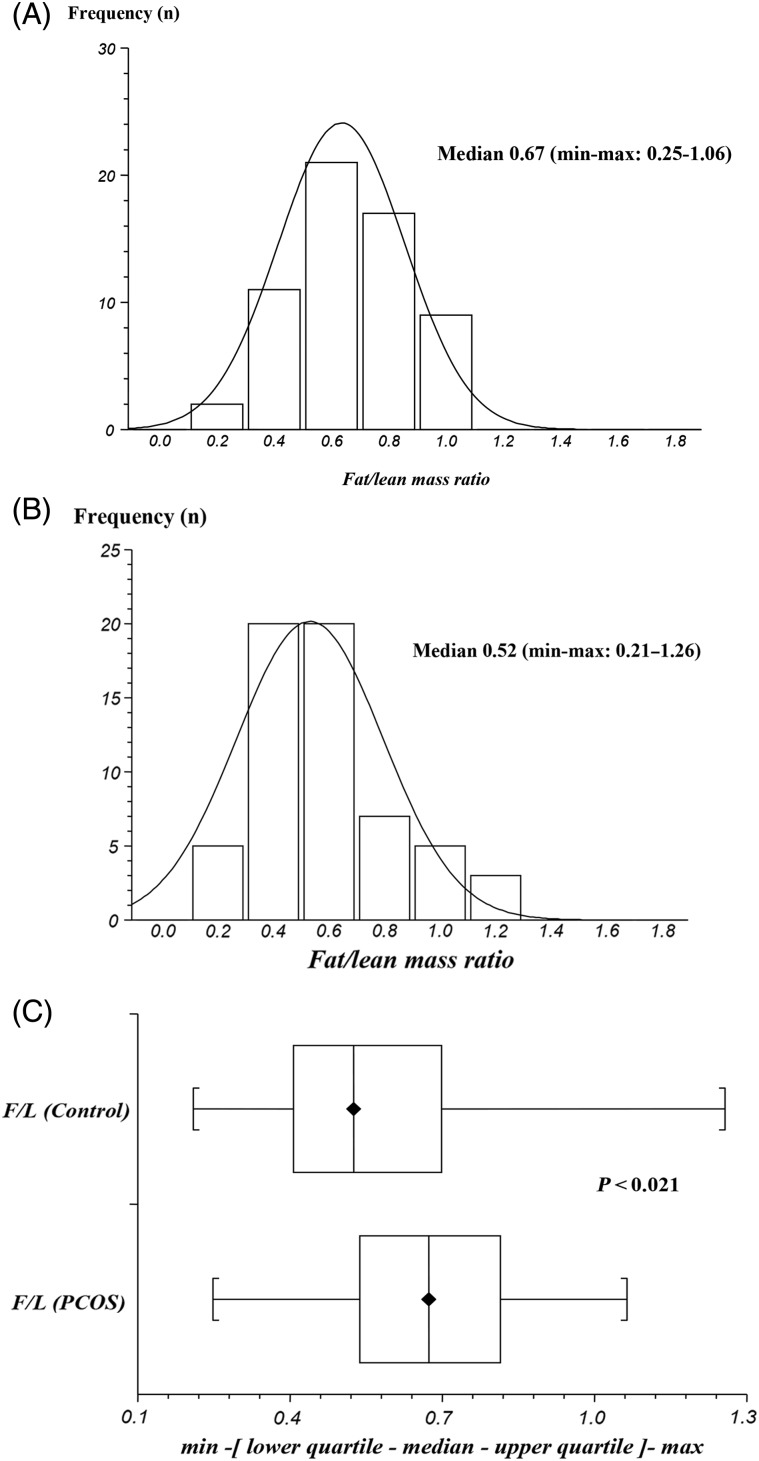
The distribution of the F/L mass ratio for PCOS and controls is depicted in Fig. 1, and appeared to reasonably follow normal distribution on the original scale. Therefore, these values were not log-transformed. Despite similar BMI values women with PCOS had a higher percentage of body fat (% BF), a higher total fat mass (FM) and higher F/L ratio than controls, while total lean mass (LM) values were similar between the two groups (Table I, Fig. 1). Similar results were obtained when FM and LM were corrected for body stature and expressed as fat BMI and lean BMI, respectively
Figure 1.
Comparison of the fat/lean mass (F/L) ratio in women with polycystic ovary syndrome (PCOS) and controls. (A) Distribution of F/L in PCOS. (B) Distribution of F/L in controls. (C) Box and whisker plot of F/L ratio in PCOS versus controls.
Association of F/L ratio, androgens and abdominal adiposity with measures of metabolic dysfunction
In bivariate analysis, the F/L ratio, free T and WHR were positively associated with measures of metabolic dysfunction (i.e. levels of fasting insulin, HOMA-IR and HOMA-%β-cell function) in PCOS and in controls (except that free T only tended to correlate with HOMA-IR and HOMA-%β-cell in the controls) (Table II). The mF-G score, and total T and DHEAS levels demonstrated no association with measures of metabolic dysfunction in either PCOS or controls (Table II). Age was also not associated with any of the measures of metabolic dysfunction in either PCOS or controls (data not shown). Models analyzing differences in metabolic function between PCOS and controls were therefore adjusted for free T and WHR as well as F/L.
Models delineating the relation of the F/L ratio to measures of metabolic dysfunction
We tested predictive models for fasting insulin levels, HOMA-IR and HOMA-%β-cell function, which included a ‘diagnostic group × F/L interaction’ term and found no significant interactions, suggesting that the relationship of F/L and the outcome variables is similar in both PCOS and controls (fasting insulin levels [P=0.841], HOMA-IR [P=0.721] and HOMA-β%-cell [P=0.989]; data not shown). Consequently, we did not stratify the subsequent multiple regressions according to diagnostic groups.
The results of multivariate models predicting differences in metabolic function parameters between PCOS and controls and differentiating the associations of the F/L ratio and WHR with measures of metabolic dysfunction are presented in Table III. We first constructed multivariate models using parameters of metabolic dysfunction (log-transformed values for fasting insulin, HOMA-IR, HOMA-%β-cell) as the dependent variable, while adjusting for diagnostic group, WHR and free T (Model 1). Significant differences were found between PCOS and controls in fasting insulin HOMA-IR, and HOMA-% β function while WHR and free T were positively and independently associated with these measures of metabolic dysfunction. To explore further possible explanations for the differences in IR between PCOS and controls and to gain better insight into the independent predictive value of the F/L ratio versus WHR, the influence of these parameters on the predictive value of the models was then evaluated .
Firstly, substituting the F/L ratio for WHR in Model 1 (i.e. Model 2) increased the R2 of the model. In addition, adding the F/L ratio to Model 1 (i.e. Model 3) not only improved the model's R2 but also blunted the β coefficient and P-value of WHR for predicting parameters of metabolic dysfunction. Finally, the F/L ratio, diagnostic group and free T were positively and independently associated with parameters of metabolic dysfunction in all the models.
To gain better insight into the independent predictive value of the F/L ratio versus WHR, the association between these two variables and the impact of their potential interactions on parameters of metabolic dysfunction were assessed. Pearson's correlation analysis indicate that WHR was positively and significantly related to the F/L ratio in both PCOS (r = 0.395; P = 0.0029) and controls (r = 0.528; P = 0.0001). Similarly, analysis by collinearity (data not shown) also revealed that these two variables are strongly confounded.
Overall, these results suggest that even though the two variables are confounded, the F/L ratio more effectively predicted metabolic dysfunction than WHR. There were no further improvements in predictability when adjusting further for total T or DHEAS levels, or mF-G score, which also showed no independent association with measures of metabolic dysfunction (data not shown).
Association of the F/L ratio and metabolic parameters generated by the FSIVGTT
Fourteen PCOS patients and 12 controls completed FSIVGTT and bioelectrical impedance analysis (BIA). Differences in basic and FSIVGTT parameters (AIRg, Si, Sg and Di) between the 12 PCOS and 12 controls of similar BMI, and race are shown in Table IV. Compared with controls of similar BMI, and race, the mean Si was less in PCOS patients than in controls while AIRG was greater. There was no difference in mean Di or Sg between PCOS and control women. Although PCOS patients were younger than controls, in those subjects that underwent FSIVGTT, there were no significant relationships between age and FSIVGTT parameters (data not shown).
Table IV.
Baseline anthropometric, endocrine and metabolic parameters of polycystic ovary syndrome (PCOS) and control subjects who underwent a frequently sampled intravenous glucose tolerance test (FSIVGTT).
| Variables | PCOS (n = 12) | Controls (n = 12) | P-value |
|---|---|---|---|
| Age (years) | 27.5 ± 1.2 | 35.8 ± 2.0 | 0.0029 |
| BMI (kg/m2) | 30.4 ± 1.5 | 28.8 ± 1.3 | 0.5512 |
| Non-Hispanic White | 5.0 (41.7%) | 6.0 (50.0%) | 0.6820 |
| Hispanic White | 5.0 (41.7%) | 3.0 (25.0%) | 0.4299 |
| Other | 2.0 (16.6%) | 3.0 (25.0%) | 0.6584 |
| AIRg (µU−1/ml) | 581.5 ± 86.0 | 340.3 ± 46.1 | 0.0449 |
| Di* | 1957.7.69 ± 314.6 | 3362.2 ± 1296.3 | 0.4428 |
| Si (min−1 × µU−1 × ml−1) | 4.03 ± 0.7 | 22.63 ± 16.1 | 0.0242 |
| Sg (min−1) | 0.023 ± 0.003 | 0.029 ± 0.004 | 0.4095 |
Analysis by Spearman correlation.
For this analysis P < 0.05 is considered significant. p values that are significant are denoted in bold.
AIRg is acute response of insulin to glucose; Di is disposition index; Sg is glucose effectiveness index and Si is insulin sensitivity index.
The association between the F/L ratio and FSIVGTT parameters is depicted in Table V. The F/L ratio was negatively associated with Si and tended to be positively associated with AIRG, in both PCOS and controls; alternatively, the F/L ratio tended to be negatively associated with Sg, although only in PCOS. No association of the F/L ratio with donor insemination was observed for either group or parameter.
Table V.
Association of the fat/lean ratio with measures of metabolic dysfunction as determined by frequently sampled intravenous glucose tolerance test (FSIVGTT) in polycystic ovary syndrome (PCOS) patients and controls.
| Variables | PCOS (n = 12) |
Controls (n = 12) |
||
|---|---|---|---|---|
| r | P-value | r | p-value | |
| AIRg (µU−1/ml) | 0.57 | 0.0555 | 0.55 | 0.0666 |
| Di | −0.24 | 0.4571 | −0.12 | 0.7495 |
| Si (min −1 µU− ml−1) | −0.62 | 0.0373 | −0.66 | 0.0240 |
| Sg (min −1) | −0.55 | 0.0793 | −0.32 | 0.3083 |
Analysis by Spearman correlation.
For this analysis P < 0.05 is considered significant. p values that are significant are denoted in bold.
AIRg is acute response of insulin to glucose; Di is disposition index; Sg is glucose effectiveness index and Si is insulin sensitivity index.
Discussion
In line with other studies, we found a greater degree of hyperandrogenism and metabolic dysfunction in women with PCOS, compared with controls (Dunaif et al., 1997; DeUgarte et al., 2005), and significant associations of increased androgen levels and abdominal fat distribution determined by WHR with decreased insulin sensitivity (Douchi et al., 1999; Coviello et al., 2006). The main and novel findings of our study are that PCOS women appear to have an adverse body composition, characterized by an increased ratio of fat to lean mass (F/L ratio), which is independently associated with differences in the values of fasting insulin, HOMA-IR and HOMA-%β-cell between PCOS and controls. PCOS women also had lower mean Si values than controls, which was negatively associated with the F/L ratio. Overall, these data suggest that the higher degrees of metabolic dysfunction in PCOS patients may be, at least in part, attributable to their higher F/L ratio.
We also observed a tendency, approaching statistical significance, for the F/L ratio to be negatively associated with glucose effectiveness (Sg) only in PCOS, which is consistent with our previous findings observing that Sg is impaired in insulin resistant PCOS women (Ezeh et al., 2013a). The effects of fat or lean mass on IR have been documented previously (Douchi et al., 1999; Kirchengaast and Huber, 2001; Svendsen et al., 2008; Carmina et al., 2009). However, this is the first study specifically designed to demonstrate whether the F/L ratio is associated with insulin action and secretion in both the steady and dynamic states in women with PCOS. Overall, our data support our previous findings which suggest that adipogenesis is abnormal in women with PCOS (Chazenbalk et al. 2010; Chen et al., 2013).
Although IR, T2DM and CVD are linked with increasing BMI, there is a growing consensus that they are more strongly correlated with the presence of abdominal or visceral obesity (Després et al. 2008; Preis et al. 2010), making WHR an important surrogate clinical measure for cardiometabolic risk. However, in our study despite adjusting for WHR and free T (each of which can predict IR), the differences in metabolic dysfunction between PCOS and controls persisted, suggesting that these parameters do not completely address IR observed in PCOS. Although the F/L ratio and WHR are highly confounded, our data indicate that the F/L ratio predicted metabolic dysfunction more effectively than WHR.
It is not surprising that our data demonstrated that an increased F/L ratio was associated with increased metabolic dysfunction, given that skeletal muscle accounts for >85% of the whole body insulin-stimulated glucose uptake via GLUT 4 (DeFronzo et al., 1985). The deleterious effects of excess body fat therefore overrides the beneficial effects of lean mass in the scenario of increased F/L ratio as in our study. Our results are supported by the reports of Lear et al. (2009) and the London Mother and Baby Study (Stanfield et al., 2012), which indicated that the greater IR (as measured by HOMA-IR) in adult and neonatal South Asian subjects, respectively, was due to their higher ratio of whole fat mass to lean mass.
Our data are also consistent with studies that demonstrated that increased lean mass or skeletal muscle alone, rather than its relationship with fat mass, is positively associated with insulin sensitivity (Miller et al., 1994; Nam et al., 2001; Van Der Heijden et al., 2010; Srikanthan and Karlamangla, 2011). However, a few studies have reported opposite results in healthy women (Kuk et al., 2008) and PCOS subjects (Comerford et al., 2012), which the investigators attributed in part to an impairment in muscle quality rather than quantity (He et al., 2001; Kuk et al., 2008; Comerford et al., 2012). However, this hypothesis runs counter to clinical evidence indicating that increases in whole body insulin sensitivity occur in parallel with increases in muscle mass following exercise (Miller et al., 1994; Nam et al., 2001) and other studies that demonstrate increased IR, mortality, T2DM and CVD with sarcopenia (Wannamethee et al., 2007).
The mechanisms underlying the adverse body composition of PCOS observed in this study are unclear. There is good evidence indicating that the presence of obesity is accompanied by an increase in muscle mass (Forbes and Welle, 1983; Lear et al. 2009). However, the increased F/L ratio observed in our study, which indicates a disproportional change in fat mass relative to lean mass, suggests a decreased ability of lean mass to increase proportionately with the changes in fat mass in PCOS. Further studies will be required to determine the time of onset of this adverse body composition, including whether it may be programmed in utero.
The present study results also highlight the limitations of BMI as the optimal measure of body composition. Despite the role of obesity as a CVD and Type 2 DM risk factor, some investigators have reported on the ‘obesity paradox’ whereby some overweight or obese individuals with established CVD have better outcomes for survival and less cardiovascular events than their normal-weight counterparts (Romero-Corral et al., 2008; Lavie et al., 2011, 2012; Florez and Castillo-Florez, 2012). Unfortunately, while we have data on lean mass, fat mass and percent fat, we do not have data on CVD and Type 2 DM mortality outcomes in our subjects, such that we cannot test the ‘obesity paradox’ concept in our population. Taken together, our data highlight the need for accurate assessment of fat and lean body tissues and the need for weight management recommendations based on the F/L ratio.
New markers of visceral adiposity, such as the Lipid Accumulation Product (LAP; calculated as [waist circumference (cm) −58] × [triglycerides (millimoles per liter)]) (Wehr et al., 2011) and Visceral Adiposity Index (VAI; based on waist circumference, BMI, triglycerides and HDL cholesterol levels) (Amato et al., 2010; Oh et al., 2013), are emerging as additional important, noninvasive and inexpensive markers of visceral adipose tissue dysfunction. Unfortunately, we did not have lipid data to be able to calculate LAP and VAI.
PCOS is characterized by an abnormal endocrine milieu, including hyperandrogenism, and hyperestrogenemia, although data on the effect of endogenous sex hormones on body composition are conflicting. High testosterone levels have been associated with increased lean mass and increased fat mass in post-menopausal women (Rariy et al., 2011), while simultaneously associated with increased lean mass but decreased fat mass among men (Bhasin et al., 1996; Rariy et al., 2011). The impact of endogenous estradiol levels on body composition and IR in both men and women is largely unknown due to insufficient prospective data (Ding et al., 2006) and the extent to which differences in estrogen levels contribute to these inconsistencies in body composition and IR remains to be investigated.
Finally, we should recognize that as for all studies, our study has potential limitations. It is cross-sectional in design, and therefore does not necessarily imply direct causality concerning the association between the F/L ratio and metabolic dysfunction. For similar reasons, we are unable to determine whether the increased levels of androgens and insulin precede the alteration in the F/L ratio or vice versa. Body composition was measured by BIA, which while useful for larger cohort or populational studies, is less sensitive and accurate when compared with more established methods such as dual-energy X-ray absorptiometry (DXA) or magnetic resonance imaging (MRI) (Lee and Gallagher, 2008). However, BIA has been shown to be a significantly more cost-effective method of measuring body composition than DXA or MRI, and provides comparable estimates of body composition while avoiding the risks of radiation, although the risk of radiation associated with DXA is very low (Wang et al., 2013). In addition, regional tissue sub-compartments, which have been shown to have independent associations with metabolic variables (Heshka et al., 2008), were not able to be measured in this study. Finally, we must recognize that paired enrollment of PCOS and control subjects matched by BMI may not have been sufficient to eliminate completely the possibility of residual confounding of the data by BMI, although we believe this residual effect to be limited at best.
In conclusion, compared with controls of similar BMI, women with PCOS demonstrate a higher amount of total body fat mass relative to lean mass (i.e. a higher F/L ratio). Differences in the F/L ratio may in part account for the observed variation in metabolic dysfunction in women with PCOS compared with controls, and may provide potential body composition correlates of metabolic features in PCOS. The physiological processes producing these deviations in tissue distribution and their metabolic dysfunction, including the mechanisms underlying noninsulin-mediated glucose transport (glucose effectiveness) and its association with the F/L ratio, warrant further investigation.
Authors’ roles
U.E. designed the study, identified and phenotyped subjects, researched data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. M.P. and R.M. identified and phenotyped subjects, obtained study samples, researched data, and reviewed and edited the manuscript. R.A. designed the study, identified and phenotyped subjects, researched data, wrote the manuscript, contributed to discussion, and reviewed and edited the manuscript. R.A. is the guarantor of this work and, as such takes responsibility for the data integrity and accuracy of the data analysis.
Funding
This work was supported by grants R01-DK073632 and R01-HD29364 from the NIH and an endowment of the Helping Hand of Los Angeles, Inc. (to R.A.).
Conflict of interest
None declared.
Acknowledgements
The authors are grateful to the PCOS patients and control subjects who participated in this study for their participation and donating their samples.
References
- Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, Galluzzo A for the AlkaMeSy Study Group. Visceral Adiposity Index; A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33:920–922. doi: 10.2337/dc09-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004a;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- Azziz R, Sanchez LA, Knochenhauer ES, Moran C, Lazenby J, Stephens KS, Taylor K, Boots LR. Androgen excess in women: Experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004b;89:453–462. doi: 10.1210/jc.2003-031122. [DOI] [PubMed] [Google Scholar]
- Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, et al. Task Force on the Phenotype of the Polycystic Ovary Syndrome of The Androgen Excess and PCOS Society. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456–488. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- Bergman RN. Lilly lecture. Toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes. 1989;38:1512–1517. doi: 10.2337/diab.38.12.1512. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- Bhattacharya SM, Jha A. Comparative study of the therapeutic effects of oral contraceptive pills containing desogestrel, cyproterone acetate, and drospirenone in patients with polycystic ovary syndrome. Fertil Steril. 2012;98:1053–1059. doi: 10.1016/j.fertnstert.2012.06.035. [DOI] [PubMed] [Google Scholar]
- Carmina E, Guastella E, Longo RA, Rini GB, Lobo RA. Correlates of increased lean muscle mass in women with polycystic ovary syndrome. Eur J Endocrinol. 2009;1:583–589. doi: 10.1530/EJE-09-0398. [DOI] [PubMed] [Google Scholar]
- Chazenbalk G, Trivax BS, Yildiz BO, Bertolotto C, Mathur R, Heneidi S, Azziz R. Regulation of adiponectin secretion by adipocytes in the polycystic ovary syndrome: role of tumor necrosis factor-alpha. J Clin Endocrinol Metab. 2010;95:935–942. doi: 10.1210/jc.2009-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazenbalk G, Chen YH, Heneidi S, Lee JM, Pall M, Chen YD, Azziz R. Abnormal expression of genes involved in inflammation, lipid metabolism, and Wnt signaling in the adipose tissue of polycystic ovary syndrome. J Clin Endocrinol Metab. 2012;97:E765–E770. doi: 10.1210/jc.2011-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Heneidi S, Lee JM, Layman LC, Stepp DW, Gamboa GM, Chen BS, Chazenbalk G, Azziz R. miRNA-93 inhibits GLUT4 and is overexpressed in adipose tissue of Polycystic Ovary Syndrome patients and women with insulin resistance. Diabetes. 2013;62:2278–2286. doi: 10.2337/db12-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow S, Shao J, Wang H. Sample Size Calculations in Clinical Research. 2nd edn. Chapman and Hall/CRC, Newyork; 2008. pp. 243–244. [Google Scholar]
- Comerford KB, Almario RU, Kim K, Karakas SE. Lean mass and insulin resistance in women with polycystic ovary syndrome. Metabolism. 2012;61:1256–1260. doi: 10.1016/j.metabol.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Coviello AD, Legro RS, Dunaif A. Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2006;91:492–497. doi: 10.1210/jc.2005-1666. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Gunnarsson R, Bjorkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in non-insulin dependent (type II) diabetes mellitus. J Clin Invest. 1985;76:149–155. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Després JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, Rodés-Cabau J, Bertrand OF, Poirier P. Abdominal obesity and the metabolic syndrome contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–1049. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- DeUgarte CM, Bartolucci AA, Azziz R. Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil Steril. 2005;83:1454–1460. doi: 10.1016/j.fertnstert.2004.11.070. [DOI] [PubMed] [Google Scholar]
- Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes:: A systematic review and meta-analysis. JAMA. 2006;295:1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- Douchi T, Yamamoto S, Oki T, Maruta K, Kuwahata R, Nagata Y. Serum androgen levels and muscle mass in women with polycystic ovary syndrome. Obstet Gynecol. 1999;94:337–340. doi: 10.1016/s0029-7844(99)00311-7. [DOI] [PubMed] [Google Scholar]
- Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- Ezeh U, Pall M, Mathur R, Dey D, Berman D, Chen IY, Dumesic DA, Azziz R, et al. Effects of endogenous androgens and abdominal fat distribution on the interrelationship between insulin and non-insulin-mediated glucose uptake in females. J Clin Endocrinol Metab. 2013a;98:1541–1548. doi: 10.1210/jc.2012-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeh U, Yildiz BO, Azziz R. Referral bias in defining the phenotype and prevalence of obesity in polycystic ovary syndrome. J Clin Endocrinol Metab. 2013b;98:E1088–E1096. doi: 10.1210/jc.2013-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah-Eways L, Reyna R, Knochenhauer ES, Bartolucci AA, Azziz R. Glucose action and adrenocortical biosynthesis in women with polycystic ovary syndrome. Fertil Steril. 2004;281:120–125. doi: 10.1016/j.fertnstert.2003.05.008. [DOI] [PubMed] [Google Scholar]
- Florez H, Castillo-Florez S. Beyond the obesity paradox in diabetes: fitness, fatness, and mortality. JAMA. 2012;308:619–620. doi: 10.1001/jama.2012.9776. [DOI] [PubMed] [Google Scholar]
- Forbes GB, Welle SL. Lean body mass in obesity. Int J Obes (London) 1983;7:99–107. [PubMed] [Google Scholar]
- He J, Watkins S, Kelley DE. Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes. 2001;50:817–823. doi: 10.2337/diabetes.50.4.817. [DOI] [PubMed] [Google Scholar]
- Heshka S, Ruggiero A, Bray GA, Foreyt J, Kahn SE, Lewis CE, Saad M, Schwartz AV. Altered body composition in type 2 diabetes mellitus. Int J Obes (Lond) 2008;32:780–787. doi: 10.1038/sj.ijo.0803802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchengaast S, Huber J. Body composition characteristics and body fat distribution in lean women with polycystic ovary syndrome. Hum Reprod. 2001;16:1255–1260. doi: 10.1093/humrep/16.6.1255. [DOI] [PubMed] [Google Scholar]
- Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83:3078–3082. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- Kuk JL, Kilpatrick K, Davidson LE, Hudson R, Ross R. Whole-body skeletal muscle mass is not related to glucose tolerance or insulin sensitivity in overweight and obese men and women. Appl Physiol Nutr Metab. 2008;33:769–774. doi: 10.1139/H08-060. [DOI] [PubMed] [Google Scholar]
- Lavie CJ, De Schutter A, Patel D, Artham SM, Milani RV. Body composition and coronary heart disease mortality—an obesity or a lean paradox? Mayo Clin Proc. 2011;86:857–864. doi: 10.4065/mcp.2011.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie CJ, De Schutter A, Patel DA, Romero-Corral A, Artham SM, Milani RV. Body composition and survival in stable coronary heart disease. Impact of lean mass index and body fat in the “obesity paradox”. J Am Coll Cardiol. 2012;60:1374–1380. doi: 10.1016/j.jacc.2012.05.037. [DOI] [PubMed] [Google Scholar]
- Lear SA, Kohli S, Bondy GP, Tchernof A, Sniderman AD. Ethnic variation in fat and lean body mass and the association with insulin resistance. J Clin Endocrinol Metab. 2009;94:4696–4702. doi: 10.1210/jc.2009-1030. [DOI] [PubMed] [Google Scholar]
- Lee SY, Gallagher D. Assessment methods in human body composition. Curr Opin Clin Nutr Metab Care. 2008;11:566–572. doi: 10.1097/MCO.0b013e32830b5f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Miller JP, Pratley RE, Goldberg AP, Gordon P, Rubin M, Treuth MS, Ryan AS, Hurley BF. Strength training increases insulin action in healthy 50- to 65-yr-old men. J Appl Physiol. 1994;77:1122–1127. doi: 10.1152/jappl.1994.77.3.1122. [DOI] [PubMed] [Google Scholar]
- Nam SY, Kim KR, Cha BS, Song YD, Lim SK, Lee HC, Huh KB. Low-dose growth hormone treatment combined with diet restriction decreases insulin resistance by reducing visceral fat and increasing muscle mass in obese type 2 diabetic patients. Int J Obes Relat Metab Disord. 2001;25:1101–1107. doi: 10.1038/sj.ijo.0801636. [DOI] [PubMed] [Google Scholar]
- Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, Grundy SM, Khera A, McGuire DK, de Lemos JA. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308:1150–1159. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH. Bioelectrical impedance analysis in body composition measurement: Proceedings of a National Institutes of Health Technology Assessment Conference. Am J Clin Nutr. 1996;64(Suppl. 3):S524–S532. doi: 10.1093/ajcn/64.3.387S. [DOI] [PubMed] [Google Scholar]
- Oh JY, Sung YA, Lee HJ. The visceral adiposity index as a predictor of insulin resistance in young women with polycystic ovary syndrome. Obesity (Silver Spring) 2013;21:1690–1694. doi: 10.1002/oby.20096. [DOI] [PubMed] [Google Scholar]
- Preis SR, Massaro JM, Robins SJ, Hoffmann U, Vasan RS, Irlbeck T, Meigs JB, Sutherland P, D'Agostino RB, Sr, O'Donnell CJ, et al. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham heart study. Obesity (Silver Spring) 2010;18:2191–2198. doi: 10.1038/oby.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rariy CM, Ratcliffe SJ, Weinstein R, Bhasin S, Blackman MR, Cauley JA, Robbins J, Zmuda JM, Harris TB, Cappola AR. Higher serum free testosterone concentration in older women is associated with greater bone mineral density, lean body mass, and total fat mass: the cardiovascular health study. J Clin Endocrinol Metab. 2011;96:989–996. doi: 10.1210/jc.2010-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Corral A, Lopez-Jimenez F, Sierra-Johnson J, Somers VK. Differentiating between body fat and lean mass—how should we measure obesity? Nat Clin Pract Endocrinol Metab. 2008;4:322–323. doi: 10.1038/ncpendmet0809. [DOI] [PubMed] [Google Scholar]
- Schutz Y, Kyle UU, Pichard C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18–98 y. Int J Obes Relat Metab Disord. 2002;26:953–960. doi: 10.1038/sj.ijo.0802037. [DOI] [PubMed] [Google Scholar]
- Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and pre-diabetes. Findings from the Third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2011;96:2898–2903. doi: 10.1210/jc.2011-0435. [DOI] [PubMed] [Google Scholar]
- Stanfield KM, Wells JC, Fewtrell MS, Frost C, Leon DA. Differences in body composition between infants of South Asian and European ancestry: the London Mother and Baby Study. Int J Epidemiol. 2012;41:1409–1418. doi: 10.1093/ije/dys139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen PF, Nilas L, Nørgaard K, Jensen JE, Madsbad S. Obesity, body composition and metabolic disturbances in polycystic ovary syndrome. Hum Reprod. 2008;23:2113–2121. doi: 10.1093/humrep/den211. [DOI] [PubMed] [Google Scholar]
- Van Der Heijden GJ, Wang ZJ, Chu Z, Toffolo G, Manesso E, Sauer PJ, Sunehag AL. Strength exercise improves muscle mass and hepatic insulin sensitivity in obese youth. Med Sci Sports Exerc. 2010;42:1973–1980. doi: 10.1249/MSS.0b013e3181df16d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JG, Zhang Y, Chen HE, Li Y, Cheng XG, Xu L, Guo Z, Zhao XS, Sato T, Cao QY, et al. Comparison of two bioelectrical impedance analysis devices with dual energy X-ray absorptiometry and magnetic resonance imaging in the estimation of body composition. J Strength Cond Res. 2013;27:236–243. doi: 10.1519/JSC.0b013e31824f2040. [DOI] [PubMed] [Google Scholar]
- Wannamethee SG, Shaper AG, Lennon L, Whincup PH. Decreased muscle mass and increased central adiposity are independently related to mortality in older men. Am J Clin Nutr. 2007;86:1339–1346. doi: 10.1093/ajcn/86.5.1339. [DOI] [PubMed] [Google Scholar]
- Wehr E, Gruber HJ, Giuliani A, Möller R, Pieber TR, Obermayer-Pietsch B. The lipid accumulation product is associated with impaired glucose tolerance in PCOS women. J Clin Endocrinol Metab. 2011;96:E986–E990. doi: 10.1210/jc.2011-0031. [DOI] [PubMed] [Google Scholar]
- Woods KS, Reyna R, Azziz R. Effect of oral micronized progesterone on androgen levels in women with polycystic ovary syndrome. Fertil Steril. 2002;77:1125–1127. doi: 10.1016/s0015-0282(02)03119-9. [DOI] [PubMed] [Google Scholar]
- Zawadzki J, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens J, Haseltine F, Marrian G, editors. Polycystic Ovary Syndrome. Current Issues in Endocrinology and Metabolism. Vol. 4. Boston: Blackwell Scientific; 1992. pp. 377–384. [Google Scholar]