Abstract
Background: Difficulties with affect regulation and impulse control have a strong influence on violence. The objective of this study was to determine whether baseline depression and impulsivity predict aggression and whether they predict differential response to antiaggressive treatment. This is important, as we lack knowledge as to the selection of antipsychotics for the treatment of aggression. Methods: Physically aggressive inpatients with schizophrenia who received an evaluation of depression and impulsivity at baseline were randomly assigned in a double-blind, parallel group, 12-week trial to clozapine, olanzapine, or haloperidol. Trait impulsivity was measured by the Barratt Impulsiveness Scale; depression by the Positive and Negative Syndrome Scale Depression factor. The number and severity of aggressive events, as measured by the Modified Overt Aggression Scale (MOAS), were the outcome measures. Results: Baseline depression and impulsivity predicted higher levels of aggression, as measured by the MOAS total score, over the 12-week treatment period across all 3 medication groups. In addition, there was a strong interaction effect between baseline depression/impulsivity and medication grouping in predicting MOAS score. In particular, when higher depression and impulsivity were present at baseline, patients on haloperidol presented with more aggression than patients on the other 3 medications. Conclusions: Depression and impulsivity are important predictors of aggression and of differential response to antiaggressive treatment. This is most likely due to the medications’ dissimilar neurotransmitter profiles. By identifying patients who will respond better to a given medication, we will be able to develop individualized strategies for the treatment of violent behavior.
Key words: depression, aggression, prediction, clozapine, olanzapine
Introduction
Depression, Impulsivity, and Aggression
Difficulties with affect regulation and impulse control have a strong influence on violent behavior in diverse subject populations, including patients with schizophrenia. Both affect and impulsivity are important determinants of action, in general, as they are related to the initiation of action or the tendency to act.1,2
Bleuler3 emphasized the importance of “affectivity,” which he defined as somatic and psychic manifestations of affect. He considered that “affectivity determines our actions” (p. 33). Individuals vulnerable to faulty regulation of negative emotion, including depression, are at risk for violence.4 Aggressive behavior is common among persons with depressive symptoms,5,6 including patients with schizophrenia.7 Van Praag and coauthors8,9 have emphasized the close connection between depression and aggression, which they attribute to an underlying serotonergic deficiency. They consider that this association lacks nosological specificity and exists in many different psychiatric disorders, including schizophrenia.
Impulsivity also plays a prominent part in violent behavior.10 Furthermore, impulsivity and depression are related to each other.11,12 These associations are thought to be related to a disturbance in serotonergic neurotransmission. A link between violent behavior and central serotonin deficit,13,14 as well as a triple link between violent behavior, suicidal behavior, and serotonin deficit15 has been demonstrated by using various measures of serotonin function. Such a deficit has also been linked to depression16,17 and to impulsivity.18,19
Thus, aggressive behaviors are often associated with dysregulation of emotion and impaired impulse control. These disturbances may be part of a broader syndrome that reflects an underlying serotonergic disturbance.
Antiaggressive Effects of the First-Generation and Second-Generation Antipsychotic Medications
Antipsychotic agents are commonly used for the control of aggression in patients with schizophrenia. First-generation antipsychotics (FGAs) have been used for a long time to treat violent behavior in these patients; they have met with partial and inconsistent success.20 The antiaggressive effects of the FGAs, such as haloperidol, have been attributed mostly to dopamine D2 antagonist activity, which results in a decrease in dopamine availability.21 These antiaggressive effects are considered to be nonspecific and may be due to a general decrease in arousal.22 Similarly, the positive relationship between dopamine levels and aggression may be explained through the effect of dopamine on general stimulus reactivity or arousal,23,24 or its ability to prevent fatigue,25 rather than directly enhancing aggression.
Some studies, in both animals and humans, even indicate that there may be a negative association between dopaminergic activity and aggression. This seems to be particularly the case for animals or humans who are more severely or persistently aggressive. Thus, agents that enhance dopamine activity disrupt rather than enhance aggression in animals with extensive aggressive experience.26–28 In some human studies, as well, a negative relationship has been reported between dopamine levels and aggression in subjects with a history of extensive violence.18,29
In contrast to the more limited neurotransmitter profile of the FGAs, the second-generation antipsychotic (SGAs) medications have binding affinity at multiple neurotransmitter binding sites, including serotonergic sites.30–32 Olanzapine and clozapine, in particular, have much greater serotonergic “behavioral activity” than dopaminergic effects.33
The SGAs are considered to have greater efficacy in treating violent behavior in patients with schizophrenia.34–36 Their greater antiaggressive effects have been linked to their long-term serotonergic action, in particular for olanzapine and clozapine.37 While the SGAs act as serotonin antagonists, their immediate effect being a decrease in available serotonin, there is evidence that chronic treatment with serotonin antagonists produces changes in the serotonin binding sites that are qualitatively and quantitatively similar to those produced by serotonin agonists.38 Chronic treatment with clozapine has been reported to decrease serotonin turnover and increase its availability in the nucleus accumbens.39
Given the fact that impulsivity and depression are strongly linked to aggression, we hypothesized that depression and impulsivity at baseline would predict aggression during the 12-week study period. Furthermore, given the different neurotransmitter profiles of the three study medications, clozapine, olanzapine, and haloperidol, we hypothesized that there would be a differential antiaggressive effect among these medications.
Methods
Subjects
The subjects were patients aged 18–60 with schizophrenia or schizoaffective disorder. Patients were required to have a confirmed episode of physical aggression during the present hospitalization and additional aggression, physical, verbal, or against property over a 2-week period following the initial incident. Research staff monitored subjects and ward documentation daily for such incidents. Patients were excluded if they had been hospitalized for more than a year; had a history of nonresponse or intolerance to clozapine, olanzapine, or haloperidol; or had received a depot antipsychotic in the prior 30 days. After complete description of the study to the subjects, written informed consent was obtained.
Design and Procedure
Patients meeting inclusion/exclusion criteria who signed informed consent were transferred to the research ward, so as to provide a uniform environment and to ensure close monitoring of aggressive incidents and of medication administration. The ward included a multicamera audio/visual system that recorded activities in public areas and allowed for constant observation of aggression. A detailed description of the ward and camera system is available.40
After completing baseline assessments, patients were randomized to clozapine (CLO), olanzapine (OLZ), or haloperidol (HAL). The study used a block randomization scheme with a block size of 3 and no baseline stratification. The 12-week trial consisted of a 6-week escalation/fixed-dose period and a 6-week variable-dose period. During the first 6 weeks, prestudy antipsychotics were gradually discontinued while doses of CLO, OLZ, and HAL were escalated to their target levels (20, 500, and 20mg/d, respectively) at which they remained fixed until the end of the first period.
During the last 6 weeks, the dose was allowed to vary within these ranges: clozapine, 200–800mg/d; olanzapine, 10–35mg/d; haloperidol, 10–30mg/d. Psychiatrists, blind to treatment group assignment, could change doses by prescribing various “levels” of medication.
Throughout the study, all patients received (double-blind) benztropine, benztropine placebo or both. Benztropine (4mg/d) was administered prophylactically to patients receiving HAL. Patients receiving mood stabilizers or antidepressants prior to study entry continued receiving these at the same dose.
Measures
All study procedures, including blood draws, were identical for all 3 groups to preserve the blind. Screening evaluations included a diagnostic interview (Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition), medical history, physical examination, and laboratory tests. Raters blind to treatment group performed all assessments.
Aggression.
The Modified Overt Aggression Scale (MOAS)41 was used to rate incidents. This scale has good psychometric properties including reliability and validity.41 It includes physical aggression against other people, verbal aggression, and physical aggression against objects—with a severity score for each type of aggression. The outcome variable, total MOAS score, was obtained by assigning a different weight for each type of aggression, through a psychometrically validated method developed by the MOAS authors,41 and summing up for each subject the weekly scores over the 12 weeks of the study.
All aggressive incidents were carefully recorded throughout the study period. There were multiple sources of information for the MOAS, including shift-to-shift reports, patient monitoring forms, and interviews with patients and staff. Nursing staff reported all behaviors contemporaneously on monitoring forms where entries were made for each patient at 30- to 60-minute intervals. Research personnel interviewed nursing staff to confirm incidents and obtain additional information. In addition, as described above,40 recordings from a multicamera system were also reviewed. The intraclass correlation coefficient (ICC) for the MOAS, established prior to the study and intermittently throughout, was above 0.90.
We also reviewed all incidents that occurred in the 4 weeks prior to study entry. This assessment was more limited because it was based only on a retrospective review of charts and incident reports. Furthermore, because the patients were on various hospital wards (prior to transfer to the research ward), it was difficult to quantify reliably the severity of incidents. Baseline aggression was therefore limited to the number of aggressive incidents during the 4-week period.
In addition, we assessed aggressive behavior outside the hospital, by interviewing patients regarding physical aggression in the community, including arrests/convictions for violent crimes. We also obtained official records of arrests, convictions, parole, and probation (“rap sheets”) from the Division of Criminal Justice Services.
Assessments of Psychiatric Symptoms.
The Positive and Negative Syndrome Scale (PANSS)42 was used to assess clinical symptoms by 2 independent raters, at baseline, week 6, and week 12 (or endpoint). This scale is widely used for the assessment of symptoms in schizophrenic and schizoaffective patients with good psychometric properties including reliability and validity. The interrater reliability of the PANSS, estimated by ICC, exceeded 0.90 in our study.
In a factor analysis study by the PANSS authors,43 one of the basic factor or “defining symptom complex” was the depression factor; it has consistently been identified in various factor analysis studies of the PANSS44,45 as one of the major factors and shows good internal consistency. The Depression factor was used for the assessment of depression, one of the 2 predictor variables in our study.
Assessment of Impulsivity.
The Barratt Impulsiveness Scale, Version 11 (BIS-11)46 is the most commonly used self-administered questionnaire of trait impulsivity with 30 items scored on a 4-point scale. It assesses long-term patterns of impulsive behaviors.
Side Effects.
Weekly white blood cell counts were done throughout the study in all patients. This was necessary for clozapine treatment and was also done for the other patients to maintain blind conditions. An electrocardiogram and physical examination were done prior to study entry and at regular intervals during the study. Adverse effects were measured weekly by the Extrapyramidal Symptom Rating Scale.47
Statistical Analysis
The relationship between aggression, depression, and impulsivity, as well as treatment group, was tested by generalized linear model analysis. As increasingly higher MOAS scores occurred with decreasing frequency (inverted “J-curve”), this analysis was based on the Poisson distribution.
The total MOAS score for the study period was the dependent variable. The independent variables included treatment assignment, baseline Depression Factor, and the BIS scores. We also included the interaction between these 2 variables and treatment assignment. Baseline violence (4-week period prior to randomization) was used as a covariate in all the analyses in order to adjust for the different levels of aggression of the patients. Gender, age, and length of participation in the study were also introduced as covariates.
We hypothesized that depression and impulsivity would predict the severity and frequency of aggression and that there would be a differential association between these 2 variables and aggression across the 3 medication groups, ie, there would be a significant interaction between treatment assignment and baseline depression/impulsivity in determining total MOAS score.
As we were interested in the statistical effect size, we investigated how combinations of low and high severity of depression and impulsivity predict violence during the treatment period in each medication group. We defined “low” as minus half an SD (−0.5 SD) from the mean and “high” as plus half an SD (+0.5 SD) from the mean.
We were also interested in the relationship between aggression and positive psychotic symptoms because these symptoms could impact on the effects of depression on aggression. We investigated therefore, in secondary analyses, the interaction between improvement in positive symptoms over the study and depression in determining total MOAS score.
Results
One hundred and one of the patients had full baseline batteries. There were 31, 36, and 34 subjects in the clozapine, olanzapine, and haloperidol groups, respectively. These constituted the study sample for the current investigation.
There were no differences among the 3 groups in the number of aggressive incidents in the 4-week period preceding the qualifying physical assault (F = 2.1; df = 100, P = .14). The mean number of aggressive incidents (±SD) during that period were 2.2 (±1.3), 2.3 (± 1.4), and 2.7± (1.8) for clozapine, olanzapine, and haloperidol, respectively.
At the end point of the first 6 weeks, the escalation and fixed-dose period, the average dose was 464.9 mg/d for clozapine (SD, 114.8mg/d; median dose, 500.0mg/d), 19.9 mg/d for olanzapine (SD, 3.1mg/d; median, 20.0mg/d), and 19.5 mg/d for haloperidol (SD, 3.9mg/d; median, 20.0mg/d). At the end of the last 6 weeks of the study, the variable-dose period, the average dose was 558.0 mg/d for clozapine (SD, 117.9mg/d; median dose, 550.0mg/d), 24.7 mg/d for olanzapine (SD, 6.1mg/d; median, 25.0mg/d), and 23.0 mg/d for haloperidol (SD, 7.1mg/d; median, 25.0mg/d).
Table 1 displays the demographic and psychiatric characteristics by treatment group. There were no differences among the 3 groups in demographic or clinical characteristics, including baseline PANSS Depression Factor and BIS-11, the 2 predictor variables.
Table 1.
Baseline Characteristics of Patients Assigned to Receive Clozapine, Olanzapine, and Haloperidola
| Characteristics | Clozapine (N = 31) | Olanzapine (N = 36) | Haloperidol (N = 34) | χ2, P |
|---|---|---|---|---|
| Categorical variables, N (%) Demographic | ||||
| Male, N (%) | 26 (83.9) | 28 (77.8) | 28 (82.4) | 0.45, .80 |
| Race/ethnicity, N (%) | ||||
| White | 5 (16.1) | 5 (13.9) | 6 (17.7) | |
| African American | 18 (58.1) | 27 (75.0) | 21 (61.8) | 6.6, .58 |
| Hispanic | 6 (19.4) | 4 (11.1) | 7 (20.6) | |
| Other | 2 (6.4) | 0 (0.0) | 0 (0.0) | |
| Diagnosis, N (%) | ||||
| Schizophrenia | 22 (71.0) | 22 (61.1) | 20 (58.8) | 1.2, .6 |
| Schizoaffective disorder | 9 (29.0) | 14 (38.9) | 14 (41.2) | |
| Violent crime in community | 17 (54.8) | 23 (63.9) | 19 (55.9) | 1.6, .4 |
| Continuous variables, M (SD) | F, P | |||
| Mean age at randomization, y | 34.5 (12.3) | 35.4 (9.5) | 32.9 (10.7) | 0.49, .61 |
| Mean duration of illness, y | 14.6 (8.7) | 16.8 (11.2) | 14.4 (11.2) | 0.51, .60 |
| Prior psychiatric hospitalizations | 12.1 (10.3) | 11.7 (9.7) | 9.0 (4.6) | 1.13, .33 |
| Mean length of hospitalization, db | 92.4 (107.2) | 74.6 (76.7) | 100.8 (127.5) | 0.56, .57 |
| PANSS scores | ||||
| Total score | 83.9 (11.0) | 83.2 (13.9) | 84.6 (13.1) | 0.09, .91 |
| PANSS depression factor | 10.6 (3.3) | 10.7 (3.2) | 10.7 (2.8) | 0.00, 1.0 |
| Barratt Impulsiveness Scale | 67.8 (10.8) | 63.1 (10.3) | 66.7 (10.1) | 1.96, .15 |
| Extrapyramidal Symptom Rating Scale (ESRS) | 22.4 (14.5) | 19.5 (11.0) | 22.8 (15.9) | 0.60, .55 |
Note: PANSS, Positive and Negative Syndrome Scale.
aFor categorical variables, the data are presented as frequencies; for continuous variables, means and SDs are provided.
bMean length of hospitalization in days upon entry into the study.
We compared the 3 groups on presence/absence of arrest and conviction for violent crime as a measure of aggression in the community. As can be seen from table 1, there were no differences with regard to the number of patients who presented with violent crime in the community.
There were no differences among these 3 groups in the proportion of subjects receiving FGAs or SGAs before randomization or in the proportion of patients receiving other psychotropic medications, including mood stabilizers or antidepressants.
During the study, there were no differences among the groups in the use of treated-as-needed medication, or in side effects, including sedation or extrapyramidal symptoms (EPS). Lack of differences in EPS was probably due to the prophylactic use of benztropine in the haloperidol group.
Baseline Depression and Impulsivity Predict Aggression Over the Study Period
The baseline Depression Factor score was a significant predictor of aggression over the 12 weeks in the total group of subjects, as assessed by the MOAS total aggression score (F = 5.2, df = 1,100, P = .03). Higher baseline depression was associated with more frequent and more severe aggression during the study.
Baseline impulsivity, as measured by the BIS-11 Total score, was also a significant predictor of aggression (F = 28.5, df = 1,100, P < .001); greater baseline impulsivity was associated with greater aggression during the treatment period. Furthermore, there was a significant interaction between depression and impulsivity in predicting the level of aggression (F = 43.3, df = 2,99, P < .001).
Depression, Impulsivity, and Aggression in the 3 Medication Groups
There were significant differences in MOAS total aggression scores among the 3 medications over the 12 weeks (F = 12.4, df = 2,99, P < .001). The mean values (±SD) were 24.8 (±30.5), 33.3 (±32.2), and 38.2 (±50.7) for clozapine, olanzapine, and haloperidol, respectively. Post-hoc paired comparisons showed that clozapine was superior to both haloperidol (P < .01) and olanzapine (P < .01), while olanzapine was superior to haloperidol (P < .01). This is in line with the results of the previous investigation that focused on aggression and psychotic symptoms.48
There was a significant 3-way interaction between medication grouping, baseline Depression Factor, and impulsivity in determining the MOAS total aggression score (F = 13.4, df = 2, 99, P < .001). Thus, while depression and impulsivity predicted aggression in all subjects, this relationship differed depending on the medication group.
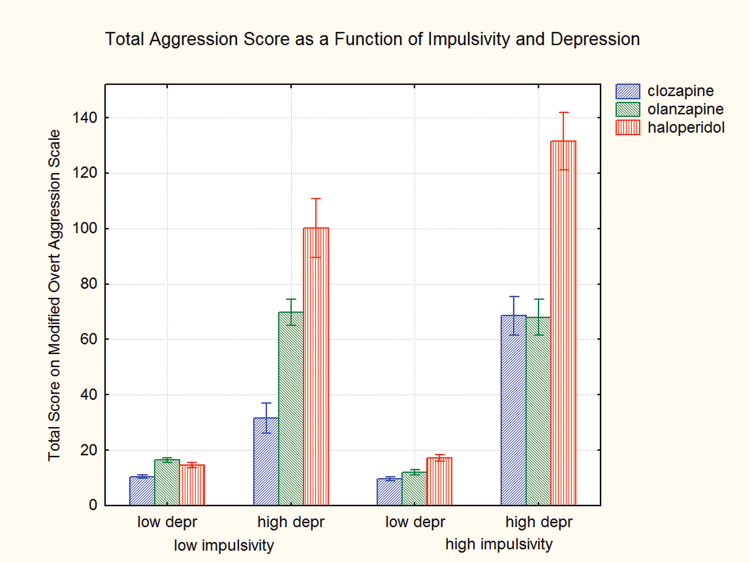
In order to assess the extent of the association between the 2 predictors and violence, we computed the Least Square (LS) Means for the MOAS total score in each medication group for low and high Depression Factor scores, first with low impulsivity and then with high impulsivity scores (table 2 and figure 1). Pairwise comparisons between medication groups were also performed (See the right-hand side of table 2.)
Table 2.
Total Modified Overt Aggression Scale Score During the Study Period in the 3 Medication Groups as a Function of Low and High Baseline Scores on the Positive and Negative Syndrome Scale Depression Factor and on the Barratt Impulsiveness, Version 11a
| Barratt Impulsiveness | Depression | Medication Groups | Pairwise Comparisons Between Medications | ||||
|---|---|---|---|---|---|---|---|
| Clozapine N = 31, M (SE) | Olanzapine N = 36, M (SE) | Haloperidol N = 34, M (SE) | CLO-OLZ | CLO-HAL | OLZ-HAL | ||
| t (P) | t (P) | t (P) | |||||
| Low | Low | 10.6 (0.7) | 16.6 (0.9) | 14.1 (1.0) | −5.6 (<.001) | −3.2 (<.01) | 1.9 (.06) |
| High | 25.9 (3.5) | 52.1 (2.7) | 66.0 (5.2) | −4.9 (<.001) | −6.1 (<.001) | −2.5 (.01) | |
| High | Low | 10.0 (0.8) | 12.1 (0.9) | 16.9 (1.0) | −1.9 (.06) | −5.6 (<.001) | −3.6 (<.001) |
| High | 47.2 (3.5) | 48.1 (3.4) | 85.9 (4.8) | −0.2 (.8) | −6.7 (<.001) | −6.4 (<.001) | |
Note: CLO, clozapine; OLZ, olanzapine; HAL, haloperidol.
aHigh and low are defined as ± half an SD from the mean.
Fig. 1.
Total Modified Overt Aggression Scale score during the study period in the 3 medication groups as a function of low and high baseline scores on the Positive and Negative Syndrome Scale Depression Factor (depr) and on the Barratt Impulsiveness, Version 11.
As can be seen from table 2 and figure 1, when there is low baseline depression, whether or not there is high impulsivity, the total MOAS scores during treatment are low (less than 20) in all 3 medication groups. When there is high depression, and especially if it is accompanied by high impulsivity, there is considerably more aggression in all 3 groups.
Specifically, when there is high depression but low impulsivity, there is a higher level of aggression in all 3 medication groups. When we compare this level of aggression to the level that is present when depression is low, we find that there is more than a 2-fold increase, more than a 3-fold increase, and almost a 5-fold increase in the CLO, OLZ, and HAL groups, respectively. In pairwise comparisons, CLO is superior to both OLZ and HAL, and OLZ is superior to HAL (table 2 and figure 1).
When high depression is also accompanied by high impulsivity, total MOAS score is nearly the same in the OLZ group as when depression was high and impulsivity low, but it is now higher in the CLO group and much higher in the HAL group. In pairwise comparisons, the level of violence is similar in the CLO and OLZ groups and it is much higher in the HAL group (table 2 and figure 1).
The Effect of Positive Psychotic Symptoms on the Relationship Between Depression and Aggression
As we saw in the previous section, when baseline depression was high, the total MOAS score was much higher and the difference among the 3 medications was more pronounced. In order to see if this relationship is modified by positive psychotic symptoms, we included both baseline positive symptoms and change in positive symptoms over the study as covariates in the analyses. The results were essentially unchanged.
In addition, we included improvement in positive psychotic symptoms over the treatment period as an additional independent variable with interaction in order to check whether improvement (ie, a decrease of one point on the PANSS Positive Symptom Factor) would modify the effect of depression on aggression. With this additional interaction, we computed once again the LS Means for Total MOAS score in each medication group for both low and high Depression Factor scores.
With improvement in positive symptoms and low baseline depression, the LS Means for Total MOAS scores were 20.9 (SE = 2.9), 10.2 (SE = 1.3), and 13.6 (SE = 1.4) for clozapine, olanzapine, and haloperidol, respectively. These values are very similar to those obtained for low baseline depression when improvement in positive symptoms was not included as an interactive variable (see table 2).
With improvement in positive symptoms and high baseline depression, aggression remained high during the study period. The LS Means for the Total MOAS scores were 30.9 (SE = 4.5), 59.9 (SE = 7.3), and 72.3 (SE = 9.1) for clozapine, olanzapine, and haloperidol, respectively. These values are similar to those reported in table 2. Thus, improvement in positive symptoms over the study did not modify the negative effect of high depression on subsequent violence or its differential effect in the 3 medication groups.
Discussion
In our study, higher depression and impulsivity were associated with more severe and frequent violence during the treatment period in all 3 medication groups. These findings corroborate the findings in the literature that violent action is influenced by both affective state and impulsivity. The strong association between depression, impulsivity, and violence may reflect a serotonergic dysfunction in some violent patients with schizophrenia.
When depression was low, the subsequent level of aggression remained low in all 3 groups, even in the presence of high impulsivity. With higher depression, and especially when it was accompanied by higher impulsivity, there was more violence. Even when positive psychotic symptoms improved with treatment, depression was still associated with high levels of violence.
However, the effect of baseline depression and impulsivity on subsequent aggression was not the same in all 3 medication groups. The relative advantage of one medication over another varied depending on the severity of baseline depression and impulsivity; hence, these baseline characteristics serve as predictors of the differential effect of medication on aggression. Furthermore, the differential association between depression and aggression is quite robust and remains unchanged when we take into consideration improvement in positive psychotic symptoms.
High baseline depression, especially when accompanied by high impulsivity, was a very strong predictor of subsequent aggression in the HAL group. These findings are consistent with the above mentioned model that depression and impulsivity indicate a serotonergic disturbance, which is also expressed in violent behavior. Thus, the superior antiaggressive effect of clozapine and, to a lesser extent, olanzapine for this type of aggression may be attributable to their serotonergic action. As mentioned above, their antiaggressive effect has been linked to their serotonergic action and may reflect normalization of serotonergic function.38,39
These findings provide us with a more differentiated picture of the advantages of the SGAs over the FGAs in the treatment of violent behavior, which may serve to explain some of the discrepancies in the literature, because some studies have not found the SGAs to be superior in treating aggression.49 Our study suggests that this superiority may depend on the type of violence or its accompanying symptoms. The SGAs may have an advantage over the FGAs in the treatment of violence that is associated with depression and impulsivity.
Given the high level of violence in the patients treated with haloperidol when high depression and impulsivity were present at baseline, it is possible that this medication not only fails to have an antiaggressive effect but may even enhance violence. Its strong D2 blockade may contribute this effect. A decrease in dopamine in schizophrenic patients has been associated with a syndrome characterized by anhedonia, dysphoria, psychomotor agitation, and impulsivity.50 Such an increase in agitation and impulsivity is likely to lead to violence in schizophrenic patients who have a predisposition to violent behavior and also evidence affective instability and poor impulse control. As mentioned above,18,26–29 a decrease in dopamine in subjects with extensive aggression was likely to be associated with increased rather than decreased aggression.
Increased aggression in patients who were receiving haloperidol has been reported in the literature. In a cross-over cohort study,51 where patients were first on placebo, then on haloperidol, and finally on either chlorpromazine or clozapine, patients were significantly more violent during haloperidol treatment than during the other 2 periods. The authors consider that the haloperidol may have induced some form of “behavioral toxicity” that may have led to an increase in aggression. It should be noted, however, that a very high dose of haloperidol, ie, 60mg/d, was used in that study.
The fact that olanzapine occupies an intermediary position when depression is higher may be due in part to the fact that olanzapine, while showing a higher 5-HT2 than D2 occupancy, has higher D2 occupancy than clozapine.31
Other factors, however, should be considered in the interpretation of our data. The dose in our study was restricted in range, and this did not allow for the comparison of olanzapine and clozapine with lower doses of haloperidol. It is possible that there would not have been as much aggression in the haloperidol group at lower doses. There is some evidence, at least with regard to psychotic symptoms, that the dose plays a role in the medication efficacy. A meta-regression analysis52 comparing SGAs with FGAs indicated that there was a greater decrease in psychotic symptoms with lower compared with higher haloperidol doses. The observed advantage in favor of the SGAs disappeared as the dose of haloperidol decreased. If this is also the case for the treatment of violent behavior, then our results might not generalize to other doses of haloperidol. However, a more recent meta-regression analysis53 challenged the above findings. In that study, there were no consistent differences between lower and higher doses of haloperidol (above or below 12 mg) in its efficacy in the treatment of psychotic symptoms.
Many clinicians select among different antipsychotic drugs to treat aggressive patients with schizophrenia on the basis of clinical lore. Our article attempts to directly address the issue of selection of antipsychotics for the treatment of aggression. By identifying patients who will respond better to a given medication, we will be able to develop individualized strategies for the treatment of violent behavior.
This study was unique in being specifically designed for the investigation of aggression with subjects who were selected on the basis of physical aggression and persistent violence. These subjects are usually not allowed to participate in clinical trials for multiple reasons, including the logistics of managing violent patients on an experimental protocol. As a result, optimal treatment strategies for violence are not systematically investigated for those who need this assessment most. The study was conducted on an inpatient research ward. This allowed for a uniform environment, careful monitoring of incidents, and high treatment compliance. These strengths, however, limit the generalizability of our results for the prediction of community violence, where factors such as poor treatment compliance, substance abuse, and adverse social environments increase the risk of violence.54
Funding
National Institutes of Health Grant Numbers (RO1 MH74767, RO1 MH85322); Eli Lilly and Company (F1D-US- IO29/4500008107); Novartis Pharmaceuticals Corporation.
Acknowledgments
Drs Krakowski and Czobor would like to thank Linda Kline, R.N., M.S., C.S., the chief coordinator of the project, the Clinical Research and Evaluation Facility (CREF) psychiatrists, Dr Fabien Tremeau, and Dr Narenda Patel; the CREF internist, Dr Surgit Dhami; Henry Epstein, the CREF team leader; the CREF nursing staff and the Nathan Kline Institute research staff. The National Institute of Mental Health had no further role in the study design; in the collection, analysis, and interpretation of the data; in the writing of the report; and in the decision to submit the article for publication. Eli Lilly and Company and Novartis Pharmaceuticals Corporation provided medications for the study. Eli Lilly and Company contributed supplemental funding for encapsulation of the medications. Overall experimental design, data acquisition, statistical analyses, and interpretation of the results were implemented with no input from any of the pharmaceutical companies. Neither Dr Krakowski nor Dr Czobor have any conflict of interest to report.
References
- 1. Barratt ES, Patton JH. Impulsivity: cognitive, behavioral, and psychophysiological correlates. In: Zuckerman M, ed. Biological Basis of Sensation-Seeking, Impulsivity and Anxiety. Hillsdale, NJ: Erlbaum; 1983:77–116 [Google Scholar]
- 2. Buchanan A, Reed A, Wessely S, et al. Acting on delusions. II: the phenomenological correlates of acting on delusions. Br J Psychiatry. 1993;163:77–81 [DOI] [PubMed] [Google Scholar]
- 3. Bleuler E. Textbook of Psychiatry (trans. Brill AA.). New York, NY: The Macmillan Company; 1924:32–35 [Google Scholar]
- 4. Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation–a possible prelude to violence. Science. 2000;289:591–594 [DOI] [PubMed] [Google Scholar]
- 5. Fava M, Bless E, Otto MW, Pava JA, Rosenbaum JF. Dysfunctional attitudes in major depression. Changes with pharmacotherapy. J Nerv Ment Dis. 1994;182:45–49 [PubMed] [Google Scholar]
- 6. Taft CT, Weatherill RP, Woodward HE, et al. Intimate partner and general aggression perpetration among combat veterans presenting to a posttraumatic stress disorder clinic. Am J Orthopsychiatry. 2009;79:461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Swanson JW, Swartz MS, Van Dorn RA, et al. A national study of violent behavior in persons with schizophrenia. Arch Gen Psychiatry. 2006;63:490–499 [DOI] [PubMed] [Google Scholar]
- 8. van Praag HM, Kahn RS, Asnis GM, et al. Denosologization of biological psychiatry or the specificity of 5-HT disturbances in psychiatric disorders. J Affect Disord. 1987;13:1–8 [DOI] [PubMed] [Google Scholar]
- 9. Apter A, van Praag HM, Plutchik R, Sevy S, Korn M, Brown SL. Interrelationships among anxiety, aggression, impulsivity, and mood: a serotonergically linked cluster? Psychiatry Res. 1990;32:191–199 [DOI] [PubMed] [Google Scholar]
- 10. Krueger RF, Caspi A, Moffitt TE, White J, Stouthamer-Loeber M. Delay of gratification, psychopathology, and personality: is low self-control specific to externalizing problems? J Pers. 1996;64:107–129 [DOI] [PubMed] [Google Scholar]
- 11. d’Acremont M, Van der Linden M. How is impulsivity related to depression in adolescence? Evidence from a French validation of the cognitive emotion regulation questionnaire. J Adolesc. 2007;30:271–282 [DOI] [PubMed] [Google Scholar]
- 12. Corruble E, Benyamina A, Bayle F, Falissard B, Hardy P. Understanding impulsivity in severe depression? A psychometrical contribution. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:829–833 [DOI] [PubMed] [Google Scholar]
- 13. Brown GL, Goodwin FK, Ballenger JC, Goyer PF, Major LF. Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry Res. 1979;1:131–139 [DOI] [PubMed] [Google Scholar]
- 14. Coccaro EF, Siever LJ, Klar HM, et al. Serotonergic studies in patients with affective and personality disorders. Correlates with suicidal and impulsive aggressive behavior. Arch Gen Psychiatry. 1989;46:587–599 [DOI] [PubMed] [Google Scholar]
- 15. Placidi GP, Oquendo MA, Malone KM, Huang YY, Ellis SP, Mann JJ. Aggressivity, suicide attempts, and depression: relationship to cerebrospinal fluid monoamine metabolite levels. Biol Psychiatry. 2001;50:783–791 [DOI] [PubMed] [Google Scholar]
- 16. Meltzer HY, Lowy MT. The serotonin hypothesis of depression. In: Meltzer HY, ed. Psychopharmacology: Third Generation of Progress. New York, NY: Raven Press; 1987:513–526 [Google Scholar]
- 17. Mulder RT, Porter RJ, Joyce PR. The prolactin response to fenfluramine in depression: effects of melancholia and baseline cortisol. J Psychopharmacol. 2003;17:97–102 [DOI] [PubMed] [Google Scholar]
- 18. Virkkunen M, De Jong J, Bartko J, Goodwin FK, Linnoila M. Relationship of psychobiological variables to recidivism in violent offenders and impulsive fire setters. A follow-up study. Arch Gen Psychiatry. 1989;46:600–603 [DOI] [PubMed] [Google Scholar]
- 19. Mehlman PT, Higley JD, Faucher I, et al. Low CSF 5-HIAA concentrations and severe aggression and impaired impulse control in nonhuman primates. Am J Psychiatry. 1994;151:1485–1491 [DOI] [PubMed] [Google Scholar]
- 20. Corrigan PW, Storzbach DM. Behavioral interventions for alleviating psychotic symptoms. Hosp Community Psychiatry. 1993;44:341–347 [DOI] [PubMed] [Google Scholar]
- 21. Allen MH, Currier GW, Hughes DH, Reyes-Harde M, Docherty JP. Expert Consensus Guideline Series. Treatment of behavioral emergencies. Post-Grad Med. 2001:1–88; quiz: 89–90 [PubMed] [Google Scholar]
- 22. de Almeida RM, Ferrari PF, Parmigiani S, Miczek KA. Escalated aggressive behavior: dopamine, serotonin and GABA. Eur J Pharmacol. 2005;526:51–64 [DOI] [PubMed] [Google Scholar]
- 23. Crowley TJ. Dose-dependent facilitation or supression of rat fighting by methamphetamine, phenobarbital, or imipramine. Psychopharmacologia. 1972;27:213–222 [DOI] [PubMed] [Google Scholar]
- 24. Miczek KA. Intraspecies aggression in rats: effects of d-amphetamine and chlordiazepoxide. Psychopharmacologia. 1974;39:275–301 [DOI] [PubMed] [Google Scholar]
- 25. Winslow JT, Miczek KA. Habituation of aggression in mice: pharmacological evidence of catecholaminergic and serotonergic mediation. Psychopharmacology (Berl). 1983;81:286–291 [DOI] [PubMed] [Google Scholar]
- 26. Hodge GK, Butcher LL. Catecholamine correlates of isolation-induced aggression in mice. Eur J Pharmacol. 1975;31:81–93 [DOI] [PubMed] [Google Scholar]
- 27. Miczek KA, Yoshimura H. Disruption of primate social behavior by d-amphetamine and cocaine: differential antagonism by antipsychotics. Psychopharmacology (Berl). 1982;76:163–171 [DOI] [PubMed] [Google Scholar]
- 28. Miczek KA, Haney M. Psychomotor stimulant effects of d-amphetamine, MDMA and PCP: aggressive and schedule-controlled behavior in mice. Psychopharmacology (Berl). 1994;115:358–365 [DOI] [PubMed] [Google Scholar]
- 29. Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin FK. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci. 1983;33:2609–2614 [DOI] [PubMed] [Google Scholar]
- 30. Bymaster FP, Calligaro DO, Falcone JF, et al. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology. 1996;14:87–96 [DOI] [PubMed] [Google Scholar]
- 31. Kapur S, Zipursky RB, Remington G, et al. 5-HT2 and D2 receptor occupancy of olanzapine in schizophrenia: a PET investigation. Am J Psychiatry. 1998;155:921–928 [DOI] [PubMed] [Google Scholar]
- 32. Nordström AL, Farde L, Nyberg S, Karlsson P, Halldin C, Sedvall G. D1, D2, and 5-HT2 receptor occupancy in relation to clozapine serum concentration: a PET study of schizophrenic patients. Am J Psychiatry. 1995;152:1444–1449 [DOI] [PubMed] [Google Scholar]
- 33. Moore NA, Leander JD, Benvenga MJ, Gleason SD, Shannon H. Behavioral pharmacology of olanzapine: a novel antipsychotic drug. J Clin Psychiatry. 1997;58:(suppl 10):37–44 [PubMed] [Google Scholar]
- 34. Glazer WM, Dickson RA. Clozapine reduces violence and persistent aggression in schizophrenia. J Clin Psychiatry. 1998;59:(suppl 3):8–14 [PubMed] [Google Scholar]
- 35. Taylor PJ, Butwell M, Gray C, et al. Schizophrenia, violence, clozapine and risperidone: A review. Br J Psychiatry. 1996;169:21–30 [PubMed] [Google Scholar]
- 36. Swanson JW, Swartz MS, Elbogen EB. Effectiveness of atypical antipsychotic medications in reducing violent behavior among persons with schizophrenia in community-based treatment. Schizophr Bull. 2004;30:3–20 [DOI] [PubMed] [Google Scholar]
- 37. Sánchez C, Arnt J, Hyttel J, Moltzen EK. The role of serotonergic mechanisms in inhibition of isolation-induced aggression in male mice. Psychopharmacology (Berl). 1993;110:53–59 [DOI] [PubMed] [Google Scholar]
- 38. Blackshear MA, Martin LL, Sanders-Bush E. Adaptive changes in the 5-HT2 binding site after chronic administration of agonists and antagonists. Neuropharmacology. 1986;25:1267–1271 [DOI] [PubMed] [Google Scholar]
- 39. Csernansky JG, Wrona CT, Bardgett ME, Early TS, Newcomer JW. Subcortical dopamine and serotonin turnover during acute and subchronic administration of typical and atypical neuroleptics. Psychopharmacology (Berl). 1993;110:145–151 [DOI] [PubMed] [Google Scholar]
- 40. Nolan KA, Czobor P, Roy BB, et al. Characteristics of assaultive behavior among psychiatric inpatients. Psychiatr Serv. 2003;54:1012–1016 [DOI] [PubMed] [Google Scholar]
- 41. Kay SR, Wolkenfeld F, Murrill LM. Profiles of aggression among psychiatric patients. I. Nature and prevalence. J Nerv Ment Dis. 1988;176:539–546 [DOI] [PubMed] [Google Scholar]
- 42. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276 [DOI] [PubMed] [Google Scholar]
- 43. Kay SR, Sevy S. Pyramidical model of schizophrenia. Schizophr Bull. 1990;16:537–545 [DOI] [PubMed] [Google Scholar]
- 44. Emsley R, Rabinowitz J, Torreman M. RIS-INT-35 Early Psychosis Global Working Group The factor structure for the Positive and Negative Syndrome Scale (PANSS) in recent-onset psychosis. Schizophr Res. 2003;61:47–57 [DOI] [PubMed] [Google Scholar]
- 45. Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry. 1997;58:538–546 [DOI] [PubMed] [Google Scholar]
- 46. Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774 [DOI] [PubMed] [Google Scholar]
- 47. Chouinard G, Ross-Chouinard A, Annable L, Jones BD. Extrapyramidal symptom rating scale. Can J Neurol Sci. 1980;72:33 [Google Scholar]
- 48. Krakowski MI, Czobor P, Citrome L, Bark N, Cooper TB. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2006;63:622–629 [DOI] [PubMed] [Google Scholar]
- 49. Swanson JW, Swartz MS, Van Dorn RA, et al. ; CATIE investigators Comparison of antipsychotic medication effects on reducing violence in people with schizophrenia. Br J Psychiatry. 2008;193:37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Strejilevich SA, Teitelbaum J, Martino DJ, Quiroz D, Kapczinski F. Dopamine sudden depletion as a model for mixed depression. Med Hypotheses. 2012;78:107–112 [DOI] [PubMed] [Google Scholar]
- 51. Herrera JN, Sramek JJ, Costa JF, Roy S, Heh CW, Nguyen BN. High potency neuroleptics and violence in schizophrenics. J Nerv Ment Dis. 1988;176:558–561 [DOI] [PubMed] [Google Scholar]
- 52. Geddes J, Freemantle N, Harrison P, Bebbington P. Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis. BMJ. 2000;321:1371–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373:31–41 [DOI] [PubMed] [Google Scholar]
- 54. Swartz MS, Swanson JW, Hiday VA, Borum R, Wagner HR, Burns BJ. Violence and severe mental illness: the effects of substance abuse and nonadherence to medication. Am J Psychiatry. 1998;155:226–231 [DOI] [PubMed] [Google Scholar]