Abstract
STUDY QUESTION
What is the relationship between semen parameters and mortality in men evaluated for infertility?
SUMMARY ANSWER
Among men undergoing an infertility evaluation, those with abnormal semen parameters have a higher risk of death, suggesting a possible common etiology between infertility and mortality.
WHAT IS KNOWN ALREADY
Conflicting data exist that suggest either an inverse relationship or no relationship between semen quality and mortality.
STUDY DESIGN, SIZE, DURATION
A study cohort was identified from two centers, each specializing in infertility care. In California, we identified men with data from 1994 to 2011 in the Stanford Reproductive Endocrinology and Infertility semen database. In Texas, we identified men with data from 1989 to 2009 contained in the andrology database at the Baylor College of Medicine Special Procedures Laboratory who were evaluated for infertility. Mortality was determined by data linkage to the National Death Index or Social Security Death Index. Comorbidity was estimated based on calculation of the Charlson Comorbidity Index or Centers for Medicare & Medicaid Services-Hierarchical Condition Categories Model.
PARTICIPANTS/MATERIALS, SETTING, METHODS
In all, 11 935 men were evaluated for infertility from 1989 to 2011. During 92 104 person years of follow-up, 69 of 11 935 men died (0.58%). The mean age at infertility evaluation was 36.6 years with a mean follow-up of 7.7 years.
MAIN RESULTS AND THE ROLE OF CHANCE
Compared with the general population, men evaluated for infertility had a lower risk of death with 69 deaths observed compared with 176.7 expected (Standardized mortality rate 0.39, 95% CI 0.30–0.49). When stratified by semen parameters, however, men with impaired semen parameters (i.e. male factor infertility) had significantly higher mortality rates compared with men with normal parameters (i.e. no male factor infertility). Low semen volume, sperm concentration, sperm motility, total sperm count and total motile sperm count were all associated with higher risk of death. In contrast, abnormal sperm morphology was not associated with mortality. While adjusting for current health status attenuated the association between semen parameters and mortality, men with two or more abnormal semen parameters still had a 2.3-fold higher risk of death compared with men with normal semen (95% CI 1.12–4.65).
LIMITATIONS, REASONS FOR CAUTION
Our cohort represents infertile men, which may limit generalizability. As comorbidity relied on administrative data, granular information on each man regarding infertility diagnosis and lifestyle factors was unavailable.
WIDER IMPLICATIONS OF THE FINDINGS
Men with impaired semen parameters have an increased mortality rate in the years following an infertility evaluation suggesting semen quality may provide a marker of health.
STUDY FUNDING/COMPETING INTEREST(S)
This study is supported in part by P01HD36289 from the Eunice Kennedy Shriver National Institute for Child Health and Human Development, National Institutes of Health (to D.J.L. and L.I.L.). The project was also partially supported by an NIH CTSA award number UL1 RR025744. None of the authors has any conflict of interest to declare.
Keywords: male infertility, oligospermia, fertility
Introduction
Approximately 15% of couples are unable to conceive after 1 year of unprotected intercourse and are labeled infertile with a male factor etiology identified in 30–50% (Thonneau et al., 1991; Chandra et al., 2005). Advances in assisted reproductive technologies have allowed men to overcome severe reproductive impairments and still father offspring. While it is thought that there may be health implications for these offspring, less is understood about the health implications of a diagnosis of impaired fecundity for a man (Davies et al., 2012).
Authors have hypothesized that genetic, hormonal, environmental/lifestyle or in utero factors could explain a link between a man's reproductive and somatic health. Indeed, as up to 15% of a man's genome is devoted to reproduction, defects in fertility may manifest as other health impairments (Matzuk and Lamb 2008). For example, defects in DNA repair will impair both meiosis and mitosis, thus conceivably affecting spermatogenesis and increasing the likelihood of carcinogenesis (Mukherjee et al., 2010). Another theory posits that hormonal aberrations may explain the association between testicular failure and mortality, as impaired androgen states have been linked to infertility and cardiovascular mortality (Andersson et al., 2004; Khaw et al., 2007).
Importantly, longitudinal studies suggest impaired health outcomes in men years after a diagnosis of male factor infertility. Groups in Europe and the USA identified an increased incidence of cancer in men with impaired fertility many years after conceptive efforts ceased (Jacobsen et al., 2000; Walsh et al., 2010). To date, there is limited data exploring mortality rates among men with a history of infertility. Investigators have demonstrated increased mortality rates among Danish men with impaired semen parameters who were evaluated for infertility between 1963 and 2001 (Jensen et al., 2009). In contrast, a German group demonstrated no change in mortality for oligospermic or azoospermic men evaluated for infertility between 1949 and 1985 (Groos et al., 2006). Thus, while infertile men are generally counseled about a higher risk of testicular germ cell tumors, the association with overall mortality is less certain.
In addition, comorbid conditions are known to impair semen quality and impact mortality (Berrington de Gonzalez et al., 2010; Sermondade et al., 2013). Thus a man's health status at the time of infertility evaluation must be examined when exploring the relationship between semen quality and mortality.
Given the biologic plausibility of this association and the conflicting data in the literature, we sought to determine if semen parameters correlated with mortality in a contemporary cohort of US men evaluated for infertility.
Methods
Study population
After Institutional Review Board approvals, a study cohort was identified from two centers, each specializing in infertility care. In California, we identified men with data from 1994 to 2011 in the Stanford Reproductive Endocrinology and Infertility semen database. The clinic evaluates and treats infertile couples with both male and female infertility. The laboratory performs a high volume of semen analyses for fertility evaluations and sperm preparations for use with assisted reproductive technologies.
In Texas, we identified men with data from 1989 to 2009 contained in the andrology database at the Baylor College of Medicine Special Procedures Laboratory who were evaluated for infertility. The Men's Health clinic (run by a urologist) evaluates and treats men with male factor infertility or men presenting for a fertility evaluation. The laboratory performs semen analyses for fertility evaluations and sperm preparation for cryopreservation or intrauterine insemination.
At both centers, men evaluated for infertility were self-referred or referred by a gynecologist, urologist or reproductive endocrinologist. For men with multiple semen analyses, only the first test was used in the present study. Men with a history of a vasectomy were excluded from the study population. The methods used for analysis of semen (sperm concentration, motility and volume) have been previously described (Coetzee et al., 1999; World Health Organization., 1999). The California cohort also included morphology scoring as described by Kruger et al. (1988). Total sperm count was calculated by multiplying volume × sperm concentration. Total motile sperm count was calculated by multiplying volume × concentration × motility. We did identify a trend toward improved semen motility in the later years in the California cohort. All other parameters in California and all parameters in Texas remained constant over the time period. Importantly, inclusion of evaluation year did not meaningfully impact our results. We limited our analysis to reproductive age men (20–50) to limit the association between age and semen quality. In addition, the California cohort was linked to administrative data to obtain information on patients' comorbidities identified using ICD-9-CM codes. The score on the Charlson Comorbidity Index (CCI) was calculated using the modifications of Quan et al. (2005). Risk adjustment for health status was also performed using Centers for Medicare & Medicaid Services-Hierarchical Condition Categories (CMS-HCC) Model developed for ambulatory patients (Pope et al., 2004). Comorbidity data were not available for the Texas cohort.
Outcome ascertainment
For the California cohort, death was identified through linkage with the Social Security Death Index (SSDI). The SSDI is a database maintained by the United States Social Security Administration which contains information about persons who had Social Security numbers and whose deaths were reported to the Social Security Administration from 1962 to the present. All eligible men in the Texas database were linked to the National Death Index (NDI). The NDI is a central computerized index of death record information compiled from data submitted to the National Center for Health Statistics (NCHS) from each state's vital statistics office.
Automated, probabilistic matching was performed using social security number, first name, last name, middle initial and date of birth. Both the SSDI and NDI provide equivalent information regarding death status and date of death with >90% case ascertainment (Williams et al., 1992; Buchanich et al., 2005).
Statistical analysis
Men accrued at risk time from their initial semen analysis until death or 31 December 2010 (the final year that complete death data was available in Texas) or 31 December 2011 (the final year that complete death data was available in California). The rate of death in our cohort was compared with the general US population after calculating the expected number of deaths by multiplying the number of years at risk by the 5-year age strata death rates from the NCHS (Murphy et al., 2013). Standardized mortality rates (SMRs) were calculated by dividing the observed number of deaths by the expected number of deaths. Analyses were performed on the entire cohort as well as subgroups of infertile men.
We also analyzed the risk of death in infertile men after stratifying by semen parameters using a Cox proportional hazards regression model while adjusting for age, year of evaluation, center of evaluation and either the CCI or CMS-HCC risk adjustment scores. Abnormal semen parameters were defined based on the WHO fifth edition of the manual on semen analyses (Cooper et al., 2010). Given that morphology data were only available in California, the analysis examining the total number of semen abnormalities was limited to semen volume, sperm concentration and sperm motility. Comparison between Kaplan–Meier curves was performed using log rank function. All P-values were two sided with P < 0.05 considered statistically significant. Analyses were performed using SAS (version 9.3, SAS Institute, Inc., Cary, NC, USA).
Results
During 92 104 person years of follow-up, 69 of 11 935 men died (0.58%). The mean age at infertility evaluation was 36.6 years with a mean follow-up of 7.7 years. The mean age at death was 44.1 years. Characteristics are listed in Table I. The California men were older than the Texas men (37.1 versus 35.0) at enrollment. Over 97% of the California men had a Charlson comorbidity score of zero.
Table I.
Characteristics of men evaluated for infertility in Texas and California.
| Characteristic | Cohort |
||
|---|---|---|---|
| Texas | California | ||
| n | 2929 | 9006 | |
| Age (years) at SA, mean (SD) | 35.03 (5.8) | 37.05 (5.1) | |
| Age (years) at SA, n (%) | 20–29 | 584 (19.9) | 648 (7.2) |
| 30–39 | 1777 (60.7) | 5812 (64.53) | |
| 40–50 | 568 (19.4) | 2546 (28.3) | |
| Age of death (years), mean (SD) | 42.7 (6.2) | 44.8 (7.8) | |
| Age at last follow-up or death (years), mean (SD) | 43.81 (7.4) | 44.4 (6.1) | |
| Age at last follow-up, n (%) | <20 | 0 | 12 (0.11) |
| 20–29 | 68 (2.2) | 49 (0.5) | |
| 30–39 | 884 (30.2) | 2183 (24.2) | |
| 40–49 | 1365 (46.6) | 5099 (56.62) | |
| 50–59 | 558 (19.1) | 1619 (18.0) | |
| 60–69 | 54 (1.8) | 56 (0.6) | |
| 70–79 | 0 | 0 | |
| Follow-up time (years), mean (SD) | 8.78 (4.6) | 7.37 (3.4) | |
| Follow-up time (years), n (%) | 0–4 | 694 (23.7) | 2645 (29.4) |
| 5–9 | 1053 (36.0) | 4133 (45.9) | |
| 10+ | 1182 (40.4) | 2226 (24.7) | |
| Year of evaluation | ≤2000 | 1191 (40.7) | 1585 (17.2) |
| 2001–2005 | 1050 (35.9) | 3848 (42.7) | |
| ≥2006 | 688 (23.5) | 3553 (39.5) | |
| Charlson Comorbidity Index | 0 | 8794 (97.7) | |
| 1+ | 81 (0.9) | ||
| 2 | 93 (1.0) | ||
| ≥3 | 38 (0.4) | ||
| Semen parameters | |||
| Volume (ml) | Mean (SD) | 2.65 (1.5) | 3.09 (1.82) |
| <1.5 ml | 552 (18.9) | 826 (9.5) | |
| Concentration (106/ml) | Mean (SD) | 29.59 (45.3) | 64.73 (51.4) |
| <15 × 106/ml | 1576 (53.8) | 1059 (12.6) | |
| Motility (%) | Mean (SD) | 35.22 (25.3) | 47.77 (23.8) |
| <40% | 1378 (47.1) | 3064 (36.5) | |
| Total sperm count (106) | Mean (SD) | 76.14 (126.9) | 193.94 (179.7) |
| <39 × 106 | 1666 (56.9) | 1226 (14.6) | |
| Total motile sperm count (106) | Mean (SD) | 41.54 (78.2) | 110.67 (126.9) |
| <9 × 106 | 1468 (50.2) | 1252 (14.9) | |
| Morphology (% normal, Kruger) | Mean (SD) | 10.61 (6.1) | |
| <14% | 4040 (71.0) | ||
Abnormal semen levels are defined by the World Health Organization reference values for human semen, fifth edition.
SA, semen analysis.
Reflecting the clinical focus differences between the clinics in Texas and California, men from Texas had significantly lower semen volumes, sperm counts and sperm motility (Table I). This was reflected in higher rates of semen abnormalities as defined by the WHO fifth edition semen analysis manual in the men from Texas compared with California (Cooper et al., 2010).
Despite these differences in patient characteristics and baseline semen parameters, the SMRs were similarly low at both centers. In the men evaluated for infertility in Texas, 21 deaths were observed compared with the 47.0 expected (SMR 0.45, 95% CI 0.28–0.68). In the California cohort, 48 deaths were observed compared with the 129.7 expected (SMR 0.37, 95% CI 0.27–0.49, Table II).
Table II.
Standardized mortality rates (SMR) stratified state of evaluation and semen parameters in men evaluated for infertility.
| Characteristic | n | Observed deaths | Expected deaths | Standardized mortality rate (95% CI) | |
|---|---|---|---|---|---|
| Center of evaluation | Texas | 2929 | 21 | 47.0 | 0.45 (0.28–0.68) |
| California | 9006 | 48 | 129.7 | 0.37 (0.27–0.49) | |
| Combined | 11 935 | 69 | 176.7 | 0.39 (0.30–0.49) | |
| Semen parameter | |||||
| Volume (ml) | <1.5 | 1378 | 15 | 21.5 | 0.70 (0.39–1.15) |
| ≥1.5 | 10 210 | 51 | 149.2 | 0.34 (0.25–0.45) | |
| Concentration (106/ml) | <15 | 2635 | 28 | 40.2 | 0.70 (0.46-1.01) |
| ≥15 | 8718 | 36 | 127.1 | 0.28 (0.20–0.39) | |
| Motility (%) | <40 | 4442 | 38 | 69.0 | 0.55 (0.39–0.76) |
| ≥40 | 6887 | 26 | 97.8 | 0.27 (0.17-0.39) | |
| Total sperm (106) | <39 | 2892 | 28 | 44.4 | 0.63 (0.42-0.91) |
| ≥39 | 8448 | 36 | 122.8 | 0.29 (0.21–0.41) | |
| Total motile sperm count (106) | <9 | 2720 | 26 | 42.0 | 0.62 (0.40–0.91) |
| ≥9 | 8590 | 38 | 124.7 | 0.30 (0.22–0.42) | |
| Morphology (% normal forms – Kruger) | <14 | 4070 | 23 | 62.1 | 0.37 (0.23–0.56) |
| ≥14 | 1662 | 8 | 20.9 | 0.38 (0.16–0.75) | |
| Number of semen abnormalities (i.e. volume, concentration, motility) | 0 | 5687 | 20 | 79.4 | 0.25 (0.15–0.39) |
| 1 | 3318 | 16 | 51.8 | 0.31 (0.18–0.50) | |
| 2+ | 2391 | 29 | 36.7 | 0.79 (0.53–1.13) |
Abnormal semen levels are defined by the World Health Organization reference values for human semen, fifth edition. Standardized mortality rates calculated based on US data (Murphy et al., 2013).
Next, we stratified the men based on semen parameters. Men with male factor infertility were identified if they possessed abnormal semen parameters as defined by the WHO fifth edition semen analysis manual (Cooper et al., 2010). Men who had been evaluated for infertility but were found to have normal volume, concentration, motility, forward progression, total sperm count and total motile sperm count (i.e. no male factor infertility) had SMRs significantly less than unity. While men evaluated for infertility who were found to have low motility, forward progression, total sperm count, total motile sperm count and morphology (i.e. male factor infertility) had SMRs less than unity, the SMR was ∼2-fold higher than men with normal semen parameters (Table II). Moreover, as the number of semen abnormalities increased, so did the SMR.
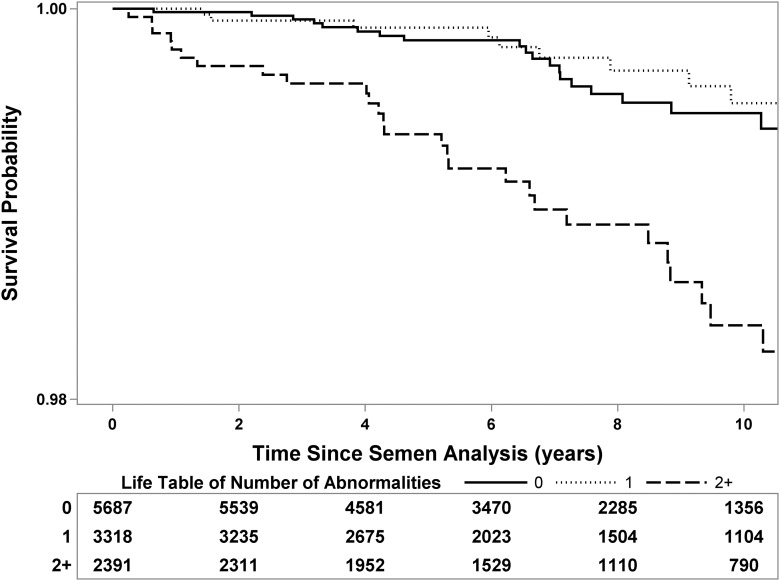
When directly comparing men with normal and abnormal semen parameters, men with abnormal parameters (except morphology) had a higher risk of death (Table III). For example, infertile men with oligospermia (concentration <15 M/ml) had 2.2 times higher risk of death compared with men with normal sperm concentration. While the hazard ratios appeared higher for the Texas men for most abnormal semen parameters, no significant difference was identified based on location. In addition, the risk of death rose as the number of semen abnormalities (i.e. semen volume, concentration and motility) increased. (Fig. 1). Because many men, particularly in the Texas cohort, had multiple semen abnormalities, the risk associated with a given abnormality may not be due to that abnormality alone since many men in the group will have other abnormalities as well. Indeed, 62% of men in the Texas cohort with abnormalities had two or more versus 30% in the California cohort illustrating the difference in semen quality between the two.
Table III.
Hazard ratio (95% CI) models represent comparison in deaths between men with normal and abnormal individual semen parameters.
| Center | Semen parameter | Model | Hazard ratio (95% CI) | |
|---|---|---|---|---|
| Texas | Volume: <1.5 versus ≥1.5 ml (Ref) (n; 552 versus 2377) |
Unadjusted | 2.21 (0.89–5.52) | 0.09 |
| 1 | 2.22 (0.89–5.53) | 0.09 | ||
| Concentration: <15 versus ≥15 × 106 /ml (Ref) (n; 1576 versus 1353) |
Unadjusted | 8.06 (1.88–34.58) | 0.01 | |
| 1 | 8.09 (1.88–34.77) | <0.01 | ||
| Motility: <40 versus ≥40% (Ref) (n; 1378 versus 1547) |
Unadjusted | 5.08 (1.71–15.098) | <0.01 | |
| 1 | 5.08 (1.71–15.10) | <0.01 | ||
| Total sperm: <39 versus ≥39 × 106 (Ref) (n; 1666 versus 1263) |
Unadjusted | 15.36 (2.06–114.44) | 0.01 | |
| 1 | 15.38 (2.06–114.63) | <0.01 | ||
| Total motile sperm count <9 versus ≥9 × 106 (Ref) (n; 1468 versus 1457) |
Unadjusted | 6.01 (1.77–20.41) | <0.01 | |
| 1 | 6.02 (1.77–20.43) | <0.01 | ||
| Number of semen parameters (volume, concentration, motility) abnormal 1 versus 0 (Ref) (n; 757 versus 928) |
Unadjusted | 4.52 (0.51–40.46) | 0.18 | |
| 1 | 4.52 (0.51–40.42) | 0.18 | ||
| Number of semen parameters (volume, concentration, motility) abnormal 2+ versus 0 (Ref) (n; 1244 versus 928) |
Unadjusted | 12.22 (1.62–92.23) | 0.02 | |
| 1 | 12.23 (1.62–92.26) | 0.02 | ||
| California | Volume: <1.5 versus ≥1.5 ml (Ref) (n; 826 versus 7833) |
Unadjusted | 2.17 (1.01–4.66) | 0.05 |
| 1 | 2.02 (0.94–4.35) | 0.07 | ||
| 2 | 1.80 (0.83–3.92) | 0.14 | ||
| 3 | 2.06 (0.96–4.45) | 0.07 | ||
| Concentration: <15 versus ≥15 × 106/ml (Ref) (n; 1059 versus 7365) |
Unadjusted | 1.89 (0.91–3.94) | 0.09 | |
| 1 | 1.87 (0.90–3.91) | 0.09 | ||
| 2 | 1.64 (0.78–3.43) | 0.19 | ||
| 3 | 1.90 (0.91–3.97) | 0.09 | ||
| Motility: <40 versus ≥40% (Ref) (n; 3064 versus 5340) |
Unadjusted | 1.41 (0.77–2.57) | 0.26 | |
| 1 | 1.37 (0.75–2.50) | 0.31 | ||
| 2 | 1.29 (0.70–2.37) | 0.42 | ||
| 3 | 1.37 (0.75–2.53) | 0.31 | ||
| Total sperm: <39 versus ≥39 × 106 (Ref) (n; 1226 versus 7185) |
Unadjusted | 1.41 (0.65–3.04) | 0.38 | |
| 1 | 1.37 (0.63–2.95) | 0.43 | ||
| 2 | 1.16 (0.53–2.52) | 0.71 | ||
| 3 | 1.39 (0.64–3.01) | 0.40 | ||
| Total motile sperm count <9 versus ≥9 × 106 (Ref) (n; 1252 versus 7133) |
Unadjusted | 1.31 (0.61–2.82) | 0.49 | |
| 1 | 1.27 (0.59–2.74) | 0.54 | ||
| 2 | 1.15 (0.53–2.49) | 0.72 | ||
| 3 | 1.29 (0.60–2.79) | 0.51 | ||
| Morphology <14 versus ≥14% normal forms (Kruger) (Ref) (n; 4070 versus 1662) |
Unadjusted | 0.89 (0.40–2.00) | 0.78 | |
| 1 | 0.87 (0.39–1.96) | 0.74 | ||
| 2 | 0.84 (0.37–1.88) | 0.67 | ||
| 3 | 0.85 (0.38–1.92) | 0.70 | ||
| Number of semen parameters (volume, concentration, motility) abnormal 1 versus 0 (Ref) (n; 2561 versus 4759) |
Unadjusted | 1.02 (0.49–2.12) | 0.95 | |
| 1 | 0.98 (0.47–2.03) | 0.96 | ||
| 2 | 0.89 (0.43–1.85) | 0.75 | ||
| 3 | 1.0 (0.48–2.08) | 0.99 | ||
| Number of semen parameters (volume, concentration, motility) abnormal 2+ versus 0 (Ref) (n; 1147 versus 4759) |
Unadjusted | 2.64 (1.30–5.35) | 0.01 | |
| 1 | 2.53 (1.25–5.14) | 0.01 | ||
| 2 | 2.29 (1.12–4.65) | 0.02 | ||
| 3 | 2.57(1.26–5.23) | <0.01 | ||
| Combined | Volume: <1.5 versus ≥1.5 ml (Ref) (n; 1378 versus 10 210) |
Unadjusted | 2.12 (1.19–3.77) | <0.01 |
| 1 | 1.80 (0.83–3.92) | 0.14 | ||
| Concentration: <15 versus ≥15 × 106/ml (Ref) (n; 2635 versus 8718) |
Unadjusted | 2.20 (1.34–3.62) | <0.01 | |
| 1 | 1.64 (0.78–3.43) | 0.19 | ||
| Motility: <40 versus ≥40% (Ref) (n; 4442 versus 6887) |
Unadjusted | 2.05 (1.24–3.37) | <0.01 | |
| 1 | 1.29 (0.70–2.37) | 0.42 | ||
| Total sperm: <39 versus ≥39 × 106 (Ref) (n; 2892 versus 8448) |
Unadjusted | 1.98 (1.20–3.26) | <0.01 | |
| 1 | 1.16 (0.53–2.52) | 0.71 | ||
| Total motile sperm count <9 versus ≥9 × 106 (Ref) (n; 2720 versus 8590) |
Unadjusted | 1.90 (1.15–3.13) | 0.01 | |
| 1 | 1.15 (0.53–2.49) | 0.72 | ||
| Morphology <14 versus ≥14% normal forms (Kruger) (Ref) (n; 4070 versus 1662) |
Unadjusted | 0.89 (0.40–2.00) | 0.78 | |
| 1 | 0.84 (0.37–1.88) | 0.67 | ||
| Number of semen parameters (volume, concentration, motility) abnormal 1 versus 0 (Ref) (n; 3138 versus 5687) |
Unadjusted | 1.21 (0.63–2.34) | 0.57 | |
| 1 | 0.89 (0.43–1.85) | 0.75 | ||
| Number of semen parameters (volume, concentration, motility) abnormal 2+ versus 0 (Ref) (n; 2391 versus 5687) |
Unadjusted | 2.96 (1.67–5.25) | <0.01 | |
| 1 | 2.29 (1.12–4.65) | 0.02 |
Abnormal semen levels are defined by the World Health Organization reference values for human semen, fifth edition. Regression models: Unadjusted; 1 – adjusted for age, year of infertility of evaluation; 2 – Model 1 + Charlson comorbidity index; 3 – Model 1 + Centers for Medicare & Medicaid Services-Hierarchical Condition Categories score. In addition the combined data were adjusted for center of evaluation. (Ref, Reference group).
Figure 1.
Kaplan–Meier curve of survival following semen analysis for infertile men (stratified by number of semen abnormalities: volume <1.5 ml, concentration <15 M/ml, motility <40%). Group 1: No semen abnormalities (i.e. no male factor infertility) (solid), Group 2: One semen abnormality (dotted), Group 3: Two or more semen abnormalities (dashed). Survival table at bottom represents number of men available for analysis at each time point.
After accounting for baseline comorbidity scores (using CCI and CMS-HCC), the association between mortality and individual abnormal semen parameters was lost (Table III). However, men with two or more semen abnormalities still had an elevated risk of death in the years after an infertility evaluation (HR 2.29, 95% CI 1.12–4.65) although the risk was attenuated.
Discussion
The current report found that a man's semen quality was inversely associated with mortality. Men with impaired sperm counts, sperm motility or semen volume (i.e. male factor infertility) had higher mortality rates compared with men with normal semen quality. Moreover, the risk of death increased with an increasing number of semen parameters in the subfertile range. While current health conditions attenuated the association between isolated semen abnormalities and mortality, men with multiple semen abnormalities continued to have an elevated risk of death. However, men who had an infertility evaluation had a lower mortality rate compared with the general population.
Our finding of an increased mortality rates for men with impaired semen parameters compared with those with normal production is consistent with a prior Danish study (Jensen et al., 2009). The authors found an increased risk of death in men with impaired semen parameters and noted an inverse linear trend with sperm morphology or motility and mortality. In contrast to the current report, no relationship between sperm count and mortality was identified. Moreover, current health status was not assessed in the Danish study.
The only other study examining the relationship between sperm parameters and mortality found no overall association among infertile men in Germany (Groos et al., 2006). However, as many of the cohort were directly involved in or affected by World War II and its aftermath, the general applicability to a contemporary group is uncertain given the length of time since the war and the powerful impact that war may have on a people and environment. The current report represents a contemporary US cohort of men evaluated for infertility.
It is important to note that the cohort had a lower mortality rate compared with the general population, similar to the findings of the Danish study. This is perhaps not surprising given that men who seek infertility care represent higher socioeconomic and educated groups (Jenkins, 2005; Hotaling et al., 2012). Indeed, socioeconomic status indicators are associated with mortality (Lantz et al., 1998). In addition, men who seek infertility services have higher rates of marriage, which is also associated with lower mortality (Martinez et al., 2006; Hotaling et al., 2012). Based on these demographic factors, one may expect lower mortality rates in men seeking an infertility evaluation compared with the general population. In addition, similar to the ‘healthy worker effect,’ observed in occupational cohorts, men attempting to conceive likely represent a healthier group than the overall population (Park, 1996).
While the etiology of the association between fertility and mortality is uncertain, several plausible explanations exist including genetic, hormonal, developmental and lifestyle/behavior. As up to 15% of the male genome is involved in reproduction, it is likely that other nonprocreative processes may also be affected by aberrations in fertility (Matzuk and Lamb 2008). For example, defects in DNA repair will impair both meiosis and mitosis, thus conceivably affecting spermatogenesis and increasing the likelihood of carcinogenesis (Mukherjee et al., 2010).
Another theory posits a hormonal explanation. Men with infertility have lower circulating testosterone levels than fertile men (Andersson et al., 2004; Meeker et al., 2007). As hypogonadism is a risk factor for cardiovascular disease and mortality, such an explanation may link offspring number to cardiovascular death (Khaw et al., 2007; Laughlin et al., 2008). In addition, researchers hypothesize that disruptions that occur during fetal life can impair normal genital development and health phenotypes (Barker, 1995; Skakkebaek et al., 2001). Finally, a shared risk factor etiology through lifestyle characteristics may link fertility to health. For example, smoking impacts both fertility and lifespan (CDC, 2010; Li et al., 2011).
The current data support the complex relationship between fertility and health. While infertility alone may be a marker of diminished fitness, it may also represent the presence of chronic diseases which themselves may lead to impaired health later in life. As health can impact semen quality, it may be covert impaired health which impairs semen production and also leads to higher mortality rates in the subsequent years. Indeed, the attenuation of the relationship between semen quality and mortality after adjusting for baseline comorbidity suggests that current health may mediate this relationship. Furthermore, a common etiology between impaired overall and reproductive health is suggested by the current analysis.
Several important limitations warrant mention. As with many analyses that rely on administrative data, granular information on each man regarding infertility diagnosis and lifestyle factors was unavailable. We did utilize medical diagnoses to estimate current comorbidity status for this relatively healthy population using two separate measures of comorbidity: CCI and CMS-HCC. We chose the CCI as it is a valid and reliable index used by health researchers to assess the impact of comorbid disease status in health care databases. However, it was developed utilizing inpatient data on older patients, so applicability in the outpatient setting among younger men is less certain. The CMS-HCC method for risk adjustment provides the advantage of incorporating a more robust sample of diagnostic codes to understand subjects' current health across ambulatory settings and incorporates data from younger patients than CCI. While both methods of comorbidity produced similar estimates, the data remain bound by the limits of retrospective, administrative data. Case ascertainment also relied on registry data and may lead to underestimates of the true number of deaths (Williams et al., 1992; Buchanich et al., 2005). Furthermore, since cause of death was not available, we could not determine if specific etiologies (e.g. cardiovascular) were present at higher rates. In addition, comorbidity data were only available for the California cohort. Given the younger age of the Texas group and the limited power of each individual cohort, it is perhaps not surprising that similar SMRs were seen between the state specific cohorts despite the fact that higher rates of abnormal semen parameters were seen in the Texas cohort. In addition, the infertility databases utilized represent men who presented for infertility evaluation rather than an unselected group of men. Indeed, data suggest that men who seek infertility care represent higher socioeconomic status than the population as a whole and are likely healthier (Hotaling et al., 2012). As such, the current findings may not apply to the general population. In addition, the patients in the cohort were relatively young with average follow-up of 7.7 years, a time when death represents a rare event.
Nevertheless, the current finding represents the first US study to demonstrate a higher risk of death in men with severely impaired semen parameters (i.e. two or more abnormal semen parameters). Our multi-centered design, including one center specializing in male infertility, provides a representative sample of men evaluated for infertility in the USA. Despite a short follow-up and the youth of the cohort, we noticed significant divergence in risk of death based on semen parameters even after accounting for baseline health. While reassuring that the absolute risk of death remains low for men evaluated for infertility in the decade after an infertility evaluation, the current report suggests that further examination of the link between mortality and semen quality is warranted.
Authors' roles
M.L.E., L.I.L. and D.J.L conceived the study. M.L.E., L.I.L., D.J.L., S.L. and D.G. collected and cleaned the data. All authors assisted in data analysis and interpretation. M.L.E. drafted the manuscript. All authors critically reviewed, revised and approved the final manuscript.
Funding
This study is supported in part by P01HD36289 from the Eunice Kennedy Shriver National Institute for Child Health and Human Development, National Institutes of Health (to D.J.L. and L.I.L.). The project was also partially supported by an NIH CTSA award number UL1 RR025744.
Conflict of interest
None declared.
References
- Andersson AM, Jorgensen N, Frydelund-Larsen L, Rajpert-De Meyts E, Skakkebaek NE. Impaired Leydig cell function in infertile men: a study of 357 idiopathic infertile men and 318 proven fertile controls. J Clin Endocrinol Metab. 2004;89:3161–3167. doi: 10.1210/jc.2003-031786. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanich JM, Dolan DG, Marsh GM, Madrigano J. Underascertainment of deaths using social security records: a recommended solution to a little-known problem. Am J Epidemiol. 2005;162:193–194. doi: 10.1093/aje/kwi178. [DOI] [PubMed] [Google Scholar]
- CDC. Racial disparities in smoking-attributable mortality and years of potential life lost—Missouri, 2003–2007. MMWR Morb Mortal Wkly Rep. 2010;59:1518–1522. [PubMed] [Google Scholar]
- Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth. Vital Health Stat. 2005;23:1–160. [PubMed] [Google Scholar]
- Coetzee K, Kruger TF, Lombard CJ. Repeatability and variance analysis on multiple computer-assisted (IVOS) sperm morphology readings. Andrologia. 1999;31:163–168. doi: 10.1046/j.1439-0272.1999.00257.x. [DOI] [PubMed] [Google Scholar]
- Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- Davies MJ, Moore VM, Willson KJ, Van Essen P, Priest K, Scott H, Haan EA, Chan A. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366:1803–1813. doi: 10.1056/NEJMoa1008095. [DOI] [PubMed] [Google Scholar]
- Groos S, Krause W, Mueller UO. Men with subnormal sperm counts live shorter lives. Soc Biol. 2006;53:46–60. doi: 10.1080/19485565.2006.9989116. [DOI] [PubMed] [Google Scholar]
- Hotaling JM, Davenport MT, Eisenberg ML, VanDenEeden SK, Walsh TJ. Men who seek infertility care may not represent the general U.S. population: data from the National Survey of Family Growth. Urology. 2012;79:123–127. doi: 10.1016/j.urology.2011.09.021. [DOI] [PubMed] [Google Scholar]
- Jacobsen R, Bostofte E, Engholm G, Hansen J, Olsen JH, Skakkebaek NE, Moller H. Risk of testicular cancer in men with abnormal semen characteristics: cohort study. BMJ. 2000;321:789–792. doi: 10.1136/bmj.321.7264.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins RL. Ensuring access to education and services on infertility for the underserved. J Natl Cancer Inst Monogr. 2005:101–103. doi: 10.1093/jncimonographs/lgi016. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Jacobsen R, Christensen K, Nielsen NC, Bostofte E. Good semen quality and life expectancy: a cohort study of 43,277 men. Am J Epidemiol. 2009;170:559–565. doi: 10.1093/aje/kwp168. [DOI] [PubMed] [Google Scholar]
- Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, Welch A, Day N. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007;116:2694–2701. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 1988;49:112–117. doi: 10.1016/s0015-0282(16)59660-5. [DOI] [PubMed] [Google Scholar]
- Lantz PM, House JS, Lepkowski JM, Williams DR, Mero RP, Chen J. Socioeconomic factors, health behaviors, and mortality: results from a nationally representative prospective study of US adults. JAMA. 1998;279:1703–1708. doi: 10.1001/jama.279.21.1703. [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93:68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lin H, Cao J. Association between socio-psycho-behavioral factors and male semen quality: systematic review and meta-analyses. Fertil Steril. 2011;95:116–123. doi: 10.1016/j.fertnstert.2010.06.031. [DOI] [PubMed] [Google Scholar]
- Martinez GM, Chandra A, Abma JC, Jones J, Mosher WD. Fertility, contraception, and fatherhood: data on men and women from cycle 6 (2002) of the 2002 National Survey of Family Growth. National Center for Health Statistics. Vital Health Stat. 2006;23:1–142. [PubMed] [Google Scholar]
- Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14:1197–1213. doi: 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Godfrey-Bailey L, Hauser R. Relationships between serum hormone levels and semen quality among men from an infertility clinic. J Androl. 2007;28:397–406. doi: 10.2164/jandrol.106.001545. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Ridgeway AD, Lamb DJ. DNA mismatch repair and infertility. Curr Opin Urol. 2010;20:525–532. doi: 10.1097/MOU.0b013e32833f1c21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 2013;61:1–167. [PubMed] [Google Scholar]
- Park RM. The healthy worker survivor effect and mortality at two automotive engine manufacturing plants. Am J Ind Med. 1996;30:655–663. doi: 10.1002/(SICI)1097-0274(199612)30:6<655::AID-AJIM2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Pope GC, Kautter J, Ellis RP, Ash AS, Ayanian JZ, Lezzoni LI, Ingber MJ, Levy JM, Robst J. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004;25:119–141. [PMC free article] [PubMed] [Google Scholar]
- Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- Sermondade N, Faure C, Fezeu L, Shayeb AG, Bonde JP, Jensen TK, Van Wely M, Cao J, Martini AC, Eskandar M, et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update. 2013;19:221–231. doi: 10.1093/humupd/dms050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- Thonneau P, Marchand S, Tallec A, Ferial ML, Ducot B, Lansac J, Lopes P, Tabaste JM, Spira A. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989) Hum Reprod. 1991;6:811–816. doi: 10.1093/oxfordjournals.humrep.a137433. [DOI] [PubMed] [Google Scholar]
- Walsh TJ, Schembri M, Turek PJ, Chan JM, Carroll PR, Smith JF, Eisenberg ML, Van Den Eeden SK, Croughan MS. Increased risk of high-grade prostate cancer among infertile men. Cancer. 2010;116:2140–2147. doi: 10.1002/cncr.25075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BC, Demitrack LB, Fries BE. The accuracy of the National Death Index when personal identifiers other than Social Security number are used. Am J Public Health. 1992;82:1145–1147. doi: 10.2105/ajph.82.8.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. 4th edn. Cambridge: Published on behalf of the World Health Organization by Cambridge University Press; 1999. [Google Scholar]