Abstract
Objectives
To determine if alpha II-spectrin breakdown products can be detected in the serum of neonates with congenital heart disease in the perioperative period.
Design
Prospective observational cohort study.
Setting
Pediatric cardiac ICU in an urban tertiary care academic center.
Patients
Neonates with congenital heart disease undergoing surgical repair or palliation.
Interventions
Serial blood sampling for measurement of 120 and 150 kDa spectrin breakdown products.
Measurements and Main Results
Fourteen neonates with congenital heart disease undergoing cardiac surgery were evaluated. Nine infants underwent open-heart surgery and five underwent closed-heart surgery. Serum spectrin breakdown products were measured with sandwich enzyme-linked immunosorbent assay preoperatively and then 6, 24, 48, 72, and 96 hours following surgery. Brain imaging was obtained as part of routine clinical care in 12 patients pre-operatively and six patients postoperatively. Six patients had normal preoperative imaging (three closed-heart surgery and three open-heart surgery), whereas six had evidence of neurologic injury prior to surgery (one closed-heart surgery and five open-heart surgery). Only one patient had a postoperative imaging study that lacked injury. All others demonstrated infarction or hemorrhage. Spectrin breakdown product 120 kDa significantly increased 24 hours after open-heart surgery compared to preoperative values and time-matched closed-heart surgery levels. Spectrin breakdown product 150 kDa significantly increased 6 hours after open-heart surgery compared to preoperative values. There was no significant change in spectrin breakdown products following closed-heart surgery. Peak spectrin breakdown products significantly increased following open-heart surgery compared to closed-heart surgery.
Conclusions
Spectrin breakdown products can be detected in the serum of neonates with congenital heart disease in the perioperative period and levels increased to a greater degree in infants following open-heart surgery. These findings suggest that, in future work, serum spectrin breakdown products may potentially be developed as biomarkers for brain necrosis and apoptosis in infants with congenital heart disease.
Keywords: biomarker, brain injury, congenital heart disease, neonate, serum, spectrin
Neurologic injury occurs commonly in infants with complex congenital heart disease (CHD) and is a major cause of neurodevelopmental impairment in this vulnerable cohort (1). Brain injury can be acquired in the perioperative period due to ischemia-reperfusion, hypoxia, and embolic phenomena associated with the use of cardiopulmonary bypass (CPB) and deep hypothermic circulatory arrest (DHCA) and can be a consequence of postoperative cardiopulmonary insufficiency (1). It is now widely recognized that neurologic injury can also manifest in patients with CHD prior to surgery due to inadequate cerebral perfusion secondary to derangements in cardiovascular physiology (1, 2). Such injury can occur in utero or in the perinatal period independent of surgery (2). Current efforts have focused on prevention of acquired brain injury via neuromonitoring and neuroprotective strategies; however, it is unclear if such approaches are effective (1). Unfortunately, identifying perioperative neurologic injury in neonates with CHD is challenging and inexact (2). Thus, there is a need to develop tools such as biochemical markers to enable early detection and quantification of brain injury in order to guide therapy and to help predict prognosis.
Alpha II-spectrin is a 280-kDa neuronal cytoskeleton protein located within axons and presynaptic terminals of the CNS (3). As a substrate for cysteine proteases, alpha II-spectrin is cleaved into characteristic fragments during neuronal cell death (3). With necrosis, calpain cleaves alpha II-spectrin into distinct 150- and 145-kDa cleavage products while apoptosis results in caspase-3-mediated cleavage of alpha II-spectrin into a 120-kDa fragment (3). Collectively, these fragments are termed “spectrin breakdown products” (SBDPs) and when detected indicate neuronal injury and provide a signature for the mechanism of cell death (4).
Both calpain- (SBDP145 and 150) and caspase-3-mediated (SBDP120) SBDPs have been shown to increase in brain parenchyma following controlled cortical impact in mature and immature rodents and are readily detectable in cerebrospinal fluid (CSF) following injury (5, 6). In adults with traumatic brain injury (TBI), levels of SBDP120, 145, and 150 increase in CSF over time and have been shown to correlate with severity of injury and neurologic outcome (7, 8). Increased SBDPs have also been demonstrated in CSF of adults suffering acute brain injury following aneurysmal subarachnoid hemorrhage and cerebral arterial vasospasm (9). SBDP145, a calpain-mediated breakdown product indicative of necrosis, was detected in the serum of infants and children following TBI and increased levels correlated with moderate to severe injury (10). In a canine model of CBP and DHCA, SBDPs increased in CSF and calpain-mediated SBDPs were associated with histology indicative of brain injury and neurologic dysfunction following DHCA (3).
Since neurologic injury occurs commonly in children with complex CHD, we aimed to determine if SBDPs could be detected in infants undergoing cardiac surgery as a first step in developing SBDPs as a potential biomarker in this clinical context. Because the blood-brain barrier can become compromised in neonates following hypoxic-ischemic insult, we hypothesized that SBDPs could be measured in the serum of neonates with CHD in the perioperative period (11). Here, we demonstrate that circulating SBDPs are readily detectable in this patient population and increase following open-heart surgery (OHS).
MATERIALS AND METHODS
This is a prospective observational cohort study and was approved by the institutional review board of Children’s National Medical Center. Informed consent was obtained from each patient’s parent or legal guardian.
Patients
Fourteen infants with CHD aged 3 to 31 days, weighing between 1.6 and 4.0 kg, admitted to the 26-bed Cardiac ICU (CICU) of a single tertiary care, academic pediatric center from July 2011 to March 2012 were studied (Table 1; Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/PCC/A79). Patients less than or equal to 31 days old with CHD requiring either OHS or closed-heart surgery (CHS) as part of their routine care were eligible for study. Patients were excluded if they had known preexisting neurological injury or known brain parenchymal disease (i.e., congenital cysts or malformations of the brain, history of perinatal asphyxia, preoperative intraventricular or intracranial hemorrhage, ischemia, or infarction). For each patient, we collected and recorded their gestational age, weight, age at enrollment and at the time of surgery, sex, cardiac diagnosis, surgical procedure, and use and duration of CPB or DHCA.
TABLE 1.
Patient Characteristics
| Variable | Value |
|---|---|
| Gestational age | |
| Full term, n (%) | 11 (78.6) |
| Premature, n (%) | 3 (21.4) |
| Weeks, median (IQR) | 36 (34–36) |
|
| |
| Sex | |
| Male, n (%) | 6 (42.9) |
| Female, n (%) | 8 (57.1) |
|
| |
| Age | |
| At enrollment, d, median (IQR) | 4.5 (3.3–11.3) |
| At surgery, d, median (IQR) | 7.5 (5.3–16.0) |
|
| |
| Weight, kg, median (IQR) | 3.1 (2.7–3.3) |
|
| |
| Cardiopulmonary bypass, n (%) | 9 (64.3) |
|
| |
| Deep hypothermic circulatory arrest, n (%) | 7 (50.0) |
|
| |
| Brain imaging | |
| Preoperative, n (%) | 12 (85.7) |
| MRI, n (%) | 11 (78.6) |
| CT scan, n (%) | 0 (0.0) |
| Ultrasound, n (%) | 1 (7.1) |
| Postoperative, n (%) | 6 (42.9) |
| MRI, n (%) | 4 (28.6) |
| CT scan, n (%) | 1 (7.1) |
| Ultrasound, n (%) | 1 (7.1) |
IQR = interquartile range.
Study Protocol
Routine clinical care was not affected or altered by this study. As is customary with neonates with CHD scheduled for cardiac surgery at Children’s National Medical Center, the majority of study patients underwent preoperative brain imaging in order to establish a baseline. Postoperative brain imaging was obtained based on clinical need. Radiologic imaging was achieved with MRI (General Electric Discovery MR750 3.0T; General Electric), ultrasound (General Electric Logiq E9; General Electric), or CT scan (Philips Brilliance 64; Philips, Andover, MA). Images were read by neuroradiologists blinded to the study and results were not reviewed by the investigators until completion of the study.
Following enrollment, 0.5 mL of blood was collected via an indwelling arterial catheter or central venous catheter prior to surgery. Subsequent blood samples (0.5 mL) were collected 6, 24, 48, 72, and 96 hours after surgery. All samples were immediately placed into collection tubes (SST Microtainer Tubes, BD and Company, Franklin Lakes, NJ) and centrifuged at 6,000 rpm for 2 minutes. Serum was removed from the collection tubes and stored at −80°C until analysis.
SBDP120 and 150 Measurement
Serum SBDP120 and 150 were measured as previously described using sandwich enzyme-linked immunosorbent assay at Banyan Biomarkers (Alachua, FL) in a blinded fashion (10, 12). Ninety-six-well plates were coated with 100 μL/well of capture antibody (rabbit polyclonal anti-SBDP120 or goat polyclonal anti-SBDP150 [Banyan Biomarkers]) overnight at 4°C. After a blocking buffer step, 10 μL of sample was added to each well and incubated for 2 hours at 27°C with gentle shaking. Amplification was achieved with biotinyl-tyramide solution (Elast Amplification Kit; Perkin Elmer, Waltham, MA) for 15 minutes at room temperature. A mouse monoclonal anti-rabbit or anti-goat antibody (Banyan Biomarkers) conjugated to horseradish peroxidase was added for colorimetric detection. Quantification was determined following generation of standard curves using recombinant glutathione S-transferase-fusion-αII-spectrin cleaved with calpain (SBDP150) or caspase-3 (SBDP120) (12).
Statistical Analysis
A sample size of 14 was chosen based on the number of patients required to detect SBDP levels three-fold higher than previously reported healthy control serum and CSF levels with a power of 80 based on an α of 0.05 (10, 12). The Friedman test with post hoc analysis for absolute rank sum differences was used to compare repeated SBDP measures within and between surgical groups over time. Preoperative SBDP levels in the neonates without radiographic evidence of preoperative brain injury were established as uninjured values. The Mann-Whitney U test was used to compare median peak SBDP levels with uninjured values and between OHS and CHS groups. Statistical significance was set at a p value of less than 0.05. Calculations were performed using Stata/IC 10.1 (Stata Corporation, College Station, TX).
RESULTS
Patient Characteristics
We prospectively evaluated a cohort of 14 neonates with CHD admitted to the CICU for surgical repair or palliation of their heart disease (Table 1; Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/PCC/A79). Eleven of the patients were full term and three were premature. Of the premature neonates, two had a gestational age of 36 weeks and one had a gestational age of 32 weeks. Nine patients underwent OHS while five underwent CHS. Of the nine neonates requiring OHS, seven required CPB with DHCA while two required CPB without DHCA. Of the five patients undergoing CHS, three underwent modified Blalock-Taussig shunt (MBTS) placement and two underwent pulmonary artery banding. Four of the nine neonates in the OHS cohort had single ventricle-type cardiac lesions while all five infants in the CHS group had single ventricle-type heart defects.
All of the patients in the CHS group had uneventful postoperative courses. On the other hand, two of the neonates in the OHS group required extracorporeal support (extracorporeal membrane oxygenation [ECMO]) in the postoperative period. ECMO was initiated in one patient following truncus arteriosus repair within hours after surgery secondary to persistent hypoxemia and hypotension without the need for chest compressions. A brain CT scan obtained on the sixth postoperative day demonstrated ischemic infarction with edema and hemorrhage and the patient did not survive. Another patient experienced a bradycardic arrest during endotracheal tube suctioning on the first postoperative day after Norwood procedure with MBTS requiring cardiopulmonary resuscitation and initiation of ECMO. This patient was successfully weaned from ECMO on the third postoperative day and survived. Initial brain ultrasound did not show any abnormalities but subsequent MRI demonstrated punctate hemorrhage. The remainder of patients in the OHS cohort had uneventful postoperative courses during the study period.
Radiologic Imaging
Eight out of the nine neonates in the OHS group and four out of the five patients in the CHS group had preoperative brain imaging performed (Table 2). Thus, the majority of the patients in the study cohort had baseline radiographic assessment of the brain prior to surgery. A total of six patients out of the entire cohort underwent postoperative brain imaging assessment (Table 2). Of the six, five patients were in the OHS group and one patient was in the CHS group. Only two patients in the entire cohort did not have any type of brain imaging performed during the study period.
TABLE 2.
Brain Imaging Assessment and Clinical Reasons for Postoperative Evaluation
| Patient No. | Surgical Cohort | Preoperative Imaging Modality | Preoperative Injury | Postoperative Imaging Modality | Postoperative Injury | Timing of Postoperative Imaging | Reason for Postoperative Imaging |
|---|---|---|---|---|---|---|---|
| 2 | CHS | MRI | Yes | – | – | – | – |
| 3 | CHS | MRI | No | – | – | – | – |
| 5 | CHS | MRI | No | – | – | – | – |
| 6 | OHS | MRI | Yes | – | – | – | – |
| 7 | OHS | Ultrasound | No | CT | Yes | POD 6 | ECMO |
| 8 | OHS | MRI | No | Ultrasound | No | POD 4 | ECMO |
| 9 | OHS | MRI | Yes | MRI | Yes | POD 15 | Seizure |
| 10 | OHS | MRI | Yes | MRI | Yes | POD 2 | Follow-up study |
| 11 | OHS | MRI | No | – | – | – | – |
| 12 | OHS | MRI | Yes | MRI | Yes | POD 15 | Seizure |
| 13 | OHS | MRI | Yes | – | – | – | – |
| 14 | CHS | MRI | No | MRI | Yes | POD 13 | Seizure |
CHS = closed-heart surgery, OHS = open-heart surgery, POD = postoperative day, ECMO = extracorporeal membrane oxygenation.
Preoperative brain ultrasound was obtained in patient 7 for prematurity.
Dashes indicate data was not obtained.
In total, six patients had imaging that demonstrated lack of brain injury (Table 2; Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/PCC/A79). Five of these neonates had normal brain MRI studies without evidence of intracranial hemorrhage, infarct, or edema. Two of these patients were in the OHS group and three were in the CHS cohort. In addition, one premature infant with truncus arteriosus had an unremarkable preoperative brain ultrasound. Three of the six patients who had radiographic evidence of lack of injury in the preoperative period also had postoperative studies performed. Two of these postoperative evaluations were abnormal, demonstrating evidence of ischemia, infarct, or hemorrhage. This small subgroup of patients included one patient from the CHS group and one infant from the OHS cohort (underwent CPB without DHCA). Only one patient in the entire cohort had an unremarkable pre- and postoperative study (Table 2). This child had hypoplastic left heart syndrome and underwent CPB with DHCA.
Six patients had abnormal preoperative brain imaging with evidence of injury including cytotoxic edema, watershed infarction, or hemorrhage (Table 2). Five of the six patients were in the OHS group while one was in the CHS cohort. These six patients were included as part of the study because their brain parenchymal injury did not manifest in overt clinical signs and symptoms (thus unknown at the time of enrollment) and the investigators were blinded to the results of preoperative imaging until completion of the study. Three out of the five OHS neonates who had an abnormal baseline MRI also had postoperative brain MRI imaging performed. All three underwent CPB with DHCA and all had abnormal postoperative studies demonstrating either acute infarction or residual injury. Thus, the majority of postoperative imaging assessments demonstrated evidence of brain injury.
SBDP Levels
Mean serum SBDP120 levels peaked 24 hours following OHS and were significantly increased compared to preoperative values and time-matched levels following CHS (Fig. 1A). After 24 hours, SBDP120 levels in the OHS cohort returned toward preoperative values (Fig. 1A). Following CHS, SBDP120 remained relatively unchanged over time (Fig. 1A). With regard to SBDP150, mean serum levels peaked 6 hours following OHS and were significantly increased compared to preoperative values (Fig. 1B). SBDP150 levels returned to baseline by 24 hours following OHS (Fig. 1B). There was a slight increase in SBDP150 72 hours after OHS; however, this increase was not statistically significant (Fig. 1B). Mean SBDP150 values did not change significantly following CHS (Fig. 1B).
Figure 1.
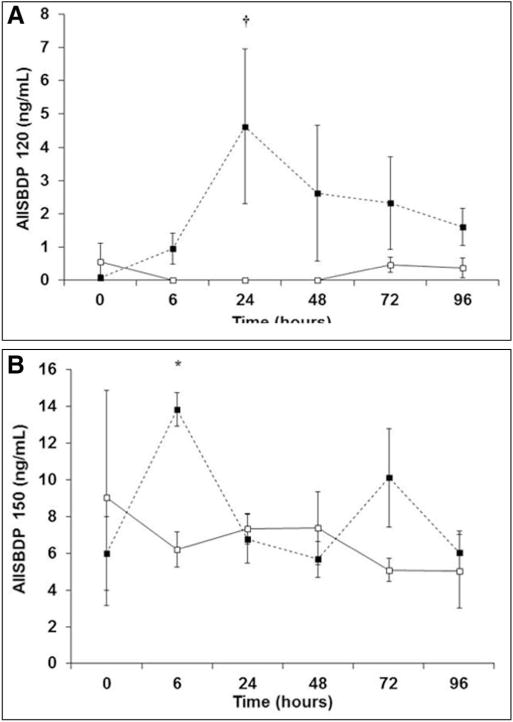
Postoperative serum alpha II-spectrin breakdown product (AIISBDP) levels over time. Levels of SBDP120 (A) and SBDP150 (B) are depicted. Preoperative values are plotted at the zero hour time point. Open squares with solid trend line represent values from patients in the closed-heart surgery (CHS) cohort (n = 5). Closed squares with dashed trend line represent values from patients in the open-heart surgery (OHS) cohort (n = 9). Values are means ± SE. *p < 0.05 versus OHS zero hour time point; †p < 0.001 versus. OHS zero hour time point and CHS 6, 24, and 48 hr time points.
Preoperative SBDP levels in the neonates without radiographic evidence of preoperative brain infarction, edema, or hemorrhage were established as uninjured values (Fig. 2). Importantly, SBDP150 values in patients without injury were consistent with previously reported serum SBDP 145 values from healthy children (10). Median peak SBDP120 and 150 levels were significantly higher following OHS compared to uninjured values and levels following CHS (Fig. 2).
Figure 2.
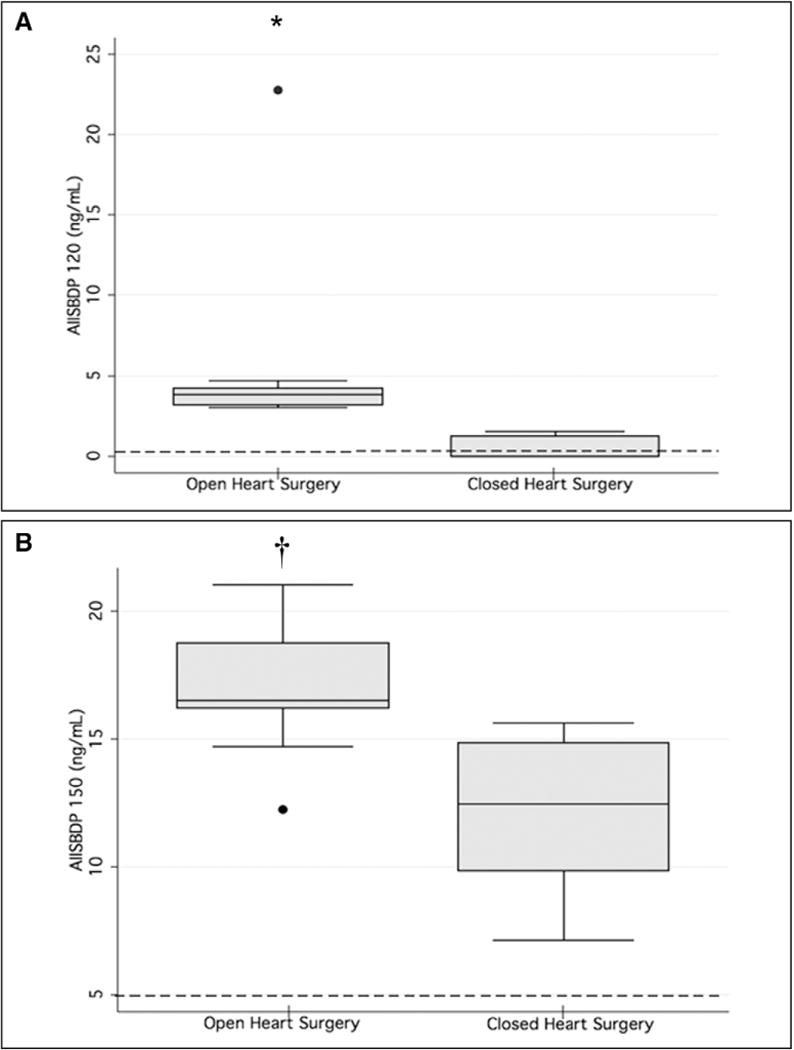
Peak postoperative serum alpha II-spectrin breakdown product (AIISBDP) levels. Box plots for SBDP120 (A) and SBDP150 (B) peak levels following open-heart surgery (n = 9) and closed-heart surgery (CHS) (n = 5) are depicted. Dashed lines represent mean SBDP values from patients who lacked radiographic evidence of brain injury. *p < 0.04 versus CHS and uninjured values; †p < 0.03 versus CHS and uninjured values. Filled circles represent outliers.
DISCUSSION
The majority of studies that have previously assessed potential biomarkers for neurologic injury in children with CHD undergoing heart surgery have focused on neuron-specific enolase (NSE) and S-100β (13–18). Although NSE is fairly specific for brain tissue, has low variability with regard to sex and age, and is rapidly detected in serum following injury, in several studies, it was unable to differentiate mild injury from absence of injury and has been shown to be released during hemolysis (12). S-100β, on the other hand, demonstrates significant age-dependent differences and is expressed to a greater degree in infancy (19). Unfortunately, these characteristics limit the potential utility of NSE and S-100β as biomarkers in the setting of cardiac surgery in infants and children. In addition, programmed cell death is a predominant feature of injury following neurologic insult at key times during brain development (19, 20). Thus, potential biomarkers for infants and children should assess for apoptotic mechanisms of cell death as well as necrosis.
Alpha II-spectrin is a potentially important protein with regard to injury in the developing brain because its cleavage products have the capacity to indicate calpain- and caspase-mediated mechanisms of neuronal cell death (3, 4, 19). Only one study has previously evaluated SBDPs in the pediatric population (10). This prior work demonstrated that the calpain-mediated breakdown product, SBDP145, was detectable in the serum of children, increased following moderate to severe TBI, and correlated with Glasgow Outcome Scale (10). SBDPs have not previously been assessed in infants and children with CHD undergoing surgical repair or palliation. However, in a canine model of CPB with and without DHCA, SBDPs were readily detected in the CSF (3). Histologically, both CPB and DHCA caused apoptosis and necrosis within different regions of the canine brain and SBDP145 and 150 increased significantly in CSF following DHCA while SBPD120 increased following CPB alone (3). Based on these prior studies and the fact that neurologic injury commonly occurs in infants and children with CHD, we hypothesized that SBDPs would be readily detectable in the serum of neonates with CHD in the perioperative period. Because accessing CSF is impractical following heart surgery, the ability to detect SBDPs in circulating blood carries importance because candidate biomarkers should be able to cross the blood-brain barrier following injury to enable ease of measurability (21). Furthermore, because alpha II-spectrin is not found in RBCs, breakdown of the protein and generation of SBDPs are not confounded by hemolysis commonly encountered during heart surgery (3). Thus, SBDPs could be useful in this clinical context if shown to be detectable in the perioperative period of infants and children with CHD.
Here, we demonstrate that SBDPs can be measured in the serum of neonates with CHD and that SBDP120 and 150 levels increased significantly following OHS. The timing of the peak increases in SBDP levels following OHS differed between SBDP120 and 150 but was consistent with prior reports (12). For example, the calpain-mediated breakdown product, SBDP145, peaked in the CSF 6 hours following TBI in an adult cohort while SBDP120 levels did not significantly increase until 24 hours postinjury (12). Thus, it appears that SBDP145 and 150 are detectable within hours following injury while SBDP120 release is delayed for at least 24 hours.
The lack of statistical significance of the repeated SBDP150 measures between the OHS and CHS cohorts may be explained by a number of factors. First, the sample size of the overall cohort was relatively small. This is a major limitation of the study. Second, 40% of patients in the CHS group demonstrated evidence of brain injury (one preoperatively and one postoperatively). One such patient was an ex-36 week premature infant with pulmonary atresia and intact ventricular septum undergoing MBTS placement. Preoperative brain MRI in this patient demonstrated cytotoxic injury and edema, and the preoperative SBDP150 level was 32.3 ng/mL. The other patient, a full-term neonate with double inlet left ventricle and right atrioventricular valve atresia, developed focal ischemic brain injury 48 hours following PA band placement and postoperative peak SBDP150 level of 14.8 ng/mL. Elevated SBDP150 in these two patients resulted in wide variation in levels in the CHS cohort causing SBDP150 levels following OHS to overlap with CHS values. Thus, there was no statistically significant difference between repeated SBDP150 measures of the two groups.
Importantly, both SBDP120 and 150 returned toward preoperative values by 96 hours following OHS and approximated uninjured levels. Related to this and because of our study design, we were unable to determine exactly when neurologic insult occurred in the neonates that demonstrated preoperative brain injury. Injury likely occurred either in utero or in the immediate perinatal period. Because brain imaging in those patients was performed 2 to 4 days prior to surgery and preoperative blood sampling occurred between 4 and 16 days of life, it is possible that our preoperative sampling missed the peak in SBDP120 and 150 following preoperative injury and levels may have already returned to preinjury values. Therefore, patients with preoperative injury would have relatively low or normal SBDP levels (false negative) based on the timing of our sampling. On the other hand, because postoperative blood sampling was performed serially following surgery, we were able to capture subsequent increases in both SBDP120 and 150 levels in the perioperative period.
We recognize that this prospective observational study has many limitations. The small sample size limits any in-depth analysis and, as such, conclusions must be drawn cautiously. Our sample size was not powered to assess for specificity or sensitivity and the study was not designed to assess for influence of age or sex on detected levels. Another important limitation is that brain imaging was not performed in every patient in the cohort. Thus, we could not assess for correlation of levels with radiographic evidence of injury. Although the majority of patients had some type of preoperative brain assessment, only a small subset underwent both pre- and postoperative imaging. Furthermore, multiple modalities of imaging were used (each with its own advantages and limitations). Because the primary aim was to determine if SBDPs could be detected in serum, our study was simply designed to serially sample blood at specific time points and to prospectively observe each patient’s perioperative course, permitting routine clinical care. Thus, brain imaging was obtained in most patients preoperatively as per routine and only postoperatively in those demonstrating a clinical need. Future studies will more rigorously use brain imaging to definitively determine if there is a correlation between magnitude of injury, neurologic outcome, and SBDP levels and peaks.
A further limitation is that this work represents an observational cohort study lacking an appropriate control group. However, because perinatal brain injury can occur in neonates independent of CHD, we opted to establish preoperative SBDP levels in those infants with CHD that lacked radiographic evidence of preoperative brain infarction, edema, or hemorrhage as uninjured values in lieu of a true control group. Importantly, SBDP150 values in the uninjured subgroup were consistent with previously reported calpain-mediated SBDP values in the serum of healthy infants (10). Thus, we were able to make some comparisons given the obvious caveats.
Another limitation of this study is that we did not prove that calpain- and caspase-mediated SBDPs in the serum result directly from acquired necrotic and apoptotic brain injury, respectively. In addition, although largely found in neurons within the CNS, alpha-II spectrin is not specific to the CNS (3, 22). This is a limitation of using SBDPs as potential biomarkers, but is not unique to alpha-II spectrin. For example, lack of CNS specificity is a disadvantage of other biomarkers such as S–100β (23). However, because MRI evidence of ischemia and infarction indicates brain necrosis and SBDP150 levels significantly increased following OHS, it is possible that perioperative brain necrosis was associated with release of this calpain-mediated SBDP into serum. On the other hand, there is currently no method to noninvasively assess for apoptosis in vivo. Thus, it is unknown if perioperative neurologic insult manifested in apoptosis or if SBDP120 is even indicative of caspase-mediated neuronal death in the developing human brain. Such a question will be difficult to answer using current noninvasive imaging modalities, but should be the focus of future investigation.
CONCLUSIONS
Despite the limitations, our work demonstrates that SBDPs are detectable in the serum of infants with CHD in the perioperative period and increase significantly following OHS. Importantly, we demonstrate that SBDPs are easily sampled and released in a time-specific sequence following injury. This study is an important first step in the development of SBDPs as potential biomarkers for infants with CHD. Future investigation will require much larger sample sizes and a more rigorous study design to determine the utility of SBDPs. If circulating SBDP150 and 120 are shown to definitively result from calpain-and caspase-mediated mechanisms of neuronal death in the developing human brain, there may be great potential for their use as biomarkers in this vulnerable cohort. Thus, with further investigation, it is possible that alpha-II SBDPs will have a role in helping to identify and prognosticate acquired neurologic injury in infants and children with CHD.
Supplementary Material
Acknowledgments
Dr. Levy is supported by Children’s National Medical Center Institutional Bridge Fund. Dr. Spaeder received support for travel from the National Center for the Study of Preparedness and Catastrophic Event Response and from the Society of Critical Care Medicine.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/pccmjournal).
The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Hirsch JC, Jacobs ML, Andropoulos D, et al. Protecting the infant brain during cardiac surgery: A systematic review. Ann Thorac Surg. 2012;94:1365–1373. doi: 10.1016/j.athoracsur.2012.05.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bokesch PM, Appachi E, Cavaglia M, et al. A glial-derived protein, S100B, in neonates and infants with congenital heart disease: Evidence for preexisting neurologic injury. Anesth Analg. 2002;95:889–892. doi: 10.1097/00000539-200210000-00018. table of contents. [DOI] [PubMed] [Google Scholar]
- 3.Weiss ES, Wang KK, Allen JG, et al. Alpha II-spectrin breakdown products serve as novel markers of brain injury severity in a canine model of hypothermic circulatory arrest. Ann Thorac Surg. 2009;88:543–550. doi: 10.1016/j.athoracsur.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Z, Larner SF, Liu MC, et al. Multiple alphaII-spectrin breakdown products distinguish calpain and caspase dominated necrotic and apoptotic cell death pathways. Apoptosis. 2009;14:1289–1298. doi: 10.1007/s10495-009-0405-z. [DOI] [PubMed] [Google Scholar]
- 5.Pike BR, Flint J, Dutta S, et al. Accumulation of non-erythroid alpha II-spectrin and calpain-cleaved alpha II-spectrin breakdown products in cerebrospinal fluid after traumatic brain injury in rats. J Neurochem. 2001;78:1297–1306. doi: 10.1046/j.1471-4159.2001.00510.x. [DOI] [PubMed] [Google Scholar]
- 6.Aikman J, O’Steen B, Silver X, et al. Alpha-II-spectrin after controlled cortical impact in the immature rat brain. Dev Neurosci. 2006;28:457–465. doi: 10.1159/000094171. [DOI] [PubMed] [Google Scholar]
- 7.Pineda JA, Lewis SB, Valadka AB, et al. Clinical significance of alphaII-spectrin breakdown products in cerebrospinal fluid after severe traumatic brain injury. J Neurotrauma. 2007;24:354–366. doi: 10.1089/neu.2006.003789. [DOI] [PubMed] [Google Scholar]
- 8.Farkas O, Polgár B, Szekeres-Barthó J, et al. Spectrin breakdown products in the cerebrospinal fluid in severe head injury—Preliminary observations. Acta Neurochir (Wien) 2005;147:855–861. doi: 10.1007/s00701-005-0559-6. [DOI] [PubMed] [Google Scholar]
- 9.Lewis SB, Velat GJ, Miralia L, et al. Alpha-II spectrin breakdown products in aneurysmal subarachnoid hemorrhage: A novel biomarker of proteolytic injury. J Neurosurg. 2007;107:792–796. doi: 10.3171/JNS-07/10/0792. [DOI] [PubMed] [Google Scholar]
- 10.Berger RP, Hayes RL, Richichi R, et al. Serum concentrations of ubiquitin C-terminal hydrolase-L1 and αII-spectrin breakdown product 145 kDa correlate with outcome after pediatric TBI. J Neurotrauma. 2012;29:162–167. doi: 10.1089/neu.2011.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baburamani AA, Ek CJ, Walker DW, et al. Vulnerability of the developing brain to hypoxic-ischemic damage: Contribution of the cerebral vasculature to injury and repair? Front Physiol. 2012;3:424. doi: 10.3389/fphys.2012.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mondello S, Robicsek SA, Gabrielli A, et al. αII-spectrin breakdown products (SBDPs): Diagnosis and outcome in severe traumatic brain injury patients. J Neurotrauma. 2010;27:1203–1213. doi: 10.1089/neu.2010.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhutta AT, Schmitz ML, Swearingen C, et al. Ketamine as a neuroprotective and anti-inflammatory agent in children undergoing surgery on cardiopulmonary bypass: A pilot randomized, double-blind, placebo-controlled trial. Pediatr Crit Care Med. 2012;13:328–337. doi: 10.1097/PCC.0b013e31822f18f9. [DOI] [PubMed] [Google Scholar]
- 14.Matheis G, Abdel-Rahman U, Braun S, et al. Uncontrolled reoxygenation by initiating cardiopulmonary bypass is associated with higher protein S100 in cyanotic versus acyanotic patients. Thorac Cardiovasc Surg. 2000;48:263–268. doi: 10.1055/s-2000-7879. [DOI] [PubMed] [Google Scholar]
- 15.Abdul-Khaliq H, Schubert S, Fischer T, et al. The effect of continuous treatment with sodium nitroprusside on the serum kinetics of the brain marker protein S-100beta in neonates undergoing corrective cardiac surgery by means of hypothermic cardiopulmonary bypass. Clin Chem Lab Med. 2000;38:1173–1175. doi: 10.1515/CCLM.2000.181. [DOI] [PubMed] [Google Scholar]
- 16.Lindberg L, Forsell C, Jögi P, et al. Effects of dexamethasone on clinical course, C-reactive protein, S100B protein and von Willebrand factor antigen after paediatric cardiac surgery. Br J Anaesth. 2003;90:728–732. doi: 10.1093/bja/aeg125. [DOI] [PubMed] [Google Scholar]
- 17.Lardner D, Davidson A, McKenzie I, et al. Delayed rises in serum S100B levels and adverse neurological outcome in infants and children undergoing cardiopulmonary bypass. Paediatr Anaesth. 2004;14:495–500. doi: 10.1111/j.1460-9592.2004.01230.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Xu Y, Li DZ, et al. Comparison of S100B and NSE between cardiac surgery and interventional therapy for children. Pediatr Cardiol. 2009;30:893–897. doi: 10.1007/s00246-009-9454-x. [DOI] [PubMed] [Google Scholar]
- 19.Kochanek PM, Berger RP, Fink EL, et al. The potential for bio-mediators and biomarkers in pediatric traumatic brain injury and neurocritical care. Front Neurol. 2013;4:40. doi: 10.3389/fneur.2013.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yakovlev AG, Faden AI. Mechanisms of neural cell death: Implications for development of neuroprotective treatment strategies. NeuroRx. 2004;1:5–16. doi: 10.1602/neurorx.1.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menascu S, Brezner A, Tshechmer SM, et al. Serum biochemical markers for brain damage in children with emphasis on mild head injury. Pediatr Neurosurg. 2010;46:82–88. doi: 10.1159/000319004. [DOI] [PubMed] [Google Scholar]
- 22.Bennett PM, Baines AJ, Lecomte MC, et al. Not just a plasma membrane protein: In cardiac muscle cells alpha-II spectrin also shows a close association with myofibrils. J Muscle Res Cell Motil. 2004;25:119–126. doi: 10.1023/b:jure.0000035892.77399.51. [DOI] [PubMed] [Google Scholar]
- 23.Bloomfield SM, McKinney J, Smith L, et al. Reliability of S100B in predicting severity of central nervous system injury. Neurocrit Care. 2007;6:121–138. doi: 10.1007/s12028-007-0008-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


