Abstract
Purpose
The purpose of this study is to test the feasibility and acceptability of a mobile phone-based peer support intervention among women in resource-poor settings to self-manage their diabetes. Secondary goals evaluated the intervention’s effectiveness to motivate diabetes-related health choices.
Methods
Women with diabetes (n=22) in Cape Town, South Africa participated in a 12-week program focused on providing and applying knowledge of health routines to manage diabetes. Women were linked with a buddy via a mobile phone for support and also questioned daily about a health behavior via text message. Women were assessed at recruitment, and then 3 and 6 months later by a trained interviewer using a mobile phone for data collection. The women were evaluated on technology uptake, reduction of BMI, blood glucose levels, and increases in positive coping and general health-seeking behaviors.
Results
Women exchanged 16739 text messages to buddies and received 3144 texts from the project. Women responded to 29% of texted questions (n=1321/14582). Women attended at least 9 of 12 possible intervention sessions; a third attended all 12 sessions (n = 8/22). Between baseline and three months, women increased their sleep and reported a higher level of positive action and social support coping, yet, blood glucose increased by 3.3 points. From 3 to 6 months, spiritual hope decreased and diastolic blood pressure increased. One year later, the 22 women continue to attend meetings.
Conclusion
Mobile phones are an easy and reliable way to provide peer support and disseminate health messages. Both positive and negative changes were observed in this pilot study.
Diabetes Buddies: Peer support through a mobile phone buddy system
South Africa is at the forefront of a global diabetes epidemic, with 2.6 million persons currently estimated to have diabetes [1] – an estimated prevalence of 5.8 - 8% [2, 3]. In 2005, diabetes was the sixth leading cause of death in South Africa, while HIV was the tenth. The number of adults with diabetes is expected to rise dramatically in the next decade [2], as South Africa’s obesity rates are high and rising. Rapid urbanization has shifted South Africans to a Westernized lifestyle, with higher fat intakes and decreased physical activity. Simultaneously, many South Africans consider being overweight desirable [4, 5]. Obesity is especially prevalent among South African women (30%) compared to men (7.5%) [6]. Black women have higher rates of obesity (31-34%) than white (18-24%), Indian (20-22%), or Coloured (26-28%) women [7].* In addition, one in five black South African children are stunted in childhood [8], which has been associated with later obesity, diabetes, and hypertension as adults [7].
South Africa’s public health system is inadequate to meet the challenge of diabetes. Clinic services are not easily accessible, acceptable, or consumer-friendly; patients frequently speak a different language than do their health care providers; and South Africans routinely report unacceptably long waiting lines and offensive attitudes by staff at community health centers [8]. Despite poverty, many black South Africans visit private health care providers (e.g., traditional healers) instead of public health clinics [8].
About one-third of those with diabetes are estimated to be undiagnosed [9]. When diabetes is diagnosed, clinic and hospital care is substandard. Diabetes management guidelines and standardized assessments are often ignored, patient education about self-care is minimal, and low control rates are reported for both blood glucose (16-49%) and blood pressure (35-39%) [8]. Doctors are reluctant to prescribe insulin, fearing that patients lack sufficient understanding to use it safely [8]. South Africa is not alone in facing a growing epidemic with inadequate resources. Low and middle income countries (LMIC) globally face similar challenges from chronic diseases. Our goal was to pilot a multi-pronged approach that comprehensively can address diabetes.
Therefore, three low-cost strategies were implemented to promote diabetes self-management: 1) link people with diabetes to clinical care; 2) maintain long-term diabetes self-management; and 3) increase and sustain social and emotional support. In particular, a robust, well-developed system of peer support could bring significant improvements in diabetes diagnosis and care. Building a program and infrastructure in which lifetime peer support (i.e., Diabetes Buddies, the pilot program discussed in this paper) is embedded in a low-cost, wrap-around system offering diagnosis, links to care, and ongoing patient education. The patient education component has tools for lifestyle change and the potential to be a sustainable approach to managing diabetes in South Africa and other LMIC. This approach is also consistent with the principle endorsed by South Africa’s Department of Health, of using paraprofessionals to extend care for chronic lifestyle diseases beyond public health sector professionals [10].
Adopting and maintaining a healthy lifestyle is key to lifelong diabetes management and achieving positive, long-term outcomes [11, 12]. In treating Type 2 Diabetes Mellitus (T2DM) in adults in the U.S., reducing body weight via a lifestyle program focused on increasing exercise and developing healthier dietary habits, has been proven to decrease insulin resistance and improve overall diabetes outcomes [12 - 14]. Weight loss associated with improvements in eating behavior, diet, and physical activity has resulted in significant reductions in fasting plasma glucose and insulin levels, hepatic glucose output, and peripheral insulin resistance, hypertension, and dyslipidemia. Three uncontrolled trials in adults with T2DM treated with oral medications have shown the benefit of weight loss associated with lifestyle modification on reducing mortality [15 - 17].
Methods
Design
The Institutional Review Boards of Stellenbosch University and UCLA, and the Board of Women for Peace, the lead NGO, approved the methods. Diabetes Buddies is a pilot program that preliminarily evaluates a package of peer support activities for diabetes management to be implemented with adult women in a Xhosa township in Cape Town, South Africa. We initiated the program by reviewing food diaries of 19 residents of Mfuleni township in 2007. We observed that women ate an average of 4.7 meals/day, over half of the times away from home. Almost all drank soda pop daily (non-diet). Women consumed foods with protein (typically meat) and ate fruits/vegetables daily. Typically, women ate three times the calories in carbohydrates than are recommended. These observations confirmed that nutrition education and changing eating habits are critical.
Sample/setting
Women receiving health services at a local health clinic were approached by their nursing sister and the project director to participate in the pilot study. Voluntary informed consent was obtained from 22 women diagnosed with diabetes.
Intervention
We adapted the Power to Prevent Program [18], an evidence-based intervention targeted towards African Americans in the U.S., into a format of peer support suited for delivery by non-governmental organizations (NGOs) in South African townships. Three nursing sisters and key informant interviews with women with diabetes informed the adaptation for Xhosa women; Table 1 outlines a 12 session psycho-educational support intervention regimen that was adapted from the U.S. to Xhosa culture.
Table 1.
Content for each of the 12 sessions of the Diabetes Buddies intervention adapted from Power to Prevent.
| Session | Content |
|---|---|
| 1 | Orientation to the intervention; expectations and rules; principles of changing behavior. |
| 2 | Being a social support buddy, using the mobile phone |
| 3 | Emotional self-awareness, coping with stress |
| 4 | Nutrition & glucose control, goal setting for better eating |
| 5 | Physical activity, strategies for maintaining daily exercise |
| 6 | Healthy cooking |
| 7 | Medical management of diabetes, interacting with your doctor |
| 8 | Assertiveness in the health care setting |
| 9 | Healthy family routines |
| 10 | Portion control |
| 11 | Relaxing daily and managing stress in uncomfortable situations |
| 12 | Relapse prevention, maintaining healthy habits |
The intervention had three components: 1) a series of 12 psycho-educational group sessions that address improving one’s lifestyle of eating, exercising, abstaining from alcohol and drugs; 2) mobile phone probes that ask about health daily; and 3) text messaging to a buddy to support lifestyle changes.
Our program, called Diabetes Buddies, offered a laddered system of peer support. The program paired volunteer peers to offer each other intensive, reciprocal support on an ongoing basis via a mobile phone. Low-cost, easy-to-use mobile phone text-messaging technology added an element of remote support for enhanced reach, effectiveness, and scalability. Two Peer Mentors with diabetes were identified: positive role models who had lost weight and increased exercise after their T2DM diagnosis. After they were trained in management of diabetes, support processes and group management by the project team, Peer Mentors received payment and functioned as peer educators, who conducted a series of 12 drop-in informational support meetings and offered support to the Diabetes Buddy-pairs.
Weekly sessions were held that included a sequence of: identifying weekly successes; learning new information about nutrition, exercise, and disease self-management; problem solving how to apply the information in daily life; managing uncomfortable emotions such as anger, anxiety, or depression; role playing new alternative strategies for coping with stress; and sharing a meal. In addition to information and support, meetings included self-check of basic diabetes markers (weight, waist circumference, blood pressure). For on-time arrival, women were given pedometers to self-monitor their number of steps daily.
Data collection procedures
Mobile phones were used to text women daily, asking about their adherence to healthy lifestyle behaviors. A list of 15 questions was identified by key informant interviews that were relevant to healthy lifestyles. These statements were translated into Xhosa and back-translated into English. Text messages from the research team were sent daily to remind women to monitor their walking and eating behaviors. Women were asked to report their behaviors by sending back a text message to the research team on one of the 15 questions daily.
DPS Health, a mobile technology company, programmed each phone so that the messages (both the question probes and the study participants’ responses) were relayed through a central server, allowing each contact to be monitored in real time. The system designed by DPS displayed instructions, items to key, and response categories on the screen of a mobile phone. Branching and skip patterns were automatic functions, based on logic models of potential responses. The quality of the data collected was further enhanced, as the mobile system provided immediate feedback to study personnel as unexpected data was entered into the computer.
Women with diabetes were randomly assigned to buddy pairs, provided mobile phones, and encouraged to call or text their buddy to support each other’s behavior change. Training of the women on the mobile phone system included: how to send and receive text messages, data entry, backup, and error resolution. Each call and text was routed through the central server so that the frequency of each contact was monitored; for text messages, the content was recorded and saved.
Assessment measures
Women were interviewed in Xhosa by a research assistant for about 1 hour each to complete the evaluation measures at recruitment, at three months (21/22 women reassessed), and at six months (21/22 women) later.
Background measures included demographics, environmental characteristics (e.g., housing), HbA1C levels, and the number of illness events.
Process measures included the number of voice or text messages exchanged by buddies, attendance at psycho-educational small group meetings, and perceived quality of the intervention.
Intermediate measures included the number of steps taken/day and number of hours slept each night (categorized as 5 hours or less, 6-9 hours, or 10 or more hours). The number of hours slept each night was collapsed into two categories for “five hours or less” and “more than five hours.” Too few participants indicated sleeping ten or more hours to analyze this category separately.
Outcomes included diabetes-specific measures such as: Body Mass Index (BMI; BMI = body weight in kilograms/height in meters squared), blood pressure, emotional distress assessed with the five item anxiety subscale of the Brief Symptom Inventory [19], and styles of coping with illness [20] composed of six subscales (positive actions, withdrawal, spiritual hope, non-disclosure, passive problem solving, and social support seeking). Computerized assessments were cleaned by the data team at UCLA, integrated into system files, and analyzed using SAS.
Data analyses
Our primary analyses examined summary statistics for uptake of text messaging, attendance at small group meetings, and perceived quality of the intervention to ascertain the feasibility and acceptability of a mobile phone-based peer support intervention.
Intervention effectiveness was evaluated by comparing differences in outcome trajectories that occurred between two time periods: baseline to three months and three to six months. Trajectories for continuous outcomes (all outcomes, except for the number of hours slept each night) were estimated by random-intercept linear regression models through the PROC MIXED procedure in SAS version 9.2. Models included time indicators for three and six months; baseline was the reference category. Significant differences between coefficients for the time indicators represented a change in the outcome trajectory. Random intercepts were included for each participant to model correlations between repeated measurements. McNemar’s test was used to test for differences in sleep between baseline and three months, as well as between three and six months.
Results
Table 2 summarizes the data obtained from interviewers at baseline, 3 and 6 months. The 22 women were an average of 53 years old (SD=12.8 years), ranging in age from 21-74 years old. Women had received less than 6 years of education. On average, women had five persons in their household; the average number of children was 4.6 per woman. Half were currently employed (46%), with 86% having had a paying job at some point in their life. Almost all had running water, electricity, and a toilet on their premises (95.5%). Two-thirds had a partner (59%); and 38% of those partners were working. About 86% lived with their children, 64% lived with their romantic partner, 5% lived with their parents, 73% live with their grandchildren, and about 25% lived with other extended family. Mean income was 3043 Rand per month per household when adding all sources of income (about 450 USD), although some have more and others are disenfranchised.
Table 2.
Summary of the Mean (M) and SD or percentage of each Bio-Behavioral Outcome at baseline, three, and 6 months.
| Baseline (n=22) |
Three Months (n=21) |
Six Months (n=22) |
Total (n=20) |
|||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| M 22 |
(SD)% | M 21 |
(SD)% | M 22 |
(SD)% | M | (SD)% | |
| Body Mass Index | 39.6 | (12.0) | 40.5 | (11.8) | 39.6 | (11.6) | 39.9 | (11.6) |
| Diastolic blood pressure | 88.9 | (13.4) | 88.8 | (13.9) | 95.5 | (11.7) | 91.1 | (13.2) |
| Systolic blood pressure | 149.0 | (24.3) | 158.2 | (31.6) | 155.8 | (19.8) | 154.3 | (25.5) |
| Blood glucose level | 8.1 | (4.0) | 11.4 | (5.8) | 10.6 | (6.0) | 10.0 | (5.4) |
| Estimated number of steps daily |
1931.8 | (1365.4) | 1972.1 | (2355.3) | 1559.8 | (2105.9) | 1823.0 | (1955.) |
| Hours of sleep each night | ||||||||
| 5 hours or less | 6 | (27.3) | 1 | (4.8) | 2 | (9.1) | 9 | (13.8) |
| 6-9 hours | 16 | (72.7) | 14 | (66.7) | 17 | (77.3) | 47 | (72.3) |
| 10 or more hours | 0 | (0.0) | 6 | (28.6) | 3 | (13.6) | 9 | (13.8) |
| Coping Style | ||||||||
| Anxiety symptoms | 3.2 | (2.1) | 4.1 | (3.1) | 3.3 | (2.5) | 3.5 | (2.6) |
| Positive action (10 items) | 29.6 | (5.4) | 40.3 | (6.0) | 37.9 | (4.3) | 35.9 | (7.0) |
| Social support (5 items) | 13/2 | (2.8) | 16.5 | (2.8) | 16.6 | (3.2) | 15.4 | (3.3) |
| Depression withdrawal (3 items) |
6.0 | (2.2) | 5.3 | (1.6) | 5.0 | (1.8) | 5.4 | (1.9) |
| Self-destructive escape | 1.0 | (0.0) | 1.0 | (0.0) | 1.1 | (0.4) | 1.0 | (0.2) |
| Spiritual hope (4 items) | 17.0 | (2.7) | 17.9 | (2.6) | 16.0 | (2.9) | 17.0 | (2.8) |
| Non-disclosure (3 items) | 8.3 | (2.8) | 8.1 | (2.5) | 7.6 | (2.6) | 8.0 | (2.6) |
| Passive problem solving | 14.5 | (3.6) | 15.8 | (4.2) | 16.5 | (4.7) | 15.6 | (4.2) |
Women had typically been diagnosed with diabetes one year ago or longer (95%). Only one woman had Type 1 diabetes, the rest were T2DM. At recruitment, half of the women had last seen their health care provider less than a month ago for their diabetes; 20% of women had monthly appointments. However, 40% of women went to their doctors twice a year for their diabetes. Only 25% did not go routinely to the doctor. Most women reported feeling comfortable talking to their doctor (95%); 50% had been hospitalized in the past due to their diabetes; and 100% were on diabetes medication.
The mean BMI at the baseline assessment was 39.6, with a SD of 12. BMIs indicated that 77% of the women were obese (BMI > 30), 18% were overweight, and one woman was underweight (BMI<18.5). On average, women walked just over 1900 steps daily, one-fifth of the recommended number of 10,000 steps daily. Half walked 1000 steps daily; an additional 30% walked up to 2000 steps daily at recruitment. About 15% reported that they never exercised in any way. For the first meeting, three people were not able to walk to the community site from their home. Yet only 5% had gone to the health clinic to change their eating habits.
Approximately 25% showed significant cardiovascular risk based on measures of peak blood flow. Women experienced many symptoms: 14% had seizures; 75% had high blood pressure; 18% had heart disease; 23% had asthma; 68% had arthritis; one person had an amputated leg; 9% had cancer; and 90% reported other serious illnesses.
Fifty-nine percent of women exceeded the norm for chronic average blood glucose level (A1C ≥ 6). The mean HbA1C was 8.1 at baseline (SD=4). The systolic blood pressure was 149 (SD=24.3) and diastolic blood pressure was 88.9 (SD=13.4). When women arrived at the psycho-educational groups, almost all were wearing their pedometers after Session 1. Several women who were unable to walk the first day to the Women for Peace site, walked regularly by Session 4.
Almost all women were trying to figure out “WHY” they have diabetes. Beliefs in retribution for sins and beliefs in witchcraft were common [21, 22]. Women reported being very religious. All (100%) trusted in God and 68% prayed often, very often, or always; 95% had spiritual experiences daily. Women reported a relatively high count of mental health symptoms: 64% shook and were nervous; 32% were often scared; 68% were tense; 41% experienced terror symptoms; and 73% reported feeling restless frequently. Most women reported sleeping about 6-9 hours nightly.
There was one text message sent daily from the research team to each participant. There was an average of 83 responses weekly, indicating that most participants responded about 54% of the time. Buddies (n = 22) exchanged an average of 123 text messages weekly, independent of the probes. The ad hoc messages were most common on weekdays but dropped each weekend. The response rate to probes from the project was highest on Wednesday; while fewer responses were texted at the beginning and end of the week.
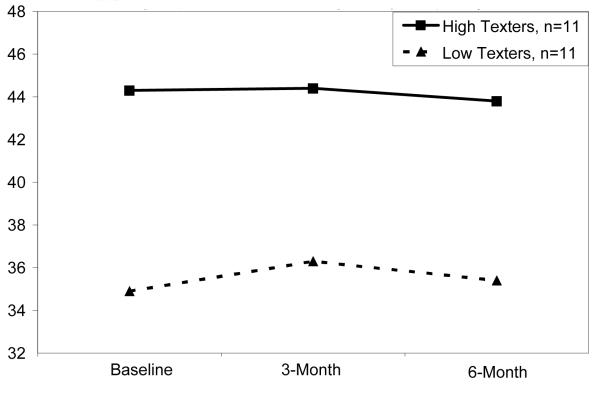
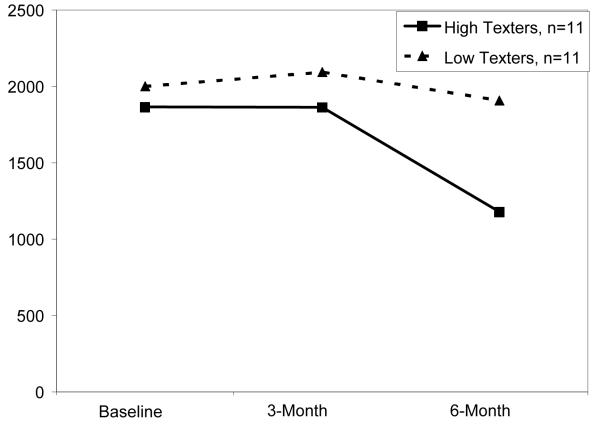
Based on a median split of 838.5 texts, women were classed as high texters (Mean number = 1002 texts over the course of the study) or low texters (mean = 754 texts). There were no significant differences over time in BMI, blood glucose, steps taken daily, and sleep between high and low texters. However, Figures 1 and 2 plot the observed differences, between the 11 high and low texters (not significantly different). High texting women appear to be those who have higher BMI, who perhaps used texting to replace walking.
Figure 1.
Body Mass Index (kg / m2), Stratified by amount of texting (high vs. low) among participating subjects.
Figure 2.
Estimated Number of Steps Daily, Stratified by Texting Amount
Table 2 also summarizes the change in specific measures over time. Based on linear regressions, BMI showed a trend towards increasing between baseline and three months and decreasing between three and six months, though neither change was significant. Similarly, blood glucose levels significantly increased between baseline and three months (B = 3.16, t = 3.36, df = 41.2, p < 0.01) and decreased, though not significantly, between three and six months. Social support (B = 3.26, t = 3.88, df = 41.9, p < 0.01) and a positive action coping style (B = 10.76, t = 6.97, df = 41.9, p < 0.01) increased significantly between baseline and three months; neither measure changed significantly between three and six months. Spiritual hope decreased significantly between three and six months (B = −1.93, t = −3.00, df = 41.3, p < 0.01). Diastolic blood pressure increased significantly between three and six months (B=6.79, t=2.05, df=41.6, p=0.05). Emotional distress (BSI), systolic blood pressure, weekly exercise, number of steps taken daily, and depression coping styles of withdrawal, self-destructive escape, non-disclosure, and passive problem solving and coping styles did not change significantly over time.
Hours slept each night increased significantly between baseline and three months (McNemar’s test, p=0.03). There was a non-significant change in sleep between three and six months (McNemar’s test, p=0.32).
Discussion
Building a structure for self-help groups has the potential to be a sustainable approach to managing diabetes in South Africa and other LMIC. Lifetime peer support (i.e. Diabetes Buddies) is embedded in a low-cost wraparound system offering diagnosis, links to care, and ongoing patient education with tools for lifestyle change. This approach is consistent with the principle, endorsed by South Africa’s Department of Health, of using paraprofessional community health workers to extend care for chronic lifestyle diseases beyond professionals in the public health sector.
Mobile phones have deeply penetrated South Africa, with more than 100% [23] of the population having mobile phone subscriptions. Mobile technologies are familiar; mobile phones are not easily broken if dropped. Unlike PDAs or laptops, there is a far smaller probability of theft, especially in townships. Data can be uploaded daily for less than ½ of a US cent, dropping the costs of field monitoring of diabetes or research on diabetes. Persons with very low education can easily use mobile phones and, rather than words, icons can be used.
The downside of mobile phones is that women may not have money to buy phone minutes. There were three weeks in which four dyads of Diabetes Buddies had trouble with their phones. These difficulties were resolved within a two week time period. Many persons consider themselves technologically challenged. Without access to technology infrastructure, phones will not be sustainable. In this study, the participants were older and seriously ill. Yet, they effectively used the mobile phones, with the women with the most serious illness utilizing them the most. Access to the resources to fix mobile phones when broken is a difficult challenge and must be a basic consideration when trying to scale interventions based on mobile technologies.
The mobile phone was used for three purposes in the current study. First, daily probes were sent to women to prompt them to increase healthy eating and exercise. Rather than endorsing and reminding a woman to do a new behavior, women were probed on one of 15 potential behaviors to assess how well they had reached their goal that day. In addition, the phones facilitated the frequency and quality of peer support. Buddies who also had diabetes supported each other using their mobile phones. The use of mobile phone technology has the potential to make this peer support program broadly scalable, by allowing for widespread distribution throughout LMIC at a cost that is already very low and is expected to decrease over time.
Finally, use of mobile phones reduces literacy issues that have been a key barrier for the adoption of previous electronic data collection platforms. Computer assisted programmed interviews (CAPI) systems have been the state of the art in data collection for about the last 20 years, typically using laptop computers. Especially in LMIC, computer literacy is low and computers are expensive. Yet, persons who find computers intimidating use mobile phones in their daily lives. Literacy is not required for mobile phone data collection; picture icons are often used.
Women continue to meet on their own with their local leaders a year later. There has been no loss of the original 22 women, and the group has gathered more women over time. Women, who could not walk to the group meetings in the beginning of the program, were able to walk by the third or fourth session to attend the meetings. The ability to monitor their own behavior, for example, the number of steps reflected on their pedometer, was an experience of empowerment, as well as motivation to observe their own behavior change over time. The qualitative impact of the program was substantial. However, the frequency of texting did not appear to be associated with changes in individuals’ symptoms of diabetes. Those with more difficulty in moving and exercising (i.e., those with higher BMI) appeared to text more. However, the frequency of texting was not related to outcomes. Overall, this study did not result in many significant changes in the women’s lives, especially in terms of their diabetes illnesses. Women slept longer and took more steps, and reported coping styles that attempted to make more positive actions to cope with stress.
Previous intervention iterations have had to consider consequences in addition to benefits, e.g. in our case, group sessions for risky cohorts have the potential to facilitate risky behavior when people “hook up.” In the current era, mobile interventions also need to consider possible consequences. A texting-based study may exacerbate the tendency for inactivity in some segments of the population it is intended to help. On the analysis side, we need to be aware of possible confounding with health outcomes from the very elements of the intervention, e.g. texting, that are meant to improve outcomes.
It is unlikely that the resources will be present within the health care system to manage the rising epidemic of diabetes and obesity-linked diseases [24,25]. Community health workers and personal self-management of family’s daily routines will be critical in the next decades [26]. This pilot reflects a model that could be a prototype of peer support and intervention that could be sustained at a community or neighborhood level. Without neighbors influencing each other, it will be difficult to manage the epidemic. Women meeting repeatedly over years have the opportunity to shift neighborhood norms regarding portion size, meal times, consistency of daily routines, and rituals. Six months is too short to observe these changes among women who are chronically ill with diabetes and have suffered serious diabetes-related symptoms for over 5 years. Yet, there is hope that over time these women’s lives and their health may shift.
In high income countries where most people have access to a viable, well-developed health care system, diabetes diagnosis would not be part of a peer support program for disease management. Thus, integrating a consumer-friendly diagnosis method into the participant recruitment process is an essential first step for any successful strategy of diabetes peer support in South Africa and other LMIC.
The challenge with pilot programs is finding the opportunities to sustain investments for new programs over time, especially in the midst of economic recessions. Researchers are unlikely to be able to mobilize the resources to network with the policy makers, who could provide funding lines for this work.
For future studies, funding is especially needed for large scale trials. The current study was not able to compare a multi-faceted intervention to other treatment groups, so it is difficult to tease out what component of the intervention had the biggest impact.
* The historical divisions of black, Coloured, Indian, and white are only used for statistical purposes in this paper.
This research study was reviewed and approved by the UCLA Institutional Review Board (SGB# 09-09-039/#10-000379) and by the Stellenbosch University Health Research Ethics Committee (#N09/10/268). All research subjects signed an Informed Consent Form prior to their participation.
Acknowledgments
This study was funded by Peers for Progress. Peers for Progress is a program of the American Academy of Family Physicians Foundation.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Statistics South Africa. Annual Report 2006/2007. Available at: http://www.statssa.gov.za/. Accessed July 11, 2011.
- 3.Levitt NS, Katzenellenbogen JM, Bradshaw D, Hoffman MN, Bonnici F. The prevalence and identification of risk factors for NIDDM in urban Africans in Cape Town, South Africa. Diabetes Care. 1993;16:601–607. doi: 10.2337/diacare.16.4.601. [DOI] [PubMed] [Google Scholar]
- 4.Kruger HS, Puoane T, Senekal M, van der Merwe MT. Obesity in South Africa: challenges for government and health professionals. Public Health Nutr. 2005;8:491–500. doi: 10.1079/phn2005785. [DOI] [PubMed] [Google Scholar]
- 5.Faber M, Kruger HS. Dietary intake, perceptions regarding body weight, and attitudes toward weight control of normal weight, overweight, and obese Black females in a rural village in South Africa. Ethn Dis. 2005;15:238–245. [PubMed] [Google Scholar]
- 6.Puoane T, Steyn K, Bradshaw D, Laubscher R, Fourie J, Lambert V, et al. Obesity in South Africa: the South African demographic and health survey. Obes Res. 2002;2002;10:1038–1048. doi: 10.1038/oby.2002.141. [DOI] [PubMed] [Google Scholar]
- 7.MT van der Merwe, Pepper MS. Obesity in South Africa. Obes Rev. 2006;7:315–322. doi: 10.1111/j.1467-789X.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- 8.Steyn K, Levitt NS. Health services research in South Africa. South African Medical Research Council; Cape Town: 2006. [Google Scholar]
- 9.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher EB, Earp JA, Maman S, Zolotor A. Cross-cultural and international adaptation of peer support for diabetes management. Fam Pract. 2010;27:i6–i16. doi: 10.1093/fampra/cmp013. doi:10.1093/fampra/cmp013. [DOI] [PubMed] [Google Scholar]
- 11.Eddy DM, Schlessinger L, Kahn R. Clinical outcomes and cost-effectiveness of strategies for managing people at high risk for diabetes. Ann Intern Med. 2005;143:251–264. doi: 10.7326/0003-4819-143-4-200508160-00006. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415. [PubMed] [Google Scholar]
- 13.Blackburn G. Effect of degree of weight loss on health benefits. Obes Res. 1995;3:211s. doi: 10.1002/j.1550-8528.1995.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 14.Maggio CA, Pi-Sunyer FX. The prevention and treatment of obesity. Application to type 2 diabetes. Diabetes Care. 1997;20:1744–1766. doi: 10.2337/diacare.20.11.1744. [DOI] [PubMed] [Google Scholar]
- 15.Wing R, Koeske R, Epstein L, Nowalk M, Gooding W, Becker D. Long-term effects of modest weight loss in type II diabetic patients. Arch Intern Med. 1987;147:1749. [PubMed] [Google Scholar]
- 16.Lean ME, Powrie JK, Anderson AS, Garthwaite PH. Obesity, weight loss and prognosis in type 2 diabetes. Diabet Med. 1990;7:228–233. doi: 10.1111/j.1464-5491.1990.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 17.Chaturvedi N, Fuller JH. Mortality risk by body weight and weight change in people with NIDDM. The WHO Multinational Study of Vascular Disease in Diabetes. Diabetes Care. 1995;18:766–774. doi: 10.2337/diacare.18.6.766. [DOI] [PubMed] [Google Scholar]
- 18.National Diabetes Education Program. Power to Prevent: A family lifestyle approach to diabetes. Available at http://www.ndep.nih.gov/media/power-to-prevent.pdf. Accessed July 13, 2011.
- 19.Derogatis LR. Brief Symptom Inventory: Administration, Scoring, and Procedures Manual. National Computer Systems, Inc.; Minneapolis, MN: 1993. [Google Scholar]
- 20.Rotheram-Borus MJ, Lee MB, Gwadz M, Draimin B. An intervention for parents with AIDS and their adolescent children. Am J Pub Health. 2001;2001;91:1294–1302. doi: 10.2105/ajph.91.8.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golooba-Mutebi F, Tollman SM. Shopping for health: Affliction and response in a South African village. Afr Soc Rev. 2007;11:64–79. [Google Scholar]
- 22.Mbeh GN, Edwards R, Ngufor G, Assah F, Fezeu L, Mbanya J-C. Traditional healers and diabetes: results from a pilot project to train traditional healers to provide health education and appropriate health care practices for diabetes patients in Cameroon. Global Health Promotion. 2010;17(2 suppl):17–26. doi: 10.1177/1757975910363925. doi: 10.1177/1757975910363925. [DOI] [PubMed] [Google Scholar]
- 23.International Telecommunication Union (ITU) Mobile cellular subscriptions 2010. Available at: http://www.itu.int/. Accessed July 11, 2011.
- 24.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Smyth S, Heron A. Diabetes and obesity: the twin epidemics. Nat Med. 2006;12:75–80. doi: 10.1038/nm0106-75. [DOI] [PubMed] [Google Scholar]
- 26.Tomlinson M. Family-centered HIV interventions: lessons from the field of parental depression. J Int AIDS Soc. 2010;2010;13(Suppl 2:S9):1–8. doi: 10.1186/1758-2652-13-S2-S9. doi: 10.1186/1758-2652-13-s2-s9. [DOI] [PMC free article] [PubMed] [Google Scholar]