Abstract
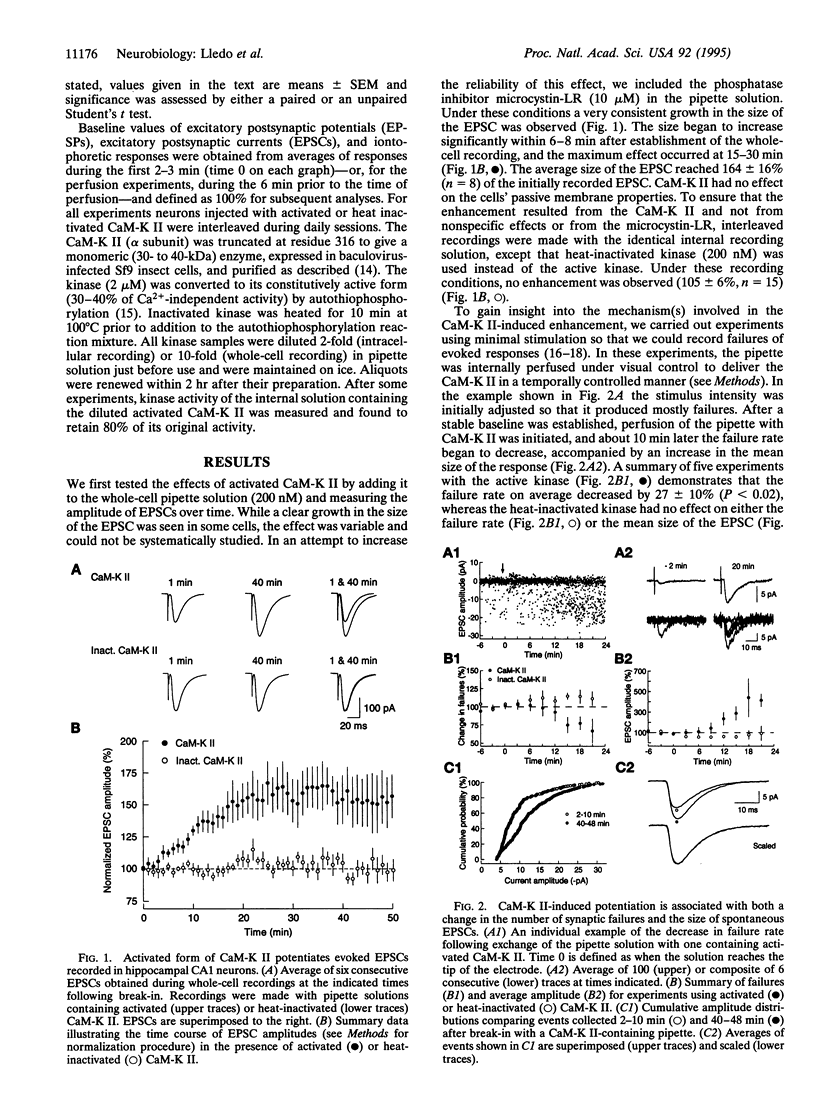
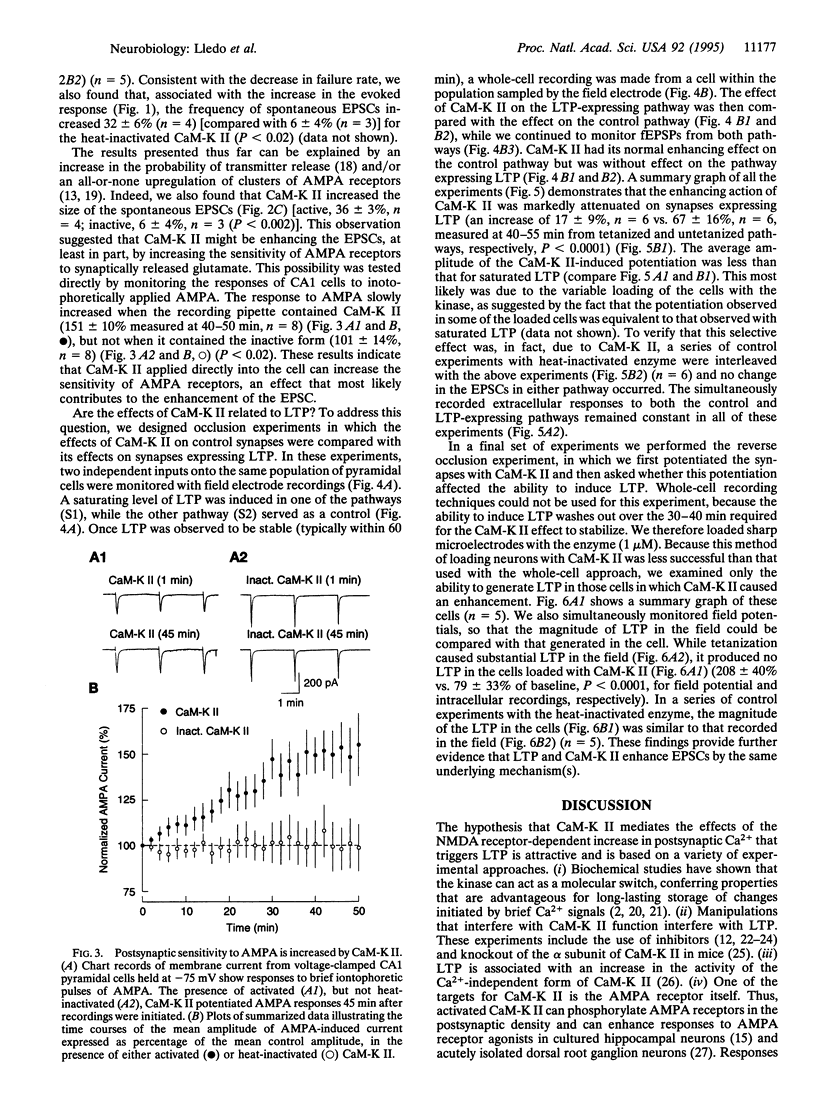
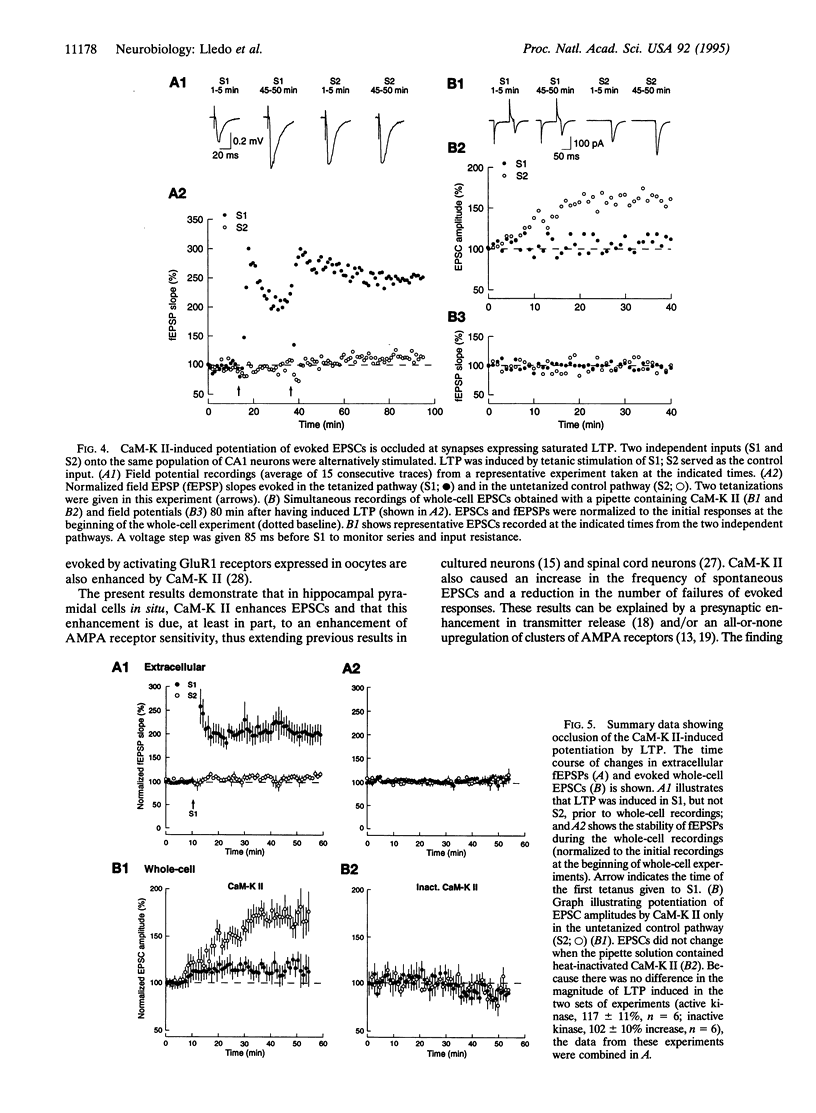
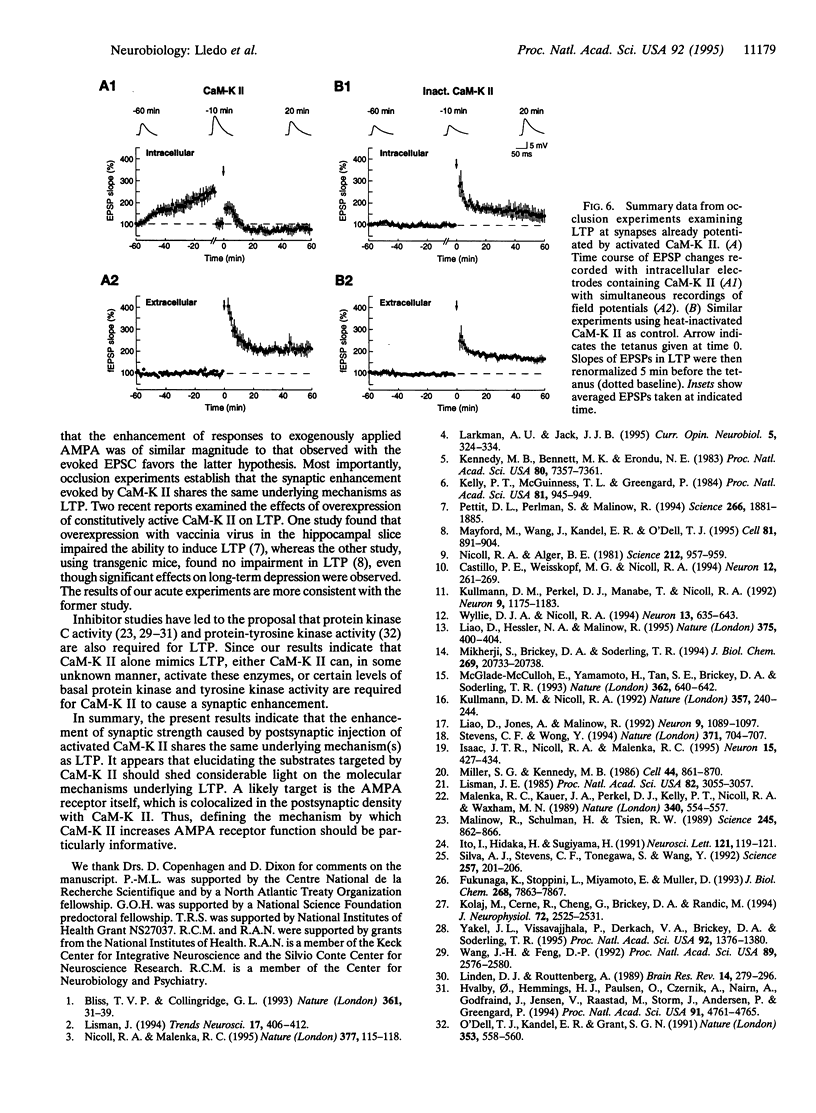
Ca(2+)-sensitive kinases are thought to play a role in long-term potentiation (LTP). To test the involvement of Ca2+/calmodulin-dependent kinase II (CaM-K II), truncated, constitutively active form of this kinase was directly injected into CA1 hippocampal pyramidal cells. Inclusion of CaM-K II in the recording pipette resulted in a gradual increase in the size of excitatory postsynaptic currents (EPSCs). No change in evoked responses occurred when the pipette contained heat-inactivated kinase. The effects of CaM-K II mimicked several features of LTP in that it caused a decreased incidence of synaptic failures, an increase in the size of spontaneous EPSCs, and an increase in the amplitude of responses to iontophoretically applied alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate. To determine whether the CaM-K II-induced enhancement and LTP share a common mechanism, occlusion experiments were carried out. The enhancing action of CaM-K II was greatly diminished by prior induction of LTP. In addition, following the increase in synaptic strength by CaM-K II, tetanic stimulation failed to evoke LTP. These findings indicate that CaM-K II alone is sufficient to augment synaptic strength and that this enhancement shares the same underlying mechanism as the enhancement observed with LTP.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Castillo P. E., Weisskopf M. G., Nicoll R. A. The role of Ca2+ channels in hippocampal mossy fiber synaptic transmission and long-term potentiation. Neuron. 1994 Feb;12(2):261–269. doi: 10.1016/0896-6273(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Fukunaga K., Stoppini L., Miyamoto E., Muller D. Long-term potentiation is associated with an increased activity of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1993 Apr 15;268(11):7863–7867. [PubMed] [Google Scholar]
- Hvalby O., Hemmings H. C., Jr, Paulsen O., Czernik A. J., Nairn A. C., Godfraind J. M., Jensen V., Raastad M., Storm J. F., Andersen P. Specificity of protein kinase inhibitor peptides and induction of long-term potentiation. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4761–4765. doi: 10.1073/pnas.91.11.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac J. T., Nicoll R. A., Malenka R. C. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995 Aug;15(2):427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Ito I., Hidaka H., Sugiyama H. Effects of KN-62, a specific inhibitor of calcium/calmodulin-dependent protein kinase II, on long-term potentiation in the rat hippocampus. Neurosci Lett. 1991 Jan 2;121(1-2):119–121. doi: 10.1016/0304-3940(91)90663-e. [DOI] [PubMed] [Google Scholar]
- Kelly P. T., McGuinness T. L., Greengard P. Evidence that the major postsynaptic density protein is a component of a Ca2+/calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1984 Feb;81(3):945–949. doi: 10.1073/pnas.81.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. B., Bennett M. K., Erondu N. E. Biochemical and immunochemical evidence that the "major postsynaptic density protein" is a subunit of a calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7357–7361. doi: 10.1073/pnas.80.23.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaj M., Cerne R., Cheng G., Brickey D. A., Randić M. Alpha subunit of calcium/calmodulin-dependent protein kinase enhances excitatory amino acid and synaptic responses of rat spinal dorsal horn neurons. J Neurophysiol. 1994 Nov;72(5):2525–2531. doi: 10.1152/jn.1994.72.5.2525. [DOI] [PubMed] [Google Scholar]
- Kullmann D. M., Nicoll R. A. Long-term potentiation is associated with increases in quantal content and quantal amplitude. Nature. 1992 May 21;357(6375):240–244. doi: 10.1038/357240a0. [DOI] [PubMed] [Google Scholar]
- Kullmann D. M., Perkel D. J., Manabe T., Nicoll R. A. Ca2+ entry via postsynaptic voltage-sensitive Ca2+ channels can transiently potentiate excitatory synaptic transmission in the hippocampus. Neuron. 1992 Dec;9(6):1175–1183. doi: 10.1016/0896-6273(92)90075-o. [DOI] [PubMed] [Google Scholar]
- Larkman A. U., Jack J. J. Synaptic plasticity: hippocampal LTP. Curr Opin Neurobiol. 1995 Jun;5(3):324–334. doi: 10.1016/0959-4388(95)80045-x. [DOI] [PubMed] [Google Scholar]
- Liao D., Hessler N. A., Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995 Jun 1;375(6530):400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- Liao D., Jones A., Malinow R. Direct measurement of quantal changes underlying long-term potentiation in CA1 hippocampus. Neuron. 1992 Dec;9(6):1089–1097. doi: 10.1016/0896-6273(92)90068-o. [DOI] [PubMed] [Google Scholar]
- Linden D. J., Routtenberg A. The role of protein kinase C in long-term potentiation: a testable model. Brain Res Brain Res Rev. 1989 Jul-Sep;14(3):279–296. doi: 10.1016/0165-0173(89)90004-0. [DOI] [PubMed] [Google Scholar]
- Lisman J. E. A mechanism for memory storage insensitive to molecular turnover: a bistable autophosphorylating kinase. Proc Natl Acad Sci U S A. 1985 May;82(9):3055–3057. doi: 10.1073/pnas.82.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. The CaM kinase II hypothesis for the storage of synaptic memory. Trends Neurosci. 1994 Oct;17(10):406–412. doi: 10.1016/0166-2236(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Kauer J. A., Perkel D. J., Mauk M. D., Kelly P. T., Nicoll R. A., Waxham M. N. An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature. 1989 Aug 17;340(6234):554–557. doi: 10.1038/340554a0. [DOI] [PubMed] [Google Scholar]
- Malinow R., Schulman H., Tsien R. W. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989 Aug 25;245(4920):862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- Mayford M., Wang J., Kandel E. R., O'Dell T. J. CaMKII regulates the frequency-response function of hippocampal synapses for the production of both LTD and LTP. Cell. 1995 Jun 16;81(6):891–904. doi: 10.1016/0092-8674(95)90009-8. [DOI] [PubMed] [Google Scholar]
- McGlade-McCulloh E., Yamamoto H., Tan S. E., Brickey D. A., Soderling T. R. Phosphorylation and regulation of glutamate receptors by calcium/calmodulin-dependent protein kinase II. Nature. 1993 Apr 15;362(6421):640–642. doi: 10.1038/362640a0. [DOI] [PubMed] [Google Scholar]
- Miller S. G., Kennedy M. B. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell. 1986 Mar 28;44(6):861–870. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- Mukherji S., Brickey D. A., Soderling T. R. Mutational analysis of secondary structure in the autoinhibitory and autophosphorylation domains of calmodulin kinase II. J Biol Chem. 1994 Aug 12;269(32):20733–20738. [PubMed] [Google Scholar]
- Nicoll R. A., Alger B. E. Synaptic excitation may activate a calcium-dependent potassium conductance in hippocampal pyramidal cells. Science. 1981 May 22;212(4497):957–959. doi: 10.1126/science.6262912. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Malenka R. C. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature. 1995 Sep 14;377(6545):115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- O'Dell T. J., Kandel E. R., Grant S. G. Long-term potentiation in the hippocampus is blocked by tyrosine kinase inhibitors. Nature. 1991 Oct 10;353(6344):558–560. doi: 10.1038/353558a0. [DOI] [PubMed] [Google Scholar]
- Pettit D. L., Perlman S., Malinow R. Potentiated transmission and prevention of further LTP by increased CaMKII activity in postsynaptic hippocampal slice neurons. Science. 1994 Dec 16;266(5192):1881–1885. doi: 10.1126/science.7997883. [DOI] [PubMed] [Google Scholar]
- Silva A. J., Stevens C. F., Tonegawa S., Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992 Jul 10;257(5067):201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Stevens C. F., Wang Y. Changes in reliability of synaptic function as a mechanism for plasticity. Nature. 1994 Oct 20;371(6499):704–707. doi: 10.1038/371704a0. [DOI] [PubMed] [Google Scholar]
- Wang J. H., Feng D. P. Postsynaptic protein kinase C essential to induction and maintenance of long-term potentiation in the hippocampal CA1 region. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2576–2580. doi: 10.1073/pnas.89.7.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie D. J., Nicoll R. A. A role for protein kinases and phosphatases in the Ca(2+)-induced enhancement of hippocampal AMPA receptor-mediated synaptic responses. Neuron. 1994 Sep;13(3):635–643. doi: 10.1016/0896-6273(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Yakel J. L., Vissavajjhala P., Derkach V. A., Brickey D. A., Soderling T. R. Identification of a Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in non-N-methyl-D-aspartate glutamate receptors. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1376–1380. doi: 10.1073/pnas.92.5.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]



