Abstract
Background: Prominent regional cortical thickness reductions have been shown in schizophrenia. In contrast, little is known regarding alterations of structural coupling between regions in schizophrenia and how these alterations may be related to cognitive impairments in this disorder. Methods: T1-weighted magnetic resonance images were acquired in 54 patients with schizophrenia and 68 healthy control subjects aged 18–55 years. Cortical thickness was compared between groups using a vertex-wise approach. To assess structural coupling, seeds were selected within regions of reduced thickness, and brain-wide cortical thickness correlations were compared between groups. The relationships between identified patterns of circuit structure disruption and cognitive task performance were then explored. Results: Prominent cortical thickness reductions were found in patients compared with controls at a 5% false discovery rate in a predominantly frontal and temporal pattern. Correlations of the left dorsolateral prefrontal cortex (DLPFC) with right prefrontal regions were significantly different in patients and controls. The difference remained significant in a subset of 20 first-episode patients. Participants with stronger frontal interhemispheric thickness correlations had poorer working memory performance. Conclusions: We identified structural impairment in a left-right DLPFC circuit in patients with schizophrenia independent of illness stage or medication exposure. The relationship between left-right DLPFC thickness correlations and working memory performance implicates prefrontal interhemispheric circuit impairment as a vulnerability pathway for poor working memory performance. Our findings could guide the development of novel therapeutic interventions aimed at improving working memory performance in patients with schizophrenia.
Key words: dorsolateral prefrontal cortex, MRI, cortical thickness, structural coupling
Introduction
Magnetic resonance imaging (MRI) morphometry studies of cortical structure in subjects with schizophrenia typically demonstrate gray matter volume reductions throughout the brain, most prominently in frontal and temporal lobes.1 Cortical volumes, however, are a product of the surface area and thickness of the cortex. Cortical surface area and thickness do not undergo the same developmental trajectories,2 due to different genetic, environmental, and cellular influences.3 Compared with cortical volumes, cortical thickness measurement is a more sensitive assay of morphological alterations in cortical brain structure, reflecting the arrangement, morphology, and density of cells.4 Neuroimaging studies have demonstrated a pattern of cortical thickness reductions in schizophrenia, also primarily in frontal and temporal brain regions.5,6 These patterns are shared among the major psychoses7 and schizophrenia subtypes.8 Cortical thickness reduction has also been related to cognitive impairment in people with schizophrenia.9 However, if schizophrenia (and other severe mental illnesses) were related to brain circuit impairment,10 understanding relationships between cortical regions may identify modifiable mechanisms underlying cognitive impairment.11
Most neuroimaging studies of cortical structure in psychiatric disorders have focused on regional analyses, reporting specific differences between patients and healthy controls. However, converging neuroimaging evidence suggests that schizophrenia is a disorder of disrupted relationships between brain regions.12,13 Studies have reported bidirectional alterations in the strength of volumetric correlations in schizophrenia.14–18 Disruption has been shown in the white matter tracts connecting brain regions, particularly in frontotemporal and interhemispheric connections.19–21 These imaging techniques have also been used to model the brain as a complex network, and others have reported altered network properties in the disease.22–24 Functional MRI studies comparing patients with schizophrenia and healthy controls have identified disruptions of functional and effective connectivity during cognitive tasks (eg, working memory tasks).25,26 In addition, patients with schizophrenia may utilize compensatory regions or networks different from controls to perform higher order cognitive tasks.27
Structural covariance analysis describes how interindividual differences in brain region structure are coordinated within communities of brain regions that fluctuate in size across the population and has been used to describe structural coupling in healthy and diseased brains.28 We used a vertex-wise approach to compare brain-wide cortical thickness correlations in patients with schizophrenia and healthy controls using regions of reduced thickness that we identified in our sample to guide our analyses. We then explored how altered structural coupling between cortical regions was related to cognitive performance. We hypothesized that we would (1) replicate the identification of cortical thickness reductions in the frontal and temporal brain regions of patients with schizophrenia; (2) detect altered structural coupling between frontotemporal and frontal interhemispheric cortical regions in patients with schizophrenia; and (3) relate this impaired structural coupling to cognitive performance, with potentially greater effect than that of regional cortical thickness.
Methods and Materials
Study Participants
Participants were recruited at the Centre for Addiction and Mental Health (CAMH) in Toronto, Canada, via referral, study registries, and advertisements. A total of 54 patients with schizophrenia aged 18–55 were matched with 68 healthy control subjects on age, gender, handedness, and highest parental level of education. We excluded individuals with previous head trauma with loss of consciousness, a neurological disorder, current substance abuse, or a history of substance dependence (urine toxicology screens were performed on all participants). A history of a primary psychotic disorder in first-degree relatives was also an exclusion criterion for controls. All participants were assessed with the Edinburgh Handedness Inventory,29 the Hollingshead index, and the Wechsler Test of Adult Reading to estimate IQ, were interviewed by a psychiatrist, and completed the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Disorders.30 Clinical symptoms were characterized with the Positive and Negative Syndrome Scale (PANSS).31 Medication histories were obtained via self-report and verified when necessary with the treating psychiatrist and chart review. The study was approved by the Research Ethics Board of CAMH, and all participants provided informed written consent.
Cognitive Testing
All participants completed a battery of cognitive tests assessing cognitive domains in which impairment has been reported in schizophrenia. Working memory was assessed with the Letter Number Sequencing (LNS) task32; executive function with the Trails B test33; and attention, immediate memory, delayed memory, language, and visuospatial ability with the Repeated Battery for the Assessment of Neuropsychological Status.34
Image Acquisition
T1-weighted MR images were acquired using an 8-channel head coil on a 1.5 Tesla GE Echospeed system (General Electric Medical Systems), which permits maximum gradient amplitudes of 40 mT/m. Axial inversion recovery–prepared spoiled gradient recalled sequence of echo time, 5.3ms; repetition time, 12.3ms; time to inversion, 300.0ms; flip angle, 20°; and number of excitations, 1 (for a total of 124 contiguous images, 1.5-mm thickness) was used to acquire images resulting in voxel size: 0.78×0.78×1.5mm3.
Image Processing
To calculate cortical thickness, the T1-weighted images were submitted to the CIVET pipeline (version 1.1.10, Montreal Neurological Institute at McGill University) to generate cortical models as described previously.35 The software package mni.cortical.statistics (Brain Imaging Centre, Montreal Neurological Institute; http://www.bic.mni.mcgill.ca) for the R environment36 was used for cortical thickness analyses.
Cortical Thickness Differences
To compare cortical thickness, a general linear model was used with diagnosis as the between-group factor and age as a covariate. Maps of t statistics for group effects on cortical thickness at each vertex were projected onto an average brain template revealing regions where thickness differed significantly between patients with schizophrenia and controls. The statistical threshold was determined by application of a 5% false discovery rate (FDR) correction.
Cortical Thickness Correlations
Mapping of anatomical correlations across the cerebral cortex was used to assess cortical thickness correlations.37 This involved assessing whether the thickness of one area of the cortex (the seed) correlates with the thickness of rest of the cortex across participants within diagnostic groups, as well as quantifying differences in correlation maps between groups. Seed vertices for this analysis were selected based on the cortical thickness difference maps. One seed was selected at the midpoint of each cluster of vertices where q < 0.01. Each seed vertex was correlated with each target vertex (40961) independently in each hemisphere within a group of subjects (with each subject contributing 1 seed and 1 target thickness value). These between-subject cortical cross-correlations were obtained using Pearson’s r. A linear regression of age was first performed at every vertex, and the residuals of that regression were substituted for the raw cortical thickness values.
Whole Brain Cortical Thickness Correlation Differences
Differences in correlations between groups were assessed with a linear interaction model that tested for differences in slope between vertex pairs using the student’s t statistic. The statistical threshold was determined by application of a 5% FDR correction.
Cognitive Performance and Cortical Thickness Correlations
Task selection from the cognitive battery was based on disrupted circuitry identified by comparing cortical thickness correlation in patients with schizophrenia and controls. The relation between cognition and cortical thickness correlations was evaluated by assessing cortical thickness correlations within the disrupted circuitry separately in those who scored above and below the median in cognitive performance. Cortical thickness correlation differences between cognitive performance groups were determined with a classic interaction linear model that tested for differences in slope between vertex pairs.
Results
Demographics and Clinical Characteristics
There were no significant differences between patients with schizophrenia and controls on the matching variables. As expected, a significant difference was present in IQ (table 1).
Table 1.
Demographic and Clinical Characteristics of Participants
| Demographic | Healthy Control, Mean (SD) | Schizophrenia, Mean (SD) |
|---|---|---|
| Age | 33 (11) | 36 (12) |
| Education (parental, highest level)a | 5 (2) | 5 (2) |
| WTAR (IQ) | 116 (9) | 106 (17) |
| Age at onset | NA | 22 (5) |
| Duration of illness | NA | 12 (12) |
| Chlorpromazine equivalent, mg | NA | 315 (254) |
| PANSS | ||
| Positive | NA | 14 (5) |
| Negative | NA | 15 (6) |
| General | NA | 26 (8) |
| Diagnosis | NA | 38 SZ, 16 SA |
| Antipsychotic treatment, n | NA | 5 1st, 40 2nd, 9 none |
| Gender, n | 28 F, 40 M | 16 F, 28 M |
| Handedness, n | 61 R, 5L, 2 A | 52 R, 2 L |
| Ethnicity, n | 55 C, 8 As, 2 Hs, 2 ME, 1 M | 34 C, 12 As, 1 Id, 4 Af, 1 Car, 2 Guy |
| Currently smoking, n | 4 | 14 |
Note: WTAR, Wechsler Test for Adult Reading; NA, not applicable; SZ, schizophrenic; SA, schizoaffective; 1st, first-generation antipsychotic; 2nd, second-generation antipsychotic; F, female; M, male; R, right handed; L, left handed; A, ambidextrous; C, Caucasian; As, Asian; Hs, Hispanic; ME, Middle Eastern; M, Mixed; Id, Indian; Af, African; Car, Caribbean; Guy, Guyanese.
aHighest level of education of participants’ parents taken from Hollingshead index (Hollingshead, 1975. Four Factor Index of Social Status; Yale University, New Haven, CT).
Cortical Thinning in Schizophrenia
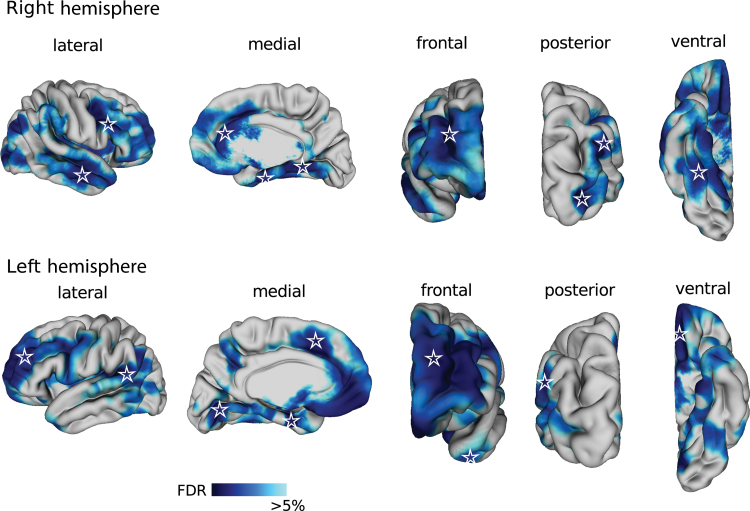
Regional cortical thinning was observed in the frontal gyri (left superior, left and right middle and right inferior), right anterior cingulate, bilateral entorhinal cortex, bilateral lingual gyrus, right middle temporal gyrus, left temporal pole, left angular gyrus and gyrus rectus, and right striate and extrastriate cortex in patients with schizophrenia patients compared with controls (figure 1). There was no vertex at which cortical thickness was significantly increased in schizophrenia.
Fig. 1.
Cortical thinning in patients with schizophrenia. Blue color map shows cortical regions that were thinner in patients with schizophrenia after correcting for multiple comparisons at a 5% false discovery rate. Cortical surface of the brain is displayed from the lateral, medial, frontal, posterior, and ventral views in the right (top) and left (bottom) hemispheres. Stars indicate vertices that were used as seeds in the cortical thickness correlation analysis.
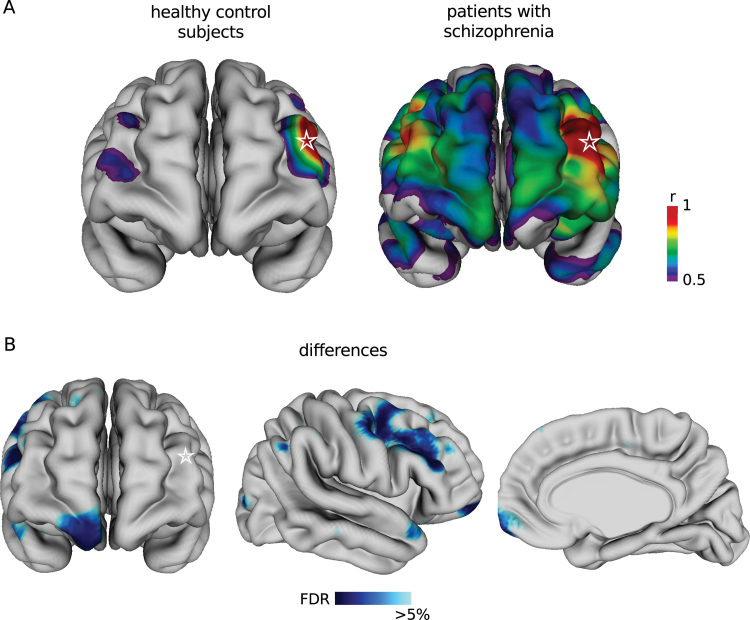
Thickness Correlations With the Left Dorsolateral Prefrontal Cortex Are Altered in Schizophrenia
Each of the regions where cortical thickness was signifi- cantly reduced in schizophrenia was used as a seed for the brain-wide correlation analysis. Of the 18 seeds, only 1 in the left middle frontal gyrus located in the dorsolateral prefrontal cortex (DLPFC) revealed significant differences in cortical thickness correlations with other brain regions, when comparing patients with schizophrenia and controls at 5% FDR. To illustrate these differences, an arbitrary correlation threshold of r > 0.5 with the left DLPFC seed was set (figure 2A). The strengths of the left-right DLPFC correlation (peak vertex slope difference = 1.01, SE = 0.13, t(118) = 4.67, P = 8.02e-06, q = 0.02) and the left DLPFC-right ventral medial prefrontal cortex (VMPFC) correlation (peak vertex slope difference = 1.03, SE = 0.25, t(118) = 4.12, P = 6.95e-05, q = 0.02) were significantly greater in patients with schizophrenia than in controls (figure 2B). Comparing the 20 patients with first-episode schizophrenia with a matched control subsample revealed similar correlation patterns and differences (see online supplementary figure 1; left-right DLPFC peak vertex slope difference = 0.55, SE = 0.26, t(36) = 2.12, P = .04).
Fig. 2.
Cortical thickness correlations and correlation differences with left dorsolateral prefrontal cortex (DLPFC) in patients with schizophrenia and healthy control subjects. (A) Spectral color map shows regions of the cortex where thickness correlates strongly (r > 0.5) with the thickness of the seed vertex in the left DLPFC (star) in healthy subjects (left) and patients with schizophrenia (right). (B) Cortical thickness correlations with the seed in the left DLPFC are different in healthy control subjects and patients with schizophrenia in the regions indicated in blue in the right hemisphere viewed from the frontal (left), lateral (middle), and medial (right) perspectives at a 5% false discovery rate.
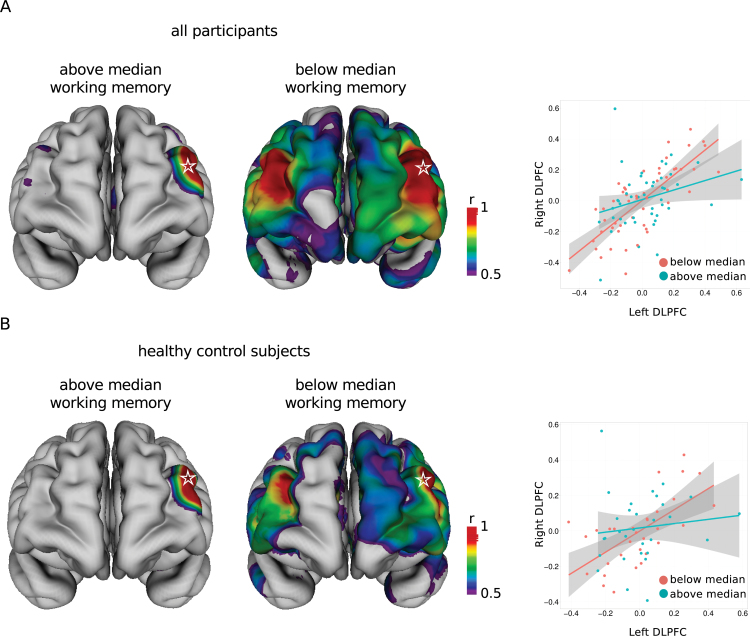
Cognitive Performance and the Interhemispheric DLPFC Circuit
Patients with schizophrenia demonstrated poorer performance in all cognitive domains (see online supplementary table 1), particularly in working memory (schizophrenia: mean LNS score = 11.96, SD = 4.58 vs controls: mean = 16.15, SD = 3.25; t(113) = 5.74, P = 8.07e-8). The DLPFC is a region critical for working memory performance; therefore, we focused on the relationship between working memory performance and left-right DLPFC thickness correlations. Overall, participants with LNS scores below the median (15) demonstrated stronger and more widespread neuroanatomic patterns of cortical thickness correlations than those with scores above the median. Specifically, the cortical thickness between the left and right DLPFC showed stronger correlation in those who scored below the median than in those who scored above the median (figure 3A) (below: Pearson’s r = 0.77 vs above: r = 0.29; slope difference = 0.51, SE = 0.17, t(92) = 2.91, P = .004). Because the DLPFC may be important for executive function in general, we conducted a similar comparison using Trails B scores and the seed in the left DLPFC. Although a similar pattern was present, the differences between those with scores below and above the median were not significant, suggesting that the altered left-right DLPFC relationship is more predictive of working memory performance than executive function in general (see online supplementary results).
Fig. 3.
Bilateral DLPFC thickness correlations differ between participants with above and below median working memory performance. Patterns of strong (r > 0.5) cortical thickness correlations with the seed vertex in left DLPFC (star) in all participants (A) and healthy control subjects (B) stratified into groups with above and below median working memory scores (left). Correlations are different between vertices in the left and right DLPFC in groups of all participants (A) and healthy control subjects (B) with above and below median working memory performance (right).
Restricting the analysis to the control group, controls with LNS scores below the median (16) showed stronger correlation between the left and right DLPFC than controls with scores above the median (below: Pearson’s r = 0.64 vs above: r = 0.12; slope difference = 0.48, SE = 0.23, t(57) = 2.06, P = .04), with controls with relatively poor working memory performers demonstrated a prefrontal interhemispheric pattern similar to the pattern demonstrated by patients with schizophrenia (figure 3B). In healthy controls, cortical thickness was not significantly correlated with working memory performance at a 5% or 10% FDR (see online supplementary methods), although DLPFC thickness was correlated with working memory when using an uncorrected P value (peak vertex left DLPFC: t(63) = 3.7, P = .00042; peak vertex right DLPFC: t(63) = 2.9, P = .0050). Using a median split in patients with schizophrenia (LNS <12 or >12), there were no statistically significant differences in cortical thickness correlations between the left and right DLPFC (slope difference = 0.15, SE = 0.28, t(39) = 0.54, P = .59). In patients with schizophrenia, the thickness of the posterior cingulate cortex (PCC), medial prefrontal cortex, and superior and middle temporal gyrus was correlated with working memory performance after residualizing for age using a 10% FDR but not a 5% FDR (see online supplementary figure 2).
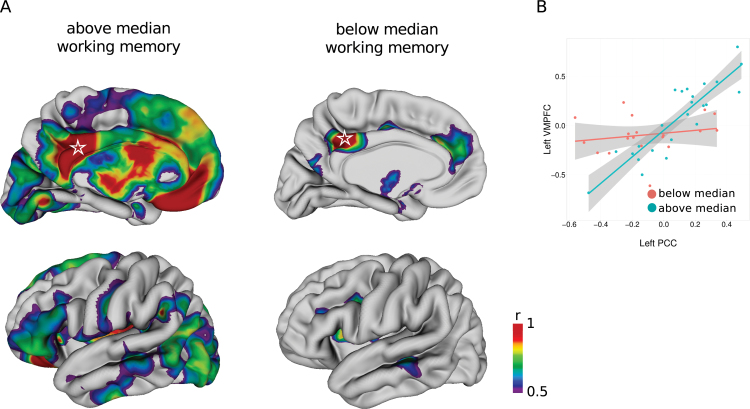
Post Hoc Analysis 1: A Compensatory Network for Working Memory Performance in Schizophrenia
Although patients with schizophrenia were characterized by both high left-right DLPFC correlations and poor working memory performance in general, this network did not explain variation in their working memory performance. Thus, we used the regions that demonstrated modest association with working memory performance (at 10% FDR) as seed regions for network exploration (see online supplementary figure 2). Seed placement in these regions revealed that a seed in the PCC demonstrated differences in thickness correlations with other regions at a 5% FDR between patients with schizophrenia with working memory performance above and below the median (see online supplementary methods). Patients with above median performance demonstrated a prominent pattern of cortical midline, as well as lateral temporal and ventrolateral prefrontal cortical thickness correlation with the PCC seed, whereas those with performance below the median demonstrated a very sparse correlation pattern mostly with the cingulate cortex (figure 4A). Differences in thickness correlations in relation to the PCC seed between the groups with performance below and above the median were most prominent in the VMPFC (figure 4B) (below: Pearson’s r = 0.20 vs above: r = 0.89; slope difference = 1.2, SE = 0.22, t(39) = 5.38, P = 3.71e-06, q = 0.004).
Fig. 4.
Cortical thickness correlations with the left posterior cingulate cortex (PCC) differ in patients with schizophrenia with above and below median working memory performance. (A) Pattern of strong (r > 0.5) cortical thickness correlations with seed vertex in left PCC (star) in patients with schizophrenia stratified into groups with above (left) and below (right) median working memory scores. Medial (top) and lateral (bottom) views of the left hemisphere are shown although correlation patterns were similar in the right hemisphere. (B) Correlations differed most between the seed in the left PCC and the left ventral medial prefrontal cortex in groups of patients with schizophrenia with above and below median working memory performance (5% false discovery rate).
Post Hoc Analysis 2: DLPFC-Hippocampal Coupling Does Not Predict Working Memory Performance
Due to the role of DLPFC-hippocampal coupling in working memory performance demonstrated in functional neuroimaging studies,38,39 we used the T1-weighted images to segment left and right hippocampus for each individual (see online supplementary methods). Correlations of left and right hippocampus volumes with the left DLPFC seed were calculated for all participants. No correlation differences were found between patients with schizophrenia and controls, or between participants with working memory performance above or below the median, either in the entire sample or within diagnostic groups (see online supplementary table 2).
Discussion
First, we identified several regions with reductions in cortical thickness in patients with schizophrenia compared with healthy controls primarily in frontal and temporal brain regions. Following seed placement in those regions, we then analyzed brain-wide cortical thickness correlations with each region in both groups and found that patients had significantly stronger correlations of left DLPFC with the right DLPFC and VMPFC compared with controls. This same relationship was found in a first-episode subsample, suggesting that the stronger correlation in the patient group was not related to duration of illness or antipsychotic medication exposure. When we analyzed all participants together, those with relatively poorer working memory performance demonstrated a significantly stronger correlation between cortical thickness in the left and right DLPFC. We found the same relationship in the control group as in the overall group. Patients with schizophrenia, however, generally had poor working memory performance and strong left-right DLPFC correlations. Although patients with better working memory performance did not have left-right DLPFC correlations different from patients with poorer working memory performance, they were characterized by a strongly correlated, potentially compensatory network, comprised of cortical midline, lateral temporal, and ventrolateral prefrontal structures.
Our finding of alterations in prefrontal interhemispheric circuitry in patients with schizophrenia is consistent with disrupted connectivity theories characterizing this disorder.12,40 Patients with schizophrenia have reduced functional connectivity between left and right inferior frontal gyri, diminished lateralization of function in the inferior frontal gyrus associated with language,30 enhanced prefrontal interhemispheric cortical effective connectivity,41 and absence of normal lateralization of prefrontal activation during working memory tasks.42 Interhemispheric disruption in patients with schizophrenia has also been shown using transcranial magnetic stimulation,43,44 and interhemispheric structure is associated with lateralization of DLPFC function in healthy subjects.45 The evidence for the role of the DLPFC in working memory performance is also considerable, emerging from multiple lines of in vivo, postmortem, cellular, and animal model investigations.46–48 In healthy subjects, activation of the DLPFC is associated with increased task demand during working memory performance.49–51 However, we found that left-right DLPFC thickness coupling is an important predictor of working memory performance, greater than that of left or right DLPFC thickness alone. In other populations with prefrontal brain susceptibility, such as healthy aging, age-related increases in frontal bilateral activation and connectivity have been shown during working memory performance,52,53 and increased frontal bilaterality in older subjects has been associated with poor memory performance.54,55
Several investigations9,41 have shown that when networks or circuits responsible for working memory performance are disrupted, other circuits or networks may serve a compensatory role. For example, subjects with schizophrenia demonstrated DLPFC and VLPFC activation during working memory performance, whereas in healthy controls only the DLPFC was activated.27 Our data demonstrate that among patients with schizophrenia, those with better working memory performance demonstrated a tight coupling among cortical midline, lateral temporal, and ventrolateral prefrontal regions. The cortical midline structures (medial prefrontal cortex, anterior cingulate, posterior cingulate, precuneus) and lateral temporal structures (temporoparietal junction, superior temporal sulcus, temporal poles) form the default mode network (DMN). This network has been shown to increase in connectivity with increases in working memory load and to demonstrate anticorrelation with working memory networks particularly as memory load increases.56 At the same time, DMN hyperconnectivity has been shown in subjects with schizophrenia compared with controls.57 In particular, the region that we seeded, the PCC, has been identified as a central hub for the DMN.58,59 It is also a region that may control the relationship between DMN, and working memory circuitry as a large study found that in patients with schizophrenia, the PCC was more deactivated than in controls across all working memory loads, but significance was no longer existed when covarying for accuracy.60 Although in need of replication, our findings align with some61 but not all62 functional neuroimaging data, suggesting that a more tightly coupled DMN plus ventrolateral PFC may collectively act as a compensatory network in patients with schizophrenia with better working memory performance.
Correlations between the thickness of cortical regions likely emerge from genetic and environmental influences on the mutually beneficial effects of trophic factors on connected regions during development,63 coordinated thinning of the cortex during adolescence,64 or modulation of brain structure by neural activity throughout adulthood.65,66 A longitudinal developmental study in healthy subjects demonstrated that regions with correlated rates of anatomical change over time showed strong convergence with cross-sectional cortical thickness correlations, suggesting that these neuroanatomical patterns arise as a result of coordinated brain maturation.67 This neurodevelopmental finding in healthy individuals supports the possibility that the enhanced bilateral coupling we observed in patients with schizophrenia is developmental in origin and may be related to reduced differentiation of the brain on the path to schizophrenia, whereby areas of the cortex do not become properly specialized. With respect to such developmental origin, the DLPFC seed overlaps with 1 of 2 heritable gray matter components identified in a multivariate, independent component analysis of schizophrenia patients and sibling pairs.68 Additionally, a large twin study found high genetic correlation between the volumes of bilateral structural homologues, and environmental influences were stronger in the same hemisphere,69 suggesting that interhemispheric correlations such as between the left and right DLPFC reported here may be largely genetically mediated. One possible mechanism of abnormal left-right prefrontal cortical relationships might occur via altered cortical midline development. The development and regulation of major axonal cortico-cortical projections that link both hemispheres through the corpus callosum is influenced by guidance molecules such as Netrin 170 and Slits.71 Robo 1, Robo 2, and GPC1 receptors for the Slit ligands are also crucially involved in the formation of midline commisures.72 Of note, ROBO1-ROBO2 variants were identified in a genome-wide association study using DLPFC activation during a working memory task as a quantitative phenotype.73
There are several limitations in our study. First, the implications of the structural alterations in relation to functional activation in the brain (ie, reduced specialization) have not been directly tested and can only be surmised based on data from other studies at this time. Second, this study cannot elucidate whether cortical thickness correlation differences are primarily neurodevelopmental or a compensatory mechanism that arises due to alterations elsewhere in the brain. A longitudinal study of high-risk subjects could address this question. Third, the differences in cortical thickness correlations identified in subjects with schizophrenia were also associated with working memory performance across all participants and in healthy subjects alone. This suggests that this anatomical pattern could be related to working memory performance in general rather than being a biomarker specific to schizophrenia. On an analytic note, a shortcoming of the use of FDR for the correction of multiple comparisons is that it was designed for collections of discrete tests,74 a condition that is not met in this study. However, it is used widely to correct for multiple comparisons in imaging studies as it is a more sensitive alternative to family wise error procedures. In addition, working memory performance is also known to be associated with frontoparietal and frontotemporal circuits75 that are also disrupted in schizophrenia.76 However, our study did not identify either altered correlations in these circuits in patients with schizophrenia at the brain-wide correction threshold employed (5% FDR) or altered correlation between DLPFC thickness and hippocampal volume identified. Finally, while a comprehensive analysis that included all subcortical regions may have revealed some additional interesting results, for subcortical analysis we only examined hippocampal-prefrontal coupling because disruptions in this circuit have been related to working memory performance in the functional imaging literature.38,39
Following identification of several regions with cortical thickness reductions, we identified an abnormal prefrontal interhemispheric circuit in patients with schizophrenia compared with healthy controls. Abnormally high coupling of thickness between left and right prefrontal cortical regions was also associated with poor working memory performance. A subset of patients with schizophrenia may have a compensatory network related to better working memory performance. Most patients with schizophrenia have working memory impairment that occurs across the lifespan,77 and this impairment is one important predictor of functional outcome.78 Therefore, emerging therapeutic approaches such as repetitive transcranial magnetic stimulation to bilateral DLPFC79 provide the potential to treat impaired cognitive performance by targeting disrupted brain circuitry in patients with schizophrenia.
Supplementary Material
Supplementary material is available at http://schizophre niabulletin.oxfordjournals.org.
Funding
Ontario Mental Health Foundation; the Canadian Institutes of Health Research; the National Institute of Mental Health (R01MH099167); the Brain and Behavior Research Foundation (formerly NARSAD); the Centre for Addiction and Mental Health Foundation.
Supplementary Material
Acknowledgments
Thanks to the Kimel family, the Koerner New Scientist Award, and the Paul E. Garfinkel New Investigator Catalyst Award. During the past 5 years, Dr Daskalakis received external funding through Brainsway Ltd and a travel allowance through Pfizer and Merck and has also received speaker funding through Sepracor Inc and has served on one advisory board for Hoffmann-La Roche Limited. Dr Mulsant currently receives medications from Bristol-Myers Squibb and Pfizer to be used in a clinical trial funded by the National Institute of Mental Health. During the past 5 years, he has also received medications from Eli-Lilly to be used in a clinical trial funded by the National Institute of Mental Health and travel support from Roche.
References
- 1. Shenton ME, Whitford TJ, Kubicki M. Structural neuroimaging in schizophrenia: from methods to insights to treatments. Dialogues Clin Neurosci. 2010;12:317–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Raznahan A, Shaw P, Lalonde F, et al. How does your cortex grow? J Neurosci. 2011;31:7174–7177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Panizzon MS, Fennema-Notestine C, Eyler LT, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parent A, Carpenter MB. Human Neuroanatomy. Baltimore, MD: Williams & Wilkins; 1995. [Google Scholar]
- 5. Goldman AL, Pezawas L, Mattay VS, et al. Widespread reductions of cortical thickness in schizophrenia and spectrum disorders and evidence of heritability. Arch Gen Psychiatry. 2009;66:467–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuperberg GR, Broome MR, McGuire PK, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–888 [DOI] [PubMed] [Google Scholar]
- 7. Rimol LM, Hartberg CB, Nesvåg R, et al. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry. 2010;68:41–50 [DOI] [PubMed] [Google Scholar]
- 8. Voineskos AN, Foussias G, Lerch J, et al. Neuroimaging evidence for the deficit subtype of schizophrenia. JAMA Psychiatry. 2013;70:472–480 [DOI] [PubMed] [Google Scholar]
- 9. Ehrlich S, Brauns S, Yendiki A, et al. Associations of cortical thickness and cognition in patients with schizophrenia and healthy controls. Schizophr Bull. 2012;38:1050–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morris SE, Cuthbert BN. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci. 2012;14:29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bassett DS, Bullmore ET. Human brain networks in health and disease. Curr Opin Neurol. 2009;22:340–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89–97 [PubMed] [Google Scholar]
- 13. Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neurosci Biobehav Rev. 2011;35:1110–1124 [DOI] [PubMed] [Google Scholar]
- 14. Bullmore ET, Woodruff PW, Wright IC, et al. Does dysplasia cause anatomical dysconnectivity in schizophrenia? Schizophr Res. 1998;30:127–135 [DOI] [PubMed] [Google Scholar]
- 15. Woodruff PW, Wright IC, Shuriquie N, et al. Structural brain abnormalities in male schizophrenics reflect fronto-temporal dissociation. Psychol Med. 1997;27:1257–1266 [DOI] [PubMed] [Google Scholar]
- 16. Mitelman SA, Buchsbaum MS, Brickman AM, Shihabuddin L. Cortical intercorrelations of frontal area volumes in schizophrenia. Neuroimage. 2005;27:753–770 [DOI] [PubMed] [Google Scholar]
- 17. Buchanan RW, Francis A, Arango C, et al. Morphometric assessment of the heteromodal association cortex in schizophrenia. Am J Psychiatry. 2004;161:322–331 [DOI] [PubMed] [Google Scholar]
- 18. Wible CG, Shenton ME, Hokama H, et al. Prefrontal cortex and schizophrenia. A quantitative magnetic resonance imaging study. Arch Gen Psychiatry. 1995;52:279–288 [DOI] [PubMed] [Google Scholar]
- 19. Kubicki M, Westin CF, Nestor PG, et al. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biol Psychiatry. 2003;54:1171–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McIntosh AM, Muñoz Maniega S, Lymer GK, et al. White matter tractography in bipolar disorder and schizophrenia. Biol Psychiatry. 2008;64:1088–1092 [DOI] [PubMed] [Google Scholar]
- 21. Voineskos AN, Lobaugh NJ, Bouix S, et al. Diffusion tensor tractography findings in schizophrenia across the adult life span. Brain. 2010;133:1494–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci. 2008;28:9239–9248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Y, Lin L, Lin CP, et al. Abnormal topological organization of structural brain networks in schizophrenia. Schizophr Res. 2012;141:109–118 [DOI] [PubMed] [Google Scholar]
- 24. van den Heuvel MP, Mandl RC, Stam CJ, Kahn RS, Hulshoff Pol HE. Aberrant frontal and temporal complex network structure in schizophrenia: a graph theoretical analysis. J Neurosci. 2010;30:15915–15926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schlösser R, Gesierich T, Kaufmann B, et al. Altered effective connectivity during working memory performance in schizophrenia: a study with fMRI and structural equation modeling. Neuroimage. 2003;19:751–763 [DOI] [PubMed] [Google Scholar]
- 26. Henseler I, Falkai P, Gruber O. Disturbed functional connectivity within brain networks subserving domain-specific subcomponents of working memory in schizophrenia: relation to performance and clinical symptoms. J Psychiatr Res. 2010;44:364–372 [DOI] [PubMed] [Google Scholar]
- 27. Tan HY, Sust S, Buckholtz JW, et al. Dysfunctional prefrontal regional specialization and compensation in schizophrenia. Am J Psychiatry. 2006;163:1969–1977 [DOI] [PubMed] [Google Scholar]
- 28. Alexander-Bloch A, Giedd JN, Bullmore E. Imaging structural co-variance between human brain regions. Nat Rev Neurosci. 2013;14:322–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113 [DOI] [PubMed] [Google Scholar]
- 30. Bleich-Cohen M, Sharon H, Weizman R, Poyurovsky M, Faragian S, Hendler T. Diminished language lateralization in schizophrenia corresponds to impaired inter-hemispheric functional connectivity. Schizophr Res. 2012;134:131–136 [DOI] [PubMed] [Google Scholar]
- 31. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276 [DOI] [PubMed] [Google Scholar]
- 32. Wechsler D. Wechsler Adult Intelligence Scale Third Edition (WAIS-III. ). San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 33. Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical. Interpretation. 2nd ed Tucson, AZ: Neuropsychology Press; 1993 [Google Scholar]
- 34. Randolph C. Repeatable Battery for the Assessment of Neuropsychological Status Manual. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- 35. Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 2005;24:163–173 [DOI] [PubMed] [Google Scholar]
- 36.R Core Team (2012). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. http://www.R-project.org/ Accessed January 2, 2013.
- 37. Lerch JP, Worsley K, Shaw WP, et al. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage. 2006;31:993–1003 [DOI] [PubMed] [Google Scholar]
- 38. Esslinger C, Kirsch P, Haddad L, et al. Cognitive state and connectivity effects of the genome-wide significant psychosis variant in ZNF804A. Neuroimage. 2011;54:2514–2523 [DOI] [PubMed] [Google Scholar]
- 39. Esslinger C, Walter H, Kirsch P, et al. Neural mechanisms of a genome-wide supported psychosis variant. Science. 2009;324:605. [DOI] [PubMed] [Google Scholar]
- 40. Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry. 2006;59:929–939 [DOI] [PubMed] [Google Scholar]
- 41. Schlösser R, Gesierich T, Kaufmann B, Vucurevic G, Stoeter P. Altered effective connectivity in drug free schizophrenic patients. Neuroreport. 2003;14:2233–2237 [DOI] [PubMed] [Google Scholar]
- 42. Walter H, Wunderlich AP, Blankenhorn M, et al. No hypofrontality, but absence of prefrontal lateralization comparing verbal and spatial working memory in schizophrenia. Schizophr Res. 2003;61:175–184 [DOI] [PubMed] [Google Scholar]
- 43. Daskalakis ZJ, Christensen BK, Chen R, Fitzgerald PB, Zipursky RB, Kapur S. Evidence for impaired cortical inhibition in schizophrenia using transcranial magnetic stimulation. Arch Gen Psychiatry. 2002;59:347–354 [DOI] [PubMed] [Google Scholar]
- 44. Fitzgerald PB, Brown TL, Daskalakis ZJ, deCastella A, Kulkarni J. A study of transcallosal inhibition in schizophrenia using transcranial magnetic stimulation. Schizophr Res. 2002;56:199–209 [DOI] [PubMed] [Google Scholar]
- 45. Voineskos AN, Farzan F, Barr MS, et al. The role of the corpus callosum in transcranial magnetic stimulation induced interhemispheric signal propagation. Biol Psychiatry. 2010;68:825–831 [DOI] [PubMed] [Google Scholar]
- 46. Arnsten AF, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76:223–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proc Natl Acad Sci U S A. 1996;93:13473–13480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Petrides M. The role of the mid-dorsolateral prefrontal cortex in working memory. Exp Brain Res. 2000;133:44–54 [DOI] [PubMed] [Google Scholar]
- 49. Callicott JH, Mattay VS, Bertolino A, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20–26 [DOI] [PubMed] [Google Scholar]
- 50. Manoach DS, Schlaug G, Siewert B, et al. Prefrontal cortex fMRI signal changes are correlated with working memory load. Neuroreport. 1997;8:545–549 [DOI] [PubMed] [Google Scholar]
- 51. Narayanan NS, Prabhakaran V, Bunge SA, Christoff K, Fine EM, Gabrieli JD. The role of the prefrontal cortex in the maintenance of verbal working memory: an event-related FMRI analysis. Neuropsychology. 2005;19:223–232 [DOI] [PubMed] [Google Scholar]
- 52. Spreng RN, Wojtowicz M, Grady CL. Reliable differences in brain activity between young and old adults: a quantitative meta-analysis across multiple cognitive domains. Neurosci Biobehav Rev. 2010;34:1178–1194 [DOI] [PubMed] [Google Scholar]
- 53. Goh JO. Functional dedifferentiation and altered connectivity in older adults: neural accounts of cognitive aging. Aging Dis. 2011;2:30–48 [PMC free article] [PubMed] [Google Scholar]
- 54. Colcombe SJ, Kramer AF, Erickson KI, Scalf P. The implications of cortical recruitment and brain morphology for individual differences in inhibitory function in aging humans. Psychol Aging. 2005;20:363–375 [DOI] [PubMed] [Google Scholar]
- 55. Duverne S, Motamedinia S, Rugg MD. The relationship between aging, performance, and the neural correlates of successful memory encoding. Cereb Cortex. 2009;19:733–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Repovš G, Barch DM. Working memory related brain network connectivity in individuals with schizophrenia and their siblings. Front Hum Neurosci. 2012;6:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76 [DOI] [PubMed] [Google Scholar]
- 58. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38 [DOI] [PubMed] [Google Scholar]
- 59. Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kim DI, Manoach DS, Mathalon DH, et al. Dysregulation of working memory and default-mode networks in schizophrenia using independent component analysis, an fBIRN and MCIC study. Hum Brain Mapp. 2009;30:3795–3811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yakushev I, Chételat G, Fischer FU, et al. Metabolic and structural connectivity within the default mode network relates to working memory performance in young healthy adults. Neuroimage. 2013;79:184–190 [DOI] [PubMed] [Google Scholar]
- 62. Whitfield-Gabrieli S, Thermenos HW, Milanovic S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kerwin RW, Murray RM. A developmental perspective on the pathology and neurochemistry of the temporal lobe in schizophrenia. Schizophr Res. 1992;7:1–12 [DOI] [PubMed] [Google Scholar]
- 64. Keshavan MS, Anderson S, Pettegrew JW. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J Psychiatr Res. 1994;28:239–265 [DOI] [PubMed] [Google Scholar]
- 65. Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312 [DOI] [PubMed] [Google Scholar]
- 66. Hyde KL, Lerch J, Norton A, et al. The effects of musical training on structural brain development: a longitudinal study. Ann N Y Acad Sci. 2009;1169:182–186 [DOI] [PubMed] [Google Scholar]
- 67. Raznahan A, Lerch JP, Lee N, et al. Patterns of coordinated anatomical change in human cortical development: a longitudinal neuroimaging study of maturational coupling. Neuron. 2011;72:873–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Turner JA, Calhoun VD, Michael A, et al. Heritability of multivariate gray matter measures in schizophrenia. Twin Res Hum Genet. 2012;15:324–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schmitt JE, Lenroot RK, Ordaz SE, et al. Variance decomposition of MRI-based covariance maps using genetically informative samples and structural equation modeling. Neuroimage. 2009;47:56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Serafini T, Colamarino SA, Leonardo ED, et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014 [DOI] [PubMed] [Google Scholar]
- 71. Bagri A, Marín O, Plump AS, et al. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248 [DOI] [PubMed] [Google Scholar]
- 72. López-Bendito G, Flames N, Ma L, et al. Robo1 and Robo2 cooperate to control the guidance of major axonal tracts in the mammalian forebrain. J Neurosci. 2007;27:3395–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Potkin SG, Turner JA, Guffanti G, et al. ; FBIRN. A genome-wide association study of schizophrenia using brain activation as a quantitative phenotype. Schizophr Bull. 2009;35:96–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chumbley JR, Friston KJ. False discovery rate revisited: FDR and topological inference using Gaussian random fields. Neuroimage. 2009;44:62–70 [DOI] [PubMed] [Google Scholar]
- 75. Takeuchi H, Taki Y, Sassa Y, et al. Verbal working memory performance correlates with regional white matter structures in the frontoparietal regions. Neuropsychologia. 2011;49:3466–3473 [DOI] [PubMed] [Google Scholar]
- 76. Voineskos AN, Felsky D, Kovacevic N, et al. Oligodendrocyte genes, white matter tract integrity, and cognition in schizophrenia.[published online ahead of print July 6, 2012]. Cereb Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rajji TK, Voineskos AN, Butters MA, et al. Cognitive performance of individuals with schizophrenia across seven decades: a study using the MATRICS consensus cognitive battery. Am J Geriatr Psychiatry. 2013;21:108–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tolman AW, Kurtz MM. Neurocognitive predictors of objective and subjective quality of life in individuals with schizophrenia: a meta-analytic investigation. Schizophr Bull. 2012;38:304–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Barr MS, Farzan F, Rajji TK, et al. Can repetitive magnetic stimulation improve cognition in schizophrenia? Pilot data from a randomized controlled trial. Biol Psychiatry. 2013;73:510–517 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.