Abstract
Autism is a neurodevelopmental disorder that has been associated with atypical brain functioning. Functional connectivity MRI (fcMRI) studies examining neural networks in autism have seen an exponential rise over the last decade. Such investigations have led to characterization of autism as a distributed neural systems disorder. Studies have found widespread cortical underconnectivity, local overconnectivity, and mixed results suggesting disrupted brain connectivity as a potential neural signature of autism. In this review, we summarize the findings of previous fcMRI studies in autism with a detailed examination of their methodology, in order to better understand its potential and to delineate the pitfalls. We also address how a multimodal neuroimaging approach (incorporating different measures of brain connectivity) may help characterize the complex neurobiology of autism at a global level. Finally, we also address the potential of neuroimaging-based markers in assisting neuropsychological assessment of autism. The quest for a biomarker for autism is still ongoing, yet new findings suggest that aberrant brain connectivity may be a promising candidate.
Keywords: autism, fMRI, functional connectivity, underconnectivity, effective connectivity, white matter integrity
Background
Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder characterized by social, communication, and behavioral disturbances (American Psychiatric Association, 2013). Today, ASD is regarded as a pediatric health issue of growing urgency given that 1 in 88 children are being identified with this disorder (Centers for Control Disease, 2012). While there has not been a definitively accepted etiology for ASD in the scientific community, research has become increasingly focused on understanding the neurobiological mechanisms underlying this disorder. A potential biological origin for ASD was first proposed in the first scientific account of autism (Kanner, 1943), which was followed by the first neurobiological account of autism (Rimland, 1964) that dispelled some myths about autism. In 1978, Damasio and Maurer (1978) observed that individuals with autism displayed behaviors that were analogous to those of persons with frontal lobe damage. They also found the dentato-thalamo-cortical pathway to be impaired in autism. This pathway plays a critical role in language and higher cognitive functions, which people with autism have difficulty with. A decade later, Horwitz et al. (1988), using positron emission tomography (PET), found a global increase in resting glucose metabolism in adults with autism, which provided indirect support as autism being linked to abnormal brain activity. These early studies were critical in providing valuable information about the alterations in brain responses and its role in the pathobiology of autism. Furthermore, genetic studies starting from the 1980s have also helped emphasize the biological origin of autism by providing strong evidence of heritability (Freitag, 2007; Geschwind and Levitt, 2007; Gillberg and Wahlstrom, 1985; Wahlstrom et al., 1986).
With the advent of modern neuroimaging techniques, the last two decades have witnessed an exponential rise in the number of studies examining the brain in autism using a wide variety of techniques, such as PET, functional magnetic resonance imaging (fMRI), electroencephalography (EEG), magnetoencephalography (MEG), diffusion tensor imaging (DTI), and proton magnetic resonance spectroscopy (1H-MRS). The majority of the first generation fMRI studies reported altered levels of brain activity in autism, relative to typical control participants. Although such studies have illuminated our understanding of regional brain function and dysfunction in autism, inconsistent findings and the limited potential of such findings to explain a distributed disorder like autism at the global level has been a topic of debate. Given its complex nature and the heterogeneity in symptoms, attempts to explain autism at a focal brain region abnormality have repeatedly fallen short. Considering this void, examining the brain at the network level has been a promising new avenue in characterizing the neurobiology of complex syndromes like autism. In these lines, there has been an array of publications, especially in the last decade, about brain connectivity in autism. In this paper, we will review the connectivity-based fMRI studies of autism by taking into account the following: conceptual origins of brain connectivity, empirical findings, methodological sophistication and concerns, the impact of brain connectivity findings in autism, and the future of this line of research. First, we discuss the conceptual bases of brain connectivity.
Functional Specialization and Integration
Cognitive processes are computationally demanding, and hence require effective allocation of the brain’s resources. This would entail optimal functioning of different brain areas, which in turn may point to two fundamental principles of brain organization: functional specialization and functional integration. Functional specialization supports the belief that different areas of the brain are specialized for different cognitive functions, while functional integration involves the coordination among brain areas to accomplish a task. Integration implies that a cortical area is specialized for some aspects of perceptual or motor processing, and that this specialization is anatomically segregated within the cortex (Friston, 2011). Early brain development involves a delicate balance between the functional specialization of specific regions as well as the formation of connections across these regions through integration. Autism is a neurodevelopmental disorder with alterations found in brain developmental trajectory (Courchesne, 2002), which may point to disrupted functional specialization and integration.
Neuroimaging techniques like fMRI has shown altered patterns of functional specialization in several domains of thinking, such as social, cognitive, linguistic, and visuospatial processing in children and adults with ASD. Findings from these studies point to several foci in the brain that had atypical response in individuals with ASD, compared with typically developing control participants (Anagnostou and Taylor, 2011; Dichter and Belger, 2008; Minshew and Keller, 2010). Despite an abundance of such findings, there are also inconsistencies in focal brain area response to different tasks in autism (Müller, 2007). Therefore, just as it is important to understand specialized functioning of a region, it is even more important to understand the integration among different brain regions. Conceptualizing autism as a network level disorder involves examining functional integration, the principle that the brain is more interactive and its regions are functionally interconnected rather than specialized. Regardless of whether the brain responds to extrinsic or intrinsic stimuli, we experience a unified, integrated conscious experience. This balance between segregation and integration is essential for the operation of distributed networks underlying cognitive function (Tononi et al., 1998).
One way to quantify functional integration among different brain areas is to examine interregional neural interactions by correlating brain activity. This measure, commonly known as functional connectivity (See Figure 1), refers to the temporal correlation between spatially remote neurophysiological events (Friston et al., 1993). Functional connectivity provides an index of the “crosstalk” among brain areas in their coordinated effort to accomplish a cognitive task. Since functional connectivity provides a systems-level approach to study brain functioning, its application to study brain disorders is quite compelling. In the last decade, the number of functional connectivity studies in autism has increased significantly with findings cutting across different tasks, brain areas, and techniques. Other measures of connectivity, such as white matter integrity from axonal connections (anatomical connectivity; See Figure 1) assessed through diffusion tensor imaging (DTI), and effective connectivity (See Figure 1), which addresses the directionality of information transfer or influence of one region on another, have provided a multilevel characterization of neural communication in autism.
Figure 1.
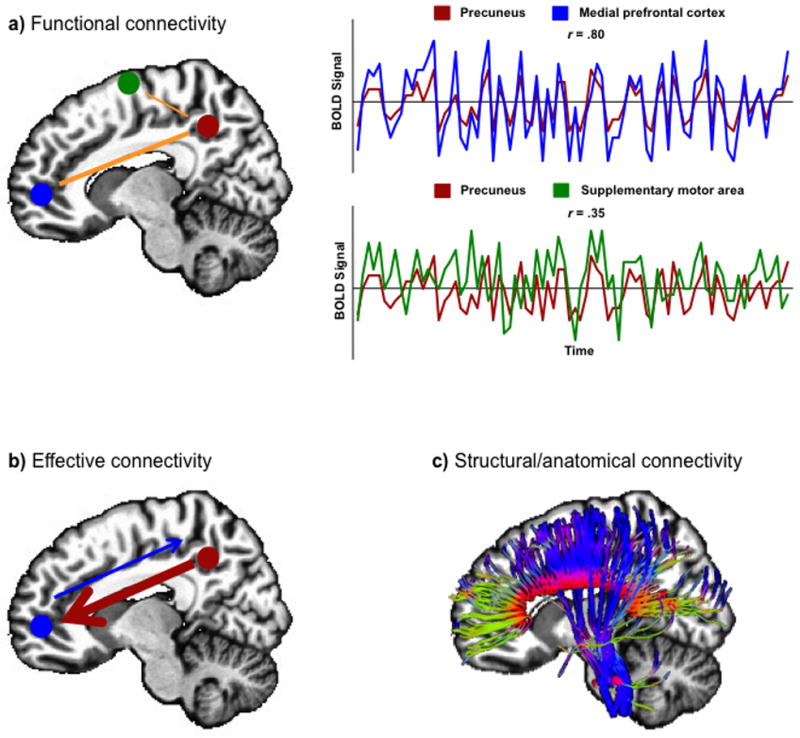
Models of brain connectivity. Sketches illustrate: a) functional connectivity between two sets of brain areas. Top panel graph: high correlation between precuneus and medial prefrontal cortex; and bottom panel graph: weaker correlation between precuneus and supplementary motor area; b) effective connectivity (information flow) between two regions; and c) structural/anatomical connectivity depicted by white matter fiber tracts.
Functional connectivity MRI (fcMRI) studies in ASD
Findings of Cortical Underconnectivity
The first empirical findings of functional connectivity in autism were reported by Just and colleagues in 2004 using a sentence comprehension task (Just et al., 2004). They found reduced functional connectivity across different centers of the brain in adults with autism. Weaker functional connectivity, also referred to as cortical underconnectivity, in autism was reported by several fMRI studies that followed using different cognitive and social tasks, such as visual imagery and language (Kana et al., 2006), working memory (Koshino et al., 2008); social and emotional tasks (Rudie et al., 2012b; Schipul et al., 2012), problem-solving (Just et al., 2007), response inhibition (Kana et al., 2007), Theory-of-Mind (Kana et al., 2009; Mason et al., 2008; Washington et al., 2013), visuospatial attention (Agam et al., 2010; Damarla et al., 2010), global processing (Liu et al., 2011), global and biological motion (Brieber et al., 2010; Freitag et al., 2008), and cognitive control (Solomon et al., 2009) (See supplementary table S1 for a detailed list of connectivity studies). Evidence of underconnectivity in ASD has also been reported in the absence of an active cognitive task (task-free resting state) (Abrams et al., 2013; Assaf et al., 2010; Cherkassky et al., 2006; Kennedy and Courchesne, 2008; Kennedy et al., 2006; Lombardo et al., 2010; Monk et al., 2009; Weng et al., 2010; Wiggins et al., 2011). The weaker connectivity reported in most of these studies was primarily between the prefrontal cortex and relatively posterior brain areas. Poor prefrontal-posterior coordination can affect higher-level processing, and may underlie the difficulty in cognitive, social and language processing seen in ASD. For example, social processing involves the coordinated functioning of the medial prefrontal cortex (MPFC) and the temporoparietal junction (TPJ, associated with ToM), the superior temporal sulcus (STS, associated with biological motion), and the fusiform gyrus (FG, associated with face processing) (Schipul et al., 2011).
Other studies have found underconnectivity in ASD in regions outside the frontal-posterior network, such as between the amygdala and temporal and frontal regions (Monk et al., 2010), between the anterior cingulate and frontal eye fields (Agam et al., 2010), insula with brain regions involved in emotional and sensory processing (Ebisch et al., 2011), within a motor network consisting of primary and supplementary motor areas, anterior cerebellum, and the thalamus (Mostofsky et al., 2009), between the prefrontal cortex and premotor and somatosensory cortices (Lombardo et al., 2010), and between the fusiform gyrus and the amygdala, the posterior cingulate and the cuneus (Kleinhans et al., 2008). Similarly, weaker functional connectivity has also been reported in cortical-subcortical networks; for instance, between the visual cortex and the thalamus and cerebellum (Villalobos et al., 2005), and between the superior frontal gyrus and the caudate nucleus (Turner et al., 2006). While these studies all reported functional underconnectivity in ASD in non-frontal posterior network, the findings vary across a wide variety of pairs of regions and across a large range of tasks, making it difficult to isolate a specific pattern of disturbance.
Establishing a relationship between the severity of the disorder, assessed by various autism diagnostic instruments, and the brain connectivity measures, has been deemed as the bridge to connect the behavioral symptoms and the underlying neuropathology. An interesting question in this context is whether the severity of the disorder could give some insights about the degree of alterations in functional connectivity. The Autism Diagnostic Observation Schedule (ADOS) and the Autism Diagnostic Interview-Revised (ADI-R), considered as the gold standard in ASD diagnosis, have shown negative relationships with functional connectivity in different networks, such as the frontal–parietal (Just et al., 2007), and inferior frontal cortex and pre-supplementary motor area (Lee et al., 2009). Other studies have also shown that individuals with ASD with poorer social functioning (high ADI-R social score) had weaker functional connectivity between the superior frontal gyrus and posterior cingulate cortex (Monk et al., 2009; Weng et al., 2010). These findings, although correlational, provide some valuable insights into brain-behavior relationships in autism.
What is the nature of an underconnected brain and how does it function? Cortical underconnectivity may reflect an inefficiency in optimizing network connections to achieve task performance (e.g., increasing the communication between task-relevant brain regions). A weak coherence among key areas of a network would mean less coordination and hence less than optimal output. Such lack of coherence may be due to several factors, such as the use of an alternative cortical route, dysfunction of key brain areas, or structural anomalies in certain areas. Subtle anatomical abnormalities, such as aberrant white matter growth early in life (Courchesne et al., 2001), atypical white matter maturation in infants and toddlers (Weinstein et al., 2011; Wolff et al., 2012), alterations in white matter volume (Herbert et al., 2004) and white matter integrity (Shukla et al., 2011) in children have been reported in ASD. Such axonal abnormalities, in some way, limit the transfer of information or “bandwidth” (the maximal rate of data transfer supported by a communication channel, taking into account the impact of noise) across different brain regions, given that cognitive functioning involves co-activation of a network of cortical areas whose activity is coordinated (synchronized), and the coordination is based on inter-regional communication using the white matter tracts that provide the anatomical connectivity (Just et al., 2012).
Thus, reports of cortical underconnectivity suggest an important aspect of brain functioning and information transfer in autism, which may explain several behavioral symptoms in individuals with autism. The social and communication impairments, which define the triad of symptoms, in autism can be explained by poor connectivity among brain areas underlying social cognition, language, and executive functions. The underconnectivity accounts have also helped develop a new way of thinking, a network-based approach to brain functioning, in understanding the neurobiology of autism spectrum disorders. Nevertheless, there have been several studies of autism where underconnectivity was not found; instead an overconnectivity among specific brain areas was reported. Below we discuss such findings and their implications in understanding autism.
Findings of Cortical Overconnectivity
While the preponderance of underconnectivity findings in ASD literature has been significant, there have also been several reports of enhanced cortical connectivity in ASD. For example, overconnectivity was found in ASD in the extrastriate cortex, frontal and temporal regions, amygdala, and parahippocampal gyri (Murphy et al., 2012; Noonan et al., 2009; Shih et al., 2011; 2010; Uddin et al., 2013a; Welchew et al., 2005). Similarly, Monk et al. (2009) reported higher functional connectivity among posterior cingulate cortex, temporal lobe, and parahippocampal gyrus using resting state. In another study, Nair and colleagues (2013) found overconnectivity in temporo-thalamic regions in individuals with ASD, which supports the previous findings of overconnected cortical-subcortical networks in ASD. The overconnectivity in thalamocortical projections, which is part of the cerebello-thalamo-cortical pathway may be related to an early reduction in the number and density of Purkinje cells in ASD (Bailey et al., 1998). In other words, the reduction in the number and density of Purkinje cells, which are inhibitory neurons, can affect the inhibition-excitation balance and hence the connectivity. Overall, the findings of overconnectivity (See supplementary table S1 for a detailed list of studies) have been interpreted to reflect hyperspecialized, rather than more efficient connectivity. Thus, inefficient connectivity may be the hallmark of ASD, with possibly overabundant connectivity between ‘non-essential’ regions, allowing for low-level cross talk resulting in increased noise in the system (Noonan et al., 2009). This may also relate to findings of early brain overgrowth in children with ASD. Impaired synaptic pruning, important for brain organization and network specialization in typical development may also play a role in creating enhanced connections (overconnectivity) in the ASD brain (Shih et al., 2011). It should be noted that, in addition to findings of exclusively overconnected regions in ASD, studies have also reported mixed patterns of both underconnectivity and overconnectivity (Di Martino et al., 2011; Lynch et al., 2013; Mizuno et al., 2006; Turner et al., 2006).
Most of these studies of overconnectivity, like the underconnectivity studies, also reported a relationship between enhanced connectivity and behavioral symptoms in ASD. For example, adolescents with ASD who had higher functional connectivity within the default mode network (DMN: the posterior cingulate cortex, retrosplenial cortex, lateral parietal cortex/angular gyrus, medial prefrontal cortex, superior frontal gyrus, and parahippocampal gyrus (Gusnard et al., 2001; Raichle et al., 2001), also had lower abilities in both verbal and non-verbal communication (Weng et al., 2010), measured by ADI and ADOS. Higher functional connectivity between the posterior cingulate cortex (PCC) and the parahippocampal gyrus (PHG) was associated with more severe repetitive behaviors (Monk et al., 2009), and higher functional connectivity between the anterior cingulate and the frontal eye fields was positively associated with restricted, repetitive behaviors (Agam et al., 2010). It was also suggested that enhanced connectivity between PCC and PHG may either be the cause or consequence of the repetitive and restrictive behaviors in ASD (Monk et al., 2009).
Overconnectivity, based on radial cytoarchitecture abnormalities has been typically seen within the frontal lobes (Courchesne et al., 2011; Courchesne and Pierce, 2005), in the lateral occipital complex in the posterior region (Pierce et al., 2001) and superior temporal gyrus (Mottron et al., 2006). An overconnected local network can be compared to a peninsula, separated from and with limited access to the rest of the brain, thereby creating long-distance underconnectivity. This may imply that the brain compensates for abnormal connectivity by incorporating areas that it has easier access to, such as the neighboring regions (Belmonte et al., 2004). An overconnected peninsula may not be confined to prefrontal cortex as an enhanced connectivity in parietal lobe in autism may result in parietal autonomy (Just et al., 2012). An interesting question in this regard is that whether frontal and parietal overconnectivity are the consequence of frontal-parietal underconnectivity or the cause of it. Considering the findings of overconnectivity and underconnectivity in ASD, perhaps a better way to characterize connection abnormalities in autism may be in terms of disrupted connectivity, which would encompass the wide array of findings involving increases and decreases.
Factors affecting Connectivity
As brain connectivity research in autism is growing, so is the use of wide variety of techniques, measuring different indices of connectivity, different levels of examination, and different topographic parameters. Thus, it is important to understand subtleties of the findings reported by different studies, as it will help in drawing common consensus as well as making better sense of inconsistent findings. In the following sections, we examine these factors and their impact on brain connectivity findings in autism.
Spatial Distance: Short-distance vs. long-distance connectivity
One of the factors that may influence the behavior of a network is how proximal or distal the nodes of that network are located. Thus, the spatial distance between two brain areas may prove critical in understanding the functional communication between them. Long distance connectivity represents interactions between distant brain regions that are likely to play a large role in perception and information processing (Sporns et al., 2000). For example, executive functioning, especially problem-solving, may involve the coordination between dorsolateral prefrontal cortex (DLPFC) and inferior parietal lobule (IPL), two regions that are spatially distant (Newman et al., 2003). Such connections have been found to be disrupted in individuals with ASD (Just et al., 2007). Conversely, studying short-distance connections may reflect neural communication between adjacent populations of neurons (Fries, 2005). It has been proposed that behavioral symptoms observed in ASD may relate to findings of increased short-distance and decreased long-distance connections in the brain (Courchesne and Pierce, 2005). However, no generic definition exists as to what constitutes “short” and “long” in terms of path length. Short-distance connectivity is often synonymously used with local connectivity. Local or short-distance connectivity can encompass different spatial scales from a few microns to millimeters and even centimeters; whether a neuron transferring information to a neighboring one through an action potential, the study of minicolumnar organization of a brain region or lobe, or simply examining time series correlations of a voxel’s time-course with its closest neighbors. Long-distance connectivity, loosely, can be labeled as the connectivity among brain regions across different lobes.
At the cellular level, postmortem studies have introduced a model where the abnormal cytoarchitecture in ASD is thought to be responsible for abnormal local circuitry. Vargas et al. (2005) reported the first microscopic evidence of maldevelopment in the frontal lobe and cerebellum, with the presence of activated astroglia with enlarged cell bodies, and panlaminar microglial activation in the dorsal and mesial regions of frontal cortex. In the cerebellum, glial activation was associated with degenerating Purkinje neurons, granule cells and axons. Glial activation persists into postnatal life in autism, and may cause the brain in the infant and toddler with autism to grow larger than normal (Courchesne and Pierce, 2005). This enlargement in brain volume due to neuroinflammation may result in excessive neuronal production causing cortical minicolumns to be numerous (Casanova et al., 2002). Since minicolumns are excitatory vertical circuits, their relative density in the autistic brain would be associated with atypically enhanced excitatory cortical function, thus affecting inhibitory systems. This will result in reduced network differentiation, i.e., “muffled” communication between regions within a functional network, and “noisy” crosstalk within non-involved regions. Thus, increased excitation/inhibition ratio might result in altered local functional differentiation (Rubenstein and Merzenich, 2003), which may point out to islets of sparing in ASD, such as the visual cortex, where minicolumns are thought to be more abundant (Keehn et al., 2013).
Local functional connectivity in ASD has been studied by correlating fMRI time series from a given voxel with the vicinity of its immediate neighbors (6, 18, and 26 voxels respectively) (Zang et al., 2004). This index of local connectivity has been labeled as the regional homogeneity (ReHo), which has been shown to be sensitive to regional abnormalities (Dai et al., 2012; Farb et al., 2013; Weaver et al., 2013; Yin et al., 2012; Zalesky et al., 2012). In the first ReHo study in ASD, Paakki and colleagues (2010) examined resting-state fMRI time series correlations of one voxel with its 26 nearest neighbors. They reported mixed findings, with increased ReHo in adolescents with ASD (compared to matched TD participants) in the right thalamus and left occipital regions and decreased ReHo in the right temporal, frontal, and insular sites. Similarly, Shukla et al. (2010), in a ReHo analysis of a voxel with its 6 immediate neighbors, reported increased ReHo in children and adolescents with ASD in right temporal regions, and decreased ReHo in numerous bilateral fronto-parietal sites. These studies provide evidence of reduced cortical functional differentiation in ASD, yet, decreased ReHo was also reported in both studies, which may be due to difference in scanner strength, nature of the tasks used, and cluster size and voxel dimension used. Thus, synchronizing the methods across studies may be a topic that future studies should pay attention to (Maximo et al., 2013). Despite the methodological differences in ReHo studies, the findings in local functional connectivity may suggest altered functional differentiation in ASD.
Contrary to the limited number of local connectivity studies, reports of long-distance connectivity are more predominant in the ASD literature. In typically developing individuals, long-distance connectivity is prominent across association areas in parietal, lateral temporal, and frontal cortices as well as paralimbic cortex including posterior cingulate (Schipul et al., 2011; Vissers et al., 2012). These regions have been previously described as important for higher-order cognitive processes, such as attention, memory, and language (Sepulcre et al., 2010), where people with autism have difficulty. In ASD fcMRI studies, disturbances in long-distance connectivity (underconnectivity and overconnectivity) have been reported in several networks, such as the fronto-parietal (Just et al., 2007; Kana et al., 2006), fronto-striatal (Silk et al., 2006), frontal-fusiform (Koshino et al., 2008), frontal inhibition network and the inferior parietal lobe (Kana et al., 2007), visual cortex and frontal (Villalobos et al., 2005), anterior cerebellum and thalamus (Mostofsky et al., 2009), thalamo-cortical (Nair et al., 2013), the prefrontal-premotor-somatosensory (Lombardo et al., 2010), areas involved in language (Dinstein et al., 2011), the fusiform-amygdala, the posterior cingulate and the cuneus (Kleinhans et al., 2008), superior frontal-caudate (Turner et al., 2006), thalamus and the cortex (Mizuno et al., 2006). These findings point to ASD as a disorder of aberrant cortical connectivity involving multiple brain networks. The long-distance connectivity impairment is supported by anatomical MRI findings of aberrant white matter growth patterns in the first few years in ASD (Courchesne et al., 2001; Hazlett et al., 2005; Sparks et al., 2002) and diffusion tensor imaging (DTI) findings of reduced white matter integrity later in life (Alexander et al., 2007; Cheung et al., 2009; Fletcher et al., 2010; Shukla et al., 2011).
Cognitive Demand: Task-based vs. Resting state functional connectivity
Traditionally, fMRI studies have relied on cognitive tasks to elicit hemodynamic changes in the brain, which represent an indirect measure of neuronal activity. In ASD research, many earlier fMRI studies reported reduced activation brain areas associated with functions like face-processing, ToM, and visual processing. Such studies served the primary purpose of understanding specialized function or dysfunction of a single region. However, studies in the last decade have begun examining the activation-driven interregional BOLD correlations in ASD (Muller et al., 2011). Thus, synchronized patterns of activation induced by a task would entail high correlation, which in turn would imply the functioning of the brain as a coordinated, coherent unit. The tasks used for task-induced fcMRI studies in ASD include cognitive (working memory, problem-solving, response inhibition), social (ToM, biological motion, face processing), language (discourse processing, prosody, pun, irony, sentence comprehension, semantic processing), visuospatial (visual imagery, visual search, embedded figures task, block design task, mental rotation), and repetitive behavior. It should be noted that these task domains would loosely represent and elicit the symptoms that underlie the triad of impairments in autism. Most of these studies have reported alterations in brain activity and/or connectivity, possibly reflecting ineffective use of neural resources and impaired coordination in the modulation of psychologically-driven activation among brain regions.
Task-based functional connectivity studies have provided great insight into understanding the neurobiology of autism and its relationship with behavioral symptoms. Nevertheless, there are points to ponder while considering their broader implications. For instance, compensatory neural routes used by individuals with ASD may result in reduced time series correlations in “typical” networks and can trigger a difference in connectivity when compared to TD participants. In addition, task-based functional connectivity studies assume the baseline connectivity in ASD and TD individuals to be at the same level, which may not be the case. Therefore, examining spontaneous low-frequency BOLD fluctuations is critical in order to determine whether the task-based alterations in connectivity in ASD is a by-product of their baseline brain functioning. The implementation of a temporal (low-pass) filter serves the purpose of isolating these spontaneous low-frequency BOLD fluctuations (0.008 < f < 0.08), which are considered to reflect network-specific intrinsic functional connectivity most robustly, even in the absence of a task (Biswal et al., 1995; Cordes et al., 2001; Fox and Raichle, 2007). An alternative to assess connectivity without relying on task-based fMRI is to compute the correlation between residual fluctuations in task activation datasets after task effects have been regressed out, i.e., the removal of task-related effects via nuisance regression and usage of a low-pass filter (Fair et al., 2007; Villalobos et al., 2005). While some of these studies found underconnectivity in autism (Jones et al., 2010), others did not (Mizuno et al., 2006; Noonan et al., 2009; Shih et al., 2011; 2010; Turner et al., 2006).
Yet another approach to study baseline connectivity is by using task-free resting state (continuous resting state) fMRI. Based on the observation that the spontaneous BOLD fluctuations during resting state are not random noise, but specifically organized pattern of signal change, resting state has opened a new avenue for investigating functional connectivity (Fox and Raichle, 2007). Resting state fMRI may also be more feasible and appealing to study ASD individuals of low cognitive ability given that they may perform relatively poorly in demanding cognitive tasks. The emergence of resting state fMRI has also facilitated data collection from younger children with ASD. Resting-state functional connectivity is usually studied in the absence of external stimulation, where participants are typically instructed to close their eyes and think of nothing in particular for a period of 5 to 10 minutes. Most of these studies (See Supplementary table S1) had similar findings of underconnectivity in regions of the DMN, but also in other social processing-related brain circuits, such as the MPFC, the amygdala, insula, and hippocampus (Gotts et al., 2012; von dem Hagen et al., 2013). Overconnectivity was also reported in some studies of resting state in autism between striatum with insula and STG (Di Martino et al., 2011), in the salience, default mode, frontotemporal, motor, and visual networks (Uddin et al., 2013a), and even in the DMN (Lynch et al., 2013). Interestingly, the findings of overconnectivity came from studies that examined fcMRI in children with ASD, while the underconnectivity was mainly seen in young adolescents and adults. Only one study did not find any significant evidence for altered resting state connectivity in their participants with autism (Tyszka et al., 2013).
While the impact of task-based fcMRI in understanding the brain functioning in autism cannot be ignored, resting state fMRI provides a unique opportunity to learn about autism at a level which was previously considered non-existent. It should be noted that resting state fcMRI is not a substitute for task-based fcMRI; instead it should supplement the findings of task-based fcMRI. While each approach has its advantages and disadvantages, all three (task-based functional connectivity, task-removed baseline connectivity, and task-free resting state functional connectivity) provide a new perspective to our understanding of brain connectivity in ASD given that each one explores a different aspect of cognitive states.
Method of Data Analyses: ROI-based vs. Whole brain analyses
An important factor that needs close examination in brain connectivity is the specific search space used for connectivity analysis, and how it differs across studies. In other words, in connectivity where you search and the way you search can have an impact on what you find. Most fcMRI studies of ASD use anatomical or functional regions of interest (ROI) to correlate signal time-courses. If the ROIs defined do not accurately represent participants with ASD and TD controls, it can create a bias, especially when ASD participants may not activate typical networks (Muller et al., 2011). Nevertheless, some studies have attempted to circumvent this by defining ROIs that are representative of both ASD and TD groups by examining activation sites shared by both groups from within-group analyses, or by combining activation clusters seen either in one or the other group (Just et al., 2007; Kana et al., 2012). Studies that report connectivity results across a selected few ROIs and not at the whole brain level need to be interpreted as specific to those ROIs. While the focus on specific ROIs may be justified by a priori hypotheses, failure to examine whole-brain effects may impede a comprehensive understanding of connectivity.
One technique that has been utilized to examine functional connectivity, especially resting state connectivity, is the independent component analysis (ICA). ICA identifies temporally coherent networks by selecting spatially independent brain areas or components whose hemodynamic time courses closely co-vary. ICA uses algorithmic constraints so that each voxel in a component that has the same time-course can be considered a functionally connected network without being limited to a priori regions. In ASD, ICA has been used to assess functional connectivity during resting state and it has revealed a pattern of underconnected regions between the precuneus, the anterior cingulate cortex and the DMN (Assaf et al., 2010); however, other studies have reported opposite findings using the same technique (Uddin et al., 2013a; Washington et al., 2013). Using ICA, Cardinale et al. (2013) examined asymmetry of functional networks, where hemispheric asymmetries were detected in ASD in components thought to be implicated in auditory, visual, sensorimotor, executive, attentional, and visuospatial processing. More recently, ICA has been used in classification analyses, where the components from functionally connected networks were used as features for predicting the group membership of a subject (ASD or TD) (Uddin et al., 2013a).
While whole brain functional connectivity methods have several advantages, such analyses must use stringent statistical corrections to account for multiple comparison issues. Thus, the statistical threshold at which these studies report their results can have a huge impact on the replicability of the findings. In addition, connectivity results reported at different levels (whole brain and a priori ROIs) can not only help conceptualizing the findings better but also in improving the replicability of findings. Thus, future studies should take the aforementioned factors into account in order to improve the reliability and replicability of connectivity studies.
Is Heterogeneity of ASD reflected in Brain Connectivity Findings?
Heterogeneity in the manifestation of autism has been an inherent challenge not only to researchers attempting to scientifically explain the disorder, but also to clinicians working towards designing appropriate intervention plans for affected individuals. Such differences have also affected the investigations to understand the neurobiological basis of ASD (Rudie et al., 2012a). The wide range of abilities and levels of functioning in ASD encompass severe cognitive impairment, no functional language skills, engagement in self-injurious behavior, poor adaptive living skills, and high intelligence with poor social skills. According to Geschwind and Levitt (2007), the neurobiological heterogeneity of ASD points to the likelihood that ASD may not be one disorder but many, both in the sense that it has myriad manifestations and because numerous etiological factors may converge on a similar outcome. Numerous genetic studies have further supported the complex genetic model in that there is no one gene that explains at least 50% of the cases of ASD and that the most frequently occurring genes appear at most 2% of cases diagnosed with autism (Abrahams and Geschwind, 2008). This implies that ASD is heterogenetic, with the located genes reflecting various mechanisms (Geschwind, 2011; Happe et al., 2006). The variation of cellular mechanisms of possible ASD-linked genes reflects the multiple pathways affected and diverse traits in autism (Geschwind, 2008; Losh et al., 2009). If the multiple pathways converged in certain brain regions, this may implicate that ASD is an integrative disorder. Thus, it is unlikely that the behavioral aspect of ASD can be equated to a single pathological entity on the neurobiological level.
Most of the empirical findings suggest that ASD is a disorder in which multiple networks are altered (not well connected or overconnected) compared to typically developing individuals, yet, most of these findings originate from only examining high-functioning individuals in the autism spectrum, which may make it difficult to generalize to the broader ASD spectrum. There is hardly any functional imaging study involving low-functioning samples of ASD, perhaps due to obvious reasons, such as stereotypies (repetitive or ritualistic movement, posture, or utterance), thus potentially not being able to remain steady in the MRI scanner. This may also result in head motion, which represents a serious confound to fcMRI studies (Satterthwaite et al., 2013; Van Dijk et al., 2012). Nevertheless, some imaging studies have addressed the heterogeneity in autism by examining intrasubject variability in their data by reporting spatial variability in activation (Muller et al., 2001) and volumetric differences in structures like amygdala (Sparks et al., 2002). Attempts to examine individuals at the lower end of the spectrum can provide valuable insights into understanding the nature and extent of neuropathology at different levels of intellectual functioning. One possible direction for this line of research, despite the challenges, is to acquire fMRI data using resting state since low-functioning individuals may not be able to fully understand or successfully complete a demanding cognitive task.
Developmental Trajectory of Brain Connectivity in Autism
Since ASD is a pervasive developmental disorder, the alterations in connectivity seen may reflect delayed and/or disrupted developmental maturation of the brain. However, the dearth of longitudinal neuroimaging studies has limited our knowledge of the developmental trajectory of brain connectivity in ASD. Most of the studies of functional connectivity in ASD in children under the age of 12 years have reported increased functional connectivity, while studies involving adolescents and adults have reported reduced functional connectivity (Uddin et al., 2013b). Such findings point to differences in critical developmental delay and/or deficits that can impact brain connectivity.
A growing body of studies has documented age-related increases in white matter volume in typical individuals (Lenroot and Giedd, 2006), which may be related to increases in long-range functional connectivity from childhood through adolescence and into adulthood (Fair et al., 2008; Kelly et al., 2009). Recent reports also suggest strengthening of structural and functional connectivity with age (Hagmann et al., 2010; Supekar et al., 2010; Uddin et al., 2011). Structural MRI studies have reported evidence of larger brains in younger children with autism (2–4 years old) compared to older children (10-year old) who had a significantly smaller brain (Courchesne et al., 2001). In addition, early overgrowth of white matter among young children with autism, followed by reduced white matter in adolescence and adulthood could underlie the reported findings of overconnectivity in children and underconnectivity in adolescents and adults (Courchesne et al., 2003; Courchesne et al., 2001; Waiter et al., 2005). This is parallel to DTI findings of increased fractional anisotropy (FA) in children with ASD (Ben Bashat et al., 2007; Cheng et al., 2010; Cheung et al., 2009; Ke et al., 2009), and reduced FA in young adolescents and adults with ASD (Barnea-Goraly et al., 2004; Fletcher et al., 2010; Sahyoun et al., 2010; Sundaram et al., 2008).
Other Indices of Brain Connectivity: Effective Connectivity & White Matter Integrity
For most part of this review, we focused on fcMRI studies as the neural connectivity models of ASD are primarily based on functional connectivity. However, a holistic understanding of the brain would also entail gathering information about the nature and route of information transfer, and axonal integrity. The number of published studies on these topics, especially effective connectivity (causal influence of one brain area on another) in autism, is relatively low compared to fcMRI studies. However, the variation in methodology and inconsistencies in fcMRI findings cannot be ignored and would suggest the need for understanding more indices of brain functioning in autism. Thus, we have included a limited discussion on structural and effective connectivity and white matter integrity.
Effective Connectivity and White matter Integrity
Functional connectivity is a method for assessing observed correlation between active brain areas, but does not provide insight into the time-lagged causality and directionality of such correlations. Effective connectivity, on the other hand, provides information about the influence one system exerts over another with respect to a given experimental context (Buchel and Friston, 2000; Friston, 2011). Only a handful of studies have examined effective connectivity in ASD. In one study, Wicker and colleagues (2008) reported abnormal patterns of effective connectivity in ASD during explicit emotion processing with the prefrontal cortex as a key site of dysfunction. In another study, Bird and colleagues (2006) found atypical flow of information between extrastriate cortex and V1 in autism during attentional modulation of social information. Yet another study revealed atypical connectivity of the imitation network (IFG, IPL, and STS) with an enhanced role of the dPFC (Shih et al., 2010). Similarly, Shen et al. (2012) found an atypically enhanced network participation of extrastriate cortex via a direct path connecting it with the IFG. More recently, Deshpande et al. (2013) found reduced effective connectivity in participants with ASD during a theory-of-mind task. These studies suggest differences in information transfer among brain areas in autism. Further studies are needed that examine both functional and effective connectivity in order to better understand the functioning of different networks in autism.
One of the obvious questions emerging from functional connectivity alterations in autism is about the quality and integrity of axonal fibers that connect different brain areas. Anatomical connectivity can be measured using DTI, which examines the water diffusion along white matter fibers in the brain. Since cooperation between distal nodes in functional brain networks rely on axonal connections, the integrity of these physical connections can prove vital. DTI studies in ASD have consistently reported abnormal FA in several white matter fiber bundles (Alexander et al., 2007; Keller et al., 2007; Lee et al., 2007) reflecting white matter damage or reduced axonal integrity. Other DTI studies, conducted on participants who are 8 years or older, have detected increased mean diffusivity and/or radial diffusivity in regions such as the corpus callosum, and arcuate fasciculus in autism (Fletcher et al., 2010; Shukla et al., 2011). Studies examining younger populations report partially divergent results. For example, Ben Bashat and colleagues (2007) found increased FA for a number of tracts (including corpus callosum) in a small sample of toddlers with ASD (Ages 1–3 years old), and in frontal lobe tracts in short-range fibers in children around 5 years old (Sundaram et al., 2008) and others (Cheng et al., 2010; Ke et al., 2009). Such heterogeneity of results may arise from the rapid developmental changes in white matter in children with ASD. Establishing anatomical connectivity bases to alterations in functional connectivity in autism has been less explored (Just et al., 2007; Kana et al., 2006), although such relationships have been reported in typical population (Greicius et al., 2009). A few recent studies have examined connectivity in ASD using simultaneous DTI and fcMRI methods (Delmonte et al., 2013; Deshpande et al., 2013; Kana et al., 2012; Mueller et al., 2013; Nair et al., 2013). These studies provide two levels of investigation, although sometimes finding no significant direct relationship between the two. Another technique that can supplement functional and anatomical connectivity is proton magnetic resonance spectroscopy (1H-MRS), which detects the concentration of brain metabolites based on chemical resonance shift. Such studies, if used in conjunction with fMRI and DTI can provide yet another layer of information on the neurobiology of autism.
Brain Connectivity Research in Autism: Implications and Future Directions
One of the primary goals of neurobiological investigations of ASD is to understand its etiology. A clear delineation of a neural marker for autism can potentially lead to aiding the diagnostic process, which currently is behavior-based. One aspect of concern in connectivity studies in ASD is the relatively medium-to-small sample sizes. Considering the high cost involved in neuroimaging, data sharing and/or multicenter projects would be quite effective (Di Martino et al., 2013); however precautions need to be taken considering the differences in protocols across sites. Despite these shortcomings, neuroimaging based brain connectivity assessment provides a promising window in identifying a neural signature of autism. While most of the studies discussed in this paper are fMRI-based assessment of functional connectivity, such measures can be complemented by other modalities of imaging, such as DTI for anatomical connectivity, EEG and MEG for connectivity with better temporal resolution, and proton magnetic spectroscopy (1H-MRS) to assess neuronal health. Each index of brain connectivity (functional, anatomical, effective) in conjunction provides a great deal of information about the nature of the neural mechanisms underlying autism. Such an approach will provide a multi-level characterization of the neurobiology of ASD.
In addition, complex network analyses such as graph theory can be used to examine both anatomical and functional connectivity, and describe complex systems as a set of nodes (i.e., brain regions) and edges (i.e., connections between nodes), and characterize the brain as a complex network with a hierarchical modular organization consisting of several major functional communities (Wang et al., 2010). Graph theory can examine functional specialization (connectivity within major networks) and functional integration (connectivity between different networks) in a sense that it can be viewed from at least two different perspectives, one based on the efficiency of global communication and the other on the ability of the network to integrate distributed information (Sporns, 2013). Recent functional connectivity studies using graph theory in ASD have found reduced modularity (a network organized into densely connected modules that are segregated from each other), global efficiency (which describes the average number of connections to be crossed to go from each voxel to every other voxel in the brain), increased betweenness centrality, (which indicates the degree to which a seed or node functions as a hub within and between networks), and altered local connection density (the number of connections of one voxel in proportion to the total number of possible connections) in several functioning networks (Maximo et al., 2013; Redcay et al., 2013; Rudie, 2013; You et al., 2013). This entails that functional differentiation may play a factor in reduced modularity and global efficiency and altered density of connections which may be reflected in brain connectivity.
Another important factor related to information processing is segregation and integration across time and task. Most fcMRI studies provide a summary of the brain dynamics over a long period. Functional connections are in constant flux and vary across time, which can be described as fluctuations in brain networks across time. These network dynamic approaches can be useful in ASD research, given that attentional deficits have been reported in ASD. Such deficits can affect in switching from one brain network to another in a fast manner (Kana et al., 2011). The integration of different modalities of neuroimaging in ASD and in other complex developmental disorders will certainly bring us a step closer to a better understanding of such disorders.
Perhaps a major outcome of neuroimaging research in neurodevelopmental disorders would be the implementation of neuroimaging-based inference to complement behavioral assessment in diagnosis. The emergence of complex computer algorithms has enabled researchers to build classifiers that are capable of predicting group membership based on neural connectivity features. Only a handful of studies have relied on this technique in classifying ASD individuals from healthy controls based on their functional connectivity patterns (Anderson et al., 2011; Deshpande et al., 2013; Murdaugh et al., 2012; Nielsen et al., 2013; Uddin et al., 2013a). These studies have found reliable classification results with relatively high accuracy, and provide a promising starting point for brain-based classification and identification of neurodevelopmental disorders. Future studies should test the accuracy, sensitivity, and specificity of classification with larger and varied samples.
While neuroimaging-based markers can aid the diagnosis of autism, such markers can also be influential in designing intervention. In the last decade, advance in cognitive neuroscience research has led to the development of intervention and treatments that rely on neurobiological principles. For example, neurofeedback (NF) training has emerged as an intervention based on operant conditioning that results in self-regulation of brain’s electrical oscillations, and it is believed that NF produces positive behavioral changes in ASD children by normalizing the aberrant connections within and between neural circuits, although its precise mechanisms are not well understood (Pineda et al., 2012). By grounding in known anatomical (e.g., mirror neuron system) and functional markers (e.g., mu rhythms) of ASD, NF training holds promise to support current treatments for this complex disorder. Some studies have also used repetitive transcranial magnetic stimulation (rTMS), a non-invasive technique for manipulating cortical activity, in individuals with ASD as an intervention (Baruth et al., 2010; Sokhadze et al., 2009). Following post-rTMS sessions, individuals with ASD showed significant improvement in discriminatory gamma activity (30–80 Hz) between visual stimuli and also showed improvement in responses on behavioral assessment. Both NF training and rTMS, in a not-so distant future, could be used as intervention tools in order to ameliorate not only behavioral deficiencies observed in ASD, but also improve aberrant connections in the brain.
Conclusions
Aberrant brain connectivity has been a relatively stable finding in neuroimaging studies of autism spectrum disorders. Despite some pitfalls, our review of such findings suggests a great deal of promise in this line of research in understanding the pathobiology of autism. Attempts to establish alterations in connectivity as potential neural signature of autism, and to target faulty neural circuitry through intervention are significant from clinical and public health perspectives. Nevertheless, lots need to be done in fine-tuning the methods before even considering to adapt neurological findings to clinical world. Future studies of autism using neuroimaging should consider data sharing to improve sample size, implement multisite studies, and examine under-studied areas, such as developmental trajectory and females with autism. The use of multimodal imaging seems another promising direction as it can uncover the complex neurobiology of autism by proving insights into multiple layers of brain organization and brain functioning.
Supplementary Material
Acknowledgments
This study was supported by the McNulty-Civitan Scientist award, the UAB department of Psychology faculty funds, and the UAB Center for Clinical and Translational Sciences grant (UL1 TR000165). The authors would like to thank Ms. Bahia Lukima for her help with the manuscript.
References
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nature reviews Genetics. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams DA, Lynch CJ, Cheng KM, Phillips J, Supekar K, Ryali S, Uddin LQ, Menon V. Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:12060–12065. doi: 10.1073/pnas.1302982110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agam Y, Joseph RM, Barton JJ, Manoach DS. Reduced cognitive control of response inhibition by the anterior cingulate cortex in autism spectrum disorders. NeuroImage. 2010;52:336–347. doi: 10.1016/j.neuroimage.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, Miller JN, Lu J, Jeong EK, McMahon WM, et al. Diffusion tensor imaging of the corpus callosum in Autism. NeuroImage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Anagnostou E, Taylor MJ. Review of neuroimaging in autism spectrum disorders: what have we learned and where we go from here. Molecular autism. 2011;2:4. doi: 10.1186/2040-2392-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JS, Nielsen JA, Froehlich AL, DuBray MB, Druzgal TJ, Cariello AN, Cooperrider JR, Zielinski BA, Ravichandran C, Fletcher PT, et al. Functional connectivity magnetic resonance imaging classification of autism. Brain : a journal of neurology. 2011;134:3742–3754. doi: 10.1093/brain/awr263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, Sahl R, O’Boyle JG, Schultz RT, Pearlson GD. Abnormal functional connectivity of default mode subnetworks in autism spectrum disorder patients. NeuroImage. 2010;53:247–256. doi: 10.1016/j.neuroimage.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter M, Lantos P. A clinicopathological study of autism. Brain : a journal of neurology. 1998;121(Pt 5):889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biological psychiatry. 2004;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Baruth JM, Casanova MF, El-Baz A, Horrell T, Mathai G, Sears L, Sokhadze E. Low-Frequency Repetitive Transcranial Magnetic Stimulation (rTMS) Modulates Evoked-Gamma Frequency Oscillations in Autism Spectrum Disorder (ASD) Journal of neurotherapy. 2010;14:179–194. doi: 10.1080/10874208.2010.501500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Bashat D, Kronfeld-Duenias V, Zachor DA, Ekstein PM, Hendler T, Tarrasch R, Even A, Levy Y, Ben Sira L. Accelerated maturation of white matter in young children with autism: a high b value DWI study. NeuroImage. 2007;37:40–47. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- Bird G, Catmur C, Silani G, Frith C, Frith U. Attention does not modulate neural responses to social stimuli in autism spectrum disorders. NeuroImage. 2006;31:1614–1624. doi: 10.1016/j.neuroimage.2006.02.037. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Brieber S, Herpertz-Dahlmann B, Fink GR, Kamp-Becker I, Remschmidt H, Konrad K. Coherent motion processing in autism spectrum disorder (ASD): an fMRI study. Neuropsychologia. 2010;48:1644–1651. doi: 10.1016/j.neuropsychologia.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Buchel C, Friston K. Assessing interactions among neuronal systems using functional neuroimaging. Neural networks : the official journal of the International Neural Network Society. 2000;13:871–882. doi: 10.1016/s0893-6080(00)00066-6. [DOI] [PubMed] [Google Scholar]
- Cardinale RC, Shih P, Fishman I, Ford LM, Muller RA. Pervasive Rightward Asymmetry Shifts of Functional Networks in Autism Spectrum Disorder. JAMA Psychiatry. 2013 doi: 10.1001/jamapsychiatry.2013.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58:428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- Centers for Control Disease. Prevalence of autism spectrum disorders: autism and developmental disabilities monitoring network, 14 sites, United States. MMWR Surveill Summ. 2012;61:12–19. [PubMed] [Google Scholar]
- Cheng Y, Chou KH, Chen IY, Fan YT, Decety J, Lin CP. Atypical development of white matter microstructure in adolescents with autism spectrum disorders. NeuroImage. 2010;50:873–882. doi: 10.1016/j.neuroimage.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17:1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Cheung C, Chua SE, Cheung V, Khong PL, Tai KS, Wong TK, Ho TP, McAlonan GM. White matter fractional anisotrophy differences and correlates of diagnostic symptoms in autism. Journal of child psychology and psychiatry, and allied disciplines. 2009;50:1102–1112. doi: 10.1111/j.1469-7610.2009.02086.x. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR American journal of neuroradiology. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Courchesne E. Abnormal early brain development in autism. Molecular psychiatry. 2002;7(Suppl 2):S21–23. doi: 10.1038/sj.mp.4001169. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA : the journal of the American Medical Association. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, Ahrens-Barbeau C, Hallet MJ, Barnes CC, Pierce K. Neuron number and size in prefrontal cortex of children with autism. JAMA : the journal of the American Medical Association. 2011;306:2001–2010. doi: 10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Current opinion in neurobiology. 2005;15:225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Dai Z, Yan C, Wang Z, Wang J, Xia M, Li K, He Y. Discriminative analysis of early Alzheimer’s disease using multi-modal imaging and multi-level characterization with multi-classifier (M3) NeuroImage. 2012;59:2187–2195. doi: 10.1016/j.neuroimage.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Damarla SR, Keller TA, Kana RK, Cherkassky VL, Williams DL, Minshew NJ, Just MA. Cortical underconnectivity coupled with preserved visuospatial cognition in autism: Evidence from an fMRI study of an embedded figures task. Autism research : official journal of the International Society for Autism Research. 2010;3:273–279. doi: 10.1002/aur.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Maurer RG. A neurological model for childhood autism. Archives of neurology. 1978;35:777–786. doi: 10.1001/archneur.1978.00500360001001. [DOI] [PubMed] [Google Scholar]
- Delmonte S, Gallagher L, O’Hanlon E, McGrath J, Balsters JH. Functional and structural connectivity of frontostriatal circuitry in Autism Spectrum Disorder. Frontiers in human neuroscience. 2013;7:430. doi: 10.3389/fnhum.2013.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, Libero LE, Sreenivasan KR, Deshpande HD, Kana RK. Identification of neural connectivity signatures of autism using machine learning. Frontiers in human neuroscience. 2013;4:670. doi: 10.3389/fnhum.2013.00670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Kelly C, Grzadzinski R, Zuo XN, Mennes M, Mairena MA, Lord C, Castellanos FX, Milham MP. Aberrant striatal functional connectivity in children with autism. Biological psychiatry. 2011;69:847–856. doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Yan CG, Li Q, Denio E, Castellanos FX, Alaerts K, Anderson JS, Assaf M, Bookheimer SY, Dapretto M, et al. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Molecular psychiatry. 2013 doi: 10.1038/mp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Belger A. Atypical modulation of cognitive control by arousal in autism. Psychiatry research. 2008;164:185–197. doi: 10.1016/j.pscychresns.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Pierce K, Eyler L, Solso S, Malach R, Behrmann M, Courchesne E. Disrupted neural synchronization in toddlers with autism. Neuron. 2011;70:1218–1225. doi: 10.1016/j.neuron.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisch SJ, Gallese V, Willems RM, Mantini D, Groen WB, Romani GL, Buitelaar JK, Bekkering H. Altered intrinsic functional connectivity of anterior and posterior insula regions in high-functioning participants with autism spectrum disorder. Human brain mapping. 2011;32:1013–1028. doi: 10.1002/hbm.21085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain’s default network. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. NeuroImage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Grady CL, Strother S, Tang-Wai DF, Masellis M, Black S, Freedman M, Pollock BG, Campbell KL, Hasher L, et al. Abnormal network connectivity in frontotemporal dementia: evidence for prefrontal isolation. Cortex; a journal devoted to the study of the nervous system and behavior. 2013;49:1856–1873. doi: 10.1016/j.cortex.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Fletcher PT, Whitaker RT, Tao R, DuBray MB, Froehlich A, Ravichandran C, Alexander AL, Bigler ED, Lange N, Lainhart JE. Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. NeuroImage. 2010;51:1117–1125. doi: 10.1016/j.neuroimage.2010.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature reviews Neuroscience. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Freitag CM. The genetics of autistic disorders and its clinical relevance: a review of the literature. Molecular psychiatry. 2007;12:2–22. doi: 10.1038/sj.mp.4001896. [DOI] [PubMed] [Google Scholar]
- Freitag CM, Konrad C, Haberlen M, Kleser C, von Gontard A, Reith W, Troje NF, Krick C. Perception of biological motion in autism spectrum disorders. Neuropsychologia. 2008;46:1480–1494. doi: 10.1016/j.neuropsychologia.2007.12.025. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends in cognitive sciences. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity: a review. Brain connectivity. 2011;1:13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal-component analysis of large (PET) data sets. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Geschwind DH. Autism: many genes, common pathways? Cell. 2008;135:391–395. doi: 10.1016/j.cell.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH. Genetics of autism spectrum disorders. Trends in cognitive sciences. 2011;15:409–416. doi: 10.1016/j.tics.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Current opinion in neurobiology. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Gillberg C, Wahlstrom J. Chromosome abnormalities in infantile autism and other childhood psychoses: a population study of 66 cases. Developmental medicine and child neurology. 1985;27:293–304. doi: 10.1111/j.1469-8749.1985.tb04539.x. [DOI] [PubMed] [Google Scholar]
- Gotts SJ, Simmons WK, Milbury LA, Wallace GL, Cox RW, Martin A. Fractionation of social brain circuits in autism spectrum disorders. Brain : a journal of neurology. 2012;135:2711–2725. doi: 10.1093/brain/aws160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Sporns O, Madan N, Cammoun L, Pienaar R, Wedeen VJ, Meuli R, Thiran JP, Grant PE. White matter maturation reshapes structural connectivity in the late developing human brain. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:19067–19072. doi: 10.1073/pnas.1009073107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe F, Ronald A, Plomin R. Time to give up on a single explanation for autism. Nature neuroscience. 2006;9:1218–1220. doi: 10.1038/nn1770. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Gilmore J, Piven J. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Archives of general psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, Sanders HA, Kennedy DN, Caviness VS., Jr Localization of white matter volume increase in autism and developmental language disorder. Annals of neurology. 2004;55:530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Grady CL, Rapoport SI. The cerebral metabolic landscape in autism. Intercorrelations of regional glucose utilization. Archives of neurology. 1988;45:749–755. doi: 10.1001/archneur.1988.00520310055018. [DOI] [PubMed] [Google Scholar]
- Jones TB, Bandettini PA, Kenworthy L, Case LK, Milleville SC, Martin A, Birn RM. Sources of group differences in functional connectivity: an investigation applied to autism spectrum disorder. NeuroImage. 2010;49:401–414. doi: 10.1016/j.neuroimage.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain : a journal of neurology. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Just MA, Keller TA, Malave VL, Kana RK, Varma S. Autism as a neural systems disorder: a theory of frontal-posterior underconnectivity. Neuroscience and biobehavioral reviews. 2012;36:1292–1313. doi: 10.1016/j.neubiorev.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain : a journal of neurology. 2006;129:2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Atypical frontal-posterior synchronization of Theory of Mind regions in autism during mental state attribution. Social neuroscience. 2009;4:135–152. doi: 10.1080/17470910802198510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high-functioning autism: decreased activation and underconnectivity in inhibition networks. Biological psychiatry. 2007;62:198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Libero LE, Hu CP, Deshpande HD, Colburn JS. Functional Brain Networks and White Matter Underlying Theory-of-Mind in Autism. Social cognitive and affective neuroscience. 2012 doi: 10.1093/scan/nss106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Libero LE, Moore MS. Disrupted cortical connectivity theory as an explanatory model for autism spectrum disorders. Physics of life reviews. 2011;8:410–437. doi: 10.1016/j.plrev.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Ke X, Tang T, Hong S, Hang Y, Zou B, Li H, Zhou Z, Ruan Z, Lu Z, Tao G, et al. White matter impairments in autism, evidence from voxel-based morphometry and diffusion tensor imaging. Brain research. 2009;1265:171–177. doi: 10.1016/j.brainres.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Keehn B, Shih P, Brenner LA, Townsend J, Muller RA. Functional connectivity for an “Island of sparing” in autism spectrum disorder: An fMRI study of visual search. Human brain mapping. 2013;34:2524–2537. doi: 10.1002/hbm.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller TA, Kana RK, Just MA. A developmental study of the structural integrity of white matter in autism. Neuroreport. 2007;18:23–27. doi: 10.1097/01.wnr.0000239965.21685.99. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Margulies DS, Castellanos FX, Milham MP. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. Functional abnormalities of the default network during self- and other-reflection in autism. Social cognitive and affective neuroscience. 2008;3:177–190. doi: 10.1093/scan/nsn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, Greenson J, Dawson G, Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain : a journal of neurology. 2008;131:1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. fMRI investigation of working memory for faces in autism: visual coding and underconnectivity with frontal areas. Cereb Cortex. 2008;18:289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Bigler ED, Alexander AL, Lazar M, DuBray MB, Chung MK, Johnson M, Morgan J, Miller JN, McMahon WM, et al. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neuroscience letters. 2007;424:127–132. doi: 10.1016/j.neulet.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Lee PS, Yerys BE, Della Rosa A, Foss-Feig J, Barnes KA, James JD, VanMeter J, Vaidya CJ, Gaillard WD, Kenworthy LE. Functional connectivity of the inferior frontal cortex changes with age in children with autism spectrum disorders: a fcMRI study of response inhibition. Cereb Cortex. 2009;19:1787–1794. doi: 10.1093/cercor/bhn209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neuroscience and biobehavioral reviews. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Liu Y, Cherkassky VL, Minshew NJ, Just MA. Autonomy of lower-level perception from global processing in autism: evidence from brain activation and functional connectivity. Neuropsychologia. 2011;49:2105–2111. doi: 10.1016/j.neuropsychologia.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Sadek SA, Pasco G, Wheelwright SJ, Suckling J, Baron-Cohen S. Atypical neural self-representation in autism. Brain : a journal of neurology. 2010;133:611–624. doi: 10.1093/brain/awp306. [DOI] [PubMed] [Google Scholar]
- Losh M, Adolphs R, Poe MD, Couture S, Penn D, Baranek GT, Piven J. Neuropsychological profile of autism and the broad autism phenotype. Archives of general psychiatry. 2009;66:518–526. doi: 10.1001/archgenpsychiatry.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CJ, Uddin LQ, Supekar K, Khouzam A, Phillips J, Menon V. Default mode network in childhood autism: posteromedial cortex heterogeneity and relationship with social deficits. Biological psychiatry. 2013;74:212–219. doi: 10.1016/j.biopsych.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RA, Williams DL, Kana RK, Minshew N, Just MA. Theory of Mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia. 2008;46:269–280. doi: 10.1016/j.neuropsychologia.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximo JO, Keown CL, Nair A, Muller RA. Approaches to local connectivity in autism using resting state functional connectivity MRI. Frontiers in human neuroscience. 2013;7:605. doi: 10.3389/fnhum.2013.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew NJ, Keller TA. The nature of brain dysfunction in autism: functional brain imaging studies. Current opinion in neurology. 2010;23:124–130. doi: 10.1097/WCO.0b013e32833782d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno A, Villalobos ME, Davies MM, Dahl BC, Muller RA. Partially enhanced thalamocortical functional connectivity in autism. Brain research. 2006;1104:160–174. doi: 10.1016/j.brainres.2006.05.064. [DOI] [PubMed] [Google Scholar]
- Monk CS, Peltier SJ, Wiggins JL, Weng SJ, Carrasco M, Risi S, Lord C. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. NeuroImage. 2009;47:764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Weng SJ, Wiggins JL, Kurapati N, Louro HM, Carrasco M, Maslowsky J, Risi S, Lord C. Neural circuitry of emotional face processing in autism spectrum disorders. Journal of psychiatry & neuroscience : JPN. 2010;35:105–114. doi: 10.1503/jpn.090085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Powell SK, Simmonds DJ, Goldberg MC, Caffo B, Pekar JJ. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain : a journal of neurology. 2009;132:2413–2425. doi: 10.1093/brain/awp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottron L, Dawson M, Soulieres I, Hubert B, Burack J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. Journal of autism and developmental disorders. 2006;36:27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Mueller S, Keeser D, Samson AC, Kirsch V, Blautzik J, Grothe M, Erat O, Hegenloh M, Coates U, Reiser MF, et al. Convergent Findings of Altered Functional and Structural Brain Connectivity in Individuals with High Functioning Autism: A Multimodal MRI Study. PloS one. 2013;8:e67329. doi: 10.1371/journal.pone.0067329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller RA. The study of autism as a distributed disorder. Ment Retard Dev Disabil Res Rev. 2007;13:85–95. doi: 10.1002/mrdd.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RA, Pierce K, Ambrose JB, Allen G, Courchesne E. Atypical patterns of cerebral motor activation in autism: a functional magnetic resonance study. Biological psychiatry. 2001;49:665–676. doi: 10.1016/s0006-3223(00)01004-0. [DOI] [PubMed] [Google Scholar]
- Muller RA, Shih P, Keehn B, Deyoe JR, Leyden KM, Shukla DK. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb Cortex. 2011;21:2233–2243. doi: 10.1093/cercor/bhq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdaugh DL, Shinkareva SV, Deshpande HR, Wang J, Pennick MR, Kana RK. Differential deactivation during mentalizing and classification of autism based on default mode network connectivity. PloS one. 2012;7:e50064. doi: 10.1371/journal.pone.0050064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy ER, Foss-Feig J, Kenworthy L, Gaillard WD, Vaidya CJ. Atypical Functional Connectivity of the Amygdala in Childhood Autism Spectrum Disorders during Spontaneous Attention to Eye-Gaze. Autism research and treatment. 2012;2012:652408. doi: 10.1155/2012/652408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Treiber JM, Shukla DK, Shih P, Muller RA. Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain : a journal of neurology. 2013;136:1942–1955. doi: 10.1093/brain/awt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SD, Carpenter PA, Varma S, Just MA. Frontal and parietal participation in problem solving in the Tower of London: fMRI and computational modeling of planning and high-level perception. Neuropsychologia. 2003;41:1668–1682. doi: 10.1016/s0028-3932(03)00091-5. [DOI] [PubMed] [Google Scholar]
- Nielsen JA, Zielinski BA, Fletcher PT, Alexander AL, Lange N, Bigler ED, Lainhart JE, Anderson JS. Multisite functional connectivity MRI classification of autism: ABIDE results. Frontiers in human neuroscience. 2013;7:599. doi: 10.3389/fnhum.2013.00599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan SK, Haist F, Muller RA. Aberrant functional connectivity in autism: evidence from low-frequency BOLD signal fluctuations. Brain research. 2009;1262:48–63. doi: 10.1016/j.brainres.2008.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paakki JJ, Rahko J, Long X, Moilanen I, Tervonen O, Nikkinen J, Starck T, Remes J, Hurtig T, Haapsamo H, et al. Alterations in regional homogeneity of resting-state brain activity in autism spectrum disorders. Brain research. 2010;1321:169–179. doi: 10.1016/j.brainres.2009.12.081. [DOI] [PubMed] [Google Scholar]
- Pierce K, Muller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI. Brain : a journal of neurology. 2001;124:2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Pineda JA, Juavinett A, Datko M. Self-regulation of brain oscillations as a treatment for aberrant brain connections in children with autism. Medical hypotheses. 2012;79:790–798. doi: 10.1016/j.mehy.2012.08.031. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, Moran JM, Mavros PL, Tager-Flusberg H, Gabrieli JD, Whitfield-Gabrieli S. Intrinsic functional network organization in high-functioning adolescents with autism spectrum disorder. Frontiers in human neuroscience. 2013;7:573. doi: 10.3389/fnhum.2013.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimland B. Infantile Autism: The Syndrome and its Implications for a Neural Theory of Behavior. New York: Appleton-Century-Crofts; 1964. [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes, brain, and behavior. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudie JD, Brown JA, Beck-Pancer D, Hernandez LM. Altered functional and structural brain network organization in autism. Neuroimage Clin. 2013:79–94. doi: 10.1016/j.nicl.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudie JD, Hernandez LM, Brown JA, Beck-Pancer D, Colich NL, Gorrindo P, Thompson PM, Geschwind DH, Bookheimer SY, Levitt P, et al. Autism-associated promoter variant in MET impacts functional and structural brain networks. Neuron. 2012a;75:904–915. doi: 10.1016/j.neuron.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudie JD, Shehzad Z, Hernandez LM, Colich NL, Bookheimer SY, Iacoboni M, Dapretto M. Reduced functional integration and segregation of distributed neural systems underlying social and emotional information processing in autism spectrum disorders. Cereb Cortex. 2012b;22:1025–1037. doi: 10.1093/cercor/bhr171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahyoun CP, Belliveau JW, Soulieres I, Schwartz S, Mody M. Neuroimaging of the functional and structural networks underlying visuospatial vs. linguistic reasoning in high-functioning autism. Neuropsychologia. 2010;48:86–95. doi: 10.1016/j.neuropsychologia.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. NeuroImage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipul SE, Keller TA, Just MA. Inter-regional brain communication and its disturbance in autism. Frontiers in systems neuroscience. 2011;5:10. doi: 10.3389/fnsys.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipul SE, Williams DL, Keller TA, Minshew NJ, Just MA. Distinctive neural processes during learning in autism. Cereb Cortex. 2012;22:937–950. doi: 10.1093/cercor/bhr162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulcre J, Liu H, Talukdar T, Martincorena I, Yeo BT, Buckner RL. The organization of local and distant functional connectivity in the human brain. PLoS computational biology. 2010;6:e1000808. doi: 10.1371/journal.pcbi.1000808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MD, Shih P, Ottl B, Keehn B, Leyden KM, Gaffrey MS, Muller RA. Atypical lexicosemantic function of extrastriate cortex in autism spectrum disorder: evidence from functional and effective connectivity. NeuroImage. 2012;62:1780–1791. doi: 10.1016/j.neuroimage.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih P, Keehn B, Oram JK, Leyden KM, Keown CL, Muller RA. Functional differentiation of posterior superior temporal sulcus in autism: a functional connectivity magnetic resonance imaging study. Biological psychiatry. 2011;70:270–277. doi: 10.1016/j.biopsych.2011.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih P, Shen M, Ottl B, Keehn B, Gaffrey MS, Muller RA. Atypical network connectivity for imitation in autism spectrum disorder. Neuropsychologia. 2010;48:2931–2939. doi: 10.1016/j.neuropsychologia.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla DK, Keehn B, Muller RA. Regional homogeneity of fMRI time series in autism spectrum disorders. Neuroscience letters. 2010;476:46–51. doi: 10.1016/j.neulet.2010.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla DK, Keehn B, Muller RA. Tract-specific analyses of diffusion tensor imaging show widespread white matter compromise in autism spectrum disorder. Journal of child psychology and psychiatry, and allied disciplines. 2011;52:286–295. doi: 10.1111/j.1469-7610.2010.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk TJ, Rinehart N, Bradshaw JL, Tonge B, Egan G, O’Boyle MW, Cunnington R. Visuospatial processing and the function of prefrontal-parietal networks in autism spectrum disorders: a functional MRI study. The American journal of psychiatry. 2006;163:1440–1443. doi: 10.1176/ajp.2006.163.8.1440. [DOI] [PubMed] [Google Scholar]
- Sokhadze EM, El-Baz A, Baruth J, Mathai G, Sears L, Casanova MF. Effects of low frequency repetitive transcranial magnetic stimulation (rTMS) on gamma frequency oscillations and event-related potentials during processing of illusory figures in autism. Journal of autism and developmental disorders. 2009;39:619–634. doi: 10.1007/s10803-008-0662-7. [DOI] [PubMed] [Google Scholar]
- Solomon M, Ozonoff SJ, Ursu S, Ravizza S, Cummings N, Ly S, Carter CS. The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 2009;47:2515–2526. doi: 10.1016/j.neuropsychologia.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, Maravilla KR, Giedd JN, Munson J, Dawson G, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- Sporns O. Network attributes for segregation and integration in the human brain. Current opinion in neurobiology. 2013;23:162–171. doi: 10.1016/j.conb.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Sporns O, Tononi G, Edelman GM. Connectivity and complexity: the relationship between neuroanatomy and brain dynamics. Neural networks : the official journal of the International Neural Network Society. 2000;13:909–922. doi: 10.1016/s0893-6080(00)00053-8. [DOI] [PubMed] [Google Scholar]
- Sundaram SK, Kumar A, Makki MI, Behen ME, Chugani HT, Chugani DC. Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cereb Cortex. 2008;18:2659–2665. doi: 10.1093/cercor/bhn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Uddin LQ, Prater K, Amin H, Greicius MD, Menon V. Development of functional and structural connectivity within the default mode network in young children. NeuroImage. 2010;52:290–301. doi: 10.1016/j.neuroimage.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Edelman GM, Sporns O. Complexity and coherency: integrating information in the brain. Trends in cognitive sciences. 1998;2:474–484. doi: 10.1016/s1364-6613(98)01259-5. [DOI] [PubMed] [Google Scholar]
- Turner KC, Frost L, Linsenbardt D, McIlroy JR, Muller RA. Atypically diffuse functional connectivity between caudate nuclei and cerebral cortex in autism. Behavioral and brain functions : BBF. 2006;2:34. doi: 10.1186/1744-9081-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyszka JM, Kennedy DP, Paul LK, Adolphs R. Largely Typical Patterns of Resting-State Functional Connectivity in High-Functioning Adults with Autism. Cereb Cortex. 2013 doi: 10.1093/cercor/bht040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Lynch CJ, Khouzam A, Phillips J, Feinstein C, Ryali S, Menon V. Salience network-based classification and prediction of symptom severity in children with autism. JAMA Psychiatry. 2013a;70:869–879. doi: 10.1001/jamapsychiatry.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Frontiers in human neuroscience. 2013b;7:458. doi: 10.3389/fnhum.2013.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar KS, Ryali S, Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Annals of neurology. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Muller RA. Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. NeuroImage. 2005;25:916–925. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers ME, Cohen MX, Geurts HM. Brain connectivity and high functioning autism: a promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neuroscience and biobehavioral reviews. 2012;36:604–625. doi: 10.1016/j.neubiorev.2011.09.003. [DOI] [PubMed] [Google Scholar]
- von dem Hagen EA, Stoyanova RS, Baron-Cohen S, Calder AJ. Reduced functional connectivity within and between ‘social’ resting state networks in autism spectrum conditions. Social cognitive and affective neuroscience. 2013;8:694–701. doi: 10.1093/scan/nss053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom J, Gillberg C, Gustavson KH, Holmgren G. Infantile autism and the fragile X. A Swedish multicenter study. American journal of medical genetics. 1986;23:403–408. doi: 10.1002/ajmg.1320230132. [DOI] [PubMed] [Google Scholar]
- Waiter GD, Williams JH, Murray AD, Gilchrist A, Perrett DI, Whiten A. Structural white matter deficits in high-functioning individuals with autistic spectrum disorder: a voxel-based investigation. NeuroImage. 2005;24:455–461. doi: 10.1016/j.neuroimage.2004.08.049. [DOI] [PubMed] [Google Scholar]
- Wang J, Zuo X, He Y. Graph-based network analysis of resting-state functional MRI. Frontiers in systems neuroscience. 2010;4:16. doi: 10.3389/fnsys.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington SD, Gordon EM, Brar J, Warburton S, Sawyer AT, Wolfe A, Mease-Ference ER, Girton L, Hailu A, Mbwana J, et al. Dysmaturation of the default mode network in autism. Human brain mapping. 2013 doi: 10.1002/hbm.22252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver KE, Chaovalitwongse WA, Novotny EJ, Poliakov A, Grabowski TG, Ojemann JG. Local functional connectivity as a pre-surgical tool for seizure focus identification in non-lesion, focal epilepsy. Frontiers in neurology. 2013;4:43. doi: 10.3389/fneur.2013.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein M, Ben-Sira L, Levy Y, Zachor DA, Ben Itzhak E, Artzi M, Tarrasch R, Eksteine PM, Hendler T, Ben Bashat D. Abnormal white matter integrity in young children with autism. Human brain mapping. 2011;32:534–543. doi: 10.1002/hbm.21042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welchew DE, Ashwin C, Berkouk K, Salvador R, Suckling J, Baron-Cohen S, Bullmore E. Functional disconnectivity of the medial temporal lobe in Asperger’s syndrome. Biological psychiatry. 2005;57:991–998. doi: 10.1016/j.biopsych.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Weng SJ, Wiggins JL, Peltier SJ, Carrasco M, Risi S, Lord C, Monk CS. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain research. 2010;1313:202–214. doi: 10.1016/j.brainres.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B, Fonlupt P, Hubert B, Tardif C, Gepner B, Deruelle C. Abnormal cerebral effective connectivity during explicit emotional processing in adults with autism spectrum disorder. Social cognitive and affective neuroscience. 2008;3:135–143. doi: 10.1093/scan/nsn007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JL, Peltier SJ, Ashinoff S, Weng SJ, Carrasco M, Welsh RC, Lord C, Monk CS. Using a self-organizing map algorithm to detect age-related changes in functional connectivity during rest in autism spectrum disorders. Brain research. 2011;1380:187–197. doi: 10.1016/j.brainres.2010.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, Botteron KN, Dager SR, Dawson G, Estes AM, et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. The American journal of psychiatry. 2012;169:589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Jin C, Eyler LT, Jin H, Hu X, Duan L, Zheng H, Feng B, Huang X, Shan B, et al. Altered regional homogeneity in post-traumatic stress disorder: a resting-state functional magnetic resonance imaging study. Neuroscience bulletin. 2012;28:541–549. doi: 10.1007/s12264-012-1261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You X, Norr M, Murphy E, Kuschner ES, Bal E, Gaillard WD, Kenworthy L, Vaidya CJ. Atypical modulation of distant functional connectivity by cognitive state in children with Autism Spectrum Disorders. Frontiers in human neuroscience. 2013;7:482. doi: 10.3389/fnhum.2013.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Egan GF, Pantelis C, Bullmore ET. The relationship between regional and inter-regional functional connectivity deficits in schizophrenia. Human brain mapping. 2012;33:2535–2549. doi: 10.1002/hbm.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. NeuroImage. 2004;22:394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


