Abstract
Rationale
The Early Pseudomonal Infection Control (EPIC) randomized trial rigorously evaluated the efficacy of different antibiotic regimens for eradication of newly identified Pseudomonas (Pa) in children with cystic fibrosis (CF). Protocol based therapy in the trial was provided based on culture positivity independent of symptoms. It is unclear whether outcomes observed in the clinical trial were different than those that would have been observed with historical standard of care driven more heavily by respiratory symptoms than culture positivity alone. We hypothesized that the incidence of Pa recurrence and hospitalizations would be significantly reduced among trial participants as compared to historical controls whose standard of care preceded the widespread adoption of tobramycin inhalation solution (TIS) as initial eradication therapy at the time of new isolation of Pa.
Methods
Eligibility criteria from the trial were used to derive historical controls from the Epidemiologic Study of CF (ESCF) who received standard of care treatment from 1995 to 1998, before widespread availability of TIS. Pa recurrence and hospitalization outcomes were assessed over a 15-month time period.
Results
As compared to 100% of the 304 trial participants, only 296/608 (49%) historical controls received antibiotics within an average of 20 weeks after new onset Pa. Pa recurrence occurred among 104/298 (35%) of the trial participants as compared to 295/549 (54%) of historical controls (19% difference, 95% CI: 12%, 26%, p<0.001). No significant differences in the incidence of hospitalization were observed between cohorts.
Conclusions
Protocol-based antimicrobial therapy for newly acquired Pa resulted in a lower rate of Pa recurrence but comparable hospitalization rates as compared to a historical control cohort less aggressively treated with antibiotics for new onset Pa.
Keywords: Cystic fibrosis, Pseudomonas aeruginosa, early intervention, randomized trial, historical controls
Introduction
The clinical impact of chronic infection with Pseudomonas aeruginosa (Pa) on morbidity and mortality is well established among individuals with cystic fibrosis (CF)(1, 2). Treatment with anti-pseudomonal antibiotics to delay or prevent chronic Pa infection is critical during the early infection period because of a limited “window of opportunity” during which Pa infection is characterized by non-mucoid phenotype, antibiotic sensitivity, and low density(3-9). Over the last decade, many studies have demonstrated the microbiologic efficacy of initial Pa eradication regimens (10-15), and the standard of care has thus transitioned to treating Pa positive cultures independent of concurrent symptoms (16-18). Treggiari et. al.(19) reported that among 146 pediatric CF clinical centers in the United States in 2003, 93% of children who received anti-pseudomonal treatment at first Pa infection were asymptomatic at the time of presentation. Only two placebo-controlled studies have been performed to assess the impact of initial eradication therapy on microbiologic outcomes(11, 12, 14), but both were limited to small sample sizes and did not evaluate impact of this therapy on clinical outcomes.
The Early Pseudomonas Infection Control (EPIC) randomized trial was designed to rigorously evaluate the impact of four different early anti-pseudomonal treatment regimens on long term clinical and microbiologic efficacy outcomes in a large cohort of CF children less than 13 years of age with recent isolation of Pa from respiratory cultures (19, 20). Over an 18-month period, the trial compared the effects of cycled antibiotic therapy administered in quarterly cycles regardless of results of quarterly respiratory cultures or symptoms with culture-based therapy administered only when Pa was isolated from quarterly cultures. All participants received an initial antibiotic course at study entry consisting of 28 to 56 days of tobramycin inhalation solution (TIS) with or without oral ciprofloxacin to promote initial Pa eradication. The trial revealed no differences between treatment regimens with respect to key microbiologic and clinical outcomes including Pa recurrence, pulmonary exacerbations, and hospitalizations (21). The overall low rate of Pa recurrence (35%) and hospitalization (26%) observed in the 18-month trial contributed to the subsequent recommendation of use of initial therapy with TIS at the time of new onset Pa followed by close microbiologic surveillance(22). However, due to the lack of a placebo-control in the trial, negative cultures following the initial cycle could have reflected spontaneous clearance rather than efficacy of the targeted therapeutic approach(22). As antimicrobial treatment based on culture positivity alone to eradicate newly isolated Pa became standard of care in the U.S. in the early 2000s, a control group receiving placebo and only symptom-based anti-pseudomonal antibiotic therapy was not considered ethical at the time of trial initiation in 2004(20). It thus remains unclear whether microbiologic and clinical outcomes observed in the clinical trial, including relatively low Pa recurrence and hospitalization rates, were different than those that would have been observed with treatment of Pa driven more heavily by the presence of respiratory symptoms than on culture positivity alone.
The objectives of the present study were to compare key outcomes between children enrolled in the EPIC trial who were treated with a standardized Pa eradication protocol and historical controls observed prior to the widespread adoption of an anti-pseudomonal eradication regimen and from an era in which treatment of Pa was driven primarily by the presence of respiratory symptoms(14). In the absence of the ability to compare to placebo, the availability of historical controls provides a unique opportunity to evaluate the effectiveness of standardized eradication therapies as has been done in a prior study of inhaled colistin(23). Our primary hypothesis was that both the frequency of Pa recurrence, defined as the first positive culture after an initial therapy period, and occurrence of hospitalizations would be significantly reduced in the clinical trial cohort who received a standardized, protocol based therapy with TIS as compared to the historic controls.
Materials and Methods
Cohort Selection
The trial cohort was comprised of 304 eligible and randomized participants in the EPIC clinical trial as previously described(20) (ClinicalTrials.gov number NCT00097773). Eligibility criteria from the clinical trial were used as the primary selection criteria for the historical control cohort (E-Table 1, Online Supplement).
The historical control cohort was obtained from the Epidemiologic Study of Cystic Fibrosis(24) (ESCF), a prospective encounter-based observational study initiated in 1994 and designed to characterize the natural history and medication usage of over 30,000 participants with CF, providing the most comprehensive historical data for comparison with the EPIC trial cohort. Controls who met the eligibility criteria outlined in E-Table 1 during the years 1995 to 1998 were selected in order to reflect treatment practices before the widespread commercial availability of TIS(25). To achieve a larger sample size and increase precision, a 2:1 matching strategy based on age and gender was used to identify 608 controls to compare with the 304 clinical trial participants.
This study was approved by the Institutional Review Board (IRB) at Seattle Children's Hospital, Seattle, Washington. Written informed consent was obtained from participants or their guardians as required by the IRBs at the participating institutions.
Study Design
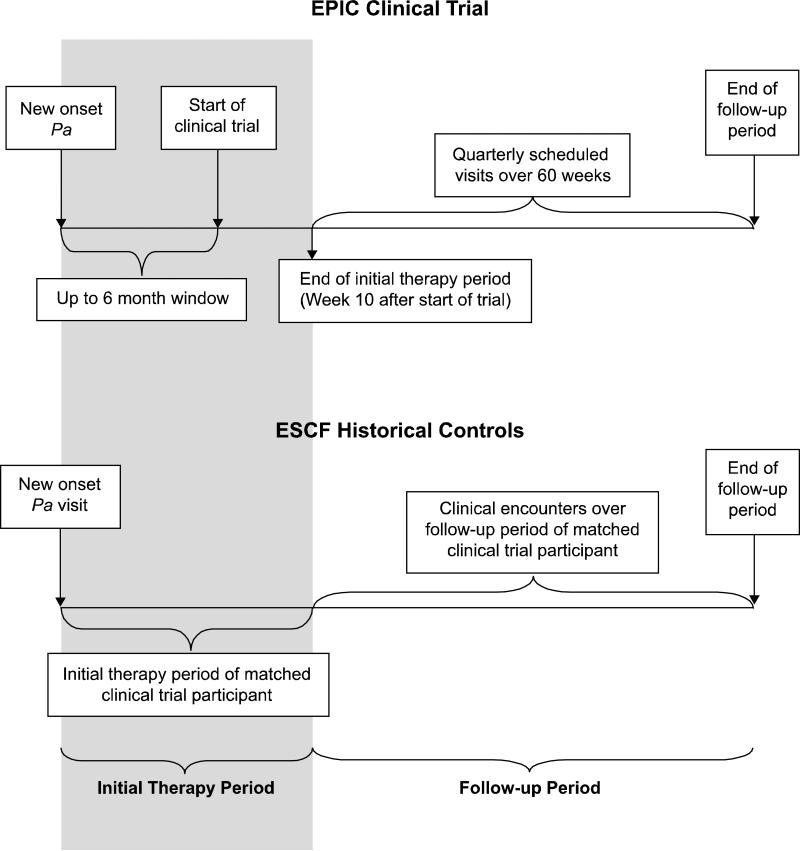
Based on the date of the new Pa culture which defined eligibility into each of the cohorts (E-Table 1), two distinct data collection periods were defined: the initial therapy period and the follow-up period (Figure 1). The initial therapy period enabled characterization of the early treatment response to antimicrobial therapy directed against new onset Pa. For EPIC clinical trial participants, the initial therapy period was defined as the time between new onset Pa and up to 10 weeks following the baseline visit in the clinical trial. The clinical trial allowed up to a 6-month window between the new onset Pa culture that defined eligibility and the baseline visit, during which time participants could receive one course of anti-pseudomonal antibiotics. After randomization and during the first quarter of the trial, all participants were given 1-2 courses of anti-pseudomonal antibiotics to promote initial Pa eradication, irrespective of treatment group assignment(20). For the ESCF controls, the length of the initial therapy period for each control was determined based on that of their matched clinical trial participant starting from the time of new onset Pa. The follow up period for each clinical trial participant was defined as the time between the end of the initial therapy period (approximately 10 weeks into the clinical trial) and the day of their final study visit at approximately 70 weeks post-randomization (Figure 1). The follow up times were derived for each EPIC clinical trial participant and similarly used to derive matched follow up periods for their ESCF historical controls.
Figure 1. Schematic of Data Collection and Timing.
The initial therapy period for the EPIC clinical trial participants was defined as the time between the Pa qualifying culture and 10 weeks post their baseline visit in the clinical trial. The clinical trial allowed up to a 6-month window between the new onset Pa that defined eligibility and the baseline randomization visit and during this time, participants were allowed limited anti-pseudomonal antibiotics.(20) For the historical controls, the length of the initial therapy period for each control was determined based on that of their matched clinical trial participant. The follow up period for each clinical trial participant was defined as the time between the end of the initial therapy period (approximately 10 weeks into the clinical trial) and the day of their final study visit at approximately 70 weeks post-randomization. The follow up times were derived for each clinical trial participant and similarly used to derive matched follow up periods for the controls.
Study Endpoints
The primary endpoint for comparison between cohorts was the proportion with recurrent Pa, defined as at least one positive respiratory culture for Pa during the follow up period, and the secondary endpoint was the proportion of participants hospitalized for any reason during the follow up period.
Statistical Methods and Precision
Demographic and baseline clinical characteristics of the cohorts were summarized descriptively in conjunction with the frequency of anti-pseudomonal antibiotic usage. The proportions of participants with Pa recurrence and with hospitalization were summarized for each cohort with corresponding 95% confidence intervals derived using the Newcombe-Wilson method(26), and p-values for differences between cohorts were obtained using a two-sided 0.05 level of significance Fisher exact test. Due to the exploratory nature of this study, no formal hypothesis testing was performed and an a priori precision estimate utilizing a confidence interval approach was derived for the primary comparison of interest. A total of 104/298 (35%) of participants in the clinical trial experienced Pa recurrence after the initial therapy period. Assuming this proportion is 10% higher among the 608 ESCF historical controls, the 95% confidence intervals corresponding to the 10% difference would be (3%, 17%). Analyses were performed using the statistical software SAS, version 9.1.3 (SAS Institute Inc., Cary, NC), R statistical package version 2.9.1 (R Foundation for Statistical Computing, Vienna, Austria), and Stata version 10.1 (StataCorp LP, College Station, TX).
Sensitivity Analysis
Under the hypothesis that the clinical trial cohort would have better outcomes than the historical controls, a sensitivity analysis was performed to assess whether a result of better outcomes among clinical trial participants could be explained merely by clinical trial participation bias. To evaluate this potential bias, the outcomes of Pa recurrence and hospitalization were also assessed among a concurrent observational control cohort not enrolled in a clinical trial and obtained from the EPIC observational study, an ancillary study to the Cystic Fibrosis Foundation National Patient Registry(20). This longitudinal observational study was conducted in parallel with the EPIC clinical trial at the same sites and enrolled 1787 children with CF <13 years of age never colonized with Pa or negative for Pa for at least 2 years prior to enrollment. Participants who turned Pa positive were offered enrollment in the EPIC clinical trial. All children (n=231) who were eligible for the clinical trial based on eligibility criteria for new onset Pa as defined for the clinical trial (E-Table 1), but did not enroll, comprised the concurrent control cohort with which sensitivity analyses were performed.
Results
Baseline Characteristics and Follow-Up Time
The EPIC clinical trial cohort was comprised of 304 participants with demographic and baseline clinical characteristics summarized in Table 1. Notably, FEV1 % predicted in the historical controls was significantly lower than the trial participants (p=0.004). The number of visits recorded during the initial therapy and follow-up periods were well balanced between the cohorts with an average of 3 visits during the 4.6 month initial therapy period in the clinical trial cohort as compared to 2.4 visits in the historical controls. During the follow-up period, an average of 5.6 visits were available from the clinical trial cohort over an average 13 months as compared to 4.9 visits from the historical controls over the comparable time period.
Table 1.
Demographic and Baseline Clinical Characteristics
| EPIC Clinical Trial (N=304) | ESCF Historical Controls (N=608) | ||
|---|---|---|---|
| Gender, n (%) | |||
| Female | 154 (50.7) | 308 (50.7) | |
| Race*, n (%) | |||
| White (non-Hispanic) | 285 (93.8) | 544 (89.5) | |
| Black (non-Hispanic) | 7 (2.3) | 19 (3.1) | |
| Hispanic | 4 (1.3) | 32 (5.3) | |
| Other/Mixed/Unknown | 8 (2.6) | 13 (2.1) | |
| Genotype, n (%) | |||
| Delta F508 Homozygous | 149 (49.0) | 266 (43.8) | |
| Delta F508 Heterozygous | 116 (38.2) | 165 (27.1) | |
| Other | 24 (7.9) | 30 (4.9) | |
| Unknown | 15 (4.9) | 147 (24.2) | |
| Age at New Onset Pa, yrs | |||
| Mean (SD) | 5.5 (3.5) | 5.5 (3.5) | |
| Min, Max | 0.1, 13.0 | 0.50, 12.96 | |
| Age Group, years, n (%) | |||
| 1-3 | 93 (30.6) | 189 (31.1) | |
| >3-6 | 91 (29.9) | 174 (28.6) | |
| >6-12 | 120 (39.5) | 245 (40.3) | |
| FEV1 (% Predicted)†* | |||
| n | 155 | 230 | |
| Mean (SD) | 96.2 (16.7) | 90.9 (18.3) | |
| Min, Max | 28.9, 130.5 | 26.9, 142.7 | |
p<0.05
Spirometry measures are only available for those old enough to perform the procedure and corresponded to the closest visit on or after new onset Pa.
Antibiotic Use in the Initial Therapy Period
There were significant differences in antibiotic usage between the cohorts during the initial therapy period (Table 2). By design, all 304 (100%) of the clinical trial participants received inhaled antibiotics, specifically TIS. In contrast, only 184/608 (30%) of the historical controls had documented use of inhaled antibiotics, of whom 129/184 (70%) used TIS. Of the 227 (75%) EPIC trial participants who received oral antibiotics, 152/227 (67%) received oral ciprofloxacin as a protocol-based therapy in combination with TIS. In contrast, 114/608 (19%) of historical controls received oral antibiotics, with the majority receiving oral quinolones (69/114 [61%]). Use of IV antibiotics during the initial therapy period among the historical controls was slightly higher than that among the EPIC trial participants with 108/608 (18%) historical controls having documented use as compared to 31/304 (10%) EPIC trial participants.
Table 2.
Summary of antibiotic therapy received during the initial therapy and follow-up periods after new onset Pa.
| EPIC Clinical Trial (N=304) | ESCF Historical Controls (N=608) | |
|---|---|---|
| INITIAL THERAPY PERIOD1 | ||
| Inhaled Antibiotics | ||
| No. (%) Participants | 304 (100%) | 184 (30.3%) |
| Oral Antibiotics | ||
| No. (%) Participants | 227 (74.7%) | 114 (18.8%) |
| IV Antibiotics | ||
| No. (%) Participants | 31 (10.2%) | 108 (17.8%) |
| Any Antibiotic | ||
| No. (%) Participants | 304 (100%) | 296 (48.7%) |
| 95% CI | (98.8%, 100%) | (44.7%,52.7%) |
| Diff. as Compared to Trial | ||
| Participants | - | −51.3% |
| 95% CI | - | (−55.3%,−47.2%) |
| p-value | <0.001 | |
| FOLLOW UP PERIOD2 | ||
| Inhaled Antibiotics | ||
| No. (%) Participants | 218 (71.7%) | 241 (39.6%) |
| Oral Antibiotics | ||
| No. (%) Participants | 276 (90.8%) | 174 (28.6%) |
| IV Antibiotics | ||
| No. (%) Participants | 62 (20.4%) | 113 (18.6%) |
| Any Antibiotic | ||
| No. (%) Participants | 289 (95.1%) | 355 (58.4%) |
| 95% CI | (92.0%,97.0%) | (54.4%,62.2%) |
| Diff. as Compared to Trial | ||
| Participants | - | −36.7% |
| 95% CI | - | (−41.1%,−31.8%) |
| p-value | <0.001 | |
IV=Intravenous; CI=Confidence Interval
The initial therapy period for the EPIC clinical trial participants was defined as the time between the Pa qualifying culture and 10 weeks post their baseline visit in the clinical trial. For the ESCF controls, the length of the initial therapy period for each control was determined based on that of their matched clinical trial participant.
The follow up period for each clinical trial participant was defined as the time between the end of the initial therapy period (approximately 10 weeks into the clinical trial) and the day of their final study visit at approximately 70 weeks post-randomization. The follow up times were derived for each clinical trial participant and similarly used to derive matched follow up periods for the controls.
Antibiotic Use in the Follow-Up Period
Similar trends in antibiotic use were observed throughout the follow up period among the clinical trial participants, with 289/304 (95%) using inhaled, oral, or IV antibiotics (Table 2). Significant differences in antibiotic use remained between the EPIC trial participants and the ESCF historical controls, with 37% fewer (95% CI: -41%, -32%) historical controls, or 58% overall, using antibiotics during the follow up period.
Pa Recurrence
While the majority of clinical trial participants had 4 or more cultures available during the follow up period, this was not the case for the historical controls and thus Pa recurrence rates are reported both overall and stratified by culture frequency (Table 3). Overall, 104/298 (35%) of trial participants experienced Pa recurrence during the clinical trial. In contrast, 295/549 (54%) of historical controls had Pa recurrence (19% difference, 95% CI: 12%, 26%, p<0.001). Importantly, the differences in Pa recurrence rates were consistent across the differing culture frequencies (Table 3). Pa recurrence rates did not significantly differ between historical controls who received antibiotics in the initial therapy period and those who did not (135/269 [50%] vs. 160/280 [57%] respectively, 7% difference, p=0.11). There was also a striking difference between EPIC trial participants and historical controls in terms of the proportion who developed persistent Pa infection, defined as at least two positive cultures during the follow-up period, with 49/292 (17%) of EPIC trial participants developing persistent Pa infection as compared to 129/396 (33%) of historical controls (16% difference, 95% CI 9%, 22%, p<0.001).
Table 3.
Proportion of participants with recurrent Pa by the number of cultures obtained during the follow-up period.
| EPIC Clinical Trial (N=304) | ESCF Historical Controls (N=608) | |
|---|---|---|
| No. (%) with Recurrent Pa | ||
| Among those w/1 culture | 0/6 (0%) | 69/153 (45.1%) |
| Among those w/2 cultures | 1/5 (20.0%) | 79/148 (53.4%) |
| Among those w/3 cultures | 1/10 (10.0%) | 47/83 (56.6%) |
| Among those w/4+ cultures | 102/277 (36.8%) | 100/165 (60.6%) |
| Overall | 104/298 (34.9%) | 295/549 (53.7%) |
| 95% CI | (29.7%,40.5%) | (49.6%,57.9%) |
| Overall Diff. as Compared | ||
| to Trial Participants | - | 18.8% |
| 95% CI | - | (11.9%,25.5%) |
| p<0.001 | ||
CI= Confidence Interval; Diff = Difference
Hospitalizations
Despite significant differences between EPIC trial participants and historical controls in early antibiotic use at new onset Pa, no significant difference in subsequent all cause hospitalization was observed. A total of 79/304 (26%) of EPIC trial participants were hospitalized during the follow up compared to 124/608 (20%) of the historical controls (difference 6%, 95% CI: 0%,12%, p=0.06). Hospitalization rates were slightly higher however among historical controls who received antibiotics during the initial therapy period with 72/296 (24%) hospitalized as compared to 52/312 (17%) historical controls who did not receive antibiotics during the initial therapy period (7%, difference, 95% CI 1%,14%, p=0.02).
Sensitivity Analyses
There were 231 children from participating sites of the clinical trial who were enrolled in the concurrent EPIC observational study, experienced new onset Pa during the trial enrollment period, and who did not enroll in the clinical trial. The mean age of the cohort was 6.2 years (SD=3.4). The primary reason for non-enrollment into the trial was the family declining to participate (105/231, 46%) with an additional 29/231 (13%) not approached for enrollment into the trial and the remainder providing unknown reasons for non-enrollment. A total of 152/231 (66%) received antibiotic therapy within the first few months following new onset Pa. Comparable to the trial participants, nearly all received at least one course of antibiotics over the follow up period (n=194/231, 84%). Pa recurrence and hospitalization outcomes were comparable between the trial participants and non-participants with 79/229 (34%, 95% CI: 28%, 40%) of non-trial participants with culture results available experiencing Pa recurrence and 49/231 (21%, 95% CI: 16%, 27%) hospitalized during the follow up period following new onset Pa. These rates were not significantly different between those receiving antibiotics during the initial therapy period and those not receiving antibiotics during the initial therapy period. Unlike the trial participants for whom the majority (91%) had 4 or more cultures during the follow-up period, only 119/231 (75%) had 4 or more cultures among the non-trial participants.
Discussion
This study provides a unique opportunity to evaluate the results from a randomized clinical trial studying the effectiveness of protocol-based anti-pseudomonal treatment regimens for new onset Pa among children with CF versus historic, less standardized treatment approaches. We hypothesized that trial participants who received anti-pseudomonal therapy in response to new onset Pa according to a study protocol would have improved outcomes in comparison to a standard of care used before the aggressive use of an initial eradication therapy. We documented that the approach to treatment of early Pa infection has changed over the past 15 years in the U.S with significantly higher use of both inhaled and oral antibiotics. This change towards earlier and more frequent antibiotic use was associated with significantly lower Pa recurrence rates in the clinical trial participants as compared to the historical controls. This result is consistent with those from other studies regarding the efficacy of inhaled antibiotics in initial eradication therapy among young children with CF(10-15, 23). Failure to initially eradicate Pa and frequent Pa recurrence has also been associated with higher risk of developing an acute pulmonary exacerbation, and thus the recurrence of Pa is an important outcome indicative of the transition to chronic Pa infection and its associated morbidity (27).
Despite significant differences in the use of antibiotics for new onset Pa between clinical trial participants and historical control cohorts, no significant differences in hospitalization rates were observed. Another clinically relevant endpoint would have been pulmonary exacerbations, as this endpoint is more specific to respiratory infections and can capture less severe events than those requiring hospitalization; however, inconsistency in the definition of exacerbation across the cohorts did not allow the use of this endpoint in this study(28). IV antibiotic treatment is often a surrogate for more severe pulmonary exacerbations. However, as with the hospitalization endpoint, it was not significantly different between the cohorts following initial treatment for new onset Pa. These results suggest that differences in Pa culture positivity between the cohorts did not translate into differences in clinical outcome as captured by hospitalizations over the course of the study follow-up.
Our sensitivity analysis utilizing concurrent controls from the EPIC observational study suggests that the protocol based therapy given during the clinical trial for Pa eradication resulted in similar clinical and microbiologic outcomes as compared to the control cohort not participating in the trial and receiving contemporary standard of care treatment. We documented that contemporary treatment with increased use of early anti-pseudomonal antibiotics was associated with a reduction in the frequency of Pa recurrence as compared with the historical controls. This is a key result needed to benchmark the outcomes observed in the clinical trial, and confirm that trial results were not due to trial participation bias.
There are several limitations to our study, in particular that differences between the trial cohort and historical controls may be attributable to other differences in care between the cohorts that have been introduced over the years including changes in infection control practices, changes in use of chronic therapy, and improvements in nutritional care. Further, antibiotic use and isolation of Pa from respiratory cultures may have been underestimated in the historical controls. Specifically, there were differences in culture frequency between the cohorts that could have resulted in under-detection of Pa among the historical controls, and thus our estimate of the difference between the cohorts in Pa recurrence rates may be an underestimate of the true difference in rates. In addition, although the historic controls were chosen based on eligibility criteria similar to those utilized in the clinical trial for the definition of new onset Pa, there were some secondary inclusion and exclusion criteria that were not available. Lack of availability of lifetime history of Pa positivity prior to the initiation of ESCF for the historical controls, and lung function for younger children, precluded us from being able to match on these potentially important factors. Thus, the historical control cohort may be different than the trial participants in terms of other unmeasurable characteristics. It is uncertain however whether any selection bias in the historic control cohort would induce better or poorer outcomes.
This study evaluates the results of a non-placebo controlled clinical trial compared to historical standard of care, and demonstrates the shift in approach to treatment of early Pa infection in the U.S. in the past 15 years and the impact of these changes on microbiologic and clinical outcomes. The use of observational registries for studies such as this is critical in the orphan disease setting and enables evaluation of the generalizability of the results of a clinical trial. The ability of this study to benchmark the EPIC clinical trial results ultimately demonstrates that the protocol based therapy received during the trial is effective in preventing Pa recurrence when compared with less aggressive antibiotic therapy following acquisition of Pa.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the EPIC Steering Committee and the ESCF Scientific Advisory Group for helpful discussions regarding the design and scientific interpretation of this study, ESCF and EPIC study investigators (see Appendix), Bruce Marshall, MD, and the Cystic Fibrosis Foundation National Patient Registry, and funding support provided from the National Institute of Health, Cystic Fibrosis Foundation, and Genentech. Most importantly, the authors wish to thank the individuals with cystic fibrosis who participated in these studies.
Acknowledgments
Financial support: The research for this article was supported in part by the Cystic Fibrosis Foundation grants number EPIC0K0 and OBSERV04K0, the National Heart Lung and Blood Institute (NHLBI) and National Institute for Digestive Disorders and Kidney (NIDDK) grant number U01-HL080310, and the National Center for Research Resources (NCRR) grant number ULI-RR025014-03. Study drugs and devices in the EPIC clinical trial were supplied free of charges by Novartis Pharmaceutical Corp. and Bayer Healthcare AG, compressors and nebulizers were provided by PARI Respiratory Equipment Inc. Funding for the Epidemiologic Study of Cystic Fibrosis and analysis by ICON Late Phase & Outcomes Research was provided by Genentech, Inc., South San Francisco, Calif.
Appendix: EPIC and ESCF Investigators
EPIC Clinical Trial and Observational Study
Cystic Fibrosis Foundation Therapeutics: Robert J. Beall, Preston W. Campbell III, Bruce C. Marshall.
Steering Committee: George Retsch-Bogart (Chair), University of North Carolina at Chapel Hill; Susan Banks-Schlegel, National Heart, Lung and Blood Institute; Robert J. Beall, Cystic Fibrosis Foundation; Preston W. Campbell, Cystic Fibrosis Foundation; Ron Gibson, University of Washington; Gavin R. Graff, The PennState Milton S. Hershey Medical Center; Hector H. Gutierrez, The University of Alabama at Birmingham; Jamshed Kanga, University of Kentucky; Richard Kronmal, University of Washington; Robert Kuhn (Ad Hoc), University of Kentucky; Thomas Lahiri, Vermont Children's Hospital at Fletcher Allen Health Care; Nicole Mayer-Hamblett, University of Washington; Catherine McKeon, National Institute of Diabetes & Digestive & Kidney Diseases; Wayne Morgan, University of Arizona; Blakeslee E. Noyes, Saint Louis University/Cardinal Glennon Children's Medical Center; Bonnie W. Ramsey, University of Washington; Margaret Rosenfeld, University of Washington; Lisa Saiman (Ad Hoc), Columbia University Medical Center, Michael Schechter, Emory University; Dennis C. Stokes, Dartmouth-Hitchcock Medical Center; Jeffrey S. Wagener, The Children's Hospital; Judy Williams, CFFT Therapeutics Development Network Coordinating Center; Marlyn Woo, Children's Hospital of Los Angeles.
Participating Sites (Site Investigators (SI) and Research Coordinators (RC)): Akron Children's Hospital/ Children's Hospital Medical Center of Akron, Akron, OH – SI: Gregory Omlor, Nathan C. Kraynack and Kimberly A. Spoonhower; RC: Deborah A. Ouellette; Albany Medical College, Albany, NY – SI: Paul G. Comber; RC: Sharon Laskoski and Julie Pursel; Alfred I. duPont Hospital for Children, Wilmington, DE – SI: Aaron Chidekel; RC: Sandra M. Budd; All Children's Hospital, St. Petersburg, FL – SI: Magdalen Gondor; RC: Stasia Lehmann and Jennifer Flanary; Children's Hospital Boston, Boston, MA, Grant #MO1-RR02172 – SI: Lindo Terry Spencer and David A. Waltz; RC: Erin Leone Thakkallapalli and Summer Adams; Children's Hospital Los Angeles, Los Angeles, CA – SI: Thomas Keens and Arnold C.G. Platzker; RC: Ricardo Ortego; Children's Hospital of Michigan Wayne State University, Detroit, MI – SI: Debbie Toder and Ibrahim Abdulhamid; RC: Catherine Van Wagnen; Children's Hospital of Pittsburgh of UPMC, Pittsburgh, PA – SI: David Orenstein; RC: Sandy Hurton and Judy Fulton; Children's Hospital of Wisconsin, Milwaukee, WI – SI: William Gershan; RC: Tami Miller; Children's Hospitals and Clinics of Minnesota, Minneapolis, MN – SI: John McNamara and Michael Pryor; RC: Mary Sachs and Sandy Landvik; Children's Memorial Medical Center, Chicago, IL – SI: Adrienne Prestridge; RC: Stacy VandenBranden; Children's Mercy Hospital, Kansas City, MO – SI: Philip Black; RC: Karie Robinson and Lora Bear; Cook Children's Health Care System, Fort Worth, TX – SI: Maynard C. Dyson and Karen D. Schultz; RC: Sara Scott; Dartmouth Hitchcock Medical Center, Lebanon, NH – SI: H. Worth Parker; RC: Stephanie Miller and Nicola Felicetti; Emory University, Atlanta, GA – SI: Michael Schechter; RC: Jeannette Peabody and Irena Kizer; Helen DeVos Children's Hospital, Grand Rapids, MI – SI: Susan L. Millard and John N. Schuen; RC: Teri L. Crumb and Tom Symington; Indiana University/Riley Hospital for Children, Indianapolis, IN – SI: Michelle Howenstine; RC: Terry Barclay; Kaiser Permanente Medical Center, Oakland, CA – SI: Greg Shay; RC: Julie Lee and Mary Seastrand; Lucile Packard Children's Hospital, Stanford University, Palo Alto, CA, Grant #1UL1RR025744 – SI: Richard Moss; RC: Colleen Dunn and Zoe Davies; Maine Medical Center, Portland, ME – SI: Anne Marie Cairns, Timothy Messitt and Thomas Mellow; RC: Mary Ellen Corrigan; Massachusetts General Hospital, Boston, MA – SI: Allen Lapey; RC: Dell Saulnier; Medical College of Georgia, Augusta, GA – SI: Margaret F. Guill and Katie McKie; RC: Julie Hall and Kathy Dyer; Memorial Miller Children's Hospital, Long Beach, CA – SI: Felice Adler-Shohet; RC: Nan O'Donnell and Candace Evans; Monmouth Medical Center, Long Branch, NJ – SI: Robert L. Zanni; RC: Bridget Marra; Nationwide Children's Hospital, Columbus, OH – SI: Karen S. McCoy; RC: Laura Raterman; Nemours Children's Clinic, Jacksonville, FL – SI: David A. Schaeffer; RC: Rena A. Sprinkle and Donna L. Pingel; New York Medical College, Valhalla, NY – SI: Allen J. Dozor, Nikhil Amin and Joseph T. Boyer; RC: Ingrid Gherson; Oregon Health Sciences University, Portland, OR – SI: Michael Wall; RC: Aaron Guzik; Rainbow Babies and Children's Hospital, Cleveland, OH – SI: Michael Konstan; RC: Colette Bucur and Cheryl Velotta; Seattle Children's, Seattle, WA – SI: Ronald L Gibson and Margaret Rosenfeld; RC: Sharon McNamara; St. Christopher's Hospital for Children, Philadelphia, PA – SI: Laurie Varlotta; RC: Joanne Gambo, Justin Overcash and Marcella Aramburo; St. Louis Children's Hospital, St. Louis, MO – SI: Thomas Ferkol; RC: Jane Quante and Patty Burks; Saint Louis University/Cardinal Glennon Children's Medical Center, St. Louis, MO – SI: Blakeslee E. Noyes; RC: Vikki L. Kociela; Schneider Children's Hospital, New Hyde Park, NY – SI: Joan DeCelie-Germana; RC: Lynn Bonitz; SUNY Upstate Medical University, Syracuse, NY – SI: Ran D. Anbar; RC: Donna M. Linder and Valoree N. Suttmore; Texas Children's Hospital, Houston, TX – SI: Peter W. Hiatt; RC: Charlene Hallmark; The Children's Hospital, Denver, CO, Grant #1UL1RR025780 – SI: Frank J. Accurso and Jeffrey S. Wagener; RC: Shelley A. Mann; The Children's Medical Center of Dayton/Wright State University, Dayton, OH – SI: Robert J. Fink and Gary A. Mueller; RC: Frederick H. Royce and Sandy R. Bartosik; The Johns Hopkins Medical Institutions, Baltimore, MD, Grant #UL1RR025005 – SI: Peter J. Mogayzel, Jr. and Pamela L. Zeitlin; RC: Carolyn G. Chapman and Karen A. Callahan; The PennState Milton S. Hershey Medical Center, Hershey, PA – SI: Gavin R. Graff and Michael S. Schwartz; RC: Diane M. Kitch and Lisa M. Allwein; The University of Alabama at Birmingham, Birmingham, AL – SI: Hector H. Gutierrez; RC: Valerie Eubanks Tarn, Lacrecia Britton and Gina Sabbatini; University of California, San Francisco, San Francisco, CA – SI: Dennis W. Nielson; RC: Diem Tran; University of Iowa, Iowa City, IA, Grant #UL1 RR0024979 – SI: Richard C. Ahrens; RC: Jean Frauenholtz, Mary Teresi and Liz Huey; University of Kentucky College of Medicine, Lexington, KY – SI: Jamshed F. Kanga and Michael I. Anstead; RC: Barbara Owsley and Catherine Dudderar; University of Massachusetts Memorial Health Care, Worcester, MA – SI: Brian P. O'sullivan; RC: Dawn Baker; University of Michigan, Ann Arbor, MI – SI: Samya Z. Nasr; RC: Dawn Kruse; University of Mississippi Medical Center, Jackson, MS – SI: Fadel Ruiz and Kim Adcock; RC: Kimberly Barfield; University of Nebraska Medical Center, Omaha, NE – SI: John Colombo; RC: Dee Acquazzino; University of North Carolina at Chapel Hill, Chapel Hill, NC, Grant #UL1RR025747 – SI: George Retsch-Bogart; RC: Carol Barlow, Diane Towle and Tracy Callahan; University of Rochester Medical Center, Rochester, NY – SI: Clement L. Ren; RC: Marissa Dixon; University of Tennessee Health Science Center, Memphis, TN – SI: Robert A. Schoumacher; RC: Teresa Knight and Barbara A. Culbreath; University of Utah, Salt Lake City, UT – SI: Barbara Chatfield, Derek Uchida and Krow Ampofo; RC: Susan Griffiths; University of Virginia, Charlottesville, VA – SI: Deborah K. Froh; RC: Robin Kelly; University of Wisconsin-Madison, Madison, WI, Grant #1UL1RR025011 – SI: Michael J. Rock; RC: Linda Makholm; Vanderbilt Children's Hospital, Nashville, TN, Grant #1UL RR024975 – SI: Elizabeth Perkett and Christopher Harris; RC: Alice Bray; Vermont Children's Hospital at Fletcher Allen Health Care, Burlington, VT – SI: Thomas Lahiri; RC: Emily Keller and Sandra Diehl.
Epidemiologic Study in Cystic Fibrosis
Advisory Group: Michael Konstan, Susanna McColley, Wayne Morgan, Alexandra Quittner, Warren Regelmann, Clement Ren, Margaret Rosenfeld, Greg Sawicki, Michael Schechter, Donald VanDevanter
ICON Statistical and Data Management group: Eric Elkin, David Pasta, Christy Guyan
ESCF Investigators: Alabama — Dana Brasfield, Raymond Lyrene, Lawrence Sindel; Alaska — Dion Roberts; Arkansas — John Carroll, Robert Warren, Louay Nassri, Paula Anderson; Arizona — Mark Brown, Amy Silverthorn, Peggy Radford, Gerald Gong, Gregory Legris; California — Gerald Greene, Reddivalam Sudhakar, Arnold Platzker, Bruce Nickerson, Karen Hardy, Ivan Harwood, Gregory Shay, Bryon Quick, Allan Lieberthal, Richard Moss, Chris Landon, Yvonne Fanous, Jay Lieberman, Eugene Spiritus, Bradley Chipps, Ruth McDonald, Mark Pian, Gerd Cropp, Nancy Lewis, Dennis Nielson, Bertrand Shapiro; Colorado — Jeff Wagener, Frank Accurso, Milene Saavedra; Connecticut — Karen Daigle, Jacob Hen, Regina Palazzo; Delaware — Kathryn Dodds, Raj Padman, John Goodill; District of Columbia — Glenna Winnie, Lea Davies; Florida — Tony Kriseman, Jorge Sallent, Joseph Chiaro, Martin Kubiet, Sue Goldfinger, Morton Schwartzman, Carlosenrique Diaz, Kevin Maupin, Eduardo Riff, David Geller, Floyd Livingston, Kunjana Mavunda, Jose Birriel, Jr., Luis Faverio, David Rosenberg, David Schaeffer, James Sherman, Mary Wagner, Michael Light, Bruce Schnapf; Georgia — Gary Montgomery, Kevin Kirchner, Mark Weatherly, Daniel Caplan, Margaret Guill, Valera Hudson; Illinois — Javeed Akhter, Donald Davison, Steven Boas, Susanna McColley, Youngran Chung, Rennee Latner, Gabriel Aljadeff, Youngran Chan, Jerome Kraut, Arvey Stone, John Lloyd Still, Girish Sharma, Lanie Eagleton, Patricia Hopkins, Umesh Chatrath, Lucille Lester, Young-Jee Kim; Indiana — Veena Anthony, Howard Eigan, Michelle Howenstine, Pushpom James, Edward Gergesha, James Harris, Robert Plant; Iowa — Veljko Zivkovich, Angela Collins, Edward Nassif, Richard Ahrens; Kansas — Daniel Doornbos, Joseph Kanarek, Richard Leff, Pamela Shaw, Elanor Demoss, Maria Riva, Leonard Sullivan; Kentucky — Michael Anstead, Jamshed Kanga, Nemr Eid, Ron Morton; Louisana — Bettina Hilman, Kim Jones, Scott Davis; Maine — Ralph Harder, Tom Lever, Anne Marie Cairns, Edgar Caldwell, Jonathan Zuckerman; Maryland — Peter Mogayzel, Beryl Rosenstein, John McQuestion, Donna Perry, Samuel Rosenberg; Massachusetts — Robert Gerstle, Andrew Colin, Mary Ellen Wohl, Allan Lapey, William Yee, Brian O'sullivan, Robert Zwerdling; Michigan — Ibrahim Abdulhamid, Adrian O’Hagan, John Schuen, Lawrence Kurlandsky, Richard Honicky, Douglas Homnick, John Marks, Bohdan Pichurko, Norma Maxvold, Samya Nasr, Richard Simon, Wan Tsai, Dana Kissner; Minnesota — John McNamara, Nancy Henry, Stephen Marker, Michael Pryor, Warren Regelmann, Lynn Walker; Mississippi — Jim Woodward, Louis Mizell, Suzanne Miller; Missouri — Daniel Rosenbluth, Philip Black, Michael McCubbin, Alan Cohen, Thomas Ferkol, George Mallory, Anthony Rejent, Bruce Rubin, Gavin Graff, Peter Konig; Nebraska — John Colombo, Peter Murphy; New Hampshire — William Boyle, H. Worth Parker; New Jersey — Chandler Patton, Robert Zanni, Arthur Atlas, Nelson Turcios, Lourdes Laraya-Cuasay, Dorothy Bisberg, Helen Aguila; New Mexico — Sarah Allen, David James, Elizabeth Perkett, Marsha Thompson; Nevada — Sonia Budhecha, Ruben Diaz; New York — Jonathan Rosen, Robert Kaslovsky, Ronald Percciacante, Drucy Borowitz, Joseph Cronin, Colin McMahon, Lynne Quittell, Robert Giusti, Rubin Cohen, Joan DeCelie-Germana, Jack Gorvoy, Kalpan Patel, Meyer Kattan, Allen Dozor, Emily DiMango, Maria Berdella, Ran Anbar, Debra Ianuzzi, James Sexton, Catherine Tayag-Kier, John McBride, Clement Ren, Karen Voter, Mary Dimaio; North Carolina — Gerald Georgitis, Joseph Marc Majure, Maria Martinez, J. Clarke McIntosh, Margaret Leigh, Michael Schechter, Hugh Black; North Dakota — James Hughes, Anand Kantak; Ohio — Robert Wilmott, Gregory Omlor, Robert Stone, Karen McCoy, James Acton, Carl Doershuk, Michael Konstan, Robert Fink, Michael Steffan, Pierre Vauthy, Patricia Joseph; Oklahoma — Santiago Reyes, John Kramer, James Royall; Oregon — Jay Eisenberg, Michael Wall; Pennsylvania — Stanley Fiel, Thomas Scanlin, Shroti Phadke, Glenna Winnie, Joel Weinberg, William Sexauer, Stephen Wolf, Douglas Holsclaw, Debra Klein, W. Stuart Warren, Robert Kinsey, Carlos Perez, Muttiah Ganeshanathan, James Shinnick, Howard Panitch, Laurie Varlotta, Cynthia Robinson; Puerto Rico — Jose Rodriguez Santana; Rhode Island — Mary Ann Passero; South Carolina — Jane Gwinn, Robert Baker, C. Michael Bowman, Patrick Flume, Daniel Brown, Roxanne Marville; South Dakota — James Wallace, Rodney Parry; Tennessee — Don Ellenburg, John Rogers, Ricky Mohon, Joel Ledbetter, Aram Hanissian, Robert Schoumacher, Preston Campbell, Christopher Harris, Bonnie Slovis, Dennis Stokes; Texas — Kathryn Hale, Marcia Katz, Dan Seilheimer, Marianne Sockrider, Allan Frank, James Daniel, James Cunningham, Iley Browning, John Bray, Amanda Dove, J. Fernando Mandujano, Larry Tremper, Martha Morse, Donna Willey-Courand, Steven Copenhaver, John Pohl, Bennie McWilliams, Marie Martine-Logvinoff, Marsh Wallace, Robert Klein, Rodolfo Amaro, Leslie Couch, Michael Brown, Claude Prestidge, Stephen Inscore, Andrew Lipton; Utah — Barbara Chatfield, Theodore Liou, Bruce Marshall; Virginia — Karl Karlson, Ignacio Ropoll, Thomas Rubio, Joel Schmidt, David Thomas, John Osborn, Deborah Froh, Benjamin Gaston, Greg Elliott; Vermont — Thomas Lahiri, Donald Swartz, Laurie Whittaker; Washington — Ronald Gibson, Bonnie Ramsey, Michael McCarthy, Lawrence Larson, David Ricker, Mark Robbins, Moira Aitken, Julia Emerson; Wisconsin — Julie Biller, Mark Splaingard, Bradley Sullivan, Paul Pritchard, Stu Adair, Peter Holzwarth, Guillermo Dopico, Keith Meyer, Christopher Green, Michael Rock; West Virginia — Stephen Aronoff, Kathryn Moffett.
Footnotes
Disclosure of Conflict of Interest. Michael Konstan, Jeffrey Wagener, and Wayne Morgan have received honoraria from Genentech for serving as members of the Scientific Advisory Group for the Epidemiologic Study of Cystic Fibrosis (ESCF) and have served as consultants to Genentech. No compensation was provided to these authors in exchange for production of this manuscript. Eric Elkin is an employee of ICON Late Phase & Outcomes Research, which was paid by Genentech for providing analytical services for this study. Jeffrey Wagener was previously an employee of Genentech.
References
- 1.Welsh MJRB, Accurso F. Cystic Fibrosis. In: Scriver CRBAL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 8th ed. McGraw-Hill; New York, NY: 2001. pp. 521–88. [Google Scholar]
- 2.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168(8):918–51. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 3.Pamukcu A, Bush A, Buchdahl R. Effects of Pseudomonas aeruginosa colonization on lung function and anthropometric variables in children with cystic fibrosis. Pediatr Pulmonol. 1995;19(1):10–5. doi: 10.1002/ppul.1950190103. [DOI] [PubMed] [Google Scholar]
- 4.Henry RL, Mellis CM, Petrovic L. Mucoid Pseudomonas aeruginosa is a marker of poor survival in cystic fibrosis. Pediatr Pulmonol. 1992;12(3):158–61. doi: 10.1002/ppul.1950120306. [DOI] [PubMed] [Google Scholar]
- 5.Kosorok MR, Zeng L, West SE, Rock MJ, Splaingard ML, Laxova A, Green CG, Collins J, Farrell PM. Acceleration of lung disease in children with cystic fibrosis after Pseudomonas aeruginosa acquisition. Pediatr Pulmonol. 2001;32(4):277–87. doi: 10.1002/ppul.2009.abs. [DOI] [PubMed] [Google Scholar]
- 6.Konstan MW, Morgan WJ, Butler SM, Pasta DJ, Craib ML, Silva SJ, Stokes DC, Wohl ME, Wagener JS, Regelmann WE, Johnson CA. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr. 2007;151(2):134–9. 9, e1. doi: 10.1016/j.jpeds.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Courtney JM, Bradley J, McCaughan J, O'Connor TM, Shortt C, Bredin CP, Bradbury I, Elborn JS. Predictors of mortality in adults with cystic fibrosis. Pediatr Pulmonol. 2007;42(6):525–32. doi: 10.1002/ppul.20619. [DOI] [PubMed] [Google Scholar]
- 8.Nixon GM, Armstrong DS, Carzino R, Carlin JB, Olinsky A, Robertson CF, Grimwood K. Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. J Pediatr. 2001;138(5):699–704. doi: 10.1067/mpd.2001.112897. [DOI] [PubMed] [Google Scholar]
- 9.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34(2):91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 10.Valerius NH, Koch C, Hoiby N. Prevention of chronic Pseudomonas aeruginosa colonisation in cystic fibrosis by early treatment. Lancet. 1991;338(8769):725–6. doi: 10.1016/0140-6736(91)91446-2. [DOI] [PubMed] [Google Scholar]
- 11.Wiesemann HG, Steinkamp G, Ratjen F, Bauernfeind A, Przyklenk B, Doring G, von der Hardt H. Placebo-controlled, double-blind, randomized study of aerosolized tobramycin for early treatment of Pseudomonas aeruginosa colonization in cystic fibrosis. Pediatr Pulmonol. 1998;25(2):88–92. doi: 10.1002/(sici)1099-0496(199802)25:2<88::aid-ppul3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 12.Gibson RL, Emerson J, McNamara S, Burns JL, Rosenfeld M, Yunker A, Hamblett N, Accurso F, Dovey M, Hiatt P, Konstan MW, Moss R, Retsch-Bogart G, Wagener J, Waltz D, Wilmott R, Zeitlin PL, Ramsey B. Significant microbiological effect of inhaled tobramycin in young children with cystic fibrosis. Am J Respir Crit Care Med. 2003;167(6):841–9. doi: 10.1164/rccm.200208-855OC. [DOI] [PubMed] [Google Scholar]
- 13.Proesmans M, Boulanger L, Vermeulen F, De Boeck K. Eradication of recent Pseudomonas aeruginosa isolation: TOBI versus colistin/ciprofloxacin (abstract). Journal of Cystic Fibrosis. 2008;7(Suppl 2):S64. [Google Scholar]
- 14.Langton Hewer SC, Smyth AR. Antiobiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis. Cochrane Database Syst Rev. 2009:CD004197. doi: 10.1002/14651858.CD004197.pub3. [DOI] [PubMed] [Google Scholar]
- 15.Ratjen F, Munck A, Kho P, Angyalosi G. Treatment of early Pseudomonas aeruginosa infection in patients with cystic fibrosis: the ELITE trial. Thorax. 2010;65(4):286–91. doi: 10.1136/thx.2009.121657. [DOI] [PubMed] [Google Scholar]
- 16.Doring G, Hoiby N. Early intervention and prevention of lung disease in cystic fibrosis: a European consensus. J Cyst Fibros. 2004;3(2):67–91. doi: 10.1016/j.jcf.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Hoiby N, Frederiksen B, Pressler T. Eradication of early Pseudomonas aeruginosa infection. J Cyst Fibros. 2005;4(Suppl 2):49–54. doi: 10.1016/j.jcf.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Hansen CR, Pressler T, Hoiby N. Early aggressive eradication therapy for intermittent Pseudomonas aeruginosa airway colonization in cystic fibrosis patients: 15 years experience. J Cyst Fibros. 2008;7(6):523–30. doi: 10.1016/j.jcf.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Treggiari MM, Rosenfeld M, Retsch-Bogart G, Gibson R, Ramsey B. Approach to eradication of initial Pseudomonas aeruginosa infection in children with cystic fibrosis. Pediatr Pulmonol. 2007;42(9):751–6. doi: 10.1002/ppul.20665. [DOI] [PubMed] [Google Scholar]
- 20.Treggiari MM, Rosenfeld M, Mayer-Hamblett N, Retsch-Bogart G, Gibson RL, Williams J, Emerson J, Kronmal RA, Ramsey BW. Early anti-pseudomonal acquisition in young patients with cystic fibrosis: rationale and design of the EPIC clinical trial and observational study'. Contemp Clin Trials. 2009;30(3):256–68. doi: 10.1016/j.cct.2009.01.003. PMCID: 2783320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Treggiari MM, Retsch-Bogart G, Mayer-Hamblett N, Khan U, Kulich M, Kronmal R, Williams J, Hiatt P, Gibson RL, Spencer T, Orenstein D, Chatfield BA, Froh DK, Burns JL, Rosenfeld M, Ramsey BW. Comparative efficacy and safety of 4 randomized regimens to treat early Pseudomonas aeruginosa infection in children with cystic fibrosis. Arch Pediatr Adolesc Med. 2011;165(9):847–56. doi: 10.1001/archpediatrics.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saiman L, Cohen MB. What Have We Learned About Early Treatment of Pseudomonas aeruginosa Infection in Infants and Children With Cystic Fibrosis? Arch Pediatr Adolesc Med. 2011;165(9):867–8. doi: 10.1001/archpediatrics.2011.133. [DOI] [PubMed] [Google Scholar]
- 23.Frederiksen B, Koch C, Hoiby N. Antibiotic treatment of initial colonization with Pseudomonas aeruginosa postpones chronic infection and prevents deterioration of pulmonary function in cystic fibrosis. Pediatr Pulmonol. 1997;23(5):330–5. doi: 10.1002/(sici)1099-0496(199705)23:5<330::aid-ppul4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 24.Morgan W, Butler SM, Johnson C, Colin AA, Fitz-Simmons SC, Geller DE, Konstan MW, Light MJ, Rabin HR, Regelmann WE, Schidlow DV, Stokes D, Wohl ME, Kaplowitz H, Wyatt MM. Epidemiologic study of cystic fibrosis: Design and implementation of a prospective, multicenter, observational study of patients with cystic fibrosis in the U.S. and Canada. Pediatr Pulmonol. 1999;28(4):231–41. doi: 10.1002/(sici)1099-0496(199910)28:4<231::aid-ppul1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 25.Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, Vasiljev KM, Borowitz D, Bowman CM, Marshall BC, Marshall S, Smith AL. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med. 1999;340(1):23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 26.Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17(8):873–90. doi: 10.1002/(sici)1097-0258(19980430)17:8<873::aid-sim779>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 27.Mayer-Hamblett N, Kronmal RA, Gibson RL, Rosenfeld M, Retsch-Bogart G, Treggiari MM, Burns JL, Khan U, Ramsey BW. Initial Pseudomonas aeruginosa treatment failure is associated with exacerbations in cystic fibrosis. Pediatr Pulmonol. 2012;47(2):125–34. doi: 10.1002/ppul.21525. PMCID: 3214247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: Epidemiology and pathogenesis. Thorax. 2007;62(4):360–7. doi: 10.1136/thx.2006.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.