Abstract
Motor actions are preceded by an efference copy of the motor command, resulting in a corollary discharge of the expected sensation in sensory cortex. These mechanisms allow animals to predict sensations, suppress responses to self-generated sensations, and thereby process sensations efficiently and economically. During talking, patients with schizophrenia show less evidence of pretalking activity and less suppression of the speech sound, consistent with dysfunction of efference copy and corollary discharge, respectively. We asked if patterns seen in talking would generalize to pressing a button to hear a tone, a paradigm translatable to less vocal animals. In 26 patients [23 schizophrenia, 3 schizoaffective (SZ)] and 22 healthy controls (HC), suppression of the N1 component of the auditory event–related potential was estimated by comparing N1 to tones delivered by button presses and N1 to those tones played back. The lateralized readiness potential (LRP) associated with the motor plan preceding presses to deliver tones was estimated by comparing right and left hemispheres’ neural activity. The relationship between N1 suppression and LRP amplitude was assessed. LRP preceding button presses to deliver tones was larger in HC than SZ, as was N1 suppression. LRP amplitude and N1 suppression were correlated in both groups, suggesting stronger efference copies are associated with stronger corollary discharges. SZ have reduced N1 suppression, reflecting corollary discharge action, and smaller LRPs preceding button presses to deliver tones, reflecting the efference copy of the motor plan. Effects seen during vocalization largely extend to other motor acts more translatable to lab animals.
Key words: predictive coding, efference copy, corollary discharge, ERPs
Introduction
People with schizophrenia often misperceive sensations and misinterpret experiences, perhaps contributing to the psychotic symptoms that characterize the illness. These misperceptions and misinterpretations might result from a basic inability to make valid predictions about expected sensations and experiences. Healthy normal people take advantage of neural mechanisms that allow them to make predictions unconsciously, facilitating processing of sensations and distinguishing the expected from the unexpected. If predictive mechanisms are dysfunctional, sensations that should have been predicted but were not might take on inappropriate salience.1,2
Abnormalities of predictive coding in schizophrenia have been reported across a broad range of experimental paradigms, from those aimed at assessing failures to extract rules from environmental patterns of events3 to those aimed at understanding failures of efference copy and corollary discharge mechanisms.4–19 Studies of rule extraction typically involve patients passively receiving input from external sources and making predictions about patterns or breaks in patterns. Studies of efference copy and corollary discharge mechanisms involve patients actively generating their own sensory experiences and adjusting actions and predictions appropriately. The latter represents a paradigm shift from the traditional stimulus-response approach, for studying normal and pathological brain function, to the more interactive response-stimulus approach.
While many investigators use “efference copy” and “corollary discharge” interchangeably, or choose one term to describe both mechanisms, some groups12,20 find it useful to distinguish between them in the following way. Every move an animal makes is preceded by an efference copy of the plan to move. The efference copy of the motor plan is sent to sensory cortex, generating a corollary discharge of the expected sensory consequence of the motor act. During the movement, expected sensations resulting from the movement are effectively subtracted from the real sensations, resulting in net suppression. The greater the match between the expected and experienced sensations, the greater the suppression of the response. Thus, the efference copy and corollary discharge form the working parts of a motor-sensory prediction system, whereby the consequences of motor actions are processed efficiently and economically. These systems are critical to survival because errors in prediction are costly.
These systems have been studied across the animal kingdom.21 They have been studied during vocalization in a variety of species, from crickets22 and bats23 to human24,25 and nonhuman primates,26 using invasive techniques. In all cases, auditory responsiveness is suppressed when the animal is producing the sounds. This is consistent with noninvasive, human electrophysiological studies employing electroencephalographic (EEG) or magnetoencephalographic (MEG) responses synchronized to vocalization onset.11,12,14,15,17,19,27–29 N1 of EEG-based event-related potentials (ERP) and M100 of MEG-based responses reveal increased activity in auditory areas during speaking, but the level of activity is lower during speaking than when recorded sounds are played back to the speaker. Reduction of N1 during talking may reflect the action of the corollary discharge mechanism. Importantly, we have found that N1 suppression during talking is reduced in schizophrenia patients,11,12,14,15,17,19 consistent with dysfunction of this predictive mechanism.
While suppression of auditory cortex may reflect the neural consequence of the corollary discharge mechanism, to observe the neural reflection of the efference copy of the motor plan to speak, we need to assess neural activity before, or at the initiation of, the action and its relationship to ultimate suppression. Using invasive recording techniques in human24 and nonhuman26 primates, suppression of auditory cortical responsiveness has been observed a split second before vocalization onset. However, relating prevocalization activity in motor areas to subsequent suppression in auditory cortex has not been attempted.
The notion that sensory suppression is triggered by efferent signals from motor planning areas is supported by Voss and colleagues,30 who used pulses of transcranial magnetic stimulation (TMS) over left primary motor cortex to delay planned finger movements in the right hand. During the motor delay, shocks were delivered to both hands. Although TMS pulses delayed finger movements, the efference copy of the motor plan to move the finger was not delayed, as evidenced by suppression of cutaneous sensations to the right hand at the intended time of movement.
Because of the millisecond timing information available in EEG data, we have been able to observe neural activity ~100 ms preceding a motor act, which we have related to the efference copy of the motor plan.12,13 In one instance, we quantified the neural activity ~100 ms before speech onset and found that intertrial phase coherence of the EEG was correlated with N1 suppression to the speech sound.12 That is, healthy control (HC) subjects who had greater prespeech synchrony were better at suppressing responses to speech sounds. Because of this association, we suggested that this prespeech neural activity was the instantiation of the motor plan to talk, or the efference copy. Importantly, there was less prespeech neural synchrony in schizophrenia patients, especially those who hallucinated. Furthermore, prespeech neural synchrony was not related to subsequent suppression of N1 to the speech sound in patients. That is, the efference copy and corollary discharge signals were decoupled in patients with schizophrenia.
There is a small but growing literature indicating that auditory cortical responses to sounds are also suppressed when they are self-delivered via a button press. That is, it appears that advance warning that a particular sound is about to occur by virtue of a button press is enough to dampen sensory cortical response to that sound even though the sound does not result from a direct motor act, as it does during talking. This can be seen both for N131–35 and subsequent P236 components. This supports the notion that an efference copy transmits information about one’s agency in bringing about sensory stimulation and that the efference copy signal prepares sensory cortex for sensations that result from self-initiated acts even when the causal chain between the act and its sensory consequence is indirect and dependent on external devices. It is important to note that N1 suppression is also seen when button presses are coincidently related to tones although suppression due to coincidence and causality were not directly compared.37
While we previously assessed premovement neural activity using time-frequency analyses of EEG,12,13 premovement neural activity has traditionally been assessed in the time-voltage domain as readiness potentials (RP). RPs can be decomposed into various subcomponents, with an early component being bilaterally symmetric and a late component being contralateral to the movement.38 To our knowledge, premovement activity associated with the delivery of a tone has not been studied, and consequently, its association with suppression of cortical responses to sensations resulting from this movement is unknown.
We aimed to extend our studies of the efference copy and corollary discharge beyond the motor act of talking by asking if we can find the same pattern of findings when subjects press a button to deliver a pure tone. We predicted that we would see suppression of auditory cortical responses to self-delivered tones, as reported by others, that this suppression will be reduced in patients with schizophrenia, and that the prepress neural activity, as measured by the late RP, would be reduced in patients and be associated with suppression in HCs but not in patients.
Method
Participants
We describe data from 26 patients with DSM-IV schizophrenia (N = 23) and schizoaffective disorder (N = 3), hereinafter referred to as schizophrenia (SZ) patients. Diagnoses were based on the Structured Clinical Interview for DSM-IV (SCID).36 Diagnostic subtypes were as follows: 1 undifferentiated schizophrenia, 18 paranoid schizophrenia, 1 residual schizophrenia, 2 disorganized schizophrenia, 3 schizoaffective disorder, and 1 schizophrenia unknown subtype. Data from SZ were compared with those from 22 HC, age and gender matched to SZ. Only right-handed subjects are included in this analysis. Clinical and demographic data for both groups are presented in table 1. Community outpatient clinicians referred SZ patients to us; both groups were recruited by advertisements and word of mouth.
Table 1.
Demographics of Healthy Controls (HC) and Schizophrenia Patients
| HC Subjects, N = 22 | Schizophrenia Patients, N = 26 | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | Minimum | Maximum | Mean | SD | Minimum | Maximum |
| Age (y)* | 42.82 | 13.12 | 20.65 | 61.04 | 44.51 | 12.26 | 19.72 | 60.64 |
| Education (y)** | 16.39 | 1.99 | 12.50 | 20.00 | 13.19 | 1.67 | 9.00 | 16.00 |
| Socioeconomic status—subject*** | 25.5 | 8.8 | 11.0 | 44.0 | 46.3 | 10.3 | 33.0 | 65.0 |
| Socioeconomic status—parent**** | 25.6 | 15.8 | 11.0 | 58.0 | 34.8 | 15.5 | 11.0 | 63.0 |
| PANSS | ||||||||
| Positive | — | — | — | — | 15.0 | 4.5 | 7.0 | 26.0 |
| Negative | — | — | — | — | 15.1 | 4.4 | 8.0 | 23.0 |
| General | — | — | — | — | 29.2 | 5.2 | 22.0 | 45.0 |
| SAPS | ||||||||
| Auditory Hallucinations | — | — | — | — | 2.0 | 1.9 | 0.0 | 5.0 |
| SANS | ||||||||
| Global avolition and apathy | — | — | — | — | 2.3 | 0.7 | 1.0 | 4.0 |
| Handedness | 22 | Right | 0 | Left | 26 | Right | 0 | Left |
| 0 | Ambidextrous | — | 0 | Ambidextrous | — | |||
| Gender | 15 | Men | 7 | Women | 20 | Men | 6 | Women |
| Antipsychotic type | — | — | — | — | 20 | Second generation | ||
| — | — | — | — | 6 | First generation | |||
| — | — | — | — | 0 | None | |||
Note: PANSS, Positive and Negative Syndrome Scale; SAPS, Scale for Positive Symptoms; SANS, Scale for Negative Symptoms. *HC vs patients, P = .65; **HC vs patients, P < .001; ***HC vs patients, P < .001; ****HC vs patients, P = .05.
Exclusion criteria for HC included a past or current DSM-IV Axis I disorder based on a SCID interview or having a first-degree relative with a psychotic disorder. Exclusion criteria for both groups included a history of a significant medical or neurological illness or a history of head injury resulting in loss of consciousness. Additionally, exclusion criteria included no substance abuse in the past 3 months for both groups and no history of substance dependence (except caffeine or nicotine) for HC, while SZ group included no substance dependence in the past year. A trained research assistant, psychiatrist, or clinical psychologist conducted all interviews. University of California at San Francisco Institutional Review Board and San Francisco Veterans Affairs Medical Center approved the study, and all participants provided written informed consent.
Clinical Ratings
Within 2 weeks of ERP assessment, a clinically trained research assistant, along with a psychiatrist or clinical psychologist, rated schizophrenia symptoms using Positive and Negative Syndrome Scale (PANSS),39 Scale for Positive Symptoms (SAPS),39 and Scale for Negative Symptoms (SANS).40 One patient had a clinical assessment within 19 days.
Task
Subjects pressed a button, every 1–2 seconds, to deliver 1000 Hz, 80 dB sound pressure level, tones with zero delay between press and tone onset (Button Tone). The task was stopped after 100 tones had been delivered. The temporal sequence of tones was preserved for play back (Play Tone). In addition, subjects pressed a button at approximately the same pace, and no sound occurred (Button Alone).
Data Acquisition and Preprocessing
EEG data were recorded from 64 scalp sites and 8 external sites using a BioSemi ActiveTwo system (www.biosemi.com). EEG data were continuously digitized at 1024 Hz and referenced off-line to averaged earlobe electrodes. After EEG was re-referenced, they were digitally bandpass filtered between 0.5 and 15 Hz. Then, EEG data were separated into 3000-ms epochs time-locked to button presses (coincident with tone onset) and were baseline corrected at −600 to −500 ms.
Electrodes placed at outer canthi of both eyes and above and below the right eye recorded vertical and horizontal electrooculogram data, which were used in a regression-based algorithm41 to correct EEG epochs for eye movements and blinks at all scalp sites. After ocular correction, data were baseline corrected again at −600 to −500 ms. Outlier electrodes were interpolated within single-trial epochs based on previously established criteria.15,19,42 EEG epochs were artifact rejected for voltages exceeding ±100 μV at all scalp sites.
Data Analysis
N1 and P2 Amplitude.
To remove button press activity from tone activity, Button Alone ERPs were subtracted from Button Tone ERPs, as is typical.31–34 N1 and P2 were measured between 80 and 100 ms and 150 and 190 ms, respectively, after tone onset, relative to baseline (−100 to 0 ms) at frontal (Fz), frontal-central (FCz), and central (Cz). They were subjected to a 3-way ANOVA for group (SZ vs HC), condition (Button Tone vs Play Tone), and region (Fz, FCz, and Cz).
For correlation analyses, N1 and P2 suppression were calculated by subtracting the value obtained during Play Tone from the value obtained during Button Tone, with greater suppression seen as a more positive value for N1 and negative value for P2.
RP Amplitude.
RP was measured as the average voltage between the button press and the 100 ms preceding it, relative to a −600 to −500 ms baseline at FC3, FC4, C3, C4, CP3, and CP4. RP amplitudes were subjected to a 4-way ANOVA for group, condition (Button Tone vs Button Alone), region [FC, C, and central-parietal (CP)], and hemisphere (left vs right).
For correlation analyses, the lateralized readiness potential (LRP) during Button Tone was calculated by subtracting the amplitude at right from left hemisphere sites, with larger prepress LRPs having more negative values.
Clinical Correlations.
We tested the relationship between SANS avolition/apathy and prepress negativity recorded from left frontal-central sites13 and between SAPS Auditory Hallucinations measure and prespeech negativity recorded from FCz.12
Behavioral Data.
We assessed button pressing pace in button alone and button tone conditions using repeated measures ANOVA for group and condition.
Results
N1 Amplitude
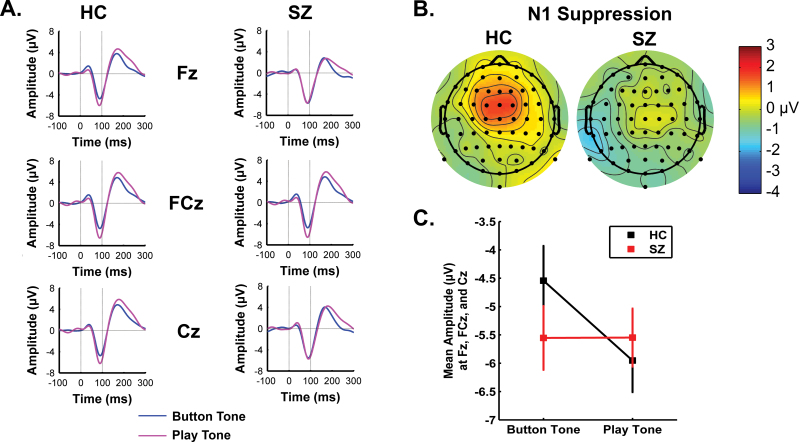
In figure 1, we show ERPs to the 1000 Hz tone for Button Tone and Play Tone conditions, scalp distributions of N1 suppression, and means.
Fig. 1.
(A) Event-related potentials to tone onset are overlaid for Button Tone (blue) and Play Tone (magenta) for healthy controls (HCs) and SZ. (B) Scalp topography maps for suppression of N1 amplitude (80–100 ms) for HC and SZ. (C) Mean N1 amplitudes, averaged across frontal, frontal-central, and central, during Button Tone and Play Tone for HC (black) and SZ (red).
N1 was suppressed during Button Tone compared with Play Tone in HC but not SZ. This was reflected in the significant condition × group interaction detailed in table 2. This was parsed by inspecting the condition effect for each group; it was significant for HC but not SZ. The interaction was also parsed by inspecting the group effect in each condition, but group was not significant in either condition. There was also a significant effect of Region, with N1 being largest at FCz.
Table 2.
ANOVA for N1 Amplitude at Frontal, Frontal-Central, and Central Regions
| Source | df | F | Significance Level |
|---|---|---|---|
| Group (HC vs SZ) | 1,46 | 0.17 | 0.68 |
| Condition (Button Tone vs Play Tone) | 1,46 | 5.12 | 0.03 |
| Condition × group | 1,46 | 5.20 | 0.027 |
| HC: condition | 1,21 | 21.28 | 0.0001 |
| SZ: condition | 1,25 | 0.00 | 0.99 |
| Button tone: group | 1,46 | 1.46 | 0.232 |
| Play tone: group | 1,46 | 0.286 | 0.595 |
| Region (frontal, frontal-central, central) | 2,92 | 3.75 | 0.03 |
| Region × group | 2,92 | 0.22 | 0.73 |
| Condition × region | 2,92 | 0.72 | 0.49 |
| Condition × region × group | 2,92 | 2.19 | 0.12 |
Note: Items in bold are significant effects at a P value of .05 or less.
P2 Amplitude
Although it appears that P2 was suppressed during button tone compared with play tone, it was not (F 1,46 = 1.78, P = .19), nor was there a condition × group interaction (F 1,46 = 1.20, P = .28).
LRP Amplitude
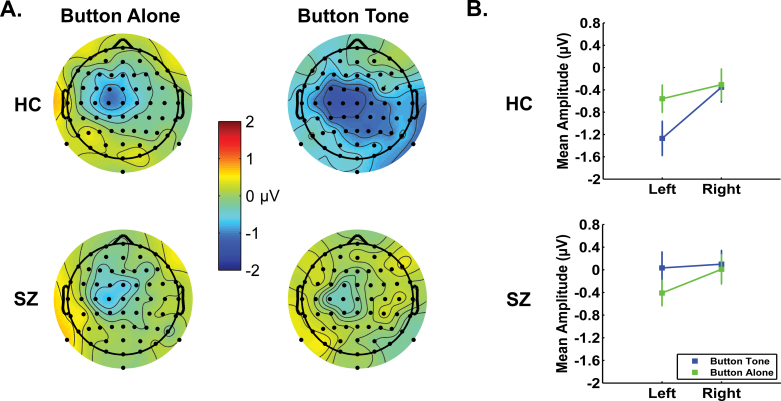
In figure 2, we show the scalp topography maps and means for button tone and button alone for HC and SZ.
Fig. 2.
(A) Scalp topography maps for readiness potential (RP) between −100 and 0 ms before the button press during Button Tone and Button Alone for healthy controls (HCs) and SZ. (B) Mean RP amplitudes averaged across frontal-central, central, and central-parietal regions for Button Tone (blue) and Button Alone (green) for HC and SZ.
There was a 2-way interaction of condition × group, as detailed in table 3. The interaction was parsed by inspecting the condition effect for HC and SZ separately. Condition was significant for HC, with RP being larger in Button Tone than in Button Alone. That is, in HC, pressing a button to deliver a tone was associated with a larger response than simple button pressing. Condition was not significant in SZ.
Table 3.
ANOVA for Readiness Potential (RP) Amplitude at Frontal-Central (FC), Central (C), and Central-Parietal (CP) Lateral Sites
| Source | df | F | Significance Level |
|---|---|---|---|
| Group (HC vs SZ) | 1,46 | 4.59 | 0.04 |
| Condition (Button Tone vs Button Alone) | 1,46 | 1.546 | 0.22 |
| Condition × group | 1,46 | 6.463 | 0.01 |
| SZ: condition | 1,25 | 2.5 | 0.10 |
| HC: condition | 1,21 | 4.43 | 0.02 |
| Button Tone: group | 1,46 | 9.97 | <0.01 |
| Button Alone: group | 1,46 | 0.34 | 0.56 |
| Site (FC, C, and CP) | 2,92 | 3.288 | 0.05 |
| Site × group | 2,92 | 0.637 | 0.51 |
| Hemisphere (left vs right) | 1,46 | 12.56 | 0.001 |
| Hemisphere × group | 1,46 | 0.031 | 0.86 |
| Condition × site | 2,92 | 1.747 | 0.19 |
| Condition × site × group | 2,92 | 0.935 | 0.39 |
| Condition × hemisphere | 1,46 | 0.214 | 0.65 |
| Condition × hemisphere × group | 1,46 | 0.719 | 0.40 |
| Hemisphere × site | 2,92 | 2.501 | 0.10 |
| Hemisphere × site × group | 2,92 | 1.7 | 0.19 |
| Condition × hemisphere × site | 2,92 | 0.435 | 0.64 |
| Condition × hemisphere × site × group | 2,92 | 7.038 | <0.01 |
| SZ: condition × hemisphere × site | 1,25 | 2.5 | 0.10 |
| HC: condition × hemisphere × site | 1,21 | 4.43 | 0.02 |
| C: condition × hemisphere | 1,21 | 0.36 | 0.56 |
| CP: condition × hemisphere | 1,21 | 0.00 | 0.95 |
| FC: condition × hemisphere | 1,21 | 5.3 | 0.03 |
| Hemisphere: button tone | 1,21 | 16.22 | 0.001 |
| Hemisphere: button | 1,21 | 1.82 | 0.19 |
Note: HC, healthy controls; SZ, schizophrenia patients. Greenhouse-Geisser corrections were used for nonsphericity in all ANOVAs. Items in bold are significant effects at a P value of .05 or less.
As can be seen in table 3, there was a 5-way interaction involving all factors. It was parsed by inspecting the 4-way interaction separately for Button Alone and Button Tone. For Button Alone, group did not interact with other variables, and no further analyses were conducted for Button Alone. For Button Tone, there was a 4-way interaction of hemisphere × area × site × group. The systematic parsing of this interaction can be seen in table 3, revealing that in HC, the RP was larger at FC3 than FC4 in the Button Tone condition.
Because this prepress period was also used as the baseline for N1, we asked whether the group × condition effects for N1 could be due effects in the baseline. The group × condition (button tone vs play tone) at midline sites for the RP was not significant (P = .94).
N1 Suppression vs LRP Amplitude
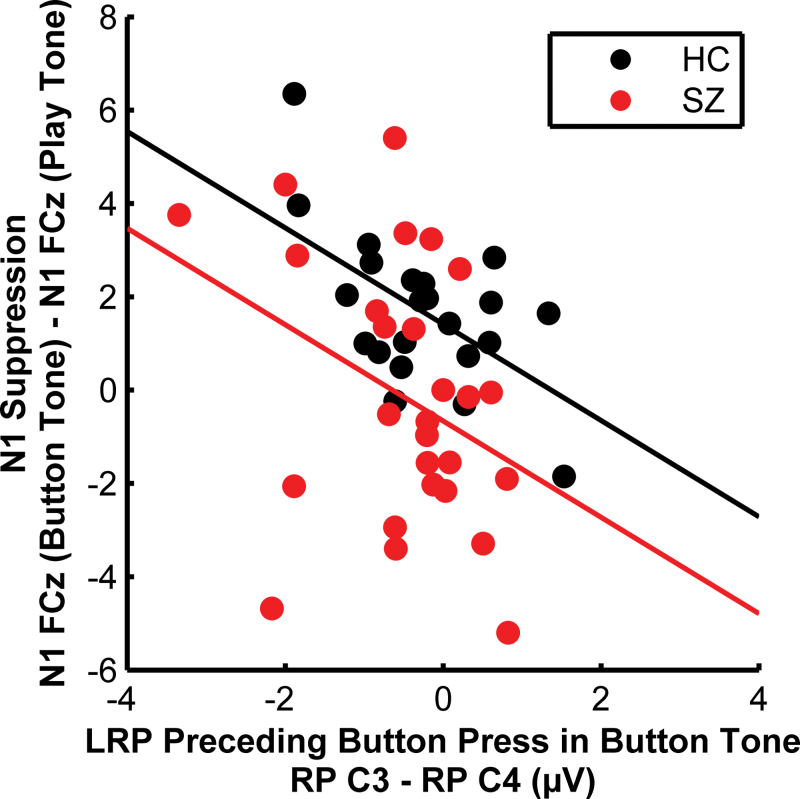
To test the association between N1 suppression and LRP amplitude, we regressed N1 suppression at FCz on LRP amplitude at C3–C4, where each was largest, with group and group × LRP as factors in the regression model. Because group × LRP was not significant (P = .87), it was dropped from the model, and a common slope assumed (figure 3). Regardless of group membership, subjects with greater N1 suppression had larger LRPs preceding the press that delivered the tones (P = .004).
Fig. 3.
Relationship between lateralized readiness potential (LRP) derived by subtracting readiness potential (RP) at C4 from C3, and N1 suppression derived by subtracting N1 at frontal-central (FC) during Play Tone from Button Tone.
Relationship Between Clinical Symptoms and ERP Measures
There were no significant relationships.
Button Pressing Pace
The mean button pressing pace was not different (P = .45) during Button Alone (2157 ms) and Button Tone (2082 ms). SZ (2343 ms) tended (P = .10) to press more slowly than HC (1896 ms). HC pressed faster in Button Tone than Button Alone (P = .003), but SZ did not (P = .53).
Discussion
We confirmed our prediction of suppression of auditory cortical responses to tones delivered via a button press. This prediction was based on the literature, rather than our own previous work, as we previously11 failed to find robust cortical suppression with self-delivery of the prerecorded personal speech sound “ah.” However, in this study, by using a pure tone, we did find suppression, as reported by others.31–34 Because we did not directly compare suppression during talking and pressing a button to deliver a tone, we cannot know whether the apparent difference is due to the different sounds or the sample. Counter to our suggestions that talking is special in its ability to produce suppression of responses to the speech sound,11 it appears that pressing a button to deliver a tone also produces robust suppression.
It is important to note that N1 is not a unitary peak,43 reflecting activity from sensory-specific and sensory-nonspecific cortical regions. Using EEG-based methods, it is difficult to tease the contributions from the different brain regions apart. However, both MEG33,37,44 and EEG45 studies have localized M100 and N1 suppression to auditory cortex, suggesting that the suppression we see in N1 during self-delivery of tones reflects auditory cortical suppression although the contribution of auditory cortex may be indirect.46 fMRI studies that could help confirm the anatomical generators of suppressed responses are lacking. Nevertheless, we suggest that N1 suppression during self-delivery of tones involves auditory cortex.
To assess the specificity of the prepress neural activity to tone delivery, we compared the final phase of the RP preceding button presses associated with the delivery of a tone (Button Tone) to the RP not associated with the delivery of a tone (Button Alone). The RP in both conditions were larger at sites contralateral to the button press, suggesting it reflects motor cortex activation preceding the button press. Importantly, neural activity immediately preceding a button press was larger and more contralateralized when the button press resulted in the delivery of a tone than when it did not. This suggested that it carried additional information, perhaps related to tone delivery.
This contralateral activity was related to suppression of cortical responses to those tones that the button press delivered. That is, people with larger LRPs preceding the press had greater suppression of the response to the tone. Importantly, this was not simply related to a person’s general RP, as RP associated with simple button pressing was not related to N1 suppression. We suggest that the LRP associated with stimulus delivery reflects the action of the motor plan to deliver a tone, or efference copy of that plan. This is consistent with our suggestions that neural activity preceding talking12 or self-paced button pressing13 is the instantiation of the efference copy.
We confirmed our prediction that patients with schizophrenia have less suppression than HCs and less activity preceding the button press when the press delivers a tone. This is consistent with our data showing that patients have less suppression to speech sounds during talking11,12,14,15,17,19 and less neural activity preceding speech onset.12 We have interpreted these data as reflecting failures of the corollary discharge and efference copy, respectively. Thus, we suggest the same interpretation holds here: even when the efference copy is indirectly related to the sound associated with it and even when the sound does not strictly come from “self,” patients with schizophrenia have failures of both the efference copy and corollary discharge.
We confirmed our prediction that premovement activity would be associated with the degree of suppression of the sensations associated with the movement, suggesting the premovement activity reflects the efference copy. However, we did not find that the relationship was stronger in controls than in patients, as we had seen with prespeech synchrony and N1 suppression to the spoken sound.12 Thus, while patients have deficits in both the efference copy and corollary discharge, the connection between them is not abnormal.
It is worth noting that, in an earlier article, we also included a visual warning, heralding the delivery of the speech sound “ah.” With this foreknowledge, N1 was modestly reduced at midline sites in controls but not in patients. Thus, there may be a general failure of predictive coding in patients with schizophrenia when predictions are based on talking to hear an “ah,” when pressing a button to get a tone or an “ah,” and when getting a visual warning of an impending “ah.” Although not involving self-delivered stimuli, predictive coding failures in schizophrenia patients can also be observed during passive listening to predictable sequences of tones. In these paradigms, patients have reduced mismatch negativities47 and P3a responses elicited by task-irrelevant deviations in predictable sequences.48
Previously, we found relationships between premovement activity and clinical symptoms. Specifically, reductions in neural synchrony preceding speech onset during talking was associated with more severe auditory verbal hallucinations,12 and reductions in lateralized prebutton press neural synchrony (not associated with the delivery of a stimulus) was associated with greater avolition and apathy.13 We were unable to demonstrate any relationships using ERP data that would have been consistent with these previous findings. We have had mixed success relating neurobiological assays of the efference copy and corollary discharge mechanisms to auditory verbal hallucinations. Perhaps a more exacting assessment of the nature of voices (eg, voices coming from inside the head vs voices coming from outside the head) than can be extracted from the SAPS and PANSS may improve the success of relating symptoms to neurobiology. Or, perhaps deficits in corollary discharge and efference copy mechanisms are persistent features in patients with a history of psychosis. For a full discussion of relating neurobiology and symptoms see Mathalon and Ford.49
Among the limitations of this study is the fact that all patients were medicated, leaving open the possibility that these effects are due to antipsychotic medications and not to the illness itself. Data collected during our talking paradigm argue against this. First, we reported deficits in cortical suppression in clinical high-risk patients, not on medications,15 and second, we see intermediate deficits in unmedicated first-degree relatives of psychotic patients.19 While medications may not be responsible for our findings, medications may have undermined our ability to relate symptoms to our neurobiological measures. For example, medications can affect the symptoms without affecting the underlying neurobiology, they can affect the neurobiology without affecting symptoms, and not every patient’s symptoms or underlying neurobiology responds similarly to medication. Another limitation is the possibility that group differences in attention may have driven the group effects on N1 suppression. Arguing against this are data showing that suppression of N1 to self-initiated tones is independent of attention.50
In conclusion, we provided evidence that pressing a button to deliver a tone produces a similar pattern of findings that we see in our talking paradigms. That is, compared with tones that are played back passively, self-delivered tones result in suppression of auditory cortical responses. The lateralized neural activity preceding that button press is related to the degree of suppression of the cortical response to that tone. Perhaps the efference copy and corollary discharge mechanisms that are invoked during vocalization are also invoked during a button press task. This suggests that these mechanisms can be studied with lab animals who do not vocalize but who can be trained to press a lever, trip a light switch, or nose-poke to deliver a tone. Moreover, neurophysiological recordings used with lab animals are similar to those used with humans, making both the paradigm and the physiological assay translatable across species. In addition, it can be argued that these paradigms have ecological validity. By involving active participation in sensory stimulation, these paradigms more closely reproduce the experience of all animals. Instead of approaching brain imaging only from the sensory side, in which animals react to input in a bottom-up fashion, these paradigms approach it from the motor side, in which animals initiate actions and interact with the environment, in a top-down manner. This is an aspect of the human experience that appears to be abnormal in schizophrenia.
Funding
Department of Veterans Affairs (I01CX000497); National Institute of Mental Health (MH-58262 to J.M.F.).
Acknowledgment
Dr Daniel Mathalon consults with Bristol-Myers Squibb.
References
- 1. Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23 [DOI] [PubMed] [Google Scholar]
- 2. Fletcher PC, Frith CD. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci. 2009;10:48–58 [DOI] [PubMed] [Google Scholar]
- 3. Servan-Schreiber D, Cohen JD, Steingard S. Schizophrenic deficits in the processing of context. A test of a theoretical model. Arch Gen Psychiatry. 1996;53:1105–1112 [DOI] [PubMed] [Google Scholar]
- 4. Feinberg I. Efference copy and corollary discharge: implications for thinking and its disorders. Schizophr Bull. 1978;4:636–640 [DOI] [PubMed] [Google Scholar]
- 5. Lindner A, Thier P, Kircher TT, Haarmeier T, Leube DT. Disorders of agency in schizophrenia correlate with an inability to compensate for the sensory consequences of actions. Curr Biol. 2005;15:1119–1124 [DOI] [PubMed] [Google Scholar]
- 6. Stirling JD, Hellewell JS, Quraishi N. Self-monitoring dysfunction and the schizophrenic symptoms of alien control. Psychol Med. 1998;28:675–683 [DOI] [PubMed] [Google Scholar]
- 7. Brébion G, Amador X, David A, Malaspina D, Sharif Z, Gorman JM. Positive symptomatology and source- monitoring failure in schizophrenia–an analysis of symptom-specific effects. Psychiatry Res. 2000;95:119–131 [DOI] [PubMed] [Google Scholar]
- 8. Shergill SS, Samson G, Bays PM, Frith CD, Wolpert DM. Evidence for sensory prediction deficits in schizophrenia. Am J Psychiatry. 2005;162:2384–2386 [DOI] [PubMed] [Google Scholar]
- 9. Turken AU, Vuilleumier P, Mathalon DH, Swick D, Ford JM. Are impairments of action monitoring and executive control true dissociative dysfunctions in patients with schizophrenia? Am J Psychiatry. 2003;160:1881–1883 [DOI] [PubMed] [Google Scholar]
- 10. Frith CD, Blakemore S, Wolpert DM. Explaining the symptoms of schizophrenia: abnormalities in the awareness of action. Brain Res Brain Res Rev. 2000;31:357–363 [DOI] [PubMed] [Google Scholar]
- 11. Ford JM, Gray M, Faustman WO, Roach BJ, Mathalon DH. Dissecting corollary discharge dysfunction in schizophrenia. Psychophysiology. 2007;44:522–529 [DOI] [PubMed] [Google Scholar]
- 12. Ford JM, Roach BJ, Faustman WO, Mathalon DH. Synch before you speak: auditory hallucinations in schizophrenia. Am J Psychiatry. 2007;164:458–466 [DOI] [PubMed] [Google Scholar]
- 13. Ford JM, Roach BJ, Faustman WO, Mathalon DH. Out-of-synch and out-of-sorts: dysfunction of motor- sensory communication in schizophrenia. Biol Psychiatry. 2008;63:736–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heinks-Maldonado TH, Mathalon DH, Houde JF, Gray M, Faustman WO, Ford JM. Relationship of imprecise corollary discharge in schizophrenia to auditory hallucinations. Arch Gen Psychiatry. 2007;64:286–296 [DOI] [PubMed] [Google Scholar]
- 15. Perez VB, Ford JM, Roach BJ, et al. Auditory cortex responsiveness during talking and listening: early illness schizophrenia and patients at clinical high-risk for psychosis. Schizophr Bull. 2012;38:1216–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frith CD. The positive and negative symptoms of schizophrenia reflect impairments in the perception and initiation of action. Psychol Med. 1987;17:631–648 [DOI] [PubMed] [Google Scholar]
- 17. Ford JM, Mathalon DH, Heinks T, Kalba S, Faustman WO, Roth WT. Neurophysiological evidence of corollary discharge dysfunction in schizophrenia. Am J Psychiatry. 2001;158:2069–2071 [DOI] [PubMed] [Google Scholar]
- 18. Ford JM, Mathalon DH, Kalba S, Whitfield S, Faustman WO, Roth WT. Cortical responsiveness during talking and listening in schizophrenia: an event-related brain potential study. Biol Psychiatry. 2001;50:540–549 [DOI] [PubMed] [Google Scholar]
- 19. Ford JM, Mathalon DH, Roach BJ, et al. Neurophysiological evidence of corollary discharge function during vocalization in psychotic patients and their non-psychotic first-degree relatives [published online ahead of print November 15, 2012]. Schizophr Bull. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miall RC, Wolpert DM. Forward models for physiological motor control Neural Networks. 1996;9:1265–79 [DOI] [PubMed] [Google Scholar]
- 21. Crapse TB, Sommer MA. Corollary discharge across the animal kingdom. Nat Rev Neurosci. 2008;9:587–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Poulet JF, Hedwig B. The cellular basis of a corollary discharge. Science. 2006;311:518–522 [DOI] [PubMed] [Google Scholar]
- 23. Suga N, Schlegel P. Neural attenuation of responses to emitted sounds in echolocating rats. Science. 1972;177:82–84 [DOI] [PubMed] [Google Scholar]
- 24. Creutzfeldt O, Ojemann G, Lettich E. Neuronal activity in the human lateral temporal lobe. II. Responses to the subjects own voice. Exp Brain Res. 1989;77:476–489 [DOI] [PubMed] [Google Scholar]
- 25. Chen CM, Mathalon DH, Roach BJ, Cavus I, Spencer DD, Ford JM. The corollary discharge in humans is related to synchronous neural oscillations. J Cogn Neurosci. 2011;23:2892–2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eliades SJ, Wang X. Neural substrates of vocalization feedback monitoring in primate auditory cortex. Nature. 2008;453:1102–1106 [DOI] [PubMed] [Google Scholar]
- 27. Curio G, Neuloh G, Numminen J, Jousmäki V, Hari R. Speaking modifies voice-evoked activity in the human auditory cortex. Hum Brain Mapp. 2000;9:183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heinks-Maldonado TH, Mathalon DH, Gray M, Ford JM. Fine-tuning of auditory cortex during speech production. Psychophysiology. 2005;42:180–190 [DOI] [PubMed] [Google Scholar]
- 29. Houde JF, Nagarajan SS, Sekihara K, Merzenich MM. Modulation of the auditory cortex during speech: an MEG study. J Cogn Neurosci. 2002;14:1125–1138 [DOI] [PubMed] [Google Scholar]
- 30. Voss M, Ingram JN, Haggard P, Wolpert DM. Sensorimotor attenuation by central motor command signals in the absence of movement. Nat Neurosci. 2006;9:26–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McCarthy G, Donchin E. The effects of temporal and event uncertainty in determining the waveforms of the auditory event related potential (ERP). Psychophysiology. 1976;13:581–590 [DOI] [PubMed] [Google Scholar]
- 32. Schafer EW, Marcus MM. Self-stimulation alters human sensory brain responses. Science. 1973;181:175–177 [DOI] [PubMed] [Google Scholar]
- 33. Martikainen MH, Kaneko K, Hari R. Suppressed responses to self-triggered sounds in the human auditory cortex. Cereb Cortex. 2005;15:299–302 [DOI] [PubMed] [Google Scholar]
- 34. Baess P, Horváth J, Jacobsen T, Schröger E. Selective suppression of self-initiated sounds in an auditory stream: An ERP study. Psychophysiology. 2011;48:1276–1283 [DOI] [PubMed] [Google Scholar]
- 35. Sowman PF, Kuusik A, Johnson BW. Self-initiation and temporal cueing of monaural tones reduce the auditory N1 and P2. Exp Brain Res. 2012;222:149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Knolle F, Schröger E, Kotz SA. Prediction errors in self- and externally-generated deviants. Biol Psychol. 2013;92:410–416 [DOI] [PubMed] [Google Scholar]
- 37. Horváth J, Maess B, Baess P, Tóth A. Action-sound coincidences suppress evoked responses of the human auditory cortex in EEG and MEG. J Cogn Neurosci. 2012;24:1919–1931 [DOI] [PubMed] [Google Scholar]
- 38. Shibasaki H, Hallett M. What is the Bereitschaftspotential? Clin Neurophysiol. 2006;117:2341–2356 [DOI] [PubMed] [Google Scholar]
- 39. Andreasen NC. Scale for the Assessment of Positive Symptoms. Iowa City, IA: University of Iowa; 1984 [Google Scholar]
- 40. Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS). Iowa City, IA: University of Iowa; 1983. [PubMed] [Google Scholar]
- 41. Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55:468–484 [DOI] [PubMed] [Google Scholar]
- 42. Nolan H, Whelan R, Reilly RB. FASTER: Fully Automated Statistical Thresholding for EEG artifact Rejection. J Neurosci Methods. 2010;192:152–162 [DOI] [PubMed] [Google Scholar]
- 43. Näätänen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24:375–425 [DOI] [PubMed] [Google Scholar]
- 44. Aliu SO, Houde JF, Nagarajan SS. Motor-induced suppression of the auditory cortex. J Cogn Neurosci. 2009;21:791–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baess P, Widmann A, Roye A, Schröger E, Jacobsen T. Attenuated human auditory middle latency response and evoked 40-Hz response to self-initiated sounds. Eur J Neurosci. 2009;29:1514–1521 [DOI] [PubMed] [Google Scholar]
- 46. SanMiguel I, Todd J, Schröger E. Sensory suppression effects to self-initiated sounds reflect the attenuation of the unspecific N1 component of the auditory ERP. Psychophysiology. 2013;50:334–343 [DOI] [PubMed] [Google Scholar]
- 47. Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76:1–23 [DOI] [PubMed] [Google Scholar]
- 48. Ford JM, Roach BJ, Miller RM, Duncan CC, Hoffman RE, Mathalon DH. When it’s time for a change: failures to track context in schizophrenia. Int J Psychophysiol. 2010;78:3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mathalon DH, Ford JM. Neurobiology of schizophrenia: search for the elusive correlation with symptoms. Front Hum Neurosci. 2012;6:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Timm J, SanMiguel I, Saupe K, Schröger E. The N1-suppression effect for self-initiated sounds is independent of attention. BMC Neurosci. 2013;14:2. [DOI] [PMC free article] [PubMed] [Google Scholar]