Abstract
Each state is autonomous in its comprehensive cancer control (CCC) program, and considerable heterogeneity exists in the program plans. However, researchers often focus on the concept of nationally representative data and pool observations across states using regression analysis to come up with average effects when interpreting results. Due to considerable state autonomy and heterogeneity in various dimensions—including culture, politics, historical precedent, regulatory environment, and CCC efforts—it is important to examine states separately and to use geographic analysis to translate findings in place and time.
We used 100 percent population data for Medicare-insured persons aged 65 or older and examined predictors of breast cancer (BC) and colorectal cancer (CRC) screening from 2001–2005. Examining BC and CRC screening behavior separately in each state, we performed 100 multilevel regressions. We summarize the state-specific findings of racial disparities in screening for either cancer in a single bivariate map of the 50 states, producing a separate map for African American and for Hispanic disparities in each state relative to whites. The maps serve to spatially translate the voluminous regression findings regarding statistically significant disparities between whites and minorities in cancer screening within states. Qualitative comparisons can be made of the states’ disparity environments or for a state against a national benchmark using the bivariate maps. We find that African Americans in Michigan and Hispanics in New Jersey are significantly more likely than whites to utilize CRC screening and that Hispanics in 6 states are significantly and persistently more likely to utilize mammography than whites. We stress the importance of spatial translation research for informing and evaluating CCC activities within states and over time.
Keywords: bivariate mapping, comprehensive cancer control, geographic disparities, spatial heterogeneity, spatial translation
INTRODUCTION
Cancer is a leading cause of death, second to heart disease (Heron et al. 2009; Edwards et al. 2010). Among all cancers, breast cancer (BC) and colorectal cancer (CRC) can be detected in early stages through effective screening methods. The 5-year survival rate for BC and CRC exceeds 90 percent if detected at an early stage and exceeds 70 percent at a regional stage (ACS 2010). However, BC and CRC screening rates are low (about 50 percent for BC and CRC in 2008) (ACS 2011), and the mortality rates remain high.
In 2009, BC was the most common cancer in women, and CRC was the third most common cancer in both men and women (Jemal et al. 2009). Both of these cancers are more common among people aged 65 or older. Specifically, BC incidence is 5 times greater and CRC incidence is 15 times greater among this age group than among younger populations (NCI 2007). Cancer morbidity and mortality in the older population become even more salient as the population size and life expectancy of older persons continue to increase (Hetzel and Smith 2001; Administration on Aging 2006). Thus, it is imperative to understand factors associated with BC and CRC screening behavior in the older population in order to promote early detection and effective reduction in cancer morbidity and mortality.
Disparities in cancer screening, morbidity, and mortality are well documented across different races or ethnicities, levels of socioeconomic status or acculturation, insurance coverage or type, and geographic location (ACS 2010; Naishadham et al. 2011). Recent evidence from the Behavioral Risk Factor Surveillance System regarding CRC screening, coupled with the National Program of Cancer Registries data, suggests that states with higher prevalence of CRC screening have lower mortality from CRC (Richardson et al. 2011). Elimination of disparities in cancer screening and outcomes has been a particular focus of comprehensive cancer control (CCC) efforts (Coughlin et al. 2006). Therefore, we focus this article on disparities in screening utilization among whites and minorities.
Several studies have demonstrated that BC or CRC screening utilization varies across states (Nelson et al. 2003; Cooper and Koroukian 2004; Schneider et al. 2009; Mobley et al. 2010). Mobley et al. (2010) reported considerable state-level variation in CRC screening rates (sigmoidoscopy or colonoscopy), ranging from 34 percent in New Mexico to 44 percent in Maryland, using 100 percent Medicare fee-for-service claims data for people aged 65 or older from 2001 through 2005.
This article focuses on racial or ethnic disparities in two types of cancer screening (BC, CRC) and how these vary across states. The article’s major contribution is in the spatial translation area. That is, we use mapping of state-specific population estimates reflecting racial or ethnic disparities in two types of cancer screening to convey the results of 100 independently estimated multilevel models all in one graphic. This efficient means of summarizing the vast amount of information allows CCC planners to easily assess how the screening behaviors of older minorities in their state compare to older whites, and how their observed disparity environments compare to a national average and to findings in other states. We stress the statistical significance of differences within states, which is the most valid comparison for disparities research. Plotting the results from all states together in one map allows for qualitative assessments of how the disparity environments differ across states. Including the national average effect estimate from a pooled model in the map legend offers a benchmark that illustrates which states are driving national estimates.
This sort of information on place-specific variation could be useful in CCC planning and interventions, under new guidelines that encourage communities to collaborate with others and to pay attention to place-based differences. More specifically, federal preventive health policy has moved toward place-based initiatives, as exemplified by a 2009 White House memo (OMB 2009). The memo promotes place-conscious planning and place-based policies and programming at the interagency level to increase the impact of public investments. To do this, planners need a way to reliably compare results across different communities and state cancer control environments. Title IV of the Affordable Care Act (ACA) of 2010 (ACA 2010) supports specific programs tailored to improving population health and integrated, place-based efforts to improve the well-being of persons and communities. The law provides substantial funding for public health and requires that the Centers for Disease Control and Prevention convene an independent Community Preventive Services Task Force to review interventions, including consideration of place-specific social, economic, and physical environments that can affect health and disease (Mueller et al. 2011).
Conceptual Model
Understanding health disparities in cancer screening requires a-priori modeling of the sources of these disparities in a geospatial context and requires an approach that explicates the social ecology of the health behaviors. As such, states should be modeled as separate systems, which allows covariates (i.e., race or ethnicity relative to whites) to have unique effects on the outcome variables (i.e., probability of BC or CRC screening) across states. Independent modeling of each state’s population in multilevel models, with person-level and community-level covariates, reveals the net effects of local-area interactions between people and their environments for each state. Thus, we estimate multilevel models to generate population-level effect estimates that are allowed to vary across states, reflecting their local population and socio-ecological differences.
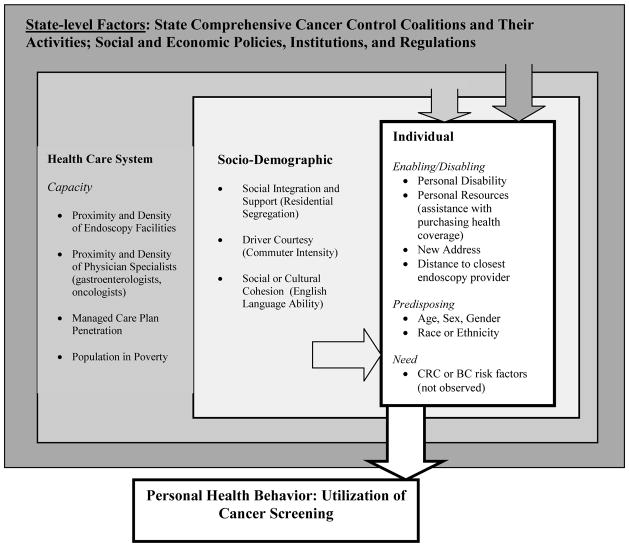
The conceptual model that we use here (Figure 1) draws from the literature and describes spatial interaction among people and characteristics of their contextual environments along the pathways to health care utilization (Mobley et al. 2008, 2010). This is a hybrid model that incorporates the behavioral model of utilization (Aday and Andersen 1974) and spatial interactions in health care access and utilization (Khan and Bhardwaj 1994). In the model, each of the U.S. states represents a unique health care environment with unique and decentralized cancer control programs that are state-specific in funding and implementation. State health care environments are governed by local and regional politics, social systems, market-level forces that determine supply factors, and community or neighborhood-level forces that determine social factors. Individuals exhibit predisposing, enabling, and need characteristics, which interact with the forces in the broader system.
Figure 1.
Socio-Ecological Model of Factors Impacting Cancer Screening
This conceptual model directs us to study state populations as separate systems and to examine racial or ethnic disparities in BC or CRC screening among each state’s population in contexts that are relevant for each state. The separate, state-specific analyses are crucial for truly understanding disparities, because racial or ethnic disparities observed in studies conducted at the national level are due in part to geographic differences rather than to differences among racial or ethnic groups within geographic areas (Chandra and Skinner 2003). Because our goal is to disentangle true disparities within states from geographic ones across the nation, we focus on individual states in separate analyses.
METHODS
Study Population
Our study population is the entire Medicare fee-for-service (FFS) population aged 65 or older and residing in the 50 U.S. states from 2001 through2005. We used the traditional definition of Medicare FFS coverage (persons with both Parts A and B coverage). We defined a FFS Medicare cohort of persons aged 65 or older in 2001 and followed them for several years to assess whether they used screening for BC (in 2001–2003 and 2003–2005) or CRC (in 2001–2005). We used annual data from 100 percent of Medicare claims to record any endoscopy or mammography use by persons over these intervals. Persons included in the cohort must remain alive during the entire period, maintain coverage of Medicare Parts A and B, and remain living in the same state. In BC multilevel statistical models we included only females, whereas in CRC models we included both males and females. Table 1 provides sample statistics by state, where the number of cohort observations per state is reported.
Table 1.
Cohort Size in Sample Population, Number of PCSAs and Counties in Each State, and U.S. Totals
| State | Number of Persons in Population Cohort, CRC | Number of Persons in Population Cohort, BC | Number of PCSAs | Number of Counties | |||||
|---|---|---|---|---|---|---|---|---|---|
| Alabama | 319,335 | 238,401 | 144 | 67 | |||||
| Alaska | 22,585 | 14,251 | 24 | 27 | |||||
| Arizona | 223,305 | 155,084 | 74 | 15 | |||||
| Arkansas | 224,275 | 158,998 | 149 | 75 | |||||
| California | 1,126,335 | 781,832 | 338 | 58 | |||||
| Colorado | 156,466 | 108,329 | 96 | 63 | |||||
| Connecticut | 245,186 | 177,375 | 71 | 8 | |||||
| Delaware | 64,072 | 44,321 | 12 | 3 | |||||
| Florida | 1,139,258 | 815,741 | 167 | 67 | |||||
| Georgia | 464,828 | 338,081 | 169 | 159 | |||||
| Hawaii | 56,573 | 37,307 | 23 | 5 | |||||
| Idaho | 82,703 | 56,394 | 57 | 44 | |||||
| Illinois | 818,437 | 586,674 | 258 | 102 | |||||
| Indiana | 471,278 | 335,827 | 172 | 92 | |||||
| Iowa | 274,939 | 197,168 | 225 | 99 | |||||
| Kansas | 211,602 | 148,392 | 162 | 105 | |||||
| Kentucky | 307,484 | 217,727 | 145 | 120 | |||||
| Louisiana | 244,130 | 184,005 | 112 | 64 | |||||
| Maine | 121,387 | 83,940 | 91 | 16 | |||||
| Maryland | 346,573 | 248,298 | 62 | 24 | |||||
| Massachusetts | 362,711 | 270,039 | 107 | 14 | |||||
| Michigan | 765,461 | 533,627 | 191 | 83 | |||||
| Minnesota | 314,019 | 225,862 | 176 | 87 | |||||
| Mississippi | 211,398 | 155,479 | 141 | 82 | |||||
| Missouri | 387,278 | 278,696 | 213 | 115 | |||||
| Montana | 79,539 | 53,217 | 71 | 56 | |||||
| Nebraska | 146,001 | 102,360 | 121 | 93 | |||||
| Nevada | 75,709 | 50,983 | 30 | 17 | |||||
| New Hampshire | 95,298 | 66,428 | 46 | 10 | |||||
| New Jersey | 567,836 | 415,796 | 139 | 21 | |||||
| New Mexico | 100,328 | 68,114 | 61 | 33 | |||||
| New York | 1,040,451 | 780,421 | 324 | 62 | |||||
| North Carolina | 587,505 | 433,969 | 207 | 100 | |||||
| North Dakota | 62,867 | 42,927 | 71 | 53 | |||||
| Ohio | 783,948 | 562,887 | 254 | 88 | |||||
| Oklahoma | 248,870 | 177,463 | 156 | 77 | |||||
| Oregon | 151,816 | 104,809 | 78 | 36 | |||||
| Pennsylvania | 819,431 | 614,838 | 296 | 67 | |||||
| Rhode Island | 50,326 | 39,362 | 14 | 5 | |||||
| South Carolina | 308,796 | 222,581 | 110 | 46 | |||||
| South Dakota | 72,116 | 49,227 | 95 | 66 | |||||
| Tennessee | 395,590 | 296,521 | 145 | 95 | |||||
| Texas | 1,118,495 | 793,778 | 414 | 254 | |||||
| Utah | 113,066 | 78,565 | 54 | 29 | |||||
| Vermont | 50,631 | 34,904 | 49 | 14 | |||||
| Virginia | 492,814 | 347,962 | 170 | 128 | |||||
| Washington | 314,345 | 214,054 | 119 | 39 | |||||
| West Virginia | 162,307 | 114,791 | 123 | 55 | |||||
| Wisconsin | 412,030 | 301,704 | 173 | 72 | |||||
| Wyoming | 37,384 | 24,669 | 41 | 23 | |||||
|
| |||||||||
| Total United States | 17,249,117 | 12,384,178 | 6,740 | 3,133 | |||||
Note: BC = breast cancer; CRC = colorectal cancer; PCSA = primary care service area
Statistical Analysis
We used the generalized estimating equations (GEE) form of multilevel models in the analysis to obtain robust population-level effect estimates for each state from individual-level data with binary response (Liang and Zeger 1986; Horton and Lipsitz 1999; Hardin and Hilbe 2003; Gekman and Hill 2007). We separately and independently estimated cancer screening models for each state and each cancer site. All models included the same set of multilevel predictors (Table 2).
Table 2.
Variables Included in Multilevel Regression
| Factors in Conceptual Model | Variables in Regression Model |
|---|---|
| Individual | |
| Enabling/disabling | Moved to a new ZIP code within state, 2001–2005 Months with state assistance to purchase Part B insurance Distance (miles) to closest screening facility |
| Predisposing | Age in 2001 Gender (only in CRC models and using female as the reference group) Race or ethnicity (five groups relative to whites) |
| Sociodemographic factors (at PCSAarea level) | |
| Social integration and support | Residential segregation (isolation) index |
| Stressor, driver courtesy | Proportion of the workforce commuting ≥60 minutes to work |
| Social or cultural cohesion | Proportion of the people aged 65 or older who speak little or no English |
| Health system factors (at County area level) | |
| Capacity | Average number of screening facilities per thousand population aged 65 or older Number of oncologists per 1,000 population aged 65 or older Medicare managed care plan penetration Proportion of people living below the federal poverty level Proportion of the county population in rural tracts |
Note: CRC = colorectal cancer; PCSA = primary care service area
The neighborhood-community level is defined as the Primary Care Service Area (Goodman et al. 2003), and the political system level is defined as the county. PCSAs are smaller and more numerous than counties (see Table 1) and may better represent local neighborhood conditions (Mobley et al. 2008). To demonstrate the deviation of state-specific estimates from a national benchmark, we needed a national estimate that we derived by estimating a national-level model that pooled all observations across states to obtain national-level population estimates.
Statistical Significance
We used extracts from 100 percent Medicare FFS population data (not survey sample data) yielding the maximal sample size possible for each state. For each state, we tested whether the effect estimate of a minority group coefficient was significantly different (higher or lower) from zero and then displayed those that were higher (positive) versus lower (negative) versus no effect (zero) for each state.
False-positive results (statistically significant effect estimates that occur by chance and reflect type 1 statistical errors) are common in the medical literature (Sainani 2009). We recognize that sampling error among small groups of Hispanics or African Americans in some states with small minority populations can create the appearance of variation in screening rates, even when none exists. In some states, the number of these individuals is relatively low and thus the power to detect a significantly different effect compared to whites may be low; thus, it is always possible that some findings are due to chance. To tightly control the probability of type 1 errors, we used a 1 percent significance level for the tests.
False positive results may also be hidden when researchers make multiple comparisons and do not properly account for the reduction in power that accompanies this practice. In our disparities research, we conducted several simultaneous tests to determine whether there were differences between various minority subgroups and whites (unless all minorities are grouped together in the model). Our model includes five subgroups (African American, Hispanic, Asian, Native American, and other) that we compared to whites in a series of tests for significance of coefficient estimates. To tightly control for type 1 error, we accounted for this multiple-testing aspect of our model (Sainani 2009) using a Bonferroni correction. The Bonferroni correction is a very conservative approach because it represents a worst-case scenario where all of the tests being conducted are assumed to be completely independent (which is not likely to be the case for these subgroup comparisons). However, the Bonferroni correction is simple to conduct and to understand, and even if it is not the best approach, using a more stringent significance level helps weed out spurious results from states with small minority populations. Thus, we used the 1 percent level of significance in translating our findings via the maps, which reflects at least a 5 percent overall level of significance according to the Bonferroni correction approach (0.05 = 0.01 × 5). Thus, the significance level for our tests is somewhere between 1 percent and 5 percent. As described next, we make only qualitative comparisons across states and between states and the nation by mapping the state-specific results to demonstrate differences across states in the state-specific population dynamics.
Spatial Translation of Findings from 100 Multilevel Regressions
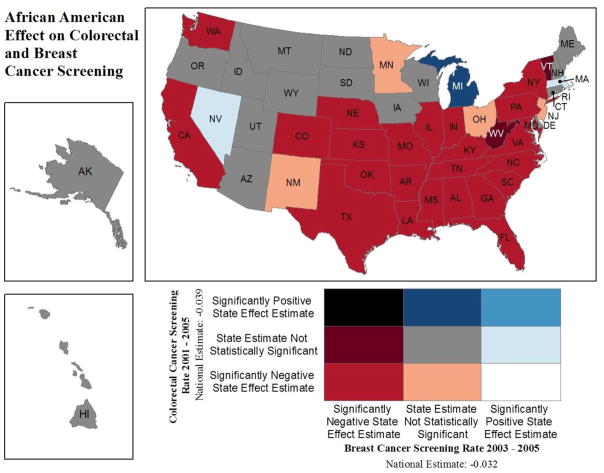
After estimation, we compiled results for the race or ethnicity effect estimates for each state and cancer site. Each state’s effect estimates for each minority group (African American and Hispanic) were classified as follows: statistically significantly different from zero (at the 1 percent level of significance) and (a) with a positive value, or (b) with a negative value, or (c) not significantly different from zero. Using these, we then translated the disparities findings using three maps (Figures 2 through 4). We used a spatial join method in ArcView 10.0 GIS software that combined the effect estimates for each cancer site and state, resulting in nine possible bivariate classes for the joined estimates. For example, one of these classes is defined as follows: a positive effect for minority group relative to whites for BC screening (horizontal legend) and also a positive effect for minority group relative to whites for CRC screening (vertical legend). All states meeting both of these criteria are classified in that cell of the map legend and colored light blue. No such states are evident for the African American disparities (Figure 2); however, for Hispanic disparities, one state (New Jersey) shows higher screening for both cancers among Hispanics relative to whites (Figure 3). For benchmarking, we display the national effect estimate for the covariate of interest in the map legend to help viewers understand which states are dominating national statistics.
Figure 2.
Positive/Negative Effect Estimates from 100 Multilevel Regressions: African American (Relative to Whites) Colorectal or Breast Cancer Screening
Figure 4.
Positive/Negative Effect Estimates from 100 Multilevel Regressions: Stability over Time of Hispanic (Relative to Whites) Breast Cancer Screening
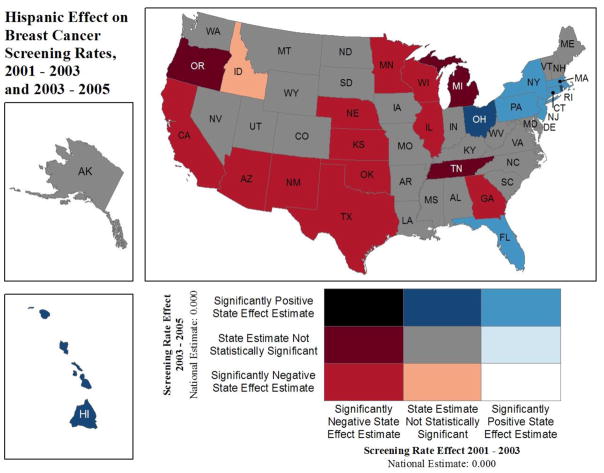
Figure 3.
Positive/Negative Effect Estimates from 100 Multilevel Regressions: Hispanic (Relative to Whites) Colorectal or Breast Cancer Screening
This innovative spatial translation of GEE modeling results allows findings from 100 separate estimating equations to be compared visually across all states in one single graphic. This demonstrates the power of mapping to aid in visualizing vast quantities of information. For example, in Figures 2 and 3, we display disparities between two minority groups (African Americans and Hispanics) and whites for each state. In some states, we see a reversal of what we might expect based on national statistics. That is, minorities seem to display an advantage over whites in terms of screening utilization. We posit that these sorts of reverse disparities reflect successful state-specific initiatives designed to reduce disparities. In Figure 4, we display the Hispanic disparity estimate relative to whites for BC screening over two time intervals to investigate whether disparity outcomes for BC screening are changing over time. These sorts of temporal comparisons may also be useful in evaluating CCC efforts.
In summary, observed differences in map colors across individual states reflect different state environments and CCC efforts and other place-specific differences across states. Multistate patches of the same color reflect similar disparity environments in particular regions, reflecting geographic disparities. For example, patches of red color across multiple states suggest regional disparities in both of the dimensions displayed in the map.
FINDINGS
Disparities Estimates from Multilevel Models
Figure 2 displays effect estimates from our GEE multilevel models, focusing on the disparity between African Americans and whites in BC screening during 2003–2005 or CRC screening during 2001–2005. As shown in Figure 2, in Michigan, African American women have no significant differences in the probability of BC screening relative to whites (“state estimate not statistically significant” category), whereas African Americans have a significantly higher probability of CRC screening than whites (“significantly positive state effect estimate” category), resulting in a dark blue color classification. Michigan is the only state exhibiting this dichotomy. In 17 states, African Americans have no significant differences in either type of screening relative to whites, whereas in the majority of states, African Americans have a significantly lower probability of BC and CRC screening than whites. This explains the national statistic often reported that African Americans are less likely to be screened for BC or CRC than whites. However, our results suggest that in Michigan, this disparity in CRC screening has been reversed for African Americans.
Figure 3 displays effect estimates from the Hispanic indicator variable, relative to whites, on probability of cancer screening. It is constructed the same way as Figure 2. In New Jersey, Hispanics have a higher probability of both BC and CRC screening than whites (light blue color, “significantly positive state effect estimate” in both dimensions). In 21 states, Hispanics have no significantly different probability of BC or CRC screening than whites (grey color). In the majority of states, Hispanics have significantly lower probability of BC and CRC screening than whites (red color). This explains the national statistic often reported that Hispanics are less likely to be screened for BC or CRC than whites. However, our results suggest that in New Jersey, this disparity in BC and CRC screening has been reversed for Hispanics.
Figure 4 displays effect estimates from the Hispanic indicator, relative to whites, on probability of BC screening over time, using bivariate mapping to compare estimates from an early period (2001–2003) and late period (2003–2005). The national estimate finds no statistically significant effect of being Hispanic on the probability of BC screening in either period, suggesting no disparity in BC screening for Hispanics relative to whites in either period. The state-specific analysis demonstrates that what seems to be the case nationally does not necessarily hold across the states. Although the majority of states exhibit no disparities, the map shows that in 6 states (New York, Pennsylvania, Massachusetts, Connecticut, New Jersey, and Florida), Hispanic women were more likely than whites to have BC screening in both periods. Conversely, in 11 states, Hispanics were significantly less likely than whites to receive BC screening in both periods (red). Seven states showed changes in disparities over time, with disparities increasing over time in only one state (Idaho) and decreasing in the remainder (Ohio, Hawaii, Rhode Island, Michigan, Oregon, and Tennessee). No states experienced a complete reversal (black) from negative to positive disparities over time (relative to whites).
DISCUSSION
In this study, we used a socio-ecological, spatial interaction framework to guide the multilevel statistical modeling of minority disparities in BC and CRC screening. The study sample is based on 100 percent FFS Medicare data and thus is fully representative of this population in all states, providing large samples that allow for robust characterization of disparities between minorities and whites in most states. No other source of BC or CRC screening data exists to date that can be used for this purpose. Sample data, such as the Behavioral Risk Factor Surveillance System, cover a broader range of adults but sparsely represent population subgroups within states. In the future, with standardized medical records and greater national health security from increased availability of competitive health insurance, population-based analyses are expected to become more prevalent. This article demonstrates the advances in disparities research that are possible with availability of population health (versus survey sample) data.
We used innovative spatial translation to present the study findings in a series of bivariate maps, which are useful for making comparisons of state-specific disparity environments across states and for revealing patterns in these disparity environments that reflect broader regional geographic disparities. Each state is treated as a separate environment, and each state’s estimates describe behaviors in that state’s FFS Medicare population. Mapping the findings for each state together in a single map of the United States enables comparisons of the disparity dynamics across the state environments, showing geographic differences in the disparities between minorities and whites. We provide a national estimate, derived from a pooled model, to use as a benchmark to demonstrate which states are driving the national estimate. We demonstrate that many states deviate from the national estimate, calling into question the utility of such a statistic for CCC efforts. The spatial translation of empirical findings that we demonstrate here is a useful tool for efficiently summarizing a tremendous amount of empirical evidence, allowing qualitative comparisons across states and over time. Observed differences across states can be used to motivate further CCC evaluation research that seeks to explain why some states exhibit disparities among minorities and whites, whereas others do not.
Although the majority of the states showed lower BC and CRC screening rates for African Americans and Hispanics than whites, consistent with the national benchmark, our findings demonstrate that minority disparities with whites are reversed in some states, such as Michigan and New Jersey. It is possible that this reflects successful efforts by the National Comprehensive Cancer Control Program (NCCCP) in some states. For example, we know that Michigan used NCCCP support to establish the Colorectal Cancer Awareness Network (CRAN) in 2002 with a mission to raise awareness about CRC and the need for screening (MCC 2002). During its first year, CRAN grew to more than 230 participants representing 145 unique organizations from every region of the state. New efforts in 2004 more fully embedded CRAN into Michigan’s communities through the development of regional CRANs in partnership with the American Cancer Society. The findings from this article suggest that Michigan’s CCC efforts may have succeeded in increasing awareness of the importance of CRC cancer screening. The state’s African Americans were more likely than whites to utilize CRC screening, suggesting that the efforts to promote screening at the church and community levels reaped positive rewards for this minority group.
The finding that Hispanics in New Jersey are significantly more likely than whites to receive both types of screening is puzzling as we found no description of CCC efforts that may help explain this phenomenon (NJCCCP 2002). Our examination of Census data from the year 2000 show that Hispanics made up 13 percent of New Jersey’s population; 33 percent of Hispanics in New Jersey were from Puerto Rico, and another 16 percent were from South America, with only 9 percent from Mexico and 7 percent from Central America. Perhaps Hispanics in New Jersey are on average wealthier or better educated; perhaps they are more firmly established over several generations and have greater social cohesion and support than Hispanics in other areas. Further research is needed to better understand this reverse disparity.
When looking at the change of BC screening rates over time for Hispanics relative to whites (see Figure 4), we found that 6 states had higher BC screening rates than whites in both periods, while the national benchmark for Hispanics was zero (no significant disparity from whites). This finding demonstrates that national statistics are not very representative of what is happening in all states. To better understand the impacts from CCC efforts in each state, analysis using state-specific data and controlling statistically for multilevel factors in each state is needed.
The geospatial research presented in this article, which spatially translates findings from state-specific multilevel models of individuals’ cancer screening behavior, may provide valuable information for state CCC programs seeking evidence that their program activities are making progress in closing disparity gaps. We have focused here on two states that exhibited reverse disparities—that is, findings that a minority group had statistically significantly higher probability of screening than whites. The majority of states exhibit the usual disparities reported in national statistics, whereas others show no disparities at all and a few show reverse disparities during the period we study.
This work demonstrates the importance of analyzing and assessing each state separately. States are separate entities in CCC and have autonomy to set insurance regulation and health promotion policies. In addition, states represent different mixtures of peoples and cultures, have different baseline and historical conditions, and are so heterogeneous that it calls into question the utility of national average statistics. With increasing availability of population data and computing capabilities, we advocate use of population data and a focus on individual states, so that meaningful interventions to improve cancer screening behavior can be implemented and evaluated.
Acknowledgments
This work was supported by a National Cancer Institute grant (1R01CA126858) and an American Recovery and Reinvestment Act (ARRA) supplement to it. The content is solely the responsibility of the authors and does not necessarily represent the official views of RTI International, Arizona State University, the National Cancer Institute, or the National Institutes of Health.
References
- [(last accessed 10 July 2011).];Affordable Care Act. 2010. The Affordable Care Act of 2010. http://www.healthcare.gov/law/introduction/index.html.
- Administration on Aging. 2006. Federal interagency forum on aging-related statistics: Older Americans update 2006: Key indicators of well-being. Washington, DC: U.S. Government Printing Office; [(last accessed 10 July 2011).]. http://www.aoa.gov/agingstatsdotnet/Main_Site/Data/2006_Documents/OA_2006.pdf. [Google Scholar]
- Aday LA, Andersen R. A framework for the study of access to medical care. Health Services Research. 1974;9:208–20. [PMC free article] [PubMed] [Google Scholar]
- American Cancer Society (ACS) Cancer Facts and Figures 2010. Atlanta, GA: American Cancer Society; 2010. [(last accessed 10 July 2011).]. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-026238.pdf. [Google Scholar]
- American Cancer Society (ACS) Cancer Prevention & Early Detection Facts & Figures 2011. Atlanta, GA: American Cancer Society; 2011. [(last accessed 10 July 2011).]. http://www.cancer.org/Research/CancerFactsFigures/CancerPreventionEarlyDetectionFactsFigures/ACSPC-029459. [Google Scholar]
- ArcView, version 10.0 [software] Redlands, CA: ESRI; [Google Scholar]
- Chandra A, Skinner J. Geography and racial health disparities. NBER Working Paper. 2003;(W9513) [Google Scholar]
- Cooper GS, Koroukian SM. Geographic variation among Medicare beneficiaries in the use of colorectal carcinoma screening procedures. American Journal of Gastroenterology. 2004;99:1544–50. doi: 10.1111/j.1572-0241.2004.30902.x. [DOI] [PubMed] [Google Scholar]
- Coughlin SS, Costanza ME, Fernandez ME, Glanz K, Lee JW, Smith SA, Stroud L, Tessaro I, Westfall JM, Weissfeld JL, Blumenthal DS. CDC-funded intervention research aimed at promoting colorectal cancer screening in communities. Cancer. 2006;107(5 Suppl):1196–204. doi: 10.1002/cncr.22017. [DOI] [PubMed] [Google Scholar]
- Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson R, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, van Ballegooijen M, Goede SL, Anderson R, Ries L. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–73. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A, Hill J. Data analysis using regression and multilevel/hierarchical models. New York, NY: Cambridge University Press; 2007. [Google Scholar]
- Goodman DC, Mick SS, Bott D, Stukel T, Chang CH, Marth N, Poage J, Carretta HJ. Primary care service areas: A new tool for the evaluation of primary care services. Health Services Research. 2003;38:287–309. doi: 10.1111/1475-6773.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin J, Hilbe J. Generalized estimating equations. New York, NY: Chapman & Hal/CRC; 2003. [Google Scholar]
- Heron M, Hoyert DL, Murphy SL, Jiaquan X, Kochanek KD, Tejada-Vera B. National Vital Statistics Report. 14. Vol. 57. Hyattsville, MD: National Center for Health Statistics; 2009. [(last accessed 10 July 2011).]. Deaths: Final data for 2006. http://www.cdc.gov/nchs/data/nvsr/nvsr57/nvsr57_14.pdf. [PubMed] [Google Scholar]
- Hetzel L, Smith A. Census 2000 brief C2KBR/01-10. Washington, DC: U.S. Census Bureau; 2001. The 65 years and over population: 2000. [Google Scholar]
- Horton N, Lipsitz S. Review of software to fit generalized estimation equation regression models. The American Statistician. 1999;53:60–169. [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun M. Cancer statistics, 2009. CA: A Cancer Journal for Clinicians. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- Khan AA, Bhardwaj SM. Access to health care: A conceptual framework and its relevance to health care planning. Evaluation & the Health Professions. 1994;17:60–76. doi: 10.1177/016327879401700104. [DOI] [PubMed] [Google Scholar]
- Liang K, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Michigan Cancer Consortium (MCC) [last accessed 10 July 2011];Statewide Colorectal Cancer Awareness Network, 2002–2004. 2002 http://www.michigancancer.org/PDFs/ColorectalCancerProjects/StatewideColoCaAwareNetwork-2002-2004/SummaryDocument.pdf.
- Mobley LR, Kuo T, Andrews LS. How sensitive are multilevel regression findings to defined area of context? A case study of mammography use in California. Medical Care Research and Review. 2008;65:315–37. doi: 10.1177/1077558707312501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley L, Kuo T, Urato M, Subramanian S. Community contextual predictors of endoscopic colorectal cancer screening in the USA: Spatial multilevel regression analysis. International Journal of Health Geographics. 2010;9:44. doi: 10.1186/1476-072X-9-44. open access online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller K, MacKinney C, Gutierrez M, Richgels J. Place based policies and public health: The road to healthy rural people and places. [(last accessed 10 July 2011)];Rural Policy Research Institute, Policy Paper. 2011 http://www.rupri.org/Forms/HHSPanels_Integration_March2011.pdf.
- Naishadham D, Lansdorp-Vogelaar I, Siegel R, Cokkinides V, Jemal A. State disparities in colorectal cancer mortality patterns in the United States. Cancer Epidemiology, Biomarkers & Prevention. 2011;20(7):1296–302. doi: 10.1158/1055-9965.EPI-11-0250. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute (NCI) [(last accessed 10 July 2011).];NCI SEER Cancer Statistics Review 1975–2007. 2007 http://seer.cancer.gov/csr/1975_2007/browse_csr.php?section=1&page=sect_01_table.25.html.
- Nelson DE, Bolen J, Marcus S, Wells HE, Meissner H. Cancer screening estimates for U.S. metropolitan areas. American journal of Preventive Medicine. 2003;24(4):301–9. doi: 10.1016/s0749-3797(03)00024-2. [DOI] [PubMed] [Google Scholar]
- New Jersey Comprehensive Cancer Control Plan (NJCCCP) [(last accessed 10 July 2011).];New Jersey Comprehensive Cancer Control Plan, 2002. 2002 http://www.nj.gov/health/ccp/ccc_plan.htm.
- Office of Management and Budget (OMB) [(last accessed 10 March 2010).];Memorandum: Developing effective place-based policies for the FY 2011 budget. 2009 Aug; http://www.whitehouse.gov/omb/assets/memoranda_fy2009/m09-28.pdf.
- Richardson L, Tai E, Rim S, Joseph D, Plescia M. Vital signs: Colorectal cancer screening, incidence, and mortality—United States, 2002–2010. [(last accessed 10 July 2011).];Morbidity and Mortality Weekly Report. 2011 60(26):884–9. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6026a4.htm?s_cid=mm6026a4_w. [PubMed] [Google Scholar]
- Sainani K. Statistically speaking: The problem of multiple testing. American Academy of Physical Medicine and Rehabilitation. 2009;1:1098–1103. doi: 10.1016/j.pmrj.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Schneider KL, Lapane KL, Clark MA, Rakowski W. Using small-area estimation to describe county-level disparities in mammography. [(last accessed 10 July 2011)];Preventing Chronic Disease. 2009 6(4) http://www.cdc.gov/pcd/issues/2009/oct/08_0210.htm. [PMC free article] [PubMed] [Google Scholar]