Abstract
The aim of this study was to investigate expression of CD147 and MMP-9 in triple-negative breast cancer (TNBC) so as to determine whether these two proteins may be correlated with poor prognosis of TNBC patients. We examined the expression levels of the CD147 and MMP-9 in 127 patients with TNBC and 30 patients with mammary gland fibroma using immunohistochemical staining before any treatments. Furthermore, we analyzed the correlation between the expression of these two proteins and various clinicopathologic factors including survival status of patients with TNBC. Positive stain of CD147 and MMP-9 was observed in all samples of TNBC. Astatistically positive correlation was observed between the expression levels of CD147 and MMP-9 in TNBC tissues. The incidences of high expression were 48.0 % for CD147 and 53.5 % for MMP-9 in 127 TNBC tissues, respectively. High expression of either CD147 or MMP-9 was significantly correlated with clinical feature and shorter progression-free survival (PFS) (PCD147 = 0.039; PMMP-9 = 0.017) and overall survival (OS) (PCD147 = 0.037; PMMP-9 = 0.023). The expression levels of CD147 and MMP-9 are positively correlated with invasion, metastasis and shorter PFS/OS of TNBC. Patients with high expression of CD147 and MMP-9 had poor prognosis than TNBC patients with low expression.
Keywords: CD147, MMP-9, Triple-negative breast cancer, Cancer prognosis
Introduction
Triple-negative breast cancers (TNBC) are characterized by lack of expression of estrogen (ER), progesterone (PR) and HER-2 receptors and comprise 15–25 % of total breast cancer incidents. Most TNBC have a basal-like molecular phenotype by gene expression profiling [1, 2]. There is also significant overlap between TNBC and germline BRCA1 mutation–associated tumors, suggesting a shared carcinogenic pathway between TNBC and BRCA1-associated breast cancer [3, 4]. In addition, TNBC are more likely to be poorly differentiated and display a higher histologic grade, and these cancers are diagnosed more frequently in younger and pre-menopausal women and have more aggressive clinical courses. Due to the lack of known specific therapeutic targets, patients with TNBC cannot benefit from conventional endocrine-targeted therapies, anti-HER2 drugs or some other types of chemotherapy. Currently, standard treatment regimens for TNBC have not been established, and our understanding for TNBC is insufficient. The mortality of patients with TNBC is high [5]. Therefore, some new prognostic indicators and approaches for the treatment of TNBC are urgently needed to be developed.
Recently, several pathways of interest have been studied, including the roles of the CD147 and Matrix metalloproteinase 9 (MMP-9). In many tumors, the expression levels of MMPs are mainly regulated by tumor-stroma interactions via tumor cell–associated extracellular MMPs inducer, CD147 (Emmprin) [6, 7]. CD147, a transmembrane glycoprotein of the immunoglobulin superfamily, is identified as a signaling receptor to extracellular CypA. CD147 is overexpressed in various tumor cells and correlates with tumor invasion, metastasis and angiogenesis [8]. CD147 has been reported to be on the cell surface of 80 % of disseminated cancer cells. Based on its role in the regulation of MMPs expression, CD147 is thought to facilitate invasion and metastasis indirectly by the induction and regulation of basement membrane– degrading proteases, in particular MMP-9 [9, 10]. MMP-9 is a secreted multidomain enzyme that regulates cell– matrix composition. It belongs to the gelatinase subfamily of the MMPs. Therefore, its main substrate is gelatin (a denatured collagen). MMP-9 is produced by selected cell types including keratinocytes, monocytes, tissue macrophages, polymorphonuclear leukocytes and a variety of malignant cells. The expression level and/or activity ofMMP-9 are also elevated in invasive tumor compared with non-invasive tumor [11]. Recent studies have demonstrated that CD147 and matrix metalloproteinase 9 (MMP-9) play an important role in the invasion and metastasis of several types of human malignancies [9, 12–15], but there are no reports aboutCD147 and MMP-9 in TNBC patients.
The aim of the present study is first to evaluate the expression levels of CD147 and MMP-9 in 127 TNBC patients coming from our affiliated hospitals of Harbin Medical University using immunohistochemistry method, and their association with various clinical parameters and prognosis of TNBC, and therefore may be useful biomarkers and in predicting outcome and prognosis in TNBC patients.
Materials and methods
Patients and tissue samples
In our present study, 127 TNBC patients were evaluated, and a complete set of follow-up data were obtained. All of the patients underwent a curative operation, such as a mastectomy or conservative surgery with axillary lymph node dissection and did not undergo pre-operative radiochemotherapy; at the same time, 30 mammary gland fibroma tissues were used as controls. All the formalin-fixed paraffin-embedded specimens used in IHC were collected from the patients undergoing surgery between January 2003 and July 2005 in the Third Affiliated Hospital of Harbin Medical University. HER2 with – and + immunohistochemical staining was considered negative expression. For cases with ++ expression, further fluorescence in situ hybridization was performed to determine negative or positive expression [16]. Each patient was treated postoperatively with suitable adjuvant therapy according to the stage of the disease. Tumors were confirmed histopathologically and were staged according to TNM classification [17]. All research involving human participants and human samples have been approved by the Ethics and Scientific Committees of the Third Affiliated Harbin Medical University, and all participants signed an Institutional Review Board–approved informed consent form to participate in the study.
Immunohistochemistry
Immunohistochemical assays were performed on formalin-fixed and paraffin-embedded sections. Sections (thickness = 4 µm) were cut and deparaffinized in xylene and rehydrated in graded alcohols. The avidin–biotin complex (ABC) method was used in the whole IHC procedure. Antigen retrieval was performed using a steamer for 20 min in 1 × EDTA buffer. Endogenous peroxidase activity was blocked by 3 % hydrogen peroxide in methanol for 20 min. Five percent non-fat milk was used for blocking (1 h). Slides were then incubated with rabbit polyclonal antibody directed against human CD147 (1:50 dilution, Zhongshan Bio., Beijing, China) or MMP-9 antibody (1:100 dilution, Zhongshan Bio., Beijing, China) for 1 h at room temperature. Then slides were reacted with the second antibody of Max Vision TM HRP-Polymer anti-Rabbit (Maixin. Bio., Fuzhou, China) and counterstained with Mayer’s hematoxylin following staining with DAB. The tumor cells with membranous or cytoplasmic staining were considered positive for expression of CD147 or MMP-9 [12, 18].
The digital images of five representative visual fields from each slide that was positive for CD147 and MMP-9 (×200) were analyzed using Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Bethesda, USA). The mean value of densitometric units of five visual fields was used as score of the expression intensity of CD147 and MMP-9. The immuno-histochemically stained tissue sections were reviewed and scored separately by two pathologists blinded to the clinical parameters. Staining for CD147 and MMP-9 was assessed using a relatively simple, reproducible scoring method [18 – 20]. The staining intensity was scored on a scale of 0–3 as negative (0), weak (1), medium (2) or strong (3). The extent of the staining, defined as the percentage of positive staining areas of cancer cells or fibroma tissue cells in relation to the whole tissue area, was scored on a scale of 0–4 as the following: 0, <10 %; 1, 10–25 %; 2, 26–50 %; 3, 50–75 %and 4, ≥76 %. The sum of the staining-intensity and staining-extent scores was used as the final staining score for CD147 and MMP-9 (0–7). For statistical analysis, final staining scores of 0–5 and 6–7 were considered to be low and high expression, respectively.
Data analysis
All data were analyzed using the Statistical Package for the Social Sciences Version 13.0 software (SPSS, Chicago, IL, USA). Correlation between expression levels was studied using the Pearson coefficient. The two-sided Pearson’s χ2 test and the Fisher’s exact test were used to compare the clinicopathological parameters between low and high expression groups. Progression-free survival (PFS) was measured from the start of therapy until the time of disease progression or until the end of the observation period in patients without a progressive disease. Overall survival (OS) was measured until death from any cause or the end of the observation period. Both PFS and OS were also measured from the date the therapy was started. Survival curves were calculated according to the Kaplan–Meier method, and the logrank test was used for analyzing differences between curves. Clinicopathologic factors known to be associated with prognosis, such as axillary lymph node metastasis (positive versus negative), histology grade (3 versus 1–2), tumor size (>2 cm versus ≤2 cm), chemotherapy regimen (taxanes versus anthracycline versus anthracycline + taxanes), menopausal status (pre-menopausal versus postmenopausal), serum CA153 and CEA levels (positive versus negative) and additionally the expression of CD147 (high versus low) and MMP-9 (high versus low) were tested in univariate analysis. Variables that were found to be significant in univariate analysis were then entered in a multivariate Cox proportional hazards regression model to identify those with independent prognostic information for PFS and OS. All statistical tests were done at the 5 % level of significance.
Results
Analysis of correlation of CD147 and MMP-9 expression in TNBC
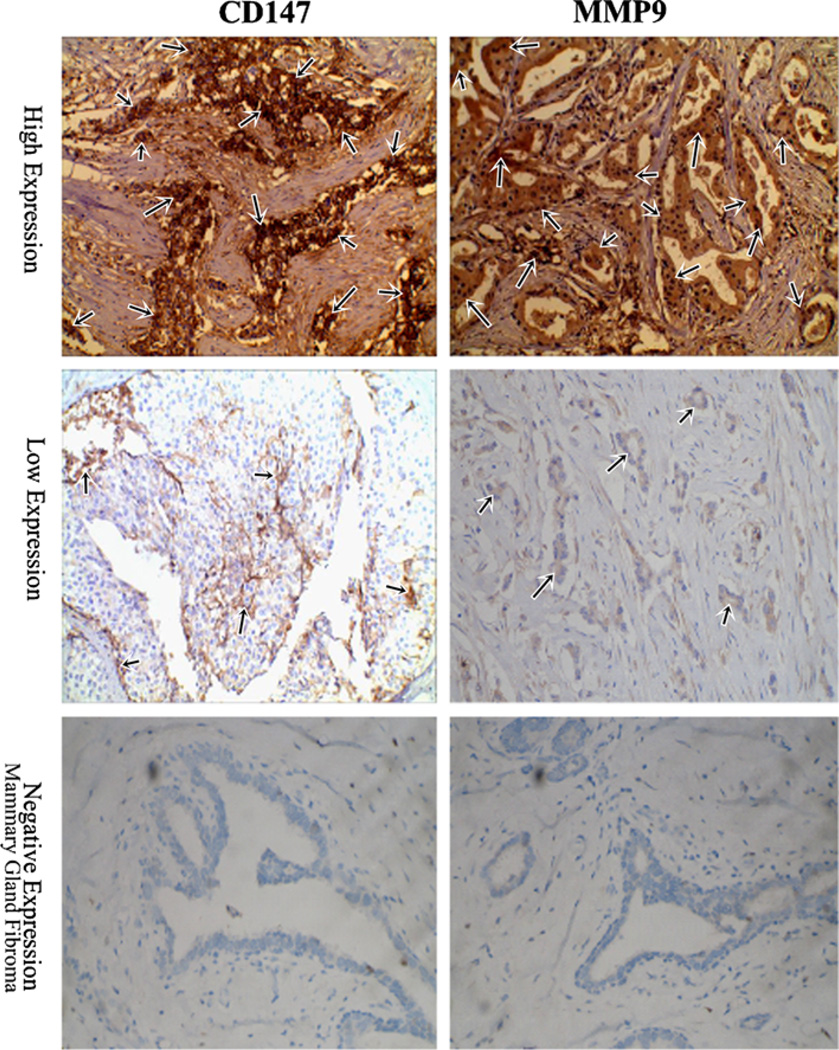
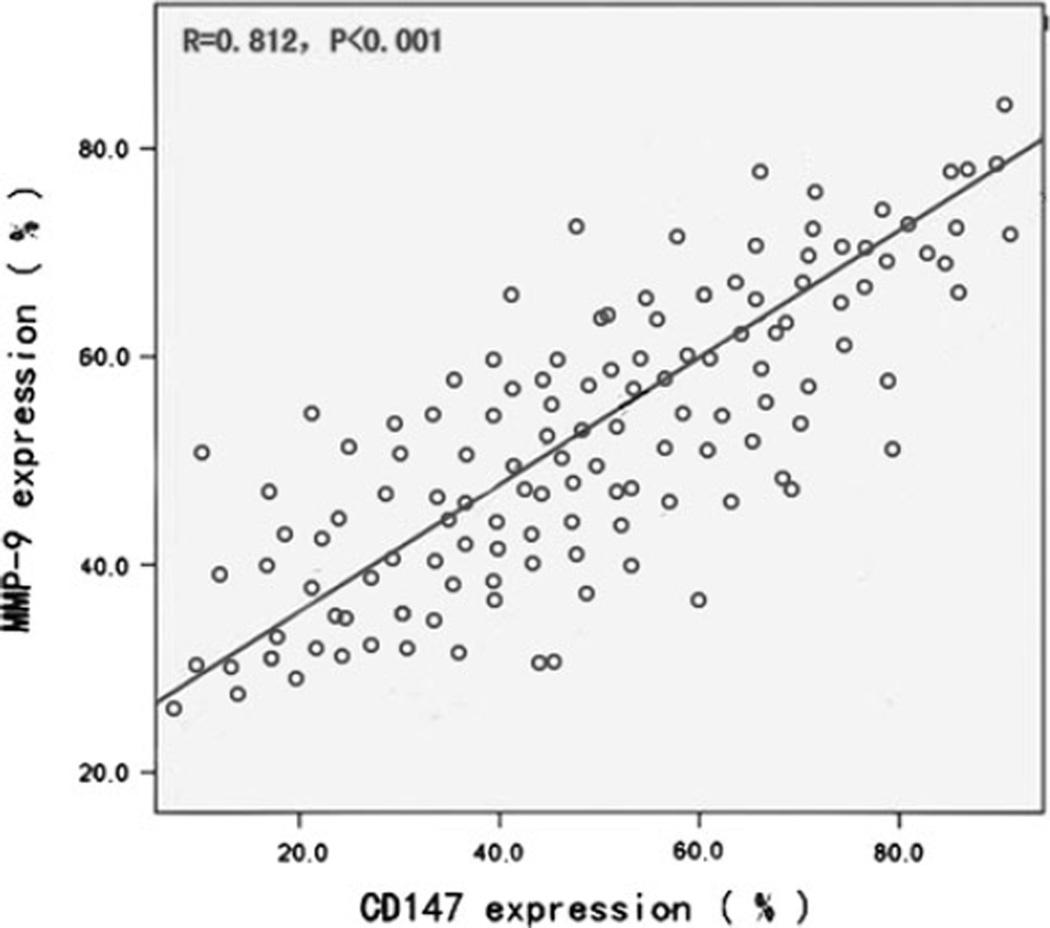
In total, 127 TNBC patients were enrolled in the present study and were analyzed for CD147 and MMP-9 expression. Patients and tumor characteristics are summarized in Table 1. All patients were women with a mean age of 49 years (range 30–68 years), The median disease-free interval of the 127 patients was 35 months (range, 10–52 months), and the median time of overall survival was 44 months (range 12–52 months). Among the 127 TNBC samples, the frequency was 48.0 % (61/127) for high expression of CD147 and 53.5 % (68/127) for MMP-9, respectively. The different intensities of the staining are shown in Fig. 1. The predominant pattern of CD147 and MMP-9 staining was cytoplasmic and the cell membrane. A statistically positive correlation was observed between CD147 and MMP-9 expression in TNBC tissues (n = 127, r = 0.812, P < 0.001; Fig. 2).
Table 1.
Patient characteristics
| Clinicopathologic characteristics |
All patients n (%) |
CD147 high expression n (%), χ2, P |
MMP-9 high expression n (%), χ2, P |
|---|---|---|---|
| Patient included | 127 (100.0) | 61 (48.0) | 68 (53.5) |
| Age (median age) | 49 (30–68) | 47 (30–66) | 54 (31–68) |
| Lymph node | |||
| 0 | 71 (55.9) | 21 (34.4) 21.97 0.000 | 20 (29.4) 41.68 0.000 |
| ≥1 | 56 (44.1) | 40 (65.6) | 48 (70.6) |
| Histology grade | |||
| 1/2 | 48 (49.0) | 24 (39.3) 6.002 0.014 | 27 (39.7) 7.645 0.006 |
| 3 | 50 (51.0) | 37 (60.7) | 41 (60.3) |
| Tumor size | |||
| ≤2 | 59 (46.5) | 21 (34.4) 6.830 0.009 | 24 (35.3) 7.332 0.007 |
| >2 | 68 (53.5) | 40 (65.6) | 44 (64.7) |
| Pathological stage | |||
| I + II | 45 (35.3) | 16 (26.2) 4.346 0.037 | 16 (23.5) 9.066 0.003 |
| III | 82 (64.5) | 45 (73.8) | 52 (76.5) |
| P53 | |||
| Negative | 57 (44.9) | 28 (45.9) 0.049 0.824 | 29 (42.6) 0.296 0.587 |
| Positive | 70 (55.1) | 33 (54.1) | 39 (57.4) |
| Ki67 | |||
| ≤30 % | 39 (30.7) | 11 (18.0) 8.864 0.003 | 17 (25.0) 7.046 0.008 |
| >30 % | 88 (69.3) | 50 (82.0) | 51 (75.0) |
| Chemotherapy regimen | |||
| Taxanes | 35 (27.6) | 20 (32.8) 3.363 0.186 | 25 (36.7) 7.393 0.125 |
| Anthracycline | 23 (18.1) | 13 (21.3) | 13 (19.1) |
| Anthracycline + taxanes | 69 (54.3) | 28 (45.9) | 30 (44.1) |
| Menopausal status | |||
| Pre-menopausal | 67 (52.7) | 27 (44.3) 3.397 0.065 | 36 (52.9) 0.002 0.964 |
| Postmenopausal | 60 (47.3) | 34 (55.7) | 32 (47.1) |
| Serum CA 15-3 levels | |||
| Positive | 31 (24.4) | 16 (26.2) 0.211 0.646 | 20 (29.4) 1.985 0.159 |
| Negative | 96 (75.6) | 45 (73.8) | 48 (70.6) |
| Serum CEA levels | |||
| Positive | 38 (29.9) | 18 (29.5) 0.010 0.922 | 24 (35.3) 2.015 0.156 |
| Negative | 89 (70.1) | 43 (70.5) | 44 (64.7) |
Fig. 1.
Immunohistochemistry (×200) for CD147 and MMP-9 in representative specimens. Positive stain of CD147 and MMP-9 was seen in all 127 cases of tumor samples. High expression of CD147 (n = 61) and MMP-9 (n = 68) was observed, respectively. The predominant pattern of CD147 and MMP-9 staining was cytoplasmic and the cell membrane. All mammary gland fibroma tissue (n = 30) was negative expression for CD147 and MMP-9
Fig. 2.
A statistical correlation was observed between CD147 and MMP-9 high expression in TNBC tissues (n = 127, r = 0.812, P < 0.001)
Analysis of correlation of CD147 and MMP-9 with clinicopathologic findings
The correlation between the protein expression of CD147 and MMP-9 and clinicopathologic variables of patients with TNBC were shown inTable 1. High expression of CD147 and MMP-9 was significantly correlated with lymph node metastasis (PCD147 < 0.001, χ2CD147 = 21.97; PMMP-9 < 0.001, χ2MMP-9 = 41.68), high pathological grade(PCD147 = 0.012, χ2CD147 = 6.002; PMMP-9 = 0.006, χ2MMP-9 = 0.014), tumor size larger than 2 cm (PCD147 = 0.009, χ2CD147 = 6.830; PMMP-9 = 0.007, χ2MMP-9 = 7.332) and high Ki67 expression (PCD147 = 0.003, χ2CD147 = 8.864; PMMP-9 = 0.008, χ2MMP-9 = 7.046), but not correlated with other index, such as different chemotherapy strategies, menstrual status, P53 expression and the values of CEA and CA153 before treatment. In the control group, only one patient with fibromas exhibited low MMP-9 expression, which was significantly different from the TNBC patients(P < 0.05).
Analysis of correlation of CD147 and MMP-9 with therapy outcome
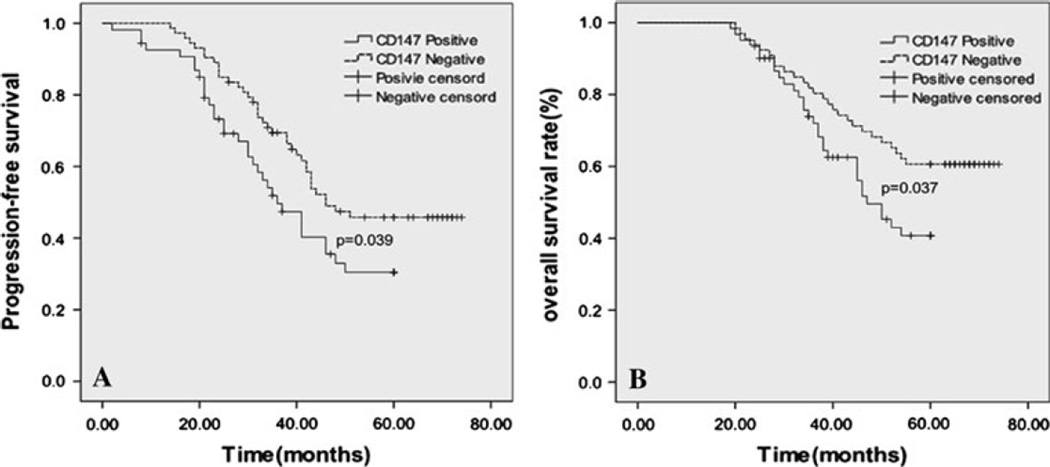
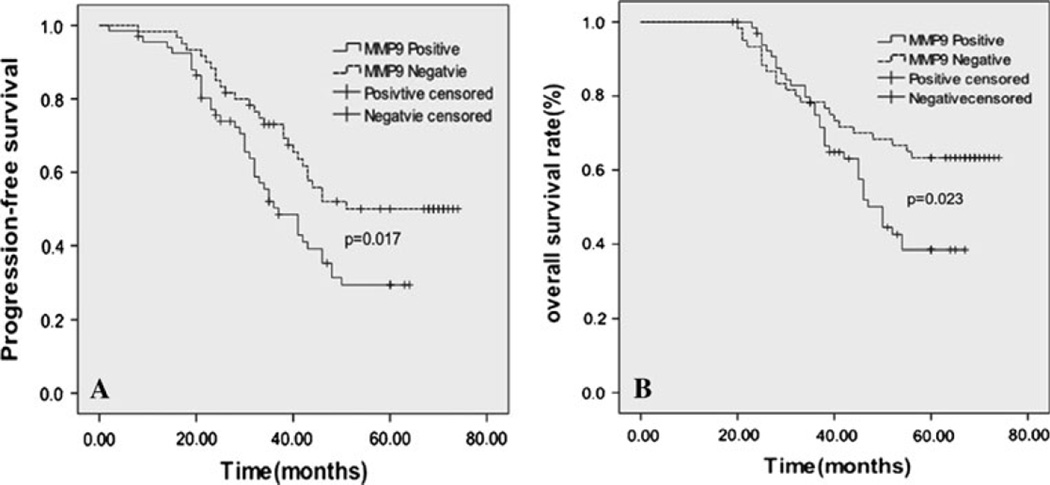
We evaluated the prognostic values of CD147 and MMP-9 on PFS and OS in all patients. None of the patients received pre-operative chemotherapy. All the patients received postoperative chemotherapy and X-ray radiation. Kaplan–Meier analysis demonstrated that TNBC patients with high expression levels of CD147 and MMP-9 had a significantly poorer PFS than TNBC patients with low expression levels of CD147 and MMP-9 (PCD147 = 0.039, median time 36.3 vs. 46.7 months; PMMP-9 = 0.017, median time 34.9 vs. 47.4 months) (Figs. 3a, 4a). TNBC patients with high expression levels of CD147 and MMP-9 also had a significantly worse OS than the patients with low expression levels of CD147 and MMP-9 (PCD147 = 0.037, median time 47.7 vs. 59.4 months; PMMP-9 = 0.023, median time 50.1 vs. 61.8 months) (Figs. 3b, 4b). Specifically, a multivariate analysis demonstrated that, in addition to tumor size, tumor grade and lymph node status, not only CD147 but also MMP-9 remained as statistically significant prognostic markers in patients with TNBC (PCD147 < 0.001; PMMP-9 < 0.001) (Table 2). These results indicated that increased expression of CD147/MMP-9 was associated with high likelihood of therapy failure in TNBC patients.
Fig. 3.
Kaplan–Meier analysis showed poorer progression-free survival (A, P = 0.039) and overall survival (B, P = 0.037) of patients with high expression of CD147
Fig. 4.
Kaplan–Meier analysis showed poorer progression-free survival (A, P = 0.017) and overall survival (B, P = 0.023) of patients with high expression of MMP-9
Table 2.
Prognostic factors by multivariate analysis for triple-negative breast cancer patients
| Parameters | Hazard ratio | P value | 95 % CI |
|---|---|---|---|
| PFS | |||
| Tumor size >2 cm | 1.733 | 0.03 | 1.054–2.848 |
| Lymph node metastases | 3.09 | 0 | 1.798–5.311 |
| Tumor grade | 1.858 | 0.01 | 1.157–2.984 |
| CD147 high expression | 2.867 | 0.023 | 1.159–7.093 |
| MMP-9 high expression | 1.824 | 0.015 | 1.122–2.965 |
| OS | |||
| Tumor size >2 cm | 1.754 | 0.023 | 1.080–2.849 |
| Lymph node metastases | 3.608 | 0 | 2.134–6.100 |
| Tumor grad | 1.72 | 0.026 | 1.066–2.776 |
| CD147 high expression | 2.683 | 0.033 | 1.082–6.656 |
| MMP-9 high expression | 1.761 | 0.02 | 1.092–2.840 |
Discussion
The recent cancer statistics show that the predicted numbers of new breast cancer cases and deaths in the United States in 2012 are at the first and second positions in the women, respectively, with an estimated 226,870 new cases diagnosed and 39,510 deaths [21]. The TNBC phenotype is heterogeneous from a histopathologic and molecular perspective, which suggests the existence of molecular subsets. Thus, the identification of molecular predictive signatures is necessary and will allow for the characterization of TNBC and the design of optimal treatment modalities. In the present study, the expression and clinical significance of the CD147 andMMP-9 were first evaluated in 127 cases of TNBC. The results showed that the status of CD147 and MMP-9 expression could be predictive factors of TNBC.
CD147 is an adhesive molecule expressed on the surface of tumor cells, especially overexpressed on the surface of malignant tumor cells. It was thought that CD 147 is a tumor cell–associated extracellular MMPs inducer and plays important roles in the malignant transformation, invasion, metastasis of tumors and angiogenesis in tumor formation [7]. It has been shown that CD147 overexpression is associated with high transcription and secretion of MMPs proteins in the invasion and metastasis of malignant tumors [10]. Transfection of antisense RNA of CD147 into hepatocellular carcinoma cells decreases the secretion of MMP-9 and inhibits tumor cells for invasion and metastasis [22]. CD147 expression in primary breast cancer tissue correlates with tumor size and staging and is predictive of poor prognosis [23]. The main function of MMP-9, a member of MMPs, is the regulation of cell matrix composition [24–27]. MMP-9 cleaves denatured collagen (gelatins) and type 4 collagen, which is the major component of the basement membranes. This cleavage helps tumor cells to enter and leave the blood and lymph circulations leading to tumor metastasis.
In our study, we found that the predominant pattern of CD147 and MMP-9 staining similar to staining of non-triple-negative breast cancer and salivary duct carcinoma tissues as described earlier [28, 29]. Both TNBC and salivary duct carcinoma which we have reported previously [29] belong to a highly aggressive adenocarcinoma. Therefore, there may be same clinical characteristics and biological behaviors between these two types of tumors. The correlation coefficient between the expression levels of MMP-9 and CD147 was 0.568 (P < 0.001). The positive correlation between the CD147 and MMP-9 expression suggested that CD147 was involved in the regulation of MMP-9 in TNBC. In our study, one patient with mammary gland fibroma was found to have the performance of low MMP-9 expression, but there are significant differences between the TNBC and mammary gland fibroma (P < 0.05), this may be caused by the heterogeneity of the mammary gland fibroma or other unknown reason.
Although this study was the first to indicate the expression levels of CD147 and MMP-9 and to determine their correlation with prognosis in TNBC, several limitations affected the results of the present study. For instance, first, this study encompassed a relatively small number of patients and was a retrospective study. Furthermore, the expression status of the above markers was not analyzed in nodal or distant metastasis sites. Therefore, we did not gather any information concerning the expression of markers at the so-called “active” sites of disease progression. Nevertheless, this study indicated that survival and prognosis in patients with TNBC could depend on expression of CD147 and MMP-9 as well as clinical factors, such as nodal stage at diagnosis. Third, this is hypothesis generating, with a strong rationale, and should be further explored. However, using a multi-center design with the cooperation of several large cancer centers, such a study would be feasible, and we have confidence that these two markers are promising biomarkers despite they can not be into clinical use at present.
In summary, the results of the present study demonstrated that TNBC could be further divided into good and poor prognosis subtypes according to the CD147 and MMP-9 expression status. We attempted to explain the mechanism of this phenomenon and suggest that poor prognosis subtypes require further evaluation. Future research should aim to include a larger sample size, and cell biology and animal studies should be combined. As some researchers [30–32] points out that inhibiting activities of CD147 and MMP-9 as potential therapeutic targets may be a good strategy for the treatment of TNBC.
Acknowledgments
This experiment was finished in the Oncobiology Key Lab of the Heilongjiang Province Common Institution of Higher Learning. This work was supported by the National Natural Science Foundation of China [grant number 81071889] and Harbin science and technology innovation young talents research funds [grant number 2007RFQXS086].
Footnotes
Conflict of interest All authors declared that there is no conflict of interest.
Contributor Information
Shu Zhao, Department of Medical Oncology, The Third Affiliated Hospital of Harbin Medical University, Haping Road 150 of Nangang District, Harbin 150081, Heilongjiang Province, China.
Wenjie Ma, Department of Medical Oncology, The Third Affiliated Hospital of Harbin Medical University, Haping Road 150 of Nangang District, Harbin 150081, Heilongjiang Province, China.
Minghui Zhang, Department of Medical Oncology, The Third Affiliated Hospital of Harbin Medical University, Haping Road 150 of Nangang District, Harbin 150081, Heilongjiang Province, China.
Dabei Tang, Department of Medical Oncology, The Third Affiliated Hospital of Harbin Medical University, Haping Road 150 of Nangang District, Harbin 150081, Heilongjiang Province, China.
Qingtao Shi, Department of Pathology, The Third Affiliated Hospital of Harbin Medical University, Harbin 150081, China.
Shanqi Xu, Department of Medical Oncology, The Third Affiliated Hospital of Harbin Medical University, Haping Road 150 of Nangang District, Harbin 150081, Heilongjiang Province, China.
Xiaosan Zhang, Department of Medical Oncology, The Third Affiliated Hospital of Harbin Medical University, Haping Road 150 of Nangang District, Harbin 150081, Heilongjiang Province, China.
Yupeng Liu, Department of Epidemiology, Public Health College of Harbin Medical University, Harbin 150040, China.
Ying Song, Department of Medical Oncology, The Third Affiliated Hospital of Harbin Medical University, Haping Road 150 of Nangang District, Harbin 150081, Heilongjiang Province, China.
Leyuan Liu, Center for Cancer and Stem Cell Biology, Institute of Biosciences and Technology, Texas A & M Health Science Center, Houston, TX 77030, USA.
Qingyuan Zhang, Email: zhma19650210@126.com, Department of Medical Oncology, The Third Affiliated Hospital of Harbin Medical University, Haping Road 150 of Nangang District, Harbin 150081, Heilongjiang Province, China.
References
- 1.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumors. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Kreike B, van Kouwenhove M, Horlings H, Weigelt B, Peterse H, Bartelink H, et al. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res. 2007;9:R65. doi: 10.1186/bcr1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reis-Filho JS, Tutt AN. Triple negative tumors: a critical review. Histopathology. 2008;52:108–118. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 4.Kang SP, Martel M, Harris LN. Triple negative breast cancer: current understanding of biology and treatment options. Curr Opin Obstet Gynecol. 2008;20:40–46. doi: 10.1097/GCO.0b013e3282f40de9. [DOI] [PubMed] [Google Scholar]
- 5.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population- based study from the California cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 6.Tang Yi, Nakada Marian T, Kesavan Prabakaran, et al. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res. 2005;65:3193–3199. doi: 10.1158/0008-5472.CAN-04-3605. [DOI] [PubMed] [Google Scholar]
- 7.Toole BP. Emmprin (CD147), a cell surface regulator of matrix metalloproteinase production and function. Curr Top Dev Biol. 2003;54:371–389. doi: 10.1016/s0070-2153(03)54015-7. [DOI] [PubMed] [Google Scholar]
- 8.Zheng HC, Takahashi H, Murai Y, et al. Upregulated EMMPRIN/CD147 might contribute to growth and angiogenesis of gastric carcinoma: a good marker for local invasion and prognosis. Br J Cancer. 2006;95:1371–1378. doi: 10.1038/sj.bjc.6603425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Ren Y, Wu QC, et al. Macrophage migration inhibitory factor enhances neoplastic cell invasion by inducing the expression of matrix metalloproteinase 9 and inter-leukin-8 in nasopharyngeal carcinoma cell lines. Chin Med J. 2004;117:107–114. [PubMed] [Google Scholar]
- 10.Hymowitz M, Zucker, Rollo EE, et al. Tumorigenic potential of extracellular matrix metalloproteinase inducer. Am J Pathol. 2001;158:1921–1928. doi: 10.1016/S0002-9440(10)64660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mira E, Lacalle RA, Buesa JM, et al. Secreted MMP9 promotes angiogenesis more efficiently than constitutive active MMP9 bound to the tumor cell surface. J Cell Sci. 2004;117:1847–1857. doi: 10.1242/jcs.01035. [DOI] [PubMed] [Google Scholar]
- 12.Yu WW, Liu JH, Xiong XL, et al. Expression of MMP-9 and CD147 in invasive squamous cell carcinoma of the uterine cervix and their implication. Pathol Res Pract. 2009;205:709–715. doi: 10.1016/j.prp.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Tang J, Zhou HW, Jiang JL, et al. ßig-h3 is involved in the HAb18G/CD147-mediated metastasis process in human hepatoma cells. Exp Biol Med. 2007;232:344–352. [PubMed] [Google Scholar]
- 14.Davidson B, Goldberg I, Berner A, et al. EMMPRIN (extracellular matrix metalloproteinase inducer) is a novel marker of poor outcome in serous ovarian carcinoma. Clin Exp Metastasis. 2003;20:161–169. doi: 10.1023/a:1022696012668. [DOI] [PubMed] [Google Scholar]
- 15.Ramos-DeSimone N, Hahn-Dantona E, Sipley J, et al. Activation of matrix metalloprotease-9 (MMP-9) via converting plasmin/stromelysin-1 cascade enhances tumor cell invasion. J Biol Chem. 1999;274:13066–13076. doi: 10.1074/jbc.274.19.13066. [DOI] [PubMed] [Google Scholar]
- 16.Alizadeh AA, Ross DT, Perou CM, Ven de Rijn M. Towards a novel classification of human malignancies based on gene expression patterns. J Pathol. 2010;195:41–52. doi: 10.1002/path.889. [DOI] [PubMed] [Google Scholar]
- 17.Kashiwagi S, et al. Significance of E-cadherin expression in triple- negative breast cancer. Br J Cancer. 2010;103:249–255. doi: 10.1038/sj.bjc.6605735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Z, Li L, Yang ZX, et al. Research article Increased expression of MMP9 is correlated with poor prognosis of nasopharyngeal carcinoma. BMC Cancer. 2010;10:270–275. doi: 10.1186/1471-2407-10-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S, Zhou J, Wang XY, et al. Down-regulated expression of SATB2 is associated with metastasis and poor prognosis in colorectal cancer. J Pathol. 2009;219:114–122. doi: 10.1002/path.2575. [DOI] [PubMed] [Google Scholar]
- 20.Masunaga R, Kohno H, Dhar DK, et al. Cyclooxygenase-2 expression correlates with tumor neovascularization and prognosis in human colorectal carcinoma patients. Clin Cancer Res. 2000;6:4064–4068. [PubMed] [Google Scholar]
- 21.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Shang P, Qian AR, et al. Inhibitory effects of antisense RNA of HAb18G/CD147 on invasion of hepatocellular carcinoma cells in vitro. World J Gastroenterol. 2003;9:2174–2177. doi: 10.3748/wjg.v9.i10.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reimers N, Za frakas K, Assmann V, et al. Expression of extracellular matrix metalloproteases inducer on micrometastatic and primary mammary carcinoma cells. Clin Cancer Res. 2004;10:3422–3428. doi: 10.1158/1078-0432.CCR-03-0610. [DOI] [PubMed] [Google Scholar]
- 24.Chakraborti S, Mandal M, Das S, et al. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253:269–285. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- 25.Opdenakker G, Van den Steen PE, Van Damme J, Gelatinase B. A tuner and amplifier of immune functions. Trends Immunol. 2001;22:571–579. doi: 10.1016/s1471-4906(01)02023-3. [DOI] [PubMed] [Google Scholar]
- 26.Opdenakker G, Van den Steen PE, Dubois B, et al. Gelatinase B functions as regulator and effector in leukocyte biology. J Leukoc Biol. 2001;69:851–859. [PubMed] [Google Scholar]
- 27.Matache C, Stefanescu M, Dragomir C, et al. Matrix metalloproteinase-9 and its natural inhibitor TIMP-1 expressed or secreted by peripheral blood mononuclear cells from patients with systemic lupus erythematosus. J Autoimmun. 2003;20:323–331. doi: 10.1016/s0896-8411(03)00037-4. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Jing X, Li C, et al. HAb18G (CD147), a cancer-associated biomarker and its role in cancer detection. Histopathology. 2009;54:677–687. doi: 10.1111/j.1365-2559.2009.03280.x. [DOI] [PubMed] [Google Scholar]
- 29.Piao S, Zhao S, Guo F, et al. Increased expression of CD147 and MMP-9 is correlated with poor prognosis of salivary duct carcinoma. J Cancer Res Clin Oncol. 2012;138:627–635. doi: 10.1007/s00432-011-1142-6. [DOI] [PubMed] [Google Scholar]
- 30.Ju XZ, Yang JM, Zhou XY, et al. EMMPRIN expression as a prognostic factor in radiotherapy of cervical cancer. Clin Cancer Res. 2008;14:494–501. doi: 10.1158/1078-0432.CCR-07-1072. [DOI] [PubMed] [Google Scholar]
- 31.Chen X, Lin J, Kanekura T, et al. A small interfering CD147-targeting RNA inhibited the proliferation, invasiveness, and metastatic activity of malignant melanoma. Cancer Res. 2006;66:11323–11330. doi: 10.1158/0008-5472.CAN-06-1536. [DOI] [PubMed] [Google Scholar]
- 32.Rao JS, Ra C, Gondi C, et al. Inhibition of invasion, angiogenesis, tumor growth, and metastasis by adenovirus-mediated transfer of antisense uPAR and MMP-9 in non-small cell lung cancer cells. Mol Cancer Ther. 2005;4:1399–1408. doi: 10.1158/1535-7163.MCT-05-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]