Abstract
Accumulating evidence indicates action naming may rely more on frontal-subcortical circuits, and noun naming may rely more on temporal cortex. Therefore, noun versus action fluency might distinguish frontal and subcortical dementias from cortical dementias primarily affecting temporal and/or parietal cortex such as Alzheimer’s disease (AD). We hypothesized patients with subcortical dementia, e.g., normal pressure hydrocephalus (NPH) and patients with dementias predominantly affecting frontal cortex, e.g., behavioral variant frontotemporal dementia (bv-FTD) and progressive nonfluent aphasia (PNFA) have more difficulty on action fluency versus noun fluency (e.g., animal naming). Patients with AD, who have temporo parietal cortical dysfunction, should have more difficulty on noun versus verb fluency. A total of 234 participants, including healthy controls (n = 20) and patients diagnosed with NPH (n =144), AD (n = 33), bv-FTD (n = 22) or PNFA (n = 15) were administered animal fluency, action fluency, and letter fluency tasks, and the Mini-Mental State Examination (MMSE, to control for dementia severity). NPH and bv-FTD/PNFA patients had significantly higher MMSE scores and animal fluency than AD patients (after adjusting for age), but their action fluency tended to be lower than in AD. Only NPH and bvFTD/PNFA patients showed significantly lower action verb than animal fluency. Results provide novel evidence that action naming relies more on frontal-subcortical circuits while noun naming relies more on temporoparietal cortex, indicating action verb fluency may be more sensitive than noun fluency, particularly for detecting frontal-subcortical dysfunction.
Keywords: Verb naming, Semantic fluency, Subcortical dementia, Frontotemporal dementia, Alzheimer’s disease, Progressive nonfluent aphasia, Category fluency
INTRODUCTION
Measures of word fluency play an important role in many cognitive and language evaluations. Word fluency tests, such as the Controlled Oral Word Association (COWA) Test (Benton, 1968) are sensitive to executive dysfunction, and have been used to evaluate change in language and cognition (Sumerall, Timmons, James, Ewing, & Oehlert, 1997). The COWA requires the production of as many words as possible (excluding proper names) beginning with a specified letter of the alphabet, in one minute. Patients are often also administered a semantic fluency task, in which they are asked to name as many items as possible within a specific category (e.g., animals or fruits), within a similar time frame. Letter and semantic (category) fluency depend on multiple cognitive processes, including searching semantic memory, selecting semantic representations, accessing the corresponding phonological representations, motor planning and articulation of selected words (Henry, & Crawford, 2004a, 2004b), as well as retaining and suppressing previously named items.
Most semantic fluency tasks evaluate retrieval of various categories of nouns (e.g., animals), but a few studies have evaluated semantic fluency with verbs. Verb fluency might provide additional insight into language dysfunction. Grammatically, there are several distinctions between nouns and verbs. For example, they can take different inflections (Maratsos, 1990). There are also a larger number of low-frequency nouns than verbs, and nouns tend to be more narrowly defined than verbs (Gentner, 1981).
Nouns and verbs may also be processed in different parts of the brain. Functional imaging studies indicate that verbs, particularly action verbs, are associated with activation in posterior frontal cortex (Perani et al., 1999; Shapiro, & Caramazza, 2003; Tranel, Damasio, & Damasio, 1997; Tranel, Adolphs, Damasio, & Damasio, 2001). Likewise, a study of repetitive transcranial magnetic stimulation (rTMS) in healthy individuals showed that suppressing the excitability of left midfrontal cortex increased response latencies for verbs, but not nouns (Cappelletti, Fregni, Shapiro, Pascual-Leone, & Caramazza, 2008). These two word classes can also be differentially affected by brain damage (Hillis, Wityk, Barker, & Caramazza, 2002b; Miceli, Silveri, Villa, & Caramazza, 1984). Noun naming deficits are often associated with posterior and anterior temporal lesions, whereas verb naming deficits are associated with frontal or subcortical lesions (Caramazza, & Hillis, 1991; Damasio, & Tranel, 1993; Mätzig, & Druks, 2006).
Furthermore, selective impairment in naming nouns versus verbs can be restricted to a single output modality. For instance, one stroke patient had a deficit in oral but not written production of verbs, and another patient had the opposite pattern – a deficit in written but not oral naming of verbs; both patients had spared oral and written naming of nouns (Hillis et al., 2002b; see also Miceli, Silveri, Nocentini, & Caramazza, 1988). Yet another patient showed the opposite dissociation – more impaired noun than verb naming – with the same stimuli (Hillis & Caramazza, 1995).
The dissociation between nouns and verbs in one output modality is also found in focal dementias (Bak, O’Donovan, Xuereb, Boniface, & Hodges, 2001). Individuals with progressive nonfluent aphasia (PNFA) are more impaired in naming pictures of verbs/actions, while those with semantic dementia (SD) are more impaired in naming nouns/objects (Hillis, Oh, & Ken, 2004). This dissociation can also be modality specific (Hillis, Tuffiash, & Caramazza, 2002b). Therefore, accuracy in naming nouns versus verbs might also provide a means of assessing subtype of primary progressive aphasia.
The most common challenge in diagnosing patients with specific dementia types is the presence of overlapping symptoms and behavioral features. For example, frontotemporal lobar degeneration (FTLD), a disorder that affects the frontal and temporal lobes asymmetrically, is commonly used as an umbrella term for three clinical syndromes: behavioral variant frontotemporal dementia (bv-FTD), progressive nonfluent aphasia (PNFA), and semantic dementia (SD). Behavioral changes and impaired social conduct are most prominent in bv-FTD, but can also occur in other subtypes. Although bv-FTD patients often have profoundly altered personality and social conduct, memory is relatively preserved. Patients with PNFA demonstrate motor speech deficits, agrammatic language, and reduced phrase length and complexity (Gorno-Tempini et al., 2006). Both bv-FTD and PNFA affect the frontal cortex, and therefore might result in more trouble naming verbs/actions than nouns. There is some support for this hypothesis in PNFA (Hillis et al., 2004). Patients with SD often have difficulty with word-finding, naming pictures and verbal definitions (Lambon Ralph, McClelland, Patterson, Galton, & Hodges, 2001) and comprehension of written, spoken, and pictoral stimuli (Hodges, Patterson, Oxbury, & Funnell, 1992; Lambon Ralph, Graham, & Patterson, 1999). Atrophy in SD is predominantly in the anterior and inferior temporal cortex, more on the left. Consistent with the proposed importance of temporal cortex in noun naming, they have more difficulty naming nouns than verbs (Hillis et al., 2004).
The ability to produce verbs versus nouns may also be disproportionately affected in subcortical dementia, common to Human Immunodeficiency Virus (HIV), Parkinson’s disease, normal pressure hydrocephalus (NPH), and other conditions. HIV-associated dementia (HAD) involves a disruption in frontal-basal ganglia circuits (Woods, Carey, Troster, Grant, & The HNRC Group, 2005). A study examining the action verb fluency and noun fluency in 97 subjects with HIV-1 infection found that subjects generated significantly fewer actions (verbs) compared to nouns, further supporting the prominent role of frontal-basal ganglia networks in verb generation (Woods et al., 2005). Selective verb impairments in patients with Parkinson’s disease are also attributed to frontal-subcortical pathophysiology. For example, action verb fluency scores discriminated demented from non-demented Parkinson’s disease (PD) patients and healthy controls, whereas tests of letter and noun semantic fluency did not (Piatt, Fields, Paolo, Koller, & Troster, 1999). Thus, action verb fluency might be more sensitive than noun semantic fluency (e.g., animal naming) in detecting frontostriatal pathology.
The syndrome of NPH is characterized by abnormal gait, cognitive deficits, and urinary incontinence (Hakim, & Adams, 1965; Raftopoulos et al., 1994; Relkin, Marmarou, Klinge, Bergneider, & Black, 2005). The cognitive impairments in NPH have been considered of the subcortical type, including recall impairment (disproportionate to recognition memory impairment), inattention, and executive dysfunction (Verrees & Selman, 2004). If verb fluency relies more on frontal-subcortical circuits, and noun fluency relies more on temporal cortex, then noun-versus verb-fluency might provide a rapid test to help distinguish subcortical dementias such as NPH from cortical dementias affecting temporal and parietal cortex such as AD.
We evaluated noun and action verb fluency in patients diagnosed with bv-FTD, PNFA, AD and NPH to test the hypothesis that patients affected by a subcortical dementia, like NPH as well as patients with frontal dementias of bv-FTD and PNFA, have more difficulty on action verb fluency in comparison to noun fluency (tested with animal naming). This finding would demonstrate that the problem with verbs is not specific to frontal-striatal dysfunction associated with dopamine depletion (as in Parkinson’s disease), but can also be observed in other subcortical or frontal dementias. In contrast, AD patients, often with temporal and parietal cortical dysfunction, might have more difficulty on noun fluency than verb fluency. Letter fluency may not distinguish between dementia types, because items from any grammatical word class can be named.
METHODS
Participants
We studied a consecutive series of consenting patients diagnosed with NPH, AD, bv-FTD or PNFA who were evaluated in an outpatient cognitive disorders referral clinic. Diagnoses were made by a senior neurologist (AH), and were based on clinical history, MRI (+ PET or SPECT) scan results, clinical neurologic examination and neuropsychological examination results. Imaging results contributed to each patient’s clinical diagnosis as well as confirmed the presence of frontal atrophy in bv-FTD and PNFA, temporo-parietal atrophy in AD and subcortical changes (ventriculomegaly) in NPH.
Patients with bv-FTD and PNFA met published criteria for these clinical syndromes (Neary et al., 1998). Patients with AD met published criteria for probable AD (McKhann et al., 1984). Patients with NPH met the clinical triad of gait disorder, urinary incontinence, and cognitive decline, and had disproportionate ventriculomegaly on imaging with normal CSF pressure by lumbar puncture; they were tested prior to treatment for NPH. Disproportionate ventriculomegaly was defined by using measured ventricle to brain volume ratio on MRI or CT scan, which correlates with cognitive performance in NPH (Sepelyak, Heidler-Gary, Davis, Newhart, Hillis, 2009).
A consecutive series of healthy controls without dementia, who were in the same age range as the patients were also enrolled. The mean age was 56.8 (SD 14.0); mean education was 17.6 (SD 3.8) years.
Exclusion criteria for all groups included neurological diseases or conditions other than dementia (e.g., symptomatic stroke, pseudotumor cerebri), other subcortical dementias (e.g., small vessel disease, atypical Parkinsonism, HIV-associated dementia, obstructive and congenital hydrocephalus, or other dementias with mixed cortical-subcortical pathology), uncorrected hearing or visual loss, or inability to understand directions of the tasks.
Clinical assessment
The Mini-Mental State Examination (MMSE; normed for age and education) was administered to screen for cognitive impairment and determine dementia severity in all patients (Folstein, Folstein, & McHugh, 1975). Other tests administered to classify patients included the: Rey Auditory Verbal Learning Test (Rey, 1964), Rey–Osterrieth Complex Figure Test (Osterrieth, 1944; Rey, 1941), Stroop Neuropsychological Screening Test (Trenerry, Crosson, DeBoe, & Leber, 1989), Trail Making Test – parts A and B (Partington, &Leiter, 1949), the Boston Naming Test (Kaplan, Goodglass, & Weintraub, 1983) and the Frontal Behavioral Inventory (Kertesz, Davidson, & Fox, 1997). Additional speech and language tests, including The Western Aphasia Battery (Kertesz, 1982), The Apraxia Battery for Adults (Dabul, 1979), and Pyramids and Palm Trees Test (Howard & Patterson, 1992) were given to confirm the diagnosis of PNFA (and distinguish it from semantic dementia). Although the Controlled Oral Word Association Test (Benton, 1968) was included in the clinical examination, it was not used to classify patients because all patients with all diagnoses can be impaired on this test; rather, it was used to assess severity of dementia.
Word fluency tasks
Semantic fluency (animal and action verb) and letter fluency tasks were administered to all patients and controls. Semantic fluency was evaluated by asking patients to orally name as many animals (noun category), or as many names of actions (verb category) as they could, for duration of 60 seconds each (task order was randomized across patients). The patient’s responses for each category were recorded separately. The total score for each category was the total number of words produced, excluding preservative and intrusive errors (e.g., words outside the target category, proper names). To evaluate letter fluency we administered the COWA test which requires patients to generate as many words as they can that began with the letter ‘F’, then ‘A’, then S for duration of 60 seconds each. Patients were told that proper nouns (e.g., names of places and people) or derivatives of the same word would not be counted. The responses for each letter category were recorded.
Normative data for age and education levels for the COWA test (Tombaugh, Kozak, & Rees, 1996), the animal fluency test (Tombaugh, Kazak, & Rees, 1999), and the action fluency test (Priatt, Fields, Paolo, & Troster, 2004) were used to compute Z-scores for each experimental task.
Statistical analysis
We combined patients with bvFTD and PNFA because (1) these clinical syndromes are often caused by the same pathology, and patients often accumulate symptoms of both and (2) both affect the frontal cortex, although PNFA is more confined to the left frontal cortex. Therefore, both groups were hypothesized to have more trouble naming action verbs than nouns.
Differences between action verb vs. noun naming Z-scores within each patient group were evaluated with paired t-tests using SPSS software (SPSS Inc., 2007). Differences between groups for age, education, and MMSE scores were assessed using ANOVA. Additionally differences between groups for MMSE, COWA, noun fluency, action verb fluency, and letter fluency, after adjusting for age, were assessed using multivariate linear regression analysis. An alpha level of p < .01 after Bonferroni correction for multiple comparisons was used to identify significant differences across groups.
RESULTS
Between group comparisons
Table 1 displays descriptive statistics for demographic variables and MMSE scores for each patient group (n = 234 patients; including 144 NPH, 33 AD, 37 bv-FTD/PNFA, 20 healthy controls). There were a relatively large number of NPH patients because our clinic serves a large NPH center. By ANOVA, there was a significant difference in MMSE scores across patients groups; F(2,202) = 40.4, p < .0001. There was no significant difference in education across patient groups; F(2,207) = 0.226, ns. There were differences in age across patient groups; F(3, 233) = 13.36, p < .0001. Not surprisingly, patients with NPH and AD were older than patients with bvFTD or PNFA.
TABLE 1.
Demographic data for patients groups
| Demographic/General clinical data | NPH Means (SD) | AD Means (SD) | bv-FTD/PNFA Means (SD) | p-Value |
|---|---|---|---|---|
| Total no. patients (Male/Female) | 144 (84/60) | 33 (11/22) | 37 (22/15) | |
| Mean (SD) age in years | 72.4 (11.2) | 72.0 (9.2) | 66.9 (9.2) | <0.0001 |
| Mean (SD) education level in years | 14.0 (3.4) | 14.3 (3.7) | 14.8 (3.1) | ns |
| MMSE | 26.2 (3.3) | 19.0 (4.8) | 23.0 (6.1) | <0.0001 |
Table 2 displays results of multivariate linear regression comparing mean scores between groups after adjusting for age, on the MMSE, noun fluency, action verb fluency, and letter fluency tasks. After age-adjustment, mean MMSE scores were significantly different across dementia groups (excluding healthy controls): patients with AD had significantly lower MMSE scores than those of patients with NPH and bv-FTD/PNFA; F(2, 199) = 40.8, p < .0001. For noun fluency there was also a significant difference between patient groups after age-adjustment; F(2, 199) = 10.17, p < .0001. Patients with AD had lower noun fluency scores than patients with NPH or bvFTD/PNFA.
TABLE 2.
Age-adjusted group means across tasks
| MMSE | COWA | Nouns (animal naming) |
Verbs (action naming) |
|
|---|---|---|---|---|
| NPH (means) | 26.6 | 8.7 | 12.7 | 7.9 |
| AD (means) | 19.4 | 7.5 | 9.6 | 8.1 |
| bv-FTD/PNFA (means) | 23.2 | 6.8 | 9.7 | 6.8 |
| Control (means) | N/A | 15.1 | 21.7 | 20.8 |
| F-Statistic (effects of dx, including all 4 groups) | N/A | F(3,229) = 17.99 | F(3,229) = 34.42 | F(3,229) = 51.65 |
| p-Values (including control groups) | N/A | <0.0001 | <0.0001 | <0.0001 |
| F-Statistic (across dementia groups, excluding control groups) | F(2,199) = 40.79 | F(2,120) = 3.31 | F(2,210) = 10.17 | F(2,210) = 1.12 |
| p-Values (across dementia groups, excluding control groups) | <0.0001 | 0.04 | <0.0001 | 0.33 |
In contrast to MMSE scores and noun fluency scores, for action verb fluency there was not a significant difference in performance between groups. However, NPH patients and bv-FTD/PNFA patients showed a trend toward lower verb fluency scores than in AD patients, even though they had significantly higher MMSE scores and higher noun fluency scores than AD patients (see Table 2). There were no significant differences between patient groups on letter fluency, after Bonferroni correction for multiple comparisons.
Healthy controls showed the highest scores on all fluency tasks. Mean age-adjusted scores were significantly higher than other groups on noun fluency, verb fluency, and letter fluency (p < .0001; Table 2).
Within group comparisons
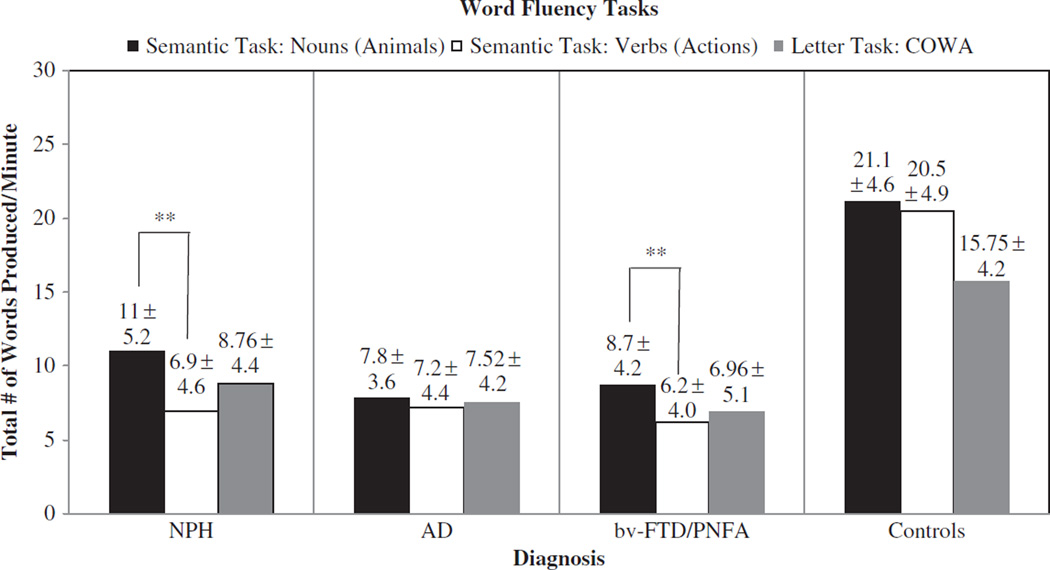
Figure 1 displays the mean raw scores on the three fluency tasks for each group. Paired t-tests revealed that patients with NPH showed significantly lower scores on verb fluency compared to noun fluency: 6.9 ± 4.6 vs. 11.0 ± 5.2 per minute; t(143) = 12.25, p < .0001. Patients with bv-FTD/PNFA also showed significantly worse performance in action verb fluency than noun fluency, 6.2 ± 4.0 vs. 8.7 ± 4.2 per minute; t(36) = 3.03, p = .004. Patients with AD showed no difference between verb and noun fluency: 7.2 ± 4.4 vs. 7.8 ± 3.6 per minute; t(32) = 0.94, p = .352. Healthy control patients also showed no significant difference in performance in verb fluency compared to noun fluency, 20.5 ± 4.9 vs. 21.1 ± 4.6 per minute; t(19) = 0.58, p = .572.
Figure 1.
Performance within patient groups for semantic fluency task and letter fluency task.
DISCUSSION
We confirmed our hypothesis that patients with bv-FTD, PNFA and NPH have worse action verb than noun (animal) fluency, consistent with the proposal that action naming relies more on frontal-subcortical circuits than does noun naming. We also hypothesized that AD patients, often with temporal and parietal atrophy, would show the opposite: more difficulty retrieving nouns versus action verbs. Although we found no difference between noun and verb fluency in patients with AD, noun fluency was more impaired in AD than in NPH or bvFTD/PNFA. The relatively poor performance on action verb vs. noun fluency of bv-FTD, PNFA and NPH patients provides additional evidence that verb retrieval may depend more on frontal and subcortical regions, whereas noun retrieval may depend more on temporal regions.
The disproportionate impairment in action verb fluency could be due to disrupted executive control, if it is assumed that greater executive resources are required for naming verbs than nouns. Executive dysfunction is associated with pathology in the prefrontal brain regions and their connections to the basal ganglia. As mentioned previously, verbs are more broadly defined and have more alternative meanings. Therefore, poor verb retrieval plausibly reflects an impaired executive system, hindering the ability to coordinate the diverse range of information that may be associated with the meanings and retrieval of a verb’s name (Grossman, 1998). Alternatively, action verb retrieval might rely on frontal and subcortical regions because action meaning might be intertwined with motor plans in the posterior frontal (motor) cortex and basal ganglia (Gallagher, 2005). Regardless of the explanation, our results indicate that action verb fluency is likely to be more sensitive than noun (e.g., animal) fluency for detecting frontal-subcortical dysfunction.
AD, a prototype of cortical dementia, is characterized by impairment of semantic memory responsible for processing meaning-related knowledge. Therefore, impaired semantic (noun and verb) fluency performance in AD can be attributed to disruptions in processing and/or organization of semantic memory (Martin & Fedio, 1983). Earlier studies have revealed deficits on both letter and semantic fluency tasks, but the deficits were more severe on semantic fluency (e.g., action or animal naming) tests (Monsch et al., 1992). There was no significant difference between action verb and noun fluency or between semantic and letter fluency in our AD patients. However, AD patients performed worse on noun retrieval compared to all other patient groups. Similar patterns have been previously observed in patients with AD, who performed worse than patients with frontotemporal dementia on an object naming task (Cappa et al., 1998). These results are consistent with the hypothesis that noun naming depends on temporoparietal circuits more than frontal-subcortical circuits, since AD patients have disproportionate temporoparietal dysfunction.
We also compared MMSE scores versus fluency scores across groups. Although the MMSE is widely used as a measure of cognitive decline for patients with different types of dementia, it does not reliably differentiate between dementia types. NPH patients and bv-FTD/PNFA patients had significantly higher MMSE scores than AD patients. The difference in MMSE scores among bv-FTD/PNFA and AD patients could be a reflection of differences involving age, disease duration or neuropathology. Because we did not control for date of onset and/or duration of illness, bv-FTD and NPH patients may have been tested earlier in their course of illness. Nevertheless, bv-FTD and NPH patients had a trend toward lower performance action verb fluency compared to AD patients, and showed significantly more impaired verb fluency compared to noun fluency (which AD patients did not show). These results raise the possibility that impaired action verb fluency with relatively normal MMSE scores and noun fluency might help rapidly identify probable frontal-subcortical dementia. Therefore, action verb fluency might be used in conjunction with MMSE scores to identify probable frontal and/or subcortical dementias. This hypothesis will need to be prospectively studied in separate populations of cortical and subcortical dementia.
Limitations of this study include the fact that patients were diagnosed clinically, without pathological confirmation. However, our results indicate that MMSE scores and brief tasks of action verb and noun fluency (one minute each) might contribute to distinguishing between groups that are typically distinguished by much lengthier neuropsychological batteries, imaging, and neurological examinations. Furthermore, we did not include other classical subcortical dementias, such as Parkinson’s disease (PD) and HIV-associated dementia. These patients are generally evaluated in separate clinics from the cognitive disorders clinic at our institution, and are more easily distinguished from AD on the basis of motor examination (in the case of PD) or blood tests (in the case of HIV).
Although the results do not show a double dissociation, the data demonstrate distinct profiles of performance across groups. Our findings provide a useful basis for future studies to evaluate the specificity and sensitivity of the identified cognitive profile in distinguishing AD from frontal and subcortical dementias. The current study established the potential usefulness of action verb fluency tests, when combined with MMSE and noun fluency, in uncovering cognitive deficits due to damaged frontal-subcortical circuits, and perhaps for distinguishing these conditions from early AD.
Acknowledgments
The research reported in this paper was supported by NIH (NIDCD), through RO1 DC 05375. We gratefully acknowledge this support and the participation of the patients.
Footnotes
Disclosure: The authors report no conflicts of interest.
REFERENCES
- Bak TH, O’Donovan DG, Xuereb JH, Boniface S, Hodges JR. Selective impairment of verb processing associated with pathological changes in Brodmann areas 44 and 45 in the motor neuron disease-dementia-aphasia syndrome. Brain. 2001;124:103–120. doi: 10.1093/brain/124.1.103. [DOI] [PubMed] [Google Scholar]
- Benton AL. Differential behavioral effects in frontal lobe disease. Neuropsychologia. 1968;6:53–60. [Google Scholar]
- Cappa SF, Binetti G, Pezzini A, Padovania A, Rozzini L, Trabucchi M. Object and action naming in Alzheimer’s disease and frontotemporal dementia. Neurology. 1998;50:351–355. doi: 10.1212/wnl.50.2.351. [DOI] [PubMed] [Google Scholar]
- Cappelletti M, Fregni F, Shapiro K, Pascual-Leone A, Caramazza A. Processing nouns and verbs in the left frontal cortex: A trancranial magnetic stimulation study. Journal of Cognitive Neuroscience. 2008;20:707–720. doi: 10.1162/jocn.2008.20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramazza A, Hillis AE. Lexical organization of nouns and verbs in the brain. Nature. 1991;349:788–790. doi: 10.1038/349788a0. [DOI] [PubMed] [Google Scholar]
- Dabul BL. Apraxia Battery for Adults. Austin, TX: Pro-Ed; 1979. [Google Scholar]
- Damasio AR, Tranel D. Nouns and verbs are retrieved with differently distributed neural systems. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:4957–4960. doi: 10.1073/pnas.90.11.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M, Folstein S, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gallagher S. Expressive movement from the beginning? In: Gallagher S, editor. How the body shapes the mind. New York: Oxford University Press Inc; 2005. [Google Scholar]
- Gentner D. Some interesting differences between nouns and verbs. Congitive Brain Theory. 1981;4:161–178. [Google Scholar]
- Gorno-Tempini ML, Ohar JM, Brambati SM, Wang P, Jeong JH, Rankin KP, et al. Anatomical correlates of early mutism in progressive nonfluent aphasia. Neurology. 2006;67:1849–1851. doi: 10.1212/01.wnl.0000237038.55627.5b. [DOI] [PubMed] [Google Scholar]
- Grossman M. Not all words are created equal: Category-specific deficits in central nervous system disease. Neurology. 1998;50:324–325. doi: 10.1212/wnl.50.2.324. [DOI] [PubMed] [Google Scholar]
- Hakim S, Adams RD. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure-obervation on cerebrospinal fluid hydrodynamics. Journal of the Neurological Sciences. 1965;2:307–327. doi: 10.1016/0022-510x(65)90016-x. [DOI] [PubMed] [Google Scholar]
- Henry JD, Crawford JR. A meta-analytic review of verbal fluency performance following focal cortical lesions. Neuropsychology. 2004a;18:284–295. doi: 10.1037/0894-4105.18.2.284. [DOI] [PubMed] [Google Scholar]
- Henry JD, Crawford JR. A meta-analytic review of verbal fluency performance in patients with traumatic brain injury. Neuropsychology. 2004b;18:621–628. doi: 10.1037/0894-4105.18.4.621. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Caramazza A. The representation of grammatical categories of words in the brain. Journal of Cognitive Neuroscience. 1995;7:396–407. doi: 10.1162/jocn.1995.7.3.396. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Tuffiash E, Caramazza A. Modality-specific deterioration in naming verbs in nonfluent primary progressive aphasia. Journal of Cognitive Neuroscience. 2002a;14:1099–1108. doi: 10.1162/089892902320474544. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Wityk R, Barker P, Caramazza A. Neural regions essential for writing verbs. Nature Neuroscience. 2002b;6:19–20. doi: 10.1038/nn982. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Oh S, Ken L. Deterioration of naming nouns versus verbs in primary progressive aphasia. Annals of Neurology. 2004;55:268–275. doi: 10.1002/ana.10812. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia: Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115:1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K. Pyramids and palm trees. Bury St. Edmunds, UK: Thames Valley Test Company; 1992. [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. Boston naming test. Philadelphia, PA: Lea and Febiger; 1983. [Google Scholar]
- Kertesz A. Western aphasia battery. New York: Grune & Stratton; 1982. [Google Scholar]
- Kertesz A, Davidson W, Fox H. Frontal behavioral inventory: Diagnostic criteria for frontal lobe dementia. The Canadian Journal of Neurological Sciences. 1997;1:29–36. doi: 10.1017/s0317167100021053. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Graham KS, Patterson K. Is a picture worth a thousand words? Evidence from concept definitions by patients with semantic dementia. Brain and Language. 1999;70:309–335. doi: 10.1006/brln.1999.2143. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, McClelland J, Patterson K, Galton CJ, Hodges JR. No right to speak? The relationship between object naming and semantic impairment: Neuropsychological evidence and a computational model. Journal of Cognitive Neuroscience. 2001;13:341–356. doi: 10.1162/08989290151137395. [DOI] [PubMed] [Google Scholar]
- Maratsos M. Are actions to verbs as objects are to nouns? On the differential semantic bases of form, class, category. Linguistics. 1990;28:1351–1379. [Google Scholar]
- Martin A, Fedio P. Word production and comprehension in Alzheimer’s disease: The breakdown of semantic knowledge. Brain and Language. 1983;19:124–141. doi: 10.1016/0093-934x(83)90059-7. [DOI] [PubMed] [Google Scholar]
- Mätzig S, Druks J. Object vs. action naming: A double dissociation? Brain and Language. 2006;99:218–219. [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;7:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Miceli G, Silveri MC, Nocentini U, Caramazza A. Patterns of dissociation in comprehension and production of nouns and verbs. Aphasiology. 1988;2:351–358. [Google Scholar]
- Miceli G, Silveri MC, Villa G, Caramazza A. On the basis for the agrammatic difficulty in producing main verbs. Cortex. 1984;20:207–220. doi: 10.1016/s0010-9452(84)80038-6. [DOI] [PubMed] [Google Scholar]
- Monsch AU, Bondi MW, Butters N, Salmon D, Katzman R, Thal LJ. Comparsions of verbal fluency tasks in the detection of dementia of the Alzheimer type. Archives of Neurology. 1992;349:1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Osterrieth PA. Le test de copie d’une figure complex: Contribution a l’étude de la perception et de la mémoire. Archiv für Psychologie. 1944;30:286–356. [Google Scholar]
- Partington JE, Leiter RG. Pathway test. Psychological Service Center Bull. 1949;1:9–20. [Google Scholar]
- Perani D, Cappa SF, Schnur T, Tettamanti M, Collina S, Rosa MM, Fazio F. The neural correlates of verb and noun processing: A PET study. Brain. 1999;122:2337–2344. doi: 10.1093/brain/122.12.2337. [DOI] [PubMed] [Google Scholar]
- Piatt AL, Fields JA, Paolo A, Koller WC, Troster AI. Lexical, semantic, and action fluency in Parkinson’s disease with and without dementia. Journal of Clinical and Experimental Neuropsychology. 1999;21:435–443. doi: 10.1076/jcen.21.4.435.885. [DOI] [PubMed] [Google Scholar]
- Priatt A, Fields J, Paolo A, Troster A. Action verbal fluency normative data for the elderly. Brain and Language. 2004;89:580–583. doi: 10.1016/j.bandl.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Raftopoulos C, Deleval J, Chaskis C, Leonard A, Cantraine F, Demyttere F, et al. Cognitive recovery in idiopathic normal pressure hydrocephalus: A prospective study. Neurosurgery. 1994;35:397–405. doi: 10.1227/00006123-199409000-00006. [DOI] [PubMed] [Google Scholar]
- Relkin N, Marmarou P, Klinge P, Bergsneider M, Black PM. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57:S4–S16. doi: 10.1227/01.neu.0000168185.29659.c5. [DOI] [PubMed] [Google Scholar]
- Rey A. L’examen psychologique dans les cas d’encéphalopathie traumatique. Archiv für Psychologie. 1941;28:286–340. [Google Scholar]
- Rey A. L’examen clinique en psychologie. Paris: Press Universitaire de France; 1964. [Google Scholar]
- Sepelyak K, Heidler-Gary J, Davis C, Newhart M, Hillis A. Speed on cognitive tests is associated with relative ventricular enlargement in normal pressure hydrocephalus. Poster session presented at the annual meeting of the American Academy of Neurology; Seattle, Washington. 2009. Apr, [Google Scholar]
- Shapiro K, Caramazza A. Grammatical processing of nouns and verbs in left frontal cortex? Neuropsychologia. 2003;41:1189–1198. doi: 10.1016/s0028-3932(03)00037-x. [DOI] [PubMed] [Google Scholar]
- Sumerall SW, Timmons PL, James AL, Ewing MJ, Oehlert ME. Expanded norms for controlled oral word association test. Journal of Clinical Psychology. 1997;53:517–521. doi: 10.1002/(sici)1097-4679(199708)53:5<517::aid-jclp14>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Damasio A. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia. 1997;35:1319–1327. doi: 10.1016/s0028-3932(97)00085-7. [DOI] [PubMed] [Google Scholar]
- Tranel D, Adolphs R, Damasio H, Damasio AR. A neural basis for the retrieval of words for actions. Cognitive Neuropsychology. 2001;18:655–670. doi: 10.1080/02643290126377. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, Kozak J, Rees L. COWA by education level in adults. In: Spreen O, Strauss E, editors. A Compendium of Neuropsychological Tests: Administration, norms, and commentary. 2nd ed. New York: Oxford University Press; 1996. [Google Scholar]
- Tombaugh T, Kazak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Archives of Clinical Neuropsychology. 1999;14:167–177. [PubMed] [Google Scholar]
- Trenerry MR, Crosson B, DeBoe J, Leber WR. Stroop Neuropsychological Screening Test. Florida: Psychological Assessment Resources; 1989. [Google Scholar]
- Verrees M, Selman WR. Management of normal pressure hydrocephalus. American Family Physician. 2004;70:1071–1078. [PubMed] [Google Scholar]
- Woods SP, Carey CL, Troster AI, Grant I The HNRC Group. Action (verb) generation in HIV-1 infection. Neuropsychologia. 2005;43:1144–1151. doi: 10.1016/j.neuropsychologia.2004.11.018. [DOI] [PubMed] [Google Scholar]