Abstract
Objectives
To examine the efficacy of sexual risk reduction interventions among South African youth.
Methods
Electronic databases were searched to identify studies published between 2007 and early 2013. Studies were eligible if they (1) targeted youth age 9–26, (2) evaluated sexual risk reduction interventions and (3) reported at least one behavioral outcome. Independent raters coded study characteristics, and intervention content. Weighted mean effect sizes were calculated; positive effect sizes indicated less sexual risk behavior and incident STIs.
Results
Ten studies (k = 11, N = 22,788; 54% female; 79% Black-African) were included. Compared to controls, interventions were successful at delaying sexual intercourse and, among sexually active youth, at increasing condom use. A single study found reductions in the incidence of herpes simplex virus-2, but not HIV.
Conclusions
Implementing behavioral interventions to delay sexual debut and improve condom use can help to reduce the transmission of HIV among South African youth.
Keywords: HIV, intervention, meta-analysis, sex, South Africa, youth
INTRODUCTION
South African youth between the ages of 15 and 24 experience the highest prevalence of HIV of any other region in the world with more than 20% of youths living with HIV.[1] Unprotected penile-vaginal sex is the primary mode of HIV transmission in South Africa. [1] School-aged girls and young women are disproportionately affected by HIV. Gender disparities in the prevalence of HIV continue as girls age; young women aged 20 to 24 continue to be disproportionately affected by HIV (21% vs. 5% of young men 20 to 24 years of age).[1]
Adolescent and adult women are more vulnerable to the transmission of HIV due to sociocultural factors (e.g., sexual coercion and violence, and multiple partnerships with older men, who are more likely to be HIV-infected) as well as biological factors (e.g., more mucosal surfaces for HIV to attach to; and reproductive changes during adolescence).[2] Partner drinking also increases young women’s risk for HIV.[3] A national survey of South Africans found 33% of young men and 11% of young women (15 to 24 years of age) report current alcohol use with 18% of young men and 3% of young women reporting hazardous or harmful levels of alcohol consumption.[4] Although the association between alcohol consumption and risky sexual risk behaviors are similar for young men and women, sexual coercion occurs most often when sex is preceded by alcohol consumption underscoring young women’s limited power in relationships [3, 5, 6] Determining the extent to which interventions can significantly impact gender inequalities, gender violence, and alcohol use among South African youth is critical for understanding the extent to which the context of sexual risk can be changed to reduce the incidence of HIV.
Prior reviews of the literature have focused on evaluating the efficacy of behavioral HIV interventions among youth in developed and developing countries. These reviews suggest that behavioral HIV interventions targeting youth are successful at delaying sexual activity, reducing condom use, and averting sexually transmitted infections in developed countries. [7, 8] Conversely, the success of youth-based interventions to reduce sexual risk behaviors in developing countries has been mixed.[9, 10] These reviews tend to focus on interventions targeting adolescents in developing countries, more broadly, or in specific geographical regions such as sub-Saharan Africa. In a narrative review of interventions for South African youth, Harrison et al. described the intervention features but, based on the range of available data, could not determine the efficacy of those interventions.[11] The efficacy of interventions specifically targeting South African youth, including factors that moderate intervention efficacy, has received limited attention. Few reviews have attended to intervention features that address gender inequalities or alcohol use. Developing, implementing, and evaluating effective HIV prevention programs for South African youth that address the context in which sexual risk occurs is a public health priority. Understanding which interventions work and why is critical for the development of effective interventions that are targeted and tailored to South African youth.
The purpose of this meta-analysis was to determine the state-of-the-science concerning the success of behavioral interventions to reduce sexual risk behaviors and the incidence of STIs among South African youth. Intervention success at modifying sexual behaviors was inferred from studies’ reports of delaying sexual activity and, among sexually active youth, increased condom use as well as reduced number of sexual partners and incident STIs. Therefore, we hypothesized that South African youth who received a behavioral sexual risk reduction or educational intervention would delay sexual intercourse and, among those who are sexually active, would increase condom use, decrease the number of sexual partners, and lower the incidence of STIs compared to control participants. We evaluated the durability of the improvements over time as well as whether these improvements were influenced by sample characteristics, intervention duration, and content. We expected that interventions (vs. controls) would be more successful in reducing sexual risk behaviors when they sampled (a) men, due to young South African women’s limited power in relationships,[12] (b) fewer alcohol users as alcohol use, including abuse and dependence, is associated with sexual risk-taking behaviors,[13, 14] and (c) youth engaging in lower levels of risk at baseline (i.e., fewer sexual partners, protected vaginal/anal sex). In evaluating the intervention content, we focused on identifying the extent to which interventions addressed contextual issues (e.g., gender inequalities, alcohol use) associated with risky sexual behavior among youth in South Africa.
METHODS
Search Strategy, Inclusion Criteria, and Study Selection
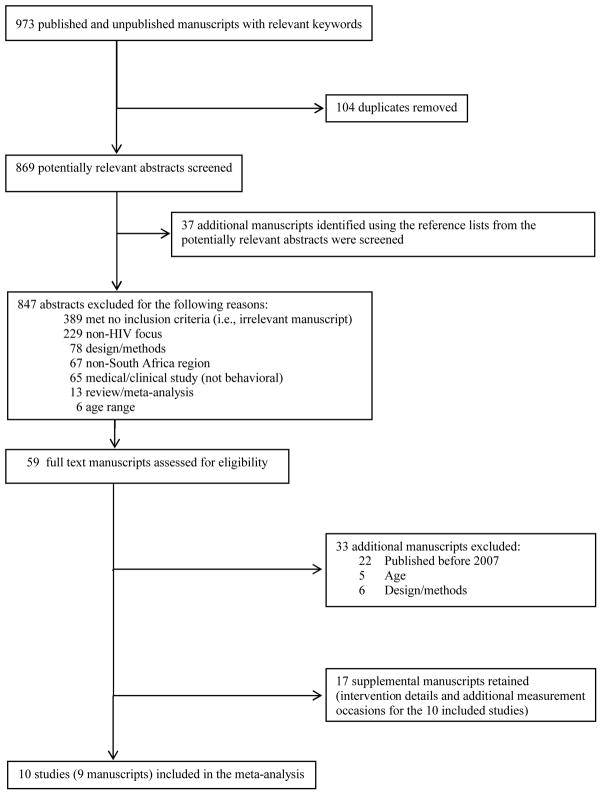
We searched electronic reference databases (PubMed, Global Health, PsycINFO, CINAHL, ERIC, Sociological Abstracts, and the Cochrane Library) using a Boolean search strategy: (South AND Africa*) AND (youth OR adolescent*) AND (alcohol OR drink* OR binge) AND (HIV OR AIDS OR (human AND immu* AND virus) OR (acquired AND immu* AND deficien* AND syndrome) OR STI OR STD OR (sexually transmitted infection*) OR (sexually transmitted disease*) OR condom OR sex* OR risk*). The electronic reference databases were searched during January 2013. Studies were included if they (1) targeted South African youth aged 9–26 with a mean age ≥12 years to ensure that the studies targeted youth rather than children, (2) evaluated a behavioral sexual risk reduction intervention (3) reported at least one risk-related outcome (e.g., unprotected sex), (4) provided sufficient information to calculate effect sizes, and (5) were published (including electronic publications) between 2007 and early 2013. Because we were interested in determining the efficacy of current behavioral HIV interventions, we included studies published in the past 5 years. Reference sections of relevant manuscripts (including published reviews obtained through the electronic reference database search) were also reviewed. Studies that fulfilled the inclusion criteria and were available through the end of December 2012 were included. When authors reported details and/or outcomes of the intervention in multiple manuscripts, the studies were linked in the database and represented as a single study. The manuscript reporting the main trial outcomes was selected as the primary study; the publication date from the primary study was used to determine eligibility. Thus, we included 10 studies (k = 11) obtained from 9 published manuscripts (Figure 1).[15–23]
Figure 1.
Selection process for study inclusion in the meta-analysis
Coding and Reliability
Two independent coders [LAJSS, PW] rated the study information, sample characteristics (e.g., gender), design and measurement specifics (e.g., recruitment strategy), and length and content of control and intervention condition (e.g., number of sessions). Study quality was assessed using 17 items (e.g., random assignment) from validated measures;[24–26] total possible quality score is 25. Inter-rater reliability was determined. For the categorical variables, raters agreed on 85% of the judgments (mean Cohen’s κ = .58). Reliability for the continuous variables (calculated using the intraclass correlation coefficient; ρ) yielded an average ρ = .97 across categories (median = 1.00). Disagreements between coders were resolved through discussion.
Study Outcomes and Effect Sizes
Effect sizes were calculated for behavioral and biological outcomes. Behavioral outcomes included abstinence/delay of sex, condom use, multiple sexual partners, and substance use (alcohol, drugs). Biological outcomes included STIs, including HIV. For each outcome, effect size estimates were calculated as the mean difference between the treatment and control group divided by the pooled standard deviation [27]. If means and standard deviations were not provided, other statistical information (e.g., odds ratio) was used to estimate the effect sizes using standard procedures [28, 29]. From the 10 studies that met the inclusion criteria, 11 interventions were analyzed. All of the studies reported at least one behavioral outcome (10 abstinence/delay of sex, 11 condom use, 4 multiple partners, 2 alcohol use, 2 drug use). One study (k = 2) reported herpes simplex virus-2 (HSV-2) and HIV incidence.[18] Multiple effect size estimates were calculated from individual studies when they reported more than one outcome variable, multiple intervention conditions, or when outcomes were separated by sample characteristics (e.g., gender). Estimates were adjusted for baseline differences when pre-intervention measures were available [30]. Effect sizes were corrected for sample size bias [31]. Positive effect size estimates indicate that an intervention was successful in reducing sexual and/or other health behaviors and lowered the incidence of STIs, including HIV, relative to controls.
Statistical Analyses
Timing of post-intervention assessments varied with the first assessment occurring between 0 to 78 weeks (k = 10), the second between 26 and 104 weeks (k = 8), and a third assessment at 52 weeks (k = 2). To avoid violating the assumption of study independence and as a strategy to examine all study assessments, effect sizes were clustered into two intervals: (a) early assessments (<52 weeks; 0 to 32 weeks, median = 26) and (b) late assessments (≥ 52 weeks; 52 to 104 weeks, median = 78). Because some studies included only a single post-intervention assessment, a final assessment interval (0 to 104 weeks, median = 52) was created to determine the overall impact of the study trials.
Data analyses were conducted with Stata 12 [32] using published macros [29, 33]. Weighted mean effect size, d+, were calculated using fixed- and random-effects procedures [29]. The 95% confidence intervals (CIs) surrounding a weighted mean effect size were calculated; CIs indicate the degree of precision as well as the significance of the mean effect size [29]. The homogeneity statistic, Q, was calculated; a significant Q indicates a lack of homogeneity and an inference of heterogeneity. To assess the extent to which outcomes were consistent across studies, the I2 index and its corresponding 95% CIs were calculated [34, 35]. I2 varies between 0 (homogeneous) and 100% (heterogeneous) [36]. If the CIs around I2 include a zero, the set of effect sizes is considered homogeneous.
To explain variability in effect size, the association between sample or intervention characteristics and the magnitude of the effects were examined using a modified weighted regression analysis (following fixed-effects assumptions) with weights equivalent to the inverse of the variance for each effect size [29, 37]. Regression analyses examined a priori determined moderators. Sample characteristics (4: proportion women, proportion alcohol users, proportion of participants with multiple partners, proportion of participants reporting protected vaginal/anal sex), intervention dose (2; number of sessions, total intervention dose, number and type of facilitators), and content (3; e.g., gender inequalities, alcohol use) were examined as potential moderators of the interventions. Weighted regression analyses were conducted only for outcomes with sufficient studies (i.e., > 5 studies).
RESULTS
Study, Sample, and Intervention Characteristics
Study, sample, and intervention characteristics of the 10 included studies are provided in Table 1. Studies were conducted in several provinces: Western Cape,[19–22] Eastern Cape,[16–18] KwaZulu-Natal,[15] Gauteng,[23] and Limpopo.[21] South African youth were typically recruited through school or college (80%); two studies recruited youth, at least in part, from the community.[18, 19] Studies were published in peer-reviewed journals between 2007 and 2013 (date of publication: November 2007 through March 2013) with a median publication date of 2011; data were collected between 2002 and 2009. Methodological quality (MQ) of the studies ranged from 9 to 22 (mean = 15, SD = 4). Neither publication date (r = .35, P = .33) nor year of data collection (r = .18, P = .62) was correlated with MQ.
Table 1.
Study, sample, and intervention characteristics of the 10 studies (k = 11) included in the meta-analysis.
| Citation | Sample | Baseline Sexual Behaviors† |
Setting | Intervention Characteristics* | Control | MQ | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Facilitators | Delivery Method |
Intervention Level |
Sessions | Total minutes |
||||||
| Cupp et al. [15, 38, 39] | N = 1,057; 53% F; Ages 13 to18; Alc Use=36% | Partners: NR PVA: 89% |
Schools in KwaZulu-Natal | Peers; Teachers | Technology-Assisted FTF | GRP | 15 | 525 | RCNM | 15 |
| Heeren et al. [16] | N=201; 53% F; Mean age=21; Alc Use = 36% | Partners: 20% PVA: NR |
University in Eastern Cape Province | Postgraduate University Student Trainees | FTF | GRP | 4 | 360 | IRCM | 22 |
| Jemmott et al.[17, 40] | N=1,057; 53% F; Mean age=12; Alc Use = NR | Partners: 14% PVA: 80% |
Schools in Mdantsane and Berlin, Eastern Cape Province | Project Staff | FTF | GRP | 12 | 720 | IRCM | 18 |
| Jewkes et al.[41–43] | N=2,776; 51% F; Ages 15 to 26; Alc Use=14% | Partners: 30% PVA: 41% |
Community Volunteers and STD Clinic Patients in Mthatha, Eastern Cape Province | Planned Parenthood Association of South Africa Staff | FTF | GRP | 17 | 3060 | RCNM | 17 |
| Mash and Mash [19, 44] | N=1,352; 64%F; Mean age=15; Alc Use = NR | Partners: NR PVA: 58% |
Church youth groups in Western Cape Province | Peers; Youth Group Leaders | FTF | GRP | 20 | 1800 | WL/NT | 9 |
| Mason-Jones et al.[20] | N=3,934; 57% F ; Ages 15 to 16; Alc Use=NR | Partners: NR PVA: 69% |
Schools in Western Cape Province | Peers | FTF | GRP/IND | NR | NR | IRCNM | 14 |
| Mathews et al.[21, 45–49] | Cohort #1 N=5352; 53% F; Mean age=13 Alc Use=NR |
Partners: NR PVA: 45% |
Schools in Cape Town, Western Cape Province | Teachers; Nurses | FTF | GRP | 16 | 1020 | WL/NT | 18 |
| Cohort #2 N=2590; 55% F; Mean age=13; Alc Use=NR |
Partners: NR PVA: 30% |
Schools in Mankweng, Limpopo Province | Teachers; Nurses | FTF | GRP | 10 | 660 | WL/NT | 17 | |
| Tibbits et al.[22, 50–53] | N=251; 51% F; Mean age=14 ; Alc Use=15% | Partners: NR PVA: 51% |
Schools in Mitchell’s Plain Township near Cape Town, Western Cape Province | Teachers | FTF | GRP | 36 | 1800 | WL/NT | 15 |
| Visser et al.[23] | N=1,918; 54% F ; Ages 13 to 20; Alc Use=26% | Partners: 12% PVA: 52% |
Schools in Tshwane, Gauteng Province | Peers; Teachers; Postgraduate University Student Trainees | FTF | GRP | NR | NR | WL/NT | 9 |
Note. NR = not reported; N, number of participants who consented to participate in the study; F, females; Alc, alcohol; PVA, protected vaginal or anal sex; FTF, face-to-face; GRP, group; IND, individual; RCM relevant content matched for time; RCNM, relevant content not matched for time; IRCM, irrelevant content matched for time; IRCNM, irrelevant content not matched for time; WL/NT, wait-list/no treatment/assessment only control; MQ, methodological quality.
In some case, intervention details (number of sessions and/or total minutes) were estimated based on range of values and/or details reported.
Baseline sexual behaviors are provided as the (1) proportion of participants with multiple sexual partners and (2) proportion of participants who reported engaging in protected vaginal or anal sex (PVA).
Of the 22,788 youth who consented to participate in the studies, more than half were female (54%), most were Black-African (79%), 14 years of age (6 out of 10 studies reported the mean age of the sample), and not sexually active (66%). Retention was 71% at follow-up. Among the participants who were sexually active at baseline, 17% reported having multiple partners (4 out of 10 studies reporting) but 52% reported any protected vaginal or anal sex (9 out of 10 studies reporting). Of the studies reporting substance use, 26% and 7% of the studies sampled youth who used alcohol or drugs, respectively. Only two studies reported sexual coercion; of these studies, 15% of participants had experienced forced or coerced sex.
Most studies randomized participants to the intervention group (70%); 3 studies used a quasi-experimental design. Interventions were typically conducted over 16 sessions (range = 4 to 36) with each session lasting a median of 66 minutes (range = 35 to 180). On average, the total dose of the intervention was a median of 17 hours (range = 6 to 51). The intervention was typically led by a single facilitator (range = 1 to 5); facilitators were professionals (e.g., teachers, nurses; 55%), peers (9%), professionals-in-training (e.g., clinical graduate student; 9%) or a combination of facilitators (27%). Facilitators delivered the interventions most often in groups with a median of 20 participants. All of the interventions provided education regarding sexual risk behaviors and HIV, 55% provided alcohol education, and 36% provided other education (e.g., reproductive health). Many interventions addressed HIV-related attitudes (63%), social norms (36%), or motivational factors (63%). Most interventions encouraged the identification of high-risk situations (82%) or barriers to safer sex (45%). Each intervention provided some skills training either in communication (82%), self-management (82%), or condoms (63%). Gender power inequalities and violence (e.g., relationship power, rape myth beliefs, intimate partner violence) were addressed in 55% of the interventions. None of the interventions reported providing alcohol skills training. Less than half (45%) reported asking youth to set risk-reduction goals. Condoms were provided in 18% of the interventions.
Control conditions were an active comparison (55%; e.g., brief form of the intervention) or an assessment-only control (45%). Active comparisons were delivered in small groups by a median of 1 facilitator over 4 sessions (range = 1 to 12) with each session lasting a median of 90 minutes (range = 35 to 150). Total dose for the active comparison conditions ranged from 2.5 to 12 hours.
Impact of Interventions Compared with Controls
The weighted mean effect sizes, d+, for the 11 studies examining differences between intervention and control conditions are provided in Table 2. South African youth participating in an intervention reported delaying sexual intercourse (fixed-effects: d+s =0.07, 0.15), increasing their condom use (fixed-effects: d+s =0.17, 0.19), and reducing the number of sexual partners (fixed-effects: d+s =0.95, 0.44) relative to those in a control condition. No differences in alcohol or drug use were found. The pattern of results was generally consistent using fixed- or random-effects assumptions except for multiple sexual partners. Differences between the common intervention effect (fixed-effects assumptions) and the estimate of the actual effect (random-effects assumptions) for multiple sexual partners is largely due to the strong treatment effects (ds >1.00) observed for Jemmott et al.[17] When the studies’ last available assessments were considered, youth participating in the interventions reported delaying sexual intercourse (fixed-effects: d+ = 0.04, 95% CI = 0.01, 0.08), increasing condom use (fixed-effects: d+ = 0.13, 95% CI = 0.09, 0.18), and reduced the number of sexual partners (fixed-effects: d+ = 0.43, 95% CI = 0.34, 0.53) compared to controls. There was an overall trend for interventions to lower the incidence of HSV-2 (k = 2, d+ = 0.17, 95% CI = 0.09, 0.25) but this was reported in only a single study reporting outcomes separated by gender (d young men = .22; d young women = .12). No differences in HIV were found between intervention and control participants.
Table 2.
Weighted mean effect sizes and homogeneity statistics by follow-up interval*
| d+ (95% CI) | d+ (95% CI) | Homogeneity of effect sizes | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Outcome | k | Fixed effects | k | Random effects | Q | P | I2 (95% CI) |
| Early Assessments (<52 weeks) | |||||||
| Abstinence/delay of sex | 6 | 0.07 (0.02, 0.12) | 6 | 0.15 (−0.05, 0.36) | 82.47 | <.001 | 94% (89, 97) |
| Condom use‡ | 7 | 0.17 (0.11, 0.23) | 7 | 0.23 (0.03, 0.44) | 56.14 | <.001 | 89% (80, 94) |
| Multiple partners | 3 | 0.95 (0.83, 1.07) | 3 | 0.31 (−0.75, 1.36) | 67.38 | <.001 | 97% (94, 98) |
| Late Assessments (≥ 52 weeks) | |||||||
| Abstinence/delay of sex | 6 | 0.15 (0.11, 0.20) | 6 | 0.19 (0.05, 0.33) | 43.79 | <.001 | 89% (78, 94) |
| Condom use‡ | 6 | 0.19 (0.13, 0.25) | 6 | 0.18 (0.01, 0.36) | 36.16 | <.001 | 86% (72, 93) |
| Multiple partners | 3 | 0.44 (0.35, 0.53) | 3 | 0.18 (−0.77, 1.14) | 181.15 | <.001 | 99% (98, 99) |
| Alcohol use | 2 | 0.06 (−0.03, 0.15) | 2 | −0.03 (−0.37, 0.30) | 9.28 | .002 | 89% (60, 97) |
| Drug use | 3 | 0.05 (−0.02, 0.12) | 3 | 0.04 (−0.16, 0.24) | 14.13 | .001 | 86% (59, 95) |
| HSV-2 | 2 | 0.17 (0.09, 0.25) | 2 | 0.17 (0.07, 0.26) | 1.31 | .253 | 23% (0, 67) |
| HIV | 2 | −0.10 (−0.19, 0.02) | 2 | −0.10 (−0.24, 0.03) | 2.56 | .110 | 61% (0, 93) |
| Last Assessment† | |||||||
| Abstinence/delay of sex | 10 | 0.04 (0.01, 0.08) | 10 | 0.12 (−0.01, 0.24) | 121.18 | <.001 | 93% (88, 95) |
| Condom use‡ | 10 | 0.13 (0.09, 0.18) | 10 | 0.17 (0.04, 0.29) | 60.68 | <.001 | 85% (74, 91) |
| Multiple partners | 4 | 0.43 (0.34, 0.53) | 4 | 0.09 (−0.75, 0.93) | 183.95 | <.001 | 98% (97, 99) |
| Alcohol use | 2 | 0.06 (−0.03, 0.15) | 2 | −0.03 (−0.37, 0.30) | 9.28 | .002 | 89% (60, 97) |
| Drug use | 3 | 0.03 (−0.04, 0.10) | 3 | 0.04 (−0.36, 0.44) | 51.90 | .001 | 96% (92, 98) |
| HSV-2 | 2 | 0.17 (0.09, 0.25) | 2 | 0.17 (0.07, 0.26) | 1.31 | .253 | 23% (0, 67) |
| HIV | 2 | −0.10 (−0.19, 0.02) | 2 | −0.10 (−0.24, 0.03) | 2.56 | .110 | 61% (0, 93) |
Weighted mean effect sizes, d+, for which the 95% confidence interval does not include a zero are significant. Effect sizes are based on fixed- and random-effects assumptions. k, number of interventions. Boldface text highlights significant values.
On average, the last assessment occurred 65 weeks post-intervention and ranged from 0 to 104 weeks (median = 78 weeks).
All of the effects were heterogeneous except for HSV-2 and HIV at last assessments. Examination of I2 confirmed moderate to high levels of heterogeneity. Moderator tests were conducted to examine whether a priori determined sample (proportion of the participants who were female, alcohol users, have multiple partners, and had protected vaginal or anal sex), intervention facilitators and dose (number and type of facilitators, number of sessions and total dose), and intervention content (gender inequalities, alcohol use, condom skills-training, and social norms) related to the variability in effect sizes (reported below). Due to insufficient sample size (k ≤ 5), moderator tests were conducted only for delay in sexual intercourse and condom use at last assessment.
Moderators of Behavioral Outcomes
Moderators of intervention impact on the delay in sexual intercourse and condom use at the last assessment are reported in Table 3. Interventions were successful in delaying sexual intercourse when (a) sampling youth who used alcohol and (b) more facilitators were used to deliver the intervention but were less successful when the (c) facilitators were professionals (e.g., teachers, nurses), (d) intervention was delivered in longer doses, and the intervention content addressed (e) social norms, (f) gender inequalities, and (g) alcohol or provided (h) condom skills training. Intervention dose was not a significant moderator of the delay in sexual intercourse after adjusting the p-value for the number of statistical tests (Bonferroni corrected p-value: P < .005). Interventions were more successful in increasing condom use when (a) sampling youth already engaging in fewer risk-taking behaviors (i.e., fewer sexual partners, protected vaginal or anal sex), (b) more facilitators were used to deliver the intervention, and (c) the intervention delivery was less intensive (i.e., delivered in fewer sessions and over a briefer period of time). Interventions that provided condom-skills training or addressed gender inequalities were more successful; including an alcohol component reduced the success of the intervention to improve condom use. None of the intervention components (i.e., condom skills-training, gender inequalities, and alcohol education/risks) were significant after adjusting the p-value for the number of statistical tests (Bonferroni corrected p-value: P < .004).
Table 3.
Moderators of delay in sexual intercourse and condom use at last assessment.
| Delay in Sex | Condom Use | |||
|---|---|---|---|---|
| β | P | β | P | |
| Sample Characteristics | ||||
| Young women (%) | .064 | .479 | .046 | .719 |
| Alcohol use, % at baseline | .996 | .000 | .292 | .566 |
| Multiple partners, % at baseline | NA | NA | −.463 | .003 |
| PVA, % at baseline | NA | NA | .406 | .002 |
|
| ||||
| Intervention Facilitators and Dose | ||||
| Facilitators (no.) | .768 | .000 | .499 | .001 |
| Paraprofessional (vs. others) | −.513 | .000 | .310 | .016 |
| Sessions (no.) | −.095 | .316 | −.545 | .000 |
| Intervention dose (total) | −.242 | .011 | −.543 | .000 |
|
| ||||
| Intervention Components | ||||
| Social norms | −.428 | .000 | −.172 | .182 |
| Skills, condom use | −.513 | .000 | .300 | .020 |
| Gender inequalities | −.315 | .001 | .345 | .007 |
| Alcohol education/risks | −.345 | .000 | −.345 | .007 |
Note. Fixed-effects regression models used the inverse of the variance for each effect size as weights. Reported coefficients (β) are standardized. Bold typeface values are significant; values underlined are significant after adjusting the p-value for the number of statistical tests performed (Bonferroni; delay in sex: P < .005; condom use: P < .004). NA, not applicable.
DISCUSSION
The HIV epidemic has had a devastating impact on South African young people who bear the heaviest HIV burden of any age group.[1] In an effort to reduce the incidence of HIV among youth, the South African government mandated an education program, Life Orientation, that encompasses a broad range of topics including HIV prevention, for all 8th grade classes in 2002.[54] Life Orientation is now required for all secondary school students, grade 8 to 11.[55] Multiple sociodemographic, cultural, and social challenges has affected the implementation quality (i.e., fidelity) of the Life Orientation program.[56, 57] Teaching safer sex practices, other than abstinence, created a moral challenge for many educators; in addition, students report being uninterested in the didactic components of the curriculum, preferring the role-plays, but these were often omitted due to time constraints. [56, 57] Alternative or supplementary school-based and community-level HIV intervention programs to reduce sexual risk-taking among South African youth have been developed, implemented, and evaluated in recent years. Studies included in the current meta-analysis were published between 2007 and 2013 with data collection occurring between 2002 and 2009. Most of these studies consisted of alternative interventions (80%) but two studies used or supplemented the mandated Life Orientation program for the intervention.[20, 22]
This meta-analysis examined 10 studies (obtained from 9 manuscripts) that evaluated a behavioral HIV intervention to reduce sexual risk among 22,788 South African youth between the ages of 9 and 26. Our results show that behavioral HIV interventions are successful at delaying sexual intercourse and increasing condom use at early and late assessments. Moreover, intervention success for delaying sexual intercourse and condom use was sustained over 104 weeks (average of 52 weeks) with effect size of small to medium magnitude (d+s = 0.04 to 0.43). The magnitude of the weighted mean effect sizes for delaying sexual intercourse and condom use were stronger than those obtained in a meta-analysis of behavioral HIV interventions among youth in sub-Saharan Africa more broadly.[9] The stronger effects observed in the current meta-analysis could be due to a number of factors such as targeted sample (i.e., South Africa vs. sub-Saharan Africa), school and/or community setting, as well as an increase in methodologically strong, theory-driven behavioral HIV interventions being implemented in South Africa. For example, Jemmott et al. [17] conducted a randomized controlled trial to assess the efficacy of a theory-driven school-based HIV intervention among sixth-grade students living in South Africa and showed that the intervention was successful in reducing unprotected vaginal sex, vaginal sex, and multiple partners. Supplemental analyses (not shown) confirm our assumption that methodologically stronger interventions were more successful in increasing condom use (P = .014).
Moderator tests suggest that sample (e.g., prior alcohol use, sexual risk behaviors at baseline) and intervention features (e.g., facilitators and dose; interventions that address social norms, condom skills, gender inequalities, and alcohol) enhance the impact of the intervention on delaying sexual intercourse or increasing condom use. Alcohol use is associated with risky sexual behavior among young people in South Africa [58, 59] and a higher incidence of HIV among adolescents and adults in Africa.[60] Because alcohol use is a risk factor for HIV, we expected that alcohol users would be less likely to delay sexual intercourse or increase condom use. Contrary to our expectations, alcohol use was associated with an increase in the delay of sexual intercourse but did not moderate the impact of the intervention on condom use. Only 5 of the 10 studies provided baseline data on alcohol use; data for these studies were collected between 2002 and 2004. More recent studies did not report the proportion of youth who consumed alcohol. Our finding that alcohol use moderated the efficacy of the intervention to delay sexual intercourse may be spurious; that is, it may be a failure of more recent studies to measure and/or report alcohol consumption. Consistent with our hypothesis, we found that youth engaging in lower levels of sexual risk-taking were more likely to use a condom following the intervention. Exposure to a behavioral HIV intervention most likely reaffirmed already established protective behaviors (cf. confirmatory bias [61]). Thus, providing an intervention to young people who are currently engaging in safer sex behaviors may serve as a “booster.”
Consistent with prior reviews [11, 62], our meta-analysis does not fully support the use of peer-led interventions. Our moderator tests also show that using professionals (e.g., health educators, teachers, nurses) alone vs. peers or a combination of professionals and peers moderated the efficacy of the intervention on both delay of sexual intercourse and condom use but the direction of our findings differed by outcome. That is, interventions that were facilitated by peers or a combination of peers and professionals are more successful at delaying sexual intercourse (peers/combo: d+ = 0.18, 95% CI, 0.11, 0.23; professionals: d+ = −0.03, 95% CI, −0.08, 0.01) but intervention facilitated by professionals (vs. peers or a combination of peers and professionals) was more successful at increasing condom use (professionals: d+ = 0.39, 95% CI, 0.28, 0.50; peers/combo: d+ = 0.06, 95% CI, −0.02, 0.13). This is a novel finding that will need to be explored in future meta-analyses with a larger set of studies. Nonetheless, our findings suggest that peers may play an important role in promoting abstinence (unrelated to peer social norms regarding HIV risk) while professionals (e.g., teachers) may be required to provide condom education and skills-training to sexually active students. Consistent with this finding, we show that interventions providing condom skills-training were more successful at increasing condom use but less successful at delaying sexual intercourse.
Gender inequality and violence have long been recognized as critical structural barriers to HIV prevention.[63] School-age girls and young women between the ages of 15 and 24 more than twice as likely to be infected by HIV than young men.[64] One important reason for the higher HIV prevalence among young women is the frequent practice of age-discrepant partnering, in which older men, who are more likely to be infected with HIV, form sexual relationships with younger women.[65–67] Young women in relationships with older men are less able to negotiate safer sex, and in fact may be subjected to violence for insisting on condom use.[3, 68] This discrepancy highlights the pervasive gender power imbalance, particularly with many young women engaging in age-discrepant sexual relationships, putting them at greater risk for HIV infection. Few interventions have systematically addressed gender inequalities and violence including relationship power and sexual coercion.[69] Of the interventions reviewed for this meta-analysis, approximately one-half (55%) addressed gender-related issues as a component of the intervention. Moderator analyses revealed that addressing gender inequalities improved the intervention impact on condom use (although, this finding was not significant after applying the Bonferroni correction) but reduced the intervention impact on the number of youth who delayed sexual intercourse. Addressing gender inequalities may reduce young men’s perpetration of sexual violence and may empower women to engage in protected sex (cf. Stepping Stones [18, 70]). Addressing gender inequalities is unlikely be to be key motivator of delaying sexual intercourse. (cf.[71]). In contrast, interventions were less successful at delaying sex and increasing condom use when the intervention provided alcohol education and/or addressed alcohol-related risks (e.g., sex under the influence of alcohol). This finding may be due to low baseline rates of alcohol use (i.e., of the 5 studies reporting baseline alcohol use, 26% reporting consuming alcohol). Nonetheless, our findings are consistent with a systematic review of interventions to prevent sexual risk-taking and substance use among youth from any region.[72] Further research is necessary to determine the efficacy of interventions that include an alcohol component, especially among youth with high rates of alcohol use.
Limitations
Several limitations should be considered when interpreting our findings. First, as with any meta-analysis, using electronic bibliographic databases to identify relevant studies are restricted by publication source and authors’ choice of keywords [73]. Second, our meta-analysis was restricted to studies sampling South African youth and thus may not be generalizable to youth in other regions (e.g., sub-Saharan Africa). Third, all outcomes, except for HSV-2 or HIV, involve self-reports, which are vulnerable to measurement, cognitive (e.g., memory), and social (e.g., self-presentation) biases.[74] Researchers typically use methods to minimize these biases and maximize data quality.[75] Fourth, few studies used a pure control condition; comparison with active conditions lessens observed effects. Fifth, only a single study measured biological outcomes.[18] It is unclear whether incident STIs, including HIV, are reduced following a behavioral HIV risk reduction intervention. Future studies will need to measure biological outcomes to determine whether behavioral changes reduce incidence of STIs. Finally, our moderator tests were limited to the data available in the individual studies. Several studies failed to measure and/or report critical participant characteristics (e.g., baseline alcohol use) that would allow us to fully explore potentially relevant moderators. Thus we were limited in our interpretation for some of the univariate moderator analyses and unable to conduct multiple moderator analyses that may elucidate our findings.
CONCLUSION
Behavioral HIV interventions are successful in reducing sexual risk-taking behaviors and the incidence of STIs. Only a single study found reductions in the incidence of herpes simplex virus-2, but not HIV. Future interventions should measure biological outcomes to determine whether behavioral changes are successful in reducing STIs, including HIV. Implementing behavioral HIV interventions to improve condom use, and ultimately reduce the transmission of HIV, should be a public health priority among South African youth. Despite the prevalence of HIV among youth, to date few interventions have been implemented among South African youth. These interventions should target youth living in the highest HIV prevalence settings (e.g., KwaZulu-Natal) and in areas where alcohol problems are pervasive (e.g., Western Cape and Eastern Cape). Addressing gender inequalities at multiple levels (e.g., individual and structural) will be important for reducing the incidence of HIV.[76, 77] Overall, interventions that exist are not yet targeted as effectively as they need to be to the sub-populations of youth who are at highest risk and in highest prevalence settings of South Africa.
Acknowledgments
Funding: This research was supported by NIH grant R01-AA021355 to Lori A. J. Scott-Sheldon and R01-AA017399 to Seth C. Kalichman.
LIST OF ABBREVIATIONS
- HSV-2
herpes simplex virus type 2
- HIV
human immunodeficiency virus
- STIs
sexually transmitted infections
Footnotes
CONFLIC OF INTEREST
None of the authors have any conflicts that might be interpreted as influencing the research.
AUTHOR CONTRIBUTIONS
- Study concept and design: Scott-Sheldon, Kalichman, Carey
- Acquisition of data: Scott-Sheldon, Walstrom
- Analysis and interpretation of data: Scott-Sheldon, Walstrom, Harrison, Kalichman, Carey
- Drafting of the manuscript: Scott-Sheldon
- Critical revision of the manuscript: Scott-Sheldon, Walstrom, Harrison, Kalichman, Carey
- Statistical analysis: Scott-Sheldon
- Obtaining funding: Scott-Sheldon, Kalichman, Carey
- Administrative, technical, or material support: Walstrom
- Study supervision: Scott-Sheldon
References
- 1.South African National AIDS Council. Global AIDS Response Progress Report. 2012 [cited 2013 March 18]. Available from: http://www.unaids.org/en/dataanalysis/knowyourresponse/countryprogressreports/2012countries/ce_ZA_Narrative_Report.pdf.
- 2.Quinn TC, Overbaugh J. HIV/AIDS in women: an expanding epidemic. Science (New York, NY) 2005;308(5728):1582–3. doi: 10.1126/science.1112489. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman S, O’Sullivan LF, Harrison A, Dolezal C, Monroe-Wise A. HIV risk behaviors and the context of sexual coercion in young adults’ sexual interactions: results from a diary study in rural South Africa. Sexually transmitted diseases. 2006 Jan;33(1):52–8. doi: 10.1097/01.olq.0000187198.77612.d8. [DOI] [PubMed] [Google Scholar]
- 4.Peltzer K, Davids A, Njuho P. Alcohol use and problem drinking in South Africa: Findings from a national population-based survey. African journal of psychiatry. 2011;14:30–7. doi: 10.4314/ajpsy.v14i1.65466. [DOI] [PubMed] [Google Scholar]
- 5.Morojele NK, Brook JS, Kachieng’a MA. Perceptions of sexual risk behaviours and substance abuse among adolescents in South Africa: a qualitative investigation. AIDS care. 2006;18(3):215–9. doi: 10.1080/09540120500456243. Publication Type: Journal Article. [DOI] [PubMed] [Google Scholar]
- 6.Kalichman SC, Simbayi LC, Kaufman M, Cain D, Jooste S. Alcohol use and sexual risks for HIV/AIDS in sub-Saharan Africa: systematic review of empirical findings. Prevention science : the official journal of the Society for Prevention Research. 2007 Jun;8(2):141–51. doi: 10.1007/s11121-006-0061-2. [DOI] [PubMed] [Google Scholar]
- 7.Johnson BT, Carey MP, Marsh KL, Levin KD, Scott-Sheldon LA. Interventions to reduce sexual risk for the human immunodeficiency virus in adolescents, 1985–2000: a research synthesis. Archives of pediatrics & adolescent medicine. 2003 Apr;157(4):381–8. doi: 10.1001/archpedi.157.4.381. [DOI] [PubMed] [Google Scholar]
- 8.Johnson BT, Scott-Sheldon LA, Huedo-Medina TB, Carey MP. Interventions to reduce sexual risk for human immunodeficiency virus in adolescents: a meta-analysis of trials, 1985–2008. Archives of pediatrics & adolescent medicine. 2011 Jan;165(1):77–84. doi: 10.1001/archpediatrics.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michielsen K, Chersich MF, Luchters S, De Koker P, Van Rossem R, Temmerman M. Effectiveness of HIV prevention for youth in sub-Saharan Africa: systematic review and meta-analysis of randomized and nonrandomized trials. AIDS. 2010 May 15;24(8):1193–202. doi: 10.1097/QAD.0b013e3283384791. [DOI] [PubMed] [Google Scholar]
- 10.Paul-Ebhohimhen VA, Poobalan A, van Teijlingen ER. A systematic review of school-based sexual health interventions to prevent STI/HIV in sub-Saharan Africa. BMC Public Health. 2008;8:4. doi: 10.1186/1471-2458-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison A, Newell ML, Imrie J, Hoddinott G. HIV prevention for South African youth: which interventions work? A systematic review of current evidence. BMC public health. 2010;10(102) doi: 10.1186/1471-2458-10-102. (26 February 2010)–(26 February ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pettifor AE, Measham DM, Rees HV, Padian NS. Sexual power and HIV risk, South Africa. Emerging infectious diseases. 2004 Nov;10(11):1996–2004. doi: 10.3201/eid1011.040252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palen LA, Smith EA, Flisher AJ, Caldwell LL, Mpofu E. Substance use and sexual risk behavior among South African eighth grade students. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2006 Nov;39(5):761–3. doi: 10.1016/j.jadohealth.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Ferrett HL, Cuzen NL, Thomas KG, Carey PD, Stein DJ, Finn PR, et al. Characterization of South African adolescents with alcohol use disorders but without psychiatric or polysubstance comorbidity. Alcoholism, clinical and experimental research. 2011 Sep;35(9):1705–15. doi: 10.1111/j.1530-0277.2011.01517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cupp PK, Zimmerman RS, Bhana A, Feist-Price S, Dekhtyar O, Karnell A, et al. Combining and adapting American school-based alcohol and HIV prevention programmes in South Africa: the HAPS project. Vulnerable Children and Youth Studies. 2008;3(2):134–42. Publication Type: Journal Article. [Google Scholar]
- 16.Heeren GA, Jemmott JB, III, Ngwane Z, Mandeya A, Tyler JC. A Randomized Controlled Pilot Study of an HIV Risk-Reduction Intervention for Sub-Saharan African University Students. AIDS and behavior. 2013 Mar;17(3):1105–15. doi: 10.1007/s10461-011-0129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jemmott JB, 3rd, Jemmott LS, O’Leary A, Ngwane Z, Icard LD, Bellamy SL, et al. School-based randomized controlled trial of an HIV/STD risk-reduction intervention for South African adolescents. Archives of pediatrics & adolescent medicine. 2010 Oct;164(10):923–9. doi: 10.1001/archpediatrics.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jewkes R, Nduna M, Levin J, Jama N, Dunkle K, Puren A, et al. Impact of stepping stones on incidence of HIV and HSV-2 and sexual behaviour in rural South Africa: cluster randomised controlled trial. BMJ (Clinical research ed) 2008;337:a506. doi: 10.1136/bmj.a506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mash R, Mash RJ. A quasi-experimental evaluation of an HIV prevention programme by peer education in the Anglican Church of the Western Cape, South Africa. BMJ open. 2012;2(2):e000638. doi: 10.1136/bmjopen-2011-000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mason-Jones A, Mathews C, Flisher A. Can Peer Education Make a Difference? Evaluation of a South African Adolescent Peer Education Program to Promote Sexual and Reproductive Health. AIDS and behavior 2011. 2011/11/01;15(8):1605–11. doi: 10.1007/s10461-011-0012-1. [DOI] [PubMed] [Google Scholar]
- 21.Mathews C, Aarø LE, Grimsrud A, Flisher AJ, Kaaya S, Onya H, et al. Effects of the SATZ teacher-led school HIV prevention programmes on adolescent sexual behaviour: cluster randomised controlled trials in three sub-Saharan African sites. International Health (RSTMH) 2012;4(2):111–22. doi: 10.1016/j.inhe.2012.02.001. Publication Type: Journal Article. Language: English. Subject Subsets: Rural Development. [DOI] [PubMed] [Google Scholar]
- 22.Tibbits MK, Smith EA, Caldwell LL, Flisher AJ. Impact of HealthWise South Africa on polydrug use and high-risk sexual behavior. Health education research. 2011 Aug;26(4):653–63. doi: 10.1093/her/cyr024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Visser MJ. HIV/AIDS prevention through peer education and support in secondary schools in South Africa. SAHARA J : journal of Social Aspects of HIV/AIDS Research Alliance/SAHARA, Human Sciences Research Council. 2007 Nov;4(3):678–94. doi: 10.1080/17290376.2007.9724891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. Journal of epidemiology and community health. 1998;52(6):377–84. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fowkes FG, Fulton PM. Critical appraisal of published research: introductory guidelines. Bmj. 1991 May 11;302(6785):1136–40. doi: 10.1136/bmj.302.6785.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller WR, Brown JM, Simpson TL, Handmaker NS, Bien TH, Luckie LF. What works? A methodological analysis of the alcohol treatment outcome literature. In: Hester RK, Miller WR, editors. Handbook of alcoholism treatment approaches: Effective alternatives. 2. Needham Heights, MA: Allyn & Bacon; 1995. pp. 12–44. [Google Scholar]
- 27.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. New York: Erlbaum; 1988. [Google Scholar]
- 28.Sanchez-Meca J, Marin-Martinez F, Chacon-Moscoso S. Effect-size indices for dichotomized outcomes in meta-analysis. Psychological Methods. 2003 Dec;8(4):448–67. doi: 10.1037/1082-989X.8.4.448. [DOI] [PubMed] [Google Scholar]
- 29.Lipsey MW, Wilson DB. Practical Meta-Analysis. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- 30.Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychological Methods. 2002 Mar;7(1):105–25. doi: 10.1037/1082-989x.7.1.105. [DOI] [PubMed] [Google Scholar]
- 31.Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. Journal of Education Statistics. 1981;6:107–28. [Google Scholar]
- 32.StataCorp. Stata/SE. 12.1 for Windows ed. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- 33.Wilson DB. Meta-analysis macros for SAS, SPSS, and Stata. 2001. [Google Scholar]
- 34.Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychological Methods. 2006 Jun;11(2):193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 35.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002 Jun 15;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 36.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. British Medical Journal. 2003 Sep 6;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hedges LV. Fixed effects models. In: Cooper H, Hedges LV, editors. The Handbook of Research Synthesis. New York: Russell Sage Foundation; 1994. pp. 285–99. [Google Scholar]
- 38.Bhana A, Zimmerman R, Cupp P, Sonja-Feist P. Preliminary findings of the HIV and Alcohol Prevention Programme (HAPS) in South African township schools. Paper presented at the 7th International AIDS Impact Conference; Cape Town, South Africa. 4–7 April; 2005. [Google Scholar]
- 39.Karnell AP, Cupp PK, Zimmerman RS, Feist-Price S, Bennie T. Efficacy of an American alcohol and HIV prevention curriculum adapted for use in South Africa: results of a pilot study in five township schools. AIDS Education & Prevention. 2006;18(4):295–310. doi: 10.1521/aeap.2006.18.4.295. Language: English. Entry Date: 20070216. Revision Date: 20091218. Publication Type: journal article. [DOI] [PubMed] [Google Scholar]
- 40.O’Leary A, Jemmott JB, III, Jemmott LS, Bellamy S, Ngwane Z, Icard L, et al. Moderation and mediation of an effective HIV risk-reduction intervention for South African adolescents. Annals of Behavioral Medicine. 2012;44(2):181–91. doi: 10.1007/s12160-012-9375-4. First Author & Affiliation: O’Leary, Ann. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jewkes R, Nduna M, Levin J, Jama N, Dunkle K, Puren A, et al. Impact of stepping stones on incidence of HIV and HSV-2 and sexual behaviour in rural South Africa: cluster randomised controlled trial. BMJ Clinical research ed. 2008;337:a506. doi: 10.1136/bmj.a506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jewkes R, Nduna M, Levin J, Jama N, Dunkle K, Khuzwayo N, et al. A cluster randomized-controlled trial to determine the effectiveness of Stepping Stones in preventing HIV infections and promoting safer sexual behaviour amongst youth in the rural Eastern Cape, South Africa: trial design, methods and baseline findings. Tropical medicine & international health : TM & IH. 2006 Jan;11(1):3–16. doi: 10.1111/j.1365-3156.2005.01530.x. [DOI] [PubMed] [Google Scholar]
- 43.Jama Shai N, Jewkes R, Levin J, Dunkle K, Nduna M. Factors associated with consistent condom use among rural young women in South Africa. AIDS care. 2010 Nov;22(11):1379–85. doi: 10.1080/09540121003758465. [DOI] [PubMed] [Google Scholar]
- 44.Mash RA. Agents of change: the implementation and evaluation of a peer education programme on sexuality in the Anglican church of the Western Cape. Stellenbosch: Stellenbosch University; 2011. [Google Scholar]
- 45.Flisher AJ, Klepp K-I. School-based HIV/AIDS prevention in Sub-Saharan Africa. 2009. [DOI] [PubMed] [Google Scholar]
- 46.Helleve A, Flisher AJ, Onya H, Mathews C, Aarø LE, Klepp KI. The association between students’ perceptions of a caring teacher and sexual initiation. A study among South African high school students. Health education research. 2011;26(5):847–58. doi: 10.1093/her/cyr031. Publication Type: Journal Article. Language: English. Number of References: 33 ref. Subject Subsets: Rural Development. [DOI] [PubMed] [Google Scholar]
- 47.Mukoma W, Flisher AJ, Ahmed N, Jansen S, Mathews C, Klepp K, et al. Process evaluation of a school-based HIV/AIDS intervention in South Africa. Scandinavian journal of public health. 2009;37:37–47. doi: 10.1177/1403494808090631. Language: English. Entry Date: 20090918. Revision Date: 20110520. Publication Type: journal article. [DOI] [PubMed] [Google Scholar]
- 48.Mũkoma W, Flisher AJ, Helleve A, Aarø LE, Mathews C, Kaaya S, et al. Development and test—retest reliability of a research instrument designed to evaluate school-based HIV/AIDS interventions in South Africa and Tanzania. Scandinavian journal of public health. 2009;37(2 suppl):7–15. doi: 10.1177/1403494809103995. [DOI] [PubMed] [Google Scholar]
- 49.Schaalma H, Aarø LE, Flisher AJ, Mathews C, Kaaya S, Onya H, et al. Correlates of intention to use condoms among Sub-Saharan African youth: the applicability of the theory of planned behaviour. Scandinavian journal of public health. 2009;37:87–91. doi: 10.1177/1403494808090632. Language: English. Entry Date: 20090918. Revision Date: 20110520. Publication Type: journal article. [DOI] [PubMed] [Google Scholar]
- 50.Caldwell L, Smith E, Wegner L, Vergnani T, Mpofu E, Flisher AJ, et al. Health wise South Africa: development of a life skills curriculum for young adults. World Leisure Journal. 2004;46(3):4–17. [Google Scholar]
- 51.Coffman DL, Smith EA, Flisher AJ, Caldwell LL. Effects of HealthWise South Africa on condom use self-efficacy. Prevention Science. 2011;12(2):162–72. doi: 10.1007/s11121-010-0196-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith E, Palen L-A, Caldwell L, Flisher A, Graham J, Mathews C, et al. Substance Use and Sexual Risk Prevention in Cape Town, South Africa: An Evaluation of the HealthWise Program. Prevention Science 2008. 2008/12/01;9(4):311–21. doi: 10.1007/s11121-008-0103-z. [DOI] [PubMed] [Google Scholar]
- 53.Wegner L, Flisher AJ, Caldwell LL, Vergnani T, Smith EA. Healthwise South Africa: cultural adaptation of a school-based risk prevention programme. Health education research. 2008 Dec;23(6):1085–96. doi: 10.1093/her/cym064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.The Department of Education South Africa; The Department of Education South Africa, editor. Revised National Curriculum Statment Grades R-9 (Schools): Overview. Pretoria, South Africa: 2002. pp. 1–36. [Google Scholar]
- 55.The Department of Education South Africa; The Department of Education South Africa, editor. Grades: 10–12. Capetown, South Africa: 2008. Revised National Curriculum Statement; pp. 1–45. [Google Scholar]
- 56.Ahmed N, Flisher AJ, Mathews C, Mukoma W, Jansen S. HIV education in South African schools: the dilemma and conflicts of educators. Scandinavian journal of public health. 2009 Jun;37( Suppl 2):48–54. doi: 10.1177/1403494808097190. [DOI] [PubMed] [Google Scholar]
- 57.Mukoma W, Flisher AJ, Ahmed N, Jansen S, Mathews C, Klepp KI, et al. Process evaluation of a school-based HIV/AIDS intervention in South Africa. Scandinavian journal of public health. 2009 Jun;37( Suppl 2):37–47. doi: 10.1177/1403494808090631. [DOI] [PubMed] [Google Scholar]
- 58.McGrath N, Nyirenda M, Hosegood V, Newell ML. Age at first sex in rural South Africa. Sexually transmitted infections. 2009 Apr;85( Suppl 1):i49–55. doi: 10.1136/sti.2008.033324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frank S, Esterhuizen T, Jinabhai CC, Sullivan K, Taylor M. Risky sexual behaviours of high-school pupils in an era of HIV and AIDS. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2008 May;98(5):394–8. [PubMed] [Google Scholar]
- 60.Fisher JC, Bang H, Kapiga SH. The association between HIV infection and alcohol use: a systematic review and meta-analysis of African studies. Sexually transmitted diseases. 2007 Nov;34(11):856–63. doi: 10.1097/OLQ.0b013e318067b4fd. [DOI] [PubMed] [Google Scholar]
- 61.Koriat A, Lichtenstein S, Fischhoff B. Reasons for confidence. Journal of Experimental Psychology: Human Learning and Memory. 1080;6:107–18. [Google Scholar]
- 62.Kim CR, Free C. Recent evaluations of the peer-led approach in adolescent sexual health education: a systematic review. Perspectives on sexual and reproductive health. 2008 Sep;40(3):144–51. doi: 10.1363/4014408. [DOI] [PubMed] [Google Scholar]
- 63.Greig A, Peacock D, Jewkes R, Msimang S. Gender and AIDS: time to act. Aids. 2008 Aug;22( Suppl 2):S35–43. doi: 10.1097/01.aids.0000327435.28538.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shisana O, Rehle T, Simbayi LC, Zuma K, Jooste S, Pillay-van-Wyk K, et al. South African national HIV prevalence, incidence, behaviour and communications survey 2008: A turning tide among teenagers? Cape Town, South Africa: HSRC Press; 2009. [Google Scholar]
- 65.Dunkle KL, Jewkes RK, Brown HC, Gray GE, McIntryre JA, Harlow SD. Gender-based violence, relationship power, and risk of HIV infection in women attending antenatal clinics in South Africa. Lancet. 2004 May 1;363(9419):1415–21. doi: 10.1016/S0140-6736(04)16098-4. [DOI] [PubMed] [Google Scholar]
- 66.Harrison A, Cleland J, Frohlich J. Young people’s sexual partnerships in KwaZulu-Natal, South Africa: patterns, contextual influences, and HIV risk. Studies in Family Planning. 2008;39(4):295–308. doi: 10.1111/j.1728-4465.2008.00176.x. Publication Type: Journal Article. Note: Special Issue: Adolescent sexual and reproductive health in Sub-Saharan Africa. Language: English. Number of References: many ref. Subject Subsets: Tropical Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mah TL. Prevalence and correlates of concurrent sexual partnerships among young people in South Africa. Sexually transmitted diseases. 2010;37(2):105–8. doi: 10.1097/OLQ.0b013e3181bcdf75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luke N. Age and Economic Asymmetries in the Sexual Relationships of Adolescent Girls in Sub-Saharan Africa. Studies in Family Planning. 2003;34(2):67–86. doi: 10.1111/j.1728-4465.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 69.Jewkes R, Morrell R. Gender and sexuality: emerging perspectives from the heterosexual epidemic in South Africa and implications for HIV risk and prevention. Journal of the International AIDS Society. 2010;13:6. doi: 10.1186/1758-2652-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jewkes R, Wood K, Duvvury N. ‘I woke up after I joined Stepping Stones’: meanings of an HIV behavioural intervention in rural South African young people’s lives. Health education research. 2010 Dec;25(6):1074–84. doi: 10.1093/her/cyq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harrison A, Smit J, Hoffman S, Nzama T, Leu CS, Mantell J, et al. Gender, peer and partner influences on adolescent HIV risk in rural South Africa. Sexual Health. 2012;9(2):178–86. doi: 10.1071/SH10150. Publication Type: Journal Article. Language: English. Number of References: 47 ref. Subject Subsets: Tropical Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jackson C, Geddes R, Haw S, Frank J. Interventions to prevent substance use and risky sexual behaviour in young people: a systematic review. Addiction (Abingdon, England) 2012 Apr;107(4):733–47. doi: 10.1111/j.1360-0443.2011.03751.x. [DOI] [PubMed] [Google Scholar]
- 73.Matt GE, Cook TD. Threats to the validity of research synthesis. In: Cooper H, Hedges LV, editors. The Handbook of Research Synthesis. New York: Russell Sage Foundation; 1994. pp. 503–29. [Google Scholar]
- 74.Schroder KE, Carey MP, Vanable PA. Methodological challenges in research on sexual risk behavior: II. Accuracy of self-reports. Annals of Behavioral Medicine. 2003 Oct;26(2):104–23. doi: 10.1207/s15324796abm2602_03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Del Boca FK, Noll JA. Truth or consequences: the validity of self-report data in health services research on addictions. Addiction. 2000 Nov;95( Suppl 3):S347–60. doi: 10.1080/09652140020004278. [DOI] [PubMed] [Google Scholar]
- 76.Shisana O, Davids A. Correcting gender inequalities is central to controlling HIV/AIDS. Bulletin of the World Health Organization. 2004 Nov;82(11):812. [PMC free article] [PubMed] [Google Scholar]
- 77.Dunkle KL, Jewkes R. Effective HIV prevention requires gender-transformative work with men. Sexually transmitted infections. 2007 Jun;83(3):173–4. doi: 10.1136/sti.2007.024950. [DOI] [PMC free article] [PubMed] [Google Scholar]