Abstract
Agaricus brasiliensis cell-wall polysaccharides isolated from fruiting body (FR) and mycelium (MI) and their respective sulfated derivatives (FR-S and MI-S) were chemically characterized using elemental analysis, TLC, FT-IR, NMR, HPLC, and thermal analysis. Cytotoxic activity was evaluated against A549 tumor cells by MTT and sulforhodamine assays. The average molecular weight (Mw) of FR and MI was estimated to be 609 and 310 kDa, respectively. FR-S (127 kDa) and MI-S (86 kDa) had lower Mw, probably due to hydrolysis occurred during the sulfation reaction. FR-S and MI-S presented ~14 % sulfur content in elemental analysis. Sulfation of samples was characterized by the appearance of two new absorption bands at 1253 and 810 cm−1 in the infrared spectra, related to S=O and C-S-O sulfate groups, respectively. Through 1H and 13C NMR analysis FR-S was characterized as a (1→6)-(1→3)-β-D-glucan fully sulfated at C-4 and C-6 terminal and partially sulfated at C-6 of (1→3)-β-D-glucan moiety. MI-S was shown to be a (1→3)-β-D-gluco-(1→2)-β-D-mannan, partially sulfated at C-2, C-3, C-4, and C-6, and fully sulfated at C-6 of the terminal residues. The combination of high degree of sulfation and low molecular weight was correlated with the increased cytotoxic activity (48 h of treatment) of both FR-S (EC50=605.6 μg/mL) and MI-S (EC50=342.1 μg/mL) compared to the non-sulfated polysaccharides FR and MI (EC50>1500 μg/mL).
Keywords: Agaricus brasiliensis, sulfated polysaccharide, chemical characterization, cytotoxic activity
1. Introduction
Agaricus brasiliensis is an edible Basidiomycete fungus belonging to the Brazilian biota and has traditionally been used to treat cancer and other diseases. In the last few decades, numerous studies have reported the cytotoxic and antitumor properties of A. brasiliensis polysaccharides, which mainly act through immunomodulatory mechanisms, but also by direct cytotoxic effects on tumor cells [1].
The species found in Brazil was originally named Agaricus blazei Murrill sensu Heinemann. Since 2005, this binominal nomenclature has been considered incorrect and replaced by two botanical names: Agaricus subrufescens Peck or Agaricus brasiliensis Wasser, with the latter being adopted for the fungus cultivated in Brazil [2–5].
Polysaccharides are a structurally diverse class of macromolecules for which physicochemical and biological properties are dependent on a combination of factors such as sugar composition, molecular weight, and chain conformation. For sulfated polysaccharides, the degree of substitution and position of sulfated groups are also important [6]. There are many reports demonstrating that sulfation improves the biological activity of polysaccharides, including anti-coagulant [7], antiviral [8], immunostimulant [9], hypoglycemic [10], anti-oxidant [11], cytotoxic [12, 13], and antitumor [14] properties.
In our previous work [15], we evaluated the anti-herpetic activity of an A. brasiliensis mycelial polysaccharide (MI) and its sulfated derivative (MI-S) and found that sulfation of MI significantly improved its antiviral activity. Since A. brasiliensis polysaccharides and sulfated derivatives have potential therapeutic applications, the goal of this work was to chemically characterize MI and MI-S as well as A. brasiliensis fruiting body polysaccharide (FR) and its sulfated derivative (FR-S). Analytical methods included scanning electron microscopy, elemental and thermogravimetric analysis, high performance gel permeation and liquid chromatography (HPGPC and HPLC), FT-IR, and NMR. The cytotoxicity of MI, MI-S, FR, and FR-S also was evaluated against tumor (A549) and non-tumor cell lines (Vero). To the best of our knowledge, this is the first report on the cytotoxic activity of sulfated derivatives of polysaccharides from this species.
2. Materials and methods
2.1 Chemical reagents
The chemical reagents were purchased from Sigma-Aldrich (USA). All the reagents used in High Performance Chromatography analysis were HPLC grade. All the others reagents were analytical grade.
2.2 Fungal materials
The fruiting bodies of Agaricus brasiliensis Wasser (syn A. subrufescens Peck) were collected in Biguaçu, Santa Catarina state, Brazil, and designed as strain UFSC 51. A voucher specimen is deposited in the FLOR Herbarium at UFSC (FLOR 11797) and at Coleção Brasileira de Micro-organismos de Ambiente e Indústria -CBMAI/UNICAMP (available at http://webdrm.cpqba.unicamp.br/catalogo/pycat/index.py, code number: 1449). The A. brasiliensis mycelium was isolated and cultivated as previously described [15].
2.3 Isolation of mycelial and fruiting body polysaccharides and sulfation method
A. brasiliensis polysaccharides were isolated as previously described [15, 16], with minor modifications. Briefly, 50 g of dried fruiting bodies or mycelial biomass from the submerge-cultivated state were blended twice with 0.5 L of distilled water, refluxed at 100°C for 3 h and filtered through a Whatman filter paper no. 42. The extracts were precipitated with three volumes of 95 % ethanol and recovered by centrifugation (2000 x g, 15 min) to obtain the crude mycelial (cMI) and fruiting body (cFR) polysaccharide fractions. Finally, the higher molecular weight polysaccharides from the mycelium and fruiting body were obtained through dialysis (5 kDa cutoff membrane - Spectrum Laboratories, New Brunswick, USA) and, after lyophilization, were designated as MI and FR, respectively. Both polysaccharides were chemically sulfated using the chlorosulfonic acid/pyridine method as described by Zhang et al. [17], generating their respective sulfated derivatives MI-S and FR-S.
2.4 Sample characterization
2.4.1 Scanning electron microscopy (SEM)
The surface morphology of gold coated samples was analyzed by scanning electron microscopy (JSM-6390 LV, Jeol, Japan).
2.4.2 Thermogravimetry combined with differential thermogravimetric analysis (TGA-DTGA)
Thermograms of samples (1 mg) were obtained in a thermogravimetric analyzer (TGA-50, Shimadzu) from room temperature up to 900°C, at a scan rate of 10°C per min.
2.4.3 High-performance gel permeation chromatography (HPGPC)
The molecular weight (Mw) determination was carried out by HPGPC using a Perkin-Elmer series 200 instrument (USA) equipped with a refractive index detector and a gel filtration column (TSK-Gel 5000 PW 7.8 × 300 mm connected to a TSK PWH 5 × 75 mm guard column; Tosoh, Japan). Samples were eluted with 0.2 M NaCl mobile phase at a flow rate of 1 mL/min. The Mw was estimated by reference to the calibration curves of standard dextrans (5, 12, 50, 150, 410, and 670 kDa).
2.4.4 Analytical methods
Total sugar content was determined using the phenol-sulfuric acid method [18] adapted to a 96 well microassay plate [19] using glucose as standard. The Bradford method [20] was used to determine the protein content using calibration curves built with bovine serum albumin. The sulfate content was determined by the BaCl2 method [21]. Results, determined from calibration curves obtained on three different days, are expressed as mean ± standard deviation (% w/w ± s.d.).
2.4.5 Elemental analysis
Elemental analysis (carbon, hydrogen, nitrogen, and sulfur) was performed with a Perkin Elmer 2400 series II elemental analyzer. The percentage of sulfur (% S) and carbon (% C) were used to calculate the degree of substitution (DS) according to the formula:
[22]
2.4.6 Monosaccharide composition analysis
The qualitative monosaccharide composition of MI and FR was evaluated by thin layer chromatography (TLC) using xylose, arabinose, mannose, glucose, and galactose as reference compounds. Briefly, the samples were hydrolyzed with 3M trifluoroacetic acid (TFA) for 4 h and analyzed on PEI-cellulose sheets (Merck, Germany), developed with n-butanol/ethyl acetate/pyridine/water (6:1:5:4). The chromatograms were visualized after spraying aniline-o-phthalic acid reagent followed by heating [23].
Quantification of monosaccharides was performed using High Performance Liquid Chromatography (HPLC) in a Perkin-Elmer series 200 instrument equipped with an UV detector, at 250 nm, according to Lv et al. [24]. Briefly, hydrolyzed samples (4 M TFA, 2 h) were derivatized with 1-phenil-3-methyl-5-pyrazolone (PMP). Calibration curves, constructed with PMP-labeled standard monomers (mannose, glucose, galactose, and glucuronic acid), were used for determining the sugar concentrations of the samples. Arabinose was employed as an internal standard. Analyses were undertaken at room temperature (25 °C) on a C18 column (4.6 mm × 250 mm, 5 μm, Perkin-Elmer, USA) using a gradient elution of 0.045 % KH2PO4 - 0.05 % triethylamine buffer (A) / acetonitrile (B) as follows: 10 % to 14 % B over 40 min, at a flow rate of 1.0 mL/min.
2.4.7 Fourier transformation infrared (FT-IR) spectroscopy
The FT-IR spectrum was recorded on a Perkin-Elmer Spectrum One spectrometer in the region between 650 and 4.000 cm−1.
2.4.8 Nuclear Magnetic Resonance (NMR) analysis
The spectra were recorded at room temperature for samples (60 mg/mL) dissolved in D2O in a Bruker Avance III 500 NMR instrument, operating at 500 MHz for 1H and 125 MHz for 13C. Chemical shifts were expressed in ppm relative to internal acetone (= 32 ppm).
2.5 Cytotoxic activity evaluation
2.5.1. Cell lines
Human lung adenocarcinoma (A549, ATCC, CCL-185) and Vero cells (ATCC, CCL-81) were grown in Minimal Essential Medium supplemented with 10 % fetal bovine serum, 100 U/mL penicillin G, 100 μg/mL streptomycin and 25 μg/mL amphotericin B in a humidified 5 % CO2 atmosphere at 37°C.
2.5.2 MTT assay
The effect on cell proliferation was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cellular viability assay [25]. In brief, 1 × 104 cells/well were seeded in 96-well microplates and incubated for 24 h. Cells were then treated with different concentrations of the samples for 48 or 72 h. Negative controls were treated with medium only. After the exposure period, the culture medium was replaced by MTT solution (1 mg/mL) and plates were further incubated for 4 h. Formazan crystals were dissolved by addition of DMSO (Merk, Germany) and the optical densities were read at 540 nm (Infinite 1200 TECAN, Austria). The 50 % effective concentration (EC50) was defined as the concentration that reduced cell proliferation by 50 % when compared to untreated controls. Paclitaxel (Glenmark, Brazil) was used as the positive control.
2.5.3 Sulforhodamine assay
The sulforhodamine assay [26] was also used to evaluate the cytotoxicity of selected samples. Briefly, A549 or Vero (ATCC: CCL 81) cells, cultured in 96-well plates, at a density of 1 × 104 cells/well, were exposed to eight concentrations of samples for 48 h. After subtracting the absorbance values of the initial cell cultures (time zero cell control), the GI50 (50 % growth inhibitory activity), TGI (total growth inhibition, cytostatic activity), and LC50 (50 % lethal concentration, cytotoxic activity) values were calculated.
2.6 Statistical analysis
GraphPad Prism 5 Software was used to calculate EC50 values and their 95 % confidence intervals through a nonlinear fit- curve (log of compound concentration versus normalized response - variable slope). GI50, TGI, and LC50 values were calculated using linear fit- curves in the same program.
3 Results and Discussion
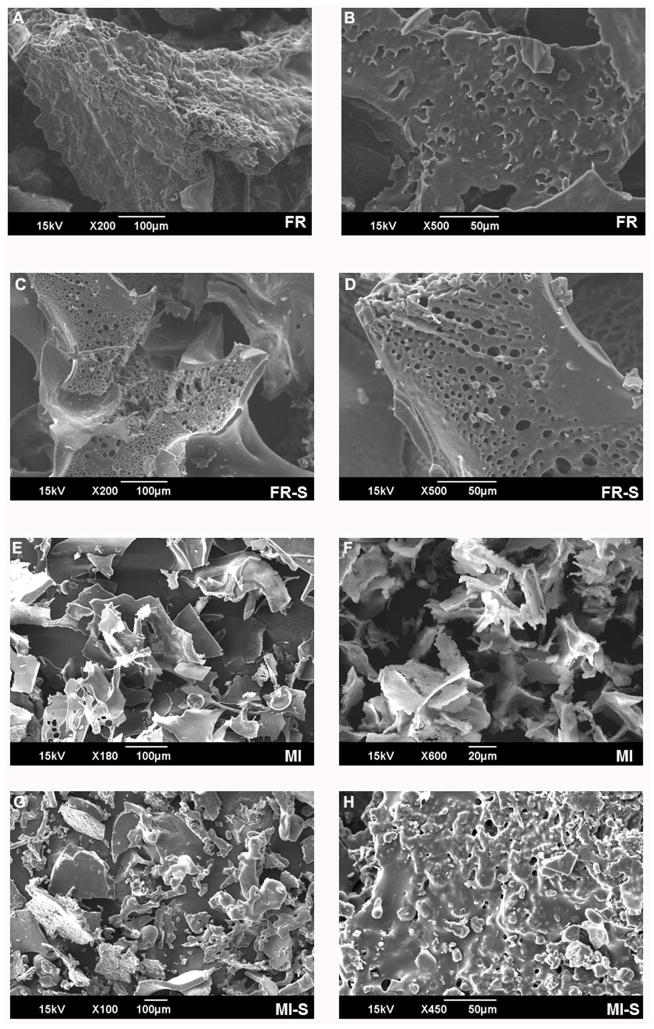
Since the introduction of sulfate groups with an appropriate degree of substitution can improve the bioactivity of polysaccharides, the goal of this study was to modify polysaccharides isolated from A. brasiliensis fruiting body and mycelium and evaluate their physicochemical properties and cytotoxic activity. Analysis of surface morphology by scanning electron microscopy (SEM) is a qualitative tool to characterize fungal polysaccharides, comparing with standards and assessing morphological differences of modified derivatives. Microstructural irregularities observed in SEMs presented in Fig. 1 show that A. brasiliensis polysaccharides are an amorphous solid. The FR polysaccharide (Fig. 1 A,) showed a rough appearance with no porosity that may be due to high molecular packing as a result of inter and intramolecular hydrogen bonds. Following sulfation, FR-S (Fig. 1 C, D) displayed a smoother surface with an internal porous structure, suggesting that the sulfate groups expanded intermolecular spaces. The MI polysaccharide (Fig. 1 E, F) consisted of flakes that were smaller in size than those of FR and FR-S. MI-S (Fig. 1 G, H) presented particles of variable size with smoother surfaces and rounded shapes. Porous structures were also observed in the MI-S preparation. These particle structures are consistent with results obtained by others. For example, an A. brasiliensis mycelial exopolysaccharide was previously reported to be an amorphous solid by Lima et al. [27]. In contrast, Hong and Choi [28] described a spherical shape for an A. blazei protein-polysaccharide complex, prepared by spray-drying process. This variation on morphology may be related to the different methods used for fungus cultivation, sample extraction and drying.
Fig. 1.
Agaricus brasiliensis polysaccharides observed under scan electron microscopy. Lyophilized samples were gold coated and analyzed by a JSM-6390 LV microscope. A and B: FR, A. brasiliensis fruiting body polysaccharide; C and D: FR-S, sulfated derivative of A. brasiliensis fruiting body polysaccharide; E and F: MI, A. brasiliensis mycelial polysaccharide; G and H: MI-S, sulfated derivative of A. brasiliensis mycelial polysaccharide.
Data from thermal analysis performed by TGA-DTGA are compiled in Table 1 and thermograms are available in Supplementary Fig. 1. According to the thermograms, polysaccharide decomposition occurred in three steps. The small initial drop in mass represents the loss of water. The second stage of decomposition began above 266°C for the non-modified compounds (FR and MI) and above 218°C for the sulfated derivatives (FR-S and MI-S) suggesting that sulfation reduced the thermal stability. The temperature peak (Tmax) recorded in the DTGA curve is characteristic of an endothermic reaction and could be attributed to thermal decomposition of the polysaccharides [29]. The Tmax obtained for FR and MI was around 300°C. Hong and Choi [28] found a similar profile for a polysaccharide-protein complex isolated from A. blazei fruiting body. A similar behavior has been described for other polysaccharides such as chitosan [30] and galactomannan [31]. Jayakumar et al. [32] also reported a slight decrease in the thermostability of chitin after sulfation.
Table 1.
Thermal analysis of Agaricus brasiliensis polysaccharides
| Sample | TGAa
|
DTGAb
|
||
|---|---|---|---|---|
| Range of temperature (°C) | Tf-Ti | Mass loss (%) | Tmax (°C) | |
| FR | 272.68 – 343.72 | 71.04 | 51.59 | 313.92 |
| FR-S | 224.96 – 277.36 | 52.40 | 39.09 | 248.89 |
| MI | 266.12 – 343.98 | 77.86 | 44.83 | 301.33 |
| MI-S | 217.21 – 260.30 | 43.09 | 27.23 | 236.32 |
FR and FR-S, A. brasiliensis fruiting body polysaccharide and its sulfated derivative;
MI and MI-S, A. brasiliensis mycelial polysaccharide and its sulfated derivative.
Thermogravimetric analysis; (Tf - Ti)= range of reaction (final temperature - initial temperature);
Differential thermogravimetric analysis; Tmax= maximal temperature of degradation.
The data on the apparent molecular weights (Mw) of A. brasiliensis polysaccharides are presented in Table 2. The polysaccharide isolated from the A. brasiliensis fruiting body (FR) was found to have a Mw of 609 kDa, which falls within the range of 390–2000 kDa previously described for similar preparations [33–36]. The Mw of 310 kDa determined for the mycelial polysaccharide (MI) showed good correspondence with the values reported by Fujimiya et al. (380 kDa) [37] and Lin and Yang (274 kDa) [38]. The sulfation process carried out in acid medium at high temperature probably induced hydrolysis of the polymers, thus accounting for the lower Mw of the sulfated derivatives (127 and 86 kDa for FR-S and MI-S, respectively). This effect has been previously described by Lanteri [39].
Table 2.
Molecular weights and chemical composition of Agaricus brasiliensis polysaccharides
| Sample | Molecular Weight (kDa) | Monosaccharides (%)
|
Content mean ± SD [% (w/w)]
|
||||
|---|---|---|---|---|---|---|---|
| Glucose | Mannose | Galactose | Total sugar | Protein | Sulfate | ||
|
|
|
||||||
| cFR | 617.93 ± 12.01 and 17.88 ± 0.98 | 60.19 ± 2.12 | 8.86 ± 3.46 | 0.94 ± 0.03 | n.t. | n.t. | n.t. |
| FR | 608.73 ± 4.11 | 63.67 ± 4.08 | 1.76 ± 0.13 | 4.55 ± 1.44 | 78.97 ± 7.52 | 1.76 ± 0.22 | n.d. |
| FR-S | 127.20 ± 6.95 | 68.98 ± 1.26 | 1.16 ± 0.34 | 2.86 ± 1.31 | 65.91 ± 9.39 | n.d. (< 0.16) | 40.25 ± 1.78 |
| cMI | 309.89 ± 2.22 and 30.20 ± 9.22 | 65.05 ± 5.12 | 9.95 ± 0.21 | n.d. | n.t. | n.t. | n.t. |
| MI | 310.11 ± 1.08 | 19.35 ± 3.12 | 58.65 ± 2.74 | n.d. | 77.33 ± 10.41 | 1.06 ± 0.06 | n.d. |
| MI-S | 85.52 ± 5.33 | 24.72 ± 4.12 | 55.28 ± 3.12 | n.d. | 76.60 ± 12.66 | 0.97 ± 0.09 | 36.07 ± 2.74 |
| DEX | 410 | n.t. | n.t. | n.t. | 75.11 ± 12.07 | n.d. (< 0.16) | n.d. |
| DEX-S | >500 | n.t. | n.t. | n.t. | 70.70 ± 10.90 | n.d. (< 0.16) | 48.67 ± 1.40 |
cFR and cMI, A. brasiliensis fruiting body and mycelial crude polysaccharide fractions, respectively.
FR and FR-S, A. brasiliensis fruiting body polysaccharide and its sulfated derivative.
MI and MI-S, A. brasiliensis mycelial polysaccharide and its sulfated derivative.
DEX and DEX-S, dextran and dextran sulfate, purchased from Sigma (USA).
n.d., not detected or the concentration is below the detection limit between parentheses.
n.t. not tested.
The monosaccharide composition of cFR and cMI was initially accessed by TLC analysis (Supplementary Fig. 2). The cFR was found to contain primarily glucose, while cMI had predominantly glucose, with minor amounts of other sugars.
The quantitative carbohydrate composition was assayed by HPLC-UV analysis of PMP derivatized samples. As shown in Table 2, the major sugar found in FR was glucose (63.67 %), while MI contained predominantly mannose (58.65 %), with significant amounts of glucose (19.35 %), which is characteristic of a heteropolymeric glucomannan. No detectable uronic acids were found in the samples since there was no peak at retention time of glucuronic acid standard. The total carbohydrate content varied from 66 % to 79 %, similar to the concentrations (80–95 %) previously reported for A. blazei polysaccharides purified by anion exchange chromatography [33, 35]. Similarly, the low percentage of protein (1–1.8 %) was comparable to values obtained previously [16, 27]. The low nitrogen content (Table 3) is in agreement with the results of protein determination by the Bradford assay (Table 2). According to Fernandes et al. [40] the temperature, solvent, and pH used for the extraction and drying procedures can break peptide bonds thereby reducing the protein content. Therefore, different extraction and drying procedures may explain the differences in protein levels reported by some groups for polysaccharides obtained from A. brasiliensis and A. blazei fruiting body [28, 35, 41, 42] and mycelium [43]. Sulfate analysis (Table 2) confirmed that the parental polysaccharides have no detectable sulfate content, whereas the derivatized samples contain over 35 % sulfate. These results were also confirmed by data from elemental analysis (Table 3).
Table 3.
Elemental analysis of polysaccharides from Agaricus brasiliensis
| Sample | Elements (% w/w)
|
||||
|---|---|---|---|---|---|
| Carbon | Hydrogen | Nitrogen | Sulfur | DSa | |
| FR | 34.42 | 5.56 | 2.50 | 0 | 0 |
| FR-S | 17.15 | 3.52 | 1.62 | 14.37 | 1.88 |
| MI | 32.07 | 4.36 | 1.63 | 0 | 0 |
| MI-S | 21.10 | 3.60 | 1.80 | 14.77 | 1.58 |
| DEX-S | 14.90 | 3.10 | 0 | 10.72 | 1.62 |
FR and FR-S, A. brasiliensis fruiting body polysaccharide and its sulfated derivative;
MI and MI-S, A. brasiliensis mycelial polysaccharide and its sulfated derivative.
DEX-S, dextran sulfate, purchased from Sigma.
Degree of substitution DS= 2.25 x (S % /C %)
The IR spectra recorded for the samples confirmed their polysaccharide constitution, disclosed by the intense stretching bands between 3200–3470 cm−1 (ν O-H) and 1075 cm−1 (ν C-O) [44]. A reduction in the intensity of the bands, observed for the derivatives, was related to the sulfation of the hydroxyl groups [45]. The absence of uronic acids was confirmed by the lack of carbonyl bands around 1700 cm−1 [16]. Polysaccharides can easily be hydrated due to their affinity for water and this is consistent with the presence of the water absorption bands at 1623–1636 cm−1. The small peaks at 1370–1540 cm−1 confirmed that the preparations contained low amounts of protein. The weak absorption bands at 876–898 cm−1 present in all the A. brasiliensis polysaccharides spectra are indicative of β stereochemistry [6]. The sulfation was confirmed by the appearance of two new absorption bands around 1200 and 800 cm−1 in FR-S and MI-S spectra, characteristic of asymmetric (S=O) and symmetric (C-O-S) vibrations, respectively [17].
The attribution of the chemical shifts from the 13C and 1H NMR spectra obtained for the native polysaccharides and the sulfated derivatives are listed in Table 4. FR was characterized as a glucan, with a majority of β-(1→6) linkages, and a minor amount of (1→3)-β-linkages. The β-(1→3)-linked H-1 of the side chain appeared as one resolved doublet centered at 4.95 ppm, whose coupling constant value (J= 8.28 Hz) designates the β-configuration [46, 47]. Although the signal around 86 ppm ascribed to C-3 of (1→3)-β-linkage was not detected in the FR spectrum, as reported by Ohno et al. [48], the remaining signals indicate the presence of a (1→3)-β-glucan component. These results show that FR consists of a backbone of (1→6)-β-D-glucan with (1→3)-β-D-glucan side chains attached to C-3, as previously described for the polysaccharides isolated from fruiting bodies of A. brasiliensis [16] as well as of A. blazei cultivated in Brazil [35] and China [48].
Table 4.
13C and 1H NMR spectroscopic data of Agaricus brasiliensis polysaccharides and its sulfated derivatives
| Sample | Residue | Chemical shift (ppm)
|
||||||
|---|---|---|---|---|---|---|---|---|
| C/H-1 | C/H-2 | C/H-3 | C/H-4 | C/H-5 | C/H-6 | C/H-6 termc | ||
|
|
||||||||
| FR | (1→6)-β-D- glucose | 103.14 (4.60–4.73) | 73.18 3.47 |
75.71 3.75 |
70.92, 68.97b 3.68 |
75.03 3.88 |
69.60 (4.06–4.13) | 63.34 (4.30–4.46) |
| FR-S | (1→6)-β-D- glucose | 103.14 (4.72–4.78) | 73.18 3.50 |
75.71 (3.70–3.90) | n.d. (76.07)a, 68.95b n.d. (4.65)a |
75.03 (4.00–4.22) | 69.60 4.39 |
n.d. (71.59)a n.d. (5.32)a |
| FR | (1→3)-β-D- glucose | 103.14 (4.96, 4.94) | 73.18 3.86 |
n.d. (3.94, 3.96) | 69.35 3.79 |
75.71 3.77 |
60.69 (3.90, 3.91) | 63.34 (4.20–4.29) |
| FR-S | (1→3)-β-D- glucose | 103.14 (4.85, 4.90) | 73.18 (3.70–3.90) | n.d. (4.00–4.22) | n.d. (74.25)a n.d. (4.59)a |
75.71 (3.70–3.90) | 60.69 3.90 (5.07)a |
n.d. (71.59)a n.d. (5.27)a |
| MI | (1→2)-β-D- mannose | 104.88, 98.55 5.30 |
78.59 (3.20–4.00) | 74.11 (3.20–4.00) | 72.26 (3.20–4.00) | 78.40 (3.20–4.00) | 63.25, 3.40 (3.20–4.00) | 60.08 (3.20–4.00) |
| MI-S | (1→2)-β-D- mannose | 105.71, 98.48 5.35 |
78.63 (3.80–4.10) | 74.00 (78.91–79.69)a 3.70 (4.30)a |
72.05 (77.64)a 3.60 (4.20)a |
78.33 3.37 |
63.21 (70.43)a (3.80–4.10) (5.46)a |
n.d. (69.10)a (3.80–4.10) (5.92)a |
| MI | (1→3)-β-D- glucose | 102.56, 94.74 (4.60–4.90) | 75.40 (3.20–4.00) | n.d. (3.20–4.00) | 72.00 (3.20–4.00) | 76.79 (3.20–4.00) | 63.40 (3.20–4.00) | 60.08 (3.20–4.00) |
| MI-S | (1→3)-β-D- glucose | 102.27, 94.60 (4.60–4.90) | 75.59 (80.87–80.93)a 3.60 (4.20)a |
86.71 (3.80–4.10) | 71.25 (77.28)a 3.48 (4.13)a |
76.72 3.37 |
63.41 (70.55)a (3.80–4.10) (5.85)a |
n.d. (69.10)a (3.80–4.10) (5.92)a |
FR and FR-S, A. brasiliensis fruiting body polysaccharide and its sulfated derivative.
MI and MI-S, A. brasiliensis mycelial polysaccharide and its sulfated derivative.
Assignments corresponding to the respective sulfation sites are shown in bold font between parentheses.
Assignment for the C4 of (1→6)-β-D-glucose linked to the side chain in C3.
term.: corresponding to the terminal sugar residues.
n.d.: not detected.
The sulfation of hydroxyl groups results in downfield shift of the carbons bearing sulfates and the protons linked to them by about 7–10 ppm and 0.5–2 ppm, respectively [49]. Hence, the 13C and 1H NMR spectra of FR-S showed downfield shifts of signals at 70.92/3.68 ppm and 63.34/4.30–4.46 ppm respectively to 76.08/4.65 and 71.59/5.32 ppm, indicating that the (1→6)-β-D-glucan portion was fully sulfated at C-4 and C-6 of the terminal residues. Similarly, the hydroxyl groups of (1→3)-β-D-glucan moiety in FR-S appear to have been fully sulfated at positions 4 and 6 of the terminal residues. Although the signal at 60.69 assigned to C-6 in the 1,3-β-chain was displayed in both FR and FR-S 13C NMR spectra, the corresponding H-6 signal (3.90 ppm) was barely detected in 1H NMR spectrum of FR-S and the appearance of a signal at 5.07 ppm most likely indicates the partial sulfation of C-6. The low reactivity of other carbon positions could be attributed to steric hindrance [50].
As expected, the signals of the hydrogens linked to carbons bearing sulfate groups experienced downfield shifts from 0.60 to 2.03 ppm with respect to the unsubstituted polysaccharides in the 1H NMR spectra of sulfated derivatives. Minor changes in chemical shifts were also observed on protons located in the vicinity of sulfation sites [51].
According to 13C and 1H NMR data, MI was characterized as a (1→3)-β-D-gluco-(1→2)-β-D-mannan, as previously described [15, 52]. The signal at 65.86 ppm probably results from the branching in C6 of the main chain. With regard to MI-S, sulfation at C-6 position of the terminal residue was nearly complete, both in the main and side chains, disclosed by the downfield shift of C-6 to a broad peak at 69.10 ppm. Partial sulfation was observed at C-3, C-4, and C-6 positions of the (1→2)-β-D-mannan moiety, as well as at C-2, C-4, and C-6 sites of the (1→3)-β-D-glucan side chain. A splitting pattern of C-3 signal (74.00 ppm) from (1→2)-β-D-mannan and C-2 (75.59 ppm) from (1→3)-β-D-glucan moiety can be attributed to the sulfation of adjacent carbons.
It is well known that sulfation of polysaccharides is responsible for changes in the original chain conformation usually resulting in alterations in their biological effects, including antiviral, cytotoxic, and antitumor activities [13, 53–59]. The inhibitory effects on A549 cell proliferation were concentration- and time-dependent and the results, expressed as EC50 values for the 48 and 72 h treatment period, are presented in Table 5. FR and MI had no cytotoxic effect after 48 h of treatment at 1,500 μg/ml. Sulfation of both preparations increased the cytotoxic activity with EC50 values of 605.6 and 342.1 μg/ml, respectively for FR-S and MI-S. Similarly DEX-S was more cytotoxic than DEX. Besides the introduction of sulfate groups, the Mw reduction of the sulfated derivatives (FR-S and MI-S) in comparison to the native polysaccharides (FR and MI) might have contributed to their higher cytotoxic effect. Similarly, Yang et al., [60] found a significantly higher cytotoxic activity for partially hydrolyzed fucoidans (Mw= 490 kDa) compared to the native polymers (Mw= 5,100 kDa).
Table 5.
Inhibitory effect of Agaricus brasiliensis polysaccharides in A549 cell proliferation (MTT assay)
| Sample | 48 h
|
72 h
|
Increase in Cytotoxicity
|
||
|---|---|---|---|---|---|
| EC50a | 95 %Confidence Interval | EC50a | 95 %Confidence Interval | EC50 48 h/ EC50 72 h | |
| FR | >1500 | - | 1147 | 1006 to 1307 | >1.3 |
| FR-S | 605.6 | 440.6 to 832.5 | 222.5 | 153 to 323.6 | 2.7 |
| MI | >1500 | - | >1500 | - | - |
| MI-S | 342.1 | 275.4 to 425.1 | 60.66 | 50.41 to 73 | 5.6 |
| DEX | >1500 | - | >1500 | - | - |
| DEX-S | 991.6 | 600.5 to 1638 | 783.1 | 560.6 to 1094 | 1.3 |
| Paclitaxel | 0.31 | 0.22 to 0.45 | 0.056 | 0.03 to 0.11 | 5.5 |
FR and FR-S, A. brasiliensis fruiting body polysaccharide and its sulfated derivative;
MI and MI-S, A. brasiliensis mycelial polysaccharide and its sulfated derivative;
DEX and DEX-S, dextran (410 kDa) and dextran sulfate (> 500 kDa), Sigma.
50 % effective concentration (μg/mL)
In order to differentiate the cytotoxic (LC50) and cytostatic (GI50 and TGI) effects, FR-S and MI-S were evaluated by the sulforhodamine assay using A549 and Vero cells. Table 6 shows that FR-S and MI-S were cytotoxic at the higher concentrations tested, with MI-S appearing to be slightly more active. Vero cells were found to be more resistant to the cytotoxic effects of FR-S and MI-S. These results raise the possibility that these sulfated polysaccharides might display selective cytotoxicity for human cancer cells, but further studies are needed to confirm this hypothesis. As observed by other authors, the antiproliferative effect of sulfated polysaccharides depends on cell type [53, 61].
Table 6.
Inhibitory effect of sulfated Agaricus brasiliensis polysaccharides in A549 and Vero cells growth by the sulforhodamine method
| Parameter
|
FR-S | MI-S | Paclitaxel |
|---|---|---|---|
|
| |||
| A549
| |||
| GI50a | 155.40 ± 24.32 | 160.90 ± 11.95 | 0.23 ± 0.03 |
| TGIb | 598.80 ± 63.89 | 488.30 ± 63.43 | 0.19 ± 0.01 |
| LC50c | 1042.00 ± 97.43 | 815.70 ± 80.59 | 0.62 ± 0.04 |
| Vero
|
|||
| GI50a | >1500 | >1500 | 0.12 ± 0.01 |
| TGIb | >1500 | >1500 | 0.46 ± 0.05 |
| LC50c | >1500 | >1500 | 1.05 ± 0.12 |
|
| |||
| SId | >1.4 | >1.8 | 1.7 |
FR-S, sulfated derivative of A. brasiliensis fruiting body polysaccharide; MI-S, sulfated derivative of A. brasiliensis mycelial polysaccharide.
Median growth inhibition;
Total growth inhibition;
Median lethal inhibition. Values represent the mean ± S.D. of two independent experiments and are expressed in μg/mL.
Selectivity index: calculated as LC50 Vero/LC50 A549.
Previous studies have shown that there is an optimum degree of sulfation (DS) to reach the maximal biological response, which varies according to the polysaccharide type. For instance, Liu et al. [54], comparing polysaccharides with similar Mw (~20 kDa), observed a stronger inhibition of Hep 2 cell growth by polysaccharides with a DS of 1.8 in comparison to those with lower DS values (1.52), while the activity was reduced when the DS was increased to 2.02. Similarly, Bao et al. [57] demonstrated that sulfated polysaccharides with low DS (0.11–0.14) were less cytotoxic than those with higher values (0.28–066), whereas further increases in DS (1.06) reduced the activity. No direct relationship between the cytotoxic activity and DS values were observed in the present work. However, it is important to note that the sulfated polysaccharides evaluated herein have different sugar compositions, chain conformations, and sulfation positions, and these features probably also contribute to the differences in their cytotoxicity.
4. Conclusions
The current study showed that the sulfation increased the cytotoxic activity of A. brasiliensis fruiting body polysaccharide, and was essential for the activity of the mycelial polysaccharide at the tested concentrations. Despite using identical conditions for chemical derivatization, distinct patterns of sulfation were obtained for MI-S and FR-S, most likely due to the differences in their native carbohydrate composition and structure. FR was determined to be a glucan with predominantly β-(1→6) linkages with some β-(1→3) linkages. FR-S was fully sulfated at C-4 and the terminal C-6. MI was found to be a (1→3)-β-D-gluco-(1→2)-β-D-mannan. Sulfation in MI-S was nearly complete at C-6 with partial sulfations at C-3, C-4, and C-6 of the mannan moiety and C-2, C-4, and C-6 of the glucan side chains.
After chemical modification, structural analysis revealed that the chain structure of the compounds was preserved. The main modifications obtained by sample derivatization besides sulfation were: 1) reduction in Mw, 2) slight decrease in thermal stability, 3) minor reduction on protein content, and 4) improvement of cytotoxic activity. The present findings increase the understanding of FR-S and MI-S structure-activity relationships and raise the possibility that these sulfated polysaccharides might display selective toxicity against tumor cells. Additional studies of these polysaccharides are clearly warranted.
Supplementary Material
Highlights.
Polysaccharides were isolated from Agaricus brasiliensis fruiting body (FR) and mycelia (MI)
The sulfated derivatives FR-S and MI-S were prepared
FR, FR-S, MI, and MI-S were chemically characterized
FR-S and MI-S presented promising cytotoxic activity against A549 lung tumor cells
The cytotoxic effect was related to the presence of sulfate groups and the lower molecular weight of the sulfated polysaccharides.
Acknowledgments
The NMR analyses were performed in the NMRFAM facility at University of Wisconsin (UW) Madison, which is supported by NIH grants P41RR002301 and P41GM103399. Equipment in the facility was purchased with funds from the UW, the NIH (P41GM66326, P41RR02301, RR02781, RR08438), the National Science Foundation (DMB-8415048, OIA-9977486, BIR-9214394), and the U.S. Department of Agriculture.
The Brazilian authors of this work thank to CAPES (MEC) and CNPq for their research fellowships. This work was also supported by NIH grant EY018597 (CRB), NIH /NEI Core Grant for Vision Research (P30-EY016665, CRB), and an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology and Visual Sciences at the UW-Madison. Authors are also grateful to Dr Aaron Crapster and Dr Matthew Kraft of the Department of Chemistry, UW-Madison, for their helpful assistance in the chemical analysis.
Footnotes
Conflict of interest
Authors disclose any actual or potential conflict of interest in this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhang M, Cui SW, Cheung PCK, Wang Q. Antitumor polysaccharides from mushrooms: a review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci Tech. 2007;18:4–19. [Google Scholar]
- 2.Largeteau M, Llarena-Hernández R, Regnault-Roger C, Savoie JM. The medicinal Agaricus mushroom cultivated in Brazil: biology, cultivation and non-medicinal valorisation. Appl Microbiol Biotechnol. 2011;92:897–907. doi: 10.1007/s00253-011-3630-7. [DOI] [PubMed] [Google Scholar]
- 3.Kerrigan RW. Agaricus subrufescens, a cultivated edible and medicinal mushroom, and its synonyms. Mycologia. 2005;97:12–24. doi: 10.3852/mycologia.97.1.12. [DOI] [PubMed] [Google Scholar]
- 4.Kerrigan RW. Inclusive and exclusive concepts of Agaricus subrufescens peck: A reply to Wasser et al. Int J Med Mushrooms. 2007;9:79–83. [Google Scholar]
- 5.Wasser SP. Molecular identification of species of the genus Agaricus. Why should we look at morphology? Int J Med Mushrooms. 2007;9:85–88. [Google Scholar]
- 6.Yang L, Zhang LM. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohydr Polym. 2009;76:349–361. [Google Scholar]
- 7.Yang J, Cai J, Wu K, Li D, Hu Y, Li G, Du Y. Preparation, characterization and anticoagulant activity in vitro of heparin-like 6-carboxylchitin derivative. Int J Biol Macromol. 2011;50:1158–1164. doi: 10.1016/j.ijbiomac.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Brandt CR, Piraino F. Mushroom antivirals. Recent Res Devel Antimicrob Agents & Chemother. 2000;4:11–26. [Google Scholar]
- 9.Yang T, Jia M, Zhou S, Pan F, Mei Q. Antivirus and immune enhancement activities of sulfated polysaccharide from Angelica sinensis. Int J Biol Macromol. 2012;50:768–772. doi: 10.1016/j.ijbiomac.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Peng Y, Wei X, Yang Z, Xiao J, Jin Z. Sulfation of tea polysaccharides: Synthesis, characterization and hypoglycemic activity. Int J Biol Macromol. 2010;46:270–274. doi: 10.1016/j.ijbiomac.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Wang J, Zhang J, Zhao B, Yao J, Wang Y. Structure-antioxidant relationships of sulfated galactomannan from guar gum. Int J Biol Macromol. 2010;46:59–66. doi: 10.1016/j.ijbiomac.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Li X, Chen Z. Sulfated modification of the polysaccharides obtained from defatted rice bran and their antitumor activities. Int J Biol Macromol. 2009;44:211–214. doi: 10.1016/j.ijbiomac.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Tao Y, Zhang Y, Zhang L. Chemical modification and antitumor activities of two polysaccharide-protein complexes from Pleurotus tuber-regium. Int J Biol Macromol. 2009;45:109–115. doi: 10.1016/j.ijbiomac.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Huang H, Wei Y, Li X, Chen Z. Characterization and anti-tumor activities of sulfated polysaccharide SRBPS2a obtained from defatted rice bran. Int J Biol Macromol. 2009;45:427–431. doi: 10.1016/j.ijbiomac.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Cardozo FTGS, Camelini CM, Mascarello A, Rossi MJ, Nunes RJ, Barardi CRM, Mendonça MM, Simões CMO. Antiherpetic activity of a sulfated polysaccharide from Agaricus brasiliensis mycelia. Antivir Res. 2011;92:108–114. doi: 10.1016/j.antiviral.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Camelini CM, Maraschin M, De Mendonça MM, Zucco C, Ferreira AG, Tavares LA. Structural characterization of beta-glucans of Agaricus brasiliensis in different stages of fruiting body maturity and their use in nutraceutical products. Biotechnol Lett. 2005;27:1295–1299. doi: 10.1007/s10529-005-0222-6. [DOI] [PubMed] [Google Scholar]
- 17.Zhang M, Zhang L, Wang Y, Cheung PCK. Chain conformation of sulfated derivatives of beta-glucan from sclerotia of Pleurotus tuber-regium. Carbohydr Res. 2003;338:2863–2870. doi: 10.1016/j.carres.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 19.Masuko T, Minami A, Iwasaki N, Majima T, Nishimura SI, Lee YC. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal Biochem. 2005;339:69–72. doi: 10.1016/j.ab.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 21.Dodgson KS, Price RG. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem J. 1962;84:106–110. doi: 10.1042/bj0840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Moura Neto E, Da J, Maciel S, Cunha PLR, De Paula RCM, Feitosa JPA. Preparation and characterization of a chemically sulfated cashew gum polysaccharide. J Brazil Chem Soc. 2011;22:1953–1960. [Google Scholar]
- 23.Jork H, Funk W, Fischer W, Wimmer H. Thin-Layer Chromatography Reagents and Detection Methods. VCH Publishers; New York: 1990. [Google Scholar]
- 24.Lv Y, Yang X, Zhao Y, Ruan Y, Yang Y, Wang Z. Separation and quantification of component monosaccharides of the tea polysaccharides from Gynostemma pentaphyllum by HPLC with indirect UV detection. Food Chem. 2009;112:742–746. [Google Scholar]
- 25.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 26.Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 27.Lima LFO, Habu S, Gern JC, Nascimento BM, Parada JL, Noseda MD, Gonçalves AG, Nisha VR, Pandey A, Soccol VT, Soccol CR. Production and characterization of the exopolysaccharides produced by Agaricus brasiliensis in submerged fermentation. Appl Biochem Biotechnol. 2008;151:283–294. doi: 10.1007/s12010-008-8187-2. [DOI] [PubMed] [Google Scholar]
- 28.Hong JH, Choi YH. Physico-chemical properties of protein-bound polysaccharide from Agaricus blazei Murill prepared by ultrafiltration and spray drying process. Int J Food Sci Technol. 2007;42:1–8. [Google Scholar]
- 29.Hatakeyama T, Quinn FX. Thermogravimetry, in: Thermal Analysis: Fundamentals and Applications to Polymer Science. John Wiley & Sons; Chichester: 1999. pp. 45–118. [Google Scholar]
- 30.Castro C, Gargallo L, Leiva A, Radić D. Interactions in blends containing chitosan with functionalized polymers. J Appl Polym Sci. 2005;97:1953–1960. [Google Scholar]
- 31.Cerqueira MA, Souza BWS, Simões J, Teixeira JA, Domingues MRM, Coimbra MA, Vicente AA. Structural and thermal characterization of galactomannans from non-conventional sources. Carbohydr Polym. 2011;83:179–185. [Google Scholar]
- 32.Jayakumar R, Nwe N, Nagagama H, Furuike T, Tamura H. Synthesis, Characterization and Biospecific Degradation Behavior of Sulfated Chitin. Macromol Symp. 2008;264:163–167. [Google Scholar]
- 33.Liu J, Zhang C, Wang Y, Yu H, Liu H, Wang L, Yang X, Liu Z, Wen X, Sun Y, Yu C, Liu L. Structural elucidation of a heteroglycan from the fruiting bodies of Agaricus blazei Murill. Int J Biol Macromol. 2011;49:716–720. doi: 10.1016/j.ijbiomac.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Wu B, Cui J, Zhang C, Li Z. A polysaccharide from Agaricus blazei inhibits proliferation and promotes apoptosis of osteosarcoma cells. Int J Biol Macromol. 2012;50:1116–1120. doi: 10.1016/j.ijbiomac.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 35.Dong Q, Yao J, Yang XT, Fang JN. Structural characterization of a water-soluble beta-D-glucan from fruiting bodies of Agaricus blazei Murr. Carbohydr Res. 2002;337:1417–1421. doi: 10.1016/s0008-6215(02)00166-0. [DOI] [PubMed] [Google Scholar]
- 36.Mizuno T, Inagaki R, Kanao T, Hagiwara T, Nakamura T, Ito H, Shimura K, Sumiya T, Asakura A. Antitumor activity and some properties of water-insoluble polysaccharides from “Himematsutake”, the fruiting body of Agaricus blazei Murill. Agric Biol Chem. 1990;54:2897–2905. [Google Scholar]
- 37.Fujimiya Y, Suzuki Y, Katakura R, Ebina T. Tumor-specific cytocidal and immunopotentiating effects of relatively low molecular weight products derived from the basidiomycete, Agaricus blazei Murill. Anticancer Res. 1999;19:113–118. [PubMed] [Google Scholar]
- 38.Lin JH, Yang SS. Mycelium and polysaccharide production of Agaricus blazei Murril by submerged fermentation. J Microbiol Immunol Infect. 2006;39:98–108. [PubMed] [Google Scholar]
- 39.Lanteri A. Processing and packaging sulfonation and sulfation technology. J Am Oil Chem Soc. 1978;55:128–133. [Google Scholar]
- 40.Fernandes MBA, Habu S, de Lima MA, Thomaz-Soccol V, Soccol CR. Influence of drying methods over in vitro antitumoral effects of exopolysaccharides produced by Agaricus blazei LPB 03 on submerged fermentation. Bioprocess Biosyst Eng. 2011;34:253–261. doi: 10.1007/s00449-010-0467-x. [DOI] [PubMed] [Google Scholar]
- 41.Fujimiya Y, Suzuki Y, Oshiman KI, Kobori H, Moriguchi K, Nakashima H, Matumoto Y, Takahara S, Ebina T, Katakura R. Selective tumoricidal effect of soluble proteoglucan extracted from the basidiomycete, Agaricus blazei Murill, mediated via natural killer cell activation and apoptosis. Cancer Immunol Immunother. 1998;46:147–159. doi: 10.1007/s002620050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonzaga MLC, Ricardo NMPS, Heatley F, Soares SDA. Isolation and characterization of polysaccharides from Agaricus blazei Murill. Carbohydr Polym. 2005;60:43–49. [Google Scholar]
- 43.Ker YB, Chen KC, Chyau CC, Chen CC, Guo JH, Hsieh CL, Wang HE, Peng CC, Chang CH, Peng RY. Antioxidant capability of polysaccharides fractionated from submerge-cultured Agaricus blazei mycelia. J Agric Food Chem. 2005;53:7052–7058. doi: 10.1021/jf0510034. [DOI] [PubMed] [Google Scholar]
- 44.Silverstein RM, Webster FX, Kiemle DJ. Identificação espectrométrica de compostos orgânicos. 7. LTC-Livros Técnicos e Científicos; Rio de Janeiro: 2005. [Google Scholar]
- 45.Mähner C, Lechner MD, Nordmeier E. Synthesis and characterisation of dextran and pullulan sulphate. Carbohydr Res. 2001;331:203–208. doi: 10.1016/s0008-6215(00)00315-3. [DOI] [PubMed] [Google Scholar]
- 46.Bubb WA. NMR spectroscopy in the study of carbohydrates: Characterizing the structural complexity. Concept Magnetic Res Part A. 2003;19:1–19. [Google Scholar]
- 47.Zakharenko AM, Kusaikin MI, Li BM, Van Huen F, Khan HH, Sova VV, Zvyagintseva TN. Catalytic properties of endo-1,3-β-D-glucanase from the Vietnamese edible mussel Perna viridis. Russ J Bioorg Chem. 2009;35:54–61. doi: 10.1134/s1068162009010075. [DOI] [PubMed] [Google Scholar]
- 48.Ohno N, Furukawa M, Miura NN, Adachi Y, Motoi M, Yadomae T. Antitumor beta glucan from the cultured fruit body of Agaricus blazei. Biol Pharm Bull. 2001;24:820–828. doi: 10.1248/bpb.24.820. [DOI] [PubMed] [Google Scholar]
- 49.Duus JÃ, Gotfredsen CH, Bock K. Carbohydrate structural determination by NMR spectroscopy: modern methods and limitations. Chem Rev. 2000;100:4589–4614. doi: 10.1021/cr990302n. [DOI] [PubMed] [Google Scholar]
- 50.Wang J, Zhao B, Wang X, Yao J, Zhang J. Structure and antioxidant activities of sulfated guar gum: Homogeneous reaction using DMAP/DCC catalyst. Int J Biol Macromol. 2012;50:1201–1206. doi: 10.1016/j.ijbiomac.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 51.Hricovíni M, Nieto PM, Torri G. NMR Spectroscopy of Glycoconjugates. Wiley-VCH Verlag GmbH & Co. KGaA; 2003. NMR of Sulfated Oligo- and Polysaccharides; pp. 189–229. [Google Scholar]
- 52.Mizuno M, Minato KI, Ito H, Kawade M, Terai H, Tsuchida H. Anti-tumor polysaccharide from the mycelium of liquid-cultured Agaricus blazei mill. Biochem Mol Biol Int. 1999;47:707–714. doi: 10.1080/15216549900201773. [DOI] [PubMed] [Google Scholar]
- 53.Zhang J, Liu Y-j, Park H-s, Xia Y-m, Kim G-s. Antitumor activity of sulfated extracellular polysaccharides of Ganoderma lucidum from the submerged fermentation broth. Carbohydr Polym. 2012;87:1539–1544. [Google Scholar]
- 54.Liu Y, Liu C, Tan H, Zhao T, Cao J, Wang F. Sulfation of a polysaccharide obtained from Phellinus ribis and potential biological activities of the sulfated derivatives. Carbohydr Polym. 2009;77:370–375. [Google Scholar]
- 55.Nie X, Shi B, Ding Y, Tao W. Preparation of a chemically sulfated polysaccharide derived from Grifola frondosa and its potential biological activities. Int J Biol Macromol. 2006;39:228–233. doi: 10.1016/j.ijbiomac.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 56.Lin Y, Zhang L, Chen L, Jin Y, Zeng F, Jin J, Wan B, Cheung PCK. Molecular mass and antitumor activities of sulfated derivatives of α-glucan from Poria cocos mycelia. Int J Biol Macromol. 2004;34:231–236. doi: 10.1016/j.ijbiomac.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 57.Bao H, Choi WS, You S. Effect of sulfated modification on the molecular characteristics and biological activities of polysaccharides from Hypsizigus marmoreus. Biosci Biotechnol Biochem. 2010;74:1408–1414. doi: 10.1271/bbb.100076. [DOI] [PubMed] [Google Scholar]
- 58.Wang X, Zhang L. Physicochemical properties and antitumor activities for sulfated derivatives of lentinan. Carbohydr Res. 2009;344:2209–2216. doi: 10.1016/j.carres.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 59.Cao Y, Ikeda I. Antioxidant activity and antitumor activity (in vitro) of xyloglucan selenious ester and surfated xyloglucan. Int J Biol Macromol. 2009;45:231–235. doi: 10.1016/j.ijbiomac.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 60.Yang C, Chung D, Shin IS, Lee H, Kim J, Lee Y, You S. Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. Int J Biol Macromol. 2008;43:433–437. doi: 10.1016/j.ijbiomac.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 61.Vishchuk OS, Ermakova SP, Zvyagintseva TN. Sulfated polysaccharides from brown seaweeds Saccharina japonica and Undaria pinnatifida: isolation, structural characteristics, and antitumor activity. Carbohydr Res. 2011;346:2769–2776. doi: 10.1016/j.carres.2011.09.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.