Abstract
Scope
Despite the solid connection between REV-ERB and obesity, the information about whether genetic variations at this locus may be associated with obesity traits is scarce. Therefore our objective was to study the association between REV-ERB-ALPHA1 rs2314339 and obesity in two independent populations.
Methods and results
Participants were 2214 subjects from Spanish Mediterranean (n = 1404) and North American (n = 810) populations. Anthropometric, biochemical, dietary, and genotype analyses were performed. We found novel associations between the REV-ERB-ALPHA1 rs2314339 genotype and obesity in two independent populations: in Spanish Mediterranean and North American groups, the frequency of the minor-allele-carriers (AA+ AG) was significantly lower in the “abdominally obese” group than in those of the “nonabdominally obese” group (p < 0.05). Minor allele carriers had lower probability of abdominal obesity than noncarriers, and the effect was of similar magnitude for both populations (OR ≈ 1.50). There were consistent associations between REV-ERB-ALPHA1 genotype and obesity-related traits (p < 0.05). Energy intake was not significantly associated with REV-ERB-ALPHA1 rs2314339. However, physical activity significantly differed by genotype. A significant interaction between the REV-ERB-ALPHA1 variant and monounsaturated-fatty-acids (MUFA) intake for obesity was also detected in the Mediterranean population.
Conclusion
This new discovery highlights the importance of REV-ERB-ALPHA1 in obesity and provides evidence for the connection between our biological clock and obesity-related traits.
Keywords: Circadian, Clock genes, Obesity, REV-ERB-ALPHA-1, Single nucleotide Polymorphism
1 Introduction
Alarge body of evidence from both human and animal studies now points to a relationship between circadian disorders and obesity, warranting a tight connection between circadian and metabolic regulatory networks [1]. This provides support to the hypothesis that genetic variation within circadian-related genes may be associated with obesity and other features of metabolic syndrome (MetS) [2]. Only a small fraction of these genes (i.e. CLOCK, BMAL1, and PERIOD) have been investigated, but results are promising. New information about a number of key genes may provide a more comprehensive picture of the circadian–obesity connection.
As one of several key genes implicated in the clock machinery function, REV-ERB-ALPHA1 (also named NR1D1) functions as a coordinator of metabolic responses that adhere to circadian patterns [3]. Circadian rhythms are generated through a feedback loop in which two components of the positive limb, BMAL1 and CLOCK activate transcription of the other components of the negative limb, CRY and PER genes. BMAL1 and PER transcription cycles display nearly opposite phases and are thus governed by different mechanisms [4]. Preitner et al. (2002) [5] identified REV-ERB-ALPHA as the major regulator of cyclic BMAL1 transcription. Therefore, REV-ERB-ALPHA is considered as a molecular link through which components of the negative limb drive antiphasic expression of components in the positive limb [5]. Currently, we know that REV-ERB-ALPHA is also a modulator of the period length and affects the phase-shifting properties of the biological clock [5,6]. Moreover, in vivo studies using targeted double knockout mice, have demonstrated that both REV-ERB isoforms together (α and β) function as integral drivers of the circadian clock, rather than simply as stabilizers of an output [7].
Interestingly, REV-ERB-ALPHA expression is induced dramatically during adipogenesis [8]. A recent study has demonstrated that REV-ERB agonists reduce fat mass in diet-induced obese mice while also reducing dyslipidemia and hyperglycemia in increased total energy expenditure [9]. Results from these studies suggest that REV-ERB may be a potential pharmacological target for novel anti-obesity therapies [8, 9]. Despite the solid connection of REV-ERB with obesity and other metabolic disorders, the information about whether genetic variations at this locus may be associated with those traits is still scarce.
The aim of the current study was to search for potential associations between a common variant rs2314339 within REV-ERB-ALPHA1 and obesity in a Mediterranean population and to seek replication in an independent European origin North American population.
2 Methods
2.1 Study participants and study design
A total of 2212 subjects from two white independent populations (Mediterranean and North American) were studied. All participants provided written informed consent.
2.1.1 Mediterranean population
The study sample consisted of 1402 subjects (82% women; age: 40 ± 12 years; BMI: 31.1 ± 5.4; mean ± SD, kg/m2) residents of Murcia (Spain) who voluntarily attended five nutrition clinics in southeast Spain with the objective of losing weight. For this purpose they followed a Mediterranean diet and supervised behavioral modification. All procedures were in accordance with good clinical practice. Patient data were codified to guarantee anonymity.
2.1.2 North American population
The study sample consisted of 810 (49.8%) women (age: 48 ± 16 years; BMI: 28.3 ± 5.6; mean ± SD, kg/m2) who participated in the Genetics of Lipid Lowering Drugs and Diet Network (GOLDN) study. GOLDN is part of the Program for Genetic Interactions Network and is funded by the NIH through the University of Alabama at Birmingham and in collaboration with the University of Utah, Washington University, Tufts University, University of Texas, University of Michigan, University of Minnesota and Fairview University of Minnesota Medical Center. The majority of participants were re-recruited from three-generational pedigrees from two National Heart, Lung, and Blood Institute Family Heart Study field centers (Minneapolis, Minnesota, and Salt Lake City, Utah) [10]. Nearly all individuals were of European descent. The details of the study are available at https://dsgweb.wustl.edu/goldn/. The protocol was approved by the institutional review boards at each of the above-referenced institutions.
2.1.3 Obesity and MetS parameters
In both populations, participants were weighed while barefoot and wearing light clothes, on a digital scale that measured to the nearest 0.1 kg, at the same time of the day (weekly in the Spanish population and once in the GOLDN population). Height was measured using a Harpenden digital stadiometer (rank 0.7–2.05). Each participant was positioned upright, and relaxed, with his or her head on the Frankfurt plane. BMI was calculated as weight (kg) divided by height (m2). Body fat distribution was assessed by anthropometric measures, including waist circumference at the level of the umbilicus, and hip circumference, with the widest circumference over the greater trochanters [11]. All measurements were made using a flexible and inextensible measuring tape.
Plasma concentrations of glucose, cholesterol, triglyceride, and lipoproteins were determined as previously described [12, 13]. High-density lipoprotein cholesterol was measured after precipitation of apoB-containing lipoproteins with dextran sulfate and magnesium. Low-density lipoprotein cholesterol was calculated as triglyceride minus high-density lipoprotein cholesterol plus very low-density lipoprotein cholesterol using the Friedewald equation, when the triglyceride was <4.52 mmol/L. Insulin was determined through a solid-phase, 2-site chemiluminescent immunometric assay (IMMULITE 2000 Insulin). Blood pressure (mm Hg) was measured with participants seated with the arm resting on a table. ATPIII 2001 guidelines were used to classify patients for MetS, which was defined by the presence of three or more of the following characteristics: (i) central obesity: waist circumference > 102 cm (men) or > 88 cm (women); (ii) high triglycerides: TG ≥ 150 mg/dL (1.7 mmol/L); (iii) low-high density lipoprotein cholesterol: < 40 mg/dL (1.03 mmol/L) (men) or < 1.29 mmol/L (50 mg/dL) (women); (iv) hypertension: blood pressure ≥ 130/85 mm Hg or taking medication; and (v) fasting plasma glucose ≥ 110 mg/dL (6.1 mmol/L) [14].
2.2 Energy intake
2.2.1 Mediterranean population
Total intake was determined by the “24-h dietary recall,” to evaluate habitual dietary intake before the treatment; this included 24-h recalls of food intake from all days of the week in all participants.
Total energy intake and macronutrient composition were analyzed with the nutritional evaluation software program Grunumur [15], on the basis of Spanish food composition tables [16, 17].
2.2.2 North American population
Dietary intake was estimated using the Diet History Questionnaire, a food frequency questionnaire developed by the National Cancer Institute. It consists of 124 food items and includes both portion size and dietary supplement questions [18]. The food list and nutrient database used with the Diet History Questionnaire are based on national dietary data [US Department of Agriculture (USDA) 1994–1996 Continuing Survey of Food Intakes by Individuals, available from the USDA Food Surveys Research Group].
2.3 Physical activity
2.3.1 Mediterranean population
To assess physical activity (PA) during the last 7 days, the International Physical Activity Questionnaire was administered with the help of a nutritionist. It was developed for adults between 18–65 years, assessing the different domains of PA (work, transport, house and garden, and leisure time). A total activity score reflecting intensity and time was calculated in MET (metabolic equivalents) minutes per week for the four domains combined. The International Physical Activity Questionnaire instrument has been validated internationally and in a Spanish population, in which good correlation with accelerometer data were obtained [19,20]. Those subjects who recorded <600 METs/week (Metabolic Equivalent of Task per week) were considered as sedentary.
2.3.2 North American population
A nonvalidated questionnaire containing questions on the number of hours/day dedicated to activities of different intensities was used [21].
2.4 DNA isolation and genotype analysis
DNA was isolated from blood (Qiagen, Hilden, Germany). For the Spanish population, we performed the genotyping for REV-ERB-ALPHA1 rs2314339 using a Taqman assay with allele-specific probes on the ABIPrism 7900HT Sequence Detection System (Applied Biosystems). For the GOLDN population, the Affymetrix Genome-Wide Human SNP Array 6.0 was used to perform genome-wide genotyping from which the rs2314339 SNP was obtained. Quality control measures were applied. Genotype frequencies were consistent with Hardy–Weinberg equilibrium in both populations. REV-ERB-ALPHA1 rs2314339 was analyzed because of its previous association with circadian rhythms and mood disorders [22–24].
2.5 Statistical analyses
Chi-square tests were used to test percentages. Normality of continuous variables was examined. Triglycerides and PA values were log-transformed. We applied ANOVA and Student’s t-test to compare crude means. We tested different genetic inheritance models and a dominant model was applied in the final analyses for REV-ERB-ALPHA1 rs2314339. We also tested the statistical homogeneity by sex, and men and women were analyzed together. To test associations between REV-ERB-ALPHA1 rs2314339 and obesity-related variables, we performed ANOVA analyses. In both populations, logistic regression models were fitted to test the REV-ERB-ALPHA1 rs2314339 association with the presence or absence of abdominal obesity and Chi-square tests were used to test differences in allele frequency with obesity.
To study gene–diet interactions in determining BMI, we used multivariate linear regression models including main effects and interaction terms. We fitted separate models for each population including the same variables for the interaction terms and for the multivariate adjustments. Monounsaturated fatty acids (MUFA) was considered as categorical (low or high) taking into account the median value. We adjusted analyses for sex, age, and clinic in the Mediterranean population. In GOLDN, additional adjustments for family relationships were undertaken as previously described [25]. All analyses were conducted with the statistical program SPSS for Windows (release 19.0; SPSS, Chicago, IL).
3 Results
We studied subjects from two independent European origin cohorts, one from a Mediterranean population in Spain and the other North American. Table 1 shows demographic, anthropometric, clinical, biochemical, dietary, and lifestyle characteristics of participants for each population. The Mediterranean population was younger and more obese, particularly for abdominal obesity, than the North American population, despite having similar energy intake. Values of plasma glucose and insulin were lower and MetS was less prevalent in the Mediterranean population as compared with the North American. Significant differences between these populations were found in the dietary fat intakes, with a higher total fat intake and a higher proportion of MUFA in the Mediterranean population than in the North American population. As expected, the intake of MUFA (% fat) was >50% in this Mediterranean population as has been previously described in populations following a Mediterranean diet [26]. The frequency of the single nucleotide polymorphism SNP was similar in both populations (Table 1).
Table 1.
General characteristics of both populations
| Mediterranean | North American | |||
|---|---|---|---|---|
|
n = 1465 |
n = 810 |
|||
| Mean | SD | Mean | SD | |
| Age (years) | 39.4 | 12.29 | 48.6 | 16.03 |
| Weight (kg) | 84.1 | 17.34 | 82.9 | 18.2 |
| Height (m) | 1.64 | 0.08 | 1.71 | 0.1 |
| BMI (kg/m2) | 31.1 | 5.38 | 28.3 | 5.6 |
| Waist circumference (cm) | 102.20 | 15.07 | 97.17 | 16.44 |
| Hip circumference (cm) | 114.1 | 10.38 | 107.9 | 11.75 |
| Triglycerides (mg/dL) | 101.9 | 53.21 | 139.9 | 99.58 |
| Total Cholesterol (mg/dL) | 193.2 | 36.58 | 191.4 | 39.74 |
| HDL-C (mg/dL) | 55.07 | 15.51 | 46.40 | 13.06 |
| LDL-C (mg/dL) | 118.19 | 31.85 | 122.86 | 31.68 |
| Fasting Glucose (mg/dL) | 85.59 | 16.35 | 101.87 | 19.58 |
| Fasting Insulin (mU/L) | 8.5 | 8.43 | 13.7 | 8.1 |
| Systolic BP (mmHg) | 115.56 | 16.30 | 115.61 | 16.14 |
| Diastolic BP (mmHg) | 71.36 | 10.642 | 68.41 | 9.35 |
| Energy intake (kcal/day) | 2066.7 | 715.4 | 2160.6 | 1258.9 |
| Total Fat (% Energy) | 42.24 | 9.55 | 35.56 | 6.76 |
| SFA (% Energy) | 10.25 | 4.07 | 11.85 | 2.63 |
| MUFA (% Energy) | 19.24 | 6.45 | 13.35 | 2.80 |
| PUFA (% Energy) | 4.72 | 1.73 | 7.69 | 2.19 |
| Total fat (g/day) | 98.31 | 44.54 | 86.25 | 53.02 |
| SFA (g/day) | 24.08 | 14.28 | 29.05 | 19.16 |
| MUFA (g/day) | 44.37 | 21.54 | 32.45 | 20.31 |
| PUFA (g/day) | 10.88 | 5.62 | 32.45 | 20.31 |
| MUFA (% Fat) | 55.5 | 8.1 | 37.48 | 2.43 |
| Sedentary (%) | 36.1a) | 57.4 | ||
| Metabolic syndromeb) (%) | 23.0 | 31.9 | ||
| Obesity (%) | 53.6 | 35.3 | ||
| Abdominal obesity (%)c) | 81.6 | 50.3 | ||
| REV-ERB-ALPHA1 rs2314339 | % | n | % | N |
| GG | 77.3 | 1133 | 76.1 | 616 |
| AA + AG | 22.7 | 332 | 22.3 | 194 |
We first examined the association between the REV-ERB-ALPHA1 rs2314339 and obesity-related traits. Men and women were analyzed together because no heterogeneity of the effect was observed by sex. We found significant and novel associations between REV-ERB-ALPHA1 rs2314339 genotype and obesity-related traits, evaluated as logistic and continuous outcomes with dominant and additive genetic models.
First, we used a logistic regression model to test the association of the REV-ERB-ALPHA1 rs2314339 with abdominal obesity and a Chi-square test to analyze differences of genotype frequencies. Abdominal obesity was dichotomized according to the definition for each sex with waist circumference > 102 cm (men) or > 88 cm (women) [14] (Table 2). For REV-ERB-ALPHA1 rs2314339 the frequency of the minor allele carriers (AA + AG) was significantly lower in the “abdominally obese” group than in those of the “nonabdominally obese” group, in both the Mediterranean and the North American populations (p < 0.05, Chi-square test). Moreover, minor allele carriers had a lower probability of abdominal obesity than noncarriers (logistic regression model). Of note, the association was of similar magnitude for both populations (OR ≈ 1.50), although it achieved significance only in the Mediterranean population (n = 1465; p = 0.010) while in the smaller North American population (n = 800) statistical significance was not reached (p = 0.086).
Table 2.
Genotype distribution of REV-ERB-ALPHA1 rs2314339 single nucleotide polymorphisms (SNPs) according to abdominal obesity in the Mediterranean and North American population
| Abdominal obesity |
|||||||
|---|---|---|---|---|---|---|---|
| Genotype | Absent |
Present |
Logistic regression | ||||
| N | % | N | % | p value | OR (95% CI) | p value | |
| Mediterraneana) | |||||||
| GG | 550 | 48.7 | 583 | 51.4 | 0.002 | 1.56 (1.004; 1.43) | 0.010 |
| AA + AG | 183 | 55.2 | 149 | 44.8 | |||
| North Americanb) | |||||||
| GG | 300 | 48.7 | 316 | 51.3 | 0.024 | 1.53 (0.942; 2.49) | 0.086 |
| AA + AG | 103 | 52.9 | 91 | 47.1 | |||
a) After adjusting for a) age, sex, and b) age, sex, and family relationships.
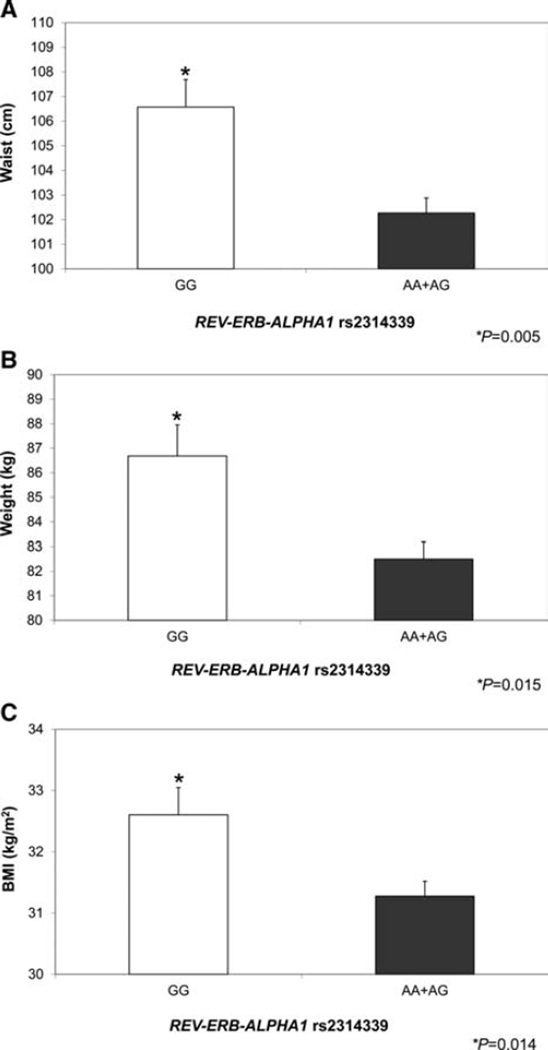
Second, we tested associations between REV-ERB-ALPHA1 rs2314339 with BMI, initial weight, and waist circumference in the Mediterranean population by ANOVA (Fig. 1). Consistent with the logistic regression analyses, there were significant associations between REV-ERB-ALPHA1 genotype and obesity-related traits, not only for total BMI but also for weight and waist circumference. In every case, minor allele carriers had lower obesity parameters. Particularly strong was the association with waist circumference in both the Mediterranean population (p = 0.005), and also in the North American population (p = 0.002). Again, minor allele carriers had lower waist circumference values in both populations. However, in contrast to the significant findings detected in the Mediterranean population using a dominant model, we used an additive model in the North American population to detect the difference in waist circumference values (cm)mean ± SD: ((83.9 ± 15.3 (AA); 94.4 ± 16.3 (AG); 98.1 ± 16.4 (GG); p = 0.002).
Figure 1.
Association of REV-ERB-ALPHA1 genotype with obesity-related traits in the Mediterranean population.
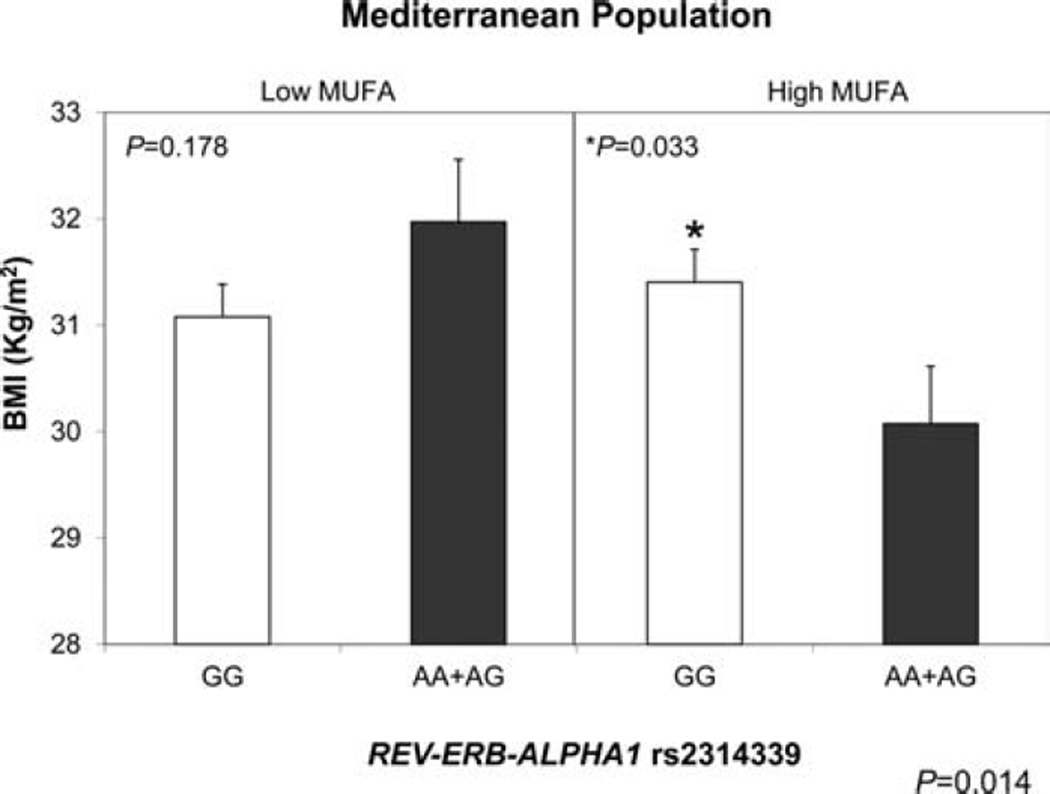
In order to better understand the novel association between REV-ERB-ALPHA1 rs2314339 genotype and obesity, we investigated whether this association could be related to differences in energy intake or expenditure. To pursue this question, we applied similar statistical methods but with the addition of a gene–diet interaction term. For evaluating gene–diet interactions, each dietary exposure was evaluated dichotomously by dividing into low and high categories based on the median population value. With respect to total energy intake, no significant associations with REV-ERB-ALPHA1 rs2314339 genotype and no interactions between genotype and energy intake were detected for the outcome of obesity. However, we identified a statistically significant interaction term (p = 0.014) between the REV-ERB-ALPHA1 rs2314339 genotype and MUFA (% of total fat) as a categorical value for the outcome of obesity in the Mediterranean population (Fig. 2), which was not replicated in the North American population. In the Mediterranean population, among those subjects with low MUFA (<55% of total fat), the REV-ERB-ALPHA1 rs2314339 variant was not significantly associated with BMI (p = 0.178). In contrast, in individuals with high MUFA (≥55% of total fat), the AA + GG genotype (minor) was associated with a lower BMI (p = 0.033).
Figure 2.
Mean (SE) BMI by rs2314339 polymorphism at the REV-ERB-ALPHA1 gene according to MUFA intakes below and above the population median (55 percentage of total fat). Estimated means were adjusted for sex, age, and nutrition centre. p Values for the interaction (p = 0.014) terms between fat intake and the rs2314339 polymorphism were obtained in the hierarchical multivariate interaction model containing MUFA intake as a categorical variable with additional control for the other covariates.
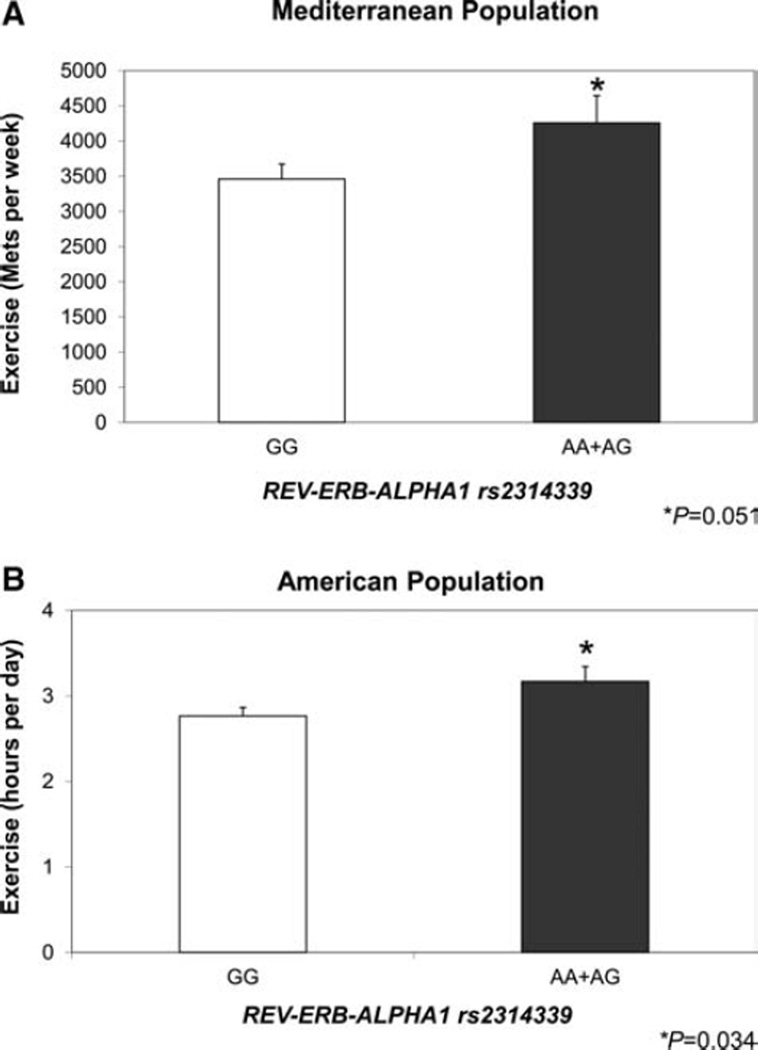
To evaluate the potential relationships between energy expenditure, REV-ERB-ALPHA1 genotype and obesity, we evaluated PA data (Fig. 3) and we detected significant associations between REV-ERB-ALPHA1 rs2314339 and PA in both populations.
Figure 3.
Association of REV-ERB-ALPHA1 genotype with PA in both populations Mediterranean and North American.
4 Discussion
We have found a significant association between the REV-ERB-ALPHA1 rs2314339 genetic variant and obesity in a Mediterranean population. More importantly, we have replicated this association in a North American, European-origin, and independent population. Based on our data, carriers of the minor allele (AA + AG) had greater protection against obesity than GG homozygotes. To better understand this relevant association, we analyzed in more depth the contribution of other significant variables such as energy intake and PA. In our populations, total energy intake was not significantly associated with REV-ERB-ALPHA1 rs2314339. However, PA differed significantly by genotype. We also detected a significant interaction for obesity between REV-ERB-ALPHA1 rs2314339 variant and MUFA intake in the Mediterranean population, demonstrating that the protection against obesity provided by the minor A allele was observed only in those who also had a high intake of MUFA (as a percentage of total fat). This study is strengthened by the replication of findings in two European-origin populations in two culturally and geographically different environments. Although genomics is providing promising insight for obesity prevention and treatment, replications are not common due to the multiple factors inherent in current experimental designs and the biological complexity. For this reason, consistency is a criterion of reliability, particularly for novel associations. This makes the current study particularly valuable because of the generalizability obtained of the results. In relation to the circadian machinery, we previously found a significant association with obesity for CLOCK SNPs that was also replicated in these same populations, despite differences in age, degree of obesity, and dietary intake [27–29]. The prior results in CLOCK and the current in REV-ERB-ALPHA1 suggest that clock-related loci are tightly and consistently connected to obesity. Along these lines, a recent study from Goumidi et al. [30] has demonstrated the impact of another REV-ERB-ALPHA1 polymorphism rs2071427, located in intron 1, on obesity phenotypes in adult and adolescent samples from three populations. However, based on the CEU (Utah residents with Northern and Western European ancestry from the CEPH collection) population, the SNP reported by Goumidi et al. is not in strong linkage disequilibrium with the SNP identified in our study. Although these two SNPs are close to each other (only 1280 base pair apart), they carry different genetic information and contribute to the phenotype separately, which indicates that the SNP reported in this paper is a novel finding.
An interesting discovery in the current study is that the frequency of the minor A allele at REV-ERB-ALPHA1 rs2314339 was significantly lower in the group characterized by abdominal obesity. Moreover, we found a significant and consistent association of REV-ERB-ALPHA1 rs2314339 with obesity-related parameters in both populations. These findings are aligned with previous results in a murine model showing that in the double knockout REV-ERB-ALPHA and REV-ERB-BETA mice, REV-ERB functions as an integral driver of the circadian clock [7]. Based on the importance of REV-ERB-ALPHA to control of the biological clock, disruption of REV-ERB-ALPHA1 function may result in a range of circadian and metabolic problems, including jet lag, sleep disorders, and obesity [7].
The recurring relationships between timing and energy metabolism are further underscored by the presence of an adipose tissue specific peripheral clock. In fact, it has been shown that in this tissue several clock genes display circadian rhythmicity ex vivo without the influence of the suprachiasmatic nucleus [31]. In particular, REV-ERB-ALPHA1 is highly expressed in adipose tissue and its expression is dramatically induced during adipogenesis, supporting a role in energy storage [32]. Additional evidence for a role of REV-ERB-ALPHA1 in energy homeostasis is suggested by the fact that human homologues of REV-ERB-ALPHA1 are encoded on the opposite strand on the thyroid receptor alpha (TR-ALPHA), which is highly implicated in energy expenditure [32, 33]. Indeed, REV-ERB-ALPHA1 and TR-ALPHA 2 mRNA products have a 269 nucleotide overlap and REV-ERB-ALPHA1 inhibits the splicing reaction that generates TR-ALPHA 2 in vitro [31, 34]. Although a direct cross-talk between REV-ERB-ALPHA and TR-alpha for the regulation of energy storage or expenditure has never been shown, the role of REV-ERB in energy storage is well known [33], while the potential effect in energy expenditure is supported by evidence that synthetic REV-ERB-a/b agonists in obese mice increase energy expenditure and decrease fat mass and body weight [9]. However, in spite of multiple potential mechanisms by which REV-ERB influence adipose tissue [32] and alters body weight, neither agonist experiments in animal models [9] nor our own study of REV-ERB-ALPHA1 rs2314339 demonstrated any relationship to energy intake.
With respect to REV-ERB and energy expenditure, the agonist studies in mice and the current study are consistent in suggesting that REV-ERB may alter the amount of energy expended. Moreover, a recent study performed in REV-ERB-ALPHA−/− mice has shown less spontaneous locomotor activity in a free-wheel exercise regimen compared to wild-type littermates [35]. Of note, REV-ERB-ALPHA was highly expressed in oxidative skeletal muscle and its deficiency in muscle led to reduced mitochondrial content and oxidative function. These cellular effects resulted in both impaired mitochondrial biogenesis and increased clearance of this organelle, leading to compromised exercise capacity [35]. These results are in line with our current findings in which we detected consistent associations between the REV-ERB-ALPHA1 rs2314339 genetic variant and PA in two populations despite the different instruments used to capture this variable.
In spite of the established role of food as a major input to circadian systems, few previous studies have examined the role of dietary factors and REV-ERB-ALPHA1 with respect to weight-related traits. In the current study, the interaction between REV-ERB-ALPHA1 rs2314339 and MUFA intake for obesity found in the Mediterranean population suggests that the type of fatty acid can modulate the association of this polymorphism with body weight. Thus, the potential protection of the minor rs2314339 allele was expressed only in those who had a high intake of MUFA. However, the presence of this interaction only in the Mediterranean population is probably related to the differential MUFA intake in North American and Mediterranean populations, (i.e. North Americans consume a significantly lower proportion of MUFA relative to the Mediterranean population), but could also be related to the greater sample size of the Spanish population. The gene*MUFA interaction for obesity outcomes was previously shown for CLOCK [28]. Taking this into account, we hypothesized that differences in red blood cells membrane MUFA content between carriers and noncarriers of CLOCK gene variants could be due to CLOCK-related changes in the circadian regulation of lipid metabolism [29]. This can also be the case for REV-ERB-ALPHA1. Indeed, it has been shown that REV-ERB-ALPHA1 influences lipid metabolism through mechanisms involving PPAR gamma, which in turn is strongly influenced by MUFA [36]. However, we cannot eliminate the possibility that other minor components of the diet may be more directly involved in driving this interaction.
One limitation of this kind of study could be the problem of multiple comparisons. However, in the current study our main goal was to look for associations between REV-ERB-ALPHA1 rs2314339 and obesity. Therefore, few comparisons were performed. Moreover, the fact that the results are replicated in two different populations diminishes the probability of false-positives. Other limitations could be the difference in measuring diets, energy intake, and PA. However, in spite of these limitations the results have been replicated which also strengths the outcomes of the current work.
In summary, this study provides the first demonstration and replication of an association between the REV-ERB-ALFA1 rs2314339 SNP and obesity. Moreover, we provide evidence suggesting that PA rather than food intake may underlie the effect of this locus. Finally, we have identified a significant interaction between this single nucleotide polymorphism, MUFA intake, and obesity. Overall, our results support the relevance of REV-ERB-ALFA1 in human obesity and provide further evidence for the strong connection between the biological clock and obesity.
Acknowledgments
This study was supported by grants from Tomás Pascual and Pilar Gómez-Cuétara Foundations, Spanish Government of Science and Innovation (BFU2011-24720), Séneca Foundation from the Government of Murcia (15123/PI/10). National Heart, Lung, and Blood Institute grants HL-54776, National Institute of Diabetes and Digestive and Kidney Diseases, Grant Number DK075030 and by contracts 53-K06-5-10 and 58-1950-9-001 from the US Department of Agriculture Research. MG designed research and wrote the paper. CES wrote the paper. PGA analyzed data and laboratory. MOM wrote the paper. YCL analyzed data and laboratory; LDP selection of the SNPs; DKA population recruitment; JMO designed research and wrote the paper. MG had primary responsibility for final content. All authors read and approved the final manuscript.
Abbreviations
- GOLDN
Genetics of Lipid Lowering Drugs and Diet Network
- MetS
metabolic syndrome
- MUFA
monounsaturated fatty acids
- PA
physical activity
Footnotes
The authors have declared no conflict of interest.
References
- 1.Garaulet M, Madrid JA. Chronobiological aspects of nutrition, metabolic syndrome and obesity. Adv. Drug Deliv. Rev. 2010;62:967–978. doi: 10.1016/j.addr.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Garaulet M, Ordovás JM, Madrid JA. The chronobiology, etiology and pathophysiology of obesity. Int. J. Obes. 2010;34:1667–1683. doi: 10.1038/ijo.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu AC, Tran HG, Zhang EE, Priest AA, et al. Redundant function of REV-ERBalpha and beta and non-essential role for Bmal1 cycling in transcriptional regulation of intracellular circadian rhythms. PLoS Genet. 2008;4:e1000023. doi: 10.1371/journal.pgen.1000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teboul M, Gréchez-Cassiau A, Guillaumond F, Delaunay F. How nuclear receptors tell time. J. Appl. Physiol. 2009;107:1965–1971. doi: 10.1152/japplphysiol.00515.2009. [DOI] [PubMed] [Google Scholar]
- 5.Preitner N, Damiola F, Lopez-Molina L, Zakany J, et al. The orphan nuclear receptor REV-ERB alpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 6.Bugge A, Feng D, Everett LJ, Briggs ER, et al. Rev-erbα and REV-ERBβ coordinately protect the circadian clock and normal metabolic function. Genes Dev. 2012;26:657–667. doi: 10.1101/gad.186858.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho H, Zhao X, Hatori M, Yu RT, et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature. 2012;485:123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar N, Solt LA, Wang Y, Rogers PM, et al. Regulation of adipogenesis by natural and synthetic REV-ERB ligands. Endocrinology. 2010;151:3015–3025. doi: 10.1210/en.2009-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solt LA, Wang Y, Banerjee S, Hughes T, et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. 2012;485:62–68. doi: 10.1038/nature11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins M, Province M, Heiss G, Eckfeldt J, et al. NHLBI Family Heart Study, objectives and design. Am. J. Epidemiol. 1996;143:1219–1228. doi: 10.1093/oxfordjournals.aje.a008709. [DOI] [PubMed] [Google Scholar]
- 11.Garaulet M, Hernández-Morante JJ, Tébar FJ, Zamora S. Anthropometric indexes for visceral fat estimation in overweight/obese women attending to age and menopausal status. J. Physiol. Biochem. 2006;62:245–252. doi: 10.1007/BF03165753. [DOI] [PubMed] [Google Scholar]
- 12.Shen J, Arnett DK, Peacock JM, Parnell LD, et al. Interleukin1beta genetic polymorphisms interact with polyunsaturated fatty acids to modulate risk of the metabolic syndrome. J. Nutr. 2007;137:1846–1851. doi: 10.1093/jn/137.8.1846. [DOI] [PubMed] [Google Scholar]
- 13.Sánchez-Moreno C, Ordovás JM, Smith CE, Baraza JC, et al. APOA5 gene variation interacts with dietary fat intake to modulate obesity and circulating triglycerides in a Mediterranean population. J. Nutr. 2011;141:380–385. doi: 10.3945/jn.110.130344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmet P, Magliano D, Matsuzawa Y, Alberti G, et al. The metabolic syndrome: a global public health problem and a new definition. J. Atheroscler. Thromb. 2005;12:295–300. doi: 10.5551/jat.12.295. [DOI] [PubMed] [Google Scholar]
- 15.Pérez-Llamas F, Garaulet M, Herrero F, Palma JT, et al. Multivalent informatics application for studies of the nutritional status of the population. Assessment of food intake. Nutr. Hosp. 2004;19:160–166. [Article in Spanish] [PubMed] [Google Scholar]
- 16.Mataix J, Mañas M, Llopis J, Martínez E. [Table of composition of Spanish foods] Tabla de composición de alimentos españoles (in Spanish) Granada, Spain: Instituto de Nutrición y Tecnología, Universidad de Granada; 1995. [Google Scholar]
- 17.Moreiras O, Carvajal A, Cabrera L. In: [Table of composition of Spanish foods] Tablas de composición de alimentos (in Spanish) Pirámide SA, editor. Madrid, Spain: EUDEMA, SA; 1995. p. 562. [Google Scholar]
- 18.Subar AF, Thompson FE, Kipnis V, Midthune D, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America’s Table Study. Am. J. Epidemiol. 2001;154:1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 19.Craig CL, Marshall AL, Sjöström M, Bauman AE, et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 20.Roman-Vinas, Serra-Majem L, Hagstrom M, Ribas-Barba L, et al. International Physical Activity Questionnaire: reliability and validity in a Spanish population. Eur. J. Sport Sci. 2010;10:297–304. [Google Scholar]
- 21.Lai CQ, Tucker KL, Parnell LD, Adiconis X, et al. PPARGC1A variation associated with DNA damage, diabetes, and cardiovascular diseases: the Boston Puerto Rican Health Study. Diabetes. 2008;57:809–816. doi: 10.2337/db07-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Partonen T. Clock gene variants in mood and anxiety disorders. J. Neural Transm. 2012;119:1133–1145. doi: 10.1007/s00702-012-0810-2. [DOI] [PubMed] [Google Scholar]
- 23.Kripke DF, Nievergelt CM, Joo E, Shekhtman T, et al. Circadian polymorphisms associated with affective disorders. J. Circadian Rhythms. 2009;7:2. doi: 10.1186/1740-3391-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campos-de-Sousa S, Guindalini C, Tondo L, Munro J, et al. Nuclear receptor REV-ERB-{alpha} circadian gene variants and lithium carbonate prophylaxis in bipolar affective disorder. J. Biol. Rhythms. 2010;25:132–137. doi: 10.1177/0748730410362713. [DOI] [PubMed] [Google Scholar]
- 25.Corella D, Arnett DK, Tsai MY, Kabagambe EK, et al. The -256T>C polymorphism in the apolipoprotein A-II gene promoter is associated with body mass index and food intake in the genetics of lipid lowering drugs and diet network study. Clin Chem. 2007;53:1144–1152. doi: 10.1373/clinchem.2006.084863. [DOI] [PubMed] [Google Scholar]
- 26.Garaulet M, Pérez-Llamas F, Pérez-Ayala M, Martínez P, et al. Site-specific differences in the fatty acid composition of abdominal adipose tissue in an obese population from a Mediterranean area: relation with dietary fatty acids, plasma lipid profile, serum insulin, and central obesity. Am. J. Clin Nutr. 2001;74:585–591. doi: 10.1093/ajcn/74.5.585. [DOI] [PubMed] [Google Scholar]
- 27.Garaulet M, Corbalán MD, Madrid JA, Morales E, et al. CLOCK gene is implicated in weight reduction in obese patients participating in a dietary programme based on the Mediterranean diet. Int. J. Obes. 2010;34:516–523. doi: 10.1038/ijo.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garaulet M, Lee YC, Shen J, Parnell LD, et al. CLOCK genetic variation and metabolic syndrome risk: modulation by monounsaturated fatty acids. Am. J. Clin. Nutr. 2009;90:1466–1475. doi: 10.3945/ajcn.2009.27536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garaulet M, Lee YC, Shen J, Parnell LD, et al. Genetic variants in human CLOCK associate with total energy intake and cytokine sleep factors in overweight subjects (GOLDN population) Eur. J. Hum. Genet. 2010;18:364–369. doi: 10.1038/ejhg.2009.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goumidi L, Grechez A, Dumont J, Cottel D, et al. Impact of REV-ERB alpha gene polymorphisms on obesity phenotypes in adult and adolescent samples. Int. J. Obes. 2013;37:666–672. doi: 10.1038/ijo.2012.117. [DOI] [PubMed] [Google Scholar]
- 31.Garaulet M, Ordovás JM, Gómez-Abellán P, Martínez JA, et al. An approximation to the temporal order in endogenous circadian rhythms of genes implicated in human adipose tissue metabolism. J. Cell. Physiol. 2011;226:2075–2080. doi: 10.1002/jcp.22531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chawla A, Lazar MA. Induction of REV-ERBA alpha, an orphan receptor encoded on the opposite strand of the alpha-thyroid hormone receptor gene, during adipocyte differentiation. J. Biol. Chem. 1993;268:16265–16269. [PubMed] [Google Scholar]
- 33.Laitinen S, Fontaine C, Fruchart JC, Staels B. The role of the orphan nuclear receptor REV-ERB alpha in adipocyte differentiation and function. Biochimie. 2005;87:21–25. doi: 10.1016/j.biochi.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Munroe SH, Lazar MA. Inhibition of c-erbA mRNA splicing by a naturally occurring antisense RNA. J. Biol. Chem. 1991;266:22083–22086. [PubMed] [Google Scholar]
- 35.Woldt E, Sebti Y, Solt LA, Duhem C, et al. REV-ERB-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat. Med. 2013;19:1039–1046. doi: 10.1038/nm.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fontaine C, Dubois G, Duguay Y, Helledie T, et al. The orphan nuclear receptor Rev-Erb alpha is a peroxisome proliferator-activated receptor (PPAR) gamma target gene and promotes PPARgamma-induced adipocyte differentiation. J. Biol. Chem. 2003;278:37672–37680. doi: 10.1074/jbc.M304664200. [DOI] [PubMed] [Google Scholar]