Abstract
FRET was used to investigate the structural and kinetic effects that PKC phosphorylations exert on Ca2+ and myosin subfragment-1 dependent conformational transitions of the cardiac thin filament. PKC phosphorylations of cTnT were mimicked by glutamate substitution. Ca2+ and S1-induced distance changes between the central linker of cTnC and the switch region of cTnI (cTnI-Sr) were monitored in reconstituted thin filaments using steady state and time resolved FRET, while kinetics of structural transitions were determined using stopped flow. Thin filament Ca2+ sensitivity was found to be significantly blunted by the presence of the cTnT(T204E) mutant, whereas pseudo-phosphorylation at additional sites increased the Ca2+-sensitivty. The rate of Ca2+-dissociation induced structural changes was decreased in the C-terminal end of cTnI-Sr in the presence of pseudo-phosphorylations while remaining unchanged at the N-terminal end of this region. Additionally, the distance between cTnI-Sr and cTnC was decreased significantly for the triple and quadruple phosphomimetic mutants cTnT(T195E/S199E/T204E) and cTnT(T195E/S199E/T204E/T285E), which correlated with the Ca2+-sensitivity increase seen in these same mutants. We conclude that significant changes in thin filament Ca2+-sensitivity, structure and kinetics are brought about through PKC phosphorylation of cTnT. These changes can either decrease or increase Ca2+-sensitivity and likely play an important role in cardiac regulation.
Keywords: PKC phosphorylation, cardiac troponin T, FRET, thin filament regulation
Introduction
In cardiac muscle, Ca2+-activation of myofilaments is linked to the thin filament, a structure composed of the heterotrimeric troponin (cTn) complex and tropomyosin (Tm) bound to the double helical actin filament [1, 2]. cTn is formed from subunits cTnC, cTnI, and cTnT, wherein cTnC binds Ca2+, cTnI binds actin and inhibits actomyosin ATPase activity in relaxed muscle, and cTnT anchors the cTn complex on the actin filament [3]. Tm forms elongated dimeric coiled-coil α-helices that wind around the actin filament and form two continuous strands on actin's surface through head-to-tail contacts occurring in a short overlap region. Each cTn is located at the overlap between adjacent Tm’s. These arrangements give rise to a stoichiometry of cTn•Tm•actin7; this is the structural regulatory unit, which not only serves as the basic repeating structure from which the thin filament is built, but also governs Ca2+-induced generation of force. In relaxed muscle and at a resting concentration of Ca2+, cTnI-Tm acts as a regulatory switch that prevents the attachment of cross-bridges to actin through steric blocking of myosin head (S1)-binding sites on actin [4–6]. To activate actomyosin ATPase, and thereby generate force, the intracellular [Ca2+] rises to saturate the regulatory sites in cTn. This induces a series of intramolecular and intermolecular structural changes in the thin filament beginning with opening of the N-domain of cTnC (N-cTnC) and subsequent interaction between N-cTnC and the switch region of cTnI (cTnI-Sr) [7, 8]. This changes the conformation for both the inhibitory region (cTnI-Ir) and cTnI-Sr [9–11] switching cTnI-Ir from interacting with actin to interacting with cTnC [12]. Furthermore, the interaction between the mobile domain of cTnI (cTnI-Md) and actin becomes disrupted [13, 14]. These structural changes in the core domain of cTn are propagated through cTnT to Tm leading to azimuthal movement of Tm on the actin surface [15] which permits strong cross-bridge binding. These Ca2+-induced protein structural transitions are thus the molecular basis of the cardiac thin filament regulation mechanism in which cTnT is crucial.
Because of its many interactions within the thin filament, cTnT has several important roles in sarcomeric regulation. It is necessary for full Ca2+-mediated activation/deactivation of the thin filament [16, 17] and for regulating cross-bridge recruitment dynamics and turnover kinetics [18, 19]. cTnT is composed of two distinct regions, TnT1 (residues 1–181) and TnT2 (residues 181–288), which can be produced from cleavage through mild chymotryptic digestion [20, 21]. TnT1 contains a highly acidic hypervariable Nterminal region, which has no biochemically or structurally verified binding partner, followed by a conserved central region, which interacts with the head-to-tail junction of axially joined Tm dimers through an N-terminal 39 residue Tm binding region [22]. The TnT2 fragment contains a short Tm binding region at its N-terminus, which anchors cTn onto Tm [22] and has been shown to bind Tm in a Ca2+-specific manner which is likely important for Ca2+-activation [23]. The C-terminal region of cTnT2 interacts with the N-domain of cTnI and the C-domain of cTnC to form the globular core domain of the cTn complex [24]. The core domain of cTn alone is able to regulate the interaction between S1 and actin–Tm [25]. Furthermore, the T2 region of cTnT is thought to modulate the Ca2+-sensitivity of thin filament activation since it directly interacts with cTnI and cTnC. In addition to acting as a Ca2+-sensitivity tuner, TnT2 is believed to be essential in maintaining the proper blocked state conformation of the thin filament as well as in conducting structural transitions initiated by cTnI-Sr switching to the T1 region and thereby to Tm allowing full ATPase activity. Highlighting the importance of TnT2 region is the fact that several mutations in this region are known to be causal in familial hypertrophic cardiomyopathy [26, 27]. Moreover, it is within the TnT2 region that all of the established protein kinase C (PKC) phosphorylation sites lie, these sites occurring at Thr-195, Ser-199, Thr-204 and Thr-285 in rat myocardium [28–30]. Phosphorylation at these sites modulates Ca2+-activated thin filament regulation [31]. For example, PKC phosphorylation of cTnT has been reported to induce a 50% reduction in maximum actomyosin Mg-ATPase activity in vitro [32] and a 30% reduction of maximum force of mouse cardiac fibers [33]. These effects reduce the heart’s ability to maintain normal function, suggesting that changes in the level of PKC phosphorylation of cTnT in the failing heart may contribute to its pathophysiology [30, 34, 35]. Hence, deeper insight into the mechanism by which PKC phosphorylation of TnT2 modulates thin filament regulation is urgently needed.
By incorporating recombinant Tn into skinned myocardium, Sumandea et al. demonstrated that among the four PKC phosphorylation sites of cTnT, Thr-204 is the functionally critical site for reduction in Ca2+-sensitivity, cooperativity, Mg-ATPase activity and maximum force generation [30]. However, the detailed molecular mechanism underlying the effects of PKC phosphorylation of Thr-204, as well as each of the other three residues and their combinations, is still elusive. In order to investigate how PKC phosphorylation of cTnT structurally and kinetically affects the conformational transitions in cTn that transduce Ca2+-signals into tropomyosin movement, we mimicked PKC phosphorylations via glutamate substitution mutants cTnT(S199E), cTnT(T204E), cTnT(S199E/T204E), cTnT(T195E/S199E/T204E) referred to as cTnT(3M), and cTnT(T195E/S199E/T204E/T285E) referred to as cTnT(4M). The distance of the cTnI-Sr to cTnC was monitored via steady-state and time-resolved Förster Resonance Energy Transfer (FRET) between either cTnI(S151CAEDANS)-cTnC(S89CDDPM) or cTnI(S167CAEDANS)-cTnC(S89CDDPM). We hypothesized that PKC phosphomimetic mutants of cTnT alter Ca2+-dependent binding of the cTnI-Sr to the hydrophobic patch of N-cTnC and concomitant release of cTnI-Ir and cTnI-Md from actin (collectively termed I-C switching) by changing cTnT-cTnI and/or cTnT-cTnC interactions. We reasoned that such structural changes should arise from either directly altered charge-charge interactions or propagated structural changes initiated at the TnT2-Tm binding region and conducted through the IT-arm of the cTn core domain [36]. By using FRET to measure the Ca2+-sensitivity and deactivation kinetics of I-C switching and the Ca2+- or Mg2+-state cTnI-cTnC distances, we tested our hypothesis by characterizing how each phosphomimetic mutant impacts the cTnI-Sr–N-cTnC, cTnI-Ir–actin, and the cTnI-Md–actin interactions.
Our results showed that the T204E mutant significantly decreased the Ca2+-sensitivity of I-C switching and slowed Ca2+-dissociation-induced structural transition kinetics, but no change in the proximity between cTnC and cTnI was apparent for this mutation. This result seems to indicate that T204E is able to greatly desensitize I-C switching to Ca2+ without altering average I-C separation. Additional pseudo-phosphorylation at residues Thr-195, Ser-199, and Thr-285 not only decreased the desensitizing effect of T204E, but even led to hypersensitization with cTnT(3M) and cTnT(4M) in the absence of S1. This Ca2+-sensitizing effect of cTnT(3M) and cTnT(4M) in the Mg2+ state was correlated with a significant decrease in Mg2+-state cTnI-cTnC distances as well as a reduction in "switching distance" (total Ca2+-induced change in distance between N-cTnC and cTnI-Sr). Finally, significant effects of cTnT pseudo-phosphorylations on the kinetics of Ca2+-dissociation-induced changes in N-cTnC–C-cTnI distances were confined to the structural changes sensed by cTnC(89CDDPM)-cTnI(167CAEDANS), suggesting a specific kinetic effect of cTnT on the C-terminal portion of the C-domain, particularly the mobile domain of cTnI (cTnI-Md), in response to thin filament deactivation, perhaps indicating a change in the orientation but not the distance between cTnC and cTnI along with a concomitant disruption of the fly casting mechanism [13, 14]. Taken together, these results indicate that T204E reduces the Ca2+-sensitivity of cTnI-cTnC switching through subtle structural changes not apparent from cTnI-cTnC distance measurements, whereas additional pseudo-phosphorylations compensated for this Ca2+-desensitizing effect in the absence of S1 by promoting increased, Ca2+-sensitizing interaction between cTnI-Sr and N-cTnC.
Materials and Methods
Protein sample preparation and characterization
To implement FRET in this study, a series of recombinant single cysteine mutants were generated from wild type rat protein clones using approaches similar to those previously reported [10, 13, 37, 38]. Briefly, using the GeneTailor™ Site-Directed Mutagenesis System (Invitrogen, Carlsbad, CA, USA), rat cDNA clones of wild-type cTnC, cTnI and cTnT sub-cloned into the plasmid pSBETa was used as a template DNA to generate single-cysteine cTnC and cTnI mutants and phosphomimetic mutants of cTnT. The mutants generated included: cTnI(S151C), cTnI(S167C), cTnC(S89C), cTnT(S199E), cTnT(T204E), cTnT(S199E/T204E), cTnT(T195E/S199E/T204E) and cTnT(T195E/S199E/T204E/T285E). Note that the cTnC mutant, endogenous cysteine residues Cys-35 and Cys-84 have each been substituted with serine; similarly, in the cTnI mutants, Cys-81 and Cys-98 have been substituted with serine and isoleucine, respectively. Recombinant cTnC, cTnI and cTnT clones were over expressed in E. coli strain BL21(DE3) cells and purified as previously described [7, 9, 12, 39]. All cTnI proteins were modified with 5-((((2-iodoacetyle)amino)ethyl)amino)naphthalene-1-sulfonic acid (AEDANS) as FRET donor according to previously described procedures [13]. cTnC(S89C) was purified and labeled with N-(4-dimethylamino-3,5-dinitrophenyl)maleimide (DDPM) as FRET acceptor by following a previously described procedure to produce cTnC(S89CDDPM) [13]. The labeling ratio was determined spectroscopically using ε325= 6000 cm−1M−1 for AEDANS and ε442=2930 cm−1M−1 for DDPM. Labeling ratios for all protein modification were >95%. Cardiac Tm was purified from bovine heart [40]; actin [41] and myosin subfragment-1 (S1) from the chymotryptic digestion of myosin [42] were obtained from rabbit skeletal muscle. AEDANS-labeled cTnI mutants and DDPM labeled cTnC(S89C) were reconstituted into thin filament samples with other thin filament proteins using a cTn:Tm:actin molar ratio of 1:1:7.5 and checked by native gel electrophoresis as previously reported [12, 13, 43]. The regulatory function of the labeled cTnI and cTnC mutants, used before in our laboratory, was also verified by testing their ability to participate in the Ca2+-dependent regulation of acto-S1 ATPase activity (data not shown). Multiple experiments were performed on prepared samples within 5 days, and no protein degradation was observed by electrophoretic analysis over this time period.
Steady-state fluorescence measurements
Steady-state FRET measurements were performed at 15 ± 0.1 °C on an ISS PC1 photon-counting spectrofluorometer equipped with a micro-titrator using a band pass of 3 nm on both the excitation and emission monochromators. Samples containing 1 µM protein dissolved in working buffer, which consisted of 30 mM MOPS pH 7.0, 5 mM MgCl2, 0.15 M KCl, 1 mM DTT, and 1 mM EGTA, were tested in the presence and absence of 3 mM CaCl2 both with and without ADP-Mg2+-S1 present. Samples were excited with 343 nm light and emission spectra of donor AEDANS of either donor-only or donor-acceptor samples at different conditions were recorded from 420 nm to 600 nm using a scanning emission monochromator. FRET between AEDANS and DDPM occurring in reconstituted samples was assessed by comparing the AEDANS fluorescence from AEDANS-DDPM samples with that of matching control, AEDANS-only samples.
Steady-state fluorescence Ca2+ titrations
To examine the Ca2+ sensitivity of changes in cTnC–cTnI distances within reconstituted thin filament samples, steady-state FRET was measured during Ca2+-titrations performed using the micro-titrator as described previously [13]. cTnC–cTnI distances were investigated by monitoring the changes in FRET efficiency between either (1) Cys-89DDPM of cTnC and Cys-151AEDANS of cTnI, or (2) Cys-89DDPM of cTnC and Cys-167AEDANS of CTnI. In a typical titration experiment, up to 100 data points were acquired after successively injecting aliquots of 3 µL of a 14 mM Ca2+ solution to 1.2 mL samples in a titration buffer containing 50 mM MOPS, pH 7.10, 2 mM EGTA, 5 mM nitrilotriacetic acid (NTA), 150 mM KCl, 5 mM MgCl2, and 1 mM DTT. Changes in FRET vs. Ca2+ concentrations were acquired by monitoring donor fluorescence intensity of samples at 490 nm with excitation at 343 nm. Data from Ca2+ titrations were fit using the Hill equation:
| (1) |
Where I represents steady state AEDANS intensity that is correlated to FRET efficiency since donor only fluorescence intensity is insensitive to Ca2+ concentrations, Imax AEDANS intensity under Ca2+ free conditions, Imin AEDANS intensity under Ca2+ saturated, pCa50 the −Log10([Ca2+]) at which apparent half occupancy of N-cTnC Ca2+-binding sites occurs, and nH the Hill coefficient/slope that represents the steepness of the Ca2+-dependent reduction in AEDANS fluorescence.
Time-resolved fluorescence measurements
To quantify the FRET observed in the steady-state measurements, fluorescence intensity decays of AEDANS of the reconstituted samples in the absence/presence of the acceptor under the same biochemical conditions as used for steady-state measurements were recorded with a FluoreCube (Horiba Jobin Yvon) lifetime system equipped with TBX pico-second photon detection modules. The decays of the donor-only samples were fitted with the multi-exponential function [44]:
| (2) |
where the αi represents the fractional amplitude associated with each lifetime τi. In the presence of the acceptor, the intensity decays of donor–acceptor samples were fit to Eq. 3:
| (3) |
Where r is a unique distance separating the donor and acceptor and Ro is the Förster critical distance at which the transfer efficiency is 0.5. The observed decay of an ensemble of donor-acceptor pairs is given by
| (4) |
P(r) is the probability distribution of distances and is assumed to be a Gaussian
| (5) |
where r̄ is the mean distance and σ is the standard deviation of the distribution. The half width at half maximum (hw) of the distribution is given by hw = 1.1772σ.
FRET stopped-flow measurements
FRET stopped-flow experiments were performed to examine the kinetics describing changes in the cTnC-cTnI proximity induced by Ca2+ dissociation using a T format KinTek stopped-flow spectrometer with a 1.8 ms dead time. Ca2+-saturated reconstituted thin filament samples were rapidly mixed with an equal volume of buffer containing an excess amount of the Ca2+ chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N’N’-tatraacetic acid (BAPTA) [45]. As in equilibrium FRET experiments, the time dependent change in AEDANS emission intensity was first determined for a donor only sample, followed by determination of the time dependent emission intensity for the corresponding donor-acceptor sample. A total of 8–20 kinetic traces were collected for each set of donor-only and donor-acceptor samples. The set of kinetic traces for each sample was averaged and then nonlinear regression was used to fit the averaged trace with an exponential function and determine the rate of conformation change.
Statistical analysis
t-tests were performed as a standard way to establish statistical significance between parameters of control (wild type cTnT) and mutations. Tabulated parameter values are given as averages over n trials as indicated in each table, and the standard errors or confidence intervals obtained from fitting are shown next to each value. Unless otherwise mentioned, parameters such as kinetic rates and pCa50 values were obtained through non-linear regression using the least squares method.
Results
Steady state fluorescence intensity measurements of the cTnC-cTnI interaction in cardiac thin filament
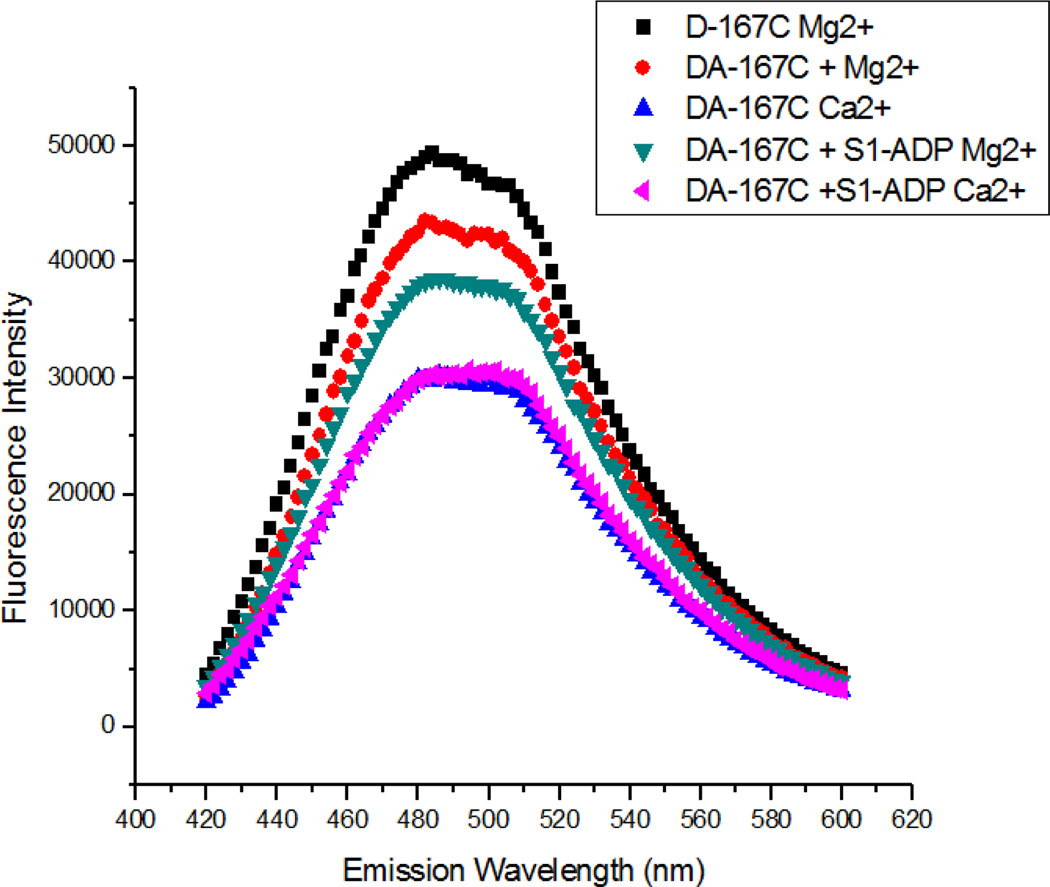
The objective of this study was to examine the structural and kinetic effects of the pseudo-phosphorylation of cTnT on thin filament regulation, specifically on the interaction between cTnC and cTnI and Ca2+-induced cTnC–cTnI switching, within reconstituted thin filaments. The proximity between cTnC and cTnI was monitored by measuring FRET between AEDANS attached to residue 151 or residue 167 of cTnI as fluorescence donor and DDPM attached to residue 89 in the central linker of cTnC as an acceptor. Fig. 1 shows typical steady-state fluorescence intensity measurements of the cTnI(S167C)AEDANS-cTnC(S89C)DDPM pair under different biochemical conditions. Peak fluorescence of cTnI(S167C)AEDANS was quenched when the acceptor, DDPM, was present in the reconstituted thin filament (comparing black curve to red curve), indicating significant FRET between the donor and acceptor. Upon Ca2+ binding to cTnC, the fluorescence was further quenched (blue trace), indicating increased FRET due to Ca2+-induced opening of the hydrophobic pocket in the N-domain of cTnC and subsequent association of this pocket with the cTnI-Sr. This brings cTnI(S167CAEDANS) and cTnI(S151CAEDANS), which are located in the cTnI-Sr, closer to cTnC(S89CDDPM), thus increasing the FRET efficiency and decreasing the fluorescence intensity of AEDANS. In the absence of Ca2+, addition of S1-ADP significantly increased FRET efficiency, indicating a decrease in separation between cTnI and cTnC (comparing red and green traces). It is known that S1 strongly binds to actin in the presence of Mg2+-ADP and that such strong-binding cross-bridges move Tm from the blocked position to the open position on actin's surface, which pushes cTnI off of actin and allows it to partially interact with cTnC [12, 46]. These structural changes are reflected by changes in fluorescence intensity (red and green curves) shown in Fig. 1. For S1-ADP containing samples, the thin filament became fully activated when saturating levels of Ca2+ were present, which is reflected by maximum quenching of donor fluorescence.
Figure 1.
Comparison of AEDANS fluorescence intensity as a function of emission wavelength for either donor-acceptor samples (DA) or donor only samples (D) under various physiological conditions. These representative traces of cTnI(S167CAEDANS)-cTnC(S89CDDPM) show substantial FRET quenching compared to donor only control in low Ca2+ conditions, with increased quenching upon addition of S1-ADP or Ca2+.
Steady state Ca2+-titrations of the cTnC-cTnI interactions within cardiac thin filament
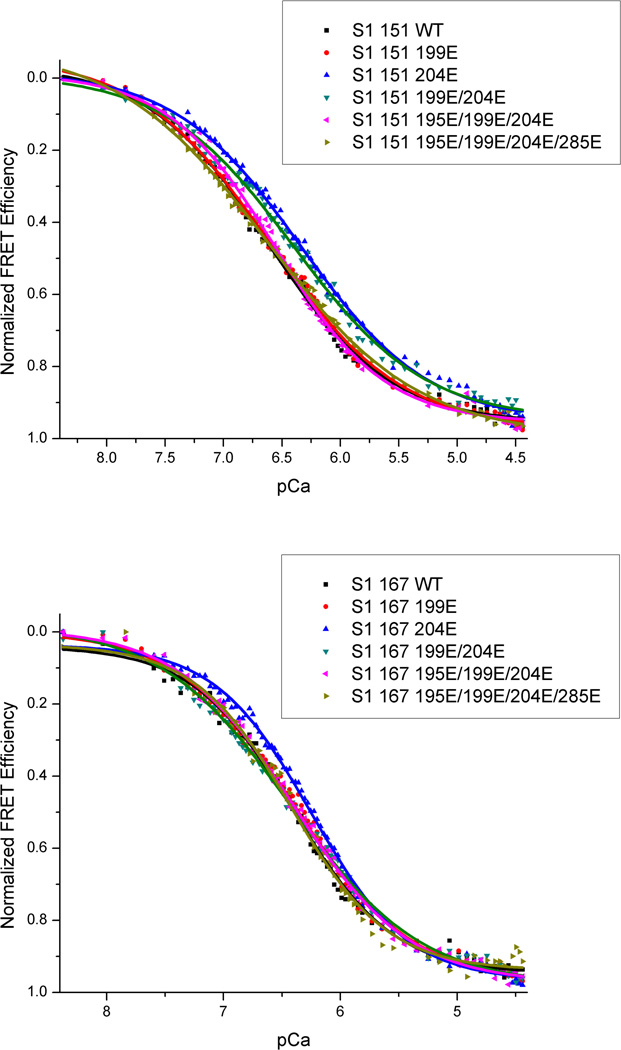
Through controlled addition of Ca2+ to thin filament samples, the change in AEDANS fluorescence intensity was monitored via spectrofluorometer as a function of [Ca2+]. Fitting these Ca2+ titrations curves with the Hill equation (Eq. 1) provided the Ca2+ sensitivity and cooperativity of the samples in the form of pCa50 and nH respectively. This allowed for comparison of the Ca2+-sensitivities and cooperativities of the various pseudo-phosphorylations of cTnT. Fig. 2 shows typical Ca2+ titration curves displayed as FRET efficiency vs. pCa (where pCa = −Log10[Ca2+]). These parameters are summarized in Table 1. It was important to establish that wild type cTnT containing thin filaments provided pCa50’s and Hill slopes in reasonable accordance with prior studies to validate the physiological relevance of our control samples and experimental conditions. Wild type cTnT containing samples yielded expected values of pCa50 while the Hill slope for the Ca2+-induced cTnC-cTnI interaction was in good agreement with previous studies from this lab, but showed relatively little cooperativity (See Table 1). The appearant lack of cooperativity seen in these reconstituted thin filaments likely results from the 7.5/1 ratio of G-actin to troponin used. This slightly higher concentration of actin ensures greater incorporation of troponin into functional regulatory units, but at the cost of cooperativity since troponin no longer occurs in every regulatory site. These same samples showed a significant increase in pCa50 upon addition of S1-ADP, consistent with previous observations [13, 38].
Figure 2.
Ca2+ titration curves for cTnI(167CAEDANS)-cTnC(89CDDPM) or cTnI(151CAEDANS)-cTnC(89CDDPM) labeled thin filaments containing either wild type or mutant cTnT. The normalized FRET efficiency increases as AEDANS and DDPM are brought into closer proximity upon addition of gradually increasing levels of Ca2+.
Table 1.
Numerical results from fitting of the Hill equation (Eq. 1) to Ca2+ titration curves for each pseudo-phosphorylation mutant and control. Parameter standard error is given to the right of each parameter value.
| cTnI:cTnT Mutants (n = 5–10) |
Thin filament | Thin filament + S1-ADP | ||
|---|---|---|---|---|
| pCa50 | nH | pCa50 | nH | |
| S151CAEDANS:wt | 6.27 ± 0.01 | 0.786 ± 0.014 | 6.50 ± 0.01 | 0.765 ± 0.014 |
| S199E | 6.29 ± 0.02 | 0.789 ± 0.019 | 6.49 ± 0.02 | 0.744 ± 0.014 |
| T204E | 6.09 ± 0.02 | 0.853 ± 0.01 | 6.22 ± 0.01 | 0.716 ± 0.018 |
| S199E/T204E | 6.18 ± 0.02 | 0.731 ± 0.012 | 6.28 ± 0.02 | 0.714 ± 0.014 |
| T195E/S199E/T204E | 6.36 ± 0.03 | 0.831 ± 0.017 | 6.43 ± 0.01 | 0.741 ± 0.016 |
| T195E/S199E/T204E/T285E | 6.47 ± 0.03 | 0.711 ± 0.019 | 6.53 ± 0.02 | 0.683 ± 0.022 |
| S167CAEDANS:wt | 6.11 ± 0.02 | 1.03 ± 0.03 | 6.28 ± 0.01 | 0.773 ± 0.017 |
| S199E | 6.03 ± 0.01 | 0.881 ± 0.024 | 6.26 ± 0.02 | 0.826 ± 0.018 |
| T204E | 5.98 ± 0.02 | 1.14 ± 0.04 | 6.12 ± 0.01 | 0.843 ± 0.016 |
| S199E/T204E | 6.11 ± 0.01 | 1.13 ± 0.04 | 6.31 ± 0.02 | 0.705 ± 0.018 |
| T195E/S199E/T204E | 6.17 ± 0.01 | 1.11 ± 0.03 | 6.28 ± 0.01 | 0.759 ± 0.017 |
| T195E/S199E/T204E/T285E | 6.21 ± 0.01 | 0.924 ± 0.013 | 6.29 ± 0.02 | 0.863 ± 0.019 |
Pseudo-phosphorylations of cTnT showed diverse site-specific effects on the Ca2+ sensitivity of the cTnC–cTnI interaction. For cTnT(S199E), little overall effect on the Ca2+ sensitivity of I-C switching was seen compared to control, with the exception of the cTnI(S167C)AEDANS-cTnC(S89C)DDPM pair in the Mg2+-state where desensitization was observed. In contrast, cTnT(T204E) consistently showed the greatest degree of Ca2+ desensitization both with and without S1-ADP for the structural transitions sensed either by cTnI(S151CAEDANS) or cTnI(S167CAEDANS). These observations are consistent with a prior study by Sumandea et al. in demembranated cardiac muscle fibers which identified Thr-204 of cTnT as a critical site for phosphorylation-induced depression of Ca2+-sensitivity and force [30]. Interestingly, in our study we observed that pseudo-phosphorylation of Ser-199 appeared to mitigate T204E-induced Ca2+-desensitization when both phosphomimetic mutations were present in cTnT. In the absence of S1-ADP, pseudo-phosphorylation of Thr-195 and Thr-285 actually elevated pCa50 values above those of control. These Ca2+-sensitizing effects were absent when strongly-bound S1-ADP was present, with Ca2+ sensitivity levels returning to those seen in control. This implied that pseudo-phosphorylation of Thr-195 and Thr-285 counteract the Ca2+-desensitizing effect of Thr-204 by promoting increased Ca2+-sensitizing interaction between cTnI-Sr and N-cTnC, as this is the same mechanism by which strong-binding cross-bridges exert an activating, positive feedback on thin filament regulation [47]. It was also observed in the study by Sumandea et al., which most closely resembles this study in the current literature, that pseudo-phosphorylations at Thr-195 and/or Thr-285 significantly reduced the desensitization caused by the T204E mutation alone. Our differing observation of a hypersensitization in the Mg2+ and Ca2+ states, an effect not observed by Sumandea et al., is explained by the fact that cross-bridge cycling, and therefore positive feedback, is was present during activation of their demembranated fibers. Thus our results with S1-ADP present would be expected to more closely resemble the findings of Sumandea et al. wherein T195E and T285E mitigated Ca2+-desensitization by T204E, which is the case.
The cooperativity of Ca2+-dependent changes in the cTnC–cTnI distances as given by nH, reflect decreased allostery for samples with S1-ADP present, as would be expected since S1-ADP is able to bind to actin in the absence of Ca2+ thus displacing cTnI from actin and moving Tm to the open position [48]. Therefore, pre-incubation with S1-ADP removes the longitudinal cooperativity usually brought about through Tm as this mechanism of cooperativity is already saturated at the start of the titration by the structural effects of S1-ADP binding [47]. Comparing the cooperativity of the PKC phosphomimetic cTnT mutants with control showed that for cTnT(T204E) containing thin filaments an increased cooperativity was seen, which was blunted both by the presence of S1-ADP as well as by pseudo-phosphorylations at additional sites. Since the presence of S1-ADP mitigated the increased cooperativity seen in cTnT(T204E) containing thin filaments, this suggests cTnT(T204E) impacts the Ca2+-binding cooperativity through the steric conduction of the structural changes occurring in the core of cTn to Tm, which would no longer be a factor upon addition of S1-ADP as discussed earlier. Meanwhile, though S1-ADP exerted a Ca2+-sensitizing effect on samples containing cTnT(T204E), the Ca2+-sensitivity of cTnT(T204E) samples was nevertheless decreased compared to control in the presence of S1-ADP, implying that this Ca2+-desensitization mechanism is not directly related to cTnI-Sr–N-cTnC interaction but is more likely related to the IT-arm, which alters the orientation of cTnI and cTnC relative to each other. For reasons we will discuss in coming sections, a more general stabilization of the blocked state conformation of cTn by cTnT(T204E) is unlikely to be involved in the mechanism underlying Ca2+-desensitization. The sites responsible for Ca2+ sensitization (Thr-195 and Thr-285) did not significantly alter the cooperativity of the cTnC-cTnI interaction.
Distance distribution analysis for the cTnC-cTnI interaction in cardiac thin filament
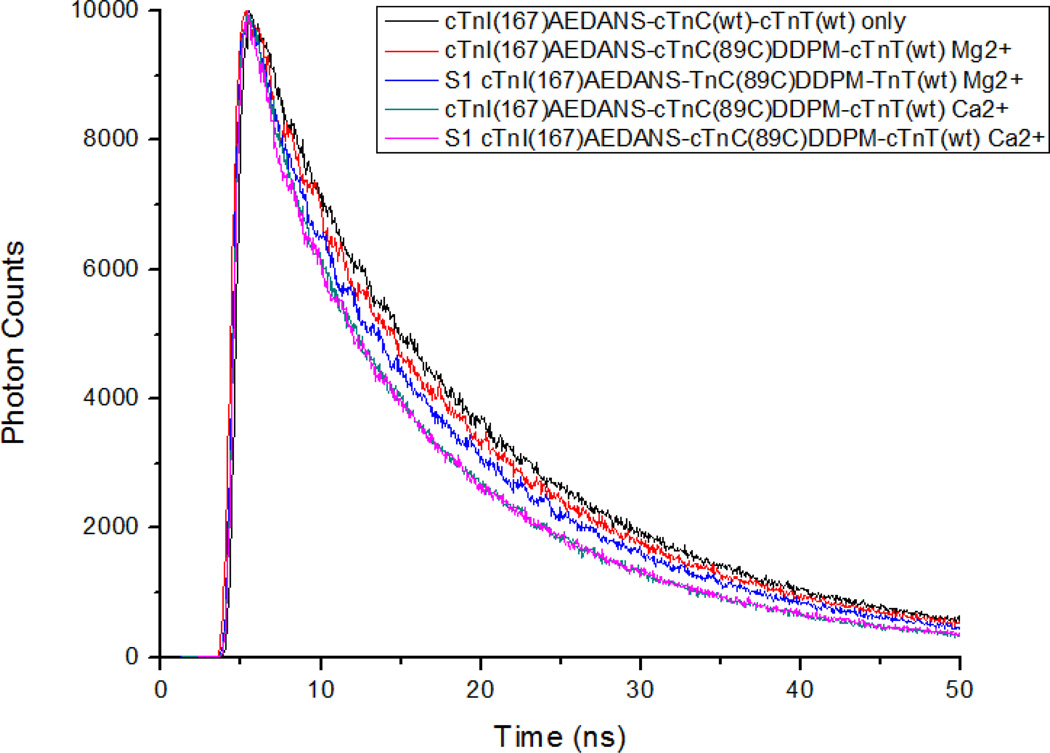
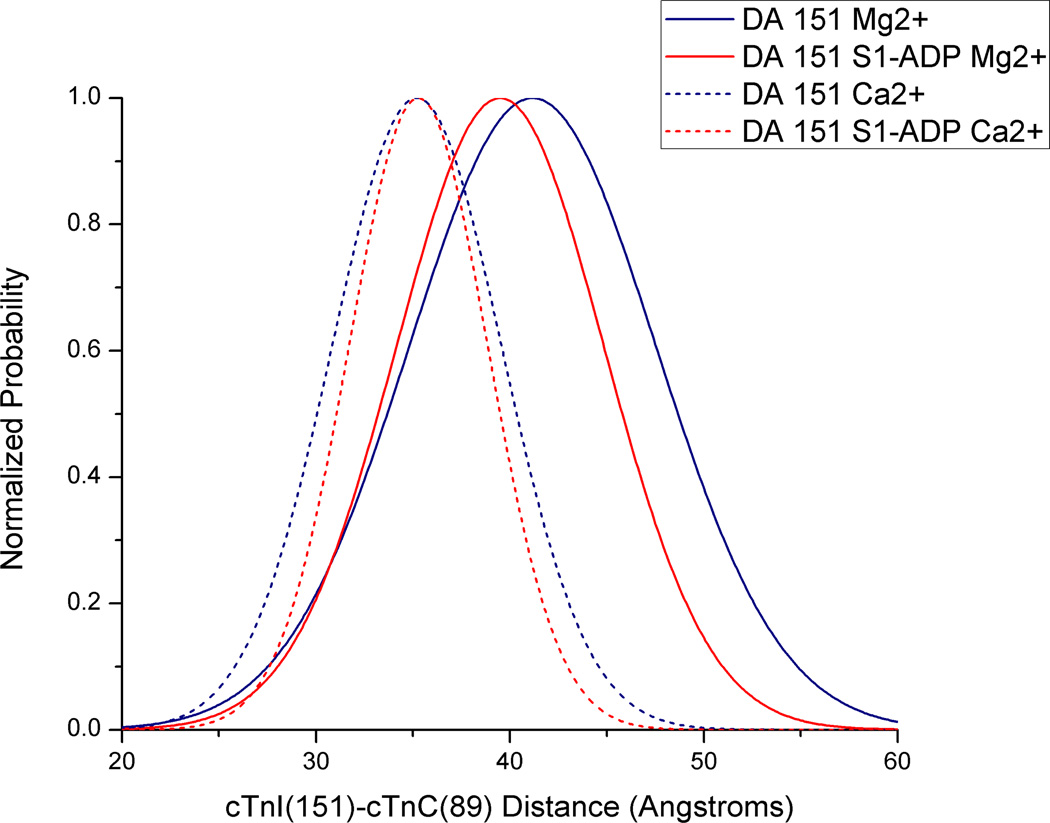
We next examined the structural effects of PKC pseudo-phosphorylations of cTnT on cTnC–cTnI distances by fitting FRET distance distributions to time-resolved fluorescence intensity decays. This was done to better understand how structural changes specific to our phosphomimetic cTnT mutations might correlate with the observed changes in Ca2+-sensitivity. Fig. 3 shows a typical set of AEDANS fluorescence intensity decays observed in reconstituted thin filament samples in the absence or presence of acceptor under different biochemical conditions. The decays underwent FRET distance distribution analysis to derive r̄ and hw values using GlobalCurve (Eq. 5) [12]. A representative distance distribution is shown in Fig. 4 for a thin filament sample containing cTnI(S151CAEDANS)-cTnC(S89CDDPM)-cTnT(wt). All other sets of time-resolved intensity decays acquired for the different PKC pseudo-phosphorylations of cTnT were analyzed, and the recovered r̄ and hw parameter values are summarized in Tables 2 and 3.
Figure 3.
Representative fluorescence decays for donor only and cTnI(S167CAEDANS)-cTnC(S89CDDPM) labeled thin filaments. Donor-acceptor decays are given for four physiological conditions; with Ca2+ or without Ca2+, and with or without S1-ADP. The donor only decay gives the longest lifetime followed by donor-acceptor (DA) without Ca2+. Addition of S1-ADP decreases the lifetime in the Ca2+ free state but not Ca2+ saturated conditions and addition of Ca2+ decreased the lifetime for all DA samples with or without S1-ADP. Donor only samples were unchanged for all conditions as was expected.
Figure 4.
Representative distance distributions derived by GlobalCurve fitting of fluoresence intensity decay data. Traces are for cTnI(S151CAEDANS)-cTnC(S89CDDPM)-cTnT(WT) samples in low Ca2+ without S1-ADP (solid blue trace), in low Ca2+ with S1-ADP present (solid red trace) and both with and without S1-ADP present under saturating levels of Ca2+ (solid red and blue traces respectively).
Table 2.
Ca2+ and S1-dependent changes in distance between cTnI(S151CAEDANS) and cTnC(S89CDDPM) in reconstituted cardiac thin filaments.
|
cTnI:cTnT Mutants (n=4–6) |
Thin filament + Mg2+ | Thin filament + S1-ADP + Mg2+ | ||
| r̄ | hw | r̄ | hw | |
| S151CAEDANS:WT | 41.2 ± 2.0 | 15.0 ± 2.2 | 39.5 ± 3.8 | 12.6 ± 4.6 |
| S199E | 40.7 ± 2.0 | 14.7 ± 2.2 | 39.0 ± 2.7 | 12.3 ± 3.2 |
| T204E | 40.3 ± 2.4 | 14.2 ± 2.4 | 38.4 ± 3.0 | 11.1 ± 3.5 |
| S199E/T204E | 39.9 ± 2.6 | 13.7 ± 2.9 | 38.9 ± 2.7 | 12.0 ± 2.9 |
| T195E/S199E/T204E | 38.8 ± 2.1* | 12.7 ± 2.5* | 37.9 ± 3.3 | 10.9 ± 3.7 |
| T195E/S199E/T204E/T285E | 38.2 ± 2.8* | 12.0 ± 3.1* | 37.2 ± 3.3* | 10.1 ± 3.5 |
| Thin filament + Ca2+ | Thin filament + S1-ADP + Ca2+ | |||
| r̄ | hw | r̄ | hw | |
| S151CAEDANS:WT | 35.2 ± 1.3 | 10.3 ± 3.4 | 35.3 ± 2.4 | 8.4 ± 2.8 |
| S199E | 34.8 ± 1.4 | 9.67 ± 3.1 | 34.8 ± 1.9 | 8.3 ± 1.9 |
| T204E | 35.1 ± 1.7 | 8.68 ± 1.8 | 34.7 ± 2.2 | 8.2 ± 2.4 |
| S199E/T204E | 34.8 ± 1.5 | 8.41 ± 1.7 | 34.8 ± 2.2 | 8.5 ± 2.1 |
| T195E/S199E/T204E | 34.4 ± 1.6 | 7.90 ± 1.8 | 34.2 ± 2.3 | 7.7 ± 2.4 |
| T195E/S199E/T204E/T285E | 34.1 ± 2.1* | 7.70 ± 2.3 | 33.6 ± 3.0* | 7.1 ± 3.4 |
Indicates a significant difference from wild type at the 90% level while
indicates significance at the 95% level.
Table 3.
Ca2+ and S1-dependent changes in distance between cTnI(S167CAEDANS) and cTnC(S89CDDPM) in reconstituted cardiac thin filaments.
|
cTnI:cTnT Mutants (n=3–6) |
Thin filament + Mg2+ | Thin filament + S1-ADP + Mg2+ | ||
| r̄ | hw | r̄ | hw | |
| S167CAEDANS:WT | 42.8 ± 1.0 | 16.3 ± 0.8 | 41.9 ± 3.0 | 14.8 ± 3.4 |
| S199E | 41.8 ± 1.1* | 15.9 ± 1.2 | 40.2 ± 2.1* | 13.3 ± 2.3 |
| T204E | 42.3 ± 0.8 | 16.3 ± 0.9 | 40.8 ± 2.5* | 14.1 ± 2.7 |
| S199E/T204E | 41.7 ± 1.2* | 15.6 ± 1.5 | 40.3 ± 2.7* | 13.5 ± 2.9 |
| T195E/S199E/T204E | 40.5 ± 1.1** | 14.2 ± 1.2** | 39.4 ± 2.6** | 11.6 ± 3.1* |
| T195E/S199E/T204E/T285E | 40.5 ± 1.6** | 14.1 ± 1.7** | 39.0 ± 2.7** | 11.2 ± 3.0* |
| Thin filament + Ca2+ | Thin filament + S1-ADP + Ca2+ | |||
| r̄ | hw | r̄ | hw | |
| S167CAEDANS:WT | 34.5 ± 1.3 | 7.71 ± 1.2 | 34.6 ± 1.2 | 7.5 ± 1.1 |
| S199E | 34.7 ± 1.1 | 7.79 ± 1.3 | 34.5 ± 1.2 | 7.8 ± 1.3 |
| T204E | 34.7 ± 1.2 | 7.86 ± 1.5 | 34.8 ± 1.1 | 8.0 ± 1.2 |
| S199E/T204E | 34.6 ± 1.1 | 7.70 ± 1.3 | 34.5 ± 1.8 | 6.2 ± 2.1* |
| T195E/S199E/T204E | 34.0 ± 1.2* | 7.22 ± 1.2** | 33.9 ± 1.6** | 7.0 ± 1.8** |
| T195E/S199E/T204E/T285E | 34.0 ± 1.3** | 7.15 ± 1.3** | 34.0 ± 1.5 | 7.2 ± 1.7* |
Indicates a significant difference from wild type at the 90% confidence level while
indicates significance at the 95% level.
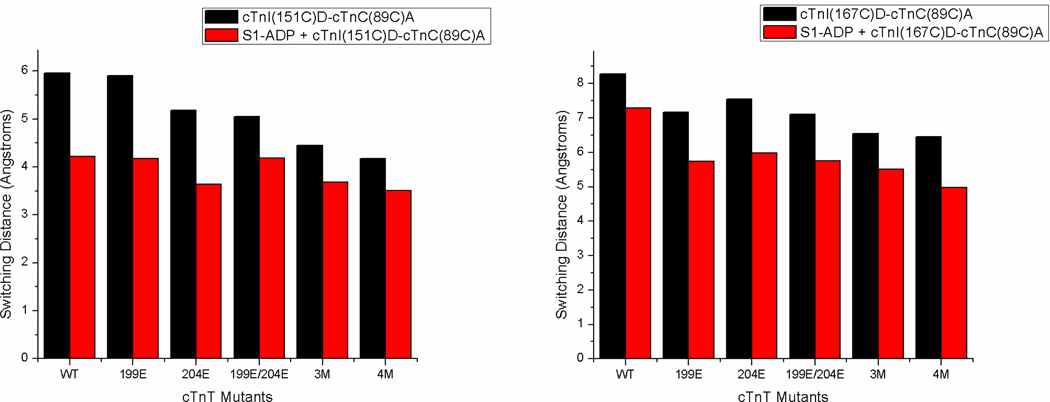
Thin filament samples containing cTnT(wt) showed the greatest Mg2+-state cTnI-cTnC separation both for cTnI(S151CAEDANS) and cTnI(S167CAEDANS). Upon addition of saturating levels of Ca2+ the mean cTnI(S151CAEDANS)–cTnC(S89CDDPM) and cTnI(S167CAEDANS)–cTnC(S89CDDPM) distances decreased by 6.0 and 8.3Å respectively. As noted above, we call this Ca2+-induced change in cTnI-cTnC separation the switching distance, and will denote it as rsw henceforth (values of rsw are displayed graphically in Fig. 5). Accompanying the Ca2+-induced reduction in cTnI-cTnC separation was a decrease in hw. Because hw is inversely related to the extent of the range of inter-probe distances experienced as cTnC and cTnI undergo conformational sampling, such that a larger hw corresponds to greater inter-probe mobility reflective of underlying protein structural flexibility, the smaller hw values observed in the Ca2+-state suggest that N-cTnC and the cTnI-Ir/cTnI-Sr intraprotein interface adopt a more rigid conformation relative to each other upon Ca2+-activation, which is consistent with previous protein dynamics studies of the C-domain of cTnI [13, 49]. Addition of strongly-bound S1-ADP to cTnT(wt) containing thin filaments reduced rsw as well as the Mg2+-state cTnI-cTnC separation and both the Mg2+- and Ca2+-state hw values, again indicating that S1-ADP disrupted cTnI-actin interactions resulting in partial association of cTnI with cTnC and subsequent reduction in inter-probe flexibility.
Figure 5.
Column graph showing switching distances for cTnT mutants with or without S1-ADP, shown in red and black respectively. The graph at left is labeled at cTnI(151) with AEDANS while the graph at right is labeled at cTnI(167), acceptor labeling at cTnC(89C) for both. This graph shows Ca2+ induced proximity changes between cTnI(151) and cTnC(89) or cTnI(167) and cTnC(89). As can be seen switching distance is greater for cTnI(167) than cTnI(151) and samples with S1-ADP all show decreased switching distances compared to the same samples without S1-ADP. The impact of cTnT pseudo-phosphorylation is seen as a decrease in switching distances, which is most clearly pronounced in cTnT(3M) and cTnT(4M). Interestingly cTnT pseudo-phosphorylation's impact is lost upon addition of S1-ADP for the cTnI(151) labeled samples.
Results in Tables 2 and 3 show that cTnI-cTnC distances decrease in thin filaments containing PKC phosphomimetic cTnT mutants compared with wild type cTnT under some conditions. The degree of distance reduction varied with pseudo-phosphorylation site and their combinations, and generally became statistically significant at the 95% confidence level only with samples containing cTnI(S167CAEDANS) and either cTnT(3M) or cTnT(4M). The reduction in distance was more significant in the Mg2+ state than in the Ca2+ state, suggesting that cTnT(3M) and cTnT(4M) destabilize especially the cTnI-Md–actin interaction associated with the blocked state of thin filament regulation, which allows for increased interaction between cTnI-Sr and N-cTnC. A decreased hw was also observed for these samples; this concurrent observation of reduced I-C separation and decreased apparent inter-probe flexibility likely derives from an increased sub-population of thin filament regulatory units in which cTnI-Sr is interacting with N-cTnC. With a larger sub-population of cTn molecules wherein cTnI-Sr is already interacting with cTnC at a given moment, the total apparent shift in ensemble-averaged cTnI-cTnC distance upon Ca2+ saturation will be decreased, explaining why samples containing cTnT(3M) and cTnT(4M) also exhibited the smallest values of rsw. Relating these structural results with the results from the Ca2+-titrations, we see that cTnT(3M) and cTnT(4M) have the greatest Ca2+-sensitivity as well as the smallest Mg2+-state I-C separation. This result is not surprising as we see the same relationship with the Mg2+-S1-ADP state; a partial association of cTnI with cTnC and a subsequent increase in Ca2+-affinity. These results differed from the case of the Ca2+-desensitizing cTnT(T204E), wherein no statistically significant change in Mg2+-state cTnI-cTnC separation was observed. This implies that the mechanism of action for the T204E mutation is completely different than that of T195E and T285E in not significantly impacting the distance between cTnI-Sr and N-cTnC; a plausible T204E structural change that might go undetected by our FRET scheme is a change in C-cTnI–N-cTnC orientation that affects the kinetics associated with I-C switching without disrupting the average position of a functional regions per se, thereby reducing Ca2+-sensitivity. Observation of such a kinetic effect was previously made in the case of the effects of PKA-mediated phosphorylation of cTnI on N-cTnC opening [37].
When S1-ADP was present, all measured distances between donor and acceptor in the Mg2+ state were decreased because of destabilization of the cTnI-Ir and cTnI-Md interactions with actin caused by the Ca2+-independent movement of Tm by strongly-bound S1, with cTnT(3M) and cTnT(4M) still showing significantly smaller Mg2+ state cTnI-cTnC distances compared to control. However, the measured distances in the Ca2+ state were not significantly affected by the presence of S1-ADP binding. It is noticed that no significant cTnT PKC pseudo-phosphorylation induced change in rsw was sensed by cTnI(151CAEDANS) samples, while decreased rsw values were observed for samples containing cTnI(167CAEDANS) with cTnT(3M) and cTnT(4M) inducing the most significant reduction (Fig. 5). These results suggest that pseudo-phosphorylation of cTnT has a different structural effect on the cTnI-Ir/cTnI-Sr compared to the cTnI-Sr/cTnI-Md, which becomes apparent in the presence of strongly-bound S1.
Kinetics of Ca2+ dissociation-induced structural changes of cTnC-cTnI interaction in cardiac thin filament
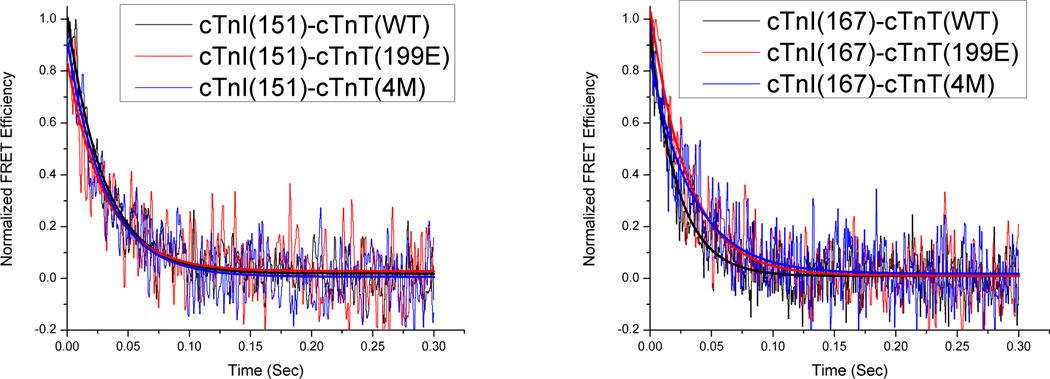
The kinetic effects of PKC phosphomimetic mutations of cTnT on thin filament regulation were characterized through FRET stopped-flow experiments. Specifically, we measured the kinetics of Ca2+-dissociation-induced structural transitions involving changes in cTnI-cTnC distances by rapidly removing Ca2+ from samples using BAPTA, a Ca2+-chelator, in a stopped flow device. Fig. 6 shows typical FRET transients generated in this manner. Fitting these transients to an exponential function enabled us to recover kinetic rates associated with the structural transitions which are given in Table 4. When samples were reconstituted with cTnT(wt), recovered kinetic rate constants were 34.1 s−1 for structural transition involving cTnI(S151CAEDANS) and a faster 46.0 s−1 for the structural transition involving cTnI(S167CAEDANS), and both rate constants were reduced in the presence of strongly-bound S1. These results are consistent with our previous observations that cTnI-Md and cTnI-Sr show faster kinetics than cTnI-Ir, and that deactivation kinetics become universally slower in the presence of strongly bound S1 [13].
Figure 6.
Representative BAPTA chelation traces for cTnT mutants S199E or 4M and control. The graph on the left shows samples using cTnI(S151CAEDANS)-cTnC(S89CDDPM) FRET pairs, while the graph on the right shows samples using cTnI(S167CAEDANS)-cTnC(S89CDDPM) FRET pairs. Deactivation kinetics at cTnI(151) are not significantly impacted by pseudo-phosphorylation mutants while at cTnI(167) deactivation kinetics are decreased.
Table 4.
Deactivation rate parameters obtained from exponential fitting of FRET kinetic transients ± 90% confidence interval. Rate constants for thin filament samples with S1-ADP are given in the right column. The top half of the table shows results obtained from cTnI(151CAEDANS)-cTnC(89CDDPM) FRET pairs, while the bottom half show results obtained from cTnI(167CAEDANS)-cTnC(89CDDPM) pairs.
| cTnI:cTnT mutants (n = 20–60) | Kinetic rate (s−1) | |
|---|---|---|
| Thin filament | Thin filament +S1- ADP |
|
| S151CAEDANS:wt | 34.1 ± 1.9 | 33.6 ± 2.1 |
| S199E | 32.5 ± 2.0 | 30.2 ± 2.1* |
| T204E | 34.5 ± 2.8 | 32.8 ± 2.7 |
| S199E/T204E | 35.1 ± 2.2 | 32.3 ± 2.7 |
| T195E/S199E/T204E | 32.8 ± 2.9 | 31.8 ± 2.8 |
| T195E/S199E/T204E/T285E | 32.2 ± 2.2 | 32.1 ± 2.4 |
| S167CAEDANS:wt | 46.3 ± 3.3 | 39.9 ± 4.0 |
| S199E | 37.9 ± 2.7** | 36.9 ± 2.9 |
| T204E | 36.1 ± 2.5** | 37.0 ± 2.2 |
| S199E/T204E | 39.1 ± 3.3** | 35.7 ± 2.7 |
| T195E/S199E/T204E | 38.8 ± 4.1** | 35.7 ± 3.4 |
| T195E/S199E/T204E/T285E | 35.1 ± 2.6** | 34.9 ± 3.3 |
Indicates a significant difference from wild type at the 90% confidence level while
indicates significance at the 95% level.
Kinetic rate constants of Ca2+-dissociaton-induced structural transitions involving cTnI(S151CAEDANS) showed negligible change between control and samples containing PKC phosphomimetic cTnT mutants, while rate constants involving cTnI(S167CAEDANS) were decreased significantly for all pseudo-phosphorylations. This trend of reduced deactivation kinetics at cTnI(S167CAEDANS) was diminished in the presence of S1-ADP. Similar to the Ca2+-titration and FRET structural measurements, these results suggest that PKC phosphorylations of cTnT may modulate thin filament regulation via changes to the Mg2+-state structure of the cTn-Tm-actin complex which manifest themselves as an altered orientation between cTnI and cTnC that particularly affects the C-terminal portion of cTnI-Sr and, by implication, cTnI-Md. These structural changes in turn alter the Ca2+-sensitivity and deactivation kinetics of I-C switching without appreciably changing the cTnC-cTnI distance; as mentioned above, similar effects were previously observed to be exerted by PKA phosphorylation of cTnI on N-cTnC opening [37]. Furthermore, because it was observed that cTnT(T204E) induces a marked decrease in the Ca2+-sensitivity of I-C switching along with reduced Ca2+-dissociation induced kinetics, we can reason that Ca2+-association-induced structural transition kinetics are also slowed by cTnT(T204E). Our kinetics data were thus also consistent with the interpretation that cTnT(T204E) through a unique propagated structural change modifies the orientation of cTnI and cTnC relative to one another such that structural transition kinetics are generally slowed without affecting the relative distance between cTnC and cTnI. This alteration in I-C switching likely propagates from cTnT(204) through the IT-arm as this is the most direct structural contact between cTnT(204) and cTnI-cTnC [36].
Similar to the case of cTnT(T204E), it is likely that cTnT(S199E) also imposed a change in cTnC-cTnI orientation that caused the slowed kinetics it exerted. However, an apparent decrease, significant at the 90% confidence level, in the cTnI(S167CAEDANS)-cTnC(S89CDDPM) distance (Table 3) may help explain why this orientational change does not lead to Ca2+-sensitization as in the case of cTnT(T204E). In the case of cTnT(S199E/T204E), cTnT(3M) and cTnT(4M), which contain the T204E mutation along with additional pseudo-phosphorylations, promotion of the Ca2+-sensitizing cTnI-Sr–N-cTnC interaction helps to compensate for the Ca2+-desensitizing hindrance on the I-C switching structural transition induced by T204E. This is especially noticeable in the 3M and 4M mutations, which showed significantly reduced I-C distances in the Mg2+-state and resensitized or hyper sensitized Ca2+-affinities accordingly. Interestingly, it can also be seen from the kinetics data that the pseudo-phosphorylation induced effects more strongly impacted the region of cTnI(S167CAEDANS) than cTnI(S151CAEDANS), which again implies that as far as cTnI is concerned, the cTnI-Md–actin interaction is most impacted by cTnT phosphorylation. Although we cannot ascertain the specific reason for this from the present FRET experiments, a likely explanation is that because cTnI-Md is the furthest away from the IT-arm, where we believe cTnI-cTnC orientational changes are arising, cTnI-Md is the most affected by the structural changes induced by PKC phosphomimetic mutations.
Discussion
In a seminal chemically-skinned cardiac muscle fiber study on the functional effects of PKC phosphorylation on thin filament regulation, Sumandea, et al. identified Thr-204 (rat sequence) as a functionally critical PKC phosphorylation site of cTnT by showing significant reductions in maximum tension, actomyosin Mg-ATPase activity, myofilament Ca2+-sensitivity, and cooperativity caused by pseudo-phosphorylation at this residue [30], which are the effects expected from studies of the role of PKC phosphorylation in cardiac physiology [50]. The same study also suggested that pseudo-phosphorylation at cTnT Thr-195 and Thr-285 partially reverse the observed Ca2+-blunting effects caused by cTnT(T204E). In the present biophysical study, the primary findings were as follows. (1) Similar to Sumandea et al.’s findings [30], cTnT(T204E) was the critical phosphorylation site to desensitize the cTnC-cTnI interaction to Ca2+. Unlike in Sumandea's study T204E Ca2+-desensitization was significantly blunted or completely replaced by sensitization in the presence of other phosphorylations. (2) Multiple PKC site pseudo-phosphorylation of cTnT structurally affected the cTnI-Md-actin and cTnI-Sr-N-cTnC interactions primarily in the Mg2+ state, this being most prominent in thin filaments containing cTnT(3M) or cTnT(4M) wherein the cTnI-cTnC separation was most significantly reduced. Promotion of increased cTnI-Sr-N-cTnC interaction in the Mg2+-state is most likely the underlying cause of the increased Ca2+-sensitivities observed for cTnT(3M) and cTnT(4M) samples. (3) The kinetics of deactivation for thin filaments with pseudo-phosphorylations were significantly slowed compared to control at cTnI(167) but not at cTnI(151), which implied a differential impact of pseudo-phosphorylation at these two positions that is most likely due to changes in the orientation between C-cTnI and N-cTnC and destabilization of the cTnI-Md-actin interaction. Taken together these findings paint a picture of cTnT in which the Ca2+-sensitivity and kinetics of the I-C switching can be significantly modulated depending on which sites on cTnT are phosphorylated by PKC. At the reconstituted thin filament level, cTnT(T204E) is able to significantly reduce the Ca2+-sensitivity of I-C switching, whereas cTnT(T195E) and cTnT(T285E) are able to mitigate Ca2+-desensitization by T204E, and can even hypersensitize the thin filament to Ca2+ in the absence of strongly bound S1. These results might appear to be at odds with the study by Sumandea et al. which showed that the T204E mutation alone had significant effects on the contractility of cardiac muscle fiber with pseudo-phosphorylations at additional PKC phosphorylation sites having only a minor impact, however such differences should be expected. They are using whole muscle fibers which contain, amongst other things, functional crossbridges and the complex and important geometrical arrangement of the sarcomere which simply is not present in reconstituted thin filaments. Furthermore, in our study we are measuring cTnI and cTnC interaction which lies upstream of force generation. Especially considering cTnT's role as a transduction unit between troponin's core domain and tropomyosin (and subsequently force generation) the effects of phosphorylation may manifest themselves differently in cTnI-cTnC interaction and force production if they alter the transduction of the former to the latter. We feel our results compliment those of Sumandea et al. by showing that the T204E mutation not only reduces force and Ca2+-sensitivity of force generation (which could be accomplished solely by altering transduction of cTnI-cTnC switching to tropomyosin) but that it also directly alters the behavior of the core domain of troponin, specifically cTnI-cTnC switching. The following paragraphs discuss the likely molecular mechanisms underlying the primary findings of this study by correlating Ca2+-titration, structural, and kinetics results for each mutation.
Sumandea’s Ca2+-desensitization mechanism for T204E suggests that a charge change at Thr-204 extends an α-helix which normally terminates at this residue, changing the position of the "fulcrum" through which structural changes associated with I-C switching propagate to Tm near the N-terminus of cTnT. This mechanism should not alter average I-C proximity, consistent with our structural results that I-C separation in the Mg2+- or Ca2+-state in the presence or absence of S1 has only a slight but statistically insignificant reduction compared to control. An important distinction between Sumandea’s study and ours is that we directly measure the distance between cTnI and cTnC that is related to the interaction between cTnI-Sr and the hydrophobic pocket of N-cTnC, and this interaction lies upstream of the force generation and Mg-ATPase measurements in Sumandea’s study (as mentioned above). Thus T204E may interfere with conduction of structural events initiated by I-C switching, as pointed out by Sumandea, as well as the I-C interaction itself as implied by our observation of decreased Ca2+-sensitivity and Ca2+-dissociation-induced kinetics of the I-C switching structural transition. The direct effect of T204E on the functionally crucial I-C switching event appears to arise from a subtle, propagated structural change which reorients cTnI and cTnC relative to one another without significantly altering average I-C separation, and/or alters cTn dynamics. Both of these mechanisms are consistent with Sumandea's notion of an α-helix extension which could alter I-C orientation (likely through the IT-arm) and cTn dynamics by changing cTnT’s flexibility. Kinetics data suggests T204E slows deactivation at the switch region/mobile domain of cTnI, a likely result of a change in I-C orientation since such a change may be expected to significantly alter the usual approach of the mobile domain of cTnI toward its binding site on actin when transitioning tropomyosin from the closed to the blocked state and thus hinder the fly casting mechanism [13]. However, we note that FRET as used in this study is able to present this argument for a change in I-C orientation resulting from an α-helix extension in cTnT only by implication; though it is beyond the scope of this study, additional structural information from a technique that can monitor orientational changes between cTnI and cTnC is needed to verify that this mechanism truly occurs.
If indeed charge modification at cTnT(204) extends an α-helix in this region, then it would seem reasonable that additional charge modifications at Serine-195 and 199 could interfere with the stability of this α-helix, even though no such interference was found by computational modeling of α-helix stability [30]. A disruption of this α-helix extension by pseudo-phosphorylation at cTnT 195 or 199 could be partially responsible for the reduced Ca2+-desensitization seen in cTnT(S199E/T204E) or the Ca2+-sensitization seen in cTnT(3M) and cTnT(4M). It has also been shown that chicken fast skeletal TnT(158–191), which corresponds to rat cTnT(188–227) has actin binding properties and plays a role in thin filament activation [20], and that in the region containing cTnT T195E, S199E and T204E there lies a Tm binding site [22]. Thus charge changes in this region could have important effects on the activation state of cTn through changes to cTnT-Tm or cTnT-actin interactions. If the actin and Tm binding properties of this region are important for the movement of Tm from the blocked to the closed state [50], or vice versa, then changes in cTnT charge in this region could significantly hinder structural transitions in such a way that Ca2+-sensitivity could be decreased or increased while Ca2+-dissociation-induced structural transition kinetics are slowed, presumably because Ca2+-association-induced structural transition kinetics are also modified.
Finally, the importance of phosphorylation at cTnT(285) should not be understated. This phosphorylation site is cardiac specific and occurs in a region of cTnT which was shown to be important in deactivation by experiments where the C-terminal 28 residues of cTnT were removed resulting in increased Ca2+-sensitivity, which was attributed to a disruption blocked state of thin filament regulation [51]. Our results are consistent with a disruption of the cTnI-Md–actin interaction that is associated with the blocked state, as the mutants containing the cTnT(T285E) mutation showed the greatest Ca2+-sensitivity, the smallest I-C separation in the Mg2+-state as well as significantly reduced deactivation kinetics at cTnI(167). The individual impact of PKC pseudo-phosphorylation at this site is difficult to determine since this study only examined this site in conjunction with pseudo-phosphorylation at the other three sites. The proximity of this site to both cTnI and the C-domain of cTnC suggest direct influence on I-C interaction likely underlies the observed blocked state destabilization, as opposed to cTnT(T195E) and cTnT(S199E) which likely work through local disruption of the cTnT-Tm or cTnT-actin interactions. Thus taken together, the above findings highlight the functional importance of the C-terminus of cTnT in modulating Ca2+-sensitivity, maintaining the stability of the blocked state of thin filament regulation, and facilitating proper transitions between the blocked and closed states.
The question should arise as to what these in vitro findings may imply for cardiac function. Previous studies have shown that, in general, PKC phosphorylation of cTnT reduces maximal force and Mg-ATPase activity [50], as well as the affinity of cTnT for Tm binding affinity [28, 52]. This decreased cTnT-Tm binding would likely uncouple the I-C switching event from force generation as well as reduce cooperativity, which reconciles our observation that cTnT(3M) and cTnT(4M) both appear to sensitize the I-C switching structural transition to Ca2+ in spite of the fact that previous studies which have shown that these mutations decrease force and Mg-ATPase activity. If the underlying mechanism for Ca2+-sensitization seen in cTnT(3M) and cTnT(4M) (and to a lesser extent cTnT(S199E/T204E)) is a reduced cTnT-Tm interaction, which we believe to exert predominantly kinetic effects, coupled with a destabilized cTnI-Md–actin interaction that allows for increased Ca2+-sensitizing cTnI-Sr–N-cTnC interaction, then it is likely the Ca2+-sensitizations seen in our study would be of secondary importance to the uncoupling of I-C switching from Tm movement due to the fact that positive feedback from strongbinding cross-bridges also involves increased cTnI-Sr–N-cTnC interaction [47]. Thus our study is consistent with the effects seen in demembranated of a variable level of Ca2+-desensitization sarcomeric force generation and decreased maximum tension, the latter of which is most likely due to the uncoupling of I-C switching from movement of Tm from the blocked to closed position that exposure of myosin biding sites on actin is reduced. One expectation based on the findings of the present study would be a decrease in diastolic function in hearts expressing cTnT(3M) or cTnT(4M). Because of the likely disruption of the cTnI-Md–actin interaction induced by these mutations, it is possible that they may result in a higher basal force and a reduced ability of the thin filament to inhibit Mg-ATPase in vivo at low levels of Ca2+; however, we note that this effect has not yet been reported [3030, 50]. Regardless, our results contribute to the case that cTnT represents another powerful avenue by which in vivo thin filament regulation can be modulated.
Highlights.
cTnT phosphorylation by PKC was mimicked in in vitro thin filaments.
I-C switching was monitored by FRET for these phospho-mimics.
Phospho-mimics altered I-C switching Ca2+-sensitivity, structure, and kinetics.
cTnT(T204E) significantly reduced Ca2+-sensitivity of I-C switching.
cTnT(3M) and (4M) increased Ca2+-sensitivity, reduced I-C separation and kinetics.
Acknowledgements
This work was partially supported by the National Institutes of Health Grant HL80186 and IR21HL109693 (W.-J. D.), as well as by the M. J. Murdock Charitable Trust (W.-J. D.). Partial support for this publication came from the NIH/NIGMS through an institutional training grant award T32-GM008336. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIGMS or NIH.
Abbreviations
- cTnT(3M)
Cardiac troponin tropomyosin binding subunit with residues T195, S199 and T204 replaced by glutamic acid
- cTnT(4M)
The same as cTnT(3M) but with residue T285 also replaced by glutamic acid
- Ir
Inhibitory region
- Md
Mobile domain
- r̄
The average FRET distance between donor and acceptor determined from lifetime decay analysis
- Sr
Switch region
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ebashi S, Endo M, Otsuki I. Q Rev Biophys. 1969;2:351–384. doi: 10.1017/s0033583500001190. [DOI] [PubMed] [Google Scholar]
- 2.Farah CS, Reinach FC. FASEB J. 1995;9:755–767. doi: 10.1096/fasebj.9.9.7601340. [DOI] [PubMed] [Google Scholar]
- 3.Jin JP, editor. Nova Biomedical. Hauppauge, NY: 2013. Conformational states and behavior of the heterotrimeric troponin complex. [Google Scholar]
- 4.Haselgrove JC. Cold Spring Harbor Symp. Qant. Biol. 1972;37:341–352. [Google Scholar]
- 5.Huxley HE. Cold Spring Harbor Symp. Qant. Biol. 1972;37:361–376. [Google Scholar]
- 6.Parry DA, Squire JM. J. Mol. Biol. 1973;75:33–55. doi: 10.1016/0022-2836(73)90527-5. [DOI] [PubMed] [Google Scholar]
- 7.Dong WJ, Xing J, Villain M, Hellinger M, Robinson JM, Chandra M, Solaro RJ, Umeda PK, Cheung HC. J. Biol. Chem. 1999;274:31382–31390. doi: 10.1074/jbc.274.44.31382. [DOI] [PubMed] [Google Scholar]
- 8.Li MX, Spyracopoulos L, Sykes BD. Biochemistry. 1999;38:8289–8298. doi: 10.1021/bi9901679. [DOI] [PubMed] [Google Scholar]
- 9.Dong WJ, Xing J, Robinson JM, Cheung HC. J. Mol. Biol. 2001;314:51–61. doi: 10.1006/jmbi.2001.5118. [DOI] [PubMed] [Google Scholar]
- 10.Dong WJ, Robinson JM, Stagg S, Xing J, Cheung HC. J Biol Chem. 2003;278:8686–8692. doi: 10.1074/jbc.M212886200. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi T, Kobayashi M, Gryczynski Z, Lakowicz JR, Collins JH. Biochemistry. 2000;39:86–91. doi: 10.1021/bi991903b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson JM, Dong WJ, Xing J, Cheung HC. J Mol Biol. 2004;340:295–305. doi: 10.1016/j.jmb.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Z, Li KL, Rieck D, Ouyang Y, Chandra M, Dong WJ. J Biol Chem. 2012;287:7661–7674. doi: 10.1074/jbc.M111.281600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman RM, Blumenschein TM, Sykes BD. J Mol Biol. 2006;361:625–633. doi: 10.1016/j.jmb.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 15.Lehman W, Craig R, Vibert P. Nature. 1994;368:65–67. doi: 10.1038/368065a0. [DOI] [PubMed] [Google Scholar]
- 16.Reinach FC, Farah CS, Monteiro PB, Malnic B. Cell Struct Funct. 1997;22:219–223. doi: 10.1247/csf.22.219. [DOI] [PubMed] [Google Scholar]
- 17.Potter JD, Zhang R, Zhao J. J Muscle Res Cell M. 1994;15:209–209. [Google Scholar]
- 18.Chandra M, Tschirgi ML, Rajapakse I, Campbell KB. Biophysical Journal. 2006;90:2867–2876. doi: 10.1529/biophysj.105.076950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gollapudi SK, Gallon CE, Chandra M. J Mol Biol. 2013;425:1565–1581. doi: 10.1016/j.jmb.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliveira DM, Nakaie CR, Sousa AD, Farah CS, Reinach FC. J Biol Chem. 2000;275:27513–27519. doi: 10.1074/jbc.M002735200. [DOI] [PubMed] [Google Scholar]
- 21.Malnic B, Farah CS, Reinach FC. J Biol Chem. 1998;273:10594–10601. doi: 10.1074/jbc.273.17.10594. [DOI] [PubMed] [Google Scholar]
- 22.Jin JP, Chong SM. Arch Biochem Biophys. 2010;500:144–150. doi: 10.1016/j.abb.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potter JD, Sheng Z, Pan BS, Zhao J. J Biol Chem. 1995;270:2557–2562. doi: 10.1074/jbc.270.6.2557. [DOI] [PubMed] [Google Scholar]
- 24.Wei B, Jin JP. Arch Biochem Biophys. 2011;505:144–154. doi: 10.1016/j.abb.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaertl S, Lehrer SS, Geeves MA. Biochemistry. 1995;34:15890–15894. doi: 10.1021/bi00049a003. [DOI] [PubMed] [Google Scholar]
- 26.Gomes AV, Barnes JA, Harada K, Potter JD. Mol Cell Biochem. 2004;263:115–129. doi: 10.1023/B:MCBI.0000041853.20588.a0. [DOI] [PubMed] [Google Scholar]
- 27.Tardiff JC. Heart Fail Rev. 2005;10:237–248. doi: 10.1007/s10741-005-5253-5. [DOI] [PubMed] [Google Scholar]
- 28.Noland TA, Jr, Kuo JF. Biochem J. 1992;288(Pt 1):123–129. doi: 10.1042/bj2880123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noland TA, Jr, Raynor RL, Kuo JF. J Biol Chem. 1989;264:20778–20785. [PubMed] [Google Scholar]
- 30.Sumandea MP, Pyle WG, Kobayashi T, de Tombe PP, Solaro RJ. J Biol Chem. 2003;278:35135–35144. doi: 10.1074/jbc.M306325200. [DOI] [PubMed] [Google Scholar]
- 31.Perry SV. J Muscle Res Cell Motil. 1998;19:575–602. doi: 10.1023/a:1005397501968. [DOI] [PubMed] [Google Scholar]
- 32.Noland TA, Jr, Kuo JF. J Mol Cell Cardiol. 1993;25:53–65. doi: 10.1006/jmcc.1993.1007. [DOI] [PubMed] [Google Scholar]
- 33.Montgomery DE, Tardiff JC, Chandra M. J Physiol. 2001;536:583–592. doi: 10.1111/j.1469-7793.2001.0583c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaminsky RA. Archives of Pathology & Laboratory Medicine. 2001;125:573–574. doi: 10.5858/2001-125-0573-PQCAER. [DOI] [PubMed] [Google Scholar]
- 35.Noguchi T, Hunlich M, Camp PC, Begin KJ, El-Zaru M, Patten R, Leavitt BJ, Ittleman FP, Alpert NR, LeWinter MM, VanBuren P. Circulation. 2004;110:982–987. doi: 10.1161/01.CIR.0000139334.43109.F9. [DOI] [PubMed] [Google Scholar]
- 36.Takeda S, Yamashita A, Maeda K, Maeda Y. Nature. 2003;424:35–41. doi: 10.1038/nature01780. [DOI] [PubMed] [Google Scholar]
- 37.Dong WJ, Jayasundar JJ, An J, Xing J, Cheung HC. Biochemistry. 2007;46:9752–9761. doi: 10.1021/bi700574n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xing J, Jayasundar JJ, Ouyang Y, Dong WJ. J Biol Chem. 2009;284:16432–16441. doi: 10.1074/jbc.M808075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong WJ, Chandra M, Xing J, Solaro RJ, Cheung HC. Biochemistry. 1997;36:6745–6753. doi: 10.1021/bi962226d. [DOI] [PubMed] [Google Scholar]
- 40.Smillie LB. Methods in Enzymology. 1982;85:234–241. doi: 10.1016/0076-6879(82)85023-4. [DOI] [PubMed] [Google Scholar]
- 41.Pardee JD, Spudich JA. Methods in Enzymology. 1982;85:164–181. doi: 10.1016/0076-6879(82)85020-9. [DOI] [PubMed] [Google Scholar]
- 42.Xing J, Cheung HC. Arch. Biochem. Biophys. 1994;313:229–234. doi: 10.1006/abbi.1994.1381. [DOI] [PubMed] [Google Scholar]
- 43.Dong WJ, Xing J, Ouyang Y, An J, Cheung HC. J Biol Chem. 2008;283:3424–3432. doi: 10.1074/jbc.M703822200. [DOI] [PubMed] [Google Scholar]
- 44.Liao R, Wang CK, Cheung HC. Biophys J. 1992;63:986–995. doi: 10.1016/S0006-3495(92)81685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong WJ, Robinson JM, Xing J, Cheung HC. J Biol Chem. 2003;278:42394–42402. doi: 10.1074/jbc.M304858200. [DOI] [PubMed] [Google Scholar]
- 46.Xing J, Chinnaraj M, Zhang Z, Cheung HC, Dong WJ. Biochemistry. 2008;47:13383–13393. doi: 10.1021/bi801492x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rieck DC, Li KL, Ouyang Y, Solaro RJ, Dong WJ. Arch Biochem Biophys. 2013;537:198–209. doi: 10.1016/j.abb.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mudalige WA, Tao TC, Lehrer SS. J Mol Biol. 2009;389:575–583. doi: 10.1016/j.jmb.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ouyang Y, Mamidi R, Jayasundar JJ, Chandra M, Dong WJ. J Mol Biol. 2010;400:1036–1045. doi: 10.1016/j.jmb.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Streng AS, de Boer D, van der Velden J, van Dieijen-Visser MP, Wodzig WK. J Mol Cell Cardiol. 2013;63:47–56. doi: 10.1016/j.yjmcc.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Stelzer JE, Patel JR, Olsson MC, Fitzsimons DP, Leinwand LA, Moss RL. Am J Physiol Heart Circ Physiol. 2004;287:H1756–H1761. doi: 10.1152/ajpheart.00172.2004. [DOI] [PubMed] [Google Scholar]
- 52.Noland TA, Jr, Kuo JF. J Biol Chem. 1991;266:4974–4978. [PubMed] [Google Scholar]