Abstract
Aims
Hemorrhagic shock leads to a higher risk of mortality and morbidity in obese patients, however the mechanisms for these outcomes are unclear. We hypothesized that following severe hemorrhage, blood pressure control in conscious obese Zucker rats (OZ) is impaired.
Main Methods
Experiments were performed in conscious lean Zucker rats (LZ) and OZ. Blood pressure, heart rate, cardiac output, total peripheral resistance (TPR), plasma renin activity (PRA), plasma antidiuretic hormone (ADH), and blood gases were measured before and after severe hemorrhage (35% of the total blood volume).
Key Findings
Basal blood pressure, cardiac output, TPR, PRA, and ADH levels were not different between LZ and OZ. Compared to LZ, OZ exhibited impaired baroreflex control of heart rate and showed higher levels of vascular adrenergic tone. One hour after the hemorrhage, LZ and OZ exhibited similar decreases in cardiac output. However, blood pressure, heart rate, TPR, PRA, and ADH levels were lower in OZ than in LZ.
Significance
These results indicate that conscious OZ has impaired blood pressure compensation after hemorrhage due to a blunted increase in TPR. This is due at least in part to an impaired regulation of vasoconstrictor hormones. To our knowledge, the current study is the first to demonstrate that hemodynamic responses and associated hormone secretion are impaired in a conscious obese model.
Keywords: Obesity, hemorrhage, cardiac output, vascular resistance, renin, antidiuretic hormone, baroreflex, and sympathetic nerve.
INTRODUCTION
Severe blood loss leads to hemodynamic instability, organ failure, and an increased inflammatory response (Schmid-Schonbein and Hugli 2005; Sharma et al. 2008). Maintenance of arterial pressure is essential to adequately perfuse vital organs. Critical physiological responses to hemorrhage-induced hypovolemia are rapid increases in both sympathetic nerve activity via baroreflex and the release of circulating vasoconstrictor hormones such as angiotensin II and antidiuretic hormone (ADH). These responses act together as powerful feedback to compensate blood pressure via increasing total peripheral resistance (TPR).
Severe hemorrhage leads to a higher risk of mortality and morbidity in obese patients (Nelson et al. 2012). However the mechanisms responsible for the increased risks are unclear. Impaired baroreflex control of sympathetic activity and heart rate in obesity (Davis 2011; Huber and Schreihofer 2010; Schreihofer et al. 2007) may blunt TPR and heart rate responses following hemorrhage. Also, an elevated basal sympathetic activity in obese individuals and animals (Eikelis et al. 2003; Kalupahana and Moustaid-Moussa 2012; Mark et al. 1999; Narkiewicz et al. 1998) may limit their ability to increase cardiovascular responses after hemorrhage (Frisbee 2006). There are virtually no studies that investigate the cardiovascular and hormonal responses to severe hemorrhage in obese subjects or animals under conscious conditions.
Determining blood pressure recovery under conscious conditions is important since anesthesia may interfere with neural and possibly other hormonal responses. Frisbee et al. showed that unconscious obese Zucker rats (OZ) exhibited significantly impaired blood pressure recovery after losing 10% of volume in 4 successive increments (Frisbee 2006). However, in our previous study, conscious OZ with a 20% loss of volume and an additional 10% loss after a 40-minute recovery did not exhibit impaired blood pressure compensation as compared to lean Zucker rats (LZ) (Xiang et al. 2012). This inconsistency raises the possibility that anesthesia interferes with the blood pressure regulation following hemorrhage differently between lean and obese rats. Additionally, there is evidence that physiologic response to hemorrhagic shock depends on rate and means of hemorrhage (Frankel et al. 2007). Therefore, the current study was designed to determine the regulation of cardiovascular hemodynamics following a single severe hemorrhage (a loss 35% of blood volume) in conscious OZ.
We hypothesized that the blood pressure compensation following severe hemorrhage is impaired in conscious OZ. OZ has been widely used as a model for central obesity with metabolic and autonomic disorders, along with cardiovascular dysfunction, similar to those conditions seen in obese subjects. In the current study, we compared hemodynamic parameters, blood gases, and hormonal responses (ADH and the renin-angiotensin system) between conscious lean Zucker rats (LZ) and OZ before and after severe hemorrhage. This data would provide important insight into the mechanism(s) and the treatment strategies in obese patients with hemorrhagic shock.
MATERIALS AND METHODS
Animals and surgical preparation
Male Zucker rats (11-13 wk), Harlan Laboratories, had mean bodyweights of 306 ± 7g for LZ and 452 ± 10g for OZ. The experimental protocols for this study were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center and were carried out according to both the “Guide for the Care and Use of Laboratory Animals” from the National Institutes of Health and also the guidelines of the Animal Welfare Act. All the rats were housed 2-3 animals per cage at 22° C (12-h light-dark cycle) with food and water ad libitum.
Animals were anesthetized with inhalation of ~4-6% of isoflurane combined with 100% O2. The neck and left hindlimb were shaved lightly and wiped with 70% ethanol. The right jugular, left carotid artery, and left femoral artery were isolated, catheterized (catheters containing 10% heparin in saline), and then exteriorized. Incisions were closed using 4-0 vicryl. After recovery from anesthesia, blood pressure and heart rate were recorded via carotid catheter (PowerLab system, Model: ML 118). Before each set of experiments, animals were allowed to equilibrate from surgery for 2-3 hours until the blood pressure and heart rate reached steady-state levels.
Baroreflex sensitivity and basal sympathetic tone
To measure baroreflex sensitivity, blood pressure and heart rate were recorded before and after administration of a bolus of the vasodilator sodium nitroprusside via jugular catheter. Three different doses of sodium nitroprusside (10, 50, and 100 μg/kg) were used for LZ. The doses used for OZ were estimated by using the mean bodyweight of the LZ, because the blood volumes of age-matched LZ and OZ are similar (Frisbee 2006; Schreihofer et al. 2005). The volume of sodium nitroprusside (SNP) for each bolus was less than 0.15 mL, so as to have minimal impact on blood volume. The SNP-induced decrease in blood pressure only lasted for 3-5 minutes. The length of time of the decreased blood pressure and the time point of the peak changes in blood pressure were variable between animals. Therefore, we compared the baroreflex control of heart rate when the maximal presser effect was achieved, similar to a previous report (Schreihofer et al. 2007). The rats were allowed to equilibrate 15 minutes or until the blood pressure and heart rate reached steady-state levels between each injection.
In an additional set of experiments, the basal adrenergic vascular tone was assessed by measuring blood pressure in LZ and OZ before and 5 minutes after administration of a bolus of prazosin (α1-receptor antagonist, 1mg/kg) via jugular catheter to estimate the basal adrenergic tone in LZ and OZ.
Hemodynamics before and after hemorrhage in LZ and OZ
Rats were placed in a Columbus Instruments metabolic cage to monitor oxygen consumption. Rats had no access to food or water during the experiment. Arterial and venous blood samples (< 0.2 mL each) were collected from femoral and jugular catheters, respectively, to measure the basal levels of blood gases (Trupoint Blood Analysis System). This minor loss of blood volume did not affect blood pressure and heart rate.
After another 30-minute equilibration in the metabolic cage, hemorrhage was induced by withdrawing 35% of total blood volume from the femoral catheter (~0.5 mL/min, 6-8 ml). The total blood volume in LZ was estimated using the formula: body weight x 0.06 + 0.77 (Boku et al. 2010; Lee and Blaufox 1985). The total blood volume in OZ was estimated by using the mean bodyweight of the LZ since there is evidence that the total blood volume is not different between age-matched LZ and OZ, despite the difference in body weight (Frisbee 2006; Schreihofer et al. 2005).
Because our goal was to measure the acute responses to hemorrhage in LZ and OZ, we chose to measure the blood pressure and heart rate both during and for one hour following hemorrhagic blood withdrawal. The first milliliter of arterial blood from the hemorrhage was collected for measurements of arterial blood gases, plasma renin activity (PRA) and ADH levels (radioimmunoassay, Diagnostic Products). The subsequent blood drawn was used to measure hematocrit by the ratio of the length of the column of blood cells relative to the length of the column containing the entire sample after being centrifuged at 3000g for 15 minutes.
After one hour of recovery, blood samples were collected again to measure blood gases, hematocrit, PRA, and ADH levels. To minimize blood loss or any other unexpected stress, we did not take blood samples during the recovery period. Using blood gas and hematocrit measurements, the arterial and venous oxygen contents (O2 ml/ ml) were derived using the following formula: hematocrit x 0.34 x 100 x 1.36 x oxygen saturation + 0.0031 x oxygen partial pressure. Based on the Fick principal, cardiac output (ml/min) = oxygen consumption x bodyweight x 100 / (arterial oxygen content – venous oxygen content). TPR (mmHg.min/ml) = mean arterial pressure / cardiac output.
Quantitative and statistical analyses
Data were compared by using two-way repeated measures ANOVA. Where significant effects occurred, individual groups were compared using the Holm-Sidak method. All the data are presented as means ± SE. A probability of P < 0.05 was accepted as statistically significant for all comparisons.
RESULTS
Baroreflex sensitivity and basal sympathetic tone
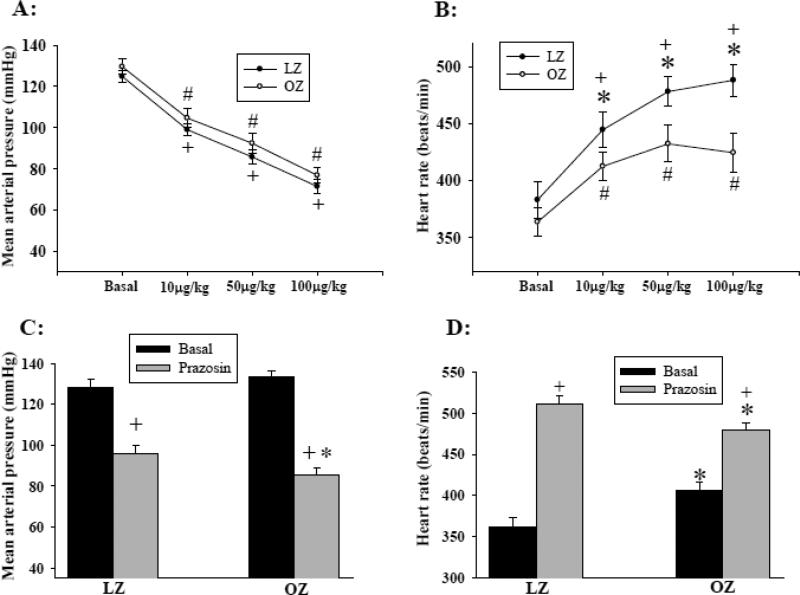
Figures 1A and 1B represent the lowest blood pressure and corresponding heart rate, respectively, after each intravenous injection of SNP. The basal blood pressure and heart rate were not significantly different between LZ and OZ. Administration of SNP caused a similar decrease in blood pressure in both LZ and OZ in a dose-dependent manner (Fig. 1A). However, the increase in heart rate in response to each dose of SNP was significantly blunted in OZ as compared to LZ (Fig. 1B). The blood pressure and heart rate returned to their baseline within 5 minutes after SNP injection.
Fig. 1.
(A) Mean arterial pressure and (B) heart rate before and after intravenous injection of sodium nitroprusside (SNP). (* P < 0.05 LZ vs. OZ; + P < 0.05 vs. basal within LZ; # P < 0.05 vs. basal within OZ; n = 8 for LZ and OZ). (C) Prazosin-induced decrease in mean blood pressure. (*: P < 0.05 LZ vs. OZ; +: P < 0.01 control vs. prazosin; n = 7 for each group). (D) Prazosin-induced increase in heart rate (*: P < 0.05 LZ vs. OZ; +: P < 0.01 control vs. prazosin; n = 7 for each group).
In a separate group of animals, prazosin injection resulted in an immediate decrease in blood pressure and increase in heart rate, which reached a steady level within 5 minutes. Therefore, the blood pressure and heart rate 5 minutes after the treatment were compared between groups. OZ exhibited a greater decrease in the basal blood pressure as compared with LZ (Fig. 1C). The increases in heart rate after the treatment were significantly blunted in OZ as compared with LZ (Figure 1D).
Blood pressure and heart rate responses to hemorrhage
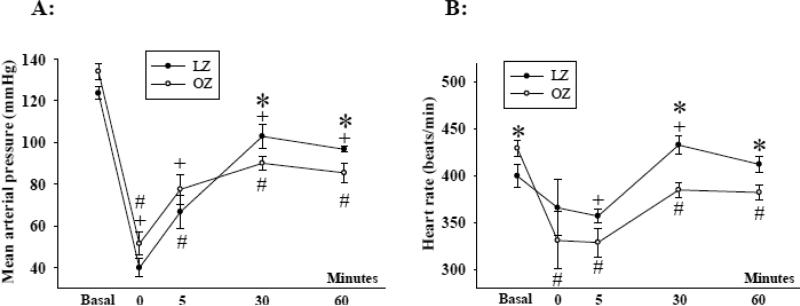
The basal blood pressure was not significantly different between LZ and OZ (Fig. 2A). Hemorrhage significantly decreased the blood pressure in all animal groups. The blood pressure was not different between LZ and OZ immediately after or 5 minutes after hemorrhage (Fig. 2). However, at 30 and 60 minutes of recovery, OZ exhibited a significantly lower blood pressure as compared with LZ.
Fig. 2.
(A) Mean arterial pressure and (B) heart rate before and during the one hour recovery period after hemorrhage. (*: P < 0.05 LZ vs. OZ; + P < 0.05 vs. basal within LZ; # P < 0.05 vs. basal within OZ; n = 8 for LZ and n = 7 for OZ).
The basal heart rate was significantly higher in the OZ than in the LZ (Fig. 2B). As compared to baseline, the heart rate in the LZ was transiently decreased 5 minutes after hemorrhage which was followed by an increase in heart rate for the remainder of the experiment. As compared to the baseline, OZ exhibited bradycardia throughout the recovery period. Heart rate was significant lower in OZ as compared to the LZ throughout recovery.
Oxygen consumption, blood gases, and cardiac output before and after hemorrhage
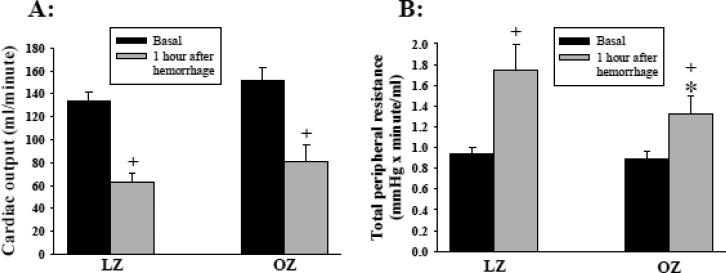
Oxygen consumption was 17 ± 1 (LZ) and 15 ± 1 (OZ) ml/min/kg before hemorrhage and 15 ± 1 (LZ) and 13 ± 1 (OZ) ml/min/kg one hour after hemorrhage. Oxygen consumption was significantly decreased in both LZ and OZ following hemorrhage (P < 0.01; basal vs. after hemorrhage). OZ exhibited significantly lower oxygen consumption before and after hemorrhage as compared to LZ (P < 0.01; LZ vs. OZ). Arterial and venous oxygen content, venous oxygen saturation, and venous partial pressure of oxygen were similarly decreased in LZ and OZ after hemorrhage (Table 1). The basal cardiac output was not different between LZ and OZ. After hemorrhage, cardiac output was decreased to similar levels in LZ and OZ (Fig. 3A).
Table 1.
Blood gases before and one hour after hemorrhage
| Arterial blood gas | Venous blood gas | |||||||
|---|---|---|---|---|---|---|---|---|
| SaO2 % | PaO2 mmHg | CaO2 O2 ml/ml | HCT % | SvO2 % | PvO2 mmHg | CvO2 O2 ml/ml | ||
| Basal | LZ | 98 ± 1 | 107± 7 | 21 ± 1 | 45 ± 1 | 81 ± 1 | 40 ± 1 | 17 ± 1 |
| OZ | 98 ± 1 | 106 ± 8 | 21± 1 | 45 ± 1 | 78 ± 1 | 41 ± 2 | 17 ± 1 | |
| Hemorrhage | LZ | 99 ± 1 | 113 ± 5 | 15 ± 1 + | 33 ± 1+ | 47 ± 5+ | 28 ± 2+ | 7 ± 1+ |
| OZ | 99 ± 1 | 116 ± 6 | 17 ± 1 + | 36 ± 1*+ | 52 ± 5+ | 29 ± 2+ | 9 ± 1+ | |
P < 0.05 LZ vs. OZ, + P < 0.05 Basal vs. Hemorrhage; n = 8 for LZ and 7 for OZ; SaO2 and SvO2 indicate arterial and venous oxygen saturation, respectively; PaO2 and PvO2 indicate arterial and venous oxygen partial pressure, respectively; CaO2 and CvO2 indicate arterial and venous oxygen content, respectively; and HCT is hematocrit.
Fig. 3.
(A) Cardiac output and (B) total peripheral resistance (TPR) before and 1 hour after hemorrhage. (*: P < 0.05 LZ vs. OZ; +: P < 0.05 basal vs. one hour after hemorrhage; n = 8 for LZ and 7 for OZ).
TPR, PRA, and ADH before and after hemorrhage
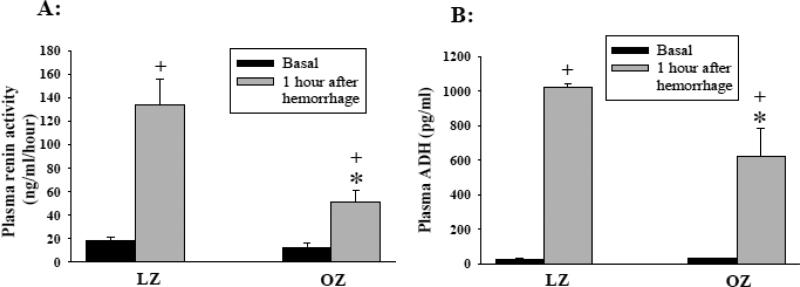
The basal TPR, PRA, and ADH levels were not different between LZ and OZ (Fig. 3B, 4A, and 4B, respectively). Hemorrhage significantly increased the TPR, PRA, and ADH in both LZ and OZ, while the increases in PRA, ADH, and TPR were significantly attenuated in OZ as compared to LZ.
Fig. 4.
(A) Plasma renin activity (PRA) and (B) plasma antidiuretic hormone (ADH) levels before and 1 hour after hemorrhage. (Figure A: *: P < 0.05 LZ vs. OZ within one hour after hemorrhage; +: P < 0.01 basal vs. one hour after hemorrhage; n = 8 for LZ and 7 for OZ); (Figure B: *: P < 0.05 LZ vs. OZ within one hour after hemorrhage; +: P < 0.01 basal vs. one hour after hemorrhage; n = 5 for LZ and OZ).
DISCUSSION
The major findings of the current study are the following: 1) despite no differences in basal blood pressure, cardiac output, and TPR between conscious LZ and OZ, the OZ exhibit blunted baroreflex sensitivity and elevated basal sympathetic vascular tone compared to LZ. 2) Following hemorrhage, OZ exhibit impaired increase in TPR and blunted recovery of blood pressure along with attenuated increases in PRA and ADH. These results suggest that emergency treatments after hemorrhage warrant more attention in obese patients.
Neural and hormonal regulation of acute blood pressure is complex and can have conflicting effects. Thus the individual contribution of each factor to regulate hemodynamics after hemorrhage is difficult to quantify, especially in conscious animals. For example, the sympathetic nervous system regulates the secretion of renin, while changes in angiotensin II levels can affect baroreflex and autonomic responses (Hiwatari et al. 1985; Struck et al. 2002). Moreover, regional differences in neural control of vascular tone and/or hormone secretions following hemorrhage makes the regulatory mechanisms more difficult to determine understand, especially under conscious conditions (Carlsson et al. 1992; Morita and Vatner 1985; Skoog et al. 1985). However, based on our knowledge, the current study is the first to demonstrate that hemodynamic responses and associated hormone secretion are impaired in a conscious obese model. These findings could provide new insight into the mechanism(s) and treatment strategies in obese patients with severe blood loss.
We found that increases in PRA after hemorrhage were significantly blunted in OZ compared to LZ. PRA is an index of the activation of the renin-angiotensin system and resultant vasoconstriction from angiotensin II after hemorrhage. Renin is released into the circulation immediately following hemorrhage and subsequently increases angiotensin II levels, which have a maximal effect within 30 minutes to increase TPR (Brough et al. 1975; Gotshall 1982). Renin is increased due to increased renal sympathetic nerve activity along with local renal mechanisms in response to large decreases in arterial pressure (Quail et al. 1987). Although OZ may have elevated basal renal sympathetic nerve activity (Morgan et al. 1995), the ability of the OZ to increase sympathetic activity after hypotension is blunted (Schreihofer et al. 2007). In the current study, basal PRA levels between LZ and OZ were not different, but PRA was attenuated in OZ one hour after severe hemorrhage as compared to LZ. Based on previous studies (Carlsson et al. 1992; Morita and Vatner 1985; Skoog et al. 1985), an impaired renal sympathetic response might be responsible for the impaired regulation of the renin-angiotensin system after hemorrhage in OZ.
Following hemorrhage circulating ADH levels were also lower in OZ as compared to LZ. ADH is released from the posterior pituitary in response to low blood volume and is regulated independently of the baroreflex (Goetz et al. 1984; Mueller 2008; Quail et al. 1987). ADH can increase 5 fold and account for 70% of blood pressure recovery after hemorrhage (Cowley et al. 1980). The maximum effect of osmolarity on ADH secretion is inferior to the stimulus of hypovolemia. Additionally, there is evidence that the osmolarity regulation of ADH secretion is not different between lean and obese subjects (Coenraad et al. 2009). To our knowledge, no study has determined obesity’s effect on renin-angiotensin II and ADH regulation during hypovolemia, especially in a conscious animal model of obesity.
The increase in heart rate in response to SNP induced decrease in blood pressure was less in the OZ as compared to the LZ, suggesting an impaired baroreflex control of heart rate in the OZ. The decrease in blood pressure was an integrative response to a SNP-induced vasodilation and sympathetic mediated-vasoconstriction. Thus, a similar blood pressure between LZ and OZ following SNP does not necessarily mean similar baroreflex-mediated vasoconstriction unless the SNP-induced vasodilation is identical between groups. Indeed, our previous study shows that SNP-induced vasodilation is impaired in OZ as compared to LZ (Xiang et al. 2005). Moreover, OZ treated with prazosin exhibited a larger decrease in blood pressure but a blunted increase in heart rates as compared to LZ. The maximal changes in blood pressure and heart rate were obtained within five minutes following prazosin or SNP treatment, minimizing the effects of other compensatory factors. Therefore, these results suggest that baroreflex is impaired in OZ, consistent with previous studies (Davis 2011; Huber and Schreihofer 2010; Schreihofer et al. 2007).
Obese patients and animals exhibit higher basal sympathetic activity levels based on observations that ganglionic blockade induces larger decreases in blood pressure compared to their lean counterparts (Carlson et al. 2000; Shibao et al. 2007). We confirmed this finding by showing that prazosin (selective α1-receptor antagonist) treatment decreased basal blood pressure more in OZ than in LZ (Fig. 1C). Previous studies have focused on the contribution of elevated sympathetic activity to the pathogenesis of obesity-induced hypertension (Eikelis et al. 2003; Hall et al. 1993; Hall et al. 2010). However, hypertension is not always present in obese subjects, and TPR can be normal or even decreased despite an overall elevated sympathetic activity (Messerli et al. 1981). In agreement with these previous findings, our data shows the basal levels of blood pressure and TPR are not different between LZ and OZ (West et al. 1987).
The elevated basal vascular adrenergic tone is not necessarily correlated with an increased heart rate. This could be explained by regional differences in neural control, impaired cardiac baroreflex, and/or a possible withdrawal of parasympathetic control of heart rate (Truett et al. 1996; Van Vliet et al. 1995). Notably, comparing basal heart rates in OZ animals across Figures 1B, 1D, and 2B shows variable values. This is likely due to impaired cardiac baroreflex and resultant blunted buffering of heart rate in obesity (Iliescu 2007; Karason et al. 1999; Windham et al. 2012). This inconsistency in basal levels should not weaken our conclusions. If comparing the delta change in heart rates, the differences between lean and obese rats are even more significant.
The basal cardiac output is not increased in OZ despite increased sympathetic activity. A previous study using a radiolabeled microsphere technique also found a similar cardiac output between LZ and OZ (West et al. 1987). In the current study, the cardiac output was measured by the Fick principal, which has been shown to be a reliable technique (Palmers et al. 2012; Robinson et al. 2003; van Herwaarden et al. 1980). Moreover, we found that the basal oxygen consumption is decreased in the OZ. These results suggest that the metabolic demand as well as cardiac output is not necessarily increased with bodyweight in obesity since the adipose tissue has a relatively low basal metabolic rate and blood flow.
Neural regulation is critical in the blood pressure compensation after hemorrhage. Subjects with high baroreflex sensitivity exhibit increased tolerance to hypovolemic shock as compared to individuals with low baroreflex sensitivity (Convertino et al. 2012). An elevated basal vascular sympathetic tone has been suggested to limit the capability of adrenergic vasoconstriction following hemorrhage (Frisbee 2006). However, there is a lack of ideal methods to quantify the impacts of impaired baroreflex and elevated basal sympathetic activity on blood pressure regulation. This is because the measurement of circulating catecholamine is elusive and sometimes unreliable, along with the probability of regional differences in neural control of vascular tone and/or hormone secretions following severe hemorrhage (Carlsson et al. 1992; Morita and Vatner 1985; Skoog et al. 1985). It is difficult to determine the direct role of sympathetic regulation using available techniques as neural regulation interacts closely with other compensatory mechanisms. Since neural regulation is critical in the blood pressure compensation, these changes in neural activity in OZ were confirmed and need to be considered as a mechanism for the impaired blood pressure compensation after hemorrhage.
The impaired baroreflex function is believed to blunt tachycardia following hemorrhage. However, the baroreflex should not further decrease the heart rate lower than baseline as was evident in OZ after hemorrhage. It is proposed that reduction in ventricular filling pressure after severe hemorrhage increases cardiac vagal outflow via myocardial mechanoreceptors (Bezold-Jarish reflex), resulting in decreased heart rate (Campagna and Carter 2003; Mark 1983). This parasympathetic nerve-mediated bradycardia following severe hemorrhage appears to be elevated in OZ and diabetic rats (Boku et al. 2010; Xiang et al. 2012). Although the mechanisms and significance of this post-hemorrhagic bradycardia are unclear, the similar cardiac output between LZ and OZ after hemorrhage suggests that a blunted increase in TPR rather than bradycardic hypotension is responsible for the impaired blood pressure compensation in OZ.
Summary and perspective
The current study demonstrates that blood pressure recovery following a severe hemorrhage is impaired in conscious OZ compared to LZ. This is consistent with clinical observations that obese subjects have increased morbidity and mortality following hemorrhage. The impaired blood pressure recovery and lower tissue perfusion in obesity could be a risk factor for a later complications and possible organ injury. Our data indicate that the impaired blood pressure compensation following hemorrhage in OZ is due to a blunted increase in TPR and vasoconstrictor hormones. The impaired blood pressure compensation in OZ was also associated with altered baseline cardiovascular function (both baroreflex sensitivity and sympathetic vascular tone). These findings would provide novel information for understanding the altered post-hemorrhagic responses in obesity.
ACKNOWLEDGMENT:
We wish to thank Drs. Thomas E. Lohmeier and Robert L. Hester for their comments. We also wish to thank Haiyan Zhang and Lynn Lee for their technical help.
GRANTS:
This work was supported by AHA-12SDG12050525, NIH HL-51971, HL-89581, and T32 HL-105324.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
We have no conflicts to disclose.
REFERENCES
- Boku A, Sugimura M, Morimoto Y, Hanamoto H, Niwa H. Hemodynamic and autonomic response to acute hemorrhage in streptozotocin-induced diabetic rats. Cardiovascular diabetology. 2010;9:78. doi: 10.1186/1475-2840-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brough RB, Jr., Cowley AW, Jr., Guyton AC. Quantitative analysis of the acute response to haemorrhage of the renin-angiotensin-vasoconstrictor feedback loop in areflexic dogs. Cardiovasc Res. 1975;9:722–33. doi: 10.1093/cvr/9.6.722. [DOI] [PubMed] [Google Scholar]
- Campagna JA, Carter C. Clinical relevance of the Bezold-Jarisch reflex. Anesthesiology. 2003;98:1250–60. doi: 10.1097/00000542-200305000-00030. [DOI] [PubMed] [Google Scholar]
- Carlson SH, Shelton J, White CR, Wyss JM. Elevated sympathetic activity contributes to hypertension and salt sensitivity in diabetic obese Zucker rats. Hypertension. 2000;35:403–8. doi: 10.1161/01.hyp.35.1.403. [DOI] [PubMed] [Google Scholar]
- Carlsson S, Skarphedinsson JO, Delle M, Hoffman P, Thoren P. Reflex changes in post- and preganglionic sympathetic adrenal nerve activity and postganglionic sympathetic renal nerve activity upon arterial baroreceptor activation and during severe haemorrhage in the rat. Acta physiologica Scandinavica. 1992;144:317–23. doi: 10.1111/j.1748-1716.1992.tb09300.x. [DOI] [PubMed] [Google Scholar]
- Coenraad MJ, Frolich M, Meinders AE. Physiologic hyperleptinemia in obesity does not affect vasopressin secretion in acute hypo- or hyperosmolality. Clin Invest Med. 2009;32:E293. doi: 10.25011/cim.v32i6.10665. [DOI] [PubMed] [Google Scholar]
- Convertino VA, Rickards CA, Ryan KL. Autonomic mechanisms associated with heart rate and vasoconstrictor reserves. Clin Auton Res. 2012;22:123–30. doi: 10.1007/s10286-011-0151-5. [DOI] [PubMed] [Google Scholar]
- Cowley AW, Jr., Switzer SJ, Guinn MM. Evidence and quantification of the vasopressin arterial pressure control system in the dog. Circulation research. 1980;46:58–67. doi: 10.1161/01.res.46.1.58. [DOI] [PubMed] [Google Scholar]
- Davis G. Baroreflex and somato-reflex control of blood pressure, heart rate and renal sympathetic nerve activity in the obese Zucker rat. Exp Physiol. 2011;96:623–34. doi: 10.1113/expphysiol.2011.057638. [DOI] [PubMed] [Google Scholar]
- Eikelis N, Schlaich M, Aggarwal A, Kaye D, Esler M. Interactions between leptin and the human sympathetic nervous system. Hypertension. 2003;41:1072–9. doi: 10.1161/01.HYP.0000066289.17754.49. [DOI] [PubMed] [Google Scholar]
- Frankel DA, Acosta JA, Anjaria DJ, Porcides RD, Wolf PL, Coimbra R, Hoyt DB. Physiologic response to hemorrhagic shock depends on rate and means of hemorrhage. Surg Res. 2007;143(2):276–80. doi: 10.1016/j.jss.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Frisbee JC. Impaired hemorrhage tolerance in the obese Zucker rat model of metabolic syndrome. Journal of applied physiology. 2006;100:465–73. doi: 10.1152/japplphysiol.01062.2005. [DOI] [PubMed] [Google Scholar]
- Goetz KL, Wang BC, Sundet WD. Comparative effects of cardiac receptors and sinoaortic baroreceptors on elevations of plasma vasopressin and renin activity elicited by haemorrhage. J Physiol (Paris) 1984;79:440–5. [PubMed] [Google Scholar]
- Gotshall RW. Role of Angiotensin II in Hemorrhagic Hypotension in the Rat. Ohio Acad Sci. 1982;82:177–81. [Google Scholar]
- Hall JE, Brands MW, Dixon WN, Smith MJ., Jr. Obesity-induced hypertension. Renal function and systemic hemodynamics. Hypertension. 1993;22:292–9. doi: 10.1161/01.hyp.22.3.292. [DOI] [PubMed] [Google Scholar]
- Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, et al. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. The Journal of biological chemistry. 2010;285:17271–6. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiwatari M, Nolan PL, Johnston CI. The contribution of vasopressin and angiotensin to the maintenance of blood pressure after autonomic blockade. Hypertension. 1985;7:547–53. doi: 10.1161/01.hyp.7.4.547. [DOI] [PubMed] [Google Scholar]
- Huber DA, Schreihofer AM. Attenuated baroreflex control of sympathetic nerve activity in obese Zucker rats by central mechanisms. J Physiol. 2010;588:1515–25. doi: 10.1113/jphysiol.2009.186387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliescu R. Increased blood pressure variability and impaired renal autoregulation in obese Zucker rats. FASEB J. 2007;21:13. [Google Scholar]
- Kalupahana NS, Moustaid-Moussa N. The adipose tissue renin-angiotensin system and metabolic disorders: a review of molecular mechanisms. Crit Rev Biochem Mol Biol. 2012;47:379–90. doi: 10.3109/10409238.2012.694843. [DOI] [PubMed] [Google Scholar]
- Karason K, Molgaard H, Wikstrand J, Sjostrom L. Heart rate variability in obesity and the effect of weight loss. Am J Cardiol. 1999;83:1242–7. doi: 10.1016/s0002-9149(99)00066-1. [DOI] [PubMed] [Google Scholar]
- Lee HB, Blaufox MD. Blood volume in the rat. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1985;26:72–6. [PubMed] [Google Scholar]
- Mark AL. The Bezold-Jarisch reflex revisited: clinical implications of inhibitory reflexes originating in the heart. Journal of the American College of Cardiology. 1983;1:90–102. doi: 10.1016/s0735-1097(83)80014-x. [DOI] [PubMed] [Google Scholar]
- Mark AL, Correia M, Morgan DA, Shaffer RA, Haynes WG. State-of-the-art-lecture: Obesity-induced hypertension: new concepts from the emerging biology of obesity. Hypertension. 1999;33:537–41. doi: 10.1161/01.hyp.33.1.537. [DOI] [PubMed] [Google Scholar]
- Messerli FH, Christie B, DeCarvalho JG, Aristimuno GG, Suarez DH, Dreslinski GR, et al. Obesity and essential hypertension. Hemodynamics, intravascular volume, sodium excretion, and plasma renin activity. Archives of internal medicine. 1981;141:81–5. doi: 10.1001/archinte.141.1.81. [DOI] [PubMed] [Google Scholar]
- Morgan DA, Anderson EA, Mark AL. Renal sympathetic nerve activity is increased in obese Zucker rats. Hypertension. 1995;25:834–8. doi: 10.1161/01.hyp.25.4.834. [DOI] [PubMed] [Google Scholar]
- Morita H, Vatner SF. Effects of hemorrhage on renal nerve activity in conscious dogs. Circulation research. 1985;57:788–93. doi: 10.1161/01.res.57.5.788. [DOI] [PubMed] [Google Scholar]
- Mueller PJ. Influence of sedentary versus physically active conditions on regulation of plasma renin activity and vasopressin. American journal of physiology Regulatory, integrative and comparative physiology. 2008;295:R727–32. doi: 10.1152/ajpregu.00144.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation. 1998;98:772–6. doi: 10.1161/01.cir.98.8.772. [DOI] [PubMed] [Google Scholar]
- Nelson J, Billeter AT, Seifert B, Neuhaus V, Trentz O, Hofer CK, et al. Obese trauma patients are at increased risk of early hypovolemic shock: a retrospective cohort analysis of 1,084 severely injured patients. Crit Care. 2012;16:R77. doi: 10.1186/cc11334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmers PJ, Vidts W, Ameloot K, Cordemans C, Van Regenmortel N, De Laet I, et al. Assessment of three minimally invasive continuous cardiac output measurement methods in critically ill patients and a review of the literature. Anaesthesiol Intensive Ther. 2012;44:188–99. [PubMed] [Google Scholar]
- Quail AW, Woods RL, Korner PI. Cardiac and arterial baroreceptor influences in release of vasopressin and renin during hemorrhage. The American journal of physiology. 1987;252:H1120–6. doi: 10.1152/ajpheart.1987.252.6.H1120. [DOI] [PubMed] [Google Scholar]
- Robinson GJ, Peyton PJ, Vartuli GM, Burfoot RB, Junor PA. Continuous measurement of cardiac output by inert gas throughflow: comparison with thermodilution. J Cardiothorac Vasc Anesth. 2003;17:204–10. doi: 10.1053/jcan.2003.48. [DOI] [PubMed] [Google Scholar]
- Schmid-Schonbein GW, Hugli TE. A new hypothesis for microvascular inflammation in shock and multiorgan failure: self-digestion by pancreatic enzymes. Microcirculation. 2005;12:71–82. doi: 10.1080/10739680590896009. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Hair CD, Stepp DW. Reduced plasma volume and mesenteric vascular reactivity in obese Zucker rats. American journal of physiology Regulatory, integrative and comparative physiology. 2005;288:R253–61. doi: 10.1152/ajpregu.00498.2004. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Mandel DA, Mobley SC, Stepp DW. Impairment of sympathetic baroreceptor reflexes in obese Zucker rats. American journal of physiology Heart and circulatory physiology. 2007;293:H2543–9. doi: 10.1152/ajpheart.01201.2006. [DOI] [PubMed] [Google Scholar]
- Sharma PK, Madan K, Garg PK. Hemorrhage in acute pancreatitis: should gastrointestinal bleeding be considered an organ failure? Pancreas. 2008;36:141–5. doi: 10.1097/MPA.0b013e318158466e. [DOI] [PubMed] [Google Scholar]
- Shibao C, Gamboa A, Diedrich A, Ertl AC, Chen KY, Byrne DW, et al. Autonomic contribution to blood pressure and metabolism in obesity. Hypertension. 2007;49:27–33. doi: 10.1161/01.HYP.0000251679.87348.05. [DOI] [PubMed] [Google Scholar]
- Skoog P, Mansson J, Thoren P. Changes in renal sympathetic outflow during hypotensive haemorrhage in rats. Acta physiologica Scandinavica. 1985;125:655–60. doi: 10.1111/j.1748-1716.1985.tb07768.x. [DOI] [PubMed] [Google Scholar]
- Struck J, Muck P, Trubger D, Handrock R, Weidinger G, Dendorfer A, et al. Effects of selective angiotensin II receptor blockade on sympathetic nerve activity in primary hypertensive subjects. Journal of hypertension. 2002;20:1143–9. doi: 10.1097/00004872-200206000-00026. [DOI] [PubMed] [Google Scholar]
- Truett AA, Borne AT, Poincot MA, West DB. Autonomic control of blood pressure and heart rate in obese hypertensive dogs. The American journal of physiology. 1996;270:R541–9. doi: 10.1152/ajpregu.1996.270.3.R541. [DOI] [PubMed] [Google Scholar]
- van Herwaarden CL, Binkhorst RA, Fennis JF, van't Laar A. Reliability of the cardiac output measurement with the indirect Fick-principle for CO2 during exercise. Pflugers Archiv : European journal of physiology. 1980;385:21–3. doi: 10.1007/BF00583910. [DOI] [PubMed] [Google Scholar]
- Van Vliet BN, Hall JE, Mizelle HL, Montani JP, Smith MJ., Jr. Reduced parasympathetic control of heart rate in obese dogs. The American journal of physiology. 1995;269:H629–37. doi: 10.1152/ajpheart.1995.269.2.H629. [DOI] [PubMed] [Google Scholar]
- West DB, Prinz WA, Francendese AA, Greenwood MR. Adipocyte blood flow is decreased in obese Zucker rats. The American journal of physiology. 1987;253:R228–33. doi: 10.1152/ajpregu.1987.253.2.R228. [DOI] [PubMed] [Google Scholar]
- Windham BG, Fumagalli S, Ble A, Sollers JJ, Thayer JF, Najjar SS, et al. The Relationship between Heart Rate Variability and Adiposity Differs for Central and Overall Adiposity. J Obes. 2012;2012:149516. doi: 10.1155/2012/149516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang L, Lu S, Fuller W, Aneja A, Russell GV, Jones LB, et al. Impaired blood pressure recovery to hemorrhage in obese Zucker rats with orthopedic trauma. American journal of physiology Heart and circulatory physiology. 2012;302:H340–8. doi: 10.1152/ajpheart.00439.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang L, Naik J, Hester RL. Exercise-induced increase in skeletal muscle vasodilatory responses in obese Zucker rats. American journal of physiology Regulatory, integrative and comparative physiology. 2005;288:R987–91. doi: 10.1152/ajpregu.00702.2004. [DOI] [PubMed] [Google Scholar]