Summary
In mammalian testis, spermatogenesis takes place in the seminiferous epithelium of the seminiferous tubule, which is composed of a series of cellular events. These include: (i) spermatogonial stem cell (SSC) renewal via mitosis and differentiation of SSC to spermatogenia, (ii) meiosis, (iii) spermiogenesis, and (iv) spermiation. Throughout these events, developing germ cells remain adhered to the Sertoli cell in the seminiferous epithelium amidst extensive cellular, biochemical, molecular and morphological changes to obtain structural support and nourishment. These events are coordinated via signal transduction at the cell-cell interface through cell junctions, illustrating the significance of cell junctions and adhesion in spermatogenesis. Additionally, developing germ cells migrate progressively across the seminiferous epithelium from the stem cell niche, which is located in the basal compartment near the basement membrane of the tunica propria adjacent to the interstitium. Recent studies have shown that some apparently unrelated proteins, such as polarity proteins and actin regulatory proteins, are in fact working in concert and synergistically to coordinate the continuous cyclic changes of adhesion at the Sertoli-Sertoli and Sertoli-germ cell interface in the seminiferous epithelium during the epithelial cycle of spermatogenesis, such that developing germ cells remain attached to the Sertoli cell in the epithelium while they alter in cell shape and migrate across the epithelium. In this review, we highlight the physiological significance of endocytic vesicle-mediated protein trafficking events under the influence of polarity and actin regulatory proteins in conferring cyclic events of cell adhesion and de-adhesion. Furthermore, these recent findings have unraveled some unexpected molecules to be targeted for male contraceptive development, which are also targets of toxicant-induced male reproductive dysfunction.
Keywords: Testis, Polarity proteins, PAR6, Cdc42, GTPase, Actin regulators, Eps8, Arp3, Anchoring junction, Ectoplasmic specialization, Seminiferous epithelial cycle
Introduction
The seminiferous tubule is the functional unit of the testis that produces spermatozoa from spermatogonia via spermatogenesis in ~58, 35 and 64 days in rats, mice, and humans respectively (de Kretser and Kerr, 1988; Franca et al., 1998). More than 100 million sperms are produced from a man each day since puberty at ~12–14 years of age throughout his entire life span (Neaves et al., 1984; de Kretser and Kerr, 1988; Johnson et al., 2001). Spermatogenesis takes place in the seminiferous epithelium (Fig. 1) which is composed of only Sertoli and germ cells. It is regulated by the pituitary hormones luteinizing hormone (LH) and follicle-stimulating hormone (FSH), as well as testosterone and estrogen that are released by Leydig cells located in the interstitial space (Sharpe, 1994; O’Donnell et al., 2001; Carreau and Hess, 2010; Verhoeven et al., 2010). However, post-meiotic germ cell development during spermiogenesis and spermiation (the release of sperm) (O’Donnell et al., 2011) is sequestered behind the blood-testis barrier (BTB), an ultrastructure established by specialized junctions between Sertoli cells near the basement membrane (Wong and Cheng, 2005; Setchell, 2008; Vogl et al., 2008; Cheng et al., 2010; Cheng and Mruk, 2010). Thus, many of the cellular events associated with spermatogenesis take place in a specialized microenvironment behind the BTB known as the apical (adluminal) compartment (Fig. 1). As such, the barrier imposed by adjacent Sertoli cells in the seminiferous epithelium prevents the access of hormones, electrolytes, ions, paracrine factors, biomolecules, water, and all other substances to germ cells undergoing meiosis I and II, and all events of post-meiotic cell development, including the release of sperm at spermiation (Mruk et al., 2008; Cheng and Mruk, 2009, 2010; Franca et al., 2011; O’Donnell et al., 2011). Despite the fact that many of the cyclic events during spermatogenesis that occur in germ cell “clones” (which are connected by cytoplasmic bridges) (Fawcett et al., 1959; Fawcett, 1961; Hamer et al., 2003) take place in a synchronized fashion, most of the necessary paracrine factors and hormones possibly needed to coordinate these events are “excluded” from the apical compartment because of the BTB. Thus, it is conceivable that germ cell development relies exclusively on Sertoli cells in the seminiferous epithelium, and these events must be coordinated via precisely regulated and coordinated “communications” at the cell-cell interface, such as via communicating gap junctions.
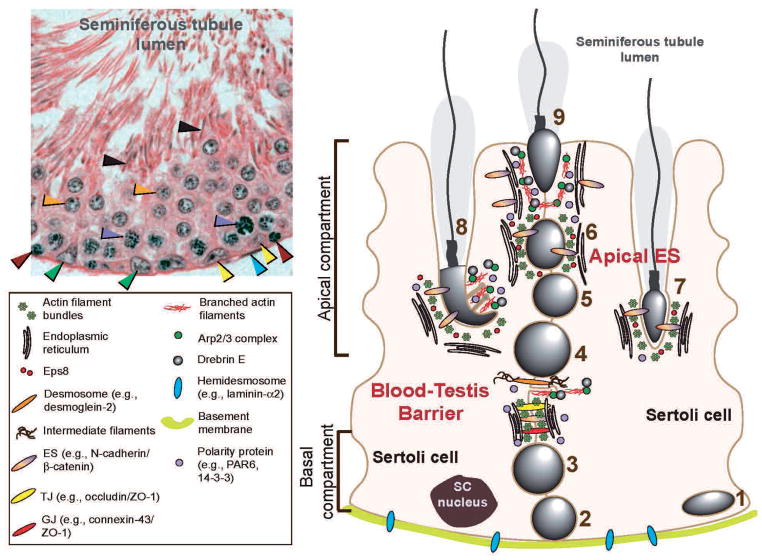
Fig. 1.
Relative distribution of polarity proteins (e.g., PAR6, 14-3-3) and actin regulatory proteins (e.g., Eps8, Arp2/3 complex, drebrin E) at the apical ES and the BTB in the seminiferous epithelium of adult mammalian testes. The left panel is the cross-section of an adult rat testis showing the seminiferous epithelium of a stage VIII seminiferous tubule. The blood-testis barrier (BTB) has physically divided the epithelium into the basal and apical or adluminal compartments as shown in the right panel. Sertoli cell nuclei (green arrowheads) are restricted to the basal compartment near the basement membrane with the peritubular myoid cells (see blue arrowhead) lying outside the basement membrane. Both spermatogonia (yellow arrowheads) and preleptotene spermatocytes (red arrowheads) are found in the basal compartment. However, preleptotene spermatocytes are in transit at the BTB at stage VIII and differentiate into pachytene spermatocytes (purple arrowheads) so that they can undergo meiosis I and II at stage XIV of the epithelial cycle. Also seen are round spermatids (step 8 spermatids, orange arrowheads) and elongated spermatids (step 19 spermatids, black arrowheads). The apical ES in the apical compartment, and the basal ES at the BTB in the basal compartment are typified by the presence of actin filament bundles (maintained by the intricate actions of Eps8, Arp2/3 complex and drebrin E) sandwiched in between cisternae of endoplasmic reticulum and the apposing plasma membranes of either the Sertoli cell and the spermatid (step 8–19 spermatids) (for apical ES) or the two Sertoli cells (for basal ES at the BTB). 1, spermatogonial stem cell (SSC) or spermatogonium (undifferenciated or differenciated type A); 2, type B spermatogonium; 3, preleptotene spermatocyte; 4, zygotene, pachytene or dyplotene spermatocyte; 5, round spermatid; 6, elongating spermatid (step 8); 7, elongating spermatid; 8, elongating spermatid; 9, elongated spermatid. Apical ES, once it appears, is the only anchoring device between the Sertoli cell and the elongating/elongated spermatid to confer cell adhesion and polarity (or orientation) to the developing spermatids until spermiation.
In the seminiferous epithelium, gap junctions (GJs, a cell-cell communication junction type) and desmosomes (an intermediate filament-based cell-cell anchoring junction type) are found at both Sertoli-Sertoli and Sertoli-germ cell interface including spermatocytes and spermatids (steps 1–8). Hemidesmosomes are intermediate filament based cell-matrix anchoring junction type at the Sertoli cell-basement membrane [note: the basement membrane is a modified extracellular matrix also known as basal lamina (Dym, 1994)] interface (Fig. 1) (Cheng and Mruk, 2002, 2010; Mruk and Cheng, 2004; Mruk et al., 2008). However, there is no “conventional” cell-cell actin-based adherens junction (AJ) in the seminiferous epithelium analogous to other epithelia. Instead, a specialized testis-specific actin-based cell-cell anchoring junction known as ectoplasmic specialization (ES) is found in the mammalian testis. ES is restricted to the interface of Sertoli cells and elongating spermatids (steps 8–19 in rats) known as apical ES and to the Sertoli-Sertoli cell interface at the BTB known as the basal ES (Fig. 1). Once apical ES appears, GJ and desmosome vanish entirely, and it becomes the only anchoring device that anchors developing spermatids (step 8–19 in rats) to the Sertoli cells in the epithelium during spermiogenesis. Interestingly, basal ES coexists with TJ and GJ, and these junctions together with desmosomes constitute the BTB in the mammalian testis (Fig. 1). Furthermore, ES is the most prominent anchoring junction in the seminiferous epithelium clearly visible under electron microscopy throughout spermatogenesis. Unexpectedly, recent studies have shown that this junction type is regulated by two groups of proteins, namely polarity and actin regulatory proteins. Recent findings also demonstrated that ES works in concert with GJ and desmosome to coordinate cellular events, in particular at the BTB, throughout spermatogenesis. While much research is needed to understand the signaling mechanism(s) underlying these events, these findings have unraveled some unexpected turns of events, revealing several molecular entities that are crucial for cell adhesion in the seminiferous epithelium, and functional studies can now be designed to probe the molecular and biochemical events pertinent to spermiogenesis and spermiation (Cheng and Mruk, 2011; O’Donnell et al., 2011).
Ectoplasmic specialization
Ectoplasmic specialization (ES) is limited to the Sertoli-Sertoli cell interface at the BTB known as the basal ES; however, ES is also found at the Sertoli-spermatid (step 8–19 spermatids) interface known as the apical ES, which is the only anchoring device once it appears during spermiogenesis (Cheng and Mruk, 2009, 2010). It is known that in adult rats each testis contains ~30×106 Sertoli cells (Berndtson and Thompson, 1990) [~4×106 Sertoli cells per testis in adult mice (Auharek and Franca, 2010)]. Sertoli cells cease to proliferate by ~15–17 days of age postpartum (Orth, 1982) and the ratio of Sertoli:germ cell is ~1:50 (Weber et al., 1983) [note: the ratio of spermatogonium:Sertoli cell was estimated to be 0.12:1 whereas spermatid: spermatogonium ratio is ~75:1 in adult rats at 150–250 days of age postpartum (Berndtson and Thompson, 1990)], illustrating the significance of ES in the seminiferous epithelium simply from a spatial distribution stand-point. While ES is basically a testis-specific cell-cell actin-based AJ type, this ultrastructure is exclusively found in the testis, typified by the presence of actin filament bundles lying parallel to the plasma membrane and sandwiched in between cisternae of endoplasmic reticulum and the apposing plasma membranes of either Sertoli-Sertoli cell interface at the basal ES or Sertoli-spermatid interface at the apical ES (Fig. 1). While these ultrastructural features are found on both sides of the Sertoli cells in the basal ES at the BTB, they are restricted to the Sertoli cell side at the apical ES, and no such ultrastructures are visible in the elongating spermatids (steps 8–19). This may be due to the fact that relatively little cytosol is present in elongating spermatids from steps 8–19 whose cell bodies are occupied almost exclusively by the nucleus and the acrosome, and thus an extensive actin filament network cannot be sustained spatially. Nevertheless, spermatids were shown to express many of the structural proteins at the apical ES (e.g., N-cadherin, E-cadherin, nectins, laminins), peripheral adaptors (e.g., β-catenin, γ-catenin, afadins) and non-receptor protein kinases (e.g., FAK, c-Src, c-Yes) (Mruk and Cheng, 2004; Zhang et al., 2005; Mruk et al., 2008) at or near the plasma membrane opposite to the actin filament bundles (Lie et al., 2010a,b, 2011; Wong et al., 2008a,b; Wong and Cheng, 2009). These findings thus suggest that adhesion protein complexes (e.g., N-cadherin/β-catenin, nectin/afadin, laminin/c-Src) residing in elongating spermatids can form putative inter-locking interactions with the corresponding domains of the adhesion complexes (e.g., N-cadherin/β-catenin, nectin/afadin, integrin/vinculin) residing in the Sertoli cell at the apical ES. Thus, developing spermatids are likely to contribute, at least in part, to the apical ES functionality without the distinctive network of actin filament bundles. This perhaps is physiologically necessary so that the metabolically inactive developing spermatids (relative to the Sertoli cells in the epithelium) do not have to express another set(s) of proteins to “de-polymerize” and “re-polymerize” the actin filament bundles similar to the Sertoli cells, but they can still “adhere” to the Sertoli cell via these putative cell adhesion protein complexes.
Interestingly, while the protein complexes that confer cell adhesion at the apical ES are better defined, the constituent protein complexes at the basal ES are less known (Table 1) because basal ES coexists with either TJ or gap junction (but not desmosome) (Mruk and Cheng, 2011b) (note: TJ, basal ES, gap junction, and desmosome constitute the BTB); thus, even if a protein is found to localize at the BTB, it is somewhat difficult to assign it to be a basal ES-, TJ- or gap junction-protein, since it is not technically possible at present to isolate basal ES without the associated TJ or gap junction for protein composition analysis. Thus, the information listed in Table 1 regarding the constituent proteins at the TJ and basal ES is largely based on earlier studies from other epithelia. If a selected protein was shown earlier to be associated with TJ (e.g., occludin) or AJ (e.g., N-cadherin) in other tissues, it would be classified accordingly at the BTB. For instance, N-cadherin would be assigned as a basal ES protein and occludin would be designated a TJ-protein.
Table 1.
Adhesion protein complexes that confer cell-cell adhesion in the seminiferous epithelium of adult testes.
| Location | Adhesion protein complex | |
|---|---|---|
| Anchoring junctions | ||
| Apical ES | Sertoli cell-spermatid (step 8–19) | α6β1-integrin-laminin α3β3γ3 N-cadherin-β-catenin Nectin-2/3-afadin JAM-C-ZO-1 CAR-ZO-1 |
| Desmosome | Sertoli cell-spermatocyte/spermatogonium Sertoli cell-spermatid (step 1–7) |
Desmoglein-desmocollin Desmoglein-desmocollin |
| Hemidesmosome | Sertoli cell-basement membrane | β1-integrin/laminin α2 |
| Communicating junctions | ||
| Gap junction | Sertoli cell-spermatid (step 1–19) | Connexin 43-plakophilin-2 |
| Blood-testis barrier | ||
| Tight junction | Sertoli-Sertoli cell | Occludin-ZO-1 N-cadherin-β-catenin JAM-A-ZO-1 JAM-B-ZO-1 CAR-ZO-1 |
| Basal ES | Sertoli-Sertoli cell | N-cadherin-β-catenin Nectin-2-afadin JAM-A-ZO-1 CAR-ZO-1 |
| Desmosome | Sertoli-Sertoli cell | Desmoglein-2-desmocollin-2 |
| Gap junction | Sertoli-Sertoli cell | Connexin 43-plakophilin-2 |
This Table was prepared based on recent reviews (Mruk and Cheng, 2004; Mruk et al., 2008; Wong et al., 2008; Cheng and Mruk, 2009; 2010; Wong and Cheng, 2009; Cheng et al., 2010; Lie et al., 2011) based on studies in the rat testis. Apical ES, basal ES, tight junction and gap junction are using actin for their attachment; desmosome and hemidesmosome are using intermediate filament for their attachment. Focal contact (or focal adhesion complex), a cell-matrix anchoring junction type using actin for its attachment is absent in the testis. CAR, coxsackievirus and adenovirus receptor; JAM, junctional adhesion molecule; ZO-1, zonula occludens-1.
It is likely that the unique ultrastructural features, such as the distinctive network of actin filament bundles at the ES, contribute to its unusual adhesive strength. The strength of ES was found to surpass that of desmosome, which is considered to be a strong adhesion junction in epidermis and in keratinocytes in vitro (Green et al., 2010; Green and Simpson, 2007; Thomason et al., 2010), as well as at the Sertoli cell-spermatid (pre-step 8 spermatids) interface in the testis (Wolski et al., 2005). For instance, the adhesive force conferred by apical ES was found to be almost twice as strong as desmosome when it was quantified by a micropipette pressure transducing system (Wolski et al., 2005), and the remarkable adhesive strength is largely the result of the extensive network of actin filament bundles at the ES. Interestingly, apical ES is also exceedingly vulnerable to toxicants (e.g., cadmium) or chemicals/drugs (e.g., adjudin) and it can be broken down by these toxicants and/or chemicals long before desmosome and gap junction are disrupted in the seminiferous epithelium (Chen et al., 2003; Yan et al., 2007; Cheng et al., 2011). Thus, earlier studies have shown that in rodents treated with toxicants, exfoliation of spermatids from the epithelium is the most typical phenotype in the testis (Boekelheide et al., 1989; Hew, et al., 1993; Wong et al., 2005; Moffit et al., 2007; Elkin et al., 2010; Wong et al., 2010a,b). The BTB is also highly sensitive to environmental toxicants (e.g., cadmium), more than other blood-tissue barriers (Setchell and Waites, 1970; Hew, et al., 1993; Wong et al., 2004). Such vulnerability of the ES to toxicants may be attributed to the hybrid nature of this junction type (Siu and Cheng, 2004; Yan et al., 2007; Wong et al., 2008). For instance, the primary molecular target of cadmium was shown to be E-cadherin because Cd2+ competed for the same binding motif as Ca2+ in E-cadherin (Prozialeck and Lamar, 1999; Prozialeck, 2000), but E-cadherin is usually shielded behind the TJ in virtually all other epithelia and/or endothelia, making the E-cadherin-based AJ less accessible to cadmium. In contrast, E-cadherin found in the basal ES “coexists” with other TJ and gap junction proteins at the BTB, making it immediately accessible to cadmium. This thus explains the unusual vulnerability of the BTB to toxicants versus other blood-tissue barriers.
Polarity proteins
Cell polarity in epithelia, such as apico-basal polarity, and cell asymmetry, is conferred by three different protein modules or complexes: (i) the partitioning defective protein (PAR) complex [e.g., PAR3/PAR6/aPKC (atypical protein kinase C)], (ii) the Crumbs complex [e.g., Crumbs-3/PALSI (protein associated with LIN7-1)/PATJ (PALS1-associated tight junction protein)], and (iii) the Scribble complex [e.g., Scribble/LGL1/2 (lethal giant larvae 1/2)/DLG1 (discs large 1)] (Assemat et al., 2008; Iden and Collard, 2008; Wong and Cheng, 2009; Cheng and Mruk, 2010). The PAR- and Crumbs-based modules are usually located at the TJ in the apical region of a cell epithelium, whereas the Scribble-based module is restricted to the basolateral domain. Since each of these protein modules recruits its own scaffolding proteins, adaptors, nonreceptor protein kinases and phosphatases, mutual exclusion of the Scribble module and the apically located Crumbs- and PAR-based modules, as well as the junctional complexes (composed of TJ-AJ plaques-desmosomes), thus confers apico-basal polarity in cell epithelium, including the seminiferous epithelium (Fig. 2). As such, TJ and basal ES at the BTB confer Sertoli cell polarity in the seminiferous epithelium, which is discernible even under light microscopy in which Sertoli cell nuclei are found localized near the basement membrane (Fig. 1). Under electron microscopy, it is noted that TJ and basal ES are restricted to the BTB near the basement membrane, whereas developing elongating spermatids are restricted to the apical compartment of the seminiferous epithelium (Fig. 1), displaying cellular asymmetry, and this polarity phenotype is the result of the polarity proteins at the BTB.
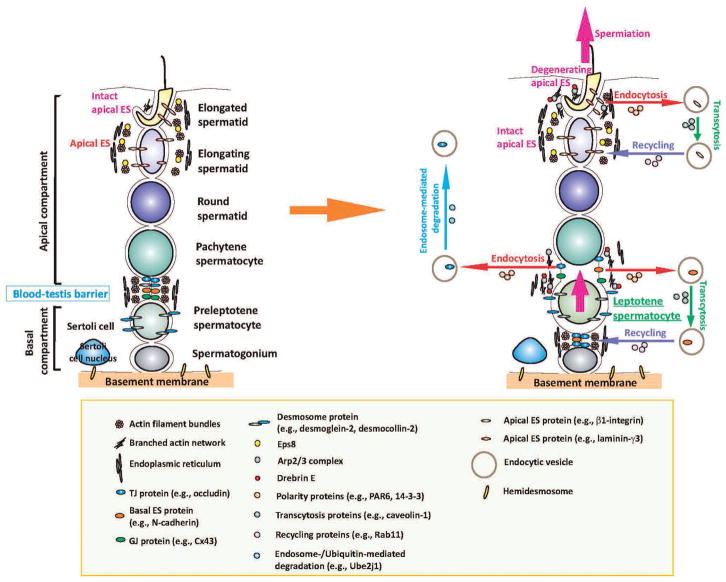
Fig. 2.
Restructuring of adhesion at the apical ES and the BTB in the seminiferous epithelium during the epithelial cycle to facilitate the transit of developing germ cell across the epithelium is regulated by the intricate actions of polarity proteins (e.g., PAR6, 14-3-3, Cdc42) and actin regulatory proteins (e.g., Eps8, Arp2/3 complex, drebrin E) as depicted in this hypothetical model. The left panel illustrates the seminiferous epithelium of a stage VII tubule in which the integrity of the apical ES and basal ES is maintained by the rigid actin filament bundles. However, at stage VIII of the epithelial cycle when preleptotene spermatocytes are in transit in “clones” at the BTB and elongated spermatids begin the process of sperm release (i.e., spermiation), endocytosis of integral membrane proteins occurs at the basal ES (at the BTB) and apical ES, which is mediated by the intricate actions of the polarity proteins, Arp2/3 protein complex and drebrin E (and a concomitant loss and/or reduced Eps8 activity) to increase the actin network plasticity to facilitate the endocytic vesicle-mediated protein trafficking events. While some of the endocytosed proteins are degraded via the endosome- or ubiquitin-mediated pathway, many of the internalized proteins undergo transcytosis and recycling, so that these proteins can be “reused” to establish the “newly” formed apical ES or basal ES and TJ/gap junction/desmosome components at the BTB. Thus, the extensive junction restructuring events that occur during the epithelial cycle do not lead to a disruption of the seminiferous epithelium.
In this context, it is of interest to note that there is no TJ at the Sertoli-spermatid (step 1–19 in rats) interface in the seminiferous epithelium to confer spermatid polarity. Yet the heads of all developing spermatids are arranged in such a way with their heads all pointing toward the basement membrane, illustrating spermatid polarity (Fig. 1). Thus, apical ES, a testis-specific AJ, was speculated to be responsible for conferring spermatid polarity, since this is the only anchoring device at the Sertoli-spermatid interface (step 8–19 spermatids) when spermatid polarity becomes apparent (Mruk and Cheng, 2004; Wong and Cheng, 2009). Indeed, recent studies have demonstrated the presence of PAR6 at the apical ES and its involvement in conferring spermatid polarity (Wong, et al., 2008). For instance, it was found that in rats treated with adjudin (Wong, et al., 2008) or cadmium (Cheng et al., 2011), the “prematurely” departing elongating spermatids found near the luminal edge of the seminiferous epithelium became “mis-oriented”, with their heads no longer pointing toward the basement membrane. More importantly, in these mis-oriented elongating spermatids, the intensely localized PAR6 usually found in the apical ES in normal rat testies reduced considerably, almost to a non-detectable level (Wong, et al., 2008). These findings thus provide the first proof that the PAR6-based polarity proteins are crucial to confer spermatid polarity at the apical ES.
Polarity proteins mediate ES-based adhesion function
The first evidence that polarity proteins confer spermatid and Sertoli cell adhesion at the apical and basal ES, respectively, comes from studies using the adjudin model in vivo, and the Sertoli cell in vitro culture system that mimics the Sertoli BTB in vivo (Cheng et al., 2005; Mruk et al., 2008; Wong and Cheng, 2009; Mok et al., 2011). It was found that within ~6–9-hr following treatment of adult rats with a single dose of adjudin (50 mg/kg b.w., by gavage), apical ES was disrupted, which was manifested by a loss of spermatid polarity, with the spermatid heads no longer pointing toward the basement membrane and a considerable loss of PAR6 staining at the apical ES. These changes were immediately followed by “premature” release of elongating spermatids from the seminiferous epithelium (Wong et al., 2008a,b) mimicking spermiation. These findings thus support the notion that polarity proteins confer spermatid adhesion in the seminiferous epithelium. This concept is further strengthened by earlier observations using Sertoli cells cultured in vitro with an established TJ-permeability barrier that functionally mimics the BTB in vivo [note: ultrastructures of TJ and basal ES are found in the Sertoli cell epithelium in vitro (Lui et al., 2001, 2003; Lee and Cheng, 2003; Siu et al., 2005], in which the knockdown of either PAR6 or PAR3 by RNAi affected the distribution of JAM-A and α-catenin (and also N-cadherin after PAR6 knockdown by RNAi) at the Sertoli-Sertoli cell interface. These proteins moved from the cell surface to cytosol, thereby destabilizing the Sertoli cell TJ-barrier function and altering cell adhesion properties (Wong, et al., 2008a,b). These initial findings were further strengthened by subsequent studies in which 14-3-3 (also known as PAR5) was also shown to be an integrated component of the basal ES and TJ at the BTB, and its knockdown by RNAi also induced mis-localization of N-cadherin and ZO-1 from the Sertoli-Sertoli cell interface to the cytosol, destabilizing the Sertoli TJ-barrier (Wong et al., 2009). But more importantly, 14-3-3 was found to regulate cell adhesion via its effects on the endocytic vesicle-mediated protein trafficking events, since a knockdown of 14-3-3 by RNAi induced a significant increase in protein endocytosis of both JAM-A and N-cadherin (Wong et al., 2009). Collectively, these findings thus illustrate that the loss of these adhesion proteins at the cell surface by RNAi destabilizes cell adhesion at the Sertoli-Sertoli cell interface.
Other studies have shown that the TGF-β3- or TNFα-induced disruption of the Sertoli cell TJ-barrier function is mediated by an increase in clathrin-mediated protein endocytosis at the BTB (Yan et al., 2008; Xia et al., 2009), to be followed by an increase in endosome- or ubiquitin-mediated protein degradation (Su et al., 2010). A recent report has shown that the cytokine-induced endocytic vesicle-mediated protein trafficking events that destabilize BTB are also regulated by polarity protein Cdc42 (Wong, et al., 2010), which is a component of the PAR-based polarity module (Iden and Collard, 2008; Wong and Cheng, 2009). Overexpression of a dominant negative mutant of Cdc42 abolished the TGF-β3-induced enhancement of protein endocytosis in the Sertoli cell epithelium (Wong et al., 2010a,b).
In short, these findings demonstrate unequivocally that polarity proteins are crucial to confer spermatid and Sertoli cell adhesion in the seminiferous epithelium at the apical and basal ES, respectively. This is likely mediated via their maintenance of the proper localization of adhesion proteins at the cell-cell interface by regulating the endocytic vesicle-mediated protein trafficking events. This, in turn, determines the levels of adhesion proteins at the cell surface to form interlocking adhesion at the Sertoli-spermatid or Sertoli-Sertoli cell interface. However, as discussed earlier, ES is structurally associated with an extensive network of actin filament bundles that contribute to the unusual “adhesive strength” of the apical ES, as shown in the study using a micropipette pressure transducing system (Wolski et al., 2005). Does this network of actin filament bundles at the apical and basal ES interfere with the endocytic vesicle-mediated protein trafficking events? If it does, what mechanism is in place in the testis to “rapidly” disassemble and reassemble this actin network during spermiogenesis? These questions will be addressed in the following sections.
Actin regulating proteins
Actin-based networks in a eukaryotic cell, such as the actin filament bundles at the ES in the Sertoli cell, are composed of filamentous actin (F-actin) (Lie et al., 2010a,b). F-actin is a polymer assembled by globular actin subunits (G-actin), with the fast-growing end known as the barbed end, and the slow-growing end called the pointed end (Pellegrin and Mellor, 2007; Bugyi and Carlier, 2010; Lie et al., 2010a,b). In the testis, the Sertoli cell is equipped with a number of actin regulatory proteins that confer actin bundling [e.g., espin (Bartles et al., 1996), α-actinin (Yazama et al., 1991), Eps8 (epidermal growth factor receptor pathway substrate 8) (Lie et al., 2009)], capping [e.g., Eps8 (Lie et al., 2009)], nucleation [or actin branching, e.g., Arp2/3 (actin-related protein 2/3) complex (Lie et al., 2010a,b)] and severing [e.g., cofilin (Guttman et al., 2004), gelsolin (Guttman et al., 2002)]. A recent study has also demonstrated an actin binding protein (also known as microfilament-associated protein) drebrin E (developmentally regulated brain protein E) in the rat testis which structurally interacts with Arp3 but not Esp8, apparently contributes to the restricted temporal and spatial expression and function of Arp3 at the ES in the testis (Li et al., 2011). The combined effects of these proteins thus induce polymerization and depolymerization of the actin filament bundles (Lie, et al., 2010), conferring the plasticity of the ES at the Sertoli-spermatid (apical ES) and Sertoli-Sertoli (basal ES) cell interface. This is also necessary for the formation and trafficking of the endocytic vesicles, such as their internalization from the plasma membrane and the subsequent cleavage from the entry site [such as by dynamins (Lie et al., 2006, 2008; Vaid et al., 2007)], as well as endosome-mediated intracellular trafficking via transcytosis or recycling (Welling and Weisz, 2010; Kelly and Owen, 2011; Mruk and Cheng, 2011a). It is conceivable that the presence of these extensive actin filament bundles at the ES would make it difficult for endocytic vesicle formation and its subsequent internalization. In fact, it is known that towards the end of spermiogenesis, such as in step 17–18 spermatids, actin nucleation occurs at stage VII of the epithelial cycle (Lie et al., 2010a,b), beginning from the concave side of the elongating spermatid heads (Lie et al., 2010a,b). This assembly of actin networks is associated with the formation of a “giant” endocytic vesicle (Young et al., 2009a,b) previously designated tubulobulbar complex (TBC) (Russell, 1979b, a), which is presently known to be a transitional ES ultrastructure undergoing degeneration to prepare for spermiation at the apical ES and the restructuring of the BTB at stage VIII of the epithelial cycle (Cheng and Mruk, 2010). Eventually, this event spreads to the entire apical ES to prepare for spermiation (i.e., the release of sperm) (Cheng and Mruk, 2010; O’Donnell et al., 2011) at the end of spermiogenesis. This event is a critical step to maintain the homeostasis of spermatogenesis, since endocytosed apical or basal ES proteins can be transcytosed and recycled to assemble “new” apical and basal ES. Thus, de novo synthesis would not be required for each component protein necessary for spermatid adhesion during spermiogenesis, considering that each Sertoli cell has to “nurture” ~30–50 developing germ cells, making this an almost impossible task from a physiological stand point (see Fig. 2). This concept is also supported by recent studies, which have shown that Eps8 (an actin barbed-end capping and bundling protein), Arp3 (an actin nucleation protein), and drebrin E (an Arp3 binding protein) display highly restricted temporal and spatial expression in the seminiferous epithelium at the apical and basal ES during the seminiferous epithelial cycle of spermatogenesis (Lie et al., 2008, 2010a,b, 2011). In short, Eps8 is being used to maintain the integrity of the actin filament bundles at the ES, whereas Arp3 is used to induce actin branching (with its action precisely regulated by an actin-binding protein drebrin E), causing the breakdown of the network of actin filament bundles at the ES to facilitate protein endocytosis, which is regulated by PAR6, 14-3-3, and Cdc42 at the site. It is through the concerted efforts of these proteins, as depicted in Fig. 2, that the integrity of the BTB can be maintained and proteins at the degenerating apical ES (in preparation for sperm release during spermiation) can be recycled to conserve protein utilization during spermiogenesis.
Coordinated effects of polarity proteins and actin regulatory proteins to modulate ES-based adhesion function
Apical ES
As depicted in Fig. 2, endocytic vesicle-mediated protein trafficking events begin at the concave side of the apical ES at later stages of spermiogenesis, such as in step 17–18 spermatids (e.g. stage VII of the epithelial cycle). This is made possible by a surge in actin branching at the site mediated by a temporal and spatial reduction in Eps8, but an increase in Arp3 and drebrin E expression, thereby diminishing the rigid actin filament bundles to increase the “plasticity” at the apical ES microenvironment to facilitate the formation of endocytic vesicles. Subsequent protein endocytosis is mediated by the combined action of PAR6, 14-3-3 and Cdc42. Internalized components (e.g., integrins, nectins, N-cadherin) from these degenerating apical ES are transcytosed and recycled to assemble “new” apical ES in newly formed elongating spermatids (step 8) via spermiogenesis. Thus, spermiation is not an entirely “proteolytic” event; instead, many physiologically important components are being “re-used” to maintain the homeostasis of the seminiferous epithelium during spermatogenesis.
Basal ES at the BTB
Since basal ES coexists with TJ and gap junction at the BTB, the events taking place at the basal ES thus affect integral membrane proteins at the TJ (e.g., occludins, JAM-A, JAM-B, claudins) and gap junction (e.g., connexin 43, connexin 33) as well. This also implies that the restructuring events that occur at the basal ES affect the integrity of the BTB. As shown in Fig. 2, besides de novo synthesis of proteins necessary to assemble “new” TJ-fibrils behind the preleptotene spermatocytes in transit at the BTB, restricted temporal and spatial expression of Arp3 and drebrin E, and a concomitant reduction in Eps8, at the site induce the loss of actin filament bundles to facilitate endocytic vesicle formation via an increase in cellular “plasticity”. This is further facilitated by a transient loss of PAR6 or 14-3-3 at the “old” BTB site, causing re-distribution of N-cadherin, occludin and JAM-A, destabilizing the “old” BTB. Internalized integral membrane proteins are transcytosed and recycled to the baso-lateral region of the transiting preleptotene spermatocytes to assemble “new” TJ-fibrils, creating a “new” BTB prior to the degeneration of the “old” BTB. This thus maintains the integrity of the BTB even during the transit of preleptotene spermatocytes that are interconnected by intercellular bridges as “clones”, and the immunological barrier will not be compromised during the cyclic events of spermatogenesis.
Concluding remarks and future respectives
While the concept depicted in Fig. 2 is a hypothetical model involving the combined efforts of polarity proteins and actin regulatory proteins to regulate cell adhesion and junction restructuring in the seminiferous epithelium during spermatogenesis, it is based on recently published findings in the field. However, much work is needed to provide some of the missing information. For instance, what triggers the temporal and spatial expression of Eps8, Arp3, and drebrin E (and other actin regulatory proteins) at the apical and basal ES during the epithelial cycle? Does this involve cytokines and/or steroids (e.g., testosterone, estradiol-17β) or their concerted expression in the microenvironment? Also, what triggers the transient loss of polarity proteins at the ES to facilitate protein endocytosis? Is there a common regulator that triggers both events? What is the mechanism that coordinates the transient loss of Eps8 and PAR-based polarity proteins but a surge in Arp3 to prepare for protein endocytosis at the site? It is expected that many of these questions will be answered in the years to come using genetic models or cell type specific knockdowns and/or overexpression of target genes.
References
- Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta. 2008;1778:614–630. doi: 10.1016/j.bbamem.2007.08.029. [DOI] [PubMed] [Google Scholar]
- Auharek SA, Franca LR. Postnatal testis development, Sertoli cell proliferation and number of different spermatogonial types in C57BL/6J mice made transiently hypo- and hyperthyroidic during the neonatal period. J Anat. 2010;216:577–588. doi: 10.1111/j.1469-7580.2010.01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartles J, Wierda A, Zheng L. Identification and characterization of espin, an actin-binding protein localized to the f-actin-rich junctional plaques of Sertoli cell ectoplasmic specializations. J Cell Sci. 1996;109:1229–1239. doi: 10.1242/jcs.109.6.1229. [DOI] [PubMed] [Google Scholar]
- Berndtson WE, Thompson TL. Changing relationships between testis size, Sertoli cell number and spermatogenesis in Sprague-Dawley rats. J Androl. 1990;11:429–435. [PubMed] [Google Scholar]
- Boekelheide K, Neely MD, Sioussat TM. The Sertoli cell cytoskeleton: A target for toxicant-induced germ cell loss. Toxicol Appl Pharmacol. 1989;101:373–389. doi: 10.1016/0041-008x(89)90188-9. [DOI] [PubMed] [Google Scholar]
- Bugyi B, Carlier MF. Control of actin filament treadmilling in cell motility. Annu Rev Biophys. 2010;39:449–470. doi: 10.1146/annurev-biophys-051309-103849. [DOI] [PubMed] [Google Scholar]
- Carreau S, Hess RA. Oestrogens and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1517–1535. doi: 10.1098/rstb.2009.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YM, Lee NPY, Mruk DD, Lee WM, Cheng CY. Fer kinase/Fer T and adherens junction dynamics in the testis: An in vitro and in vivo study. Biol Reprod. 2003;69:656–672. doi: 10.1095/biolreprod.103.016881. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82:825–874. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. An intracellular trafficking pathway in the seminiferous epithelium regulating spermatogenesis: A biochemical and molecular perspective. Crit Rev Biochem Mol Biol. 2009;44:245–263. doi: 10.1080/10409230903061207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nature Rev Endocrinol. 2010;6:380–395. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. Regulation of spermiogenesis, spermiation and blood-testis barrier dynamics: Novel insights from studies on eps8 and arp3. Biochem J. 2011;435:553–562. doi: 10.1042/BJ20102121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD, Silvestrini B, Bonanomi M, Wong CH, Siu MKY, Lee NPY, Lui WY, Mo MY. AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: A review of recent data. Contraception. 2005;72:251–261. doi: 10.1016/j.contraception.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Wong EWP, Yan HHN, Mruk DD. Regulation of spermatogenesis in the microenvironment of the seminiferous epithelium: New insights and advances. Mol Cell Endocrinol. 2010;315:49–56. doi: 10.1016/j.mce.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Wong EWP, Lie PPY, Li MWM, Su L, Siu ER, Yan HHN, Mannu J, Mathur PP, Bonanomi M, Silvestrini B, Mruk DD. Environmental toxicants and male reproductive function. Spermatogenesis. 2011;1:2–13. doi: 10.4161/spmg.1.1.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kretser D, Kerr J. The cytology of the testis. In: Knobil E, Neill J, Ewing L, Greenwald G, Markert C, Pfaff D, editors. The Physiology of Reproduction. Vol. 1. Raven Press; New York: 1988. pp. 837–932. [Google Scholar]
- Dym M. Basement membrane regulation of sertoli cells. Endocr Rev. 1994;15:102–115. doi: 10.1210/edrv-15-1-102. [DOI] [PubMed] [Google Scholar]
- Elkin ND, Piner JA, Sharpe RM. Toxicant-induced leakage of germ cell-specific proteins from seminiferous tubules in the rat: Relationship to blood-testis barrier integrity and prospects for biomonitoring. Toxicol Sci. 2010;117:439–448. doi: 10.1093/toxsci/kfq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett DW. Intercellular bridges. Exp Cell Res. 1961;8:174–187. doi: 10.1016/0014-4827(61)90347-0. [DOI] [PubMed] [Google Scholar]
- Fawcett DW, Ito S, Slautterback D. The occurrence of intercellular bridges in groups of cells exhibiting synchronous differentiation. J Biophys Cytol. 1959;5:453–460. doi: 10.1083/jcb.5.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franca LR, Ogawa T, Avarbock MR, Brinster RL, Russell LD. Germ cell genotype controls cell cycle during spermatogenesis in the rat. Biol Reprod. 1998;59:1371–1377. doi: 10.1095/biolreprod59.6.1371. [DOI] [PubMed] [Google Scholar]
- Franca LR, Auharek SA, Hess RA, Dufour JM, Hinton BT. Morphofunctional and immunological aspects of the blood-testis and blood-epididymal barriers. In: Cheng CY, editor. Biology and regulation of blood-tissue barriers. Landes Bioscience and Springer Science + Business Media, LLC; Austin, TX: 2011. (in press) [PubMed] [Google Scholar]
- Green KJ, Simpson CL. Desmosomes: New perspectives on a classic. J Invest Dermatol. 2007;127:2499–2515. doi: 10.1038/sj.jid.5701015. [DOI] [PubMed] [Google Scholar]
- Green KJ, Getsios S, Troyanovsky S, Godsel LM. Intercellular junction assembly, dynamics, and homeostasis. Cold Spring Harb Perspect Biol. 2010;2:a000125. doi: 10.1101/cshperspect.a000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman J, Janmey P, Vogl A. Gelsolin - evidence for a role in turnover of junction-related actin filaments in Sertoli cells. J Cell Sci. 2002;115:499–505. doi: 10.1242/jcs.115.3.499. [DOI] [PubMed] [Google Scholar]
- Guttman J, Obinata T, Shima J, Griswold M, Vogl A. Non-muscle cofilin is a component of tubulobulbar complexes in the testis. Biol Reprod. 2004;70:805–812. doi: 10.1095/biolreprod.103.022723. [DOI] [PubMed] [Google Scholar]
- Hamer G, Roepers-Gajadien HL, Gademan IS, Kal HB, De Rooij DG. Intercellular bridges and apoptosis in clones of male germ cells. Int J Androl. 2003;26:348–353. doi: 10.1111/j.1365-2605.2003.00436.x. [DOI] [PubMed] [Google Scholar]
- Hew K, Ericson W, Welsh M. A single low cadmium dose causes failure of spermiation in the rat. Toxicol Appl Pharmacol. 1993;121:15–21. doi: 10.1006/taap.1993.1123. [DOI] [PubMed] [Google Scholar]
- Hew KW, Heath GL, Jiwa AH, Welsh MJ. Cadmium in vivo causes disruption of tight junction-associated microfilaments in rat sertoli cells. Biol Reprod. 1993;49:840–849. doi: 10.1095/biolreprod49.4.840. [DOI] [PubMed] [Google Scholar]
- Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization. Nature Rev Mol Cell Biol. 2008;9:846–859. doi: 10.1038/nrm2521. [DOI] [PubMed] [Google Scholar]
- Johnson L, Staub C, Neaves WB, Yanagimachi R. Live human germ cells in the context of their spermatogenic stages. Human Reprod. 2001;16:1575–1582. doi: 10.1093/humrep/16.8.1575. [DOI] [PubMed] [Google Scholar]
- Kelly BT, Owen DJ. Endocytic sorting of transmembrane protein cargo. Curr Opin Cell Biol. 2011;23:404–412. doi: 10.1016/j.ceb.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Lee NPY, Cheng CY. Regulation of Sertoli cell tight junction dynamics in the rat testis via the nitric oxide synthase/soluble guanylate cyclase/3′,5′-cyclic guanosine monophosphate/protein kinase G signaling pathway: An in vitro study. Endocrinology. 2003;144:3114–3129. doi: 10.1210/en.2002-0167. [DOI] [PubMed] [Google Scholar]
- Li MWM, Xiao X, Mruk DD, Lam YL, Lee WM, Lui WY, Bonanomi M, Silvestrini B, Cheng CY. Actin binding protein drebrin E is involved in junction dynamics during spermatogenesis. Spermatogenesis. 2011;1:123–136. doi: 10.4161/spmg.1.2.16393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PPY, Xia W, Wang CQF, Mruk DD, Yan HHN, Wong CH, Lee WM, Cheng CY. Dynamin II interacts with the cadherin- and occludin-based protein complexes at the blood-testis barrier in adult rat testes. J Endocrinol. 2006;191:571–586. doi: 10.1677/joe.1.06996. [DOI] [PubMed] [Google Scholar]
- Lie PPY, Mruk DD, Cheng CY. Dynamins, spermatogenesis and contraceptive development. Immun Endocr Metab Agents Med Chem. 2008;8:51–58. [Google Scholar]
- Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009;23:2555–2567. doi: 10.1096/fj.06-070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PPY, Mruk DD, Lee WM, Cheng CY. Cytoskeletal dynamics and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010a;365:1581–1592. doi: 10.1098/rstb.2009.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci USA. 2010b;107:11411–11416. doi: 10.1073/pnas.1001823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PPY, Cheng CY, Mruk DD. The biology of the desmosome-like junction: A versatile anchoring junction and signal transducer in the seminiferous epithelium. Int Rev Cell Mol Biol. 2011;286:223–269. doi: 10.1016/B978-0-12-385859-7.00005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui WY, Lee WM, Cheng CY. Transforming growth factor-β3 perturbs the inter-Sertoli tight junction permeability barrier in vitro possibly mediated via its effects on occludin, zonula occludens-1, and claudin-11. Endocrinology. 2001;142:1865–1877. doi: 10.1210/endo.142.5.8116. [DOI] [PubMed] [Google Scholar]
- Lui WY, Lee WM, Cheng CY. Transforming growth factor-β3 regulates the dynamics of sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol Reprod. 2003;68:1597–1612. doi: 10.1095/biolreprod.102.011387. [DOI] [PubMed] [Google Scholar]
- Moffit JS, Bryant BH, Hall SJ, Boekelheide K. Dose-dependent effects of Sertoli cell toxicants 2,5-hexanedione, carbendazim, and mono-(2-ethylhexyl) phthalate in adult rat testis. Toxicol Pathol. 2007;35:719–727. doi: 10.1080/01926230701481931. [DOI] [PubMed] [Google Scholar]
- Mok KW, Mruk DD, Lie PPY, Lui WY, Cheng CY. Adjudin, a potential male contraceptive, exerts its effects locally in the seminifeorus epithelium of mammalian testes. Reproduction. 2011;141:571–580. doi: 10.1530/REP-10-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. The myotubularin family of lipid phosphatases in disease and in spermatogenesis. Biochem J. 2011a;433:253–262. doi: 10.1042/BJ20101267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Desmosomes in the testis. Moving into an unchartered territory. Spermatogenesis. 2011b;1:47–51. doi: 10.4161/spmg.1.1.15443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: Role in contraceptive development. Pharmacol Rev. 2008;60:146–180. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neaves WB, Johnson L, Porter JC, Parker CRJ, Petty CS. Leydig cell numbers, daily sperm production, and serum gonadotropin levels in aging men. J Clin Endocrinol Metabol. 1984;59:756–763. doi: 10.1210/jcem-59-4-756. [DOI] [PubMed] [Google Scholar]
- O’Donnell L, Robertson KM, Jones ME, Simpson ER. Estrogen and spermatogenesis. Endocr Rev. 2001;22:289–318. doi: 10.1210/edrv.22.3.0431. [DOI] [PubMed] [Google Scholar]
- O’Donnell L, Nicholls PK, O’Bryan MK, McLachlan RI, Stanton PG. Spermiation: The process of sperm release. Spermatogenesis. 2011;1:14–35. doi: 10.4161/spmg.1.1.14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth JM. Proliferation of sertoli cells in fetal and postnatal rats: A quantitative autoradiographic study. Anat Rec. 1982;203:485–492. doi: 10.1002/ar.1092030408. [DOI] [PubMed] [Google Scholar]
- Pellegrin S, Mellor H. Actin stress fibers. J Cell Sci. 2007;120:3491–3499. doi: 10.1242/jcs.018473. [DOI] [PubMed] [Google Scholar]
- Prozialeck W. Evidence that E-cadherin may be a target for cadmium toxicity in epithelial cells. Toxicol Appl Pharmacol. 2000;164:231–249. doi: 10.1006/taap.2000.8905. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Lamar PC. Interaction of cadmium (Cd2+) with a 13-residue polypeptide analog of a putative calcium-binding motif of E-cadherin. Biochem Biophys Acta. 1999;1451:93–100. doi: 10.1016/s0167-4889(99)00077-4. [DOI] [PubMed] [Google Scholar]
- Russell LD. Further observations on tubulobulbar complexes formed by late spermatids and Sertoli cells in the rat testis. Anat Rec. 1979a;194:213–232. doi: 10.1002/ar.1091940204. [DOI] [PubMed] [Google Scholar]
- Russell LD. Spermatid-Sertoli tubulobulbar complexes as devices for elimination of cytoplasm from the head region in late spermatids of the rat. Anat Rec. 1979b;194:233–246. doi: 10.1002/ar.1091940205. [DOI] [PubMed] [Google Scholar]
- Setchell BP. Blood-testis barrier, junctional and transport proteins and spermatogenesis. In: Cheng CY, editor. Molecular mechanisms in spermatogenesis. Landes Bioscience/Springer Science+Business Media, LLC; Austin, TX: 2008. pp. 212–233. [DOI] [PubMed] [Google Scholar]
- Setchell BP, Waites GMH. Changes in the permeability of the testicular capillaries and of the “blood-testis barrier” after injection of cadmium chloride in the rat. J Endocrinol. 1970;47:81–86. doi: 10.1677/joe.0.0470081. [DOI] [PubMed] [Google Scholar]
- Sharpe RM. Regulation of spermatogenesis. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven press; New York: 1994. pp. 1363–1434. [Google Scholar]
- Siu MKY, Cheng CY. Dynamic cross-talk between cells and the extracellular matrix in the testis. BioEssays. 2004;26:978–992. doi: 10.1002/bies.20099. [DOI] [PubMed] [Google Scholar]
- Siu MKY, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem. 2005;280:25029–25047. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- Stofikitis N, Giotitsas N, Tsounapi P, Baltogiannis D, Giannakis D, Pardalidis N. Hormonal regulation of spermatogenesis and spermiogenesis. J Steroid Biochem Mol Biol. 2008;109:323–330. doi: 10.1016/j.jsbmb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Su L, Mruk DD, Lee WM, Cheng CY. Differential effects of testosterone and TGF-β3 on endocytic vesicle-mediated protein trafficking events at the blood-testis barrier. Exp Cell Res. 2010;316:2945–2960. doi: 10.1016/j.yexcr.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason HA, Scothern A, McHarg S, Garrod DR. Desmosomes: Adhesive strength and signalling in health and disease. Biochem J. 2010;429:419–433. doi: 10.1042/BJ20100567. [DOI] [PubMed] [Google Scholar]
- Vaid KS, Guttman JA, Babyak N, Deng W, McNiven MA, Mochizuki N, Finlay BB, Vogl AW. The role of dynamin 3 in the testis. J Cell Physiol. 2007;210:644–654. doi: 10.1002/jcp.20855. [DOI] [PubMed] [Google Scholar]
- Verhoeven G, Willems A, Denolet E, Swinnen JV, De Gendt K. Androgens and spermatogenesis: Lessons from transgenic mouse models. Phil Trans R Soc Lond B Biol Sci. 2010;365:1537–1556. doi: 10.1098/rstb.2009.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogl A, Vaid K, Guttman J. The sertoli cell cytoskeleton. In: Cheng CY, editor. Molecular mechanisms in spermatogenesis. Landes Bioscience/Springer Science+Business Media, LLC; Austin, TX: 2008. pp. 186–211. [Google Scholar]
- Weber JE, Russell LD, Wong V, Peterson RN. Three dimensional reconstruction of a rat stage V Sertoli cell: Ii. Morphometry of Sertoli-Sertoli and Sertoli-germ cell relationships. Am J Anat. 1983;167:163–179. doi: 10.1002/aja.1001670203. [DOI] [PubMed] [Google Scholar]
- Welling PA, Weisz OA. Sorting it out in endosomes: An emerging concept in renal epithelial cell transport regulation. Physiology. 2010;25:280–292. doi: 10.1152/physiol.00022.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolski KM, Perrault C, Tran-Son-Tay R, Cameron DF. Strength measurement of the Sertoli-spermatid junctional complex. J Androl. 2005;26:354–359. doi: 10.2164/jandrol.04142. [DOI] [PubMed] [Google Scholar]
- Wong CH, Cheng CY. The blood-testis barrier: Its biology, regulation and physiological role in spermatogenesis. Curr Topics Dev Biol. 2005;71:263–296. doi: 10.1016/S0070-2153(05)71008-5. [DOI] [PubMed] [Google Scholar]
- Wong EWP, Cheng CY. Polarity proteins and cell-cell interactions in the testis. Int Rev Cell Mol Biol. 2009;278:309–353. doi: 10.1016/S1937-6448(09)78007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CH, Mruk DD, Lui WY, Cheng CY. Regulation of blood-testis barrier dynamics: An in vivo study. J Cell Sci. 2004;117:783–798. doi: 10.1242/jcs.00900. [DOI] [PubMed] [Google Scholar]
- Wong CH, Mruk DD, Siu MKY, Cheng CY. Blood-testis barrier dynamics are regulated by α2-macroglobulin via the c-jun N-terminal protein kinase pathway. Endocrinology. 2005;146:1893–1908. doi: 10.1210/en.2004-1464. [DOI] [PubMed] [Google Scholar]
- Wong EWP, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochem Biophys Acta. 2008a;1778:692–708. doi: 10.1016/j.bbamem.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EWP, Mruk DD, Lee WM, Cheng CY. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008b;105:9657–9662. doi: 10.1073/pnas.0801527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EWP, Sun S, Li MWM, Lee WM, Cheng CY. 14-3-3 protein regulates cell adhesion in the seminiferous epithelium of rat testes. Endocrinology. 2009;150:4713–4723. doi: 10.1210/en.2009-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EWP, Mruk DD, Lee WM, Cheng CY. Regulation of blood-testis barrier dynamics by TGF-β3 is a Cdc42-dependent protein trafficking event. Proc Natl Acad Sci USA. 2010a;107:11399–11404. doi: 10.1073/pnas.1001077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EWP, Yan HHN, Li MWM, Lie PPY, Mruk DD, Cheng CY. Cell junctions in the testis as targets for toxicants. In: McQueen CA, Hoyer PB, Richburg JH, editors. Comprehensive toxicology. 2. Vol. 11. Oxford: Academic press, Elseiver; 2010b. pp. 167–188. Reproductive and endocrine toxicology. [Google Scholar]
- Xia W, Wong EWP, Mruk DD, Cheng CY. TGF-β3 and TNFα perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: A new concept of BTB regulation during spermatogenesis. Dev Biol. 2009;327:48–61. doi: 10.1016/j.ydbio.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HHN, Mruk DD, Lee WM, Cheng CY. Ectoplasmic specialization: A friend or a foe of spermatogenesis? BioEssays. 2007;29:36–48. doi: 10.1002/bies.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HHN, Mruk DD, Lee WM, Cheng CY. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008;22:1945–1959. doi: 10.1096/fj.06-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazama F, Sawada H, Hirosawa K, Hayashi Y, Nishida T. Deep-etch visualization of the Sertoli cell (blood-testis) barrier in the boar. Tissue Cell. 1991;23:235–246. doi: 10.1016/0040-8166(91)90078-8. [DOI] [PubMed] [Google Scholar]
- Young JS, Guttman JA, Vaid KS, Vogl AW. Tubulobulbar complexes are intercellular podosome-like structures that internalize intact intercellular junctions during epithelial remodeling events in the rat testis. Biol Reprod. 2009a;80:162–174. doi: 10.1095/biolreprod.108.070623. [DOI] [PubMed] [Google Scholar]
- Young JS, Guttman JA, Vaid KS, Vogl AW. Cortactin (CTTN), n-WASP (WASL), and clathrin (CLTC) are present at podosome-like tubulobulbar complexes in the rat testis. Biol Reprod. 2009b;80:153–161. doi: 10.1095/biolreprod.108.070615. [DOI] [PubMed] [Google Scholar]
- Zhang J, Mruk DD, Cheng CY. Myotubularin phosphoinositide phosphatases, protein phosphatases, and kinases: Their roles in junction dynamics and spermatogenesis. J Cell Physiol. 2005;204:470–483. doi: 10.1002/jcp.20303. [DOI] [PubMed] [Google Scholar]