To the Editor
Host response against infectious challenge is typically mediated by specialized subsets of CD4+ effector T helper cells. Th1 T-cells producing IFNγ mediate cellular immunity against intracellular pathogens, while Th2 T-cells producing IL-4 and IL-13 mediate humoral immunity against parasites and helminthes. Th17 T-cells producing IL-17 confer early protection against pathogenic insult in epithelial surfaces by inducing neutrophil-mediated immune responses, and enhanced production of anti-microbial peptides (AMPs) such as β-defensins, lipocalin, and S100 proteins.1, 2
Atopic dermatitis (AD) and psoriasis vulgaris are inflammatory skin diseases that share some similarities, i.e. acanthotic epidermis and abundant dermal mononuclear infiltrate, in the chronic phase. However, one striking difference between these two diseases is the high frequency of recurrent skin infections in AD3 versus an increased resistance to infections in psoriasis.4 AD skin is heavily colonized by toxin-producing Staphylococcus aureus,5 and is more prone to cutaneous viral infections, such as eczema vaccinatum and eczema herpeticum.6 This has been attributed to the relative deficiency of innate-defense molecules, including AMPs,7 primarily regulated by IL-17.2
The role of Th17 cells and IL-17 in AD is still unclear. Increased IL-17+ T-cells have been identified in tissue sections of acute AD lesions relative to normal skin.8 Our previous work on chronic AD lesions also demonstrated increased IL-17 expression compared to normal skin, but the over-all expression of the IL-23/Th17 pathway is much reduced when compared to psoriasis.9 To clarify whether AMP deficiency in AD was due to (1) absence of cognate IL-17 receptors, (2) defective signaling, or (3) Th2 suppression, we obtained lesional and non-lesional skin from 18 chronic AD patients and 15 healthy volunteers under a Rockefeller University IRB-approved protocol. Biopsy samples were processed for histology, RT-PCR analysis or generation of primary keratinocyte (KC) cultures. Normal human keratinocytes were also obtained as previously described2 (see OR Methods for details).
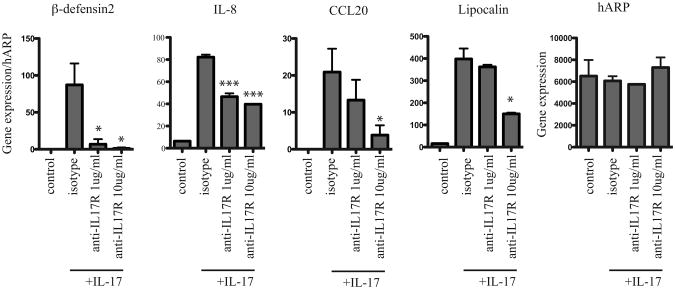
We have previously shown that IL-17 strongly induces keratinocyte expression of innate defense molecules. To determine whether functional IL-17 receptors were essential for the epidermal response to IL-17, we cultured pooled human keratinocytes with 1μg/ml or 10 μg/ml monoclonal IL-17RA blocking antibody prior to stimulation with IL-17. After 24 hours, keratinocytes were harvested and examined for expression of known IL-17-induced innate defense genes. Figure 1 demonstrates that functional blockade of IL-17RA substantially inhibits expression of β-defensin2 (DEFB4), interleukin 8 (IL-8), chemokine C-C motif ligand 20 (CCL20), and lipocalin2 (LCN2) (Figure 1). While all four genes were substantially inhibited by 10 μg/ml of IL-17RA blocking antibody, 1μg/ml of IL-17RA blocking antibody was sufficient to suppress keratinocyte mRNA expression of DEFB4 and IL-8, with less effective inhibition of CCL20 and LCN2 expression, compared to isotype-blocked keratinocytes (Figure 1). The housekeeping gene human acidic ribosomal protein (hARP) was used to control for general keratinocyte gene expression effects. These results demonstrate that fully functional IL-17 receptors are necessary for IL-17-mediated barrier defense. The differential sensitivity of AMP expression to IL-17RA blockade necessitates further investigation. One possibility for residual expression of AMP despite IL-17RA blockade is signaling through human IL-17RC, another receptor from the IL-17R family that can bind IL-17.
Figure 1. IL-17R blockade dampens keratinocyte expression of IL-17 induced innate defense genes.
Quantitative RT-PCR analysis of β-defensin2, IL-8, CCL20, lipocalin and hARP in keratinocytes cultured with IL-17 ± IL-17RA blocking antibody. Data normalized to hARP housekeeping gene and expressed as mean and SEM; *p<0.05, **p<0.01, ***p<0.005 compared to IL-17 + isotype control.
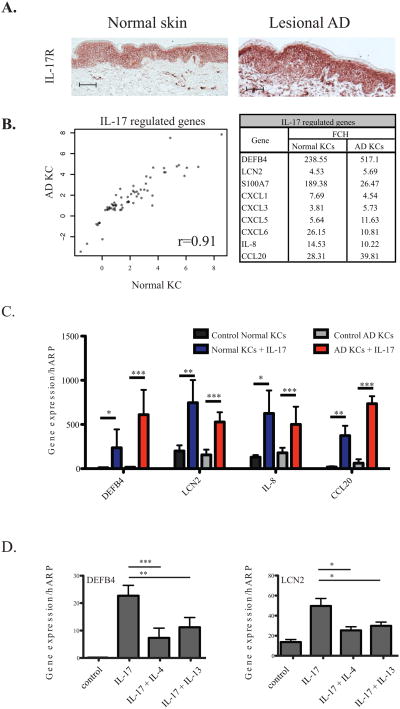
We noted strong cell-surface expression of IL-17R in lesional AD KCs(n=5) by immunohistochemistry, with a staining pattern that is consistent with normal KCs (n=5) (Figure 2A). In addition to KCs, scattered dermal cells were noted to express IL-17R as previously described.2 Consistent with immunohistochemical findings, gene expression levels for IL-17R measured by quantitative RT-PCR were similar between AD and normal KCs (Supplementary Figure 1).
Figure 2.
(A) IL-17 receptors are expressed by normal and atopic dermatitis (AD) keratinocytes (KCs). (B) Genomic analysis demonstrates linear correlation between Normal and AD KCs after IL-17 stimulation. Table shows modulation of selected genes. (C) RT-PCR analysis confirm up-regulation of anti-microbial peptides (AMP) by AD KCs after IL-17 stimulation. (D) Th2 cytokines IL-4 and IL-13 suppress IL-17-induced AMP production.
After establishing surface expression of IL-17R in AD KCs, we assessed whether these cells can sufficiently respond to IL-17 stimulation by genomic analysis (see OR Methods). Genes with fold change (FCH) >1.5 and false discovery rate (FDR) <0.1 in IL-17-treated versus untreated AD KCs were considered differentially expressed (Supplementary table 1). The AD gene set was subsequently compared against previously-published IL-17-modulated genes in normal KCs.2
We found strong positive correlation between IL-17 modulated gene sets generated from AD KCs and normal KCs (r=0.91) (Figure 2B). The close similarity between AD and normal KC gene sets was further established by the computed connectivity score10, CS=0.904 (p value < 10−16), wherein a perfect agreement between the two gene sets would yield a value of 1, while a perfect disagreement would be (-1). Importantly, AD keratinocytes stimulated with IL-17 had robust up-regulation of AMPs β-defensin2 (DEFB4) (517-fold from control) and lipocalin2 (LCN2) (5.7-fold from control), similar to normal keratinocytes (Figure 2B). Pro-inflammatory neutrophil chemoattractants, CXCL1, 3, 5, 6 and 8, as well as CCR6+ lymphocyte chemoattractant CCL20, were likewise strongly induced in both AD and normal KCs (Figure 2B). This was verified by quantitative RT-PCR analysis showing that IL-17 significantly upregulated DEFB4, LCN2, IL-8 and CCL20 expression in AD KCs (p<0.005) as it would in normal KCs (Figure 2C).
Despite increased IL-17 levels compared to normal skin,8, 9, 11 and equivalent capacity to respond to cytokine stimulation, AD lesions still have minimal DEFB4 and LCN2 gene expression.9 To determine whether the Th2 cytokine milieu within AD lesions may have an effect towards IL-17 induced AMP expression, we cultured normal keratinocytes with IL-17 (200 ng/ml) alone or in combination with IL-4 (200 ng/ml) or IL-13 (200 ng/ml), and examined for gene expression of the aforementioned AMPs. We found that both IL-4 and IL-13 can significantly antagonize IL-17 induction of DEFB4 and LCN2 in keratinocytes (Figure 2D) as previously reported.12 Thus, decreased innate defense molecule expression in AD is derived, not from an inherent inability to respond to Th17 cytokine stimulation, but likely from low ligand levels, compounded by further suppressive action from the Th2 microenvironment. Our findings have interesting implications for translational research as future therapeutics directed at increasing signaling through intact IL-17 receptors, despite low ligand expression, may potentially reverse this relative AMP deficiency in AD. One such approach might be Th2 blockade, allowing existing IL-17 to fully function without suppression, potentially restoring AMP expression and alleviating microbial load in AD.
Supplementary Material
Acknowledgments
This publication was made possible by grant number 5UL1RR024143-03 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Abbreviations
- AD
Atopic dermatitis
- AMP
Anti-microbial peptide
- CS
Connectivity score
- hARP
Human acidic ribosomal protein
- KC
Keratinocyte
- RT-PCR
Reverse transcriptase polymerase chain reaction
Footnotes
The authors state no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iwakura Y, Nakae S, Saijo S, Ishigame H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev. 2008;226:57–79. doi: 10.1111/j.1600-065X.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- 2.Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suarez-Farinas M, Cardinale I, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008;159:1092–102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leung DY, Bieber T. Atopic dermatitis. Lancet. 2003;361:151–60. doi: 10.1016/S0140-6736(03)12193-9. [DOI] [PubMed] [Google Scholar]
- 4.Henseler T, Christophers E. Disease concomitance in psoriasis. J Am Acad Dermatol. 1995;32:982–6. doi: 10.1016/0190-9622(95)91336-x. [DOI] [PubMed] [Google Scholar]
- 5.Breuer K, S HA, Kapp A, Werfel T. Staphylococcus aureus: colonizing features and influence of an antibacterial treatment in adults with atopic dermatitis. Br J Dermatol. 2002;147:55–61. doi: 10.1046/j.1365-2133.2002.04872.x. [DOI] [PubMed] [Google Scholar]
- 6.Beck LA, Boguniewicz M, Hata T, Schneider LC, Hanifin J, Gallo R, et al. Phenotype of atopic dermatitis subjects with a history of eczema herpeticum. J Allergy Clin Immunol. 2009;124:260–9. 9 e1–7. doi: 10.1016/j.jaci.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 8.Toda M, Leung DY, Molet S, Boguniewicz M, Taha R, Christodoulopoulos P, et al. Polarized in vivo expression of IL-11 and IL-17 between acute and chronic skin lesions. J Allergy Clin Immunol. 2003;111:875–81. doi: 10.1067/mai.2003.1414. [DOI] [PubMed] [Google Scholar]
- 9.Guttman-Yassky E, Lowes MA, Fuentes-Duculan J, Zaba LC, Cardinale I, Nograles KE, et al. Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. J Immunol. 2008;181:7420–7. doi: 10.4049/jimmunol.181.10.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamb J, Crawford ED, Peck D, Modell JW, Blat IC, Wrobel MJ, et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313:1929–35. doi: 10.1126/science.1132939. [DOI] [PubMed] [Google Scholar]
- 11.Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, et al. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol. 2009;123:1244–52. doi: 10.1016/j.jaci.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eyerich K, Pennino D, Scarponi C, Foerster S, Nasorri F, Behrendt H, et al. IL-17 in atopic eczema: linking allergen-specific adaptive and microbial-triggered innate immune response. J Allergy Clin Immunol. 2009;123:59–66. doi: 10.1016/j.jaci.2008.10.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.