Abstract
Recent years have seen considerable progress in epidemiological and molecular genetic research into environmental and genetic factors in schizophrenia, but methodological uncertainties remain with regard to validating environmental exposures, and the population risk conferred by individual molecular genetic variants is small. There are now also a limited number of studies that have investigated molecular genetic candidate gene-environment interactions (G × E), however, so far, thorough replication of findings is rare and G × E research still faces several conceptual and methodological challenges. In this article, we aim to review these recent developments and illustrate how integrated, large-scale investigations may overcome contemporary challenges in G × E research, drawing on the example of a large, international, multi–center study into the identification and translational application of G × E in schizophrenia. While such investigations are now well underway, new challenges emerge for G × E research from late-breaking evidence that genetic variation and environmental exposures are, to a significant degree, shared across a range of psychiatric disorders, with potential overlap in phenotype.
Key words: schizophrenia, gene-environment interaction, psychosis, epidemiology, genetics
The Environment and Schizophrenia: Evidence Beyond Reasonable Doubt?
Over the past decades, substantial and consistent evidence has accrued that implicates environmental factors in the development of schizophrenia. Numerous studies have consistently reported an increased incidence of schizophrenia in urban areas1–8 as well as in migrant and minority ethnic groups.4,7,9–12 Evidence further suggests cannabis use13–17 and childhood adversity18–20 confer substantial risk for psychotic disorder. For these environmental factors, pooled effects sizes from meta-analyses in the range of a 2- to 4-fold increase in risk,4,5,8–10,13–17,20 evidence of dose-response gradient,10,20–26 and population attributable risk fractions of 20%–35%20,27 have been reported. These advances notwithstanding, a number of methodological uncertainties remain in validating environmental exposures, including risk of systematic information bias, confounding by genetic and other factors, and possible reverse causality.19,28–32
Recent Gene Discoveries in Schizophrenia: (Some) More Light in the Dark
While the initial surge for the molecular genetic basis of schizophrenia was characterized by slow progress and methodological concerns,32,33 recent years have seen more rapid advances through large-scale collaboration in genome-wide association studies (GWAS), which have generated replicated findings on a number of common risk alleles.34–38 Recent advances have further produced consistent findings that rare copy number variants (CNVs) increase schizophrenia risk substantially and to a greater extent than individual common risk alleles identified by GWAS.39–42
However, the common variants identified to date explain only a small proportion of the genetic risk of schizophrenia and a large number of common risk alleles (with small effects) remain to be identified.35,41,43 Also, heritability estimates of the overall contribution of common genetic variants based on molecular genetic data are considerably smaller (ie 23%–33%)38,44 than heritability estimates from twin studies (ie 81%).45,46 What is more, while the reported effect sizes for CNVs tend to be much larger, they are rare and therefore contribute even less to total risk.40–42 There are several potential explanations that may account for this pattern in molecular genetic findings, but, given the consistent evidence that environmental factors confer substantial, and much greater risk than individual common genetic risk variants, it seems reasonable that gene-environment interactions (G × E) play an important role.
Gene-Environment Interactions: Contemporary Challenges
The G × E approach posits that the effect of an individual’s genotype depends on environmental exposure and, vice versa, the effect of environmental exposure on risk depends on an individual’s genotype.32,47 Since both environmental and genetic factors have consistently been implicated in etiology, but there is considerable variation in phenotype, in so far as not all individuals exposed to environmental risk or carrying genetic risk variants go on to develop the disorder, G × E appears to be particularly relevant in schizophrenia.29,48 G × E would also plausibly account for the large discrepancy in heritability estimates from twin and molecular genetic studies.44–46 This heritability gap may come about because G × E involving shared environmental factors within families are included in heritability estimates of twin studies, but not molecular genetic studies of unrelated subjects.46
While long ignored in molecular genetic analyses, and still an emerging field, the beginning of this century has seen a limited number of G × E studies on candidate genes in schizophrenia.29,49,50 These studies have tested individual, a priori selected single-nucleotide polymorphisms (SNPs), with very few attempts at replication49–52 and limited evidence on the potential mechanisms underlying G × E in schizophrenia.31,32 Indeed, there remain a number of conceptual and methodological challenges in contemporary molecular G × E research. These include (a) the validation of environmental exposures, consistently measured in sufficiently large, epidemiologically characterized samples for G × E analysis33,46; (b) selecting optimal strategies for (1) the use of complex GWAS data, (2) a priori, hypothesis-based vs exploratory approaches, and (3) the type of genetic variation to be used in G × E analysis; (c) a relative paucity of validated and scalable experimental methods for investigating modifiable mechanisms underlying G × E in schizophrenia; (d) the different phenotypic levels of schizophrenia at which G × E may impact, including intermediate phenotypes, prodrome, onset, severity, and course of schizophrenia; (e) statistical modeling of the likely simultaneous presence of G × E, G × G and E × E interactions; (f) ethical issues that may arise if G × E analyses produce evidence of substantial risks to be leveraged in risk assessment and early prediction; and (g) the need for translation of G × E findings to clinical practice.
It has repeatedly been noted that current challenges in molecular genetic G × E research warrant integrated, large-scale investigations that bring together international experts at the forefront of research in epidemiology, genetics, experimental psychiatry, statistics, social psychiatry, brain imaging, and clinical psychiatry.31–33,47,50,53 While in molecular genetic research large-scale collaborations such as the International Schizophrenia Consortium35 or the Psychiatric Genomics Consortium54 are increasingly common, there are only few examples in G × E research; one is “The European Network of National Networks studying Gene-Environment Interactions in Schizophrenia” (EU-GEI)32,47 (see also www.eu-gei.eu).
The European Network of National Schizophrenia Networks Studying Gene-Environment Interactions (EU-GEI)
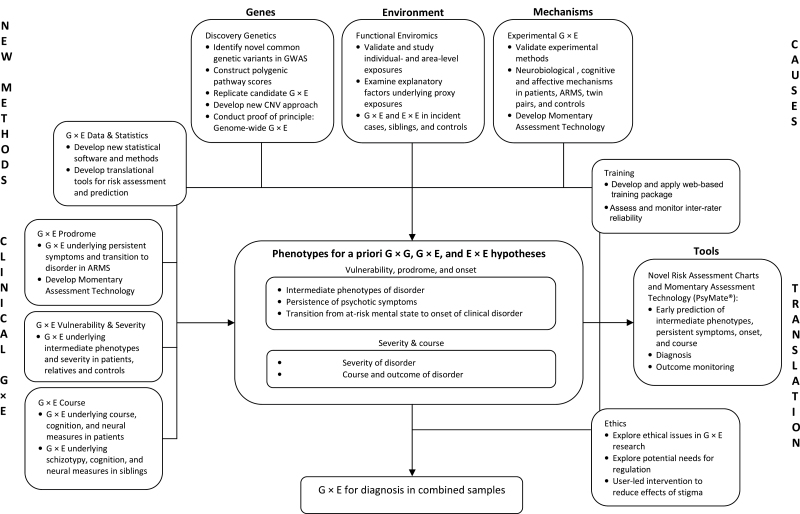
EU-GEI is a large, international, multi–center study of G × E in schizophrenia using family-based, multidisciplinary research paradigms in more than 15 countries (the Netherlands, the UK, Germany, Turkey, Spain, France, Belgium, Greece, Austria, Switzerland, Hong Kong (China), Brazil, Australia, Ireland, Italy, and other European and non-European countries represented by EU-GEI affiliated centers) for testing a priori G × E hypotheses. The overall aim of EU-GEI is the identification and translational application of clinical, genetic, and environmental interactions in the development, severity, and course of schizophrenia in patients and their families. To this end, several work packages are currently underway that amalgamate expertise from multiple disciplines for addressing contemporary challenges in G × E research (see figure 1).
Fig. 1.
General approach and overview of European Network of National Networks studying Gene-Environment Interactions in Schizophrenia.
The “Functional Enviromics” work package has developed and currently applies methods for the detailed assessment of candidate, individual- and area-level environmental exposures of public health relevance (ie with the largest attributable fractions and most relevant to the EU study population).12 The work package employs a number of strategies for validating environmental exposures by using an optimum, family-based, case-control design in a diverse range of settings across Europe, drawing on corroborative sources of information in the assessment of childhood and adult adversity to minimize recall bias, and taking account of potential confounding by direct and indirect measures of genetic risk as well as other relevant factors. In doing so, “Functional Enviromics” aims to investigate the impact of hypothesized individual- and area-level environmental exposures on risk of first episode psychosis and to identify proximal explanatory factors that account for high rates of psychotic disorder in urban areas and in migrant and ethnic minority groups. The work package further aims, together with “Discovery Genetics” and “G × E Data & Statistics,” to examine evidence for hypothesized G × E and environment × environment (E × E) interactions.
The “Discovery Genetics” work package aims to identify novel genes and biological pathways and implement new approaches for CNVs that will allow, jointly with all other work packages, to test specific, a priori G × E hypotheses. Specifically, this work package combines available with newly generated GWAS data to identify common variants, showing robust genome-wide evidence for association, to test specific, a priori SNP-based G × E hypotheses. “Discovery Genetics” further constructs, in collaboration with “G × E Data & Statistics,” polygenic pathway scores based on pathway-wide evidence for association of SNPs in genes that are involved in specific biological pathways underlying environmental risks. In addition, this work package targets previously identified candidate G × E for replication, develops and implements new approaches to G × E analysis with CNVs, and conducts proof of principle for genome-wide G × E analysis, with the aim of identifying novel risk-environment interplays that are not predicated upon the existence of observed genetic main effects.
In the “Experimental G × E” work package, validated and scalable experimental methods have been developed for investigating neural, cognitive, and affective mechanisms underlying the interplay of genetic and environmental factors under experimental conditions, controlling for measured and unmeasured confounding factors, including genetic factors (and, thereby, gene-environment correlation). Work by the experimental G × E work package also supports the view that epidemiologically validated risk factors such as migration or urban living and upbringing have a social component, as proposed by the social defeat hypothesis.55 Specifically, findings from this work package suggest a specific impact of social stress on activation in a perigenual cingulate-amygdalar circuit in healthy populations exposed to urban living and upbringing56 and migration.57 This suggests that this circuit may be a core convergence region for risk of mental disorders arising through social stressors.58 The experimental G × E approach is also of considerable potential value for generating translational knowledge. Therefore, this work package has developed innovative Momentary Assessment Technology (ie the PsyMate) to investigate stress sensitivity in daily life as an important affective mechanism underlying environmental and genetic risk in the development of schizophrenia.59
The “G × E Prodrome,” “G × E Vulnerability and Severity,” and “G × E Course” work packages take into account the different phenotypes and clinical stages of disorder at which gene-environment interactions may impact, including intermediate phenotypes, prodrome, onset, severity, and course of schizophrenia. These work packages aim to investigate clinical, environmental, and genetic determinants as well as G × E at all these levels, with initial evidence of candidate SNP × cannabis interaction for psychosis liability.60,61 The “G × E Data & Statistics” work package provides coordination and support for statistical methodology to examine, jointly with all other work packages, G × E, G × G, and E × E interactions underlying disease risk, course, and outcome of schizophrenia. The work package further develops novel statistical software and methodology for examining G × E interactions. In the “Training” work package, a web-based training environment has been developed for addressing a key issue in large multi–national collaborations, i.e. inter-rater reliability in the assessment of environmental exposures, diagnosis, intermediate phenotypes, prodrome, onset, severity, and course. Given the potential ethical issues raised by G × E research in schizophrenia, the “Ethics” work package explores these, detects potential needs for regulation and, in collaboration with the “Dissemination” work package, will include ethical and legal perspectives in the dissemination activities. Lastly, the entire project is coordinated by the “Management” work package.
Evidence on G × G, G × E, and E × E generated by EU-GEI will aggregate in the development of risk prediction algorithms that will be implemented in innovative, translational risk assessment charts and momentary assessment technology for early prediction of intermediate phenotypes, persistent symptoms, transition to, as well as onset, severity, course, and outcome of, schizophrenia. Application of these tools will allow the targeting of prevention, treatment and resources to modifiable mechanisms as well as subgroups of individuals with the greatest vulnerability, highest risk of developing persistent symptoms and psychotic disorder and, once diagnosed, to those with greatest severity and highest risk of poor course and outcome.
Conclusion and Future Prospects
Recent years have seen significant advances in epidemiological and molecular genetic research, consistently implicating environmental and genetic factors in the etiology of schizophrenia. However, methodological uncertainties remain with regard to validating environmental exposures and the population risk conferred by the molecular genetic variants identified to date remains small. While G × E may account for the latter, so far, replication of the limited number of molecular genetic candidate G × E findings is rare. Important conceptual and methodological challenges of G × E research in schizophrenia are currently being addressed in integrated, large-scale investigations, such as EU-GEI.
While EU-GEI is now well underway, new challenges emerge at the horizon of G × E research. It now appears increasingly likely that genetic variation44 and environmental exposures (such as childhood adversity)18 are, to a significant degree, shared across a range of psychiatric disorders, with some emerging evidence of overlap in phenotypes.62 Therefore, as for mono-disciplinary, epidemiological and molecular-genetic research, the study of G × E needs to be extended beyond individual disorders to investigations of all major psychiatric disorders in order to unpick the complex interplay of genes, environment, and underlying mechanisms that push some people along a pathway to psychosis, whilst others to non-psychotic or no disorder. Not only cross-discipline, but also large-scale cross-disorder investigations are now required to more fully realize the potential of G × E research in elucidating the etiology of, and, ultimately, improving prevention and treatment for, schizophrenia.
Funding
European Community’s Seventh Framework Program under grant agreement (HEALTH-F2-2009–241909, Project EU-GEI).
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
European Network of National Networks studying Gene-Environment Interactions in Schizophrenia (EU-GEI)
Authors
Jim van Os1,2, Bart P. Rutten1, Inez Myin-Germeys1, Philippe Delespaul1,3, Wolfgang Viechtbauer1, Catherine van Zelst1, Richard Bruggeman1,4, Ulrich Reininghaus1,5, Craig Morgan5, Robin M. Murray2, Marta Di Forti2, Philip McGuire2, Lucia R. Valmaggia6, Matthew J. Kempton2, Charlotte Gayer-Anderson5, Kathryn Hubbard5, Stephanie Beards5, Simona A. Stilo2,5, Adanna Onyejiaka5, Francois Bourque5, Gemma Modinos2, Stefania Tognin2, Maria Calem2, Michael C. O’Donovan7, Michael J. Owen7, Peter Holmans7, Nigel Williams7, Nicholas Craddock7, Alexander Richards7, Isla Humphreys7, Andreas Meyer-Lindenberg8, F. Markus Leweke8, Heike Tost8, Ceren Akdeniz8, Cathrin Rohleder8, J. Malte Bumb8, Emanuel Schwarz8, Köksal Alptekin9, Alp Üçok10, Meram Can Saka11,12, E. Cem Atbaşoğlu11,12, Sinan Gülöksüz1,13, Guvem Gumus-Akay12, Burçin Cihan14, Hasan Karadağ15, Haldan Soygür16, Eylem Şahin Cankurtaran15, Semra Ulusoy17, Berna Akdede9, Tolga Binbay9, Ahmet Ayer18, Handan Noyan19, Gülşah Karadayı10, Elçin Akturan10, Halis Ulaş9, Celso Arango20, Mara Parellada20, Miguel Bernardo21, Julio Sanjuán22, Julio Bobes23, Manuel Arrojo24, Jose Luis Santos25, Pedro Cuadrado26, José Juan Rodríguez Solano27, Angel Carracedo28, Enrique García Bernardo29, Laura Roldán20, Gonzalo López20, Bibiana Cabrera21, Sabrina Cruz22, Eva Mª Díaz Mesa23, María Pouso24,28,30, Estela Jiménez25, Teresa Sánchez20, Marta Rapado20, Emiliano González20, Covadonga Martínez20, Emilio Sánchez29, Mª Soledad Olmeda29, Lieuwe de Haan31, Eva Velthorst31, Mark van der Gaag32,33, Jean-Paul Selten1,34, Daniella van Dam31, Elsje van der Ven1,34, Floor van der Meer31, Elles Messchaert31,34, Tamar Kraan31,33, Nadine Burger31,33, Marion Leboyer35–38, Andrei Szoke35–38, Franck Schürhoff35–38, Pierre-Michel Llorca38–40, Stéphane Jamain36– 38, Andrea Tortelli36,41, Flora Frijda41, Jeanne Vilain35–38, Anne-Marie Galliot36, Grégoire Baudin35,36, Aziz Ferchiou35,36, Jean-Romain Richard36,38, Ewa Bulzacka35, Thomas Charpeaud38,39,40, Anne-Marie Tronche38,39,40, Marc De Hert42, Ruud van Winkel1,42, Jeroen Decoster43, Catherine Derom44,45, Evert Thiery45,46, Nikos C. Stefanis47, Gabriele Sachs48, Harald Aschauer48, Iris Lasser48, Bernadette Winklbaur48, Monika Schlögelhofer48, Anita Riecher-Rössler49, Stefan Borgwardt50, Anna Walter50, Fabienne Harrisberger50, Renata Smieskova50, Charlotte Rapp49, Sarah Ittig49, Fabienne Soguel-dit-Piquard49, Erich Studerus49, Joachim Klosterkötter51, Stephan Ruhrmann51, Julia Paruch51, Dominika Julkowski51, Desiree Hilboll51, Pak C. Sham52, Stacey S. Cherny52, Eric Y. H. Chen53, Desmond D. Campbell52, Miaoxin Li52, Carlos María Romeo-Casabona54, Aitziber Emaldi Cirión54, Asier Urruela Mora55, Peter Jones56, James Kirkbride56,57, Mary Cannon58, Dan Rujescu59, Ilaria Tarricone60, Domenico Berardi60, Elena Bonora61, Marco Seri61, Thomas Marcacci60, Luigi Chiri60, Federico Chierzi60, Viviana Storbini60, Mauro Braca60, Maria Gabriella Minenna62, Ivonne Donegani62, Angelo Fioritti62, Daniele La Barbera63, Caterina Erika La Cascia63, Alice Mulè64, Lucia Sideli63, Rachele Sartorio63, Laura Ferraro64, Giada Tripoli63, Fabio Seminerio64, Anna Maria Marinaro64, Patrick McGorry65, Barnaby Nelson65, G. Paul Amminger65, Christos Pantelis66, Paulo R. Menezes67,68, Cristina M. Del-Ben68,69, Silvia H. Gallo Tenan68,69, Rosana Shuhama68,69, Mirella Ruggeri70, Sarah Tosato70, Antonio Lasalvia70, Chiara Bonetto70, Elisa Ira70, Merete Nordentoft71, Marie-Odile Krebs72, Neus Barrantes-Vidal73–76, Paula Cristóbal73, Thomas R. Kwapil75, Elisa Brietzke77, Rodrigo A. Bressan77, Ary Gadelha77, Nadja P. Maric78, Sanja Andric78, Marina Mihaljevic78, and Tijana Mirjanic78
Affiliations
1Department of Psychiatry and Neuropsychology, School for Mental Health and Neuroscience, South Limburg Mental Health Research and Teaching Network, Maastricht University Medical Centre, Maastricht, The Netherlands; 2Department of Psychosis Studies, Institute of Psychiatry, King’s College London, London, UK; 3Mondriaan Mental Health Trust, South Limburg, Maastricht/Heerlen, Heerlen, The Netherlands; 4University Centre of Psychiatry, Rob Giel Clinical Research, University of Groningen, University Medical Centre Groningen, Groningen, The Netherlands; 5Department of Health Service and Population Research, Institute of Psychiatry, King’s College London, London, UK; 6Department of Psychology, Institute of Psychiatry, King’s College London, London, UK; 7Medical Research Council (MRC) Centre for Neuropsychiatric Genetics and Genomics, Cardiff University Cardiff, UK; 8Department of Psychiatry and Psychotherapy, Central Institute of Mental Health, Mannheim, Germany; 9Department of Psychiatry, School of Medicine, Dokuz Eylül University, Konak, Turkey; 10Psychotic Disorders Research Unit, Department of Psychiatry, Istanbul Faculty of Medicine, Istanbul University, Istanbul, Turkey; 11Department of Psychiatry, School of Medicine, Ankara University, Cebeci Hospital, Mamak cad, Ankara, Turkey; 12Ankara University Brain Research Center, Ankara University, Ankara, Turkey; 13Department of Psychiatry, Yale University Medical School, Department of Psychiatry, New Haven, CT; 14Department of Psychology, Middle East Technical University ODTÜ Üniversiteler Mah., Ankara, Turkey; 15Dışkapı Y.B. Research and Training Hospital, İrfan Baştuğ Cad, Dışkapı, Ankara, Turkey; 16Turkish Federation of Schizophrenia Associations, Ankara, Turkey; 17Psychiatry Clinic, Ataturk Training and Research Hospital, Ankara, Turkey; 18Manisa Mental Health Hospital, Manisa, Turkey; 19Department of Advanced Neurological Sciences, Institute for Experimental Medical Research, Istanbul Faculty of Medicine, Istanbul University, Istanbul, Turkey; 20Department of Child and Adolescent Psychiatry, Hospital General Universitario Gregorio Marañón, School of Medicine, Universidad Complutense, IiSGM (CIBERSAM), Madrid, Spain; 21Department of Psychiatry, Hospital Clinic, IDIBAPS, Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), Universidad de Barcelona, Barcelona, Spain; 22Department of Psychiatry, School of Medicine, Universidad de Valencia, Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), Valencia, Spain; 23Department of Medicine, Psychiatry Area, School of Medicine, Universidad de Oviedo, Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), Oviedo, Spain; 24Department of Mental Health and Drug-addition assistance, Health Service of Galicia, Psychiatric Genetic Group IDIS, Hospital Clínico Universitario de Santiago de Compostela, affiliated center to Centro de Investigación Biomédica en Red de Salud Mental, (CIBERSAM), Servicio Gallego de Salud. Edificio Administrativo de San Lázaro s/n 15706 Santiago de Compostela, Spain; 25Department of Psychiatry, Servicio de Psiquiatría Hospital “Virgen de la Luz,” C/Hermandad de Donantes de Sangre, Cuenca, Spain; 26Villa de Vallecas Mental Health Department, Villa de Vallecas Mental Health Centre, Hospital Universitario Infanta Leonor/Hospital Virgen de la Torre, Madrid, Spain; 27Puente de Vallecas Mental Health Department, Hospital Universitario Infanta Leonor/Hospital Virgen de la Torre, Centro de Salud Mental Puente de Vallecas, Madrid, Spain; 28Fundación Pública Galega de Medicina Xenómica, Hospital Clínico Universitario, Santiago de Compostela, Spain; 29Department of Psychiatry, Hospital General Universitario Gregorio Marañón, School of Medicine, Universidad Complutense, Madrid, Spain; 30Hospital Psiquiatrico de Conxo. ext 251951, Santiago de Compostela, A Coruña, Spain; 31Department of Psychiatry, Early Psychosis Section, Academic Medical Centre, University of Amsterdam, Amsterdam, The Netherlands; 32Department of Clinical Psychology, VU University and EMGO Institute of Health and Care Research, Amsterdam, The Netherlands; 33Department of Psychosis Research, Parnassia Psychiatric Institute, The Hague, The Netherlands; 34Rivierduinen Psychiatric Institute, Leiden, The Netherlands; 35AP-HP, Groupe Hospitalier “Mondor”, Pôle de Psychiatrie, Créteil, France; 36INSERM, U955, Equipe 15, Créteil, France; 37Faculté de Médecine, Université Paris-Est, Créteil, France; 38Fondation Fondamental, Créteil, France; 39CMP B CHU, BP 69, 63003 Clermont-Ferrand, Cedex 1, France; 40Université d’Auvergne, EA 7280, Clermont-Ferrand, France; 41EPS Maison Blanche, Paris, France; 42 UPC KU Leuven, Campus Kortenberg, Department of Neurosciences, UPC, Kortenberg, Belgium; 43Research Group Psychiatry, Department of Neurosciences, UPC, Leuven, Belgium; 44Department of Human Genetics, University Hospital Gasthuisberg, Katholieke Universiteit Leuven, Leuven, Belgium; 45Association for Scientific Research in Multiple Births, Ghent, Belgium; 46Department of Neurology, Ghent University, Ghent University Hospital, Ghent, Belgium; 47National and Kapodistrian University of Athens, Medical School Eginition Hospital, Athens, Greece; 48Department of Psychiatry and Psychotherapy, Medical University of Vienna, Vienna, Austria; 49Center for Gender Research and Early Detection, Psychiatric University Clinics Basel, Basel, Switzerland; 50Diagnostic and Crisis Intervention Centre, Psychiatric University Clinics Basel, Basel, Switzerland; 51Department of Psychiatry and Psychotherapy, University of Cologne, Cologne, Germany; 52Centre for Genomic Sciences, State Key Laboratory of Brain and Cognitive Sciences and Department of Psychiatry, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, PR China; 53State Key Laboratory of Brain and Cognitive Sciences and Department of Psychiatry, Li Ka Shing Faculty of Medicine, The University of Hong Kong, New Clinical Building, Queen Mary Hospital, Hong Kong SAR, PR China; 54Inter-University Chair in Law and the Human Genome (Provincial Government of Biskay) in University of Deusto, University of the Basque Country, Bilbao, Bizkaia, Spain; 55Criminal Law, University of Zaragoza, Zaragoza, Spain; 56Department of Psychiatry, University of Cambridge, Hercel Smith Building for Brain & Mind Sciences, Cambridge, UK; 57Division of Psychiatry, University College, Charles Bell House, London, UK; 58Department of Psychiatry, Royal College of Surgeons in Ireland, Education and Research Centre, Beaumont Hospital, Dublin 9, Ireland; 59Division of Molecular and Clinical Neurobiology, Department of Psychiatry, Ludwig-Maximilians University, Munich, Germany; 60Department of Medical and Surgical Science, Psychiatry Unit, Alma Mater Studiorium Università di Bologna, Bologna, Italy; 61Department of Medical and Surgical Science, Genetic Unit, Alma Mater Studiorium Università di Bologna, Bologna, Italy; 62Department of Mental Health and Pathological Addictions, Local Health Trust, Bologna, Italy; 63Department of Experimental Biomedicine and Clinical Neuroscience, Section of Psychiatry, University of Palermo, Palermo, Italy; 64Unit of Psychiatry, “P. Giaccone” General Hospital, Palermo, Italy; 65Centre for Youth Mental Health, University of Melbourne Parkville, Victoria, Australia; 66Melbourne Neuropsychiatry Centre, University of Melbourne, Carlton South, Victoria, Australia; 67Departamento de Medicina Preventiva, Faculdade de Medicina, Universidade de São Paulo, Avenida Doutor Arnaldo 455, CEP 01246-903, São Paulo, Brazil; 68Núcleo de Pesquina em Saúde Mental Populacional, Universidade de São Paulo, São Paulo, Brazil; 69Departamento de Neurociências e Ciencias do Comportamento, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, Brazil; 70Section of Psychiatry, Department of Public Health and Community Medicine, University of Verona, Verona, Italy; 71Copenhagen University Hospital, Research Unit, Mental Health Centre Copenhagen, Copenhagen, Denmark; 72Hôpital Sainte-Anne, Service Hospitalo-Universitaire, Faculté de Médecine Paris Descartes, University Paris Descartes, Paris, France; 73Departament de Psicologia Clínica i de la Salut, Universitat Autònoma de Barcelona, Barcelona, Spain; 74Departament de Salut Mental, Sant Pere Claver-Fundació Sanitària, Barcelona, Spain; 75Department of Psychology, University of North Carolina at Greensboro, Greensboro, NC; 76Spanish Mental Health Research Network, CIBERSAM, Spain; 77PRISMA Early Intervention Program, Department of Psychiatry, Federal University of São Paulo, São Paulo, Brazil; 78School of Medicine, University of Belgrade, Beograd, Serbia
Correspondence
Bart P. Rutten, Department of Psychiatry and Neuropsychology, School for Mental Health and Neuroscience, South Limburg Mental Health Research and Teaching Network, Maastricht University Medical Centre, PO Box 616, 6200 MD Maastricht, The Netherlands; tel: 31-43-388-1263, fax: 31-43-388-4086, e-mail: b.rutten@maastrichtuniversity.nl
Ulrich Reininghaus, Department of Psychiatry and Neuropsychology, School for Mental Health and Neuroscience, South Limburg Mental Health Research and Teaching Network, Maastricht University Medical Centre, PO Box 616, 6200 MD Maastricht, The Netherlands; tel: 31-43-388-3896, fax: 31-43-388-4122, e-mail: u.reininghaus@maastrichtuniversity.nl
References
- 1. Allardyce J, Boydell J. Review: the wider social environment and schizophrenia. Schizophr Bull. 2006;32:592–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heinz A, Deserno L, Reininghaus U. Urbanicity, social adversity and psychosis. World Psychiatry. 2013;12:187–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kelly BD, O’Callaghan E, Waddington JL, et al. Schizophrenia and the city: A review of literature and prospective study of psychosis and urbanicity in Ireland. Schizophr Res. 2010;116:75–89 [DOI] [PubMed] [Google Scholar]
- 4. Kirkbride JB, Errazuriz A, Croudace TJ, et al. Incidence of schizophrenia and other psychoses in England, 1950–2009: a systematic review and meta-analyses. PloS one. 2012;7:e31660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krabbendam L, van Os J. Schizophrenia and urbanicity: a major environmental influence–conditional on genetic risk. Schizophr Bull. 2005;31:795–799 [DOI] [PubMed] [Google Scholar]
- 6. March D, Hatch SL, Morgan C, et al. Psychosis and place. Epidemiol Rev. 2008;30:84–100 [DOI] [PubMed] [Google Scholar]
- 7. McGrath J, Saha S, Welham J, El Saadi O, MacCauley C, Chant D. A systematic review of the incidence of schizophrenia: the distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Med. 2004;2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vassos E, Pedersen CB, Murray RM, Collier DA, Lewis CM. Meta-analysis of the association of urbanicity with schizophrenia. Schizophr Bull. 2012;38:1118–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bourque F, van der Ven E, Malla A. A meta-analysis of the risk for psychotic disorders among first- and second-generation immigrants. Psychol Med. 2011;41:897–910 [DOI] [PubMed] [Google Scholar]
- 10. Cantor-Graae E, Selten JP. Schizophrenia and migration: a meta-analysis and review. Am J Psychiatry. 2005;162:12–24 [DOI] [PubMed] [Google Scholar]
- 11. Kirkbride JB, Fearon P, Morgan C, et al. Heterogeneity in incidence rates of schizophrenia and other psychotic syndromes: findings from the 3-center AeSOP study. Arch Gen Psychiatry. 2006;63:250–258 [DOI] [PubMed] [Google Scholar]
- 12. Morgan C, Charalambides M, Hutchinson G, Murray RM. Migration, ethnicity, and psychosis: toward a sociodevelopmental model. Schizophr Bull. 2010;36:655–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arseneault L, Cannon M, Witton J, Murray RM. Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry. 2004;184:110–117 [DOI] [PubMed] [Google Scholar]
- 14. Henquet C, Murray R, Linszen D, van Os J. The environment and schizophrenia: the role of cannabis use. Schizophr Bull. 2005;31:608–612 [DOI] [PubMed] [Google Scholar]
- 15. Minozzi S, Davoli M, Bargagli AM, Amato L, Vecchi S, Perucci CA. An overview of systematic reviews on cannabis and psychosis: discussing apparently conflicting results. Drug Alcohol Rev. 2010;29:304–317 [DOI] [PubMed] [Google Scholar]
- 16. Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328 [DOI] [PubMed] [Google Scholar]
- 17. Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19:187–194 [DOI] [PubMed] [Google Scholar]
- 18. Matheson SL, Shepherd AM, Pinchbeck RM, Laurens KR, Carr VJ. Childhood adversity in schizophrenia: a systematic meta-analysis. Psychol Med. 2013;43:225–238 [DOI] [PubMed] [Google Scholar]
- 19. Morgan C, Fisher H. Environment and schizophrenia: environmental factors in schizophrenia: childhood trauma–a critical review. Schizophr Bull. 2007;33:3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Varese F, Smeets F, Drukker M, et al. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull. 2012;38:661–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. D’Souza DC, Perry E, MacDougall L, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572 [DOI] [PubMed] [Google Scholar]
- 22. Eaton WW, Mortensen PB, Frydenberg M. Obstetric factors, urbanization and psychosis. Schizophr Res. 2000;43:117–123 [DOI] [PubMed] [Google Scholar]
- 23. Harrison G, Fouskakis D, Rasmussen F, Tynelius P, Sipos A, Gunnell D. Association between psychotic disorder and urban place of birth is not mediated by obstetric complications or childhood socio-economic position: a cohort study. Psychol Med. 2003;33:723–731 [DOI] [PubMed] [Google Scholar]
- 24. Morrison PD, Zois V, McKeown DA, et al. The acute effects of synthetic intravenous Delta9-tetrahydrocannabinol on psychosis, mood and cognitive functioning. Psychol Med. 2009;39:1607–1616 [DOI] [PubMed] [Google Scholar]
- 25. Mortensen PB, Pedersen CB, Westergaard T, et al. Effects of family history and place and season of birth on the risk of schizophrenia. New Engl J Med. 1999;340:603–608 [DOI] [PubMed] [Google Scholar]
- 26. Pedersen CB, Mortensen PB. Evidence of a dose-response relationship between urbanicity during upbringing and schizophrenia risk. Arch Gen Psychiatry. 2001;58:1039–1046 [DOI] [PubMed] [Google Scholar]
- 27. Kirkbride JB, Jones PB. The prevention of schizophrenia–what can we learn from eco-epidemiology? Schizophr Bull. 2011;37:262–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fisher HL, Craig TK, Fearon P, et al. Reliability and comparability of psychosis patients’ retrospective reports of childhood abuse. Schizophr Bull. 2011;37:546–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Arch Gen Psychiatry. 2005;62:473–481 [DOI] [PubMed] [Google Scholar]
- 30. Susser E, Widom CS. Still searching for lost truths about the bitter sorrows of childhood. Schizophr Bull. 2012;38:672–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468:203–212 [DOI] [PubMed] [Google Scholar]
- 32. van Os J, Rutten BP, Poulton R. Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr Bull. 2008;34:1066–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McGrath JJ, Mortensen PB, Visscher PM, Wray NR. Where GWAS and epidemiology meet: opportunities for the simultaneous study of genetic and environmental risk factors in schizophrenia. Schizophr Bull. 2013;39:955–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Corvin A. Schizophrenia at a genetics crossroads: where to now? Schizophr Bull. 2013;39:490–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. International Schizophrenia Consortium. Purcell SM, Wray NR, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O’Donovan MC, Craddock N, Norton N, et al. Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat Gen. 2008;40:1053–1055 [DOI] [PubMed] [Google Scholar]
- 37. Owen MJ, Craddock N, O’Donovan MC. Suggestion of roles for both common and rare risk variants in genome-wide studies of schizophrenia. Arch Gen Psychiatry. 2010;67:667–673 [DOI] [PubMed] [Google Scholar]
- 38. Ripke S, O’Dushlaine C, Chambert K, et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Gen. 2013;45:1150–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grozeva D, Kirov G, Ivanov D, et al. Rare copy number variants: a point of rarity in genetic risk for bipolar disorder and schizophrenia. Arch Gen Psychiatry. 2010;67:318–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guha S, Rees E, Darvasi A, et al. Implication of a rare deletion at distal 16p11.2 in schizophrenia. JAMA Psychiatry. 2013;70:253–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee SH, DeCandia TR, Ripke S, et al. Estimating the proportion of variation in susceptibility to schizophrenia captured by common SNPs. Nat Gen. 2012;44:247–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Malhotra D, Sebat J. CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell. 2012;148:1223–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Owen MJ. Implications of genetic findings for understanding schizophrenia. Schizophr Bull. 2012;38:904–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cross-Disorder Group of the Psychiatric Genomics Consortium. Lee SH, Ripke S, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Gen. 2013;45:984–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–1192 [DOI] [PubMed] [Google Scholar]
- 46. Uher R. Gene-environment interactions in common mental disorders: an update and strategy for a genome-wide search. Soc Psychiatry Psychiatr Epidemiol. 2014;49:3–14 [DOI] [PubMed] [Google Scholar]
- 47. European Network of Schizophrenia Networks for the Study of Gene-Environment Interactions. Schizophrenia aetiology: do gene-environment interactions hold the key? Schizophr Res. 2008;102:21–26 [DOI] [PubMed] [Google Scholar]
- 48. Vassos E, Collier DA, Holden S, et al. Penetrance for copy number variants associated with schizophrenia. Hum Mol Gen. 2010;19:3477–3481 [DOI] [PubMed] [Google Scholar]
- 49. Iyegbe C, Campbell D, Butler A, Ajnakina O, Sham P. The emerging molecular architecture of schizophrenia, polygenic risk scores and the clinical implications for GxE research. Soc Psychiatry Psychiatr Epidemiol. 2014;49:169–182 [DOI] [PubMed] [Google Scholar]
- 50. Modinos G, Iyegbe C, Prata D, et al. Molecular genetic gene-environment studies using candidate genes in schizophrenia: a systematic review. Schizophr Res. 2013;150:356–365 [DOI] [PubMed] [Google Scholar]
- 51. Decoster J, van Os J, Myin-Germeys I, De Hert M, van Winkel R. Genetic variation underlying psychosis-inducing effects of cannabis: critical review and future directions. Curr Pharm Des. 2012;18:5015–5023 [DOI] [PubMed] [Google Scholar]
- 52. Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am J Psychiatry. 2011;168:1041–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reininghaus U, Morgan C. Integrated models in psychiatry: the state of the art. Soc Psychiatry Psychiatr Epidemiol. 2014;49:1–2 [DOI] [PubMed] [Google Scholar]
- 54. Sullivan PF. The psychiatric GWAS consortium: big science comes to psychiatry. Neuron. 2010;68:182–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Selten JP, Cantor-Graae E. Social defeat: risk factor for schizophrenia? Br J Psychiatry. 2005;187:101–102 [DOI] [PubMed] [Google Scholar]
- 56. Lederbogen F, Kirsch P, Haddad L, et al. City living and urban upbringing affect neural social stress processing in humans. Nature. 2011;474:498–501 [DOI] [PubMed] [Google Scholar]
- 57. Akdeniz C, Tost H, Streit F, et al. Neuroimaging evidence for a role of neural social stress processing in ethnic minority associated environmental risk. JAMA Psychiatry. In press. 10.1001/jamapsychiatry.2014.35. [DOI] [PubMed] [Google Scholar]
- 58. Meyer-Lindenberg A, Tost H. Neural mechanisms of social risk for psychiatric disorders. Nat Neurosci. 2012;15:663–668 [DOI] [PubMed] [Google Scholar]
- 59. Myin-Germeys I, Birchwood M, Kwapil T. From environment to therapy in psychosis: a real-world momentary assessment approach. Schizophr Bull. 2011;37:244–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Genetic Risk and Outcome in Psychosis Investigators. Evidence that familial liability for psychosis is expressed as differential sensitivity to cannabis: an analysis of patient-sibling and sibling-control pairs. Arch Gen Psychiatry. 2011;68:138–147 [DOI] [PubMed] [Google Scholar]
- 61. van Winkel R; Genetic Risk and Outcome of Psychosis (GROUP) Investigators. Family-based analysis of genetic variation underlying psychosis-inducing effects of cannabis: sibling analysis and proband follow-up. Arch Gen Psychiatry. 2011;68:148–157 [DOI] [PubMed] [Google Scholar]
- 62. Reininghaus U, Priebe S, Bentall RP. Testing the psychopathology of psychosis: evidence for a general psychosis dimension. Schizophr Bull. 2013;39:884–895 [DOI] [PMC free article] [PubMed] [Google Scholar]