Abstract
Background: The study of individuals at clinical high risk (CHR) for psychosis provides an important opportunity for unraveling pathological mechanisms underlying schizophrenia and related disorders. A small number of diffusion tensor magnetic resonance imaging (DTI) studies in CHR samples have yielded anatomically inconsistent results. The present study is the first to apply tract-based spatial statistics (TBSS) to perform a whole-brain DTI analysis in CHR subjects. Methods: A total of 28 individuals meeting CHR criteria and 34 healthy controls underwent DTI. TBSS was used for a group comparison of fractional anisotropy (FA), as well as axial, radial, and mean diffusivity (AD, RD, and MD). Conversion to psychosis was monitored during a mean follow-up period of 12.3 months. Results: The rate of conversion to psychosis was relatively low (4%). TBSS revealed increased MD in several clusters in the right hemisphere, most notably in the superior longitudinal fasciculus (SLF), posterior corona radiata, and corpus callosum (splenium and body). Increased RD was restricted to a smaller area in the posterior parietal lobe. Conclusion: We present further evidence that white matter microstructure is abnormal in CHR individuals, even in a sample in which the vast majority do not transition to psychosis over the following year. In accord with previous studies on CHR individuals and patients with early-onset schizophrenia, our findings suggest an important pathological role for the parietal lobe and especially the SLF. The latter is known to undergo particularly dynamic microstructural changes during adolescence and early adulthood, a critical phase for the development of psychotic illness.
Key words: schizophrenia, prodrome, TBSS, radial, diffusivity, mean, diffusivity, parietal lobe
Introduction
In recent years, there has been increasing interest in the study of individuals at higher than normal risk for developing psychosis. Motives include the potential benefits of early intervention, as well as the opportunity to study the illness under reduced influences of confounding factors, such as aging, medication, and chronicity.
Different levels of psychosis risk can be distinguished: First, “familial/genetic high risk” refers to the presence of relatives with psychosis. Second, there are risk states defined by the presence of symptoms in the person concerned. For example, the ultra high risk (UHR) approach corresponds to a relatively narrow set of criteria primarily emphasizing positive symptoms.1,2 The Criteria of Prodromal States (COPS),3 which are based on the Scale of Prodromal Symptoms (SOPS),4 are similar to, but not identical with, the UHR criteria. The UHR/COPS syndromes have been called “late prodromal” syndromes, whereas “early prodromal” symptoms are more subtle and require wider criteria for definition,5 including the so-called basic symptoms6,7 and also schizotypal personality disorder.5,8
In the present study, inclusion criteria incorporated these different approaches (see table 1 for detailed definitions). We use the term “clinical high risk” (CHR) to denote our sample, in accordance with the terminology recently used in the North American Prodrome Longitudinal Study (NAPLS).8 However, it should be noted that our inclusion criteria were broader; the NAPLS’s inclusion criteria approximately correspond to our “late prodromal” criteria (see table 1).
Table 1.
Inclusion Criteria for Clinical High Risk (CHR) Individuals
| 1. “Late Prodromal Phase” Inclusion Criteria, corresponding to the Criteria of Prodromal States.3 |
| 1.1. Genetic Risk and Deterioration Syndrome (GRDS; also referred to as “trait and state” risk factors): Schizotypal personality disorder or first-degree relative with a psychotic disorder; 30% or greater drop in GAF score within the last month compared with the person’s highest GAF in the prior 12 mo. |
| 1.2. Attenuated Positive Symptoms Syndrome (APSS): Severity rating of 3, 4, or 5 on one or more of the 5 SOPS positive symptom scales; symptom occurs at the above severity level at an average frequency of at least once per week in the past month; symptom(s) must have begun in the past year or currently rate at least 1 point higher than if rated a year ago. |
| 1.3. Brief Intermittent Psychotic Syndrome: Severity rating of 6 (psychotic intensity) on any of the 5 positive symptom scales of the SOPS; symptom is present at least several minutes per day at a frequency of at least once a month; symptom(s) must have reached a psychotic intensity in the past 3 mo; symptom(s) have never occurred at least 1h per day at a minimum average frequency of 4 d per week over 1 mo and been seriously disorganizing or dangerous. |
| 2. “Early Prodromal Phase” Inclusion Criteria |
| 2.1. If under the age of 19 and meets DSM-IV-TR criteria for schizotypal personality disorder. |
| 2.2. Modified GRDS Criteria: First-degree relative with psychosis and a 10-point drop in the GAF score compared with a year ago (premorbid level); must be sustained over the past 3 mo. |
| 2.3. Modified APSS Criteria: The presence of at least 1 attenuated positive symptom (defined as a score of 3, 4, or 5 on any one of the 5 positive symptoms of the SOPS) and must occur at an average frequency of at least twice per month over the past 3 mo. |
| 2.4. Clinical High Risk Negative Symptoms Syndrome: The presence of at least 2 negative symptoms (defined as a score of 3 or above on any 2 of the 6 negative symptoms of the SOPS) and must occur at an average frequency of at least once per week for the past month in the context of all positive symptom ratings below a moderate degree of severity (severity rating < 3). |
| 2.5. Basic Symptoms Syndrome: Occurrence of one or more of the cognitive-perceptual symptoms,7 ie, the 10 basic symptoms that were found to have the best predictive validity for a future psychotic disorder.6 Their occurrence is defined as a rating of 3 (several times in a month or weekly) or higher, rated over the past 3 mo, on an abbreviated version of the SPI-A. The 10 symptoms are thought interference, thought perseveration, thought blockages, thought pressure, disturbances of receptive language, decreased ability to discriminate between ideas and perception or fantasy and memory, derealization, unstable ideas of reference, visual, and acoustic perception disturbances. |
In CHR individuals, only a small number of diffusion tensor magnetic resonance imaging (DTI) studies have been conducted thus far (see table 2 for overview).9–14 For example, in a recent longitudinal DTI study,10 there was a group by time interaction effect (in a cluster around the left anterior limb of the internal capsule) such that CHR individuals who converted to psychosis during the study showed a more negative fractional anisotropy (FA) trajectory than CHR subjects who did not convert. At baseline, these 2 subgroups did not show differences in FA, axial diffusivity (AD), or radial diffusivity (RD). A baseline comparison of CHR, first-episode (FE) psychosis patients, and healthy controls revealed relatively widespread effects for FA, AD, and RD such that diffusivity values of the CHR individuals were intermediate between the 2 other groups.
Table 2.
Overview of Diffusion Tensor Imaging (DTI) Literature on CHR
| Citation | Imaging Analysis Method Used | Baseline FA Differences Between CHR and NC | Baseline FA Differences Between CHR-P and CHR-NP |
|---|---|---|---|
| Peters et al12 | Tractography | Negative | (Not tested) |
| Karlsgodt et al11 | TBSS and ROI masking using atlas | SLF (collapsed over both hemispheres) | Negative |
| Peters et al14 | Voxel-wise whole-brain analysis | r superior frontal lobe, l middle frontal lobe | (Not tested) |
| Peters et al13 | Tractography | Negative | (Not tested) |
| Bloemen et al9 | Voxel-wise whole-brain analysis | r superior frontal lobe, l superior frontal lobe | CHR-NP > CHR-P: lateral to r putamen (UF, IFOF, SLF), l superior temporal lobe (SLF, ILF, IFOF); CHR-P > CHR-NP: l medial temporal lobe (PTR, ILF, IFOF) |
| Carletti et al10 | Voxel-wise whole-brain analysis | Splenium and body of corpus callosum, l ILF, l SLF, l IFOF, r EC, r retrolenticular IC, r PCRa | Negative |
Note: FA, fractional anisotropy; CHR, clinical high risk; NC, normal controls; CHR-P, CHR individuals later converting to psychosis; CHR-NP, CHR individuals who did not later convert psychosis; TBSS, tract-based spatial statistics; ROI, region of interest; r, right; l, left; SLF, superior longitudinal fasciculus; UF, uncinate fasciculus; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; PTR, posterior thalamic radiation; EC, external capsule; IC, internal capsule; PCR, posterior corona radiata.
aDifferences were not significant between the CHR and the NC group, but there was an overall significant effect of group such that NC had the highest FA, CHR had intermediate FA, and first-episode psychotic patients had the lowest FA.
Although findings for some brain regions, such as the superior longitudinal fasciculus (SLF), have been reproduced more than once,9–11 DTI results obtained from CHR samples appear rather heterogeneous, perhaps largely due to the diversity of methods, sample sizes, and hence, power. Results are anatomically diverse even among studies using similar methodology, eg, between different voxel-wise whole-brain studies, in which one group found FA changes bilaterally only in the frontal lobe,9,14 whereas a recent study found FA changes in several regions excluding frontal areas.10 Accordingly, we adopted a whole-brain approach in the present study, without a priori hypothesis about the anatomical location of any findings. In particular, we employed tract-based spatial statistics (TBSS), a relatively recent method for intersubject registration of DTI data.15
Apart from being a whole-brain method not requiring a priori anatomical hypotheses, TBSS has several other advantages.15 More specifically, in comparison with previously established voxel-based morphometry (VBM), TBSS improves intersubject registration by way of a 2-step process. The first step is a nonlinear registration similar to that used in VBM, whereas the second step includes the projection of locally maximum FA values onto a white matter “skeleton,” which is then used for group statistics. This skeletonization process also reduces partial volume effects because only the central course of white matter tracts is compared between groups, and the skeleton is FA thresholded to contain only white matter voxels.
As diffusivity parameters of interest, we chose FA, AD, RD, and mean diffusivity (MD). FA is a measure of the overall “directedness” of the Brownian movement of water molecules, AD represents the movement in the direction of maximum diffusion, and RD represents the movement perpendicular to this main axis. In the white matter of the brain, the direction of maximum diffusion is generally along the axons, whereas diffusion in the perpendicular direction is hindered by axon membranes and myelin.16 MD is a measure of overall diffusion.
Our hypotheses were based on the existing literature on schizophrenia and CHR samples: in schizophrenia, FA reduction17,18 and MD increase18 have most often been observed, and there is some evidence that these are primarily based on increased RD and not on changes in AD.19,20 In CHR samples, the only study thus far examining FA, AD, and RD together found decreased FA with a corresponding increase in RD, but bidirectional AD changes.10 Accordingly, we hypothesized for our sample that the CHR group, compared with controls, would show decreased FA attended by increased RD and MD, and unchanged AD.
Methods
Participants
The sample in this study was part of the ongoing Boston Center for Intervention Development and Applied Research (CIDAR) study (http://bricweb.bidmc.harvard.edu/bostoncidar/). Individuals with CHR symptoms (n = 33) were recruited from outpatient treatment settings and the general community in the Boston metropolitan area. Healthy controls (n = 34) were recruited from the general community and group matched to the CHR subjects on sex, age, handedness, years of education, and parental socioeconomic status (PSES).
In both CHR individuals and controls, CHR criteria were assessed with the SOPS,4 as well as the Diagnostic Interview of Personality Disorders,21 and the 10 items from the Bonn Scale for the Assessment of Basic Symptoms that were identified as having high predictive validity for the development of psychosis6,7 and are implemented in the Schizophrenia Proneness Instrument, Adult Version.22
Inclusion criteria for the CHR individuals were age 13–35 and meeting diagnostic criteria as specified in table 1 (see online supplementary methods S1 for a detailed description of exclusion criteria).
The study was approved by the local institutional review board committees at each institution, including Beth Israel Deaconess Medical Center, Massachusetts General Hospital, and Brigham and Women’s Hospital, and written informed consent was obtained from all subjects.
Magnetic Resonance Imaging Data Acquisition
All participants were scanned on a 3 T magnetic resonance imaging (MRI) scanner (TimTrio, Siemens Medical Solutions, Erlangen, Germany) at Massachusetts General Hospital, Boston, MA, using a standard circular- polarized head coil. DTI was acquired in axial orientation using a diffusion-weighted spin-echo single-shot echo-planar imaging sequence (echo time (TE) = 84 ms, repetition time (TR) = 9400 ms, flip angle = 90°, 10 images with b = 0 s/mm2 and 60 noncollinear directions with b = 700 s/mm2, 75 contiguous axial slices with 2 mm thickness, field of view (FOV) = 256, matrix size = 128 × 128, resulting in 2 mm isotropic voxels). MRI scans were carefully evaluated to rule out structural abnormalities or artifacts. Five CHR individuals were excluded because of major artifacts in DTI, leaving 28 CHR and 34 control subjects in the sample. During the course of the study, the MRI scanner was disassembled and reassembled in another room within the hospital. To monitor any resulting bias, we assessed the effect of “pre- vs postdisassembly” on the diffusivity indices measured (see below).
Image Preprocessing
First, the diffusion data were corrected for effects of motion and eddy currents through affine registration to the first b0 volume. This was done using the Linear Image Registration Tool (FLIRT), part of the Oxford Centre for Functional MRI of the Brain Software Library (FSL, http://fsl.fmrib.ox.ac.uk/fsl/). Diffusion gradients were rotated accordingly.
In order to avoid any bias due to head motion in the scanner, we computed the relative motion index as proposed by Ling et al.23 This parameter was then compared between groups using a t test. In addition, we included this index as a covariate in the statistical analysis (see below).
In order to separate brain and nonbrain areas, we first generated a brain mask using an automated method in 3Dslicer (www.slicer.org). This mask was then manually edited to avoid any misregistration due to inaccurate masking.
Next, the diffusion tensor was estimated for each voxel, using a weighted least-squares method in 3D Slicer. From these tensor volumes, scalar measures were computed for each voxel, namely FA, AD, RD, and MD.
Tract-Based Spatial Statistics
The TBSS procedure is described in detail elsewhere.15 In short, FA images from all subjects were coregistered into a template and then linearly aligned into Montreal Neurological Institute (MNI) 152 space. The Johns Hopkins University ICBM-DTI-81 white matter labels atlas24 was used to locate anatomical structures in the MNI152 space.
Because our sample included individuals as young as 13 years old that do not match the standard template, a study-specific template was created. The mean FA image of all coregistered subjects then underwent a thinning process to create a white matter skeleton, subsequently thresholded to contain only voxels with FA > 0.2. Along this skeleton, neighboring voxels were searched for the maximum local FA values, which were then projected onto the skeleton. Thus, without prior perfect coregistration, the central course of each subject’s fiber tract is represented on the skeleton. In order to analyze group differences in the other scalars of interest (AD, RD, and MD), we applied the nonlinear warps obtained from the FA registration, as well as the skeleton projection of the FA data, to the other diffusion scalar volumes.
Statistical Analysis
Group comparisons for each voxel on the skeleton were performed by cluster-based permutation tests (Randomise, FSL).25 We used threshold-free cluster enhancement, which means that clusters are created without any single arbitrary cutoff for the t value. Randomise, thus, yielded a P value that is family wise error corrected, corresponding to a 1-sided t test. Because we aimed at 2-sided testing, we set the threshold for significance to P < .025. We chose 2-sided testing because we wanted to be conservative regarding type I error and also because there have been some reports of reverse effects on diffusivity parameters in psychosis or risk samples, eg, increased FA,26 although the vast majority of studies in schizophrenia have reported decreased FA.17,18
In our statistical model, we sought to rigorously control for any potential confounders. In particular, a bias could have arisen if the diffusivity indices were systematically higher or lower after disassembly and reassembly of the MRI machine. Scanning pre- vs postdisassembly was not equally distributed between groups (χ2(1) = 3.6, P = .07, see table 3) such that controls were relatively more often scanned after reassembly.
Table 3.
Demographic and Clinical Information
| Healthy Controls (n = 34) | CHR Subjects (n = 28) | Test Statistic | P | |
|---|---|---|---|---|
| Number of females, n (%) | 18 (53) | 10 (36) | χ2(1) = 1.84 | .207 |
| Age, mean (SD, minimum, maximum) | 20.4 (4.0, 13, 29) | 20.6 (3.9, 14, 31) | t(60) = −0.193 | .847 |
| Handedness (right/either/left), n | 32/0/2 | 24/2/2 | Fisher’s exact test | .358 |
| PSES, median, mean (SD) (1–5 scale, 1 highest) | 1, 1.62 (0.817) | 2, 1.93 (0.766) | MWU = 353.5 | .058 |
| Years of education, mean (SD) | 13.2 (3.2) | 12.4 (2.6) | t(60) = 1.01 | .319 |
| WRAT, standardized reading score, mean (SD) | 120.1 (15.5) | 112.4 (16.7) | t(60) = 1.88 | .066 |
| Estimated current IQ, mean (SD) | 122.2 (13.5) | 117.9 (13.4) | t(59) = 1.25 | .216 |
| Global Assessment of Functioning, mean (SD) | 85.6 (7.2) | 51.5 (11.6) | t(43.2) = 13.5a | <.001 |
| CHR syndrome | ||||
| APSS | — | 20 | ||
| APSS and GRDS | — | 1 | ||
| APSS and SPD | — | 2 | ||
| GRDS | — | 1 | ||
| MAPSS | — | 2 | ||
| CHR− | — | 2 | ||
| Current treatment with any psychiatric medication, n (%) | — | 15 (54) | ||
| Current treatment with antipsychotic medication, n (%) | — | 6 (21) | ||
| Follow-up period in months, mean (minimum, maximum) | — | 12.3 (10, 17) | ||
| Subjects showing conversion to DSM-IV diagnosis of schizophrenia within follow-up period, n (%) | — | 1 (3.6) | ||
| Relative motion index in mm, mean (SD) | 0.69 (0.17) | 0.75 (0.17) | t(60) = −1.283 | .205 |
| Scan before vs after scanner disassembly/ reassembly, n | 8/26 | 13/15 | χ2(1) = 3.60 | .067 |
Note: Abbreviations of CHR syndromes are explained in table 1. PSES, parental socioeconomic status; WRAT, Wide-Range Achievement Test; SPD, schizotypal personality disorder.
Estimation of current IQ was based on Wechsler Adult Scale of Intelligence (WASI), Vocabulary and Block Design. WASI information was missing for 1 subject.
aDegrees of freedom adjusted for unequal variances.
We tested whether diffusivity values were systematically different between scans acquired before and after scanner disassembly/reassembly. This was done in a statistical model including diagnosis and a “pre- vs postdisassembly” binary variable, where we computed the main effect of this latter variable.
We further minimized the risk of bias by adding “pre- vs postdisassembly” as a covariate when making between group comparisons. Additional covariates were motion, age, and gender to ensure that any group effect seen would not be due to confounding effects of these variables.
Results
Demographic and Clinical Characteristics
Demographic and clinical characteristics of the 2 groups are displayed in table 3. There were no significant differences between groups in age, sex, handedness, years of education, or PSES.
Although there was a trend toward a group difference in Wide-Range Achievement Test reading score, a proxy of premorbid intelligence, groups did not significantly differ on the Vocabulary and Block Design subtests of the Wechsler Adult Scale of Intelligence, which are commonly used as an estimate of current intelligence. It is notable that both groups exceeded average estimated current IQ by about 1 SD. As expected, groups differed strongly on the Global Assessment of Functioning Scale.27
Eighty-two percent of CHR individuals were included based on the attenuated positive symptoms syndrome (see table 1). Slightly more than half of the CHR individuals were receiving psychotropic medication; among these, only a minority (n = 6) was receiving antipsychotic medication. A majority of CHR individuals also had Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV), diagnoses of nonpsychotic, ie, “comorbid,” disorders, mostly affective and anxiety disorders (see online supplementary table S1). Follow-up information was available for 26 CHR subjects (93%). Only 1 CHR individual converted to a DSM-IV diagnosis of psychotic illness (in this case, schizophrenia), in a mean follow-up period of 12.3 months (see table 3).
Diffusivity Parameters
The relative motion index23 did not differ significantly between groups (see table 3), suggesting that motion alone was unlikely to cause group differences in diffusivity indices. Further, there was no significant or trend-level effect of “pre- vs postdisassembly” on diffusivity indices.
Comparing the CHR group with controls, we detected significant increases of MD and RD (see figure 1 and table 4). In addition, there was a nonsignificant tendency toward decreased FA (P > .068). There were no significant AD changes.
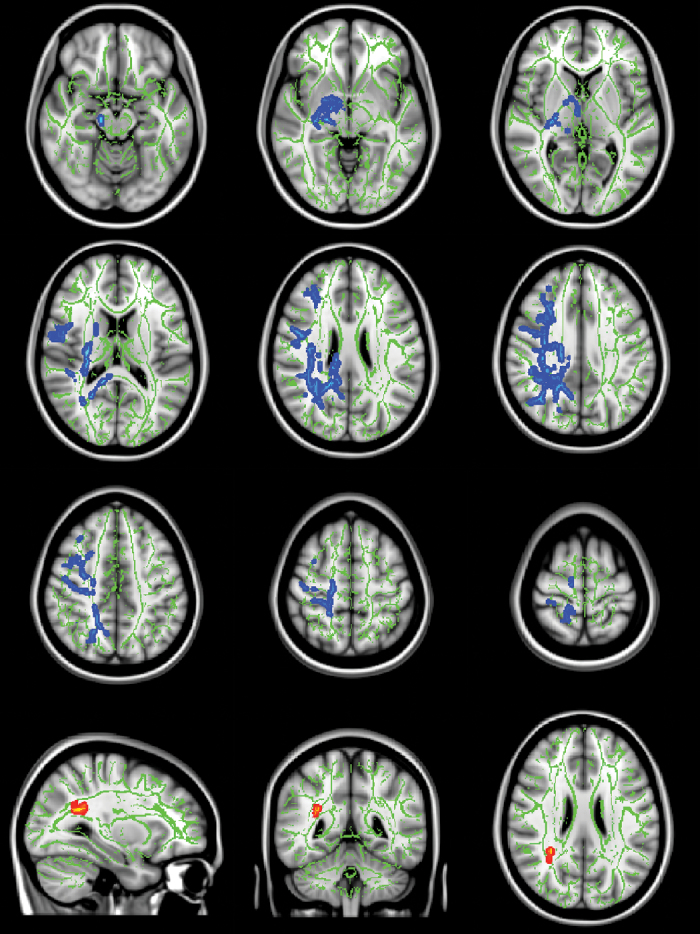
Fig. 1.
Significant increases in mean diffusivity (MD, blue) and radial diffusivity (RD, red-yellow) in clinical high risk (CHR) individuals. Significance threshold was set to P < .025 (family wise error corrected, 1-sided). In the background, the tract-based spatial statistics (TBSS) white matter skeleton (green) is shown on top of a T1-weighted template image. Slices shown are at Montreal Neurological Institute (MNI) coordinates Z = −15, −5, 5, 15, 25, 35, 45, 55, 65 for MD, and X = 31, Y = −46, and Z = 25 for RD. Left side on figure corresponds to right hemisphere.
Table 4.
MNI Coordinates of Clusters of Significant (P < .025, Family Wise Error Corrected, One-Sided) Group Difference in MD and RD (CHR > Controls)
| Diffusion Tensor Imaging Index | Cluster Size (mm2) | MNI Coordinates of Peak Voxel | White Matter Tracts Overlapping With the Clusters (Size of Overlap in mm3)a | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| MD | 9 169 | 24 | −50 | 20 | r SLF (703), r PCR (501), splenium of corpus callosum (409), r PLIC (322), r SCR (240), r cerebral peduncle (161), r RLIC (158), r cingulum (cingulate part, 130), body of corpus callosum (125), r PTR (69), r ALIC (50), r fornix/stria terminalis (38), r EC (16), r tapetum (2) |
| 5 | 12 | −26 | 71 | ||
| 2 | 24 | 29 | 35 | ||
| 1 | 29 | 50 | 25 | ||
| RD | 52 | 31 | −51 | 22 | r PCR (51) |
| 8 | 32 | −53 | 26 | ||
Note: Abbreviations are explained in the first footnote to table 2. MNI, Montreal Neurological Institute; MD, mean diffusivity; RD, radial diffusivity; PLIC, posterior limb of internal capsule; SCR, superior corona radiata; RLIC, retrolenticular part of internal capsule; ALIC, anterior limb of internal capsule.
aWhite matter tracts as defined with the Johns Hopkins University White Matter Label Atlas.24
The RD and MD findings were lateralized to the right hemisphere. The group differences in MD were widespread in posterior regions, mainly including the SLF (all previously described28 subcomponents, see online supplementary material S2), posterior and superior corona radiata, posterior thalamic radiation, posterior internal capsule, body and splenium of corpus callosum, and cingulum bundle. However, rostral and subcortical areas were also affected, including the anterior limb of the internal capsule, fornix, and cerebral peduncle. The significant effects on RD were more spatially limited and located within the posterior corona radiata.
The CHR individual later converting to schizophrenia was not an outlier that could have biased the group difference in MD or RD (see online supplementary result S3).
See online supplementary material S4 for further analyses regarding correlations of diffusivity indices with clinical variables, which showed an association between MD in SLF and level of functioning within the control group but not the CHR group. This could be due to additional confounding factors, such as medication, in the CHR group. Lastly, we saw a nonsignificant tendency toward FA increase with age over both groups, whereas there was no age-by-group interaction (see online supplementary material S5).
Discussion
We detected white matter alterations in a sample of individuals meeting psychosis CHR criteria. Hypothesis-free whole-brain comparison using TBSS revealed group differences in RD and MD (CHR > controls), which were most prominent in the right parietal lobe.
The SLF was the white matter tract most widely affected, which corresponds to some (but not all) previous DTI studies of CHR samples9–11 (see table 2). It is noteworthy, however, that parietal regions are not among the most consistent findings in the DTI literature on schizophrenia, which is the case rather for frontal and temporal white matter.17,18
However, more posterior, especially parietal, abnormalities may play an important role in the early phase of schizophrenia. For example, a DTI study in early-onset schizophrenics revealed bilateral parietal, but not frontal or temporal, alterations in FA.29
Furthermore, the meta-analysis cited above17 revealed that right frontoparietal white matter volume (including fibers of the SLF) is increased in FE patients, both in comparison with healthy controls and chronic patients, which would be compatible with a more acute pathology in this region in the early stages of psychotic illness. Another study, assessing the longitudinal gray matter loss in very early-onset schizophrenia, found a striking posterior to anterior dynamic, with gray matter volumes decreasing in the parietal lobe first, and only later in the “classical” schizophrenia regions, the frontal and temporal lobes.30
In light of these findings, it is highly noteworthy that the SLF (a major frontoparietal connection) seems to be one of the most dynamically changing white matter tracts during adolescence and early adulthood, according to a recent meta-analysis of TBSS studies assessing normal development of FA.31
Taken together, these data suggest that the parietal lobe and the SLF may play a crucial role in the development and early phase of schizophrenia, an illness with typical onset in late adolescence and early adulthood.
The finding of increased RD without significant FA decrease is probably related to the fact that FA is a nonlinear mapping of RD and AD. If RD increases, but AD does not change, this will be reflected in decreased FA. However, a concurrent (nonsignificant) increase in AD can “compensate” the negative effect of increased RD on FA. The direction of AD change in pathological states appears heterogeneous: a recent DTI study found both increases and decreases of AD in CHR individuals,10 and increases in AD have also been reported in schizophrenia.18 In our study, there was indeed a nonsignificant tendency toward increased AD in the CHR group (data not shown). This could explain the lack of FA findings in the context of significant effects on RD.
Nonsignificant increases in AD could also explain why the MD differences were considerably more widespread than the differences in RD. (This is because MD is the mean of the 3 eigenvalues, AD is the first eigenvalue and RD is the mean of the second and third eigenvalues—therefore, a nonsignificant increase in AD would add to the positive effect of RD on MD.)
The biological correlates of these DTI parameters are far from obvious. In particular, it is very difficult to dissect the relative contributions of axonal membranes and myelin sheathing to the anisotropy of water diffusion, although axonal membranes seem to play the primary role.16 There have been combined imaging-histopathology animal studies indicating that RD increase might be a more specific marker of myelin pathology (and AD decrease of axonal damage),32 although other data have suggested that RD increases also in the context of reduced density of axonal membranes.33
Thus, the RD and MD differences seen in our sample cannot be simply equated to any histological dimension. Nevertheless, myelin changes remain a plausible candidate, particularly in light of the histopathological data about myelin damage in schizophrenia.34 We cannot, however, discount the fact that the white matter changes observed could also be secondary to gray matter loss, although we did not perform volumetric analyses to test for such correlations.
The right-lateralized findings are also difficult to interpret. Brain asymmetry seems to be associated with different factors, including gender and pathological states such as schizophrenia.35 We did not have sufficient numbers of subjects within the sex subgroups to explicitly test such interaction effects. Larger studies in CHR samples, containing larger gender subgroups, are therefore warranted.
When interpreting our results, it should be noted that DTI abnormalities in CHR subjects, if at all present, are probably more subtle than those in FE subjects, which is consistent with a recent study comparing patients with FE schizophrenia, CHR subjects, and controls.10 Indeed, in that study, which had sample sizes very similar to our study, the diffusivity differences between the CHR group and controls were themselves nonsignificant.10
Moreover, methodological considerations need to be taken into account. In particular, we used TBSS, which restricts analysis to the central part (the “skeleton”) of the white matter tracts. Although this likely reduces partial volume effects, abnormalities in peripheral white matter might be overlooked. Also, and importantly, the skeleton projection step in TBSS does not correct all remaining misalignment after the initial coregistration step.36
For an interpretation of our findings, it is also necessary to note the clinical particularities of our sample: Conversion rate in this study was 4% within a mean follow-up of 12.3 months, which is low compared with some other studies (eg, 41%,1 35%,2 and 54%37 in 12 months; a recent meta-analysis38 demonstrated 22% conversion rate within 12 months). Consequently, if our sample included many individuals who were never going to transition to psychosis (or only in the relatively distant future), neurobiological markers (including DTI) might also be closer to normal than in other CHR samples.
The low conversion rate seen here, however, cannot solely be due to the inclusion criteria used in this study because over 86% of CHR individuals included met 1 or more of the COPS criteria (“late prodromal” symptoms). Rather, it is possible that there is a trend toward lower conversion rates in newer studies (eg, 26% in 400 days),39 which could be for different reasons. First, higher awareness among healthcare providers and potentially patients and families themselves may cause more individuals to become part of CHR studies, with the proportion of “truly prodromal” individuals decreasing. Second, pharmacological and psychotherapeutic treatment in CHR individuals might prevent or mitigate the outbreak of frank psychosis. Moreover, it is obviously unknown how many CHR individuals convert after the follow-up periods. To some extent, the low conversion rate might also be explained by the fact that the CHR individuals were up to 31 years old, which is older than the peak age for schizophrenia onset. We also noted a higher-than-average intelligence in both groups (see table 3), and there is evidence that cognitive function is inversely correlated with transition to psychosis in CHR populations.40 If our CHR sample, on average, has good cognitive resources, this would be another explanation for the rather subtle DTI abnormalities seen.
We note another issue concerning the external validity and comparability of this and other studies: there was a high level of comorbidity in our sample, mostly consisting of mood and anxiety disorders. This finding is in itself not surprising: Woods et al39 reported 69% of a large sample of CHR individuals (n = 377) to have at least 1 anxiety and/or affective comorbid diagnosis. If mood and anxiety symptoms often coexist with CHR symptoms, this raises the question of whether they are themselves also predictive of psychosis. For mood disorders, there is some evidence that this is the case,1,2 although data are thus far inconclusive regarding anxiety symptoms.1,2
It would be very interesting to compare comorbidity rates and diagnoses with other CHR DTI studies, but this information has not been given in the reports to date.9–14
As an important methodological limitation, we note that the scanner had been disassembled and reassembled while the study was ongoing. Diagnosis groups were not optimally matched between pre- and postdisassembly. Therefore, we cannot entirely rule out the possibility that group differences are, in part, the result of this bias. However, we note that there was no effect of “pre- vs postdisassembly” on the diffusivity values, and including this factor into the statistical model did not negate the significant group differences in the diffusivity values.
In conclusion, our findings further support the hypothesis that changes in white matter microstructure are present in CHR states, thus confirming earlier studies.9–11,14 Moreover, our findings are compatible with a prominent role of the parietal lobe and its connecting tracts, especially the SLF, in very early stages of psychosis pathology.
Supplementary Material
Supplementary material is available at http://schizophr eniabulletin.oxfordjournals.org.
Funding
National Institutes of Health [P50 MH 080272 to R.W.M., M.E.S., and L.J.S.; UO1 MH081928 to L.J.S.; R01M H074794 to O.P., M.K., and M.E.S.]; the Commonwealth Research Center of the Massachusetts Department of Mental Health (SCDMH82101008006 to L.J.S.); Veterans Affairs Merit Awards to M.E.S. and R.W.M.; Veterans Affairs Schizophrenia Center to R.W.M. and M.E.S.; National Alliance for Research in Schizophrenia and Depression Distinguished Investigator Award to M.E.S. and O.P.; National Alliance for Research in Schizophrenia and Depression Young Investigator Grant from the Brain & Behavior Research Foundation to O.P.; Clinical Translational Science Award (UL1RR025758) and General Clinical Research Center Grant (M01RR01032) from the National Center for Research Resources to Harvard University and Beth Israel Deaconess Medical Center; the National Center for Research Resources (P41RR14075); Shared Instrumentation Grants (1S10RR023401, 1S10RR019307, 1S10RR023043). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Supplementary Material
Acknowledgments
We thank all subjects for their participation in the study. We also thank Psychiatry Neuroimaging Laboratory Software Engineer, Ryan Eckbo, MSc, Research Assistants, including Kathryn Hawley, BA, Kelsey Smith, BA, and Paula Pelavin, BA, for their help and support. We also thank the clinical and data management staff from the Boston CIDAR study, including Matcheri Keshavan, MD, Joanne Wojcik, PhD, APRN, Ann Cousins, PhD, APRN, Michelle Friedman-Yakoobian, PhD, Anthony J. Giuliano, PhD, Andréa Gnong Granato, MSW, Lauren Gibson, EdM, Sarah Hornbach, BA, Julia Schutt, BA, Kristy Klein, PhD, Maria Hiraldo, PhD, Grace Francis, PhD, Corin Pilo, LMHC, Rachael Serur, BS, Grace Min, EdM, Alison Thomas, BA, and Molly Franz, BA. We also thank Nikos Makris, MD, PhD, for neuroanatomical advice. This work is part of CCvH’s doctorate thesis (Dr Med.). The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Yung AR, Phillips LJ, Yuen HP, et al. Psychosis prediction: 12-month follow up of a high-risk (“prodromal”) group. Schizophr Res. 2003;60:21–32 [DOI] [PubMed] [Google Scholar]
- 2. Yung AR, Phillips LJ, Yuen HP, McGorry PD. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67:131–142 [DOI] [PubMed] [Google Scholar]
- 3. Woods SW, Miller TJ, McGlashan TH. The “prodromal” patient: both symptomatic and at-risk. CNS Spectr. 2001;6:223–232 [DOI] [PubMed] [Google Scholar]
- 4. Miller TJ, McGlashan TH, Woods SW, et al. Symptom assessment in schizophrenic prodromal states. Psychiatr Q. 1999;70:273–287 [DOI] [PubMed] [Google Scholar]
- 5. Olsen KA, Rosenbaum B. Prospective investigations of the prodromal state of schizophrenia: review of studies. Acta Psychiatr Scand. 2006;113:247–272 [DOI] [PubMed] [Google Scholar]
- 6. Klosterkötter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58:158–164 [DOI] [PubMed] [Google Scholar]
- 7. Schultze-Lutter F, Klosterkötter J, Picker H, Steinmeyer E-M, Ruhrmann S. Predicting first-episode psychosis by basic symptom criteria. Clin Neuropsych. 2007;4:11–22 [Google Scholar]
- 8. Addington J, Cadenhead KS, Cornblatt BA, et al. North American Prodrome Longitudinal Study (NAPLS 2): overview and recruitment. Schizophr Res. 2012;142:77–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bloemen OJ, de Koning MB, Schmitz N, et al. White-matter markers for psychosis in a prospective ultra-high-risk cohort. Psychol Med. 2010;40:1297–1304 [DOI] [PubMed] [Google Scholar]
- 10. Carletti F, Woolley JB, Bhattacharyya S, et al. Alterations in white matter evident before the onset of psychosis. Schizophr Bull. 2012;38:1170–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karlsgodt KH, Niendam TA, Bearden CE, Cannon TD. White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biol Psychiatry. 2009;66:562–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peters BD, de Haan L, Dekker N, et al. White matter fibertracking in first-episode schizophrenia, schizoaffective patients and subjects at ultra-high risk of psychosis. Neuropsychobiology. 2008;58:19–28 [DOI] [PubMed] [Google Scholar]
- 13. Peters BD, Dingemans PM, Dekker N, et al. White matter connectivity and psychosis in ultra-high-risk subjects: a diffusion tensor fiber tracking study. Psychiatry Res. 2010;181:44–50 [DOI] [PubMed] [Google Scholar]
- 14. Peters BD, Schmitz N, Dingemans PM, et al. Preliminary evidence for reduced frontal white matter integrity in subjects at ultra-high-risk for psychosis. Schizophr Res. 2009;111:192–193 [DOI] [PubMed] [Google Scholar]
- 15. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505 [DOI] [PubMed] [Google Scholar]
- 16. Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455 [DOI] [PubMed] [Google Scholar]
- 17. Bora E, Fornito A, Radua J, et al. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res. 2011;127:46–57 [DOI] [PubMed] [Google Scholar]
- 18. Fitzsimmons J, Kubicki M, Shenton ME. Review of functional and anatomical brain connectivity findings in schizophrenia. Curr Opin Psychiatry. 2013;26:172–187 [DOI] [PubMed] [Google Scholar]
- 19. Scheel M, Prokscha T, Bayerl M, Gallinat J, Montag C. Myelination deficits in schizophrenia: evidence from diffusion tensor imaging. Brain Struct Funct. 2013;218:151–156 [DOI] [PubMed] [Google Scholar]
- 20. Seal ML, Yücel M, Fornito A, et al. Abnormal white matter microstructure in schizophrenia: a voxelwise analysis of axial and radial diffusivity. Schizophr Res. 2008;101:106–110 [DOI] [PubMed] [Google Scholar]
- 21. Zanarini MC, Frankenburg FR, Chauncey DL, Gunderson JG. The diagnostic Interview for Personality Disorders: interrater and test-retest reliability. Compr Psychiatry. 1987;28:467–480 [DOI] [PubMed] [Google Scholar]
- 22. Schultze-Lutter F, Addington J, Ruhrmann S, Klosterkötter J. The Schizophrenia Proneness Instrument, Adult Version (SPI-A). Rome, Italy: Giovanni Fioriti Editore; 2007. [Google Scholar]
- 23. Ling J, Merideth F, Caprihan A, Pena A, Teshiba T, Mayer AR. Head injury or head motion? Assessment and quantification of motion artifacts in diffusion tensor imaging studies. Hum Brain Mapp. 2012;33:50–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mori S, Wakana S, Nagae-Poetscher LM, van Zijl PCM. MRI Atlas of Human White Matter. Amsterdam, the Netherlands: Elsevier; 2005. [Google Scholar]
- 25. Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoptman MJ, Nierenberg J, Bertisch HC, et al. A DTI study of white matter microstructure in individuals at high genetic risk for schizophrenia. Schizophr Res. 2008;106:115–124 [DOI] [PubMed] [Google Scholar]
- 27. Hall RC. Global assessment of functioning. A modified scale. Psychosomatics. 1995;36:267–275 [DOI] [PubMed] [Google Scholar]
- 28. Makris N, Kennedy DN, McInerney S, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15:854–869 [DOI] [PubMed] [Google Scholar]
- 29. Kyriakopoulos M, Vyas NS, Barker GJ, Chitnis XA, Frangou S. A diffusion tensor imaging study of white matter in early-onset schizophrenia. Biol Psychiatry. 2008;63:519–523 [DOI] [PubMed] [Google Scholar]
- 30. Thompson PM, Vidal C, Giedd JN, et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci U S A. 2001;98:11650–11655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peters BD, Szeszko PR, Radua J, et al. White matter development in adolescence: diffusion tensor imaging and meta-analytic results. Schizophr Bull. 2012;38:1308–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harsan LA, Poulet P, Guignard B, et al. Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. J Neurosci Res. 2006;83:392–402 [DOI] [PubMed] [Google Scholar]
- 33. Concha L, Livy DJ, Beaulieu C, Wheatley BM, Gross DW. In vivo diffusion tensor imaging and histopathology of the fimbria-fornix in temporal lobe epilepsy. J Neurosci. 2010;30:996–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Konrad A, Winterer G. Disturbed structural connectivity in schizophrenia primary factor in pathology or epiphenomenon? Schizophr Bull. 2008;34:72–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rentería ME. Cerebral asymmetry: a quantitative, multifactorial, and plastic brain phenotype. Twin Res Hum Genet. 2012;15:401–413 [DOI] [PubMed] [Google Scholar]
- 36. Zalesky A. Moderating registration misalignment in voxelwise comparisons of DTI data: a performance evaluation of skeleton projection. Magn Reson Imaging. 2011;29:111–125 [DOI] [PubMed] [Google Scholar]
- 37. Miller TJ, McGlashan TH, Rosen JL, et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159:863–865 [DOI] [PubMed] [Google Scholar]
- 38. Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69:220–229 [DOI] [PubMed] [Google Scholar]
- 39. Woods SW, Addington J, Cadenhead KS, et al. Validity of the prodromal risk syndrome for first psychosis: findings from the North American Prodrome Longitudinal Study. Schizophr Bull. 2009;35:894–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Giuliano AJ, Li H, Mesholam-Gately RI, Sorenson SM, Woodberry KA, Seidman LJ. Neurocognition in the psychosis risk syndrome: a quantitative and qualitative review. Curr Pharm Des. 2012;18:399–415 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.