Abstract
Luciferase reporter gene assays are one of the most common methods for monitoring gene activity. Because of their sensitivity, dynamic range, and lack of endogenous activity, luciferase assays have been particularly useful for functional genomics in cell-based assays, such as RNAi screening. This unit describes delivery of two luciferase reporters with other nucleic acids (siRNA /dsRNA), measurement of the dual luciferase activities, and analysis of data generated. The systematic query of gene function (RNAi) combined with the advances in luminescent technology have made it possible to design powerful whole genome screens to address diverse and significant biological questions.
Keywords: Luciferase, reporter gene assay, high-throughput screening, RNAi
INTRODUCTION
This unit describes the utilization of luciferase as a genetic reporter system for both Drosophila and mammalian adherent cells. Promoters, enhancers, or other putative cis-acting sequences from a gene of interest can be fused to the coding sequence of an unrelated gene whose activity can be measured. After introduction into cells by transfection or stable integration, the reporter gene then provides an indirect way of measuring how the regulatory sequences influence eukaryotic gene expression.
Luminescent reporter gene assays are based on the light generated by luminescence in a reaction catalyzed by luciferase (de Wet et al., 1987). Historically, luciferase-based reporter gene assays have been used for promoter characterization or gene expression, especially in combination with small molecule screening. Stable cell lines expressing the reporter constructs can be generated (e.g. Cherbas and Cherbas, 2000; Kingston, 2001) as they have the advantage of producing robust assay results (e.g. Chen et al., 2009; Zhao et al., 2012).
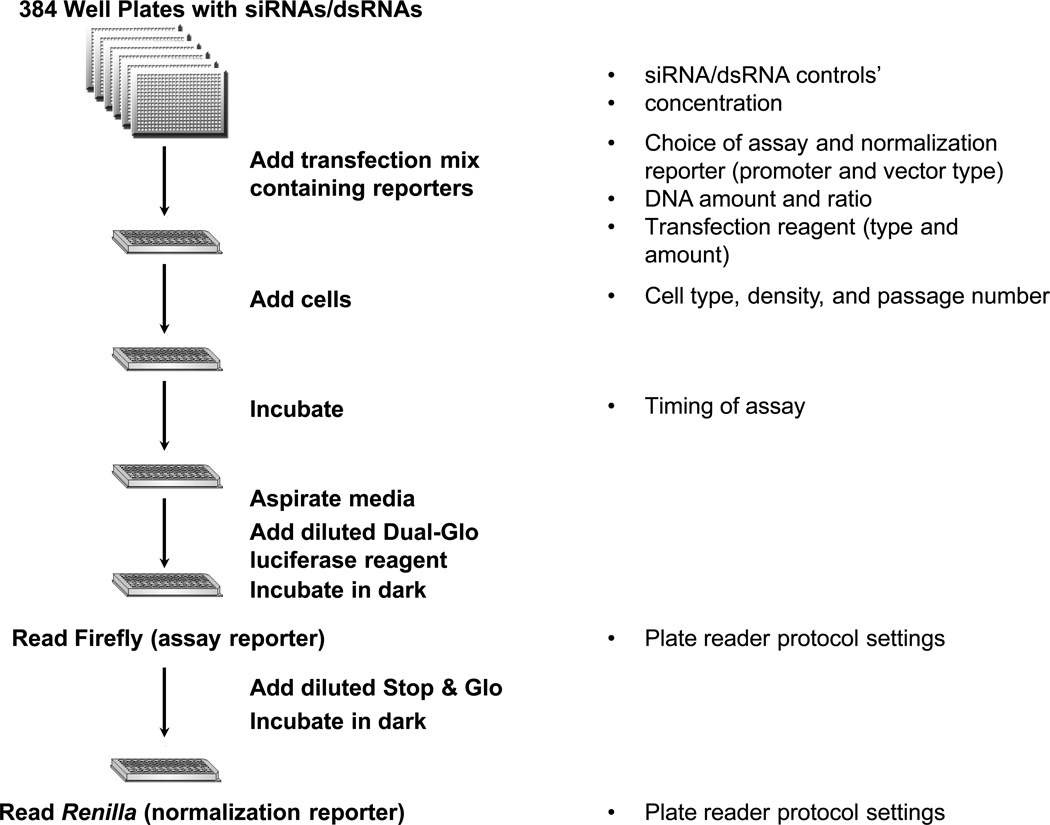
This unit primarily focuses on the utility of luciferase as a cell-based assay for functional genomics, in particular RNAi screening, in both Drosophila and mammalian cells (Fig. 1).
Figure 1.
Outline of an RNAi high-throughput screen using luciferase. Each well in a 384-well assay plate stores a reagent that targets a single gene. A transfection mix with firefly assay and Renilla normalization reporters, and transfection reagent is added to the assay plates, followed by mammalian or Drosophila cells. After incubation, media is aspirated, followed by reading of both firefly and Renilla luciferase activities using the Dual-Glo luciferase assay kit (Promega) and a plate reader. Some factors that influence the each step are listed on the right.
Basic Protocol 1: Reverse transfection of Drosophila cells in 384-well plates describes how to introduce firefly reporter, Renilla normalization reporter, and inducer DNAs along with dsRNAs in a 384-well plate format. Transfections are performed in a reverse format where the nucleic acids and transfection reagents are complexed first, followed by plating of cells.
Alternate Protocol 1: Reverse transfection of HEK293T cells in 384-well plates describes a similar procedure as the basic protocol but with HEK293T cells as an example of mammalian cells.
Both Basic Protocol 1 and Alternate Protocol 1 can be modified to use stable cell lines, and compound treatment alone or in combination with RNAi.
Basic Protocol 2: Measuring firefly and Renilla luciferase activities in Drosophila and mammalian tissue culture cells describes how the luciferase reagent is used and provides suggestions for data analysis. A dual luciferase reagent is directly added to the media to both lyse cells and act as substrates for both firefly and Renilla luciferases.
BASIC PROTOCOL 1: REVERSE TRANSFECTION OF CLONE8 CELLS IN 384-WELL PLATES
In reverse transfection, nucleic acids (plasmid DNAs, dsRNA, siRNA) are complexed with transfection reagent(s), followed by the addition of adherent cells. The order of addition of nucleic acids and cells is reversed compared to conventional transfection. Reverse transfection is a highly efficient method for delivery of nucleic acids into cells and is particularly suitable for high throughput formats where screening libraries (cDNA/ORF, dsRNA/siRNA) are stored in 96 or 384-well plates.
This particular protocol describes the use of Effectene tranfection reagent from Qiagen to transfect dsRNAs and DNAs into the Drosophila imaginal-disc derived Clone8 epithelial cells (an adherent Drosophila cell line) in a 384-well plate format. Use of laboratory automation is not described for this Basic Transfection Protocol or for the Alternative Protocol, but both can be automated using standard instruments such as plate fillers and automated pipettors instead of multi-channel pipets (Rudnicki and Johnston, 2009) if multiple experimental plate are prepared for screening. Both Basic Protocol 1 and the Alternative Protocol 1 can be modified for 96 well plates along with use of stable cell lines and small molecule treatment.
Materials
dsRNAs of interest (~0.016-0.050 ug/ul dsRNA in water)
Drosophila Clone8 cells
Shields and Sang M3 Insect Medium (Sigma S3652)
Firefly luciferase reporter DNA (0.1ug/ul stock) (e.g. Promega pGL3/4 plasmid)
Renilla luciferase normalization DNA (0.1ul/ul stock) (e.g. Promega pRL plasmid)
Inducer DNA (0.1ul/ul stock) (Optional)
Effectene transfection reagent (Qiaqen, cat. No. 1054250)
Compounds from small-molecule libraries (Optional)
384-well white solid bottom plates (e.g. Corning #3570)
20- and 200ul pipets and tips
Adhesive foil (e.g. Corning #6570)
Cell scraper (e.g. BD Falcon 353086)
10ml serological pipets
15mL conical tube (e.g., Falcon)
Hemocytometer
Light microscope
Benchtop centrifuge equipped with conical and plate adapters (e.g. Eppendorf centrifuge 5810 with swing-bucket rotor A-4-81-MTP/Flex)
1.5 ml microcentrifuge tubes
Vortex mixer
Multichannel pipette (capable of 15ul-40ul transfers)
Reagent reservoirs (e.g. Corning #4870)
25°C incubator with wet paper towels (humidified chamber)
Prepare experimental plate
-
1.
Add 5ul of dsRNAs (total dsRNA ~80–200ng per well) to the appropriate wells in a 384-well white solid bottom plate (Corning #3570). Seal plate with adhesive foil, and set aside.
Protocols for dsRNA synthesis are described in Boutros et al. (2004), Ramadan et al. (2007), and listed in Internet Resources. The amount of dsRNA in each well is ~80–200ng. Sealed plates with pre-aliquoted dsRNAs can also be frozen at −20°C. Before use, thaw these plates at room temperature for ~2 hours, centrifuge 2 min at 290 x g, (room temperature), and carefully remove adhesive foil.
White, solid-bottom plates such as Corning #3570 provide the best performance for luminescence assays since they maximize signal and prevent light leakage through the sides of the plate.
A minimum of triplicate transfections is recommended for each sample condition.
Dissociate cells from tissue culture plates/flasks
-
2.
Detach cells using cell scraper or tap the side of the vessel. Triturate the cells in the existing 10mL media by pipetting several times with a serological pipette to remove clumps and obtain single-cell suspension.
-
3.
Transfer into a 15mL conical tube (e.g., Falcon), and spin the cells down in a benchtop centrifuge (e.g. Eppendorf centrifuge 5810) for 3 min at 290 x g, (room temperature). Gently aspirate most of the media from top of pellet.
Some media can be left to ensure that cells are not lost.
-
4.
Resuspend the cell pellet with 10ml of fresh media by pipetting up and down with a serological pipette 10 times.
Ensure that there are no cell clumps.
-
5.
Use a hemocytometer to count the cells and dilute the Clone8 cells into fresh media (Shields and Sang M3 Insect Medium) at final concentration of 40 × 103 cells per 40µl of media. Set aside the cells until step 11.
The concentration of other Drosophila cell types may require optimization (For S2R+, SL2, and Kc167 cells, it is recommended to use 20 × 103 cells per 40µL). Standardized numbers of cells is critical for consistent transfection efficiency.
Prepare transfection mixes
Note that volumes are given on a per-well basis for a 384-well plate.
-
6.
In a tissue culture hood, combine in a 15 ml conical tube: 25ng per well Firefly luciferase reporter DNA, 25ng per well Renilla control DNA, and 50ng per well inducer DNA.
The total DNA per well is 100ng or 0.1ug in a volume of 1ul per well. All stock DNAs are stored at 0.1µg/µL. The inducer DNA represents an expression vector, which is optional depending on the assay (see DasGupta et al. (2005) for use of this assay including an inducer DNA construct, such as pMK33-Wg to activate the Wingless/Wnt signaling pathway). If an inducer DNA is not used or a stable cell line is used for one of the reporters, carrier DNA can be substituted or the amount of Enhancer should be modified as described in step 8. Luminescence from the firefly luciferase should not exceed 100 times more than the luminescence from the Renilla luciferase to ensure that the firefly luciferase activity is completely quenched. DNA amounts and choice of reporter should be modified so that the firefly luciferase activity does not contribute to the Renilla signal.
-
7.
Add 13ul per well of EC buffer (component of the Effectene transfection kit) to the DNAs. Mix by pipetting 2–3 times.
EC buffer is a DNA condensation buffer from Qiagen that provides optimal salt conditions for efficient DNA condensation.
-
8.
Add 0.8µL per well of Enhancer (component of the Effectene transfection kit) to the DNA/EC buffer; mix by pipetting 10X or vortex 10 secs. Wait 2 mins.
The Enhancer is the DNA-condensing reagent that is used with EC buffer and Effectene reagent. The ratio of DNA to Enhancer should always remain 1:8. Here, 0.1ug of DNA is used with 0.8ul of Enhancer. If the DNA amounts change, the amount of Enhancer should change accordingly.
-
9.
Add 0.25µL of Effectene per well to DNA/EC/Enhancer; mix by pipetting 10X or vortex 10 secs. This is the Transfection Mix.
Note that this protocol uses ~10 times less Effectene reagent than that described in the Qiagen protocol. Higher concentrations of Effectene were found to be toxic for Clone8 cells.
Transfection
-
10.
Carefully remove adhesive foil from the 384-well experimental plate containing the dsRNA library. Moving quickly and using a multichannel pipette and a reagent reservoir, add 15µL per well of the Transfection Mix to the pre-aliquoted dsRNA. Wait 5–10 minutes at room temperature.
-
11.
Gently rock the conical tube containing Clone8 cells from step 5, making sure they are resuspended well.
-
12.
Using a multichannel pipette and a reagent reservoir, add 40ul of the Clone 8 cells to each well of the 384-well plate. Mix the plate gently by rocking.
Transfection efficiency decreases if the Transfection Mix sits for longer than 20 minutes before use, so the Transfection Mix and cells should be added in a timely fashion.
Each well contains a total volume of 60µL (5µL dsRNA+ 15µL Transfection Mix + 40µL of cells).
If the entire plate is not used, fill empty wells with media and/or cells to minimize any edge effects due to uneven evaporation of sample wells.
-
13.
Centrifuge the plate for 1 min at 200 x g, (room temperature) using a benchtop centrifuge with plate adapters (e.g. Eppendorf centrifuge 5810 with swing-bucket rotor A-4-81-MTP/Flex).
-
14.
Incubate the plate in a 25°C incubator with wet paper towels (humidified chamber) until ready to proceed to the luciferase assay (Basic Protocol 2).
Incubation is typically for 3–5 days; the length of incubation varies depending on the assay protocol and knockdown efficiency. Optional: Small molecules can be added at day 3–5, after ensuring knockdown of intended targets (which should be empirically determined in a given cell line), in order to perform modifier screens to identify compounds that can enhance or suppress an RNAi phenotype. Such screens have proved to be beneficial in synthetic lethal screens or for the identification of small molecule modifiers of tumor suppressor/oncogenic pathways (Gonsalves et al., 2011; Castoreno et al., 2010).
ALTERNATE PROTOCOL 1: REVERSE TRANSFECTION OF HEK293T CELLS IN 384-WELL PLATES
Alternate protocol 1 describes reverse transfection of HEK293T cells, but can be modified for other adherent mammalian cell types.
Materials
siRNAs (Ambion/Life Technologies, Dharmacon/ThermoFisher, Sigma, Qiagen)
HEK 293T cells (ATCC, cat. no. CRL-3216)
Phosphate-buffered saline (PBS; Life Technologies, cat. no. 10010-023)
Trypsin (Life Technologies, cat. no. R001–100)
Dulbecco’s modified eagle medium (DMEM; Life Technologies, cat. No 11965167)
Fetal bovine serum (FBS; Life Technologies, cat.no.16000044)
Penicillin-Streptomycin (Life Technologies, cat. no. 15140148)
Firefly luciferase reporter DNA (0.1ug/ul stock) (e.g. Promega pGL3/4 plasmid)
Renilla luciferase normalization DNA (0.1ul/ul stock) (e.g. Promega pRL plasmid)
Opti-MEM 1 Reduced Serum Media (Life Technologies, cat. no. 31985-062)
Lipofectamine 2000 Transfection reagent (Life Technologies, cat. no. 11668-019)
Compounds from small-molecule libraries (Optional)
384-well white solid bottom plates (e.g. Corning #3570)
20- and 200ul pipets and tips
10ml serological pipets
15mL /50ml conical tube (e.g., Falcon)
Benchtop centrifuge equipped with conical and plate adapters (e.g. Eppendorf centrifuge 5810 with swing-bucket rotor A-4-81-MTP/Flex)
Hemocytometer
Light microscope
37°C incubator with 5% CO2
1.5 ml microcentrifuge tubes
Multichannel pipette (capable of 15ul-40ul transfers)
Reagent reservoirs (e.g. Corning #4870)
Prepare experimental plate and cells
-
1.
Add 5ul of siRNA (equivalent to 1.5pmol) to the appropriate wells in a 384-well white, solid-bottom plate (Corning #3570). Seal plate with adhesive foil, and set aside.
In a final well volume of 50ul, 1.5pmol represents a 30nM concentration of siRNA. Concentration of siRNA can be varied due to differences in potency and knockdown efficiency. Sealed plates with prealiquoted siRNAs can be frozen at −20°C. Before use, thaw these plates at room temperature, centrifuge 2 min at 290 x g, (room temperature), and carefully remove adhesive foil.
White solid bottom plates such as Corning #3570 provide the best performance for luminescence assays since they maximize signal and prevent light leakage through the sides of the plate.
A minimum of triplicate transfections is recommended for each sample condition.
Note: split HEK293 cells 1:2 or 1:3 a day before setting up the knockdown experiment
Dissociate cells from tissue culture plates (split the day before)
-
2.
Remove media from 10 cm tissue culture dish with log-phase HEK293T cells (~80% confluency).
Healthy cells at a low passage are important for successful transfection.
For long-term experiments, cell aliquots are frozen and thawed when needed so that the same passage number of cells is used throughout the course of a screen.
-
3.
Rinse cells with 5ml PBS and then remove PBS. Add 3ml of Trypsin to each 10cm tissue culture dish and incubate at 37°C with 5% CO2 for ~ 3 minutes or until cells detach.
Incubation time depends on cell type. Cell detachment is visible after gently tapping the sides of the dish.
-
4.
4. Add 7 ml of DMEM (complete with 10% Fetal Bovine Serum (FBS) and penicillin/streptomycin) and gently triturate cells into a suspension by pipetting 9–10 times with a serological pipette.
-
5.
Transfer the entire cell suspension to a 15ml conical tube (e.g., Falcon), and spin 5 min at 290 × g, (room temperature).
-
6.
Aspirate supernatant and resuspend cell pellet gently in 10ml DMEM+ 10% FBS without penicillin/streptomycin..
Lipofectamine 2000 requires that no antibiotics be added during transfection. Antibiotic addition results in inefficient transfection and cell death.
-
7.
Count cells using a hemocytometer and dilute into fresh DMEM without penicillin/streptomycin at final concentration of 3000 cells per 40ul of medium.
Appropriate densities for other cell types might require optimization. A standardized number of cells across experiments with the same cell line is critical for consistent transfection efficiency.
-
8.
8. Place the conical tube containing the diluted cells on its side in 37°C incubator until ready to add cells for transfection.
Cells can also be prepared during the 20 minute incubation step in step 15.
Prepare transfection mixes
Note that volumes are given on a per well basis for a 384-well plate.
-
9.
In a tissue culture hood, mix the transfection reagent Lipofectamine 2000 gently before use, then into a 1.5 ml microcentrifuge tube, add 0.075ul per well Lipofectamine 2000 to 6ul per well of Opti-MEM 1 Reduced Serum Media. Mix gently by pipetting up and down.
-
10.
In a different 1.5 ml microcentrifuge tube, combine 50ng per well of Firefly luciferase reporter DNA with 25ng per well of Renilla luciferase control DNA and 6.25ul per well of Opti-MEM 1 Reduced Serum Media.
All stock DNAs are stored at 0.1µg/µL so the total volume of the DNAs and Opti-MEM 1 Reduced Serum Media in the tube is 7ul per well. Note: If a stable cell line is used for one of the reporters, carrier DNA can be substituted or the amount of Lipofectamine 2000 may need to be reduced.
Luminescence from the firefly luciferase should not exceed 100 times more than the luminescence from the Renilla luciferase to ensure that the firefly luciferase activity is completely quenched. DNA amounts and choice of reporter should be modified so that the firefly luciferase activity does not contribute to the Renilla signal. Multiple options are available to drive expression of the Renilla luciferase reporter, including CMV, SV40, TK (thymidine kinase), and heat shock (hs) promoters.
-
11.
Mix gently by pipetting up and down and add to appropriate wells of the experimental plate containing siRNAs that was prepared in Step 1. Mix gently by rocking the plate by hand.
-
12.
Incubate the plate for 5 min at room temperature. Spin at 1 min at 290 x g, (room temperature).
-
13.
Add 6ul of the diluted Lipofectamine 2000 to each well.
-
14.
Mix gently by rocking the plate by hand, and spin 1 min at 290 x g, (room temperature).
-
15.
Incubate for 20 min at room temperature.
-
16.
Using a multichannel pipette and a reagent reservoir, add 40ul of the HEK293T cells resuspended in media without antibiotics from step 8 to each well of the 384-well plate. Mix the plate gently by rocking.
If the entire plate is not used, fill empty wells with media and/or cells to minimize any edge effects due to uneven evaporation of sample wells.
-
17.
Centrifuge the plate for 1 min at 200 x g, (room temperature) using a benchtop centrifuge with plate adapters (e.g. Eppendorf centrifuge 5810 with swing-bucket rotor A-4–81-MTP/Flex).
-
18.
Incubate the plate in a 37°C incubator with 5% CO2 until ready to proceed to the luciferase assay.
Incubation is typically for 2–4 days; the length of incubation varies depending on the assay protocol and knockdown efficiency. Small molecule libraries can also be added after siRNA incubation for RNAi-based sensitized chemical genetic screens.
BASIC PROTOCOL 2: MEASURING FIREFLY AND RENILLA LUCIFERASE ACTIVITIES IN DROSOPHILA AND MAMMALIAN TISSUE CULTURE CELLS
This protocol describes the use of Dual-Glo luciferase reagent for the measurement of both firefly and Renilla luciferase in fly and mammalian cells. The optimal time for performing the luciferase assay after transfection will vary based on cell type and DNA sequence of interest driving luciferase expression. The Dual-Glo assay is preferred because of the long-term stability of the luminescence signal (half life of ~2hrs), which allows the reading of multiple microtiter plates (up to 25 plates) prepared in one screening session. Moreover, this assay reads luminescence from the entire cell population within each well, thereby reducing well-to-well variability.
Materials
Dual-Glo Luciferase Assay System (Promega, cat. no. E2920)
Serum-free media
Plate(s) from Basic Protocol 1 or Alternate Protocol 1
15mL conical tube (e.g., Falcon)
10ml serological pipets
Benchtop centrifuge equipped with plate adapters (e.g. Eppendorf centrifuge 5810 with swing-bucket rotor A-4-81-MTP/Flex)
20ul, 200ul, 1000ul pipets and tips
24-channel wand (e.g. V&P Scientific VP 186L-1)
Multichannel pipette
Luminometer or plate reader equipped to read luminescence (e.g. EnVision from Perkin Elmer)
Reagent preparation and Luciferase readings
-
1.
Thaw an aliquot of Dual-Glo luciferase assay reagent in a dark container (e.g. covered ice bucket) filled with room temperature water. Mix well after thawing. Keep the reagent covered and equilibrated to room temperature before use.
After following the directions for reagent preparation by Promega, store aliquots of the Dual-Glo luciferase assay reagent in 15ml conical tubes, covered with foil at −20°C. Limit freeze/thaws of each aliquot to fewer than four times since freeze/thawing affects the stability of the assay reagents.
-
2.
Centrifuge the experimental plate(s) from Basic Protocol 1 or Alternate Protocol 1 for 1 min at 290 x g, (room temperature).
-
3.
Prepare a mix of 20ul of Dual-Glo luciferase reagent and 20ul serum-free media for each well.
Equilibrate all components of the Dual-Glo luciferase assay kit to room temperature before use. The experimental plate(s) should sit at room temperature for 20–30 minutes before adding the Dual-Glo luciferase reagents. This ensures consistent results across each plate, and eliminates a big source of edge effects.
-
4.
Carefully aspirate the media from the assay plate with a 24-channel wand (e.g. V&P Scientific VP 186L-1) or multichannel pipette.
Take care not to aspirate the cells; leaving some media behind in each well is fine. If using a wand, use a low vacuum and make sure that the pins do not touch the bottom of the well.
-
5.
Add 40ul per well of the diluted Dual-Glo reagent and rock the plate to mix so that the cells lyse completely.
-
6.
Incubate plate in the dark for 10 minutes.
When exposed to ambient light, the white solid bottom plates used for luminescence experiments can autofluoresce. Incubating the plate in a dark environment will minimize plate autofluorescence, and therefore help prevent background luminescence.
-
7.
Read the firefly luciferase (FF) in a luminometer or plate reader equipped to read luminescence (e.g. EnVision from Perkin Elmer).
The firefly luciferase values will reflect activity of the reporter. Background readings for firefly luciferase should be made on wells containing cells not transfected with the reporter constructs, but treated under the same experimental conditions (e.g. same sample volume and media/sera conditions) and also with the Dual-Glo reagent. The background readings should be subtracted from the experimental readings as part of the plate reader instrument protocol. It is also highly advisable to run the instrument protocol for cross-talk correction that eliminates contaminating luminescence reads from neighboring wells.
-
8.
Prepare 20ul per well of a 1:100 dilution of Stop & Glo substrate reagent in the Stop & Glo dilution buffer.
Prepare the Stop & Glo assay reagent immediately before use.
-
9.
Add 20ul of diluted Stop & Glo to each well and rock the plate.
The Stop & Glo reagent simultaneously quenches the firefly luciferase activity and begins the Renilla luciferase reaction.
-
10.
Incubate plate in the dark for at least 10 minutes.
Do not exceed 30 minutes because the luminescent signal from Renilla luciferase activity will slowly decay after 30 mins.
-
11.
Read the Renilla Luciferase (RL) in a luminometer or plate reader equipped to read luminescence.
The Renilla luciferase values will reflect activity of the normalization reporter. Background readings for Renilla luciferase should be made on wells containing cells not transfected with any luciferase construct, but treated under the same experimental conditions (eg same sample volume and media/sera conditions) and also with the Dual-Glo reagents. The background readings should be subtracted from the experimental readings as part of the plate reader instrument protocol.
Analysis of Firefly and Renilla data
The suggested data analysis protocols described below were used in DasGupta et al. (2005). Other useful references are listed in the Key References.
-
12.
Calculate the Normalized Value (N) for each well, where N = (firefly luciferase value)/Renilla luciferase value).
The Renilla luciferase acts as an internal control to minimize experimental variability due to pipetting errors, cell viability, and transfection efficiency.
Wells with high N should be examined for low Renilla values which would represent a false positive if looking for increases in reporter activity. This is more evident in situations where the linear range of the Renilla luciferase no longer overlaps with the linear range of the firefly luciferase, especially at the lower end of the Renilla luminescence curve. Plotting N versus the Renilla Luciferase value (RL) for each well is a way to visualize this easily.
-
13.
Calculate Z score of N, which is the number of standard deviations the Normalized Value for a particular well is from the plate average for Normalized Values of all wells.
The z score is defined as =(Nwell-Nplate)/sdplate
Nplate = Mean of N (FF/RL) for all 384 wells for a given screening plate
sdplate = Standard deviation calculated for the entire plate based on the N of each of the 384 wells.
Z score assumes a normal distribution of experimental values.
Alternatively, in many cases it is better to use robust Z-score, which is calculated based on the plate median and the median absolute deviation instead of plate mean and standard deviation (Birmingham et al., 2009). This is especially useful where the experimental values do not represent a normal distribution because of inherent properties of the cell biological process/pathway under study, or the nature of the cell based assay itself (baseline screens versus induced/modifier screens).
-
14.
Calculate log(N) which is log transformation of N, to view both increases and decreases in a linear progression.
-
15.
Calculate the log (N/plate average of N) as a measure of fold change (FC) of a normalized well value from the plate average.
This is especially useful while visualizing up- versus downregulation of reporter activities compared to baseline or untreated samples. For example, while a 2-fold increase is equivalent to a 100% increase, a 2-fold decrease represents a 50% decrease. Therefore depending on the stringencies of the cut-off for “hit” selection, the genes that result in decreased reporter activity upon their RNAi-mediated knockdown may get treated as false negatives, and thereby be eliminated from the hit-list. Log transformation of fold changes (FC) with respect to the baseline control or plate mean/median allows equal weightage to both increases and decreases in assay readout (for example, log (FC of 2) is 0.3 and log (FC of ½) is −0.3).
-
16.
Display data from steps 12–15 graphically by plotting a plate-well series scatter plot and heatmaps with boxplot statistics. Carefully examine behavior of positive and negative controls to help determine cut-off values for candidate hits as well as intra- and interplate well reproducibility.
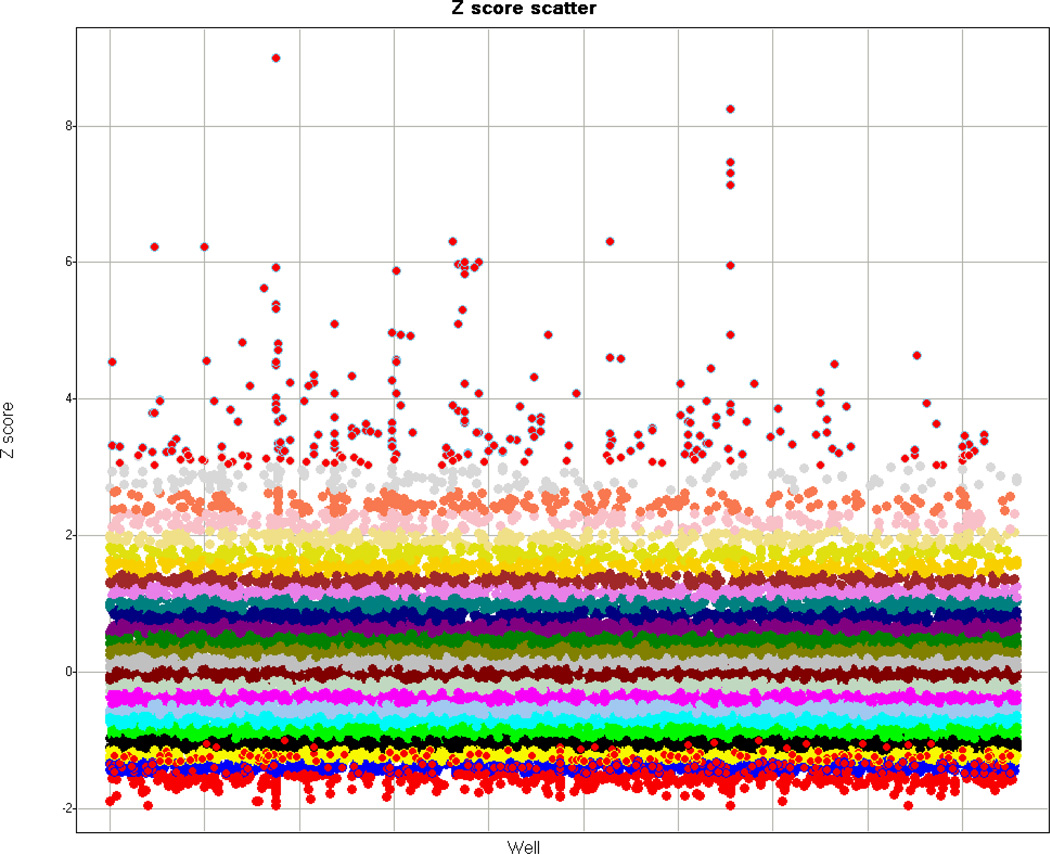
A plate-well series scatter plot displays values well by well and plate by plate in an experiment (Zhang et al., 2006). This type of plot displays plate variability, the experimental scatter, potential candidate hits, and data features that may be common to multiple plates (Fig. 2).
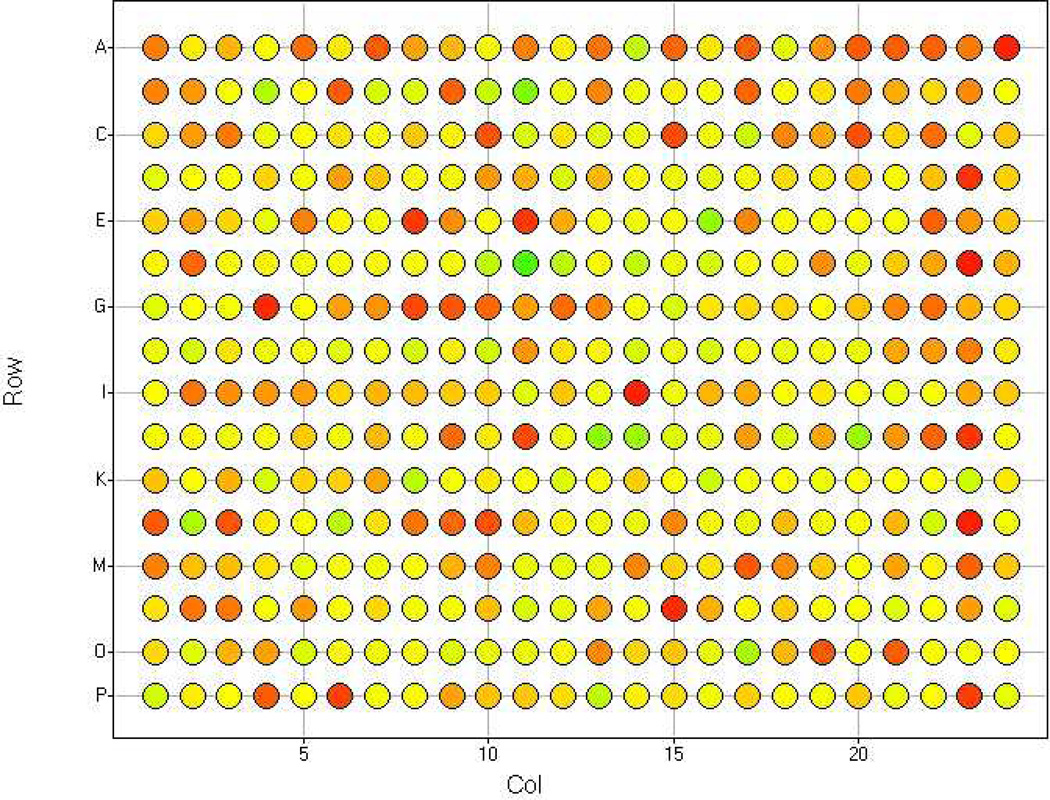
A heat map incorporating boxplot statistics of each plate is highly recommended to reveal any positional effects in the assay plate (Boutros et al., 2004; Fig. 3).
Figure 2.
Example of plate-well series scatter plot. Experimental Z scores are plotted against the wells of each assay plate. The binned Z score values are grouped by color.
Figure 3.
Example of heat map with boxplot statistics for Z score for a single plate. Note that there are no obvious positional or edge effects.
Post-screening experiments
-
17.
Repeat the assay to confirm positives from the primary screen in a smaller scale experiment (e.g. 96-well plate format).
-
18.
Repeat the assay with candidate hits using a reporter with mutated binding sites, a different type of reporter, or in a different cell type.
These experiments serve as controls for the luciferase promoter, the luciferase itself, and the cell line used.
It is also important to repeat the assay after swapping the firefly and renilla luciferase reporters, i.e. using the firefly luciferase as a normalization control and the Renilla as a pathway/process-specific reporter. Such screens eliminate false positives that may non-specifically influence the activity of either the firefly or Renilla luciferase activity.
Mutated binding site: controls for specificity of the effect on the reporter activity to the transcription factor binding sites present in a given reporter
Alternative pathway reporter: controls for the specificity of the effect of knockdown of a particular gene on the activity of one or more signaling pathways (pleiotropic effects, etc) Cell line specificity: controls for the effect of knockdown of a particular gene for a cell type versus a pathway. Ideally, if the pathway under study is active in multiple cell lines, then knockdown of a conserved regulatory gene should result in an identical effect on the reporter, provided it is expressed and active in the different cell lines.
-
19.
Confirm expression and knockdown of candidate dsRNA/siRNA hits by querying ENCODE/modENCODE or online gene expression databases and by carrying out quantitative RT-PCR.
-
20.
Rank and categorize candidate hits based on strength and putative function. Design secondary assays to test functional relevance.
Secondary assays are critical to confirm both the specificity and biological function of the candidate hits from the luciferase assay. They can be performed in a different cell type or species (to assess evolutionarily conserved functions) and/or independent cell-based assasy such as those with imaging readouts. High content imaging of cell-based assays provide information on responses of individual cells and are complementary to the luciferase assay described here, which provides information about the responses of the cell population in each well.
COMMENTARY
Background Information
Luciferase assays are one of the most common types of reporter gene assays performed. Since the cloning of luciferase in 1985 (de Wet et al., 1985), it is been a valuable tool in monitoring gene activity. When luciferin, ATP, Mg2+, and molecular oxygen are combined, luciferase catalyzes a bioluminescent reaction resulting in a flash of light. The emitted light signal is detected using a luminometer or plate reader. Historically a major limitation of luciferase technology was the stability of the signal. Advances in expression vectors (Leclerc et al., 2000; Dougherty and Sanders, 2005), enzyme optimization (Gould et al., 1987; Keller et al., 1987), and reagents have made it more suitable for screening. Luciferase assays are straightforward to perform and highly sensitive with a broad dynamic range.
This unit describes reverse transfection methods for Drosophila and mammalian cultured cells as well as measuring luciferase activities from both cell types. During reverse transfection, nucleic acids are complexed with transfection reagent, followed by the addition of cells. The advantages of reverse transfection compared to typical transfection to introduce expression of nucleic acids into cells include standardized seeding conditions, increased efficiency of delivery, and speeding up workflow especially for systematic high-throughput screens (HTS) that entail simultaneous screening of many experimental conditions.
The luciferase protocol described utilizes the Dual-Glo Luciferase Assay system from Promega which is designed for high throughput assays. In this dual reporter system, both firefly (Photinus pyralis) and Renilla reniformis (sea pansy) luciferase activities are measured in succession within the same well as both are functional enzymes immediately after translation (Wood et al, 1984; De Wet et al 1985). The Firefly luciferase reporter should be engineered to be under the regulatory control of a cloned sequence of interest while the Renilla luciferase is driven by a constitutively active promotor (e.g. CMV, SV40, TK). The Renilla luciferase acts as an internal control for normalization of cell number per well. Cell number might vary from well to well at the time of initial cell plating or due to treatments during the experimental protocol that are cytotoxic or non-specifically perturb transcription or translation. Advantages to the Dual Luciferase protocol include that there is no endogeneous luciferase activity to interfere with assay signal, that it is a simple protocol with few steps and no wash steps (e.g. the Dual-Glo reagent both lyses cells and acts as a substrate for firefly luciferase), and that it produces relatively stabilized luminescence signals for both firefly and Renilla luciferases. In addition, data generated from luciferase assays are relatively straightforward to analyze. The primary disadvantage in performing luciferase assays is the reagent expense.
Alternate methods of monitoring gene expression such as mRNA quantitation are not ideally suited for HTS, especially when screening on a large scale. High content imaging of protein expression is a more indirect method as transcript expression changes are not always clearly reflected at the protein level. Fluorescent reporter assaysare often plagued by high background, issues of a limited linear range, difficulties in detecting both small and large changes (Friedman and Perrimon (2006) describes an EGFR/MAPK whole genome screen that resulted in over 1000 hits in the primary screen). In small molecule screening, fluorescent reporter assays are confounded by fluorescent compounds that can lead to false screening positives (Thorne et al., 2010).
A recent PubMed search using the terms “Luciferase reporter assay” resulted in over 11,000 publications since 1987. This technique is commonly used in promoter structural/function analysis; one example includes characterization of the 5’-flanking promoter of mouse Tcf3 (Solberg et al., 2012), an important effector of the Wnt signaling pathway. Other applications of this approach have also been used in the analysis of signaling pathways (DasGupta et al., 2005; Nybakken et al., 2005) and identification of splicing regulators (Warzecha et al., 2009).
Critical parameters and Troubleshooting
Choice of promoter and cell type
The choice of the regulatory sequence to fuse to the firefly luciferase reporter and of the constitutive control promoter driving Renilla luciferase production is critical. The firefly reporter should reflect activity of the gene or pathway of interest, which can be assessed by including positive and negative controls (e.g. siRNA, dsRNA, compound treatment, mutated binding sites within the promoter) as experimental conditions. In many instances specific artificial reporters need to be constructed that harbor multiple transcription factor binding sites for the pathway/process of interest (Korinek et al., 1998a; Korinek et al., 1998b). Such reporter constructs help increase sensitivity of the assay, eliminate issues related to repressive elements within endogenous promoters, and provide for a “clean” readout of transcriptional activity dependent on a given transcription factor or a cell biological process. That said, secondary screens using endogenous promoters are typically utilized to functionally validate candidate genes identified in HTS. The control Renilla luciferase reporter should always be driven under a promoter which is otherwise not influenced by the activity of the pathway under study, and simply reflects the robustness of cell viability, and transfection efficiency. For example, PolIII-RL, SV40-RL, pCMV-RL, and pIZT-RL are commonly used for normalization.
The cell type used for the luciferase assay should be representative of the biological pathway or process. For example, if screening for modulators of a specific cell signaling pathway, all of the critical components of the pathway should be expressed in the chosen cell line such that the pathway can be activated robustly (high signal to background ratio) that then allows screening for novel components that may regulate the activity of the pathway-specific reporter. Finally, cross-species validation for evolutionarily conserved function using cell lines from a different species (e.g. Drosophila, mouse and human) add tremendous value to the primary screen performed in any given cell type (Gonsalves et al., 2011).
Finally the cells should be efficiently transfectable if planning to use dsRNA or siRNA reagents for screening. Otherwise, for RNAi screening, using short-hairpin RNA (shRNA) libraries should be considered. Furthermore, for cells that are not easily transfectable, it is not practical for luciferase reporters to be introduced by transient transfection as part of the assay protocol; stable luciferase reporter cell lines should be produced and characterized in advance.
Optimization of transfection
Different cell lines are differentially sensitive to a given transfection reagent. Many transfection reagents can be toxic or simply ineffective/inefficient in transfecting nucleic acids into a specific cell type. Therefore choice and concentration of transfection reagent needs to be optimized for every cell type. Another important aspect to keep in mind in the context of these protocols is that while several transfection reagents are optimized for either DNA or siRNAs, there are only a few that work for both simultaneously. This becomes important in siRNA/dsRNA screens with luciferase reporter assays where efficient transfection of both RNA and DNA reagents is essential for robust reporter activity and knockdown.
Determining an optimal cell number for each assay is important for cells that are contact-inhibited versus those that are not. One must determine the optimal number of cells that produce efficient transfection and that provide robust assay readout-plating large numbers of cells can saturate the reporter activity depending on the rate of cell division; if too few cells are plated, reporter signal might be difficult to differentiate from background. Cell passage number is also important. Using a similar low passage of healthy cells for each experiment will help in the reducing overall assay variability.
The quality of DNA also contributes to successful transfection, and the ratio normalization to reporter DNA should be determined empirically.
Systematic processing and assay timing
The use of a master mix for both transfection set-up and the luciferase assay will increase consistency between wells. About 10% more than the calculated required volumes for each reagent and total volume should be prepared to account for pipetting errors.
When dealing with multiple plates, it is important to be mindful of the plate order and timing of each step. Plates should be numbered, and processed in the same order for each step. Consistency is important, especially for comparison of plate sets processed on different days. If screening more than a few plates per session, lab automation such as plate fillers and automated pipettors should be implemented (Rudnicki and Johnston, 2009) to decrease assay variability.
The timing of the luciferase assay-the interval between reporter transfection, RNAi transfection, or compound addition and the luciferase assay-is another variable that should be optimized. The number of days post-transfection/cell perturbation may affect protein expression or accumulation, recovery from transfection, and post-translational modifications. In general most luciferase assays are performed between 2–5 days post-transfection or cell perturbation.
Conditions affecting luciferase reagents and assay
The Dual-Glo luciferase reagent is sensitive to freezing and thawing, with about a 15% loss in firefly relative light units after 5 freeze-thaw cycles (Promega). Aliquots of varying volumes can be stored, and the number of freeze-thaw cycles should be noted. All reagents, including the cells in assay plates should be equilibrated to room temperature before the assay is run since the optimal temperature for firefly and Renilla luciferase is about 20–25°C.
A consistent incubation of less than 20 minutes for both the Dual-Glo luciferase and Stop & Glo reagent should be followed since the luminescent signals will decay over time and at different rates. Background readings, using cells not expressing luciferase reporters, but treated the same experimentally as other samples in the assay, should be subtracted as part of the instrument protocols.
Design of post-screening experiments
Careful consideration should take place when designing experiments to differentiate between false positives generated by assay-dependent features (e.g. due to effects specific to one cell type) and candidate hits that may be biologically relevant. Secondary assays should be designed to be robust, at least medium throughput, and shed some light on the biological function of the candidate hits from the luciferase assay.
Anticipated Results
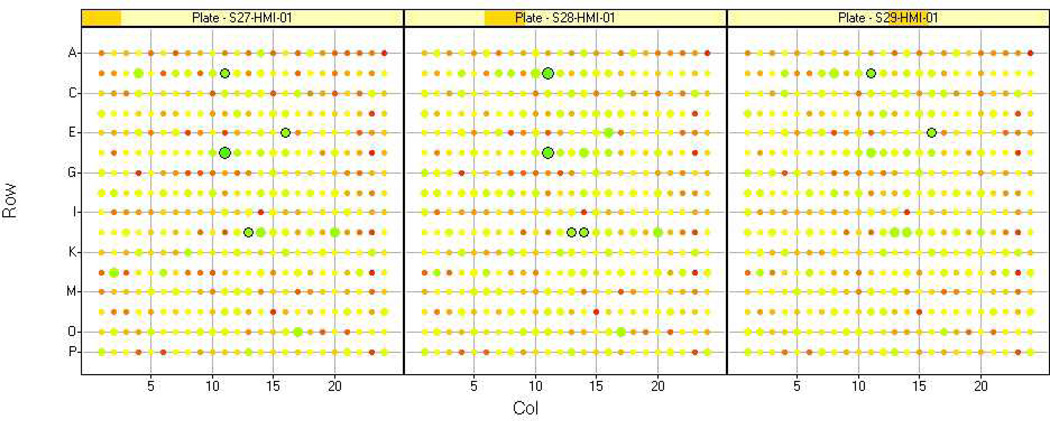
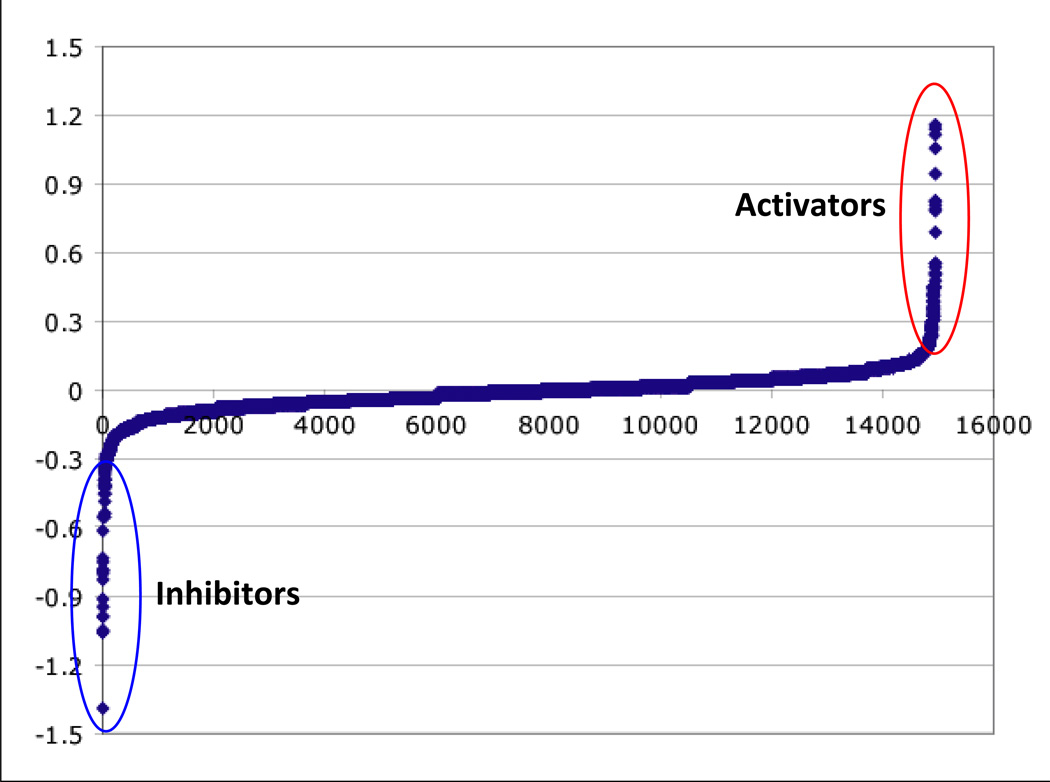
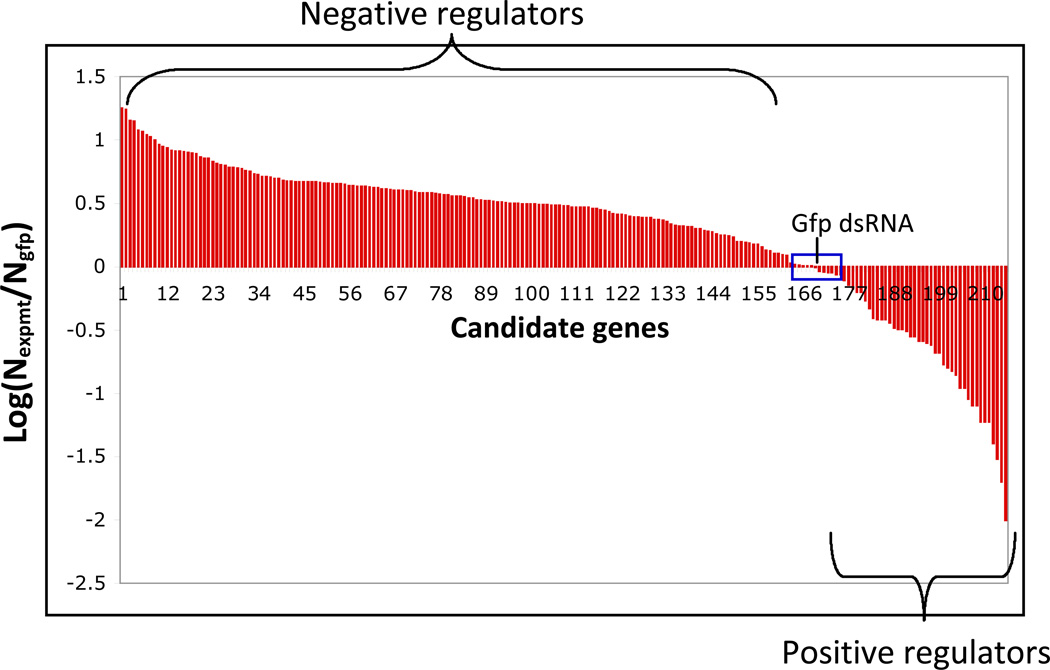
Initially two raw values (firefly and Renilla luciferase readings) are generated per well. The raw values should be normalized and then analyzed by a variety of methods to determine what type of analysis method works for the dataset. Depending on the number of assay plates, data can be analyzed in Excel; however data visualization programs (e.g. Spotfire) are useful for data triage, and in defining cutoffs for identifying screening positives (Fig. 4, Fig. 5) . The number of candidate hits will vary based on the stringency of cut-offs applied and functional clustering. This number will be also be influenced by the capacity of the investigators to perform followup experiments.
Figure 4.
Example of heat map comparison of replicate plates. The Z score value for each well is represented by color and size. Note that the values are highly reproducible between the replicates.
Figure 5.
Examples of distribution of luciferase data from a modifier screen (A.) and a secondary luciferase reporter assay (B.)
Time Considerations
This protocol can be easily completed in less than a week, depending on the interval between transfection and luciferase assay. Reverse transfection using a multi-channel pipet is not recommended for more than a few plates due to the increased chance of errors. A liquid dispenser (e.g. Wellmate from Matrix Technologies; Rudnicki and Johnston, 2009) can be placed inside a tissue culture hood to dispense reagents and cells quickly and accurately. Using this type of lab automation, twenty or more 384-well plates can be processed at a time.
Acknowledgements
We would like to thank Foster Gonsalves, and members of the DasGupta lab, and NYU RNAi Core staff for useful discussions. We gratefully acknowledge the support from the Helen L. and Martin S. Kimmel Center for Stem Cell Biology, NYUCI Center Support Grant, “NIH/NCI 5 P30 CA016087-32”, NIH/NCI #1R01CA155125-01 (R.D) and NYSTEM contract C026719.
Footnotes
INTERNET RESOURCES
http://www.flyrnai.org/DRSC-TOO.html
This site links to the On-Line Tool Box from the Drosophila RNAi Screening Center at Harvard Medical School.
http://www.flyrnai.org/DRSC-PRS.html
This links to protocols for dsRNA synthesis from the Drosophila RNAi Screening Center at Harvard Medical School.
http://miare.sourceforge.net/HomePage
This link for the Minimum Information About an RNAi Experiment provides information about proposed reporting guidelines for data exchange and comparison between datasets.
Contributor Information
Chi Yun, Email: chi.yun@nyumc.org, New York University School of Medicine, NYU RNAi Core, Department of Biochemistry and Molecular Pharmacology, Skirball Institute, Lab 3-7, 540 First Avenue, New York, NY 10016, Ph. (212) 263-9080, Fax (212) 283-7984.
Ramanuj DasGupta, Email: ramanuj.dasgupta@nyumc.org, New York University School of Medicine, NYU Cancer Institute, Department of Biochemistry and Molecular Pharmacology, Smilow Research Building, Rm 1211, New York, NY 10016, Ph. (212) 263-9247, Fax (212) 263-9210.
LITERATURE CITED
- Birmingham A, Selfors LM, Forster T, Wrobel D, Kennedy CJ, Shanks E, Santoyo-Lopez J, Dunican DJ, Long A, Kelleher D, Smith Q, Beijersbergen RL, Ghazal P, Shamu CE. Statistical methods for analysis of high-throughput RNA interference screens. Nat Methods. 2009;6:569–575. doi: 10.1038/nmeth.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros M, Kiger AA, Armknecht S, Kerr K, Hild M, Koch B, Haas SA, Consortium HF, Paro R, Perrimon N. Genome-wide RNAi analysis of growth and viability in Drosophila cells. Science. 2004;303:832–835. doi: 10.1126/science.1091266. [DOI] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, Roth MG, Amatruda JF, Chen C, Lum L. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbas L, Cherbas P. In: Drosophila cell culture and transformation. In Drosophila Protocols. Sullivan W, Ashburner M, Hawley RS, editors. New York: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- DasGupta R, Kaykas A, Moon RT, Perrimon N. Functional genomic analysis of the Wnt-wingless signaling pathway. Science. 2005;308:826–833. doi: 10.1126/science.1109374. [DOI] [PubMed] [Google Scholar]
- de Wet JR, Wood KV, Helinski DR, DeLuca M. Cloning of firefly luciferase cDNA and the expression of active luciferase in Escherichia coli . Proc Natl Acad Sci U S A. 1985;82:7870–7873. doi: 10.1073/pnas.82.23.7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet JR, Wood KV, DeLuca M, Helinski DR, Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987;7:725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DC, Sanders MM. Comparison of the responsiveness of the pGL3 and pGL4 luciferase reporter vectors to steroid hormones. Biotechniques. 2005;39:203–207. doi: 10.2144/05392ST02. [DOI] [PubMed] [Google Scholar]
- Friedman A, Perrimon N. A functional RNAi screen for regulators of receptor tyrosine kinase and ERK signalling. Nature. 2006;444:230–234. doi: 10.1038/nature05280. [DOI] [PubMed] [Google Scholar]
- Gonsalves FC, Klein K, Carson BB, Katz S, Ekas LA, Evans S, Nagourney R, Cardozo T, Brown AM, DasGupta R. An RNAi-based chemical genetic screen identifies three small-molecule inhibitors of the Wnt/wingless signaling pathway. Proc Natl Acad Sci U S A. 2011;108:5954–5963. doi: 10.1073/pnas.1017496108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould SG, Keller GA, Subramani S. Identification of a peroxisomal targeting signal at the carboxy terminus of firefly luciferase. J Cell Biol. 1987;105:2923–2931. doi: 10.1083/jcb.105.6.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller GA, Gould S, Deluca M, Subramani S. Firefly luciferase is targeted to peroxisomes in mammalian cells. Proc Natl Acad Sci U S A. 1987;84:3264–3268. doi: 10.1073/pnas.84.10.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston RE. Stable Transfer of Genes into Mammalian Cells. Current Protocols in Immunology. 2001;12:10.17.1–10.17.6. doi: 10.1002/0471142735.im1017as12. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genetics. 1998a;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Willert K, Molenaar M, Roose J, Wagenaar G, Markman M, Lamers W, Destree O, Clevers H. Two members of the Tcf family implicated in Wnt/beta-catenin signaling during embryogenesis in the mouse. Mol Cell Biol. 1998b;18:1248–1256. doi: 10.1128/mcb.18.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc GM, Boockfor FR, Faught WJ, Frawley LS. Development of a destabilized firefly luciferase enzyme for measurement of gene expression. Biotechniques. 2000;29:590–591. doi: 10.2144/00293rr02. 594–6, 598 passim. [DOI] [PubMed] [Google Scholar]
- Nybakken K, Vokes SA, Lin TY, McMahon AP, Perrimon N. A genome-wide RNA interference screen in Drosophila melanogaster cells for new components of the Hh signaling pathway. Nat Genet. 2005;37:1323–1332. doi: 10.1038/ng1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan N, Flockhart I, Booker M, Perrimon N, Mathey-Prevot B. Design and implementation of high-throughput RNAi screens in cultured Drosophila cells. Nature Protocols. 2007;2:2245–2264. doi: 10.1038/nprot.2007.250. [DOI] [PubMed] [Google Scholar]
- Rudnicki S, Johnston S. Overview of liquid handling instrumentation for high-throughput screening applications. Current Protocols in Chemical Biology. 2009;1:43–54. doi: 10.1002/9780470559277.ch090151. [DOI] [PubMed] [Google Scholar]
- Solberg N, Machon O, Krauss S. Characterization and functional analysis of the 5’-flanking promoter region of the mouse Tcf3 gene. Mol Cell Biochem. 2012;360:289–299. doi: 10.1007/s11010-011-1068-y. [DOI] [PubMed] [Google Scholar]
- Thorne N, Auld DS, Inglese J. Apparent activity in high-throughput screening: origins of compound-dependent assay interference. Curr Opin Chem Biol. 2010;14:315–324. doi: 10.1016/j.cbpa.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warzecha CC, Sato TK, Nabet B, Hogenesch JB, Carstens RP. ESRP1 and ESRP2 are epithelial cell-type-specific regulators of FGFR2 splicing. Mol Cell. 2009;33:591–601. doi: 10.1016/j.molcel.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KV, de Wet JR, Dewji N, DeLuca M. Synthesis of active firefly luciferase by in vitro translation of RNA obtained from adult lanterns. Biochem Biophys Res Commun. 1984;124:592–596. doi: 10.1016/0006-291x(84)91595-x. [DOI] [PubMed] [Google Scholar]
- Zhang XHD, Yang XC, Chung NJ, Gates A, Stec E, Kunapuli P, Holder DJ, Ferrer M, Espeseth AS. Robust statistical methods for hit selection in RNA interference high-throughput screening experiments. Pharmacogenomics. 2006;7:299–309. doi: 10.2217/14622416.7.3.299. [DOI] [PubMed] [Google Scholar]
- Zhao H, Lin W, Kumthip K, Cheng D, Fusco DN, Hofmann O, Jilg N, Tai AW, Goto K, Zhang L, Hide W, Jang JY, Peng LF, Chung RT. nA functional genomic screen reveals novel host genes that mediate interferon-alpha’s effects against hepatitis C virus. J Hepatol. 2012;56:326–333. doi: 10.1016/j.jhep.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
KEY REFERENCES
- Gonsalves FC, DasGupta R. High-throughput RNAi screen in Drosophila . Methods Mol Biol. 2008;469:163–184. doi: 10.1007/978-1-60327-469-2_13. This review describes the advantages and limitations of high-throughput RNAi screening in Drosophila, using a screen for novel modifiers of the Wnt/wg signaling pathway as an example.
- Xhang XD. Optimal High-Throughput Screening: Practical Experimental Design and Data Analysis for Genome-Scale RNAi Research. Cambridge: Cambridge University Press; 2011. This book provides a comprehensive discussion of data analysis for high-throughput RNAi screening.
- Mohr S, Bakal C, Perrimon N. Genomic Screening with RNAi: Results and Challenges. Annu Rev Biochem. 2010;79:37–64. doi: 10.1146/annurev-biochem-060408-092949. This chapter is an excellent review of high throughput RNAi screening and provides a useful summary table of results from genome-scale cell-based high-throughput screens in Drosophila and mammalian cells.