Abstract
We have developed herein a quantitative mass spectrometry-based approach to analyze the etiology-related alterations in fucosylation degree of serum haptoglobin in patients with liver cirrhosis and hepatocellular carcinoma (HCC). The three most common etiologies, including infection with hepatitis B virus (HBV), infection with hepatitis C virus (HCV), and heavy alcohol consumption (ALC), were investigated. Only 10 μL of serum was used in this assay in which haptoglobin was immunoprecipitated using a monoclonal antibody. The N-glycans of haptoglobin were released with PNGase F, desialylated, and permethylated prior to MALDI-QIT-TOF MS analysis. In total, N-glycan profiles derived from 104 individual patient samples were quantified (14 healthy controls, 40 cirrhosis, and 50 HCCs). A unique pattern of bifucosylated tetra-antennary glycan, with both core and antennary fucosylation, was identified in HCC patients. Quantitative analysis indicated that the increased fucosylation degree was highly associated with HBV- and ALC-related HCC patients compared to that of the corresponding cirrhosis patients. Notably, the bifucosylation degree was distinctly increased in HCC patients versus that in cirrhosis of all etiologies. The elevated bifucosylation degree of haptoglobin can discriminate early stage HCC patients from cirrhosis in each etiologic category, which may be used to provide a potential marker for early detection and to predict HCC in patients with cirrhosis.
Keywords: Hepatocellular carcinoma, liver cirrhosis, biomarker, haptoglobin, fucosylation, MALDI
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and is the third leading cause of cancer-related mortality.1 Approximately 80–90% of HCC patients have underlying liver cirrhosis, which is characterized by tissue fibrosis and the conversion of normal liver architecture into structurally abnormal nodules.2 The major risk factors for developing cirrhosis and HCC are infection with hepatitis B (HBV) or hepatitis C (HCV) virus and excessive alcohol consumption (ALC).3 A strong positive correlation has been demonstrated between HCC incidence rates and liver cirrhosis, showing that patients with HBV-related cirrhosis have a 1000-fold greater risk of developing HCC, whereas HCV-related cirrhosis carries the highest risk of developing HCC in the United States.1,4 Therefore, it is important to identify biomarkers for the early detection of HCC and prediction of disease progression in patients suffering from cirrhosis.
Glycosylation changes in serum proteins are highly associated with the progression of liver disease and have been explored as diagnostic indicators for HCC.5 The most notable change is the increased core fucosylation in alpha-fetoprotein (AFP).6 AFP is a serum glycoprotein marker commonly used to diagnose HCC and to monitor the development of this malignancy. However, the diagnostic power of serum AFP is restricted because of a lack of specificity, which is elevated only in 60–70% of HCC patients; the elevation also occurs under non-HCC circumstances, such as chronic hepatitis and cirrhosis.7,8 In contrast, AFP-L3, produced as the result of aberrant core fucosylation of AFP, is more specific for HCC than AFP and is negative for most benign liver diseases.9 AFP-L3, approved as a tool for the detection of HCC by the FDA in 2005, may provide a new generation of cancer biomarkers. Exploiting cancer-associated glycosylation aberration in specific serum glycoproteins provides a promising strategy to discover specific and sensitive biomarkers for discrimination between HCC and cirrhosis.
Serum haptoglobin (Hp) has attracted particular attention because of its potential as a reporter molecule for aberrant glycosylation in liver disease.10,11 Haptoglobin is one of the acute phase proteins (APP) secreted by the liver, which binds to hemoglobin and plays an important role in response to inflammation and malignancy.12 It consists of β, α-1, and α-2 chains, with four potential N-glycosylation sites on its β chain.13 Increasing evidence of elevated fucosylation in haptoglobin has been observed in pancreatic cancer,14,15 prostate cancer,16 colon cancer,17 and liver cancer,10,11 suggesting that it may serve as a promising biomarker for cancer diagnosis. In HCC, the elevation of haptoglobin fucosylation has been investigated using a variety of approaches such as lectin-ELISA, lectin blotting, and mass spectrometric analysis.10,11,18 A MALDI-QIT-TOF analysis of N-glycans of serum Hp revealed that a biantennary fucosylated glycan was highly increased in patients with cirrhosis and HCC compared to healthy controls.11 Recently, a quantitative liquid chromatography–mass spectrometry multiple reaction monitoring analysis of site-specific glycoforms of Hp showed that multiply fucosylated glycoforms increased significantly in the liver disease group compared to that in healthy controls.18 However, in these studies, mass spectrometric analyses of haptoglobin glycans were performed using either pooled sera or a limited number of cases of liver diseases. The comparison of fucosylation alteration in haptoglobin between HCC and cirrhosis patients of various etiologies has not been investigated. The correlation between altered fucosylation of haptoglobin and the etiology could provide an indicator for distinguishing HCC from cirrhosis with different etiologies.
Herein, we have performed a comprehensive study of fucosylation changes in serum Hp from healthy donors and patients with liver cirrhosis and HCC of the three most common etiologies, HBV, HCV, and alcohol abuse, using a mass spectrometry-based glycomics approach. Haptoglobin was extracted from IgG-depleted serum by immunoprecipitation using an antibody coupled with Protein A/G agarose beads followed by deglycosylation, desialylation and extraction of the N-glycans. Permethylation was applied to improve the sensitivity of MS detection and to yield more informative fragmentation patterns. The MALDI-QIT-TOF MS analysis was performed to study the fucosylation patterns of the glycans in MS and MS/MS modes for all disease-related samples and normal controls. The most distinct change was observed in the level of bifucosylated N-glycans with both core and antennary fucosylation, which was highly associated with HCC patients of different etiologies. Bifucosylation degree of haptoglobin N-glycans was significantly elevated in early stage HCC patients compared to that in cirrhosis in each etiology, suggesting that it may serve as a potential marker for early diagnosis and prediction of HCC in cirrhosis patients induced by HBV or HCV infection and excessive alcohol consumption.
Experimental Methods
Materials
N-glycosidase F (PNGase F) was purchased from New England Biolabs Inc. (Ipswich, MA). Neuraminidase, sodium hydroxide, methyl iodide, β-mercaptoethanol, chloroform, dimethyl sulfoxide (DMSO), HPLC-grade acetonitrile (ACN), water, and porous graphitized carbon tips (PGC tips) were purchased from Sigma-Aldrich (St. Louis, MO). The MALDI matrix, 2,5-dihydroxybenzoic acid (2,5-DHB), was purchased from Thermo Scientific (Rockford, IL). Anti-human haptoglobin antibody was purchased from Abcam (Cambridge, MA), and the anti-human haptoglobin ELISA kit was from Genway (San Diego, CA).
Serum Samples
One hundred and ten serum samples of patients and healthy donors were provided by the University Hospital, Ann Arbor, Michigan, according to IRB approval, which includes 50 HCC cases (9 HBV-, 21 HCV-, and 20 ALC-related), 46 cirrhosis cases (10 HBV-, 18 HCV-, and 18 ALC-related), and 14 normal controls. Because of the limited access to HBV-related liver disease samples in the United States, only 9 HBV-related HCC and 10 HBV-related cirrhosis samples were included in this study. The clinical features of patients with HCC and cirrhosis are summarized in Table 1. The cancer group consisted of 50 primary HCCs at different clinical stages (TNM classification): stage I (n = 7), stage II (n = 24), stage III (n = 11), and stage IV (n = 8). Among the 31 early stage HCC patients (TNM stages I and II), there were 6 HBV-, 15 HCV-, and 10 ALC-related HCCs. Among the 19 advanced HCC patients (TNM stages III and IV), there were 3 HBV-, 6 HCV-, and 10 ALC-related HCCs. Samples were aliquoted and stored at −80 °C until further use.
Table 1. Summary of Sample Population Characteristics.
| disease diagnosisa | HCC | cirrhosis |
|---|---|---|
| number | 50 | 46 |
| etiology % (HBV/HCV/ALC)b | 18/42/40 | 22/39/39 |
| gender % (M/F) | 56/44 | 65/35 |
| age (mean ± SD) | 60.3 ± 12 | 58.4 ± 9 |
| AFP levelc (median, ng/mL) | 11.85 | 2.7 |
| MELDd score | 9.7 ± 4.7 | 9.2 ± 2.8 |
| TNM stage % (I/II/III/IV) | 14/48/22/16 | NA |
| BCLC stage % (A/B/C/D) | 62/26/10/2 | NA |
Samples were provided by the Division of Gastroenterology, University of Michigan.
HBV, hepatitis B virus; HCV, hepatitis C virus; ALC, alcohol consumption.
AFP level was provided by the Division of Gastroenterology, University of Michigan.
MELD, model for end-stage liver disease.
Measurement of Serum Haptoglobin Abundance
The abundance of serum Hp was measured by ELISA assay (Genway, San Diego, CA) according to the manufacturer’s instructions. The absorbance values were read on a microplate reader (BioTek, Winooski, VT) at a wavelength of 450 nm.
Purification of Haptoglobin from Serum
Haptoglobin was immunoprecipitated from 10 μL of human serum by using the Cross-link IP kit (Pierce Scientific, Rockford, IL) as described previously.15 Serum was thawed and diluted to 250 μL in coupling buffer (10 mM sodium phosphate, 150 mM sodium chloride, pH 7.2). IgG, the most abundant glycoprotein in serum, was depleted prior to haptoglobin capture using Protein A/G agarose beads (Pierce Scientific, Rockford, IL). The IgG-depleted serum dilution was spun in a centrifuge at 1000g for 1 min. Immunoprecipitation was performed using the Cross-link IP kit according to the manufacturer’s instruction. Briefly, 10 μg of monoclonal anti-human haptoglobin antibody (Abcam, Cambridge, MA) was bound to 20 μL of a Protein A/G plus agarose slurry at room temperature for 30 min and then cross-linked with the beads by using the cross-linking reagent, disuccinimidyl suberate (DSS). The antibody-conjugated beads were then incubated with IgG-depleted serum at 4 °C overnight. After four washes with the coupling buffer, haptoglobin was eluted off the beads in 60 μL of elution buffer and dried in a SpeedVac concentrator (Labconco, Kansas City, MO). The haptoglobin was then redissolved in 10 μL of water followed by desalination using 75 μL of Zeba desalting spin columns (Pierce Scientific, Rockford, IL).
The yield and purity of haptoglobin eluent were evaluated by gel electrophoresis and mass spectrometric analysis after rapid on-plate digestion.15 One-fifth of the haptoglobin eluent was run on a 4–20% SDS-PAGE gel (Bio-Rad, Hercules, CA) and visualized by silver staining using ProteoSilver Plus silver stain kit (Sigma) following the manufacturer’s instruction. In addition, rapid on-plate digestion and mass spectrometric analysis were performed by depositing the desalted haptoglobin (0.5 μL) on a MALDI plate that was subsequently allowed to air dry followed by depositing 0.5 μL of a trypsin solution in 50 mM NH4HCO3 with 20% acetonitrile on top of the haptoglobin spot. The plate was then placed in a covered humid chamber at 37 °C for 10 min, and the digested peptides were analyzed using an Axima MALDI quadrupole ion trap TOF instrument (Shimadzu Biotech, Manchester, UK). Ionization was performed with a pulsed N2 laser (337 nm) at 5 Hz. Helium was used to cool the trapped ions, and Argon was used for CID fragmentation. The TOF detector was calibrated using calibration standards prior to analysis. The peptide peaks were searched against the Mascot database.
Deglycosylation and Desialylation of Haptoglobin
Ten microliters of haptoglobin solution was denatured by adding 1 μL of denaturing solution (0.2% SDS, 100 mM 2-mercaptoethanol) and incubated at 60 °C for 30 min. Ammonium bicarbonate solution was then added to make a final concentration of 15 mM. One unit of PNGase F was added and incubated with the sample at 37 °C for 18 h. The action of PNGase F was quenched through heating the reaction mixture at 95 °C for 10 min. Subsequently, the mixture was dried and reconstituted in 20 mM ammonium acetate followed by desialylation with neuraminidase (40 mU) (Sigma-Aldrich, St. Louis, MO) at 37 °C for 20 h. The mixture of desialylated glycans and the protein was dried in a SpeedVac and redissolved in 10 μL of water (with 0.1% TFA). Glycans were extracted using porous graphitized carbon tips (PGC tips) (Sigma-Aldrich, St. Louis, MO), according to a procedure described previously.15
Permethylation of Glycans
The glycans were permethylated according to the procedure of Kang.19 Briefly, the sample was suspended in 20 μL of DMSO, and 3 mg of grounded NaOH powder, 3.8 μL of methyl iodide, and 0.2 μL of water were added. After mixing for 10 min at room temperature, the permethylated glycans were extracted with chloroform. Ice-cold water was first added to the derivatization mixture, which was placed in an ice bath prior to the addition of chloroform. The aqueous layer was then discarded, and the chloroform layer was washed with water five times to eliminate residual NaOH, methyl iodide, and any side products. Finally, the permethylated glycans were dried under vacuum and redissolved in 2 μL of 20% acetonitrile for mass spectrometric analysis.
MALDI-QIT-TOF Analysis
The DHB matrix (10 mg/mL DHB in 50% acetonitrile with 1 mM sodium acetate) was spotted on the MALDI plate and allowed to air dry. Then, permethylated glycans were mixed with an equal amount of the DHB matrix and deposited on the dried matrix layer. The TOF detector was calibrated with 1 nmol/μL peptide mixtures of angiotensin II (m/z 1046.54), angiotensin I (m/z 1296.68), substance P (m/z 1347.74), bombesin (m/z 1619.82), ACTH 1-17 (m/z 2093.09), ACTH 18-39 (m/z 2465.20), and somatostatin 28 (m/z 3147.47) prior to data acquisition. The mass accuracy with calibration was 30 ppm. All glycans were sodiated and analyzed in positive ion mode in this study. GlycoMod tool (http://www.expasy.org/tools/glycomod) was utilized to predict the glycan composition. Only glycan structures included in the GlycoSuite database (http://glycosuitedb.expasy.org/glycosuite/glycodb) were selected. The glycan compositions were further confirmed by collision-induced dissociation (CID) MS/MS analysis.
Data Analysis
The MALDI MS data were acquired and processed in Launchpad software (Karatos, Manchester, UK). The m/z values and intensities were exported as ASCII files and plotted in SigmaPlot (San Jose, CA), and peak intensities were scaled with the highest peak as 100%. Glycan peak area integration was performed with Matlab (Natick, MA) using the same approach described in our previous study.20 The peak area of each glycan was the addition of both the permethylated glycan peak and the most abundant underpermethylated glycan peak detected 14 Da lower than the fully permethylated peak. The abundance of each glycan was normalized by the sum of all glycan abundances identified in each sample. For data visualization, a column scatter plot of the calculated fucosylation/bifucosylation index was generated with GraphPad Prism 6 (La Jolla, CA). The significance of the difference between groups was evaluated using Tukey’s multiple comparisons test in ANOVA. The receiver operating characteristics (ROC) curves of the total fucosylation and bifucosylation levels between the study groups were generated with Prism 6 (La Jolla, CA). The 2D plot of the bifucosylation degree of serum Hp and the clinical AFP values in cirrhosis and HCC patients was generated with SPSS 13 (IBM, Armonk, NY). The combined ROC analysis of AFP and the bifucosylation level of haptoglobin was performed on the basis of the predicted probabilities that were generated with binary logistic regression using the AFP and bifucosylation level of haptoglobin as covariates. The comparison of the model fit between the combined model and the AFP-alone model was examined by likelihood ratio (LR) test using SAS 9.3 PROC GENMOD type 3 analysis (SAS Institute Inc., Cary, NC).
We performed a power analysis to determine the power of our experiments by using GraphPad StatMate 2 (La Jolla, CA). At the given sample size, the variance of total fucosylation or bifucosylation degree values, and the difference that we want to detect (two-tailed, 0.05), the power of the experiment was calculated. The powers at the calculated differences of the means (delta mean) of fucosylation/bifucosylation level in comparison groups are higher than 95%, which provide the statistical support for the number of samples included in our study.
Results and Discussion
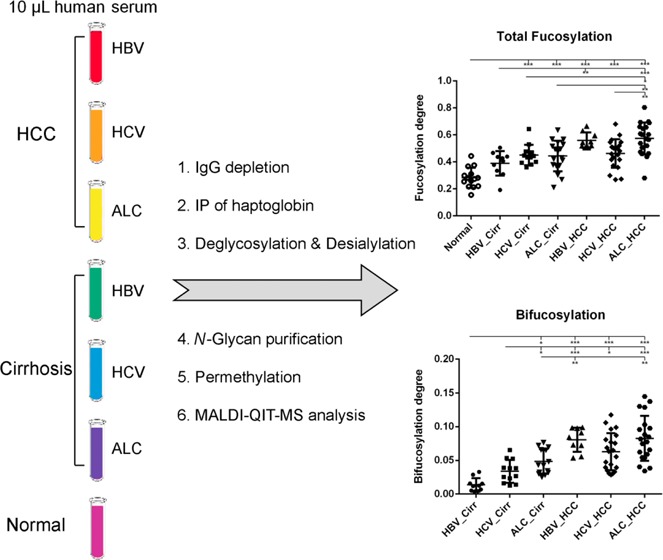
Changes in N-linked fucosylation have been highly associated with the development of HCC. Significantly increased fucosylation has been found in both total serum21 and serum Hp10 in patients with HCC compared to that in patients with liver cirrhosis and healthy subjects. However, it is not clear if the fucosylation profiles are similar in HCC patients of different etiologies or are unique to specific etiologies. Thus, the aim of this study is to compare fucosylation in serum Hp between HCC and cirrhosis patients with respect to the type of etiology and cancer stages in order to identify unique Hp fucosylation patterns discriminating HCC from cirrhosis of each etiology. For this purpose, we performed N-linked fucosylation analysis on serum Hp from HCC and cirrhosis patients induced by the three major risk factors, HBV, HCV, and ALC, based on our quantitative glycomic methodology.15 The workflow is outlined in Figure 1. First, IgG was depleted from serum prior to Hp capture because of the large quantities of immunoglobulins in human serum that normally contain fucose resides. Subsequently, haptoglobin was immunoprecipitated from IgG-depleted serum using an antibody coupled with Protein A/G agarose beads. The purified haptoglobin was then deglycosylated and desialylated followed by glycan extraction using the PGC tips. Finally, the glycans were permethylated prior to mass spectrometry analysis. Analysis of fucosylation degree was performed by Matlab and visualized using Prism 6.
Figure 1.
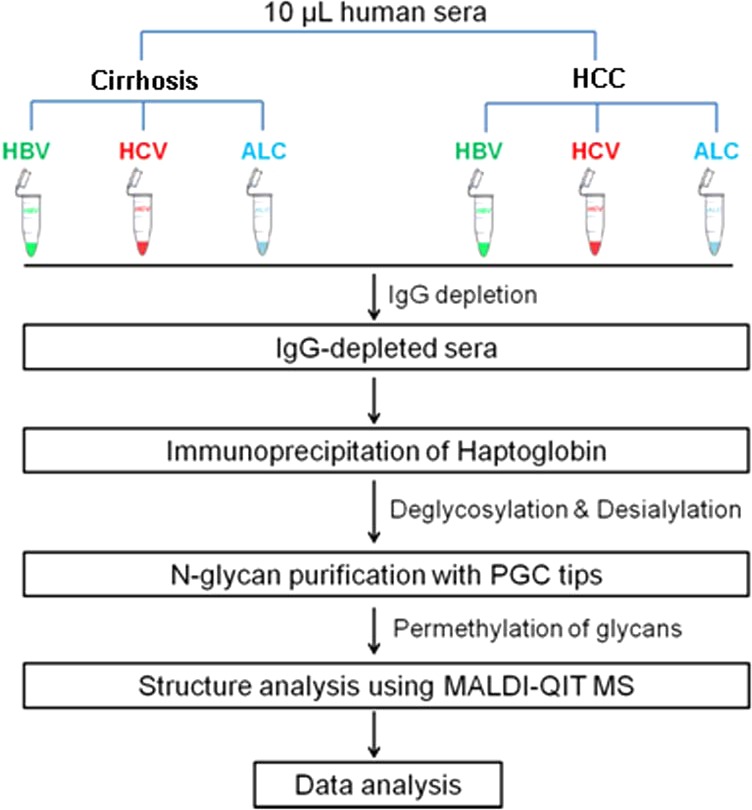
Workflow of N-glycan profiling of haptoglobin and fucosylation changes between hepatocellular carcinoma and liver cirrhosis of the three most common etiologies, infection with hepatitis B virus (HBV), infection with hepatitis C virus (HCV), and heavy alcohol consumption (ALC).
Purification of Serum Haptoglobin
A total of 50 HCC cases (9 HBV-, 21 HCV-, and 20 ALC-related), 46 cirrhosis cases (10 HBV-, 18 HCV-, and 18 ALC-related), and 14 normal controls were investigated by glycomic analysis. There is a distinct geographic variation in the etiology, with the majority of HBV-associated liver disease in Africa, Asia, and the western Pacific region, whereas the HCV-associated liver disease is found mainly in Europe, North America, and Japan.4 Because of the limited access to HBV-related liver disease samples in the United States, only 9 HBV-related HCC and 10 HBV-related cirrhosis samples were included in this study.
Prior to immunoprecipitation, the haptoglobin abundance in sera of patients with HCC and cirrhosis and healthy donors was measured by ELISA to evaluate the protein abundance variation among different groups. As shown in Supporting Information Figure S1A, the serum concentration of total Hp (mean ± SD) in the cancer, cirrhosis, and normal groups was 1195 ± 140, 882 ± 115, and 1043 ± 169 μg/mL, respectively. Thus, there is no significant difference in serum Hp level between HCC and cirrhosis or normal controls. However, when the cancer and cirrhosis samples were divided into three different subgroups according to the etiology (i.e., HBV, HCV, and ALC), a significant increase in Hp abundance was observed in HBV-related HCC compared to HBV-related cirrhosis (p < 0.05, Supporting Information Figure S1B). The result showed that the protein level of haptoglobin remained the same between HCC and cirrhosis with various etiologies except for HBV infection. In the following quantitative mass spectrometry analyses, the abundance of each N-glycan of Hp was normalized by the sum of all N-glycan abundances identified in each sample. Thus, the changes in the fucosylation level of serum Hp investigated in this study are due to the specific glycosylation alteration rather than protein abundance variation. ELISA showed that the abundance of Hp was extremely low in 6 of 18 patients with HCV-related cirrhosis, which was further confirmed by gel electrophoresis (data not shown). These six samples were then removed from the sample set.
After immunoprecipitation of Hp from IgG-depleted serum, the yield and purity of Hp was evaluated by gel electrophoresis in combination with silver staining and by mass spectrometry analysis after rapid on-plate digestion.15 A representative image of SDS-PAGE corresponding to Hp captured from sera of HCC and cirrhosis patients, affected by HBV, HCV, and ALC, respectively, is shown in Figure 2. One-fifth of the Hp eluent was loaded on the gel, and 3 μL of the Kaleidoscope protein marker (Bio-Rad, Hercules, CA) was applied to estimate the yield of haptoglobin. As shown in Figure 2, Hp β chain (∼42 kDa), α-2 chain (∼18 kDa), and α-1 chain (∼13 kDa) were all observed. The composition of α chain varied in patient sera with different liver diseases; however, the β chain remained the same. This is consistent with known work in which Hp is characterized by a genetic polymorphism that arises from differences in α chains, whereas the β chains are identical in all Hp types.13 No contamination from other proteins was observed in the eluted haptoglobin (Figure 2). The total yield of haptoglobin β chain was estimated to be around 1 to 2 μg per 10 μL of serum. The yield of immunoprecipitation was limited by the starting volume of serum and the efficiency of the haptoglobin antibody. In this method, 1 μg of protein is sufficient for subsequent glycan analysis. Rapid on-plate digestion and MALDI-QIT-TOF analysis further confirmed the identity and purity of haptoglobin. The mass spectrum, shown in Supporting Information Figure S2, was searched against the Mascot database, which showed human haptoglobin as the only significant protein, having 11 matched peptides.
Figure 2.
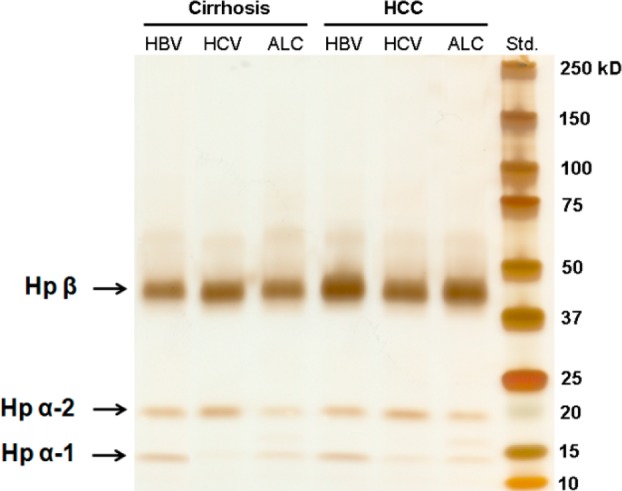
Purification of haptoglobin from 10 μL of serum from patients with HCC and cirrhosis, induced by HBV, HCV, and ALC, respectively. One-fifth of each individual immunoprecipitation eluent was subjected to 4–20% SDS-PAGE followed by silver staining. Hp α-1, α-2, and β chains were observed. Although the composition of Hp α chains varies among patients with different liver diseases, the β chains are identical.
N-Glycan Profiles of Haptoglobin with Desialylation
After purification of Hp from serum, the N-glycans were released from haptoglobin using PNGase F. To quantitate the variation in fucosylation level of Hp in patients with HCC and cirrhosis as well as healthy donors, sialic acids were removed from the glycans. This was performed such that sialylated glycans can easily lose a significant amount of sialic acid in the ion source or after the ion extraction from the ion source, considerably distorting the glycan profiles. Also, the removal of sialic acids eliminates the complicated heterogeneity of sialic acids so that the glycan spectrum is simplified. In addition, removal of sialic acids can combine glycans with differences in their sialic acid content into one peak so that the sensitivity is highly improved. Thus, glycans were treated with neuraminidase to remove sialic acids. After treatment with neuraminidase, no peaks for glycans with sialic acids were observed in the MALDI-TOF-MS spectra, indicating that sialic acids were completely removed by enzymatic cleavage.
The desialylated N-glycans were then subjected to permethylation. The approach requires methylation of all of the hydroxyl groups on saccharides. Permethylation is useful for in-depth analysis of glycans because it provides information on linkages and branches that supplement tandem mass spectrometry for structural determination. The improved signal intensity enables us to determine the relative quantities of each fucosylated glycan present in the complex glycan profile. The procedure of permethylation for N-glycans has been well-established by Kang,19 which is widely used because of its simplicity and robustness. The factors that affect the efficiency of in-solution permethylation have been discussed in our previous study.20 Although permethylation is simple and robust, special attention should be paid to sample handling, including using fine sodium hydroxide powder, adding ice-cold water after the reaction, and performing washes until the top water layer is neutral. According to the optimization procedure, in this study, we achieved a highly efficient permethylation of the released N-glycans. The MALDI-MS spectra illustrated that all N-glycans were fully permethylated with minimal side reaction peaks, giving a yield of greater than 95% based on the peak area. The major side product is the underpermethylated glycan, which is 14 Da smaller than the fully permethylated one. To obtain the accurate peak area of each glycan, both the fully permethylated glycan and its underpermethylated counterpart at 14 Da less were included in the peak integration. All permethylation reactions were performed in the same manner to reduce inconsistency.
A typical desialylated N-glycan profile of human haptoglobin from healthy controls and cirrhosis and HCC patients is shown in Figure 3. In total, 8 glycan structures were identified, as listed in Table 2, including nonfucosylated bi-, tri-, and tetra-antennary glycans (m/z 2070.07, 2519.28, and 2968.49, respectively), monofucosylated bi-, tri-, and tetra-antennary glycans (m/z 2244.13, 2693.40, and 3142.69, respectively), and bifucosylated tri- and tetra-antennary glycans (m/z 2867.48 and 3316.69, respectively). The glycan composition and core/antennary fucosylation were further confirmed by MALDI-QIT-TOF MS/MS analysis, as shown in Supporting Information Figure S3.
Figure 3.
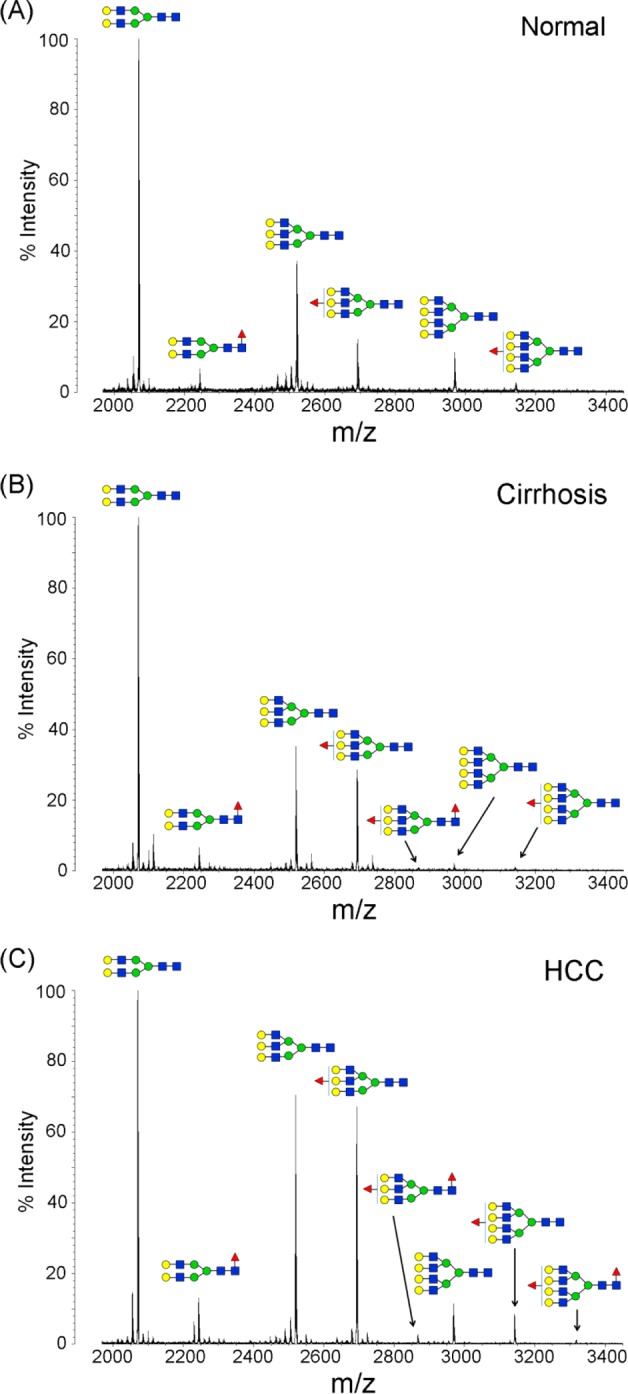
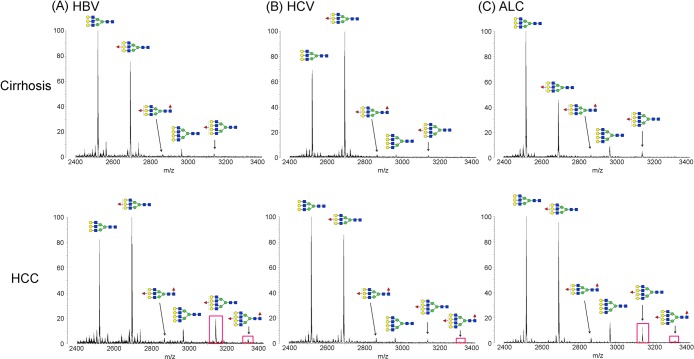
Representative MALDI-QIT-TOF MS spectra of desialylated haptoglobin N-glycans in sera of healthy subjects (A) and patients with liver cirrhosis (B) and HCC (C). Glycans were desialylated by neuraminidase, purified with PGC tips, and permethylated. The nonfucosylated bi- and triantennary complex-type glycans (m/z 2070.07 and 2519.28) were the most abundant structures in healthy subjects and patients with cirrhosis and HCC. Both the triantennary and tetra-antennary glycans were highly elevated in HCC compared to their abundance in normal and cirrhosis samples. The bifucosylated glycans, with both core and antennary fucosylation, were predominantly identified in HCCs but were absent in healthy subjects. Patients with cirrhosis showed a distinctly low abundance of bifucosylated triantennary (m/z 2867.48) glycan. The composition of each glycan is confirmed with both MS/MS analysis and knowledge of their biosynthesis pathways (red triangle, Fuc; blue square, GlcNAc; green circle, Man; yellow circle, Gal).
Table 2. Desialylated N-Glycans Identified in Haptoglobin from Patient Sera with Hepatocellular Carcinoma and Liver Cirrhosis.
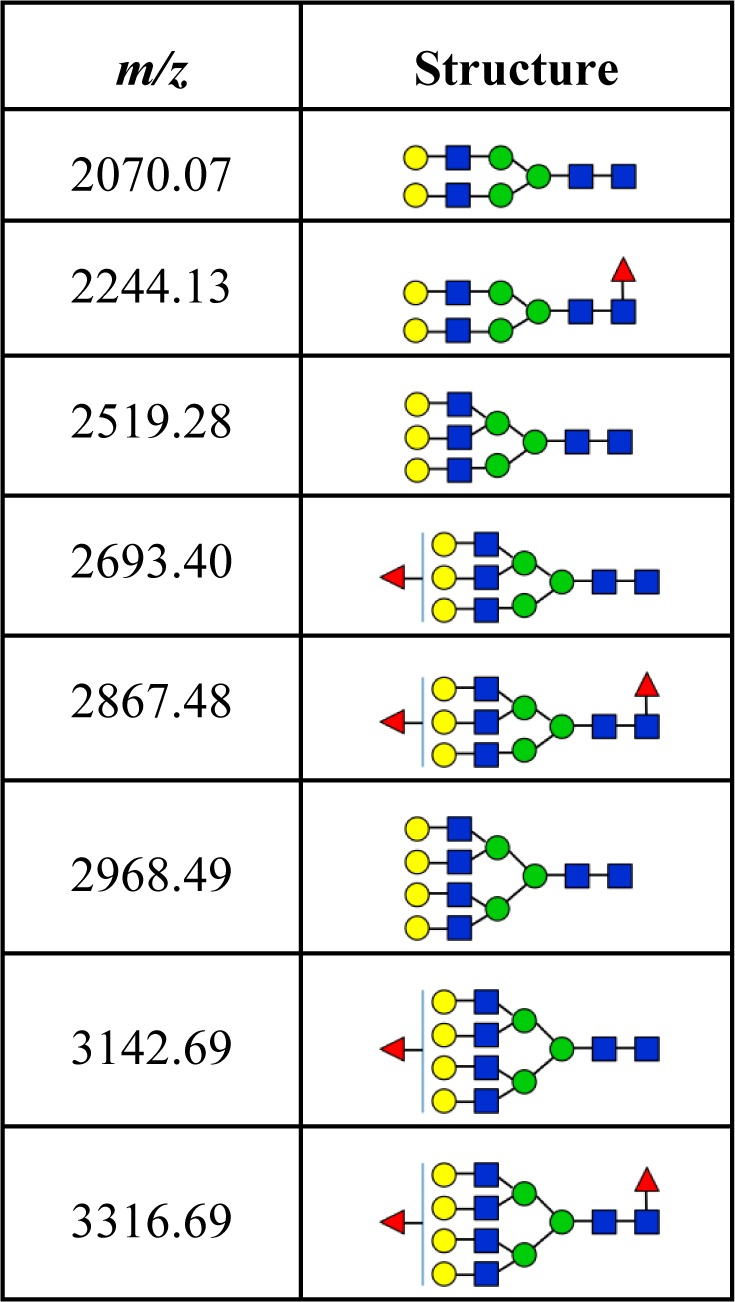
As Figure 3A shows, the nonfucosylated bi- and triantennary complex-type glycans were the most abundant structures in serum Hp of healthy subjects, accounting for more than 65% of the total N-glycan pool. The fucosylated glycans were distinctly increased in patients with cirrhosis and HCC (Figure 3B,C) compared to that in healthy subjects. No bifucosylated glycans were observed in serum Hp of healthy subjects (Figure 3A), whereas a low-abundance bifucosylated triantennary glycan was observed in cirrhosis (Figure 3B) that was distinctly increased in HCC (Figure 3C), so this glycan can distinguish HCC and cirrhosis from normal subjects. Notably, the bifucosylated tretra-antennary glycans, with both core and antennary fucosylation, were predominantly present in HCC, suggesting that this glycan may serve as a possible distinctive marker for HCC. In addition, both the triantennary and tetra-antennary glycans were highly elevated in HCC compared to their abundance in healthy and cirrhosis samples. The result is consistent with a previous study11 that showed an increased level of triantennary glycans in serum Hp of HCC patients.
Elevated Fucosylation and Tetra-Antennary Glycans in HCC
Both the fucosylated and branched glycans were highly elevated in HCC compared to their levels in healthy controls and cirrhosis patients. Figure 4 represents a zoomed-in comparison of tri- and tetra-antennary structures of serum Hp in cirrhosis and HCC, induced by HBV, HCV, and ALC, respectively. The peaks of nonfucosylated (m/z 2968.49) and monofucosylated (m/z 3142.69) tetra-antennary glycans highly increased in HBV- and ALC-related HCCs compared with the corresponding cirrhosis; however, no significant alteration of these two glycans was observed in the group of HCC and cirrhosis affected by HCV. The elevated intensity of the peak (m/z 2867.48) corresponding to bifucosylated triantennary glycan was observed in all HCCs compared to that in liver cirrhosis. Notably, a unique peak of the bifucosylated tetra-antennary glycan (m/z 3316.69) was predominantly present in HCC samples but not in liver cirrhosis except for a small proportion of ALC-related cirrhosis. This structure was observed in 8 of 18 ALC-related cirrhosis, where peak intensity was generally weaker compared to that in HCC. The result illustrates that the bifucosylated tetra-antennary structure can discriminate HCC from cirrhosis, with further distinction of HBV- and ALC-related HCC from HCV-related HCC based on the monofucosylated tetra-antennary structure.
Figure 4.
MALDI-QIT-TOF MS spectra showing the difference of fucosylation in tri- and tetra-antennary N-glycans of haptoglobin between HCC and cirrhosis in relation to the etiology, HBV (A), HCV (B), and ALC (C), respectively. The bifucosylated tetra-antennary (m/z 3316.69) glycan was predominantly present in HCC samples but not in liver cirrhosis. The tetra-antennary glycans were highly elevated in HBV- and ALC-related HCC compared with the corresponding levels in cirrhosis; however, no significant difference in tetra-antennary glycans was observed between HCV-related HCC and cirrhosis. The elevated presence of fucosylated tetra-antennary glycans in HCC samples compared to that in cirrhosis of each etiology is highlighted with a red rectangle.
Fucosylation Correlation with Etiology
We further calculated the relative abundance of each glycan by using a quantitative glycomics method described in our previous study.20 Glycan peak area integration was performed with Matlab. The peak area of each glycan was the addition of the fully permethylated glycan peak and the most abundant underpermethylated glycan peak. The abundance of each glycan was normalized by the sum of all glycan abundances identified in each sample. MALDI MS analysis of permethylated N-glycans has been demonstrated to be highly quantitative, making it possible to evaluate reliably the N-glycan profiles of haptoglobin from sera of HCC patients and to compare them with profiles from sera of cirrhosis and healthy subjects.
In total, we were able to quantify N-glycan profiles in 104 individual patient samples, including 50 HCC cases (9 HBV-, 21 HCV-, and 20 ALC-related), 40 cirrhosis cases (10 HBV-, 12 HCV-, and 18 ALC-related), and 14 healthy controls. Relative abundance changes in the N-glycan profile of haptoglobin, with a focus on the five fucosylated N-glycans, in sera of healthy controls as well as in cirrhosis and HCC patients with different etiologies are shown in Figure 5.
Figure 5.
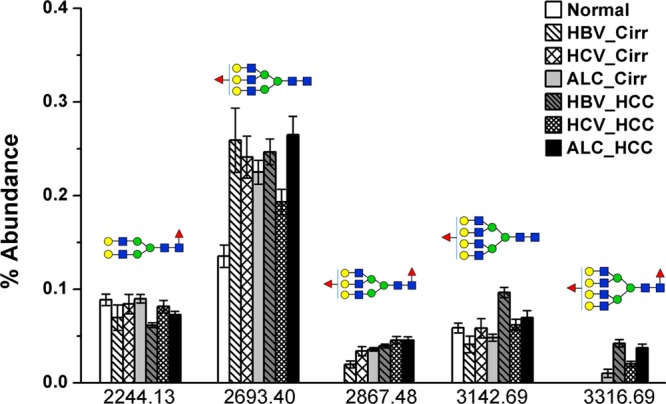
Relative abundance changes of five fucosylated N-glycans in haptoglobin from sera of healthy controls and patients with cirrhosis and HCC of different etiologies. Fucosylation level was significantly increased in liver cirrhosis and HCC compared to healthy individuals. No bifucosylated N-glycans were observed in serum haptoglobin of healthy controls. The monofucosylated tetra-antennary N-glycan was highly increased in HBV- and ALC-related HCC compared to that in the corresponding cirrhosis patients, but it was not increased in HCV-related HCC. Notably, bifucosylated tetra-antennary glycan was predominantly identified in HCC of all etiologies (HBV_Cirr, HBV-related cirrhosis; HCV_Cirr, HCV-related cirrhosis; ALC_Cirr, ALC-related cirrhosis; HBV_HCC, HBV-related HCC; HCV_ HCC, HCV-related HCC; ALC_ HCC, ALC-related HCC).
As shown in Figure 5, in healthy controls, the mean level of the monofucosylated bi-, tri-, and tetra-antennary glycans in the N-glycan profiles was 8.84, 13.52, and 5.84%, respectively. ANOVA analysis showed that no significant difference in the abundance of monofucosylated biantennary glycan was observed between normal subjects, cirrhosis, and HCC. However, the monofucosylated triantennary N-glycan was distinctly increased in sera of patients with cirrhosis and HCC compared to that from healthy subjects, ranging from 19.3 to 26.49% of the glycan profiles. The monofucosylated tetra-antennary N-glycan was significantly increased in serum Hp of HCC induced by HBV infection, which can discriminate HBV-related HCC from HBV-related cirrhosis (p < 0.001).
It should be noted that no bifucosylated glycans were observed in serum Hp of healthy subjects. The mean level of bifucosylated triantennary glycan ranged from 1.95 to 4.55% in serum Hp of patients with cirrhosis and HCC, which could be used to distinguish HCC from HBV-related cirrhosis patients (p < 0.001). The unique pattern of bifucosylated tetra-antennary glycan was predominantly identified in HCC patients, ranging from 2.01 to 4.21% of the glycan profiles, but it was not present in HBV- and HCV-related cirrhosis. It was observed in 8 of 18 ALC-related cirrhosis patients, with a low abundance of 1.01 ± 0.35%. The results showed that the bifucosylation tetra-antennary structure can discriminate HCC from cirrhosis, which may allow it to serve as a possible marker for HCC.
Statistical Analysis of Total Fucosylation and Bifucosylation Degrees
To quantify the degree of total fucosylation, a fucosylation index developed by Imre and co-workers22 was applied to haptoglobin N-glycans to discriminate between healthy, cirrhosis, and HCC patients with different etiologies. The total fucosylation index gives the average number of fucose units for a group of oligosaccharides. It is defined as
where glycan F1 denotes the sum of abundances of singly fucosylated glycans, glycan F2 denotes that of bifucosylated glycans, and ∑glycans represents the sum of abundances of all glycans. In this study, we used this index to characterize the total fucosylation level of serum Hp and to illustrate the differences between HCC and cirrhosis in relation to etiology. A comparison of total fucosylation degree among healthy and cirrhosis and HCC induced by HBV, HCV, and ALC, respectively, is shown in Figure 6A. The receiver operating characteristics (ROC) curves of the total fucosylation level between the HCC and cirrhosis patient groups are shown in Figure 6C.
Figure 6.
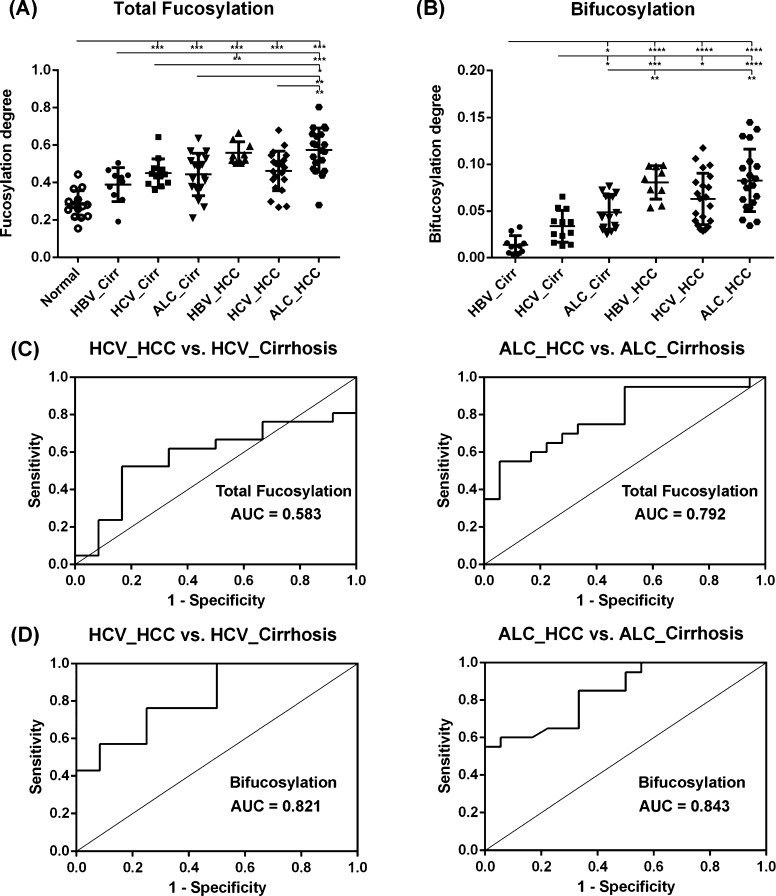
(A) Scatter plot of the total fucosylation degree of haptoglobin N-glycans in healthy subjects and cirrhosis and HCC patients induced by HBV, HCV, and ALC, respectively. The total fucosylation levels are distinctly elevated in HCC and cirrhosis patients compared to that in healthy controls. The elevated fucosylation degree can discriminate HCC from cirrhosis patients induced by HBV infection (p < 0.01) or alcohol abuse (p < 0.01), but it cannot do so in the HCV-related HCC and cirrhosis cases. (B) Scatter plot of the bifucosylation degree of haptoglobin N-glycans in cirrhosis and HCC patients of each etiology, which shows a significantly increased bifucosylation level in HCC of all etiologies compared to that in the corresponding cirrhosis. Notably, the bifucosylation level is distinctly elevated in all HBV-related HCC patients compared to that in HBV-related cirrhosis in this sample set (p < 0.0001), suggesting good diagnostic power of bifucosylation degree in discriminating these liver diseases. (C, D) Receiver operating characteristics (ROC) curves of the total fucosylation degree (C) and bifucosylation degree (D) to differentiate HCC from cirrhosis cases induced by HCV and ALC, respectively (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001).
The overall fucosylation degree ranges from 0.15 to 0.83 for all samples. The non-cancer groups (normal and cirrhosis affected by HBV and ALC, respectively) have a low fucosylation degree (the mean is 0.28, 0.39, and 0.42, respectively), but the fucosylation degree is significantly elevated in HBV- and ALC-related HCCs with a mean of 0.57 and 0.58, respectively (Figure 6A). ANOVA analysis showed a statistically significant increase in the fucosylation degree in HBV-related HCC and ALC-related HCC compared to that in the corresponding cirrhosis samples (p < 0.01). The total fucosylation degree was elevated in all HBV-related HCC patients compared to that for HBV-related cirrhosis in this sample set, suggesting good diagnostic performance of the total fucosylation degree in distinguishing these two diseases. The ROC curve analysis between ALC-related HCC and ALC-related cirrhosis resulted in an AUC value of 0.792 with a specificity of 75% at a sensitivity of 72% (Figure 6C). However, no significant change in the total fucosylation degree was observed in the group of HCC and cirrhosis induced by HCV infection (the mean is 0.45 and 0.46, respectively). The ROC curve analysis between HCV-related HCC and HCV-related cirrhosis showed an AUC of only 0.583 (Figure 6C). The statistical elevation of the total fucosylation level provides a potential marker to discriminate HCC with ALC and HBV etiologies from their corresponding cirrhosis.
We also evaluated the bifucosylation degree where this showed the greatest differences between HCC and cirrhosis in the mass spectra. The bifucosylation degree in this study is calculated by the ratio of the sum of abundances of bifucosylated glycans to all glycans, which is
A comparison of the bifucosylation degree between cirrhosis and HCC in relation to etiology is shown in Figure 6B. The ROC curves of the bifucosylation level between the patient groups are shown in Figure 6D.
The overall bifucosylation degree ranges from 0.005 to 0.14 for all cirrhosis and HCC samples. The cirrhosis groups caused by HBV, HCV and ALC have a degree of low fucosylation (the mean is 0.014, 0.033, and 0.048, respectively), but the bifucosylation degree is highly elevated in the corresponding HCCs, with a mean of 0.081, 0.063, and 0.082, respectively. The distinctly increased bifucosylation degree in HBV- and ALC-related HCCs was consistent with the elevated total fucosylation level in these subgroups. ANOVA analysis showed statistically significant differences between HCC samples and the corresponding cirrhosis samples of all etiologies (Figure 6B). The power analysis showed that the power at the calculated differences of the means (delta mean) of the bifucosylation level in the comparison groups is higher than 95%, which provides statistical support for the number of samples included in this study.
Notably, a distinctly elevated bifucosylation degree was observed in all HBV-related HCC patients compared to that in HBV-related cirrhosis, with a low p value (p < 0.0001). The ROC curve analysis resulted in an AUC of 1, indicating the diagnostic power of bifucosylation degree in distinguishing subjects from these two diseases in this sample set. Interestingly, the ROC curve analysis between HCV-related HCC and HCV-related cirrhosis yielded an AUC of 0.821 with a specificity of 75% at a sensitivity of 76% (Figure 6D). Bifucosylation degree has an AUC of 0.843 in differentiating ALC-related HCC from ALC-related cirrhosis samples, with a specificity of 70% at a sensitivity of 85% (Figure 6D). The significant elevation of bifucosylation degree in HCC patients of all etiologies compared to the corresponding cirrhosis cases suggested that it may serve as a promising predictor to track the disease progression of patients suffering from cirrhosis.
The core-fucosylation degree was also evaluated by the ratio of the sum of the abundance of core-fucosylated glycans to all glycans (data not shown), which showed a significantly elevated core-fucosylation level between HBV-related HCC and HBV-related cirrhosis samples (p < 0.05). However, no significant difference of core fucosylation was observed in HCV-related or ALC-related HCC samples compared to that of the corresponding cirrhosis in this sample set.
Validation of Reproducibility of the Quantitation Method
To evaluate the reproducibility of the method, four aliquots of an HCV-related HCC sample were processed, and the total fucosylation and bifucosylation degrees of each aliquot were calculated (Supporting Information Table S1A). The relative standard deviation (RSD) of the total fucosylation and bifucosylation degrees is 4.3 and 8.3%, respectively, for the four replicates. The result showed the high analytical reproducibility of the method, which is able to provide reproducible quantitative data for fucosylation aberration analysis.
We further investigated the effect of serum sample amount on quantification of the total fucosylation and bifucosylation levels. Three different volumes, 8, 10, and 20 μL, of the same serum sample from an ALC-related HCC patient were tested. The glycan spectra showed that all 8 glycans were identified in these three different volumes and no differences were observed in the spectra (Supporting Information Figure S4). The total fucosylation and bifucosylation degrees in these three different volumes are consistent (Supporting Information Table S1B), and the RSD is 3.9 and 7.3%, respectively, for the total fucosylation and bifucosylation degrees. The result suggests that, for a given patient, the sample volume does not exert significant effects on the quantification of fucosylation and bifucosylation degrees.
To measure the range of our quantitation method, we performed a test on a human haptoglobin standard in sequential aliquots of 0.1, 0.3, 0.5, 1, 2, 5, 10, 15, and 20 μg. The limit of detection (LOD) was found to be 0.3 μg. As shown in Supporting Information Figure S5, no significant differences in glycan distribution were observed in the 8 glycan spectra from 0.3 to 20 μg. Although the total intensity of the spectrum increased with the sample amount, the fucosylation degree is consistent for the 8 sequential aliquots, and the RSD is 9.8%. The result demonstrated the consistency of the quantitation of fucosylation degree in a human haptoglobin standard within a range of 0.3 to 20 μg.
Validation of Total Fucosylation Degree by Lectin Blot
An AAL lectin blot was performed to validate the total fucosylation level in patients with HCC and cirrhosis induced by HBV, HCV, and ALC, respectively. As shown in Supporting Information Figure S6, equal amounts of haptoglobin purified from 6 HCC and 6 cirrhosis patients were loaded on a 4–15% SDS-PAGE gel and evaluated by AAL lectin blot. The result verified that the total fucosylation level of haptoglobin was significantly increased in HCC patients with HBV and ALC etiologies compared to that in the corresponding cirrhosis, which was consistent with the MS result.
Although LCA and AOL bind specifically to core fucose, there is no lectin with a strict binding specificity to the bifucosylated glycan structures identified by mass spectrometry. Moreover, LCA binds not only to core fucose but also to mannose residues in N-glycans. MALDI-TOF MS is more specific to evaluate individual glycan structures than is a lectin blot, providing detection of specific glycan structures. In this case, as shown in Figure 4, the larger peaks in the mass spectrum at m/z 2693.40 and 3142.69 that contain fucose will overwhelm any differences in signal that are due to changes in the bifucosylation peaks at m/z 2867.48 and 3316.69 that are much smaller. The bifucosylation change can be confirmed only with mass spectrometric detection. Our next step is to confirm our findings in a larger cohort of patients with diverse ethnicity and etiology of liver disease.
Combinatorial Analysis of Bifucosylation Degree with AFP
Because the bifucosylation degree of serum haptoglobin was distinctly elevated in HCC compared to that in cirrhosis patients, we further performed a 2D plot of the bifucosylation degree and the clinical AFP value in cirrhosis and HCC patients with different etiologies. As shown in Figure 7, most HCC patients (represented by solid icons) were found in the upper panel of the plot, with distinctly increased bifucosylation degrees of haptoglobin. In contrast, the cirrhosis patients (represented by hollow icons) clustered in the lower left panel of the plot, indicating low levels of both bifucosylation degree of haptoglobin and AFP value. When combining the AFP value with the bifucosylation degree of haptoglobin, the performance of the clinical marker, AFP, was significantly increased. The likelihood ratio (LR) test p value was calculated to evaluate the significance in the improvement between the combination model of Hp bifucosylation degree with AFP and the AFP alone model. For example, the combination of AFP and bifucosylated haptoglobin had an AUC of 1 to distinguish between HBV-related liver diseases in this sample set, showing a distinctively improved diagnostic performance compared to that of AFP alone (AUC = 0.815, LR test p value < 0.0001). The combination of bifucosylated haptoglobin and AFP also showed improved performance in differentiating HCV-related HCC from HCV-related cirrhosis samples (AUC=0.896) compared to that of AFP alone (AUC = 0.750) (LR test p value = 0.0024). It should be noted that the bifucosylated haptoglobin had an AUC of 0.821, which outperforms AFP in distinguishing HCV-related HCC from the corresponding cirrhosis patients. With the combination of bifucosylated haptoglobin and AFP, the AUC value between ALC-related HCC and the corresponding cirrhosis increased to 0.936, which is a significant improvement over AFP alone (AUC = 0.868, LR test p value = 0.0004). This improvement is mainly due to the enhanced sensitivity, which increased from 72 to 91%.
Figure 7.
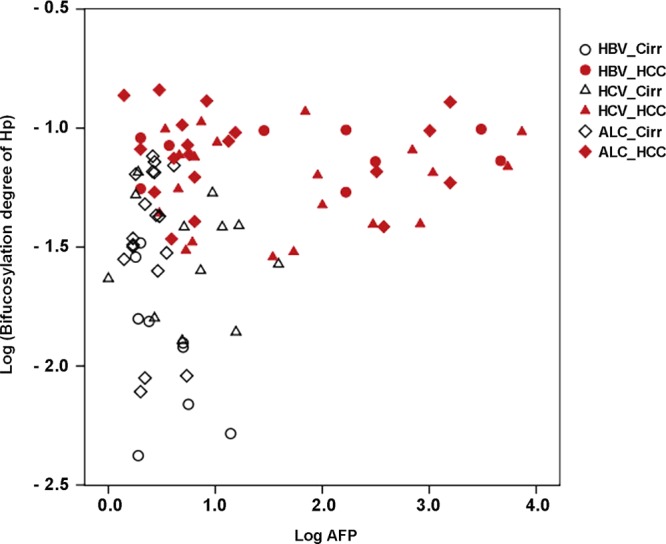
Two-dimensional plot of the bifucosylation degree of serum Hp and the clinical AFP value in cirrhosis (hollow icons) and HCC (solid icons) patients with different etiologies. Each spot represents an individual patient. For the plot, log-transformed values of each marker are used.
Bifucosylation Degree for the Early Detection of HCC
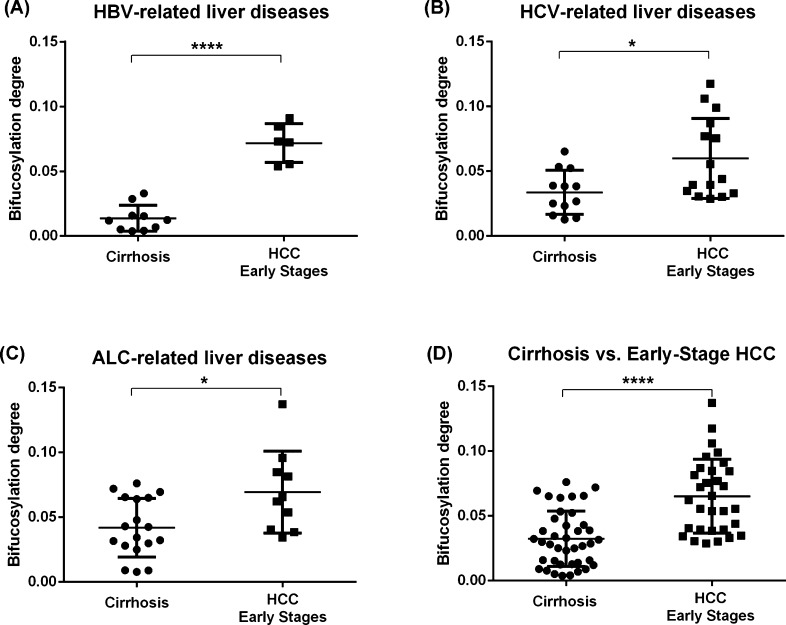
We further evaluated the performance of the bifucosylation degree of haptoglobin to distinguish early stage HCC patients from cirrhosis cases with different etiologies. As shown in Figure 8, the level of bifucosylated haptoglobin was significantly increased in HCC patients at early stages compared to the corresponding level in cirrhosis patients in each etiology (p < 0.05). The most notable difference was observed between early stage HBV-related HCC and HBV-related cirrhosis samples (p < 0.0001, Figure 8A). When combining the three etiologies together, a statistically significant difference in bifucosylation degree of haptoglobin was observed among all early stage HCCs and cirrhosis patients (p < 0.0001, Figure 8D). The ROC curve analysis showed that bifucosylated haptoglobin shows an improved performance (AUC = 0.834) in differentiating early stage HCC from cirrhosis patients when compared with AFP (AUC = 0.764) in this sample set. Because the majority of patients with HCC have underlying cirrhosis, the bifucosylation degree of serum haptoglobin may provide a potential marker for the early diagnosis and prediction of HCC in patients with cirrhosis induced by HBV, HCV, and ALC. Compared to early stage HCC samples, no significant difference in bifucosylation degree was found in advanced HCC samples in each etiologic group, mainly because of limited cases of advanced-stage samples of each etiology. However, when combining the three etiologies together, both the total fucosylation and bifucosylation degrees showed a significant difference between advanced and early stages (p < 0.05). This is a preliminary finding over a limited sample size and will require validation with a larger cohort of patients.
Figure 8.
Scatter plot of the bifucosylation degree of haptoglobin N-glycans in HCC patients at early stages and cirrhosis cases with different etiologies, HBV (A), HCV (B), and ALC (C), and combined etiologies (D). Bifucosylation degrees are significantly elevated in HCC patients at early stages versus that in the corresponding cirrhosis of different etiologies. When combining the three etiologies together, a significant difference in bifucosylation degree of haptoglobin was also observed between all early stage HCCs and cirrhosis patients (p < 0.0001) (*, p < 0.05; ****, p < 0.0001).
Conclusions
Because 80–90% of patients with HCC have an established background of liver cirrhosis and the development of HCC typically occurs after long periods of chronic liver disease,1 it is important to find biomarkers to monitor and predict disease progression in patients suffering from cirrhosis. In this study, a comprehensive comparison of fucosylation aberration in serum Hp was performed between HCC and cirrhosis patients of the three most common etiologies, infection with hepatitis B virus (HBV), infection with hepatitis C virus (HCV), and heavy alcohol consumption (ALC). We have described herein a quantitative mass spectrometry-based approach to determine fucosylation alterations of serum Hp in 104 individual patients, including 50 HCC, 40 cirrhosis, and 14 healthy controls. MALDI-QIT-TOF MS analysis showed that both fucosylated and branched N-glycan structures were distinctly increased in patients with cirrhosis and HCC compared to healthy subjects, which is consistent with previous studies.11 Singly fucosylated triantennary glycan, with antennary fucosylation, was found to be significantly increased in HCC and cirrhosis. A similar result has been reported in a study of serum N-glycomic changes in HCC patients infected with HBV, showing that a branched (α-1,3)-fucosylated triantennary glycan was found to be more abundant in patients with HCC than those with cirrhosis and healthy subjects.23 No bifucosylated glycans were observed in serum Hp of healthy subjects. The most notable change is a unique bifucosylated tetra-antennary glycan that was predominantly present in HCC patients, suggesting that this glycan may serve as a possible distinctive marker for HCC.
Quantitative analysis of the total fucosylation degree and bifucosylation degree of serum Hp revealed their diagnostic potential in discriminating HCC from cirrhosis patients of different etiologies. Compared to the corresponding cirrhosis patients in each etiologic category, the total fucosylation degree was significantly increased in HBV- and ALC-related HCC but not in HCV-related HCC patients. Interestingly, bifucosylation degree, corresponding to the level of bifucosylated Hp, was distinctly elevated in HCC of all etiologies compared to that in the corresponding cirrhosis cases. The significantly elevated bifucosylation degree was observed in all HBV-related HCC patients compared to that in HBV-related cirrhosis in this sample set (p < 0.0001), suggesting the diagnostic power of bifucosylation degree in distinguishing these two diseases. The performance of bifucosylation degree in discriminating HCV- or ALC-related HCC from the corresponding cirrhosis patients was evaluated, resulting in an AUC value of 0.821 and 0.843, respectively, which outperforms AFP to distinguish these liver diseases. With the combination of bifucosylated haptoglobin, the performance of the clinical marker AFP was significantly improved in distinguishing HCC from cirrhosis cases of each etiology. Furthermore, the elevated bifucosylation is present in all early stage HCC samples in this study, suggesting that it may serve as a promising predictor for early detection of HCC in patients with cirrhosis. The next step is to confirm our findings in a larger cohort of patients with diverse ethnicity and etiology of liver disease.
Acknowledgments
We acknowledge the support of this work from the National Cancer Institute under grant nos. 1R01 CA160254 (D.M.L.) and 1R01 CA154455 (D.M.L.) and from the National Institutes of Health under grant R01 GM 49500 (D.M.L.).
Supporting Information Available
Scatter plot of serum Hp concentration measured by ELISA assay in groups of HCC, cirrhosis, and normal patients as well as in the subgroups divided according to etiology, HBV, HCV, and ALC; representative MALDI-QIT-TOF MS spectrum of tryptic peptides of haptoglobin resulting from 10 min on-plate digestion; representative MALDI-QIT-TOF MS/MS spectra of glycans at m/z 2244.13, 2867.48, 2693.40, and 3142.69 for glycan composition and core/antennary fucosylation assignment; MALDI-QIT-TOF MS spectra of N-glycans from an ALC-related HCC serum sample evaluated at three different volumes; MALDI-QIT-TOF MS spectra of N-glycans from a human haptoglobin standard processed in sequential aliquots of 0.3, 0.5, 1, 2, 5, 10, 15, and 20 μg; AAL lectin blot analysis of fucosylation level of haptoglobin in patients with HCC and cirrhosis induced by HBV, HCV, and ALC; reproducibility test of four aliquots of an HCV-related HCC sample; and influence of sample amount on the quantitation of fucosylation and bifucosylation degrees evaluated at three different volumes. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Shariff M. I.; Cox I. J.; Gomaa A. I.; Khan S. A.; Gedroyc W.; Taylor-Robinson S. D. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis and therapeutics. Expert Rev. Gastroenterol. Hepatol. 2009, 3, 353–367. [DOI] [PubMed] [Google Scholar]
- Davis G. L.; Dempster J.; Meler J. D.; Orr D. W.; Walberg M. W.; Brown B.; Berger B. D.; O’Connor J. K.; Goldstein R. M. Hepatocellular carcinoma: management of an increasingly common problem. Proceedings (Baylor Univ. Med. Center) 2008, 21, 266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Serag H. B. Hepatocellular carcinoma: an epidemiologic view. J. Clin. Gastroenterol. 2002, 35, S72–S78. [DOI] [PubMed] [Google Scholar]
- Sanyal A. J.; Yoon S. K.; Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist 2010, 15, 14–22. [DOI] [PubMed] [Google Scholar]
- Wang M.; Long R. E.; Comunale M. A.; Junaidi O.; Marrero J.; Di Bisceglie A. M.; Block T. M.; Mehta A. S. Novel fucosylated biomarkers for the early detection of hepatocellular carcinoma. Cancer Epidemiol., Biomarkers Prev. 2009, 18, 1914–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D.; Mallory T.; Satomura S. AFP-L3: a new generation of tumor marker for hepatocellular carcinoma. Clin. Chim. Acta 2001, 313, 15–19. [DOI] [PubMed] [Google Scholar]
- Arrieta O.; Cacho B.; Morales-Espinosa D.; Ruelas-Villavicencio A.; Flores-Estrada D.; Hernandez-Pedro N. The progressive elevation of alpha fetoprotein for the diagnosis of hepatocellular carcinoma in patients with liver cirrhosis. BMC Cancer 2007, 7, 28-1–28-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H. T.; Chang Y. H.; Chen Y. Y.; Lee T. H.; Tai D. I.; Lin D. Y. AFP-L3 in chronic liver diseases with persistent elevation of alpha-fetoprotein. J. Chin. Med. Assoc. 2007, 70, 310–317. [DOI] [PubMed] [Google Scholar]
- Kumada T.; Toyoda H.; Tada T.; Kiriyama S.; Tanikawa M.; Hisanaga Y.; Kanamori A.; Tanaka J.; Kagebayashi C.; Satomura S. High-sensitivity Lens culinaris agglutinin-reactive alpha-fetoprotein assay predicts early detection of hepatocellular carcinoma. J. Gastroenterol. 2014, 49, 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang I. L.; Poon T. C.; Lai P. B.; Chan A. T.; Ngai S. M.; Hui A. Y.; Johnson P. J.; Sung J. J. Study of serum haptoglobin and its glycoforms in the diagnosis of hepatocellular carcinoma: a glycoproteomic approach. J. Proteome Res. 2006, 5, 2691–2700. [DOI] [PubMed] [Google Scholar]
- Zhang S.; Shu H.; Luo K.; Kang X.; Zhang Y.; Lu H.; Liu Y. N-linked glycan changes of serum haptoglobin beta chain in liver disease patients. Mol. BioSyst. 2011, 7, 1621–1628. [DOI] [PubMed] [Google Scholar]
- Dobryszycka W. Biological functions of haptoglobin–new pieces to an old puzzle. Eur. J. Clin. Chem. Clin. Biochem. 1997, 35, 647–654. [PubMed] [Google Scholar]
- Kurosky A.; Barnett D. R.; Lee T. H.; Touchstone B.; Hay R. E.; Arnott M. S.; Bowman B. H.; Fitch W. M. Covalent structure of human haptoglobin: a serine protease homolog. Proc. Natl. Acad. Sci. U.S.A. 1980, 77, 3388–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama N.; Ide Y.; Nakano M.; Nakagawa T.; Yamanaka K.; Moriwaki K.; Murata K.; Ohigashi H.; Yokoyama S.; Eguchi H.; Ishikawa O.; Ito T.; Kato M.; Kasahara A.; Kawano S.; Gu J.; Taniguchi N.; Miyoshi E. Fucosylated haptoglobin is a novel marker for pancreatic cancer: a detailed analysis of the oligosaccharide structure and a possible mechanism for fucosylation. Int. J. Cancer 2006, 118, 2803–2808. [DOI] [PubMed] [Google Scholar]
- Lin Z.; Simeone D. M.; Anderson M. A.; Brand R. E.; Xie X.; Shedden K. A.; Ruffin M. T.; Lubman D. M. Mass spectrometric assay for analysis of haptoglobin fucosylation in pancreatic cancer. J. Proteome Res. 2011, 10, 2602–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T.; Shinohara Y.; Tissot B.; Pang P. C.; Kurogochi M.; Saito S.; Arai Y.; Sadilek M.; Murayama K.; Dell A.; Nishimura S.; Hakomori S. I. Glycosylation status of haptoglobin in sera of patients with prostate cancer vs. benign prostate disease or normal subjects. Int. J. Cancer 2008, 122, 39–49. [DOI] [PubMed] [Google Scholar]
- Park S. Y.; Yoon S. J.; Jeong Y. T.; Kim J. M.; Kim J. Y.; Bernert B.; Ullman T.; Itzkowitz S. H.; Kim J. H.; Hakomori S. I. N-glycosylation status of beta-haptoglobin in sera of patients with colon cancer, chronic inflammatory diseases and normal subjects. Int. J. Cancer 2010, 126, 142–155. [DOI] [PubMed] [Google Scholar]
- Sanda M.; Pompach P.; Brnakova Z.; Wu J.; Makambi K.; Goldman R. Quantitative liquid chromatography-mass spectrometry-multiple reaction monitoring (LC-MS-MRM) analysis of site-specific glycoforms of haptoglobin in liver disease. Mol. Cell. Proteomics 2013, 12, 1294–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang P.; Mechref Y.; Klouckova I.; Novotny M. V. Solid-phase permethylation of glycans for mass spectrometric analysis. Rapid Commun. Mass Spectrom. 2005, 19, 3421–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z.; Lubman D. M. Permethylated N-glycan analysis with mass spectrometry. Methods Mol. Biol. 2013, 1007, 289–300. [DOI] [PubMed] [Google Scholar]
- Comunale M. A.; Wang M.; Hafner J.; Krakover J.; Rodemich L.; Kopenhaver B.; Long R. E.; Junaidi O.; Bisceglie A. M.; Block T. M.; Mehta A. S. Identification and development of fucosylated glycoproteins as biomarkers of primary hepatocellular carcinoma. J. Proteome Res. 2009, 8, 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imre T.; Kremmer T.; Heberger K.; Molnar-Szollosi E.; Ludanyi K.; Pocsfalvi G.; Malorni A.; Drahos L.; Vekey K. Mass spectrometric and linear discriminant analysis of N-glycans of human serum alpha-1-acid glycoprotein in cancer patients and healthy individuals. J. Proteomics 2008, 71, 186–197. [DOI] [PubMed] [Google Scholar]
- Liu X. E.; Desmyter L.; Gao C. F.; Laroy W.; Dewaele S.; Vanhooren V.; Wang L.; Zhuang H.; Callewaert N.; Libert C.; Contreras R.; Chen C. N-glycomic changes in hepatocellular carcinoma patients with liver cirrhosis induced by hepatitis B virus. Hepatology 2007, 46, 1426–1435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.