Abstract
This study involves a re-analysis of spoken vocabulary outcomes of children with intellectual disabilities who were randomly assigned to receive Milieu Communication Teaching (MCT) at low (one 1-hour session per week) or high (five 1-hour sessions per week) dose frequency over nine months (Fey, Yoder, Warren, & Bredin-Oja, in press). Non-Down syndrome (NDS) and Down syndrome (DS) subgroups were matched on intelligence, mental age, and chronological age. A growth model including intercept, slope, and quadratic revealed that children in the NDS group had significantly more growth in spoken vocabulary than children in the DS group independent of dose frequency manipulations. Subsequent etiological subgroup analyses demonstrated that in the DS subgroup, children receiving MCT at the higher dose frequency had more spoken vocabulary growth than children receiving MCT at the lower dose frequency. Subgroup analyses also supported our previous findings that high dose frequency of MCT yielded greater vocabulary production outcomes than low dose frequency for children who played functionally with a range of objects, regardless of etiology.
Keywords: Down syndrome, intellectual disability, language, vocabulary, intervention, intensity, dose frequency
There is increasing interest in the role of etiology in spoken language as clinicians and researchers attempt to explain the variability in response to intervention across all children with intellectual disabilities (ID). Understanding how etiology impacts treatment response will ultimately allow us to personalize treatment planning to optimize spoken language outcomes for this heterogeneous population (Hart, 1996; Rondal & Edwards, 1997). Prior work has confirmed that Down syndrome (DS) etiology accounts for a significant amount of the variability in spoken language ability of young children with ID (e.g., Warren et al., 2008; Yoder & Warren, 2004). It also makes it possible to predict individual differences in response to early communication intervention (Yoder & Warren, 2002). Therefore, we anticipated that DS etiology may also be associated with a differential response to dose frequency manipulations in our recent randomized controlled trial (RCT) (Fey, Yoder, Warren, & Bredin-Oja, 2013) on the effects of an early communication intervention, Milieu Communication Teaching (MCT; Fey et al., 2006; Warren, et al., 2008; Yoder & Warren, 1999a, 1999b, 2002). In this study, MCT was delivered at one hour per week (low dose frequency) versus five hours per week (high dose frequency) in children with ID.
In a previous report, we observed that dose frequency effects varied according to child characteristics (Fey et al., 2013). Specifically, children who played in functional ways with many different objects at the onset of intervention made greater gains in spoken vocabulary if they received the high dose frequency than the low dose frequency MCT. On the other hand, we did not detect either a main effect of dose frequency or a differential effect of dose frequency on spoken vocabulary as a function of presence or absence of Down syndrome (DS). In the present study, we re-examine the data set from the Fey et al. (2013) report to evaluate whether our failure to find the aforementioned effects may be related to the analytical approach employed in the prior work.
Potential for Factors to Explain Variability in Response to Intervention
Possibility of an effect of dose frequency on outcomes
Clinicians, educators, and parents often presume that more intervention is better. One might expect more sessions per week (i.e., higher dose frequency) to result in greater gains than fewer sessions per week due to an increase in teaching and learning opportunities. However, inconsistency in findings related to dose frequency manipulations across the extant literature (Al Otaiba, Schatschneider, & Silverman, 2005; Barratt, Littlejohns, & Thompson, 1992; Denton et al., 2011; McGinty, Breit-Smith, Fan, Justice, & Kaderavek, 2011; Ukrainetz, Ross, & Harm, 2009) suggests that more treatment may not always be better for all children(Yoder, Fey, & Warren, in press). If increased dose frequency does not have a consistent effect across all children, then it is quite possible that the effect of dose frequency differs according to child variables (Fey et al., 2013). For children with ID, one such variable is the presence or absence of DS as the etiology of ID.
Probability of effects related to DS etiology
We suspected that diagnosis of DS may moderate the effects of dose frequency manipulations on spoken vocabulary outcomes for a number of reasons. First, children with DS display a distinctive profile, wherein spoken language delays are excessive relative to severity of ID (see Abbeduto, Warren, & Conners, 2007; Martin, Klusek, Estigarribia, & Roberts, 2009 for recent reviews). This is most clearly the case for some domains of spoken language, such as expressive syntax and morphology (Chapman, Seung, Schwartz, & Kay-Raining Bird, 1998; Eadie, Fey, Douglas, & Parsons, 2002; Vicari, Caselli, & Tonucci, 2000). However, several studies indicate that inordinate deficits in spoken language also extend to expressive vocabulary skills of children with DS (Cardoso-Martins, Mervis, & Mervis, 1985; Caselli, Monaco, Trasciani, & Vicari, 2008; Miller, 1992, 1999; Warren, et al., 2008).
Though a few studies have failed to detect a dissociation between vocabulary production and nonverbal cognitive ability in DS (Caselli et al., 1998; Galeote, Soto, Checa, Gomez, & Lamela, 2008; Vicari, et al., 2000), the majority of reports suggest a pattern of slow early lexical development followed by later spoken vocabulary deficits that are disproportionate in comparison to degree of global cognitive impairment in children with DS (Cardoso-Martins, et al., 1985; Caselli, et al., 2008; Miller, 1992, 1999; Warren, et al., 2008). One recent study confirmed that young children with DS display slower growth in expressive vocabulary in comparison with children with ID of non-DS etiology matched on mental age (MA) (Warren, et al., 2008). Another investigation found lower levels of spoken vocabulary in toddlers with DS relative to children with ID not due to DS, even after controlling for chronological age (CA), MA, and IQ (Yoder & Warren, 2004). Unfortunately, inordinate deficits in spoken language may persist despite children with DS receiving early intervention services (Brady, Bredin-Oja, & Warren, 2008). Thus, we suspected that presence of DS may impact spoken vocabulary growth and outcomes in our present sample of young children with ID.
We also suspected that dose frequency of MCT could differentially affect our DS and non-Down syndrome ID (NDS) subgroups. In part, this was because communication outcomes varied according to the presence or absence of DS in a previous RCT of an earlier version of MCT – Responsivity Education and Prelinguistic Milieu Teaching (Yoder & Warren, 2002). However, neither logic nor the extant literature provided sufficient information to predict which subgroup would derive greater benefit from increased dose frequency. On one hand, the NDS subgroup may be expected to benefit to a greater degree from more treatment. Our prior work suggests that the spoken language skills of children with NDS may be more readily (and favorably) influenced by MCT (Yoder & Warren, 2002). Additionally, non-linguistic characteristics of children with DS may limit their response to treatments, such as MCT (Fidler, 2005). For example, some studies have suggested a tendency for task resistance in children with DS (Fidler, Philofsky, Hepburn, & Rogers, 2005; Ruskin, Mundy, Kasari, & Sigman, 1994; J. Wishart, 1993). If children with DS are indeed predisposed to avoid difficult tasks, more frequent treatment sessions that include prompts for production of deficient skills may be more likely to promote acts of non-compliance, protest, or withdrawal in the DS group than in the NDS group. On the other hand, given their special difficulties with spoken language, children with DS may simply require more treatment and thus benefit from high dose frequency to a greater extent than their NDS peers. Thus, one could hypothesize that dose frequency effects may produce differential effects in favor of either etiological subgroup.
Rationale for Re-analysis of Dose Frequency Effects on Spoken Vocabulary
We have subsequently considered whether our failure to find effects related to dose frequency and DS etiology in our most recent study (Fey et al., 2013) may be related to our previous statistical plan. The Fey et al. (2013) study used a highly interpretable but simple linear growth model that quantified only outcomes and mean rate of spoken vocabulary growth across the treatment period. This linear model was fit across a group of children with DS and a NDS group; that is, any effects of dose frequency were examined only in the sample that pools across etiological subgroups.
Consideration of a complex growth model
It is possible that more complex growth models may better fit the spoken vocabulary growth of at least some children with ID, thus boosting our potential to detect effects related to DS etiology and treatment dose frequency. For example, a growth model that includes a quadratic parameter quantifies acceleration and deceleration in growth rate over time. The typical developmental trajectory is characterized by an initial period of slow and seemingly stable acquisition of first words at ages of approximately 16 – 20 months, followed by an acceleration in growth rate that has been characterized as the “vocabulary burst” (Harris, Yeeles, Chasin, & Oakley, 1995; Mervis & Bertrand, 1995). This burst leads to approximately 300 words by 24 months among TD children. At least some children with ID follow the same growth pattern, albeit with a later onset and less steep acceleration (Miller, 1999).
Increasing the frequency of sessions to create a more intensive treatment regimen may provide greater consistency and repetition to speed up the learning process in children with ID. Increased frequency of treatment sessions (i.e., dose frequency) directly translates to more regular interactions with a communication partner who has expertise in scaffolding spoken language development. More frequent treatment may thus induce an acceleration in learning rate over time, at least for some children (Warren, Fey, & Yoder, 2007). Alternatively, it is conceivable that a quadratic term may reveal an atypical deceleration in early lexical acquisition in children with ID. Therefore, several plausible scenarios support consideration of a quadratic term in modeling spoken language growth of young children with ID.
Potential necessity of etiological subgroup analyses
It is also possible that lack of a dose frequency effect within one etiological subgroup masked a significant effect present in another subgroup when growth was modeled across etiological subgroups in our previous report (Fey et al., in press). Evaluating spoken language growth of participants with ID within a model inclusive of both DS and NDS subgroups may influence the selection of the best-fitting growth model and the ability to detect main effects of dose frequency within etiological subgroups. For example, significant celeration (i.e., acceleration or deceleration) limited to one etiological subgroup could unduly influence the decision to include a quadratic term in the across-etiology growth model. This, in turn, would affect the reliability of the outcome estimates and could result in loss of power to detect subsequent effects. Additionally, lack of a dose frequency effect within one etiological subgroup may mask a significant effect of treatment dose frequency present in another subgroup if the effect of dose frequency is investigated only in the sample that pools across etiological subgroups.
Summary and Research Questions
This report is a follow-up to Fey et al (2013); it is focused on the effects of dose frequency on spoken vocabulary growth of children with and without DS. In this new analysis, we considered more complex growth curves than were used in the original report; specifically, we evaluated models that included a quadratic parameter reflecting possible deceleration or acceleration in the rate of growth over time. We also conducted within-etiological subgroup analyses, when warranted, to determine whether effects of dose frequency were present in both etiological groups or limited to one etiological subgroup. We paid special attention to any differences in findings between the present work and our original report (Fey et al., 2013) that might require us to re-evaluate our conclusion from that study that the effects of treatment dose frequency in young children with ID were moderated by children's interest in playing with a range of objects. Specific research questions included: (a) Is a growth model that quantifies celeration a better fit to spoken vocabulary growth of children with ID than a growth model that quantifies growth as a straight line?; (b) Do children with DS grow more slowly in spoken vocabulary than children with mixed etiology ID when CA, MA, and global intellectual ability are matched or controlled?; (c) Using the best-fitting growth model, does the dose frequency effect on spoken vocabulary vary depending on whether the child has DS?; (d) Using the best-fitting growth model and controlling for effects of global intellectual ability, is there more favorable growth in spoken vocabulary when MCT is administered in a high dose frequency than when it is administered in a low dose frequency?
Method
Overview of Study Design
Young children with ID with and without DS were randomly assigned to receive MCT at either one 1-hour session per week (low dose frequency, LDF) or five 1-hour sessions per week (high dose frequency, HDF) over nine months. Parents of all participants also received nine sessions of Responsivity Education over the first three months of MCT intervention, regardless of their child's assigned treatment dose frequency. Measures of spoken language use were administered at entry to the study (Time 1), following three months of treatment (Time 2), following six months of treatment (Time 3), and following nine months of treatment (Time 4). The methods indicated below are detailed in the Fey et al. (2013) report. Essential elements are repeated here for the reader's convenience.
Participants
Inclusion criteria for children were: (a) chronological age between 18 and 27 months; (b) expressive vocabulary less than or equal to 20 signed or spoken words excluding animal sounds, caregiver names, and routinized games and activities as reported by a primary caregiver on the MacArthur-Bates Communicative Development Inventories: Words and Gestures vocabulary checklist (MB-CDI; Fenson et al., 2003); (c) Mental Development Index between 55 and 75 on the Bayley Scales of Infant and Toddler Development: 3rd Edition (Bayley-III); (d) score less than or equal to 2.75 on the Screening Tool for Autism in 2-year olds (STAT) confirming low-risk for autism; (e) normal hearing in at least one ear confirmed by sound field hearing screening; (f) normal or corrected to normal vision; (g) sufficient motor skill to sit unsupported and interact with an interventionist; and (h) primarily English-speaking household.
A total of 76 children meeting inclusion criteria were identified and enrolled at Vanderbilt University and the University of Kansas. A computer program using a random number generator was utilized to assign children to either LDF or HDF MCT intervention. Twelve participants (six each from LDF and HDF groups) were excluded from analyses due to failure to attend at least two-thirds of intervention sessions or early withdrawal from the study. A per protocol approach was employed in this study because inclusion of all participants who were randomized, regardless of compliance with treatment regimen, could have compromised detection of dose frequency effects. Thus, the final sample was comprised of 31 children assigned to LDF intervention and 33 children assigned to HDF intervention. Of the 31 participants completing the LDF MCT protocol, 16 were diagnosed with DS, and 15 did not have DS. Of the 33 children who completed the HDF MCT protocol, 19 children were diagnosed with DS, and 14 children were not diagnosed with DS. Diagnoses of children with ID not due to DS included: No Diagnosis (16), Premature birth (4), Seizure Disorder (1), Cerebral Palsy (1), Beckwith-Wiedemann syndrome (1), Shwachman-Diamond Syndrome (1), Dandy-Walker syndrome (1), Septo-Optic Dysplasia (de Morsier's Syndrome (1), Chromosome 8 Abnormality (1), Cornelia de Lange Syndrome (1), and mitochondrial disorder (1).
Pre-treatment group comparisons
The LDF and HDF groups did not significantly differ in proportion of children with DS (p = .63). As indicated in Table 1, the dose frequency groups and diagnostic groups were non-significantly different on several other important variables, including expressive spoken vocabulary. Group comparisons on 34 additional variables of interest confirmed that randomization successfully produced LDF and HDF groups with highly similar characteristics at entrance to the study (p > .08), d < |.45| (Fey et al., 2013).
Table 1.
Pre-experimental Characteristics by Dose Frequency Groups and Diagnoses.
| Pre-treatment characteristic | Group | DS M(SD) | NDS M(SD) | d for dose frequency | d for diagnosis |
|---|---|---|---|---|---|
| Chronological age in months | LDF | 22.50(3.09) | 23.54(3.50) | ||
| HDF | 21.62(2.61) | 22.10(2.92) | |||
| Total | 22.02(2.83) | 22.85(3.26) | .39 | -.27 | |
|
| |||||
| Bayley III mental age in months | LDF | 12.69(1.66) | 12.20(2.54) | ||
| HDF | 12.05(2.09) | 13.36(1.86) | |||
| Total | 12.34(1.91) | 12.76(2.28) | -.08 | -.20 | |
|
| |||||
| Bayley III CC | LDF | 65.94(6.38) | 63.33(6.73) | ||
| HDF | 63.95(7.18) | 68.93(5.25) | |||
| Total | 64.86(6.80) | 66.03(6.60) | -.21 | -.17 | |
|
| |||||
| Number of words spoken on MB-CDI | LDF | .94(1.34) | 2.73(6.91) | ||
| HDF | 1.37(1.61) | .86(1.03) | |||
| Total | 1.17(1.48) | 1.83(5.03) | .19 | -.19 | |
LDF = low dose frequency. HDF = high dose frequency. DS = Down syndrome. NDS = Non-Down syndrome intellectual disability. CC = Cognitive Composite standard score.
Participation in non-project treatment
Parent reports indicated that most children enrolled in the project did participate in early intervention programs that involved speech-language pathology services over the course of the study; however, LDF and HDF groups did not differ in the mean number of hours spent in non-experimental interventions (LDF M = 2.7 hours/month, SD = 2.4; HDF M = 3.2 hours/month, SD = 2.4), t(62) = 1.5, p > .13.
Treatment
All children received MCT comprising Prelinguistic Milieu Teaching (Yoder & Warren, 1998), Milieu Language Teaching (Hancock & Kaiser, 2006), and Responsivity Education (Yoder & Warren, 2002). A detailed description of MCT is provided in Fey et al. (2013) and Warren et al. (2008). Research staff provided MCT in homes or child care centers according to parent preference. Interventionists were para-professionals who were supervised by licensed and certified speech-language pathologists. Details of interventionist training have been described in Fey et al. (2013).
Fidelity of dose, dose frequency, and treatment duration
Thirty minutes of one 60-minute treatment session per month were coded from media files by the supervisor at the site opposite the clinician whose session was being evaluated. Treatment fidelity was estimated on a random sample of 5% of HDF and 23% of LDF treatment sessions. Absolute agreement intra-class correlation coefficients ranged from .91 to .98 for the number of correctly implemented teaching episodes. The average rate of correct teaching episodes per min (M = 1.10, SD = .30) did not differ across groups, F(1, 60) = 1.69, p >.20. Children in the LDF (M = 35.67 SD = 2.31) group experienced, on average, 4.19 times less cumulative teaching episodes than the HDF (M = 149.3, SD = 12.17) group, F(1, 60) = 2609.23, p < .001. Finally, intervention lasted almost exactly 9 months for both groups (M = 8.94 months, SD = .10; M = 8.98 months, SD = 0.10, for LDF and HDF groups respectively). Thus, the clinicians at both sites successfully delivered the intervention at the planned dose, dose frequency, and intervention duration.
Measures
Dependent variable
Expressive spoken vocabulary was measured via the MacArthur-Bates Communicative Development Inventories: Words and Gestures (MB-CDI; Fenson, et al., 2003) vocabulary checklist. Parents were asked to indicate whether their child either “understands” or “understands and says” several hundred early lexical items in semantic categories such as action words, household items, and animal names by checking the appropriate column on the checklist. The dependent variable for the present study, number of words said, included the raw number of words that parents indicated their child “understands and says” with a few specified exceptions. Our principle long-term goal was to facilitate children's use of words to promote referential communication. As such, we were less concerned with children's use of “social” words or rote-learned “formulas.” Thus, to ensure that we were measuring gains in referential language, sound effects, animal sounds (e.g., “woof woof” or “moo”), caregiver names (e.g., “mommy” and “nana”), and items associated with routinized games and activities (e.g., “thank you”, “hi”) from the MB-CDI were not counted in our spoken vocabulary measure.
Intellectual ability
At Time 1, the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley III; Bayley, 2006) Cognitive Composite (CC) standard score was used to quantify the degree to which cognitive ability was delayed relative to chronological age expectations. The Bayley III is an individually administered, comprehensive developmental assessment comprising three scales assessed via direct child-examiner interaction (cognitive, motor, language), as well as two scales evaluated via caregiver report (social-emotional, adaptive behavior). This measure is among the most widely used instruments for evaluating intellectual abilities of infants and toddlers (Anderson, De Luca, Hutchinson, Roberts, & Doyle, 2010). Although there have been reports that the CC underestimates the intelligence of children under a certain cut point (i.e., 70; Anderson, et al., 2010), the relative degree of developmental delay within the current study sample should not be affected by the Bayley III's absolute underestimation of developmental delay.
Object interest
At Time 1, an adapted version of the Developmental Play Assessment (DPA; Lifter, 2000) was administered to obtain pre-treatment levels of children's functional and symbolic play skills. In this procedure, an examiner presented three different standard sets of toys for a period of 5 minutes each. The examiner served as a responsive play partner by commenting on participants' actions, imitating participants' play behaviors, and responding to requests. They provided little or no scaffolding of play skills, however. For example, they may have presented items to the child one at a time, if necessary, but they did not suggest, prompt, or model novel play acts. Object Interest was operationally defined as the number of different toys with which the participant demonstrated functional or symbolic play over the course of the 15-minute session. This variable was coded with the Playcoder software program (Tapp & Yoder, 2003). Each session was coded by a primary coder who was blind to participants' group assignment as well as the sessions that would be coded for reliability. Approximately 20% of the sessions were randomly selected for independent coding by a secondary observer who was also blind to group assignment. The intraclass correlation coefficient obtained for Time 1 Object Interest was .97.
Statistical Analysis Plan
Mixed level modeling was conducted with the Hierarchical Linear Modeling (HLM) software program (Raudenbush & Bryk, 2002) to model spoken vocabulary growth across the treatment period. Dependent variables were base 10 logarithmic (log10) transformed due to detected violations in homoscedasticity. Time in Study was centered at Time 4 so that the intercept represented outcome at the post-treatment period.
At Level 1, spoken language growth and outcome of each participant were modeled over the four assessment periods with times nested within person. Modeling was conducted systematically by progressing from the most parsimonious to the most complex Level 1 model. The following sequence was tested: (a) fixed-intercept, fixed-slope model; (b) random-intercept, fixed-slope model; (c) random-intercept, random-slope model; (d) random-intercept, random-slope, fixed-quadratic model; and (e) random-intercept, random-slope, random-quadratic model. At each step, a more complex model was only accepted if the model comparison test (i.e., difference chi square) indicated a significant improvement in fit to the data over the more parsimonious model. Formal tests for homogeneity of Level 1 variance and monitoring of covariance between factors were consistently conducted throughout model building to detect possible violations of statistical assumptions (i.e., heteroscedasticity or multicollinearity).
At Level 2, predictors were added in step-wise fashion to examine whether Bayley III CC, diagnosis (presence or absence of DS), or treatment group (LDF or HDF) accounted for a significant amount of the variance in trajectories. Finally, the product term (diagnosis × treatment group) was added at Level 2 to determine if presence of DS moderated the effects of MCT treatment dose frequency on spoken language growth.
Modeling across and within etiological subgroups
Statistical analysis was first conducted with both DS and NDS subgroups included in the growth model. Exploratory modeling was subsequently conducted separately for DS and NDS subgroups if preliminary modeling in the across-etiology model inclusive of both DS and NDS subgroups indicated either (a) a DS etiology effect on a parameter of the growth model (i.e., intercept, slope, etc.), or (b) a statistical interaction between DS etiology and dose frequency. The presence of either type of effect indicates that growth over time and/or growth related to dose frequency assignment differ according to etiology. In these instances, subgroup analyses may allow us to best evaluate the fit of growth models and the differential effects of dose frequency for each etiological subgroup.
For each subgroup analysis, Level 1 modeling was conducted according to the steps previously delineated in the statistical plan. At Level 2, predictors were added in step-wise fashion to examine whether Bayley III CC or dose frequency group (HDF or LDF) accounted for a significant amount of the variance in growth trajectories.
Results
Across-Etiology Level 1 Growth Model
When growth was modeled across etiological subgroups of children with DS and NDS, the model that quantified celeration was a better fit to the spoken vocabulary growth of children with ID than a simple growth model that quantified growth as a straight line. Significant fixed effects of slope and intercept in the simple linear model were obtained (ps < .005), confirming that the mean outcome and mean rate of growth across the treatment period were significantly different from zero. Subsequent tests of the random effects of all three parameters in the more complex model including the quadratic term revealed significant variation (p values < .05). Thus, a Level 1 model including a random-intercept, random-slope, and random-quadratic best fit the data on spoken vocabulary growth across etiological subgroups (Table 2). Substantively, this means that we can account for more variance in the number of words that children with ID produced after 9 months of treatment if we add a random quadratic term (i.e., consider individual variability in the celeration in growth over the treatment period) to our simple model that included only random intercept and slope terms (i.e., considered only individual variability in outcomes and mean rate of growth across the treatment period).
Table 2.
Systematic comparison of level 1 models of spoken vocabulary growth.
| Analysis Type | Model 1 | Model 2 | Deviance statistic | p |
|---|---|---|---|---|
| Across | RIFS | RIRS | χ2(2) = 33.25 | <.001 |
| Etiological | RIRS | RIRSFQ | χ2(1) = 15.52 | <.001 |
| Subgroups | RIRSFQ | RIRSRQ | χ2(3) = 30.32 | <.001 |
|
| ||||
| Within | RIFS | RIRS | χ2(2) = 34.41 | <.001 |
| DS | RIRS | RIRSFQ | χ2(1) = 2.00 | 1.53 |
| Subgroup | RIRS | RIRSRQ | χ2(3) = 7.54 | 1.09 |
|
| ||||
| Within | RIFS | RIRS | χ2(2) = 31.37 | <.001 |
| NDS | RIRS | RIRSFQ | χ2(1) = 1.31 | .251 |
| Subgroup | RIRS | RIRSRQ | χ2(4) = 15.44 | .004 |
Abbreviations reference model type including: (a) random-intercept, fixed-slope model (RIFS); (b) random-intercept, random-slope model (RIRS); (c) random-intercept, random-slope, fixed-quadratic model (RIRSFQ); and (d) random-intercept, random-slope, random-quadratic model (RIRSRQ). NDS = non-Down syndrome (mixed-etiology ID) subgroup, DS= Down syndrome subgroup.
Across-Etiology Level 2 Model
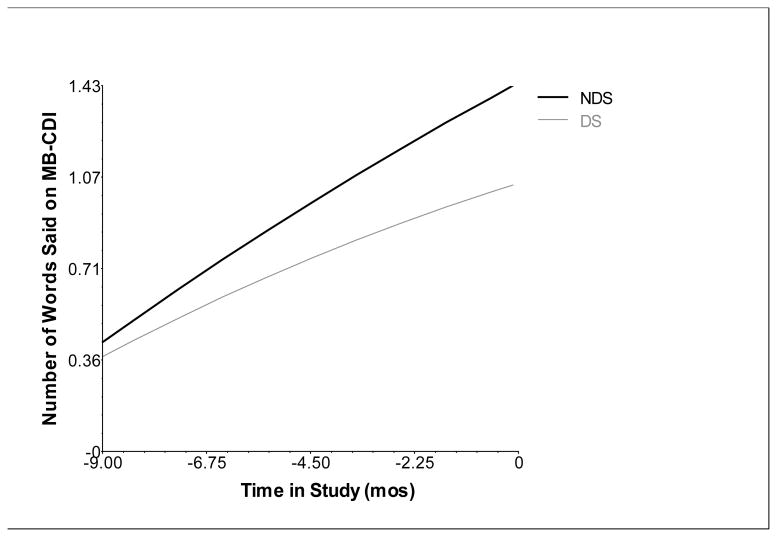
At Level 2, we added predictors to our final across-etiology growth model, which contained random intercept, slope, and quadratic parameters. Bayley III CC did not account for a significant amount of variance in the rate of growth in words spoken at Time 4, t(62) = 1.052, p = .297 or celeration in growth across the treatment period, t(62) = -.973, p = .334. However, the CC did account for significant variance in the number of words said at the post-treatment period, t(62) = 4.158, p < .001, and was thus retained as a covariate in the across-etiology, Level 2 model. Diagnosis of DS also did not significantly predict the rate of growth in words spoken at Time 4, t(61) = -1.448, p = .153 or celeration in growth over time, t(61) = -.161, p = .873. However, DS etiology significantly predicted spoken vocabulary outcome at Time 4, t(61) = -2.599, p < .05 (g = -.66). That is, children with DS were reported by their parents to say fewer words than children without DS at the end of the treatment phase (Figure 1; Table 3). Specifically, children with DS produced an average of 9.6 words at post-treatment, whereas children in the NDS group produced 28.3 words, approximately 3 times more than the DS group. Finally, we evaluated the main effect of treatment dose frequency and the interaction between dose frequency and diagnosis in our model that included both DS and NDS subgroups. Neither treatment dose frequency nor the interaction between treatment dose frequency and diagnosis had significant effects on the final outcome, growth rate at Time 4, or celeration in growth for the number of words said (p values >.05).
Figure 1.
Average growth in the number of words spoken as a function of diagnosis controlling for Bayley III Cognitive Composite standard score. Y-axis reflects log10 transformed values (Log 10 (x+1)) for the number of words children were reported to say on the MacArthur-Bates Communicative Development Inventory. X-axis denotes time in study (in months) centered at post-treatment (completion of the 9-month treatment protocol). NDS = non-Down syndrome (mixed-etiology ID) subgroup, DS= Down syndrome subgroup.
Table 3.
Fixed effects estimates for across-etiology model predicting growth of spoken vocabulary.
| Parameter | Level 2 predictor | Coefficient | SE | t(df) | p | 95% CI* |
|---|---|---|---|---|---|---|
| Intercept | ||||||
| Mean | 1.440 | .124 | 11.577(61) | <.001 | [1.196,1.684] | |
| Down syndrome | -.392 | .151 | -2.599(61) | .012 | [-.688, -.096] | |
| Bayley III CC | .042 | .010 | 4.137(61) | <.001 | [.022, .062] | |
| Slope | ||||||
| Mean | .097 | .022 | 4.338(61) | <.001 | [.053, .140] | |
| Down syndrome | -.041 | .028 | -1.448(61) | .153 | ||
| Bayley III CC | .002 | .002 | .968(61) | .337 | ||
| Quadratic | ||||||
| Mean | -.002 | .002 | -.799(61) | .427 | ||
| Down syndrome | -.0004 | .003 | -.161(61) | .873 | ||
| Bayley III CC | -.0002 | .0002 -1.006(61) | .318 | |||
95% confidence intervals have been included for significant parameters in final models. CC = Cognitive Composite standard score.
Within-Etiology Subgroup Models
The effect of DS on the intercept is a type of cross-level interaction which suggests that the growth of spoken vocabulary in children with ID receiving MCT is different in children with DS than in children with NDS. This effect warrants the consideration of Level 1 growth trajectories and Level 2 effects in etiological subgroups defined by the presence of DS etiology.
DS subgroup
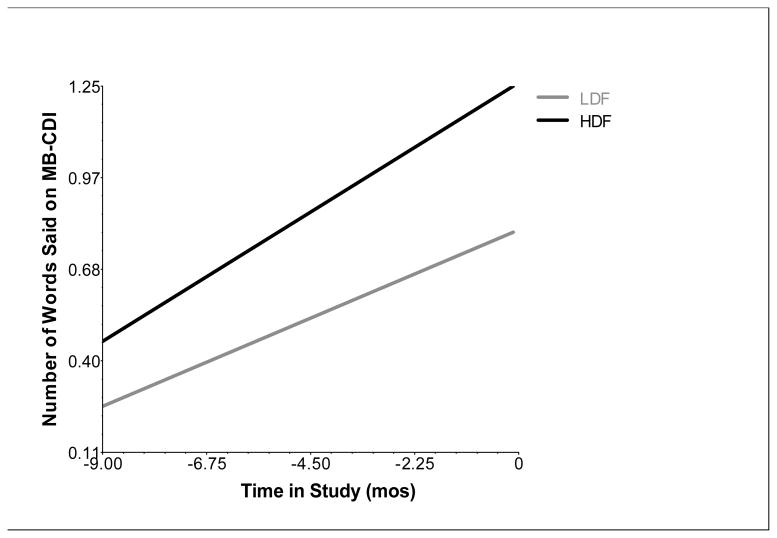
The growth model that quantified celeration was not a better fit to spoken vocabulary growth of children with DS than models that quantified growth as a straight line (see Table 2 for further information regarding comparison of level 1 models of spoken vocabulary growth). Significant fixed effects of slope and intercept in the simple linear model were obtained (ps < .001), confirming that the mean outcome and average rate of growth over the treatment period were significantly different from zero. Subsequent tests of random effects revealed significant variation in the intercept and slope parameters (p values < .001). However, the fixed and random effects of the quadratic term were not significant (t(69) = -1.439, p = .155, and χ2(34) = 43.721, p =.123 respectively). Inclusion of a quadratic term to the Level 1 growth model did not result in significant improvement in model fit for the DS subgroup, whether the parameter was fixed or random, (ps > .05). Therefore, the simple linear model (random-intercept, random-slope) was retained as the Level 1 model of spoken vocabulary growth for the DS subgroup. Bayley III CC accounted for a significant amount of the variance in the outcome (i.e., intercept) and mean rate of growth (i.e., slope) observed for spoken vocabulary, and it significantly improved fit to the data according to the model comparison test (ps < .001). Using the best-fitting random-intercept, random-slope model and controlling for the effect of Bayley III CC, we observed a significant effect of dose frequency on the number of words spoken at the end of the 9-month treatment (i.e., the intercept). In contrast with the across-etiology model, there was more growth on the spoken vocabulary outcome when MCT was administered in a high dose frequency than when it was administered in a low dose frequency for children with DS, t(32) = 2.926, p < .01 (g = .55; see Table 4). At the sample-average Bayley III CC level, children with DS in the HDF group produced an average of 17.3 words at post-treatment, while children with DS in the LDF group used only 5.4 words on average after completing the 9-month treatment protocol (Figure 2). The effect of dose frequency on the mean rate of growth across the treatment period approached, but did not reach, statistical significance, t(32) = 1.946, p = .06.
Table 4.
Fixed effects estimates for within-etiology model predicting growth of spoken vocabulary for children in the DS subgroup.
| Parameter | Level 2 predictor | Coefficient | SE | t(df) | p | 95% CI* |
|---|---|---|---|---|---|---|
| Intercept | ||||||
| Mean | .803 | .101 | 7.979(32) | <.001 | [.605, 1.001] | |
| Dose frequency | .458 | .157 | 2.926(32) | .006 | [.151, .766] | |
| Bayley III CC | .057 | .012 | 4.626(32) | <.001 | [.033, .081] | |
| Slope | ||||||
| Mean | .061 | .007 | 8.655(32) | <.001 | [.047, .074] | |
| Dose frequency | .028 | .015 | 1.946(32) | .06 | ||
| Bayley III CC | .005 | .001 | 3.603(32) | .001 | [.003, .007] | |
95% confidence intervals have been included for significant parameters in final models. CC = Cognitive Composite standard score.
Figure 2.
Effects of dose frequency (DF) on spoken vocabulary growth controlling for Bayley III Cognitive Composite standard score in children with Down syndrome (DS). Y-axis reflects log10 transformed values (Log 10 (x+1)) for the number of words children were reported to say on the MacArthur-Bates Communicative Development Inventory. X-axis denotes time in study (in months) centered at post-treatment (completion of the 9-month treatment protocol). LDF = low dose frequency, HDF = high dose frequency.
Note: Controlling for Time 1 number of words said did not affect the interpretation of the dose frequency effects.
We conducted follow-up analyses to confirm that the dose frequency effects in the DS subgroup could not be explained by differences in Time 1 spoken vocabulary level. The difference between treatment dose frequency groups in Time 1 spoken vocabulary (Figure 2) was statistically non-significant, t(34) = -.85, p = .40. Additionally, when spoken vocabulary and Bayley III CC at Time 1 were statistically controlled, the positive dose frequency effect on Time 4 spoken vocabulary levels (i.e., intercept) remained statistically significant, t(31) = 2.55, p = .016. Therefore, the observed difference in outcomes does not appear to be the result of Time 1 vocabulary status. In fact, with both Bayley III CC and Time 1 spoken vocabulary controlled, the effect of dose frequency on rate of spoken vocabulary growth across the treatment period (i.e., slope) also became statistically significant, t(31) – 2.06, p = .047. Thus, once we controlled for pre-treatment spoken vocabulary level and Bayley III CC, the higher dose frequency of MCT resulted in better spoken vocabulary growth and outcomes for children with DS.
NDS subgroup
The model that quantified celeration was a better fit to spoken vocabulary growth of children in the NDS group than models that quantified growth as a straight line (see Table 2 for further information regarding comparison of level 1 models of spoken vocabulary growth). The random-intercept, random-slope, random-quadratic model significantly improved model fit in comparison to more parsimonious models (ps < .005), indicating that there was significant variance in outcomes, rate of growth at the post-treatment period, and change in rate of growth over time in the NDS subgroup. However, Bayley III CC did not account for a significant amount of the variance in any parameter of the complex model or improve fit according to the model comparison test (ps > .05). Additionally, even when employing the best-fitting growth model, a main effect of dose frequency was not observed for the number of words said in the NDS subgroup (p > .05). Thus, the individual differences in trajectories of spoken vocabulary growth of children with mixed etiology ID did not vary systematically according to either their general intellectual ability or their dose frequency assignment.
Analysis of Alternative Explanations for the Findings
Given the finding from our original study that the effects of dose frequency were moderated by children's interest in playing with different objects (Fey et al., 2013), it is also reasonable to question whether differences in object interest across etiological subgroups might explain the results of our re-analysis. For example, despite our random participant assignment, our sample of children with DS who received HDF MCT could have been especially high in object interest. This could then account for the main effect of dose frequency in the DS subgroup analysis suggesting that children with DS benefit significantly more from HDF versus LDF interventions. Additionally, if the children in the NDS group were uniformly low in object interest, they may not have been able to capitalize on potential benefits of the HDF intervention.
We tested these possibilities using ANOVAs with Time 1 Object Interest as the dependent variable. These analyses confirmed that the DS and NDS groups did not differ, F(1,60) = .01, p = .93, and that there was no interaction between DS and dose frequency group, F(1, 60) = .99, p = .33, in Time 1 Object Interest. In other words, object interest was fairly evenly distributed across etiological subgroups and levels of intervention dose frequency. Thus, there are no subgroup differences in Time 1 Object Interest that could potentially account for the significant etiology-based effects reported in the present paper.
Despite our finding that etiological subgroups did not differ in level of object interest, our discovery of different treatment dose frequency effects within DS and NDS groups in the present analysis still begs the question as to whether the effects of object interest observed in our original article (Fey et al., 2013) were the same across the subgroups. To address this question, we conducted separate etiological subgroup analyses to evaluate whether Time 1 Object Interest moderated the effects of dose frequency on spoken vocabulary growth in each etiological subgroup (i.e., DS and NDS subgroups), utilizing our best-fitting Level 1 growth models (the random-intercept, random-slope model for the DS subgroup and the random-intercept, random-slope, random-quadratic for the NDS subgroup). There was a significant interaction between Time 1 Object Interest and dose frequency group on the intercept for both the DS subgroup, t(31) = 2.256, p = .031, and the NDS subgroup, t(24) = 2.384, p = .026. Furthermore, Time 1 Object Interest moderated the effect of dose frequency on spoken vocabulary outcomes similarly in each etiological subgroup. Consistent with our previous report, whether or not they had DS, children with ID who engaged in functional play with more objects at the onset of treatment produced more spoken words at post-treatment if they received MCT at a HDF than a LDF.
Discussion
This follow-up of previously reported data (Fey et al., 2013) re-examined the effects of treatment dose frequency of an early communication intervention, MCT, on spoken vocabulary outcomes in young children with ID with and without DS. The present work has important implications for researchers investigating interventions in diverse populations: how we model growth may impact whether we detect significant treatment effects present in our data. By considering more complex growth models and conducting subgroup analyses, we discovered that etiology of ID had a critical influence on growth and treatment response in our cohort of children with ID. The results highlight the need for researchers to further consider the role of etiology in outcomes by: (a) demonstrating that growth trajectories and predictors of treatment outcome vary according to diagnosis, (b) suggesting that effects of treatment intensity manipulations differ based on DS etiology in young children with ID, and (c) confirming that the presence or absence of DS covaries with spoken language outcomes.
The Impact of Etiology on Modeling and Detection of Treatment Effects
Our findings indicate that child factors, such as etiology, may affect both the selection of best-fitting growth models and the detection of treatment effects in intervention studies involving diverse populations, such as children with ID. In the present study, changes in rate of growth (i.e., significant celeration) within the NDS group seemed to drive the selection of Level 1 models when growth was collectively modeled for participants with and without DS for spoken vocabulary. Additionally, lack of a significant dose frequency effect in the NDS group apparently masked the dose frequency effect on spoken vocabulary outcomes of children with DS in the across-etiological subgroup analysis. Thus, the most informative models for testing dose frequency effects were those within the etiological subgroups (i.e., within DS or NDS).
Dose Frequency Effect on Spoken Vocabulary in DS
The within-etiological subgroup models demonstrated that dose frequency manipulations affected spoken vocabulary outcomes of children with DS. Increased dose frequency resulted in a greater number of words said at post-treatment for children with DS. Although there is insufficient evidence to conclude that increased dose frequency will result in children with DS “catching up” with NDS peers, there is sufficient evidence to suggest that HDF MCT produced more optimal spoken vocabulary outcomes for children with DS than LDF treatment.
Notably, the effects of dose frequency were only discerned for children with DS after controlling for the effect of Bayley III CC. That is, general intellectual ability was a powerful covariate that increased the statistical power of the test of dose frequency. This occurred because of its low correlation with dose frequency group due to random assignment, and its high association with growth of spoken vocabulary. This high correlation of general intellectual ability with spoken vocabulary, although logical, is not always observed in studies of predictors of spoken language growth in young children with DS (Mundy, Kasari, Sigman, & Ruskin, 1995; Yoder & Warren, 2004). The present finding suggests that general intellectual ability may predict growth in spoken vocabulary, perhaps because it reflects, among other factors, learning capacity in children with DS.
On one hand, the relative lack of significant effects of dose frequency and general intellectual ability on spoken vocabulary in the NDS subgroup is somewhat surprising. The failure of this study to find effects of intellectual ability and intensity manipulations on spoken vocabulary of children with NDS may be attributed to the heterogeneity inherent in a mixed-etiology subgroup. A greater variety of constraining and enabling factors may be expected to exert effects on linguistic growth within this group of participants than in the DS subgroup. Thus, further work is necessary to fully evaluate the impact of dose frequency manipulations within single-etiology subgroups of children with intellectual disability (e.g., Fragile X syndrome, Williams syndrome). Unfortunately, such single-etiology subgroup comparisons are very difficult to conduct due to low prevalence of subgroups other than DS.
On the other hand, in the present study we confirmed that interest in objects was a moderator of the effects of treatment dose frequency for both DS and NDS subgroups. Thus, there are some stable findings across etiological subgroups with respect to response to MCT dose frequency manipulations: those that benefit most from increased treatment dose frequency tended to display functional play with many objects prior to the onset of intervention.
Effects of DS Etiology on Spoken Vocabulary
The current investigation confirms a DS disadvantage for growth in the use of spoken words. Modeling across etiological subgroups showed that the presence of DS predicted significantly lower spoken vocabulary in young children with ID, when dose frequency was not considered. This finding occurred even when intellectual ability was controlled both by matching and statistical methods. This result is consistent with several extant studies (Martin, et al., 2009; Yoder & Warren, 2004). Therefore, we are increasingly confident that the presence of an additional 21st chromosome contributes to the disproportionate deficit in spoken language development seen in children with DS above and beyond the presence and severity of ID.
The mechanisms by which this chromosomal composition causes language impairments remain unclear, however. Candidate factors consistently observed among individuals with DS include auditory memory deficits, co-morbid hearing loss, and poor oral motor skills (Kumin, 2006; Marcell, Ridgeway, Sewell, & Whelan, 1995; Roizen, 1997). Several of these factors are likely involved, although they are not within the exclusive domain of the 21st chromosome. For example, the vocabulary and morphosyntactic language impairments of children with DS are qualitatively similar to those of children with specific language impairment (SLI) (Caselli, et al., 2008; Chapman, Hesketh, & Kistler, 2002; Chapman, et al., 1998; Eadie, et al., 2002). As in children with DS, working memory limitations are common and account for significant proportions of variance in measures of language ability among children with SLI (Leonard, Ellis Weismer, Miller, Francis, Tomblin, & Kail, 2007), who have no 21st chromosome anomalies. Additional characteristics associated with DS may also contribute to or perpetuate disproportionate impairments in spoken language. For example, a tendency towards task resistance noted even in young children with DS may manifest as an avoidance of potentially therapeutic interactions, particularly when the target of treatment is an especially challenging skill (Fidler, 2005; J. G. Wishart, 1993). Additionally, social-emotional skill, a relative strength in children with DS, may serve as a compensatory mechanism, allowing children with DS to successfully navigate social situations despite inordinate spoken language deficits (Freeman & Kasari, 2002). Further investigation is needed to evaluate the impact of these and other variables on the delayed and different patterns of language acquisition in DS.
Clinical Significance of Benefits of Dose Frequency
The children with DS who received the HDF and the LDF MCT had back-transformed means on the MB-CDI of 17 and 5 words, respectively, after 9 months of intervention. When we characterize this difference simply as 12 words, the effect seems rather small. We could also say that the estimated mean vocabulary size of children with DS in the HDF group was 300% more than that of the LDF group, in which case the difference seems rather large. However, these two approaches to thinking about the benefit of more therapy only reflect average differences, not the spread around the mean. The effect size, g, more appropriately quantifies the difference between groups in terms of variability around the mean. The g that reflects the difference in spoken vocabulary in our study was about .5 SD, much the same as what we observed for the moderating effect of object interest (Fey et al., in press). Cohen's (1988) benchmarks are problematic when applied generally to all types of interventions and outcomes. Nevertheless, judging this as a “medium” effect appears to us to be about right. Are these medium-sized effects sufficiently meaningful to be worth the cost in time and money needed to provide over four times more intervention as was the case for the HDF MCT we provided? This question is of crucial importance, and we suspect it will be the substance of many discussions among professionals, parents, and third-party payers. It is exceedingly difficult to answer, however, for two reasons. First, it must be recalled that the words reported in our study included only those that have grammatical significance, such as nouns, verbs, adjectives, and adverbs. Terms of politeness and other social words, animal noises, interjections, and other non-referential words were excluded. Gains observed in the DS group, then, reflected change in the types of words we were attempting to facilitate with MCT and that the children can use to their great benefit in multi-word constructions. Second, as Kazdin (1999) argued, clinical significance of change must be evaluated in light of the severity of disabilities that participants experience. The spoken vocabulary delay that children with DS experience, in general, is severe and disproportionate relative to generalized degree of ID. Therefore, it is possible that an effect size of about .5 could be considered an “important” difference in this population, considering how important vocabulary development is to the rest of the language system.
Limitations
The major limitations of the study were discussed in detail in Fey et al. (in press). One of the most important is the absence of a business as usual control group, which might have enabled a conclusion that both dose frequencies of MCT were facilitative of spoken vocabulary relative to no MCT. This omission prevents such a conclusion. A second major limitation is that we cannot discern whether there are variables, such as deficits in phonological working memory, correlated with DS that provide more direct explanations for the slow development of spoken vocabulary in the children with DS compared to other children with ID. Our current data does not provide information on these more proximal explanatory variables.
A third limitation involves our reliance on parent report in evaluating spoken vocabulary of our cohort. Though parent report is one of the most valid and reliable options for measurement in young children, informants, usually the child's mother, cannot be kept blind to the purposes of the investigation or the point during the study at which the inventory is presented (e.g., at the beginning or end of therapy). This could have led to biased reporting due to increased attention to the constructs of interest and/or anticipation of gain related to treatment. Two factors suggest that this bias had limited effects on our results. First, both groups experienced treatment, including the parent education/training component. Thus, we might expect similar bias across both experimental groups. Nonetheless, it's true that the biasing effects could still be greater in the HDF group versus the LDF group because the parents' investment in time and travel was relatively higher in the HDF group. However, it seems improbable that such effects could account for the present findings, given that the effect of dose frequency was observed only within the DS subgroup.
Summary
We conducted a re-analysis of the Fey et al. (2013) data because we suspected that a more complex growth model and within-etiological subgroup analyses might reveal dose frequency effects that were undetected in our previous analysis. Etiological-subgroup analysis revealed that increased dose frequency led to greater spoken vocabulary growth of children with DS when general intellectual ability was controlled. Our re-analysis also confirms the moderating influence of object interest in both subgroups of children with ID. Across-etiological subgroup analyses confirmed that young children with DS, on average, have poorer spoken vocabulary skills than their peers with ID not due to DS. We hope that these results will generate further investigation into (a) how treatments may be successfully altered to maximize the potential of all children with ID and (b) why DS correlates with a disadvantage in spoken language learning.
Acknowledgments
This research was supported in part by grant R01DC007660 and Center Grant P30-DC005803 from the National Institute on Deafness and Other Communication Disorders and Center Grant P30 NICHD HD 002528 from the National Institute on Child Health and Human Development. The authors acknowledge the significant contributions of Shelley L. Bredin-Oja, Jayne Brandel, Catherine Bush, Debby Daniels, Elizabeth Gardner, Nicole Thompson and Peggy Waggoner.
Contributor Information
Paul J. Yoder, Special Education, Vanderbilt University
Tiffany G. Woynaroski, Hearing and Speech Sciences, Vanderbilt University, Vanderbilt University
Marc E. Fey, Hearing and Speech Department, University of Kansas Medical Center
Steven F. Warren, Institute for Life Span Studies, University of Kansas
References
- Abbeduto L, Warren SF, Conners FA. Language development in Down syndrome: from the prelinguistic period to the acquisition of literacy. Mental Retardation and Developmental Disabilities Research Reviews. 2007;13(3):247–261. doi: 10.1002/mrdd.20158. [DOI] [PubMed] [Google Scholar]
- Al Otaiba S, Schatschneider C, Silverman E. Tutor-assisted intensive learning strategies in kindergarten: how much is enough? Exceptionality. 2005;13(4):195–208. doi: 10.1207/s15327035ex1304_2. [DOI] [Google Scholar]
- Anderson PJ, De Luca CR, Hutchinson E, Roberts G, Doyle LW. Underestimation of developmental delay by the new Bayley-III Scale. Archives of Pediatric and Adolescent Medicine. 2010;164(4):352–356. doi: 10.1001/archpediatrics.2010.20. 164/4/352[pii]10.1001/archpediatrics.2010.20. [DOI] [PubMed] [Google Scholar]
- Barratt J, Littlejohns P, Thompson J. Trial of intensive compared with weekly speech therapy in preschool children. Archives of Disease in Childhood. 1992;67(1):106–108. doi: 10.1136/adc.67.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley N. Bayley Scales of Infant and Toddler Development. San Antonio, TX: The Psychological Corporation; 2006. [Google Scholar]
- Brady NC, Bredin-Oja SL, Warren SF. Prelinguistic and early language interventions for children with Down syndrome or fragile X syndrome. In: Roberts JE, Chapman RS, Warren SF, editors. Speech and language development and intervention in Down syndrome and fragile X syndrome. Baltimore; Brookes: 2008. pp. 173–192. [Google Scholar]
- Cardoso-Martins C, Mervis CB, Mervis CA. Early vocabulary acquisition by children with Down syndrome. American Journal of Mental Deficiency. 1985;90(2):177–184. [PubMed] [Google Scholar]
- Caselli MC, Monaco L, Trasciani M, Vicari S. Language in Italian children with Down syndrome and with specific language impairment. Neuropsychology. 2008;22(1):27–35. doi: 10.1037/0894-4105.22.1.27. 2008-00382-005[pii]10.1037/0894-4105.22.1.27. [DOI] [PubMed] [Google Scholar]
- Caselli MC, Vicari S, Longobardi E, Lami L, Pizzoli C, Stella G. Gestures and words in early development of children with Down syndrome. Journal of Speech Language and Hearing Research. 1998;41(5):1125–1135. doi: 10.1044/jslhr.4105.1125. [DOI] [PubMed] [Google Scholar]
- Chapman RS, Hesketh LJ, Kistler DJ. Predicting longitudinal change in language production and comprehension in individuals with Down syndrome: hierarchical linear modeling. Journal of Speech Language and Hearing Research. 2002;45(5):902–915. doi: 10.1044/1092-4388(2002/073). [DOI] [PubMed] [Google Scholar]
- Chapman RS, Seung HK, Schwartz SE, Kay-Raining Bird E. Language skills of children and adolescents with Down Syndrome: II. production deficits. Journal of Speech Language and Hearing Research. 1998;41(4):861–873. doi: 10.1044/jslhr.4104.861. [DOI] [PubMed] [Google Scholar]
- Denton CA, Cirino PT, Barth AE, Romain M, Vaughn S, Wexler J, Fletcher JM. An experimental study of scheduling and duration of “Tier 2” first-grade reading intervention. Journal of Research on Educational Effectiveness. 2011;4(3):208–230. doi: 10.1080/19345747.2010.530127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eadie PA, Fey ME, Douglas JM, Parsons CL. Profiles of grammatical morphology and sentence imitation in children with specific language impairment and Down syndrome. Journal of Speech Language and Hearing Research. 2002;45(4):720–732. doi: 10.1044/1092-4388(2002/058). [DOI] [PubMed] [Google Scholar]
- Fenson L, Dale P, Reznick J, Thal D, Bates E, Hartung J, Reilly J. MacArthur communicative development inventories: User's guide and technical manual. Baltimore, MD: Paul H. Brookes; 2003. [Google Scholar]
- Fey ME, Warren SF, Brady N, Finestack LH, Bredin-Oja SL, Fairchild M, Yoder PJ. Early effects of responsivity education/prelinguistic milieu teaching for children with developmental delays and their parents. Journal of Speech Language and Hearing Research. 2006;49(3):526–547. doi: 10.1044/1092-4388(2006/039). [DOI] [PubMed] [Google Scholar]
- Fey ME, Yoder PJ, Warren SF, Bredin-Oja S. Is more better? Milieu communication teaching in toddlers with intellectual disabilities. Journal of Speech Language and Hearing Research. 2013 doi: 10.1044/1092-4388(2012/12-0081). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler DJ. The Emerging Down Syndrome Behavioral Phenotype in Early Childhood: Implications for Practice. Infants & Young Children. 2005;18(2):86–103. [Google Scholar]
- Fidler DJ, Philofsky A, Hepburn SL, Rogers SJ. Nonverbal requesting and problem-solving by toddlers with Down Syndrome. American Journal of Mental Retardation. 2005;110(4):312–322. doi: 10.1352/0895-8017(2005)110[312:NRAPBT]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SF, Kasari C. Characteristics and qualities of the play dates of children with Down syndrome: emerging or true friendships? American Journal of Mental Retardation. 2002;107(1):16–31. doi: 10.1352/0895-8017(2002)107<0016:CAQOTP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Galeote M, Soto P, Checa E, Gomez A, Lamela E. The acquisition of productive vocabulary in Spanish children with Down syndrome. Journal of Intellectual and Developmental Disability. 2008;33(4):292–302. doi: 10.1080/13668250802441870. 905608481[pii]10.1080/13668250802441870. [DOI] [PubMed] [Google Scholar]
- Hancock TB, Kaiser AP. Enhanced milieu teaching. In: McCauley RJ, Fey ME, editors. Treatment of language disorders in children. Baltimore: Paul H. Brookes; 2006. pp. 203–236. [Google Scholar]
- Harris M, Yeeles C, Chasin J, Oakley Y. Symmetries and asymmetries in early lexical comprehension and production. Journal of Child Language. 1995;22(1):1–18. doi: 10.1017/s0305000900009600. [DOI] [PubMed] [Google Scholar]
- Hart B. The initial growth of expressive vocabulary among children with Down Syndrome. Journal of Early Intervention. 1996;20(3):211–221. doi: 10.1177/105381519602000305. [DOI] [Google Scholar]
- Kazdin AE. The meanings and measurement of clinical significance. Journal of Consulting and Clinical Psychology. 1999;67(3):332–339. doi: 10.1037//0022-006x.67.3.332. [DOI] [PubMed] [Google Scholar]
- Kumin L. Speech intelligibility and childhood verbal apraxia in children with Down syndrome. Down Syndrome Research and Practice. 2006;10:10–22. doi: 10.3104/reports.301. [DOI] [PubMed] [Google Scholar]
- Lifter K. Linking assessment to intervention for children with developmental disabilities or at-risk for developmental delay: The developmental play assessment (DPA) instrument. In: Gitlin-Weiner K, Sandgrund A, Shafer C, editors. Play diagnosis and assessment. 2nd. New York: John Wiley & Sons; 2000. pp. 228–261. [Google Scholar]
- Marcell MM, Ridgeway MM, Sewell DH, Whelan ML. Sentence imitation by adolescents and young adults with Down's syndrome and other intellectual disabilities. Journal of Intellectual Disability Research. 1995;39:215–232. doi: 10.1111/j.1365-2788.1995.tb00504.x. [DOI] [PubMed] [Google Scholar]
- Martin GE, Klusek J, Estigarribia B, Roberts JE. Language characteristics of individuals with Down syndrome. Topics in Language Disorders. 2009;29(2):112–132. doi: 10.1097/tld.0b013e3181a71fe1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty AS, Breit-Smith A, Fan X, Justice LM, Kaderavek JN. Does intensity matter? Preschoolers' print knowledge development within a classroom-based intervention. Early Childhood Research Quarterly. 2011;26(3):255–267. doi: 10.1016/j.ecresq.2011.02.002. [DOI] [Google Scholar]
- Mervis CB, Bertrand J. Early lexical acquisition and the vocabulary spurt: a response to Goldfield & Reznick. Journal of Child Language. 1995;22(2):461–468. doi: 10.1017/s0305000900009880. [DOI] [PubMed] [Google Scholar]
- Miller J. Development of speech and language in children with Down syndrome. In: Lott IT, McCoy EE, editors. Down syndrome: advances in medical care. New York: Wiley-Liss; 1992. [Google Scholar]
- Miller J. Profiles of language development in children with Down Syndrome. In: Miller J, Leddy M, Leavitt L, editors. Improving the communication of people with Down Syndrome. Baltimore: Brookes; 1999. pp. 11–40. [Google Scholar]
- Mundy P, Kasari C, Sigman M, Ruskin E. Nonverbal Communication and Early Language Acquisition in Children With Down Syndrome and in Normally Developing Children. Journal of Speech, Language, & Hearing Research. 1995;38(1):157–167. doi: 10.1044/jshr.3801.157. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2nd. London: Sage; 2002. [Google Scholar]
- Roizen NJ. Hearing loss in children with Down syndrome: a review. Down Syndrome Quarterly. 1997;2:1–4. [Google Scholar]
- Rondal JA, Edwards S. Language in mental retardation. Philadelphia, PA: Whurr Publishers; 1997. [Google Scholar]
- Ruskin EM, Mundy P, Kasari C, Sigman M. Object mastery motivation of children with Down Syndrome. American Journal of Mental Retardation. 1994;98(4):499–509. [PubMed] [Google Scholar]
- Tapp J, Yoder P. Playcoder (Version 1.1.2) Nashville, TN: Vanderbilt University; 2003. [Google Scholar]
- Ukrainetz TA, Ross CL, Harm HM. An investigation of treatment scheduling for phonemic awareness with kindergartners who are at risk for reading difficulties. Language Speech and Hearing Services in the Schools. 2009;40(1):86–100. doi: 10.1044/0161-1461.(2008/07-0077). [DOI] [PubMed] [Google Scholar]
- Vicari S, Caselli MC, Tonucci F. Asynchrony of lexical and morphosyntactic development in children with Down Syndrome. Neuropsychologia. 2000;38(5):634–644. doi: 10.1016/s0028-3932(99)00110-4. S0028-3932.(99)00110-4[pii] [DOI] [PubMed] [Google Scholar]
- Warren SF, Fey ME, Finestack LH, Brady NC, Bredin-Oja SL, Fleming KK. A randomized trial of longitudinal effects of low-intensity responsivity education/prelinguistic milieu teaching. Journal of Speech Language and Hearing Research. 2008;51(2):451–470. doi: 10.1044/1092-4388.(2008/033). [DOI] [PubMed] [Google Scholar]
- Warren SF, Fey ME, Yoder PJ. Differential treatment intensity research: a missing link to creating optimally effective communication interventions. Mental Retardation and Developmental Disabililities Research Reviews. 2007;13(1):70–77. doi: 10.1002/mrdd.20139. [DOI] [PubMed] [Google Scholar]
- Wishart J. Learning the hard way: Avoidance strategies in young children with Down syndrome. Down Syndrome Research and Practice. 1993;1:47–55. [Google Scholar]
- Wishart JG. The development of learning difficulties in children with Down's syndrome. Journal of Intellectual Disabilities Research. 1993;37:389–403. doi: 10.1111/j.1365-2788.1993.tb00882.x. [DOI] [PubMed] [Google Scholar]
- Yoder PJ, Fey M, Warren SF. Studying the impact of intensity is important but complicated. International Journal of Speech-Language Pathology. doi: 10.3109/17549507.2012.685890. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder PJ, Warren SF. Maternal responsivity predicts the prelinguistic communication intervention that facilitates generalized intentional communication. Journal of Speech Language and Hearing Research. 1998;41(5):1207–1219. doi: 10.1044/jslhr.4105.1207. [DOI] [PubMed] [Google Scholar]
- Yoder PJ, Warren SF. Maternal responsivity mediates the relationship between prelinguistic intentional communication and later language. Journal of Early Intervention. 1999a;22(2):126–136. doi: 10.1177/105381519902200205. [DOI] [Google Scholar]
- Yoder PJ, Warren SF. Facilitating self-initiated proto-declaratives and proto-imperatives in prelinguistic children with developmental disabilities. Journal of Early Intervention. 1999b;22(4):337–354. doi: 10.1177/105381519902200408. [DOI] [Google Scholar]
- Yoder PJ, Warren SF. Effects of prelinguistic milieu teaching and parent responsivity education on dyads involving children with intellectual disabilities. Journal of Speech Language and Hearing Research. 2002;45(6):1158–1174. doi: 10.1044/1092-4388.(2002/094). [DOI] [PubMed] [Google Scholar]
- Yoder PJ, Warren SF. Early predictors of language in children with and without Down Syndrome. American Journal on Mental Retardation. 2004;109(4):285–300. doi: 10.1352/0895-8017(2004)109<285:epolic>2.0.co;2. [DOI] [PubMed] [Google Scholar]