Abstract
Although CD4 T cells are required for host resistance to Mycobacterium tuberculosis, they may also contribute to pathology. In this study, we examine the role of the inhibitory receptor PD-1 and its ligand PD-L1 during M. tuberculosis infection. After aerosol exposure, PD-1 knockout (KO) mice develop high numbers of M. tuberculosis-specific CD4 T cells but display markedly increased susceptibility to infection. Importantly, we show that CD4 T cells themselves drive the increased bacterial loads and pathology seen in infected PD-1 KO mice, and PD-1 deficiency in CD4 T cells is sufficient to trigger early mortality. PD-L1 KO mice also display enhanced albeit less severe susceptibility, indicating that T cells are regulated by multiple PD ligands during M. tuberculosis infection. M. tuberculosis-specific CD8 T cell responses were normal in PD-1 KO mice, and CD8 T cells only had a minor contribution to the exacerbated disease in the M. tuberculosis-infected PD-1 KO and PD-L1 KO mice. Thus, in the absence of the PD-1 pathway, M. tuberculosis benefits from CD4 T cell responses, and host resistance requires inhibition by PD-1 to prevent T cell-driven exacerbation of the infection.
Mycobacterium tuberculosis is one of the most important human pathogens and infects 9.3 million people each year leading to 1.3 million deaths (1). The success of M. tuberculosis as a pathogen is due to its ability to establish a lifelong persistent infection in the lungs and other tissues of immunocompetent hosts. It is clear that CD4 T cells are required to slow the growth of the tuberculosis bacilli, as CD4 T cell lymphopenic HIV patients are highly susceptible to M. tuberculosis and CD4 T cell-deficient mice rapidly succumb to uncontrolled bacterial replication. Nonetheless, in all species examined, T cell responses induced in naive hosts are unable to clear M. tuberculosis. Furthermore, vaccination against M. tuberculosis generates anamnestic CD4 T cell responses that can enhance control of M. tuberculosis after challenge, but no vaccination strategy tested to date leads to complete elimination of the bacteria. In fact, mice cured of M. tuberculosis with antibiotics show only modest protection upon reexposure (2), and even mice injected with large numbers of M. tuberculosis-specific differentiated Th1 cells prior to exposure are unable to clear the subsequent infection (3). The inability of T cells to eliminate this infection likely indicates that T cell responses are subject to potent immunoregulation during tuberculosis. Whereas there is extensive data concerning immune evasion mechanisms used by M. tuberculosis to persist within macrophages (4, 5), little is known about the role of endogenous host immunomodulatory molecules that regulate CD4 and CD8 T cell responses during M. tuberculosis infection. Understanding host factors that control T cell function during M. tuberculosis infection may lead to strategies for boosting immune function during chemotherapy or therapeutic vaccination and to a better fundamental understanding of the pathogenesis of tuberculosis.
PD-1 (CD279/Pdcd1) is a cell surface inhibitory receptor expressed on activated T and B cells upon Ag receptor engagement (6). Binding of PD-1 to either of its two ligands, PD-L1 (B7-H1/CD274) and PD-L2 (B7-DC/CD273) inhibits T cell proliferation and cytokine secretion. Most work on the role of PD-1 in infectious disease has focused on chronic viral infections where it has been shown to play a major role in regulating CD8 T cell function. In vivo blockade of the PD-1 pathway during LCMV infection in mice (7) and SIV infection in nonhuman primates (8) results in increased T cell proliferation and effector function and enhances viral control. In humans infected with HIV (9–11), hepatitis C virus (12), and hepatitis B virus (13), in vitro PD-1 blockade significantly enhances virus-specific T cell responses. Indeed, PD-1 is now recognized as a major regulator of pathogen-specific T cell responses.
Although negative T cell regulation through PD-1 can contribute to poor pathogen control, this inhibition can also be essential for limiting immunopathology. PD-1 knockout (KO) mice infected with adenovirus clear the infection more quickly but develop a more severe hepatitis than do wild-type (WT) mice (14). Administration of anti–PD-L1 blocking Ab during HSV-1 infection of the cornea leads to exacerbated herpetic stromal keratitis (15). Furthermore, PD-L1–deficient mice infected with a persistent strain of LCMV (clone-13) rapidly succumb to T cell-mediated immunopathology (7, 16). Disrupting the PD-1 pathway during infection, however, does not always result in enhanced disease. PD-L1 KO mice infected with a less virulent strain of LCMV (Armstrong) do not show the rapid mortality seen during LCMV clone-13 infection. Also, after intranasal inoculation with Histoplasma capsulatum, PD-1 KO mice show less signs of disease and rapidly clear the infection, whereas WT mice all eventually succumb to disseminated histoplasmosis (17). Therefore, the trade-off between pathogen clearance and T cell-mediated immunopathology resulting from blockade of the PD-1 pathway will likely depend on factors, such as the target tissue and, perhaps more importantly, the infectious agent in question.
In this study, we examine the role of PD-1 during M. tuberculosis infection. We show that PD-1 KO mice mount greatly increased M. tuberculosis-specific CD4 T cell responses and have high levels of inflammatory cytokines in their lungs. However, rather than showing enhanced bacterial control, PD-1 KO mice developed necrotic pulmonary lesions with high numbers of bacilli and rapidly succumbed postinfection. Adoptive transfer studies demonstrate that CD4 T cells promote tuberculosis when released from normal inhibition through PD-1. Therefore, perturbation of a single inhibitory receptor leads to active tuberculosis driven by unrestrained T cell responses. These data demonstrate how a fine balance between positive and negative CD4 T cell regulation is fundamentally important for optimal control of M. tuberculosis infection and implicate PD-1 as a central regulator inhibiting tuberculosis-promoting T cell responses.
Materials and Methods
Mice
C57BL/6 and TCRa KO mice were purchased from Taconic Farms (Germantown, NY). CD45.1 congenic mice were purchased from The Jackson Laboratory (Bar Harbor, ME). PD-1 KO and PD-L1 KO mice were generated by Dr. Arlene Sharpe (Harvard Medical School, Boston, MA). P25 TCR transgenic (Tg) CD4 T cells, originally generated by Takatsu and colleagues, were provided by Dr. Joel Ernst (New York University School of Medicine, New York, NY) and were crossed to PD-L1 KO mice in our laboratory. All animals were bred and housed at an Association for the Assesment and Accreditation of Laboratory Animal Care-approved facility at the National Institute of Allergy and Infectious Diseases/National Institutes of Health according to the National Research Council Guide for the Care and Use of Laboratory Animals. Mice were used according to an animal study proposal approved by the National Institute of Allergy and Infectious Diseases Animal Care and Use Committee.
Histopathology
Lungs were fixed by infusing 4% formaldehyde. Sectioning, H&E staining, and Ziehl-Neelsen staining for acid fast bacilli was performed by Histoserv (Germantown, MD).
Mycobacterial infections and measurement of bacterial loads
Mice were exposed to ~100 CFU of the H37Rv strain of M. tuberculosis in a nose-only aerosol machine (CH Technologies). Bacterial loads were measured in tissue homogenates by serial dilution on 7H11 agar plates supplemented with oleic acid-albumin-dextrose-catalase (Difco).
Cytokine ELISA and NO quantification
Levels of cytokines in bronchoalveolar lavage (BAL) fluids were determined with IFN-γ, TNF-α, IL-12/23 p40, IL-1α, IL-1β, IL-6, IL-17A, IL-1Ra, and IL-10 R&D Duoset ELISA kits (R&D Systems, Minneapolis, MN). BAL and serum NO was measured using the Total NO/nitrite/nitrate kit from R&D Systems.
In vivo T cell depletions, cell purifications, and adoptive transfers
For T cell depletion experiments, mice were injected i.p. with 500 μg αCD4 (GK1.5) or αCD8 (53.6.72) (BioXCell, West Lebanon, NH). For long-term CD4 depletion, mice were treated with the depleting Ab every third day from day 0 to 30. CD4 and CD8 T cell purifications from spleen and lymph nodes were performed with MACS magnetic beads and columns from Miltenyi Biotec (Auburn, CA) according to the manufacturer’s instructions. TCRα KO mice were reconstituted with 4 × 106 cells of each indicated population 1–10 d prior to M. tuberculosis infection. For P25 adoptive transfer experiments, WT and PD-L1 KO P25 CD4 T cells were MACS purified and stained with 1 μM CFSE at 1 × 106 cells/ml for 10 min at room temperature. Then, 1 × 106 cells of each population were adoptively transferred i.v. into CD45.1 congenic recipient mice infected with M. tuberculosis 6 d previously.
In vitro stimulation assays
For T cell stimulations, cells were incubated with ESAT-61–20 peptide (Rv3875) at 2 μg/ml, Mtb32c93–102 (Rv0125) and TB10.3/44–11 (Rv3019c and Rv0288) peptides at 0.2 μg/ml, purified protein derivative (Statens Serum Institut, Copenhagen, Denmark) at 20 μg/ml, or soluble anti-CD3 at 1 μg/ml for 5 h in the presence of brefeldin A or monensin. For CD107 degranulation assays, FITC-labeled CD107a and CD107b Abs were also included during the 5 h stimulation at a 1:100 dilution. Bone marrow-derived macrophages were generated as previously described (18) and were stimulated overnight with irradiated H37Rv (Colorado State University, Fort Collins, CO) and recombinant murine IFN-γ (PeproTech, Rocky Hill, NJ) at the indicated doses.
MHC tetramers, Abs, and flow cytometry
Anti-CD4 (RM4-4), CD8 (53-6.7), CD45.1 (A20), CD45.2 (104), CD90.1 (H1S51), IFN-γ (XMG1.2), TNF-α (MP6-XT22), CD107a (1D4B), CD107b (M3/84), CD44 (IM7), T-bet (eBio4B10), Foxp3 (FJK-16s), CTLA-4 (UC10-4F10-11), lag3 (C97B7W), I-Ab (M5/114.15.2), CD11c (HL3), PD-L1 (MIH5), PD-L2 (TY25), and PD-1 (RPM1-30) were purchased from eBioscience (San Diego, CA), Biolegend (San Diego, CA), and BD Pharmingen (San Jose, CA). UV-fixable live/dead stain and streptavidin-Qdot605 were purchased from Molecular Probes-Invitrogen (San Diego, CA). I-AbESAT-61–20, KbTB10.3/44–11, and DbMtb32c93–102 MHC tetramers were produced by the National Institute of Allergy and Infectious Diseases Tetramer Core Facility (Emory University, Atlanta, GA). For staining with I-AbESAT-61–20 tetramer, cells were incubated with tetramer at 1:50 dilution in complete media containing 10% FCS and monensin at a 1:1000 dilution for 1 h at 37°C prior to staining with surface Abs. Cells were stained with MHC class I tetramers at 1:400 dilution at 10°C for 30 min. All samples were acquired on an LSRII flow cytometer (Becton Dickinson, Franklin Lakes, NJ) and analyzed with FlowJo software (Tree Star, Ashland, OR).
Weight loss calculation and statistical analysis
Weight loss was calculated as: percentage weight change = ([current weight/initial weight] × 100) − 100. The statistical significance between groups was analyzed by nonparametric Student t test and survival curves by the Mantel–Cox test using GraphPad Prism software version 5.0c. The p values are indicated in figures as follows: *p < 0.05; **p < 0.01; and ***p < 0.001.
Results
PD-1 is required for control of aerosol M. tuberculosis infection
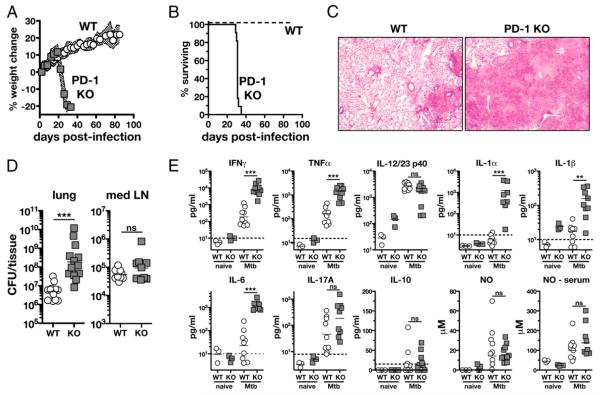
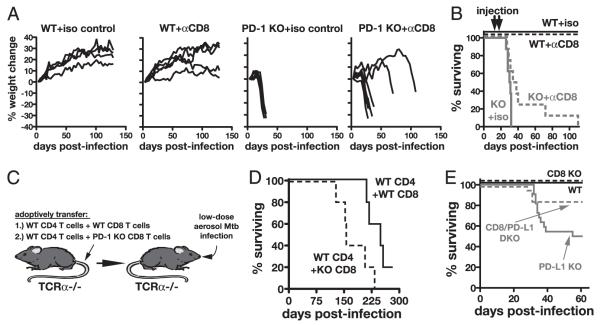
To evaluate the role of the inhibitory receptor PD-1 in regulating immunity to M. tuberculosis, we infected WT and PD-1 KO mice via aerosol exposure. At about day 20 postinfection, PD-1 KO mice began to rapidly lose weight (Fig. 1A), and all of these mice succumbed by day 35 postinfection, whereas all WT mice survived (Fig. 1B). Surprisingly, the lungs of PD-1 KO mice displayed large necrotic lesions (Fig. 1C) and contained greatly elevated bacterial loads compared with lungs of WT mice at 4 wk postinfection (Fig. 1D). In contrast, the mediastinal lymph nodes of WT and PD-1 KO mice contained similar numbers of bacteria (Fig. 1D). These data are in agreement with a recent report demonstrating that PD-1–deficient mice are unusually susceptible to M. tuberculosis (19).
FIGURE 1.
PD-1 is required for control of M. tuberculosis infection. A and B, Weight loss (A) and survival (B) after low-dose aerosol infection with H37Rv. n = 15 WT and n = 11 PD-1 KO animals. Traveling error bars in A indicate the SD. C, H&E staining of lung sections on day 26 postinfection. Original magnification ×50. D, Bacterial loads in lung homogenates and mediastinal lymph nodes on day 26 postinfection. E, Cytokines were measured by ELISA and NO by nitrate reductase assay in the bronchoalveolar lavage fluid in naive WT and PD-1 KO mice and on day 26 of infection. NO is also shown in the serum. Data in D and E are pooled from two to three independent experiments.
We next examined the expression of inflammatory cytokines and NO in the BAL fluid of WT and PD-1 KO mice. On day 26 postinfection, we found that the normally host-protective mediators IFN-γ (20, 21), TNF-α (22, 23), IL-1α, IL-1β (18, 24, 25), and IL-6 (26) were all highly upregulated in the BAL fluid of PD-1 KO compared with that of WT mice, whereas the levels of IL-12/23p40 (27, 28) and NO (29, 30) were similar in WT and PD-1 KO animals (Fig. 1E). In addition, the levels of IL-17A (31) or IL-10 (32) (both of which have been shown to lead to exacerbated disease or poor control during mycobacterial infections) were also the same in WT and PD-1 KO mice (Fig. 1E). Thus, the susceptibility of the PD-1 KO mice to M. tuberculosis infection is not associated with a defect in the expression of a critical cytokine or NO or with the overproduction of IL-17A or IL-10. In summary, these data indicated that deficiency of a single inhibitory receptor, PD-1, rather than leading to enhanced bacterial control, results in acute mortality with extensive pulmonary necrosis and high bacterial burden.
M. tuberculosis infects and persists within macrophages, and although PD-1 is principally expressed on T cells, several reports in other model systems have indicated a role for PD-1 in regulating cytokine secretion by nonlymphoid cells (33). Therefore, we next asked if PD-1 might regulate cytokine production by macrophages exposed to M. tuberculosis. To do so, we stimulated WT and PD-1 KO bone marrow-derived macrophages with increasing doses of irradiated H37Rv and doses of rIFN-γ (a potent inducer of PD-1 ligand expression) and measured cytokine production. We found no difference under any condition in the in vitro production of NO, TNF-α, IL-12/23p40, or IL-10 (Supplemental Fig. 1), indicating that PD-1 KO macrophages respond normally to M. tuberculosis stimulation. This suggests that the increased levels of innate inflammatory cytokines in the PD-1 KO mice may not be due to direct regulation of macrophages by PD-1 but rather to the higher bacterial loads occurring in these animals.
PD-1–deficient mice develop increased frequencies of M. tuberculosis-specific CD4 but not CD8 T cells
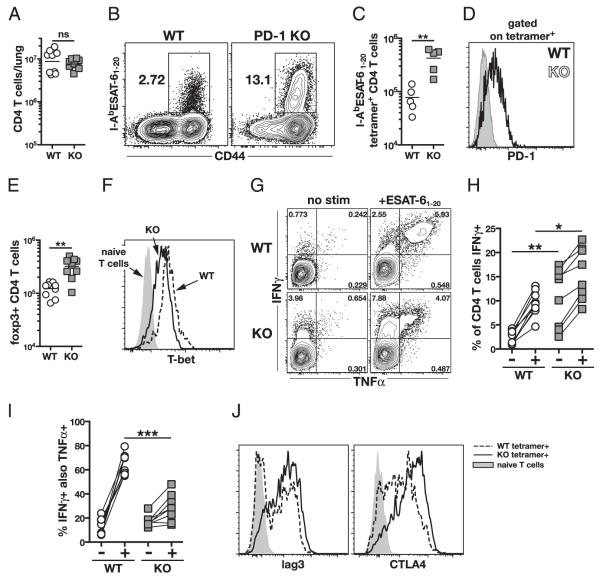
Containment of M. tuberculosis infection requires CD4 T cells (34–36), so we next compared CD4 T cell responses in the lungs of WT and PD-1 KO mice. On day 26 postinfection, WT and PD-1 KO mice had similar numbers of total CD4 T cells in their lungs (Fig. 2A). An immunodominant CD4 T cell response to M. tuberculosis in H-2b mice is directed against the bacterial ESAT-6 protein, so we next used I-AbESAT-61–20 MHC class II tetramers to visualize directly and enumerate M. tuberculosis-specific CD4 T cells. We found that ~13% of CD4 T cells in the infected PD-1 KO mice stained with this tetramer versus only ~3% in the infected WT mice (Fig. 2B). This corresponded with ~5-fold increase in the total number of Ag-specific CD4 T cells in the lungs of PD-1 KO compared with that of WT mice on day 26 post-infection (Fig. 2C). Accordingly, ESAT-61–20-specific CD4 T cells from WT mice expressed significant levels of surface PD-1 (Fig. 2D).
FIGURE 2.
M. tuberculosis-specific CD4 T cells in lung express PD-1 and are increased in PD-1 KO mice. A, Numbers of total CD4 T cells in the lungs on day 26 postinfection. Data are pooled from two independent experiments. B and C, Representative plots of IAbESAT-61–20 MHC class II tetramer staining of CD4 T cells in the lung on day 26 postinfection (B) and numbers of tetramer+ CD4 T cells in the lung (C). Data in B and C are representative of three independent experiments. D, PD-1 expression on IAbESAT-61–20 tetramer+ CD4 T cells from WT and PD-1 KO mice. E, Number of Foxp3+ CD4 T cells in the lungs on day 26. Data are pooled from two independent experiments. F, Representative histograms of T-bet expression on IAbESAT-61–20 tetramer+ CD4 T cells from WT and PD-1 KO mice on day 26 postinfection. G and H, Representative plots of IFN-γ and TNF-α staining on lung CD4 T cells after ESAT-61–20 peptide restimulation (G) and summary data pooled from two independent experiments (H). I, Percentage of IFN-γ–producing CD4 T cells that co-produced TNF-α. Data are pooled from two independent experiments. J, Lag3 and intracellular CTLA-4 expression by IAbESAT-61–20 tetramer+ lung CD4 T cells from WT and PD-1 KO mice.
It has been shown that regulatory T cells can play a role in limiting the number of effector CD4 T cells in the lungs during the early phase of M. tuberculosis infection (37), so we next examined the numbers of Foxp3+ CD4 T cells. We found ~2- to 3-fold increase in the number of Foxp3+ CD4 T cells in PD-1 KO mice (Fig. 2E). This parallels the elevation in effector CD4 T cells and indicates that the increased number of M. tuberculosis-specific CD4 T cells in the PD-1 KO mice is not due to a deficiency in pulmonary Foxp3+ cells.
The transcription factor T-bet (38) and the cytokine IFN-γ (20) are required for control of M. tuberculosis, so we next measured their expression in CD4 T cells. Notably, we found decreased levels of T-bet expression in PD-1 KO relative to WT tetramer+ CD4 T cells (Fig. 2F). Nonetheless, PD-1 KO mice displayed increased frequencies of CD4 T cells expressing IFN-γ without additional in vitro stimulation relative to those of WT mice (Fig. 2G, 2H), which is consistent with the higher levels of IFN-γ in the BAL fluid of PD-1 KO animals (Fig. 1E). We also found increased frequencies of IFN-γ–producing CD4 T cells in PD-1 KO mice compared with those in WT mice after in vitro restimulation with ESAT-61–20 peptide (Fig. 2G, 2H). In contrast, lower frequencies of the IFN-γ–producing CD4 T cells from PD-1 KO mice co-produced TNF-α compared with that in WT mice (Fig. 2I). We also observed greatly increased levels of two other inhibitory receptors, lag3 and CTLA-4, on tetramer+ CD4 T cells in PD-1 KO mice (Fig. 2J). These data indicate that the inability of PD-1 KO mice to control M. tuberculosis is not due to a lack of Th1 responses. Furthermore, the finding that the observed simultaneous increase of both lag3 and CTLA-4 cannot compensate for the absence of PD-1 underscores the role for PD-1 as a key regulator of T cell responses in M. tuberculosis infection.
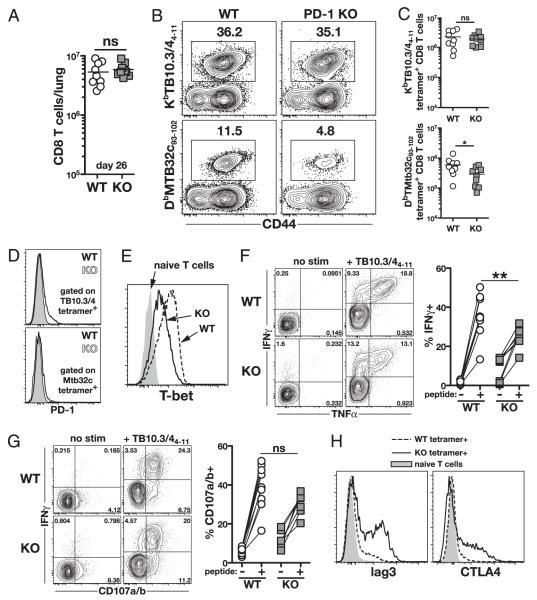
We next compared the CD8 T cell responses of M. tuberculosis-infected WT and PD-1 KO mice. We observed no differences in the total number of CD8 T cells in the lungs 26 d postinfection (Fig. 3A). Two major CD8 T cell responses to M. tuberculosis in C57BL/6 mice are directed against the TB10.3/44–11 peptide in the context of Kb and the Mtb32c93–102 peptide bound to Db. We therefore used the corresponding MHC class I tetramers to measure M. tuberculosis-specific CD8 T cell responses. WT and PD-1 KO mice displayed similar frequencies and numbers of KbTB10.3/44–11-specific CD8 T cells in the lung, whereas DbMtb32c93–102-specific cells were ~2 fold reduced in the KO animals (Fig. 3B, 3C). Notably, only low levels of PD-1 expression were detected on the immunodominant KbTB10.3/44–11-specific cells, and no PD-1 was found on the subdominant DbMtb32c93–102-specific cells (Fig. 3D). As was observed with the M. tuberculosis-specific CD4 T cells, we observed decreased levels of T-bet in KbTB10.3/44–11-specific CD8 T cells in PD-1 KO mice (Fig. 3E). To analyze CD8 T cell function, we measured IFN-γ and TNF-α production by intracellular cytokine staining. We observed increased levels of cytokine production with no exogenous peptide restimulation in vitro, and stimulation with TB10.3/44–11 peptide revealed only a slight decrease in the frequency of IFN-γ–secreting cells in PD-1 KO mice (Fig. 3F, 3G). To measure the ability of CD8 T cells to degranulate, we examined CD107a/b surface expression upon peptide restimulation and compared it with intracellular IFN-γ staining. We found that CD8 T cells from WT and PD-1 KO mice displayed similar levels of degranulation (Fig. 3G). We next measured the expression of lag3 and CTLA-4 on KbTB10.3/44–11-specific CD8 T cells from WT and PD-1 KO mice. Ag-specific CD8 T cells displayed increased levels of both lag3 and CTLA-4 in PD-1 KO mice, albeit to a lesser extent than what was observed on CD4 T cells (Fig. 3H). Together, these data indicated that with the exception of a compensatory increase in other inhibitory receptors, M. tuberculosis-specific CD8 T cell expansion and effector functions are normal or even slightly reduced in the PD-1 KO mice. This is in direct contrast with the increased M. tuberculosis-specific CD4 T cell responses observed in the same animals.
FIGURE 3.
Immunodominant M. tuberculosis-specific CD8 T cells express low levels of PD-1 and are not increased in PD-1 KO mice. A, Numbers of total CD8 T cells in the lungs on day 26 postinfection. Data are pooled from two independent experiments. B and C, Representative plots of KbTB10.3/44–11 and DbMtb32c93–102 MHC class I tetramer staining (B) and total numbers of tetramer+ CD8 T cells in the lungs of WT and PD-1 KO mice (C). Data in C are pooled from two independent experiments. D, PD-1 expression on KbTB10.3/44–11 and DbMtb32c93–102 tetramer+ CD8 T cells in the lungs of WT and PD-1 KO mice. E, T-bet expression in WT and PD-1 KO KbTB10.3/44–11 tetramer+ CD8 T cells. F, Representative plots and summary data for IFN-γ and TNF-α production by lung CD8 T cells after TB10.3/44–11 peptide restimulation. G, Costaining for surface CD107a/b expression (a surrogate for degranulation) and intracellular IFN-γ production by lung CD8 T cells after TB10.3/44–11 peptide restimulation. Pooled data in F and G are from two independent experiments. H, Lag3 and intracellular CTLA-4 expression by KbTB10.3/44–11 tetramer+ CD8 T cells in the lung on day 26 postinfection.
PD-1–PD-L1 interactions partially account for the requirement for PD-1 in control of M. tuberculosis infection
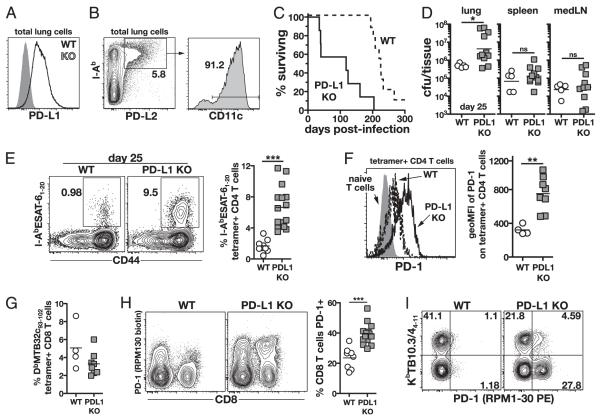
Two B7 family ligands have been identified for PD-1, PD-L1/B7-H1 (39, 40) and PD-L2/B7-DC (41, 42), and they differ greatly in their cellular distribution. On day 25 postinfection, PD-L1 was found to be widely expressed on all hematopoietic cells recovered from the lung (Fig. 4A). In contrast, PD-L2 was expressed on ~5% of cells in the lung, which were all MHC class II-expressing cells and greater than 90% CD11c+ (Fig. 4B). Because PD-L1 is a much more abundant ligand for PD-1 compared with PD-L2 and previous studies in chronic LCMV infection have implicated a role for PD-L1 in regulating lethal immunopathology (7, 16), we assessed the outcome of M. tuberculosis infection in PD-L1 KO mice. Approximately 50% of the PD-L1 KO mice succumbed before day 50 during the acute phase of infection, with 100% mortality by about day 200 postinfection (Fig. 4C). This represented a median survival time of 118 d for the PD-L1 KO mice compared with 225 d for the WT mice. On day 25 postinfection, PD-L1 KO mice contained higher numbers of bacteria in their lungs (but not in spleen or mediastinal lymph node) compared with those in WT mice (Fig. 4D). Notably, a subset of the PD-L1 KO mice contained much higher bacterial loads and may correspond with the mice that succumb during early infection. Thus, although PD-L1 serves as a ligand for PD-1 during M. tuberculosis infection, PD-L1 deficiency can only partially account for the phenotype of infected PD-1 KO mice indicating the involvement of PD-L2 or as an yet to be described alternative ligand.
FIGURE 4.
PD-L1 KO mice are susceptible to M. tuberculosis infection and display elevated M. tuberculosis-specific CD4 T cell responses. A, PD-L1 expression on total viable lung cells in WT and PD-L1 KO mice on day 25 postinfection. B, PD-L2 expression by I-Ab bright CD11c+ cells in the lung on day 28 postinfection. C, Survival of WT and PD-L1 KO mice after M. tuberculosis infection. p < 0.001. n = 9 WT and n = 7 PD-L1 KO mice. Data are representative of three independent experiments. D, Bacterial loads in lung, spleen, and mediastinal lymph nodes in WT and PD-L1 KO mice on day 25 postinfection. Data are pooled from two independent experiments. E, Representative plots and summary data of IAbESAT-61–20 MHC class II tetramer staining on lung CD4 T cells from WT and PD-L1 KO mice on day 25 postinfection. Data in summary graph are pooled from two independent experiments. F, PD-1 expression on tetramer+ CD4 T cells in the lung on day 25 postinfection. Data are representative of three independent experiments. G, DbMtb32c93–102-specific CD8 T cells in the lungs on day 25 postinfection. Data are representative of two independent experiments. H, PD-1 expression on lung CD8 T cells in WT and PD-L1 KO mice on day 25. Summary data are pooled from two independent experiments. I, Representative plots of costaining lung CD8 T cells for KbTB10.3/44–11 tetramers and PD-1. Data are representative of three experiments.
We next measured Ag-specific CD4 and CD8 T cell responses on day 25 of infection in WT and PD-L1 KO mice using MHC class II tetramers. Similar to the PD-1 KO mice, PD-L1 KO mice displayed greatly elevated I-AbESAT-61–20-specific CD4 T cell responses compared with that of WT mice (Fig. 4E). In addition, the cell surface expression of PD-1 was increased on tetramer+ CD4 T cells in the PD-L1 KO mice compared with that in WT mice (Fig. 4F) indicating that PD-1–PD-L1 interactions may lead to the downregulation of PD-1. Although PD-L1 KO mice showed normal or slightly reduced Ag-specific CD8 T cell responses (Fig. 4G), a large increase in PD-1–expressing CD8 T lymphocytes was seen in the absence of PD-L1 (Fig. 4H). Notably, this reflected the expansion of a population of CD8 T cells that were not specific for either DbMtb32c93–102 or KbTB10.3/44–11 (Fig. 4I). Because these cells were identified by their high expression of PD-1, this expansion could not be quantified in the PD-1 KO mice. This may indicate a dramatic shift in the immunodominance hierarchy of the CD8 T cell response and is consistent with the extremely low expression of PD-1 on DbMtb32c93–102- and KbTB10.3/44–11-specific CD8 T cells.
Although PD-L1 can inhibit responses by interacting with PD-1 on T cells, it has also been shown that PD-L1 on T cells can interact with B7-1 and deliver an inhibitory signal itself (43). To examine the inhibitory role of PD-L1 on T cells, we generated PD-L1 KO Ag85b-specific TCR Tg CD4 T cells. Thy1.1+ WT and Thy1.2+ PD-L1 KO TCR Tg CD4 T cells were CFSE labeled and co-transferred into CD45.1+ congenic recipients that had been infected with M. tuberculosis 6 d previously (Supplemental Fig. 2A). WT and PD-L1 KO CD4 T cells in the pulmonary lymph node and lungs displayed identical CFSE dilution on day 20 postinfection (Supplemental Fig. 2B). Upon restimulation with cognate peptide, no difference was seen in the percentage of cells staining for IFN-γ when the WT and PD-L1 KO CD4 T cells were compared (Supplemental Fig. 2B). These data indicate that PD-L1 on T cells is unlikely to play a major role in regulating T cell responses during M. tuberculosis infection.
PD-1 deficiency in CD8 T cells plays a minor role in susceptibility to M. tuberculosis infection
Although we did not observe a marked alteration in M. tuberculosis-specific CD8 T cell responses in PD-1 KO mice, we nonetheless examined the possible contribution of CD8 T cells to the susceptibility of PD-1 KO mice to M. tuberculosis infection. In the first approach, we treated WT and PD-1 KO mice with CD8 T cell-depleting mAbs on day 17 and 25 of infection. As expected, CD8 depletion had no effect on the wasting or survival of WT mice (Fig. 5A, 5B). However, the weight loss was slightly delayed in PD-1 KO mice depleted of CD8 T cells, and their median survival was extended from 30.5 to 36 d (Fig. 5A, 5B). To examine further the role of CD8 T cells in the mortality of the PD-1 KO mice, we used an adoptive transfer system where TCRa KO mice were reconstituted with different combinations of T cells prior to M. tuberculosis exposure. Without CD4 T cells, mice rapidly succumbed to M. tuberculosis infection, and reconstitution of TCRa KO with either WT or PD-1 KO CD8 T cells alone failed to protect these CD4 T cell-deficient animals (data not shown). For this reason, a second series of experiments was performed in which TCRa KO mice were reconstituted with WT CD4 T cells in combination with either WT or PD-1 KO CD8 T cells (Fig. 5C). The transferred PD-1 KO CD8 T cells significantly accelerated the mortality of the reconstituted mice (median survival time of 250 d for recipients of WT CD4 plus WT CD8 T cells and only 150 d for recipients of WT CD4 plus PD-1 KO CD8 T cells) (Fig. 5D). As a final approach to addressing the role of CD8 T cells, we generated PD-L1/CD8 double-KO mice and compared their survival with PD-L1 single-KO mice after M. tuberculosis infection. We found that by day 60 postexposure, 50% of the PD-L1 single-KO mice survived the acute phase of infection versus 83% of the PD-L1/CD8 double-KO mice (Fig. 5E). Collectively, the results of these three experimental approaches indicated that the loss of PD-1 expression on CD8 T cells can make only a minor contribution to the increased susceptibility of PD-1 KO mice to M. tuberculosis infection.
FIGURE 5.
CD8 T cells play a minor role in the increased susceptibility of PD-1 KO mice to M. tuberculosis infection. A and B, Weight loss (A) and survival (B) of WT and PD-1 KO mice treated with CD8 T cell-depleting Abs on days 20 and 25 postinfection with M. tuberculosis. Data are representative of two independent experiments. C and D, TCRα KO mice were reconstituted with WT CD4 T cells and either WT CD8 T cells or PD-1 KO CD8 T cells and then infected with M. tuberculosis (C) and survival was monitored (D). p < 0.05. Data are representative of two independent experiments. E, Survival of M. tuberculosis-infected WT (n = 10), CD8 KO (n = 10), PD-L1 KO (n = 22), and PD-L1/CD8 double-KO (n = 18) mice.
CD4 T cell depletion rescues PD-1 KO mice from early mortality during tuberculosis
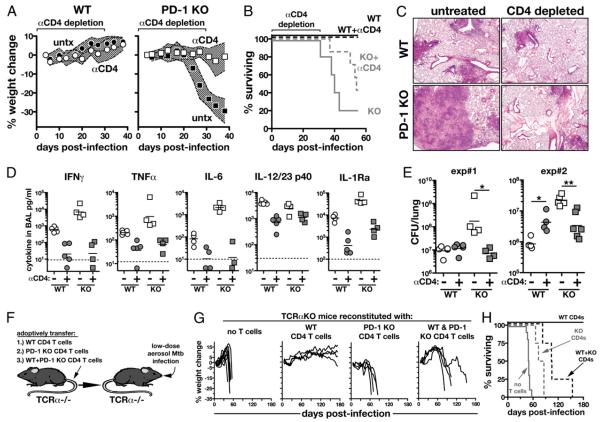
As shown in Fig. 2, PD-1 KO mice display greatly increased IAb ESAT-61–20-specific CD4 T cell responses. Although CD4 T cells are critical for containment of M. tuberculosis, we next tested the possibility that CD4 T cells are also capable of promoting tuberculosis in the absence of PD-1 inhibition. To do so, WT and PD-1 KO mice were infected with M. tuberculosis and administered CD4 T cell-depleting mAbs from day 0 to 30 postinfection. CD4 T cell ablation during the first month of infection had little impact on weight loss and survival in WT animals but completely rescued PD-1 KO mice from the early weight loss and prolonged survival (Fig. 6A, 6B). When lung pathology was examined on day 30 post-infection, we found that control PD-1 KO mice displayed large, highly necrotic lesions (as also shown in Fig. 1C), whereas the lungs of CD4 T cell-depleted mice were comparable with those of WT mice (Fig. 6C). CD4 T cell depletion also decreased the levels of cytokines (IFN-γ, TNF-α, IL-6, IL-12/23 p40, and IL-1Ra) in the BAL fluid of both WT and PD-1 KO mice (Fig. 6D). Importantly, in two independent experiments CD4 T cell depletion reduced the bacterial loads in the PD-1 KO mice to the levels found in depleted WT mice (Fig. 6E). These data indicate that CD4 T cells drive severe lung pathology and promote M. tuberculosis infection when released from negative regulation by PD-1.
FIGURE 6.
CD4 T cells induce severe pathology and promote M. tuberculosis infection in the absence of PD-1. A and B, Weight loss (A) and survival (B) of WT and PD-1 KO mice treated with CD4-depleting Abs from day 0 to 30 postinfection with M. tuberculosis. Traveling error bars in A represent the SD. (n = 5 WT no treatment, n = 5 WT plus αCD4, n = 5 PD-1 KO no treatment, and n = 7 PD-1 KO plus αCD4.) C, H&E sections of lungs on day 30 postinfection. Original magnification ×50. D, Cytokines were measured in the BAL fluid on day 30 postinfection in untreated and CD4-depleted WT and PD-1 KO mice. Data are representative of two independent experiments. E, Bacterial loads in the lungs on day 30 of infection. F, TCRα KO mice were reconstituted with WT, PD-1 KO, or a mixture of WT and PD-1 KO CD4 T cells and then infected with M. tuberculosis. G and H, Weight loss (G) and survival (H) of mice shown in F. Data are representative of three independent experiments.
PD-1 deficiency in CD4 T cells is sufficient to trigger increased susceptibility to M. tuberculosis
To test if PD-1 deficiency in CD4 T cells was sufficient to enhance disease during M. tuberculosis infection, we reconstituted T cell-deficient mice with WT or PD-1 KO CD4 T cells and then infected them with M. tuberculosis (Fig. 6F). Unreconstituted TCRa KO mice rapidly lost weight and all succumbed by day 60 post-infection, whereas mice reconstituted with WT CD4 T cells all survived for >180 d (Fig. 6G, 6H). Strikingly, mice reconstituted with PD-1 KO CD4 T cells underwent wasting, and all succumbed by ~90 d postinfection (Fig. 6G, 6H), indicating that the loss of PD-1 on CD4 T cells is sufficient to lead to acute susceptibility to M. tuberculosis infection. We next examined M. tuberculosis infection of TCRa KO mice reconstituted with equal numbers of CD90.2+ WT and CD90.1+ PD-1 KO CD4 T cells. We found that these doubly reconstituted mice first began to lose weight around day 60 postinfection and all succumbed by about day 150 (Fig. 6G, 6H). This finding demonstrates that even in the presence of WT CD4 T cells, PD-1 KO CD4 T cells actively promote disease during M. tuberculosis infection. Equal numbers of donor cells were recovered in WT and PD-1 KO CD4 T cell recipients, and in mice that received a mixture of both types of cells the numbers of PD-1 KO CD4 T cells were <2-fold increased compared with the WT T cells (Supplemental Fig. 3A) indicating that PD-1 KO CD4 T cells did not suppress the expansion of the WT CD4 T cells and did not dramatically overexpand. As measured by intracellular cytokine staining for IFN-γ and TNF-α, purified protein derivative-specific responses of WT and PD-1 KO CD4 T cells were similar in each group of recipient mice (Supplemental Fig. 3B, 3C). Therefore, the PD-1 KO CD4 T cells did not suppress cytokine production by the WT CD4 T cells. Collectively, these data indicate that PD-1 promotes host resistance by suppressing disease-enhancing CD4 T cells.
Discussion
In this study, we have investigated the role of the cell surface inhibitory receptor PD-1 in regulating T cell responses during aerosol M. tuberculosis infection. Although CD4 T cells are required to slow the growth and spread of M. tuberculosis, we now show that PD-1–mediated inhibition is also required to prevent CD4 T cells from promoting severe disease. Loss of PD-1 on CD4 T cells is sufficient to lead to rapid wasting and mortality during the clonal expansion phase of the T cell response to M. tuberculosis. In fact, PD-1 KO mice succumb to M. tuberculosis infection more rapidly than T cell-deficient mice.
It has recently been shown that human T cell epitopes are highly conserved across globally distinct isolates of M. tuberculosis (44), strongly suggesting that the bacteria benefits from T cell responses. This may reflect a critical role of CD4 T cells in the transmission of M. tuberculosis (45). One outcome of active tuberculosis in immunocompetent hosts is the formation of cavities in the lung, and individuals with this form of tissue pathology are the most infectious (46). In HIV patients, the number of CD4 T cells correlates with the probability of cavitation, with severely immunocompromised patients often displaying mediastinal adenopathy but rarely cavitary disease (47, 48), suggesting that T cells are involved in cavity formation. Therefore M. tuberculosis may benefit from CD4 T cell-induced tissue destruction that promotes escape of bacilli into the large airways and efficient transmission to new hosts. Our data support the hypothesis that M. tuberculosis can benefit from T cell responses and raise the additional possibility that enhanced CD4 T cell responses resulting from lower expression of inhibitory receptors may actually promote bacterial replication and tissue destruction in those patients who progress to active tuberculosis.
It is not clear what T cell effector functions are important for promoting M. tuberculosis in the absence of PD-1 or if the increased bacterial loads are from quantitative and/or qualitative changes in T cell function. Although we found decreased levels of T-bet in PD-1 and PD-L1 KO T cells, Th1 responses were not defective. Furthermore, we were unable to detect appreciable IL-4, IL-17A, IL-22, IL-9, or IL-10 from M. tuberculosis-specific PD-1 KO T cells by intracellular cytokine staining (data not shown) indicating that they had not been skewed toward an alternate Th phenotype. Recently, it has been shown that during influenza infection, Ag-specific Th1 cells drive the production of inflammatory cytokines and chemokines by innate cells independently of IFN-γ or TNF-α production (49). Similarly, we found that depletion of CD4 T cells in WT mice dramatically reduced the levels of innate inflammatory cytokines and in PD-1 KO mice prevented the exacerbated production of the same cytokines. Although the precise mechanism is unclear, our data suggest that PD-1 may regulate Th1-driven innate inflammation.
It is also not clear if the increase in bacterial loads induced by PD-1–deficient CD4 T cells is the cause or effect of the necrotic pathology. PD-1 KO CD4 T cells may directly promote bacterial growth within macrophage, but it is also possible that they induce tissue destruction, which allows for high levels of extracellular bacterial growth. Indeed, we see high numbers of extracellular acid-fast organisms within the necrotic debris of granulomas in the lungs of PD-1 KO animals (data not shown), and it has been shown that M. tuberculosis replicates more efficiently in macrophages undergoing necrotic death (50). Although the mortality of the PD-1 KO mice could be directly due to tissue damage mediated by CD4 T cells, it is likely that the wasting and extensive inflammation induced by the necrosis and high bacterial loads also are contributing factors. Indeed, the levels of innate cytokines in the airways were tightly correlated with the bacterial loads in the lung (data not shown), and in preliminary experiments antibiotic treatment prior to the onset of weight loss rescues the PD-1 KO mice from early mortality.
Apart from the insights into the pathogenesis of M. tuberculosis infection, our data have implications for PD-1 as a target of immunotherapeutic interventions. In numerous preclinical studies, it has been shown that boosting immune function through PD-1 blockade clearly holds tremendous potential for the treatment of malignancies and chronic viral infections. In particular, there is much interest in the use of PD-1 blockade in conjunction with other therapeutic approaches during HIV infection. It has been noted in previous studies that PD-1 blockade has the potential for inducing immunopathology. The data presented in this study now indicate that PD-1 blockade may have major deleterious consequences during M. tuberculosis infection. This may be of particular relevance to clinical trials involving PD-1 blockade in HIV infection where an estimated one-third of all HIV patients are co-infected with M. tuberculosis. Indeed, an ironic implication of our data are that in patients with tuberculosis, it may be more useful to agonize rather than antagonize the PD-1 pathway as an approach to limiting T cell-mediated pathology.
Supplementary Material
Supplemental Figure 1. PD-1 does not regulate nitric oxide or cytokine production by macrophage in response to Mtb stimulation. WT and PD-1 KO bone marrow derived macrophage were stimulated overnight with increasing doses of recombinant murine IFNγ and irradiated Mtb (H37Rv). Nitrate was determined by Griess assay and TNFα, IL-12/23 p40 and IL-10 were measured by ELISA in supernatants.
Supplemental Figure 2. PD-L1 expression on Mtb-specific CD4 T cells does not regulate their expansion or IFNγ expression. (A) CD45.1+ mice were infected with Mtb and on day 6 post-infection a mixture of CFSE labeled CD45.2+ thy1.1+ WT P25 (Ag85b specific TCR Tg CD4 T cells) and CD45.2+ thy1.2+ PD-L1 KO P25. (B) On day 20 post-infection, donor T cells in mediastinal lymph nodes and lungs were analyzed for CFSE dilution and IFNγ production after peptide restimulation.
Supplemental Figure 3. PD-1 KO CD4 T cells do not suppress WT CD4 T cells during Mtb infection. (A) Recovery of WT and PD-1 KO donor CD4 T cells the lungs of recipient mice shown in Fig. 6F on day 70 post-infection. (B) Representative plots of IFNγ and TNFα production by lung CD4 T cells in (A) after stimulation with PPD. (C) Percentage of lung CD4 T cells producing IFNγ after restimulation with PPD or αCD3. Data are pooled from 2 independent experiments.
Acknowledgments
We thank Dr. Bruno Andrade and Kevin Shenderov for helpful discussions. We are also grateful to Sandy White, Patricia Caspar, and Sara Hieny for technical assistance and Marsha Abramson, Fernando Carvajal Borda, and Sharon Fong for performing necropsies and tissue harvests from M. tuberculosis-infected mice under aBSL3 conditions.
This work was supported by the National Institute of Allergy and Infectious Diseases intramural research program.
Abbreviations used in this article
- BAL
bronchoalveolar lavage
- KO
knockout
- LCMV
lymphocytic choriomeningitis virus
- Tg
transgenic
- WT
wild-type
Footnotes
Disclosures D.L.B. and A.H.S. have patents and receive patent royalties related to PD-1.
The online version of this article contains supplemental material.
References
- 1.World Health Organization . Tuberculosis Fact Sheet. WHO; Geneva, Switzerland: 2010. Number 104. [Google Scholar]
- 2.Jung YJ, Ryan L, LaCourse R, North RJ. Properties and protective value of the secondary versus primary T helper type 1 response to airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 2005;201:1915–1924. doi: 10.1084/jem.20050265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallegos AM, Pamer EG, Glickman MS. Delayed protection by ESAT-6-specific effector CD4+ T cells after airborne M. tuberculosis infection. J. Exp. Med. 2008;205:2359–2368. doi: 10.1084/jem.20080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tischler AD, McKinney JD. Contrasting persistence strategies in Salmonella and Mycobacterium. Curr. Opin. Microbiol. 2010;13:93–99. doi: 10.1016/j.mib.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flynn JL, Chan J. Immune evasion by Mycobacterium tuberculosis: living with the enemy. Curr. Opin. Immunol. 2003;15:450–455. doi: 10.1016/s0952-7915(03)00075-x. [DOI] [PubMed] [Google Scholar]
- 6.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 8.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 10.Trautmann L, Janbazian L, Chomont N, Said EA, Wang G, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 11.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J. Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 2007;81:4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J. Exp. Med. 2003;198:39–50. doi: 10.1084/jem.20022235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jun H, Seo SK, Jeong HY, Seo HM, Zhu G, Chen L, Choi IH. B7-H1 (CD274) inhibits the development of herpetic stromal keratitis (HSK) FEBS Lett. 2005;579:6259–6264. doi: 10.1016/j.febslet.2005.09.098. [DOI] [PubMed] [Google Scholar]
- 16.Mueller SN, Vanguri VK, Ha SJ, West EE, Keir ME, Glickman JN, Sharpe AH, Ahmed R. PD-L1 has distinct functions in hematopoietic and nonhematopoietic cells in regulating T cell responses during chronic infection in mice. J. Clin. Invest. 2010;120:2508–2515. doi: 10.1172/JCI40040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lázár-Molnár E, Gácser A, Freeman GJ, Almo SC, Nathenson SG, Nosanchuk JD. The PD-1/PD-L costimulatory pathway critically affects host resistance to the pathogenic fungus Histoplasma capsulatum. Proc. Natl. Acad. Sci. USA. 2008;105:2658–2663. doi: 10.1073/pnas.0711918105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayer-Barber KD, Barber DL, Shenderov K, White SD, Wilson MS, Cheever A, Kugler D, Hieny S, Caspar P, Núñez G, et al. Caspase-1 independent IL-1beta production is critical for host resistance to Mycobacterium tuberculosis and does not require TLR signaling in vivo. J. Immunol. 2010;184:3326–3330. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lázár-Molnár E, Chen B, Sweeney KA, Wang EJ, Liu W, Lin J, Porcelli SA, Almo SC, Nathenson SG, Jacobs WR., Jr Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc. Natl. Acad. Sci. USA. 2010;107:13402–13407. doi: 10.1073/pnas.1007394107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenzweig SD, Holland SM. Defects in the interferon-gamma and interleukin-12 pathways. Immunol. Rev. 2005;203:38–47. doi: 10.1111/j.0105-2896.2005.00227.x. [DOI] [PubMed] [Google Scholar]
- 22.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, Schreiber R, Mak TW, Bloom BR. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 23.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 24.Yamada H, Mizumo S, Horai R, Iwakura Y, Sugawara I. Protective role of interleukin-1 in mycobacterial infection in IL-1 alpha/beta double-knockout mice. Lab. Invest. 2000;80:759–767. doi: 10.1038/labinvest.3780079. [DOI] [PubMed] [Google Scholar]
- 25.Fremond CM, Togbe D, Doz E, Rose S, Vasseur V, Maillet I, Jacobs M, Ryffel B, Quesniaux VF. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J. Immunol. 2007;179:1178–1189. doi: 10.4049/jimmunol.179.2.1178. [DOI] [PubMed] [Google Scholar]
- 26.Ladel CH, Blum C, Dreher A, Reifenberg K, Kopf M, Kaufmann SH. Lethal tuberculosis in interleukin-6-deficient mutant mice. Infect. Immun. 1997;65:4843–4849. doi: 10.1128/iai.65.11.4843-4849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper AM, Magram J, Ferrante J, Orme IM. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J. Exp. Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altare F, Durandy A, Lammas D, Emile JF, Lamhamedi S, Le Deist F, Drysdale P, Jouanguy E, Döffinger R, Bernaudin F, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432–1435. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 29.Chan J, Tanaka K, Carroll D, Flynn J, Bloom BR. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect. Immun. 1995;63:736–740. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. USA. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cruz A, Fraga AG, Fountain JJ, Rangel-Moreno J, Torrado E, Saraiva M, Pereira DR, Randall TD, Pedrosa J, Cooper AM, Castro AG. Pathological role of interleukin 17 in mice subjected to repeated BCG vaccination after infection with Mycobacterium tuberculosis. J. Exp. Med. 2010;207:1609–1616. doi: 10.1084/jem.20100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lang R, Rutschman RL, Greaves DR, Murray PJ. Autocrine deactivation of macrophages in transgenic mice constitutively overexpressing IL-10 under control of the human CD68 promoter. J. Immunol. 2002;168:3402–3411. doi: 10.4049/jimmunol.168.7.3402. [DOI] [PubMed] [Google Scholar]
- 33.Yao S, Wang S, Zhu Y, Luo L, Zhu G, Flies S, Xu H, Ruff W, Broadwater M, Choi IH, et al. PD-1 on dendritic cells impedes innate immunity against bacterial infection. Blood. 2009;113:5811–5818. doi: 10.1182/blood-2009-02-203141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caruso AM, Serbina N, Klein E, Triebold K, Bloom BR, Flynn JL. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J. Immunol. 1999;162:5407–5416. [PubMed] [Google Scholar]
- 35.Scanga CA, Mohan VP, Yu K, Joseph H, Tanaka K, Chan J, Flynn JL. Depletion of CD4(+) T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon gamma and nitric oxide synthase 2. J. Exp. Med. 2000;192:347–358. doi: 10.1084/jem.192.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saunders BM, Frank AA, Orme IM, Cooper AM. CD4 is required for the development of a protective granulomatous response to pulmonary tuberculosis. Cell. Immunol. 2002;216:65–72. doi: 10.1016/s0008-8749(02)00510-5. [DOI] [PubMed] [Google Scholar]
- 37.Shafiani S, Tucker-Heard G, Kariyone A, Takatsu K, Urdahl KB. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J. Exp. Med. 2010;207:1409–1420. doi: 10.1084/jem.20091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sullivan BM, Jobe O, Lazarevic V, Vasquez K, Bronson R, Glimcher LH, Kramnik I. Increased susceptibility of mice lacking T-bet to infection with Mycobacterium tuberculosis correlates with increased IL-10 and decreased IFN-gamma production. J. Immunol. 2005;175:4593–4602. doi: 10.4049/jimmunol.175.7.4593. [DOI] [PubMed] [Google Scholar]
- 39.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 41.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 42.Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J. Exp. Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Comas I, Chakravartti J, Small PM, Galagan J, Niemann S, Kremer K, Ernst JD, Gagneux S. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat. Genet. 2010;42:498–503. doi: 10.1038/ng.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flynn JL, Chan J. What’s good for the host is good for the bug. Trends Microbiol. 2005;13:98–102. doi: 10.1016/j.tim.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Rodrigo T, Caylà JA, García de Olalla P, Galdós-Tangüis H, Jansà JM, Miranda P, Brugal T. Characteristics of tuberculosis patients who generate secondary cases. Int. J. Tuberc. Lung Dis. 1997;1:352–357. [PubMed] [Google Scholar]
- 47.Mukadi Y, Perriëns JH, St Louis ME, Brown C, Prignot J, Willame JC, Pouthier F, Kaboto M, Ryder RW, Portaels F, et al. Spectrum of immunodeficiency in HIV-1-infected patients with pulmonary tuberculosis in Zaire. Lancet. 1993;342:143–146. doi: 10.1016/0140-6736(93)91346-n. [DOI] [PubMed] [Google Scholar]
- 48.Perlman DC, el-Sadr WM, Nelson ET, Matts JP, Telzak EE, Salomon N, Chirgwin K, Hafner R, The Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA). The AIDS Clinical Trials Group (ACTG) Variation of chest radiographic patterns in pulmonary tuberculosis by degree of human immunodeficiency virus-related immunosuppression. Clin. Infect. Dis. 1997;25:242–246. doi: 10.1086/514546. [DOI] [PubMed] [Google Scholar]
- 49.Strutt TM, McKinstry KK, Dibble JP, Winchell C, Kuang Y, Curtis JD, Huston G, Dutton RW, Swain SL. Memory CD4+ T cells induce innate responses independently of pathogen. Nat. Med. 2010;16:558–564. doi: 10.1038/nm.2142. 1p following 564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Behar SM, Divangahi M, Remold HG. Evasion of innate immunity by Mycobacterium tuberculosis: is death an exit strategy? Nat. Rev. Microbiol. 2010;8:668–674. doi: 10.1038/nrmicro2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. PD-1 does not regulate nitric oxide or cytokine production by macrophage in response to Mtb stimulation. WT and PD-1 KO bone marrow derived macrophage were stimulated overnight with increasing doses of recombinant murine IFNγ and irradiated Mtb (H37Rv). Nitrate was determined by Griess assay and TNFα, IL-12/23 p40 and IL-10 were measured by ELISA in supernatants.
Supplemental Figure 2. PD-L1 expression on Mtb-specific CD4 T cells does not regulate their expansion or IFNγ expression. (A) CD45.1+ mice were infected with Mtb and on day 6 post-infection a mixture of CFSE labeled CD45.2+ thy1.1+ WT P25 (Ag85b specific TCR Tg CD4 T cells) and CD45.2+ thy1.2+ PD-L1 KO P25. (B) On day 20 post-infection, donor T cells in mediastinal lymph nodes and lungs were analyzed for CFSE dilution and IFNγ production after peptide restimulation.
Supplemental Figure 3. PD-1 KO CD4 T cells do not suppress WT CD4 T cells during Mtb infection. (A) Recovery of WT and PD-1 KO donor CD4 T cells the lungs of recipient mice shown in Fig. 6F on day 70 post-infection. (B) Representative plots of IFNγ and TNFα production by lung CD4 T cells in (A) after stimulation with PPD. (C) Percentage of lung CD4 T cells producing IFNγ after restimulation with PPD or αCD3. Data are pooled from 2 independent experiments.