Abstract
DNA methylation, one of the main epigenetic mechanisms to regulate gene expression, appears to be involved in the development of schizophrenia (SZ). In this study, we investigated 7562 DNA methylation markers in blood from 98 SZ patients and 108 healthy controls. A linear regression model including age, gender, race, alcohol, nicotine and cannabis use status, and diagnosis was implemented to identify C-phosphate-G (CpG) sites significantly associated with diagnosis. These CpG sites were further validated using an independent data set. Sixteen CpG sites were identified with hyper- or hypomethylation in patients. A further verification of expression of the corresponding genes identified 7 genes whose expression levels were also significantly altered in patients. While such altered methylation patterns showed no correlation with disorganized symptoms and negative symptoms in patients, 11 CpG sites significantly correlated with reality distortion symptoms. The direction of the correlations indicates that methylation changes possibly play a protective mechanism to lessen delusion and hallucination symptoms in patients. Pathway analyses showed that the most significant biological function of the differentially methylated CpGs is inflammatory response with CD224, LAX1, TXK, PRF1, CD7, MPG, and MPO genes directly involved in activations of T cells, B cells, and natural killer cells or in cytotoxic reaction. Our results suggest that such methylation changes may modulate aspects of the immune response and hence protect against the neurobiological substrate of reality distortion symptoms in SZ patients.
Key words: hyper- or hypo, methylation, gene expression, reality distortion symptom, inflammatory response
Introduction
It has been generally accepted that both genetic and environmental factors play a significant role in schizophrenia (SZ). However, as a complex genetic disorder, the epigenetic role in the development of SZ has been recognized in recent years, after first being proposed and modeled by Petronis.1 DNA methylation as one of the main epigenetic mechanisms regulates the expression of genes across the life span. The best known mechanism involves methylation of C-phosphate-G (CpG) dinucleotides within the promoter region of a gene, which silences or downregulates the expression of the gene. It has shown functions in embryonic development,2 X-chromosome inactivation, genomic imprinting,3 and preservation of chromosome stability.4 It is not surprising that DNA methylation may mediate environmental influences on gene expression related to SZ5–8 and a epigenetic model may integrate diverse empirical data into a powerful SZ pathogenetic synthesis.9 In addition to several gene-specific methylation studies of SZ using postmortem brain tissue,10–14 a methylome-wide study of the frontal cortex strongly supported the epigenetic theory of major psychosis, in particular SZ.15
It is important to know whether, or to what extent, the easily accessible peripheral tissues such as blood can act as a surrogate to study the intersubject phenotypic variation, ie, whether methylation change in blood can indicate the status of SZ patients. A recent study by Davies et al16 analyzed DNA methylome-wide profiling across tissues and found that the interindividual differences of DNA methylation are significantly correlated across blood and brain. The correlation between blood and cerebellum was 0.76, and it was 0.66 between blood and cortex. This study together with other studies11,17–20 supports the validity for the investigation of blood DNA methylation in SZ.
Few studies have used blood DNA methylation to identify potential biomarkers for SZ disease status.21,22 Global hypomethylation in leukocytes has characterized SZ patients in 2 studies,23,24 and some genes with hyper- or hypomethylation in blood were also reported21,23 in SZ patients. Yet, the epigenetic study of SZ is still in its early phase. The mechanism by which methylation interacts with genetic and environment factors to regulate or reflect to the progress of SZ is far from clear. We believe that by incorporating methylation, gene expression, and psychopathology related to SZ we can derive informative findings to help understand the role of methylation change in SZ. In this study, we have tested SZ diagnostic effect on DNA methylation at each CpG site across the genome in 98 SZ patients and 108 healthy controls. Independent data sets were used to validate methylation changes and to investigate the corresponding gene expression changes. Furthermore, we evaluated the association of altered methylation patterns with symptoms of patients.
Methods
Participants
Participants in this study come from the Mind Clinical Imaging Consortium, a collaborative effort of 4 research sites. Site information and enrollment for SZ patients and healthy controls are explained in online supplementary text. Ninety-eight SZ patients and 108 healthy controls were analyzed here. As listed in table 1, no significant difference was observed in age and gender between patients and controls. Significantly more African Americans were enrolled as patients than controls, which we controlled for in our regression model. Alcohol and cannabis use were assessed by 2 categories: (1) abuse or dependence (not active during the month previous to the study) and (2) never abused or dependent. Nicotine use was measured by calculated Pack_Years, as done by Cullen et al.25 Summaries of symptom measures and medication status for patients are also listed in table 1. Patients symptoms were assessed by the Scale of the Assessment of Positive Symptoms and the Scale of the Assessment of Negative symptoms.26,27 Reality distortion symptom score is the sum of the global ratings of delusions and hallucinations, and disorganized symptom score is the sum of the global ratings of bizarre behavior and positive formal thought disorder. The sum of the global ratings of affective flattening, alogia, avolition-apathy, and anhedonia-asociality is the negative symptom score.
Table 1.
Demographic Information of Subjects Analyzed in This Study
| Subjects | Male/Female | Age | Race (White/Black/ Asian/Others) | Alcohol Abuse/Dependent | Cannabis Abuse/Dependent | Nicotine Pack_Year | |
|---|---|---|---|---|---|---|---|
| SZ (98) | 73/25 | 34±11 | 73/17/3/5 | 32 | 30 | 8.8±11.8 | |
| Healthy controls (108) | 70/38 | 32±11 | 97/3/4/4 | 0 | 0 | 1.0±4.8 | |
| Patient information | |||||||
| Reality distortion symptoms | Range: 0–10; mean: 4.84±2.80 | ||||||
| Disorganized symptoms | Range: 0–10; mean: 1.85±1.93 | ||||||
| Negative symptoms | Range: 0–20; mean: 8.11±3.97 | ||||||
| Age of onset | Range: 13–46; mean: 22±6 | ||||||
| Antipsychotic medication | 90 patients in use; 3 not in use; 5 with missing data | ||||||
| Use of haloperidol, risperidone, clozapine, olanzapine, quetiapine, and aripiprazole | 7, 32, 15, 13, 11, 18 patients for each type, respectively | ||||||
| Use of HDAC inhibitor | 6 patients on divalproex sodium; 1 on valproic acid | ||||||
Note: HDAC, histone deacetylases; SZ, schizophrenia.
DNA Methylation Assay
DNA from blood samples was assessed by the Illumina Infinium Methylation27 Assay. A methylation value, beta (β), represents the ratio of the methylated probe intensity to the total probe intensity. A series of quality controls on the beta values were applied to remove bad samples and probes (see online supplementary text). Then, 7562 “variable probes”21 in autosomes were selected for association analyses. Variable probes are defined by calculating the mean of the SD of beta values of all probes and selecting those with SD larger than the mean SD value.21
Genomic Methylation Analyses for SZ Association
Methylation has been shown to be affected by the interplay between genetic and environmental factors and has shown to differ under various conditions, including age,28,29 gender,30 race,31 diet, and lifestyle.32–34 The impact of nicotine and alcohol use on DNA methylation has been specifically studied, and both global change and changes in specific genes have been reported in various tissues.35–44 Albeit there have been no direct studies of cannabis use on methylation, it is conceivable and has been hypothesized45 that a similar effect may occur. Considering such potential confounding factors, we applied to each CpG site a linear regression model: β = α1 Age + α2 Gender + α3 Race + α4 Alcohol_Use + α5 Cannabis_Use + α6 Pack_Years + α7 Diagnosis + ε. We calculated the significance of diagnosis effect and its effect size: a delta-β (Δβ) value represents the mean difference in methylation between SZ patients and controls after correcting for the covariates. To identify the CpG sites associated with SZ diagnosis, we applied similar criteria as in the study of Dempster et al,21 requiring both significance P value <.0001 and effect size Δβ > 0.02.
Validation on Methylation Changes
A public Gene Expression Omnibus (GEO) data GSE 41037 were used for validation. These data include DNA methylation profiling of whole blood in 325 SZ patients and 394 healthy subjects, assessed by the same Illumina Infinium Methylation27 Assay. This sample comprises 446 males and 273 females with an average age of 37 ± 16 years. Without substance use information, a simple linear regression with diagnosis, age, and gender was used to validate SZ association of identified methylation sites in our data. A P value less than .05 for diagnosis effect was required for validation.
Gene Expression Verification
A public GEO data, GSE38484, were used for verification of gene expression changes. These data include gene expression profiles in whole blood of 106 SZ patients and 96 controls. The verification was only applied to validated methylation sites, using a linear regression model with age, gender, and diagnosis as independent variables, followed by a permutation test. The details are proved in online supplementary text.
Medication and Symptoms Tests
Medication measures include the current chlorpromazine equivalent dosage,46 and 7 separate binary variables indicating the current use of haloperidol, risperidone, clozapine, olanzapine, quetiapine, aripiprazole, and histone deacetylases inhibitors (divalproex and valproic acid). In addition, duration of illness was also tested to capture the effect of chronicity. Symptoms included reality distortion symptoms, disorganized symptoms, negative symptoms, and age of onset. Correlations between methylation values of validated sites and such medication and symptom variables were calculated. A P value less than .05 was considered significant for any potential correlation. Furthermore, we conducted permutation tests, through randomly selecting a set of CpG sites equal in number to the validated sites and calculating the chance the number of correlated CpG sites was the same or higher than that found from the validated methylation sites.
Pathway and Network Analysis
Interactive pathway analyses (IPA) from Ingenuity Systems (http://www.ingenuity.com) was used to assess the validated methylation-altered genes for the enrichment of gene networks, canonical pathways, and biological processes. Enrichment for specific pathways, networks, or functions was determined relative to the Ingenuity Knowledge Base, a repository of biological interactions and functional annotations created from millions of individually modeled relationships between proteins, genes, complexes, cells, tissues, drugs, and diseases.
Results
In our data of 98 SZ patients and 108 healthy controls, we identified 20 CpG sites associated with SZ diagnosis (P < .0001, and absolute Δβ > 0.02), after controlling for age, gender, race, alcohol, cannabis, and nicotine use. During the validation test, 14 CpG sites showed patient vs control group differences with P < .05 and 2 sites showed marginal difference with P values <.06. These 16 sites presented the same altered hyper- or hypomethylation in patients in the validation data as in our discovery data, as shown by Δβ (SZ patients − controls) in table 2. Hereafter, we consider these 16 sites from 16 genes as validated methylation-altered sites, on which further gene expression verification, medication effect, symptom association, and pathway analyses were conducted. The full names of these genes are provided in online supplementary text.
Table 2.
Results From the 16 C-phosphate-G Sites Associated With SZ
| Gene | Target ID | Discovery | Validation | Gene expression | Reality Distortion Symptoms | ||||
|---|---|---|---|---|---|---|---|---|---|
| Δβa | P value | Δβa | P value | Δb | P value | r c | P value | ||
| CD244 | cg11939496 | −0.03 | 4.75E-05 | −0.04 | 6.47E-10 | −0.07 | 1.91E-02 | 0.26 | 8.50E-03 |
| LAX1 | cg12022621 | 0.02 | 4.04E-05 | 0.01 | 6.18E-02* | −0.10 | 5.67E-03 | −0.26 | 1.06E-02 |
| ESPNL | cg09039163 | −0.03 | 1.28E-05 | −0.01 | 2.52E-03 | −0.02 | 5.47E-02 | 0.11 | 2.84E-01 |
| TXK | cg20981615 | 0.03 | 3.36E-05 | 0.01 | 1.24E-04 | 0.02 | 3.34E-01 | −0.36 | 3.00E-04 |
| PRF1 | cg09914304 | 0.02 | 2.50E-05 | 0.04 | 3.69E-07 | −0.46 | 2.29E-10 | −0.20 | 4.47E-02 |
| MS4A1 | cg06806711 | 0.02 | 9.26E-06 | 0.01 | 6.10E-04 | 0.02 | 3.78E-01 | −0.15 | 1.27E-01 |
| TCN1 | cg20018806 | −0.02 | 3.65E-05 | −0.01 | 1.20E-04 | 0.23 | 2.23E-03 | 0.22 | 2.84E-02 |
| APBA2 | cg21917349 | 0.03 | 3.64E-05 | 0.01 | 1.67E-02 | −0.01 | 9.11E-01 | −0.27 | 6.50E-03 |
| FAM173A | cg09830866 | 0.03 | 9.66E-05 | 0.04 | 1.53E-07 | −0.08 | 2.72E-02 | −0.31 | 2.30E-03 |
| CBFA2T3 | cg13745346 | −0.02 | 1.11E-05 | −0.03 | 4.10E-07 | −0.09 | 1.93E-06 | 0.27 | 7.70E-03 |
| MPG | cg16003913 | −0.03 | 8.87E-05 | −0.01 | 5.89E-02* | 0.00 | 7.32E-01 | 0.31 | 1.60E-03 |
| CD7 | cg02473123 | 0.02 | 6.05E-05 | 0.02 | 1.11E-03 | −0.22 | 2.41E-05 | −0.22 | 2.76E-02 |
| MPO | cg04988978 | −0.03 | 9.15E-05 | −0.01 | 6.93E-05 | 0.08 | 2.54E-01 | 0.18 | 7.30E-02 |
| SLC25A10 | cg07845392 | −0.02 | 2.59E-05 | −0.04 | 9.50E-10 | 0.01 | 5.48E-01 | −0.03 | 7.51E-01 |
| CKM | cg19154438 | 0.02 | 3.33E-05 | 0.03 | 6.63E-09 | 0.00 | 4.75E-01 | −0.21 | 4.18E-02 |
| H1F0 | cg07141002 | −0.03 | 8.38E-06 | −0.04 | 2.92E-06 | −0.08 | 1.22E-01 | 0.14 | 1.58E-01 |
aΔβ of SZ patients—controls.
bΔ, a gene expression difference of SZ patients—controls.
c r, correlation between reality distortion symptom scores and methylation values.
*Marginal significant P values of ~.06.
From the gene expression verification test, 7 genes showed marked expression changes with P < .05 (shown in bold in the gene expression column in table 2) with CD244, LAX1, PRF1, FAM173A, CBFA2T3, and CD7 upregulated and TCN1 downregulated in patients. According to the 100 000 permutation test on randomly selected 16 genes, the chance of having 7 or more genes with significant SZ-related up- or downregulation is less than 0.05. Five of these changes followed the direction of hypomethylation to upregulation and hypermethylation to downregulation, eg, SZ patients showed hypomethylation at the CpG site in TCN1 and corresponding upregulation of TCN1 gene expression. Two genes, CD244 and CBFA2T3, presented downregulation with hypomethylation.
When testing medication effects, the methylation of gene MS4A1 presented a significant association with current chlorpromazine equivalent dosage (P = .01). Higher dos age correlated to higher methylation values, leading to the same direction as SZ-related hypermethylation. Thus, MS4A1 hypermethylation observed in patients may be due to medication. Therefore, we removed MS4A1 from the genetic pathway analyses of SZ methylation-altered genes. Out of 7 types of psychiatric medications, only quetiapine showed an effect on methylation patterns of CD244, PRF1, APBA2, FAM173A, CBFA2T3, and CD7, where use of quetiapine was linked to methylation change counteracting the SZ related hyper- or hypomethylation. A post hoc test showed that the use of quetiapine was not correlated to any symptom scores (P > .38). Regarding illness duration, genes MPG and SLC25A10 showed significant correlations with P = .015 and P = .003, respe ctively. The longer the duration of illness, the higher the methylation value. This hypermethylation effect is opposite to the hypomethylation observed in patients, suggesting that illness duration may reflect a mixture of prolonged medication exposure and/or a saturation effect.
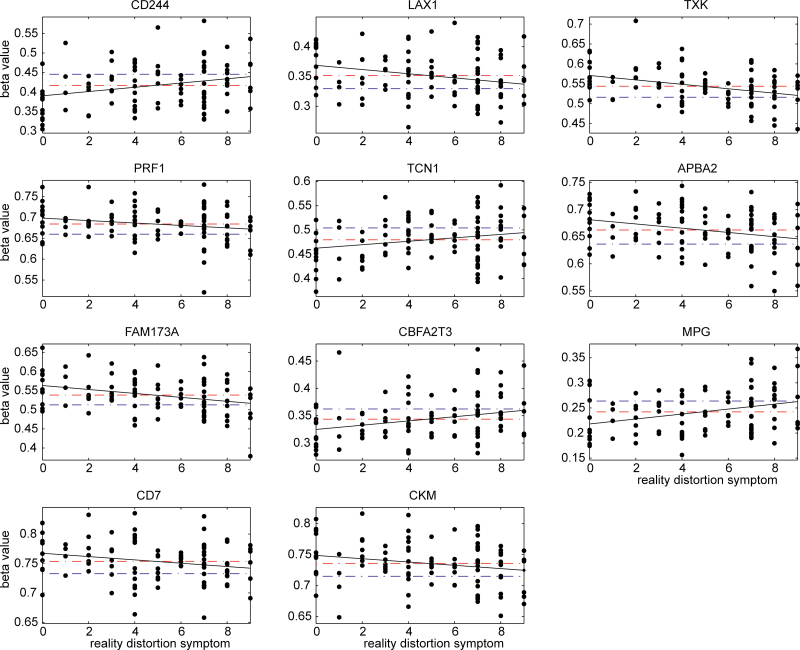
Out of the 16 sites, none were correlated with disorganized symptoms or negative symptoms. In contrast, 11 CpG sites were significantly correlated with reality distortion symptoms, which are shown as P values in bold in the last column in table 2. The 100 000 permutation test showed that the chance of having 11 or more sites associated with reality distortion symptoms is less than 1.0 × 10−6. We plotted the slopes between reality distortion symptom scores and methylation beta values in figure 1, as well as the mean values for both patients and controls. Seven sites from genes LAX1, TXK, PRF1, APBA2, FAM173A, CD7, and CKM, showed that SZ patients were on average hypermethylated with higher mean beta values than controls; the higher the beta values patients had, the lower the reality distortion symptom scores were. In the other 4 CpG sites from genes CD244, TCN1, CBFA2T3, and MPG, SZ patients were on average hypomethylated with lower mean beta values than controls, and the lower the beta values, the lower the reality distortion symptom scores. Therefore, from the relative position of mean differences and the slope direction, it can be seen that the altered hyper- or hypomethylation observed in SZ patients relates to lower reality distortion symptom scores. In addition, methylation of CBFA2T3 also correlated to age of onset (P = .028); late onset was linked to low methylation values, suggesting that hypomethylation in patients delays symptom onset.
Fig. 1.
Methylation beta values of 11 C-phosphate-G (CpG) sites in schizophrenia (SZ) patients sorted by their reality distortion symptom scores; x axis plots reality distortion symptoms and y axis plots the measured beta values. Red dashed lines are the mean beta values of patients, while blue dashed lines are the mean beta values of healthy controls. The black line presents the slope between reality distortion symptom score and methylation beta value in patients.
Removing 1 gene, MS4A1, IPA revealed the most significant network of hematological development and function from methylation-altered genes, involving activation of lymphocytes (CD244, CD7, LAX1, and PRF1), activation of T cells (CD7, LAX1, PRF1, and TXK), activation of natural killer cells (CD224 and PRF1), activation of B cells (LAX1), and differentiation of granulocytes (CBF2T3). These genes together with genes MPO and MPG, involved in cytotoxic reaction, formed the most significant biological function, the inflammatory response (P = 7.0 × 10−6).
Discussion
Given the known effect on methylation from age, gender, ethnicity, substance use, and comorbidity between SZ and substance abuse, we implemented a full regression model to account for possible confounding factors. The 16 CpG sites not only presented significant association with SZ diagnosis after controlling for confounding factors in our data but also presented the same hyper- or hypomethylation in patients in an independent data set with a significance P value close to or less than .05. The significance criteria used in our discovery data are not strictly Bonferroni corrected. Instead, it is a combination of P < .0001 and effect size Δβ > 0.02. This practice21 provides a balanced control for false-positive and false-negative results. Using our data as an example, 2 CpG sites associated with diagnosis in the discovery group (P = 4.6 × 10−6 and 5.5 × 10−6, passing Bonferroni correction, and Δβ < 0.02) could not be replicated with the validation group with P = .28 and .39. These 2 sites are likely false positives. In contrast, 1 CpG site (cg03636183) residing in F2RL3 showed an association with Pack_Years of P = 2.1 × 10− 5, failing Bonferroni correction, but its connection with smoking has been reported and replicated in different studies.38,40 Thus, strict Bonferroni correction will likely cause a false-negative finding in this case. We believe that by using combined P value and effect size as criteria, plus validation tests, we are able to identify with confidence the altered methylation associated with SZ diagnosis.
To further probe the biological effect of altered methylation patterns, we tested their corresponding gene expression changes. Seven genes were significantly up- or downregulated in SZ patients with P < .05. Five CpG sites were located near to transcription start sites (TSS), ranging from 144 to 705 base pairs away, and their corresponding gene expression changes followed the direction of hypermethylation to downregulation and hypomethylation to upregulation, which reflects a common methylation regulation effect. The other 2 CpG sites in genes CD244 and CBFA2T3, located 915 and 1121 base pairs away from their TSS, respectively, were hypomethylated in patients, and their gene expression were downregulated. Given only 1 CpG site for each gene was measured in our data, we were unable to explore the exact mechanisms of gene expression change. However, the changes that we observed provides further evidence that altered methylation patterns in patients may affect downstream cellular change and further influence behavioral or clinical symptoms.
Our correlation tests with symptoms showed pronounced associations of methylation with reality distortion symptoms, while no connection with disorganized and negative symptoms. Eleven CpG sites were correlated with reality distortion symptoms, which occurred well below chance (P < 1.0 × 10−6). As illustrated in figure 1, for the 7 sites, which were hypermethylated in patients, increased methylation was associated with lower reality distortion symptom scores. For the 4 sites, which were hypomethylated in patients, decreased methylation was associated with lower reality distortion symptom scores. The 11 sites present that the altered methylation is related to a lower reality distortion symptom score. Though we cannot prove causality, the results are consistent with methylation changes being a responsive or protective biological reaction to counteract delusion and hallucination symptoms. The same suggestion can be drawn from the correlation of CBFA2T3 hypomethylation with age of onset, whereby the SZ-related hypomethylation delays illness onset.
This suggestion is further strengthened by the genetic function analyses. In particular, genes involved directly in the inflammatory response provide a possible biological pathway to react to or participate in the pathological changes of SZ. Our findings that the methylation of these genes, related to inflammatory processes and reality distortion symptoms in the SZ group, converges with several lines of evidence regarding the importance of inflammatory processes in SZ. Doorduin et al,47 using positron emission tomography, documented increased microglial activation in the hippocampus of SZ subjects with significant positive symptoms. Moreover, antipsychotic drugs have been reported to modulate microglial activation and inhibit the production of various cytokines in the brain.48,49 In addition, a variety of anti-inflammatory drugs, like celocoxib, minocycline, and aspirin, appear to have antipsychotic properties.50–53 It is possible that the immune-modulating effects on psychotic symptoms are in part through gene expression change (specifically downregulation confirmed by our study) regulated by hypermethylation of LAX1, TXK, PRF1, CD7, and CKM and hypomethylation of CD244, CBFA2T3, and MPG. It is also noteworthy that gene TCN1 encodes a vitamin B12-binding protein that facilitates the transport of B12 into cells. Our data suggest that upregulation of TCN1 expression by hypomethylation in SZ patients reduces reality distortion symptoms, consistent with the observation of B12 deficiency-induced SZ-like symptoms.54,55
Regarding medication influence, our test showed that hypermethylation of MS4A1 in patients was significantly influenced by medication dosage. Therefore, it was removed for genetic analyses. The use of quetiapine in patients decreased the SZ-associated hyper- or hypomethylation and was not related to symptoms. Therefore, we believe that the hyper- or hypomethylation acting against reality distortion symptoms in patients is not an effect of the medication.
The cross-sectional nature of this study limits causal inferences. Recently, Davies et al16 reported a set of genes in neurodevelopment and neuronal differentiation functional pathways differentially methylated across blood, brain, and regions of brain in a healthy population.16 We tested whether this set of genes better relates to SZ or SZ symptoms. We found no significant results (for details see online supplementary text), suggesting that these genes are more sensitive to healthy brain development than to SZ. We speculate that the methylation patterns we identified in blood related to inflammation may correspond to similar methylation patterns in brain tissue, as demonstrated by high correlation of interindividual variation across brain and blood.16 Future postmortem methylation studies and longitudinal studies assessing the relationship between symptoms and methylation would provide important mechanistic information.
Our study comes with a number of limitations. One main limitation is that methylation value derived from whole blood presents a mixture of various leukocyte subtypes. As reported by Reinius et al,56 methylation differs between leukocyte subtypes, and methylation patterns in whole blood can be different from that in specific cell types. Our findings regarding methylation in whole blood can be partially confounded by various proportions of leukocyte subtypes. Further study is necessary to confirm the relation between methylation of inflammation genes with SZ. Second, measures from Illumina Methylation27 Assay were not confirmed by a different platform due to inadequate DNA quantity. However, Breitling and colleagues38 utilized Sequenom MALDI-TOF mass spectrometry to verify the pattern detected by Illumina Methylation27 Assay and arrived at similar results. Alternatively, we used an independent sample to validate the methylation pattern. Third, gene expression data to verify the methylation functional effect were drawn from different samples. It may reduce the direct connection between methylation and gene expression change, but the expression change we have observed in a different sample still provides, to an extent, evidence of methylation regulation effect. Fourth, we investigated DNA methylation of peripheral blood sample, where methylation of brain tissue may be more directly related to schizophrenic symptoms. The last limitation is that methylation can be potentially affected by many environmental factors, such as diet, medication, and lifestyle. We do not have accurate chronic medication dosage measures to precisely assess chronic medication effect though we have used current medication dosage and illness duration to provide some indications. We have tested the effect of body mass index (BMI) by adding BMI into the regression model, which did not change our results. We also tested the interaction terms between alcohol, cannabis, and nicotine use and diagnosis, but neither interaction term changed results.
In summary, DNA methylation of some genes, mainly related to inflammation, in blood is significantly altered in SZ patients, and this hyper- or hypomethylation pattern also protects against reality distortion symptoms. Our data provide evidence for the functional impact of methylation in SZ, and a potential pathway through epigenetic regulation, which has been hypothesized earlier.
Supplementary Material
Supplementary material is available at http://schizophrenia bulletin.oxfordjournals.org.
Funding
National Institutes of Health (R01EB005846 to V.C. and R33DA027626 to J.L.).
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Petronis A. The origin of schizophrenia: genetic thesis, epigenetic antithesis, and resolving synthesis. Biol Psychiatry. 2004;55:965–970 [DOI] [PubMed] [Google Scholar]
- 2. Haaf T. Methylation dynamics in the early mammalian embryo: implications of genome reprogramming defects for development. Curr Top Microbiol Immunol. 2006;310:13–22 [DOI] [PubMed] [Google Scholar]
- 3. Paulsen M, Ferguson-Smith AC. DNA methylation in genomic imprinting, development, and disease. J Pathol. 2001;195:97–110 [DOI] [PubMed] [Google Scholar]
- 4. Tuck-Muller CM, Narayan A, Tsien F, et al. DNA hypomethylation and unusual chromosome instability in cell lines from ICF syndrome patients. Cytogenet Cell Genet. 2000;89:121–128 [DOI] [PubMed] [Google Scholar]
- 5. Thibaut F. Why schizophrenia genetics needs epigenetics: a review. Psychiatr Danub. 2012;24:25–27 [PubMed] [Google Scholar]
- 6. Malaspina D, Perrin M, Kleinhaus KR, Opler M, Harlap S. Growth and schizophrenia: aetiology, epidemiology and epigenetics. Novartis Found Symp. 2008;289:196–203; discussion 203,–197 238–140 [DOI] [PubMed] [Google Scholar]
- 7. Müller N, Dursun SM. Schizophrenia genes, epigenetics and psychoneuroimmunology therapeutics: all make sense now? J Psychopharmacol. 2011;25:713–714 [DOI] [PubMed] [Google Scholar]
- 8. Akbarian S. The molecular pathology of schizophrenia–focus on histone and DNA modifications. Brain Res Bull. 2010;83:103–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maric NP, Svrakic DM. Why schizophrenia genetics needs epigenetics: a review. Psychiatr Danub. 2012;24:2–18 [PubMed] [Google Scholar]
- 10. Abdolmaleky HM, Cheng KH, Russo A, et al. Hypermethy lation of the reelin (RELN) promoter in the brain of schizophrenic patients: a preliminary report. Am J Med Genet B Neuropsychiatr Genet. 2005;134B:60–66 [DOI] [PubMed] [Google Scholar]
- 11. Abdolmaleky HM, Cheng KH, Faraone SV, et al. Hypom ethylation of MB-COMT promoter is a major risk factor for schizophrenia and bipolar disorder. Hum Mol Genet. 2006;15:3132–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grayson DR, Jia X, Chen Y, et al. Reelin promoter hypermethylation in schizophrenia. Proc Natl Acad Sci U S A. 2005;102:9341–9346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iwamoto K, Bundo M, Yamada K, et al. DNA methylation status of SOX10 correlates with its downregulation and oligodendrocyte dysfunction in schizophrenia. J Neurosci. 2005;25:5376–5381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peedicayil J. The role of epigenetics in altered gene expression involved in GABAergic transmission in the cerebellum of schizophrenia patients. Am J Psychiatry. 2009;166493; author reply 493–494 [DOI] [PubMed] [Google Scholar]
- 15. Mill J, Tang T, Kaminsky Z, et al. Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am J Hum Genet. 2008;82:696–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davies MN, Volta M, Pidsley R, et al. Functional annotation of the human brain methylome identifies tissue-specific epigenetic variation across brain and blood. Genome Biol. 2012;13:R43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Teschendorff AE, Menon U, Gentry-Maharaj A, et al. An epigenetic signature in peripheral blood predicts active ovarian cancer. PLoS One. 2009;4:e8274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Widschwendter M, Apostolidou S, Raum E, et al. Epigenotyping in peripheral blood cell DNA and breast cancer risk: a proof of principle study. PLoS One. 2008;3:e2656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hsiung DT, Marsit CJ, Houseman EA, et al. Global DNA methylation level in whole blood as a biomarker in head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2007;16:108–114 [DOI] [PubMed] [Google Scholar]
- 20. Nohesara S, Ghadirivasfi M, Mostafavi S, et al. DNA hypomethylation of MB-COMT promoter in the DNA derived from saliva in schizophrenia and bipolar disorder. J Psychiatr Res. 2011;45:1432–1438 [DOI] [PubMed] [Google Scholar]
- 21. Dempster EL, Pidsley R, Schalkwyk LC, et al. Disease-associated epigenetic changes in monozygotic twins discordant for schizophrenia and bipolar disorder. Hum Mol Genet. 2011;20:4786–4796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen Y, Zhang J, Zhang L, Shen Y, Xu Q. Effects of MAOA promoter methylation on susceptibility to paranoid schizophrenia. Hum Genet. 2012;131:1081–1087 [DOI] [PubMed] [Google Scholar]
- 23. Melas PA, Rogdaki M, Ösby U, Schalling M, Lavebratt C, Ekström TJ. Epigenetic aberrations in leukocytes of patients with schizophrenia: association of global DNA methylation with antipsychotic drug treatment and disease onset. FASEB J. 2012;26:2712–2718 [DOI] [PubMed] [Google Scholar]
- 24. Shimabukuro M, Sasaki T, Imamura A, et al. Global hypomethylation of peripheral leukocyte DNA in male patients with schizophrenia: a potential link between epigenetics and schizophrenia. J Psychiatr Res. 2007;41:1042–1046 [DOI] [PubMed] [Google Scholar]
- 25. Cullen KR, Wallace S, Magnotta VA, et al. Cigarette smoking and white matter microstructure in schizophrenia. Psychiatry Res. 2012;201:152–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andreasen NC. Scale for the assessment of positive symptoms (SAPS). Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- 27. Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Iowa City: University of Iowa; 1983. [Google Scholar]
- 28. Bocklandt S, Lin W, Sehl ME, et al. Epigenetic predictor of age. PLoS One. 2011;6:e14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Christensen BC, Houseman EA, Marsit CJ, et al. Aging and environmental exposures alter tissue-specific DNA methylation dependent upon CpG island context. PLoS Genet. 2009;5:e1000602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu J, Morgan M, Hutchison K, Calhoun VD. A study of the influence of sex on genome wide methylation. PLoS One. 2010;5:e10028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu J, Hutchison K, Perrone-Bizzozero N, Morgan M, Sui J, Calhoun V. Identification of genetic and epigenetic marks involved in population structure. PLoS One. 2010;5:e13209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cordero P, Gomez-Uriz AM, Campion J, Milagro FI, Martinez JA. Dietary supplementation with methyl donors reduces fatty liver and modifies the fatty acid synthase DNA methylation profile in rats fed an obesogenic diet. Genes Nutr. 2013;8:105–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Niculescu MD, Zeisel SH. Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. J Nutr. 2002;132:2333S–2335S [DOI] [PubMed] [Google Scholar]
- 34. Lim U, Song MA. Dietary and lifestyle factors of DNA methylation. Methods Mol Biol. 2012;863:359–376 [DOI] [PubMed] [Google Scholar]
- 35. Philibert RA, Gunter TD, Beach SR, Brody GH, Madan A. MAOA methylation is associated with nicotine and alcohol dependence in women. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:565–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bleich S, Lenz B, Ziegenbein M, et al. Epigenetic DNA hypermethylation of the HERP gene promoter induces down-regulation of its mRNA expression in patients with alcohol dependence. Alcohol Clin Exp Res. 2006;30:587–591 [DOI] [PubMed] [Google Scholar]
- 37. Chang HW, Ling GS, Wei WI, Yuen AP. Smoking and drinking can induce p15 methylation in the upper aerodigestive tract of healthy individuals and patients with head and neck squamous cell carcinoma. Cancer. 2004;101:125–132 [DOI] [PubMed] [Google Scholar]
- 38. Breitling LP, Salzmann K, Rothenbacher D, Burwinkel B, Brenner H. Smoking, F2RL3 methylation, and prognosis in stable coronary heart disease. Eur Heart J. 2012;33:2841–2848 [DOI] [PubMed] [Google Scholar]
- 39. Wong CC, Mill J, Fernandes C. Drugs and addiction: an introduction to epigenetics. Addiction. 2011;106:480–489 [DOI] [PubMed] [Google Scholar]
- 40. Breitling LP, Yang R, Korn B, Burwinkel B, Brenner H. Tobacco-smoking-related differential DNA methylation: 27K discovery and replication. Am J Hum Genet. 2011;88:450–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ehrlich S, Walton E, Roffman JL, et al. Smoking, but not malnutrition, influences promoter-specific DNA methylation of the proopiomelanocortin gene in patients with and without anorexia nervosa. Can J Psychiatry. 2012;57:168–176 [DOI] [PubMed] [Google Scholar]
- 42. Philibert RA, Plume JM, Gibbons FX, Brody GH, Beach SR. The impact of recent alcohol use on genome wide DNA methylation signatures. Front Genet. 2012;3:54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhou FC, Balaraman Y, Teng M, Liu Y, Singh RP, Nephew KP. Alcohol alters DNA methylation patterns and inhibits neural stem cell differentiation. Alcohol Clin Exp Res. 2011;35:735–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ouko LA, Shantikumar K, Knezovich J, Haycock P, Schnugh DJ, Ramsay M. Effect of alcohol consumption on CpG methylation in the differentially methylated regions of H19 and IG-DMR in male gametes: implications for fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2009;33:1615–1627 [DOI] [PubMed] [Google Scholar]
- 45. Morris CV, DiNieri JA, Szutorisz H, Hurd YL. Molecular mechanisms of maternal cannabis and cigarette use on human neurodevelopment. Eur J Neurosci. 2011;34:1574–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167:686–693 [DOI] [PubMed] [Google Scholar]
- 47. Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med. 2009;50:1801–1807 [DOI] [PubMed] [Google Scholar]
- 48. Kato T, Monji A, Hashioka S, Kanba S. Risperidone significantly inhibits interferon-gamma-induced microglial activation in vitro. Schizophr Res. 2007;92:108–115 [DOI] [PubMed] [Google Scholar]
- 49. Hou Y, Wu CF, Yang JY, et al. Effects of clozapine, olanzapine and haloperidol on nitric oxide production by lipopolysaccharide-activated N9 cells. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1523–1528 [DOI] [PubMed] [Google Scholar]
- 50. Müller N, Riedel M, Scheppach C, et al. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. Am J Psychiatry. 2002;159:1029–1034 [DOI] [PubMed] [Google Scholar]
- 51. Miyaoka T, Yasukawa R, Yasuda H, Hayashida M, Inagaki T, Horiguchi J. Minocycline as adjunctive therapy for schizophrenia: an open-label study. Clin Neuropharmacol. 2008;31:287–292 [DOI] [PubMed] [Google Scholar]
- 52. Levkovitz Y, Mendlovich S, Riwkes S, et al. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry. 2010;71:138–149 [DOI] [PubMed] [Google Scholar]
- 53. Laan W, Grobbee DE, Selten JP, Heijnen CJ, Kahn RS, Burger H. Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2010;71:520–527 [DOI] [PubMed] [Google Scholar]
- 54. Cornish S, Mehl-Madrona L. The role of vitamins and minerals in psychiatry. Integr Med Insights. 2008;3:33–42 [PMC free article] [PubMed] [Google Scholar]
- 55. Petrie WM, Ban TA. Vitamins in psychiatry. Do they have a role? Drugs. 1985;30:58–65 [DOI] [PubMed] [Google Scholar]
- 56. Reinius LE, Acevedo N, Joerink M, et al. Differential DNA methylation in purified human blood cells: implications for cell lineage and studies on disease susceptibility. PLoS One. 2012;7:e41361 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.