Abstract
Integrin-signaling complexes play important roles in cytoskeletal organization and cell adhesion in many species. Components of the integrin-signaling complex have been linked to aging in both Caenorhabditis elegans and Drosophila melanogaster, but the mechanism underlying this function is unknown. Here, we investigated the role of integrin-linked kinase (ILK), a key component of the integrin-signaling complex, in lifespan determination. We report that genetic reduction of ILK in both C. elegans and Drosophila increased resistance to heat stress, and led to lifespan extension in C. elegans without majorly affecting cytoskeletal integrity. In C. elegans, longevity and thermotolerance induced by ILK depletion was mediated by heat-shock factor-1 (HSF-1), a major transcriptional regulator of the heat-shock response (HSR). Reduction in ILK levels increased hsf-1 transcription and activation, and led to enhanced expression of a subset of genes with roles in the HSR. Moreover, induction of HSR-related genes, longevity and thermotolerance caused by ILK reduction required the thermosensory neurons AFD and interneurons AIY, which are known to play a critical role in the canonical HSR. Notably, ILK was expressed in neighboring neurons, but not in AFD or AIY, implying that ILK reduction initiates cell nonautonomous signaling through thermosensory neurons to elicit a noncanonical HSR. Our results thus identify HSF-1 as a novel effector of the organismal response to reduced ILK levels and show that ILK inhibition regulates HSF-1 in a cell nonautonomous fashion to enhance stress resistance and lifespan in C. elegans.
Keywords: aging, C. elegans, heat-shock response, HSF-1, integrin signaling, PAT-4/ILK
Introduction
Integrin-linked kinase (ILK) is a central intracellular component of the integrin-signaling complex, a multicomponent protein complex clustered at the plasma membrane. Integrin-mediated cell–matrix and cell–cell adhesion play critical roles in numerous biological processes, including cell migration, survival, and proliferation (Wu & Dedhar, 2001). Accordingly, dysregulation of ILK has been observed in many human pathologies, including cancers and cardiomyopathies (Knoll et al., 2007; McDonald et al., 2008). ILK is also essential for the early development of model organisms such as C. elegans, Drosophila, zebrafish, and mice (Williams & Waterston, 1994; Zervas et al., 2001; Postel et al., 2008; Lange et al., 2009).
Although complete deficiency of ILK confers lethality, analysis of animals with moderately reduced levels of ILK or other components of the integrin-signaling complex has revealed a novel function in aging. Specifically, genetic screens using RNA interference (RNAi) have shown that depletion of pat-4/ILK and its conserved binding partner pat-6/Parvin extends lifespan in C. elegans (Hansen et al., 2005). Similarly, a heterozygous mutation in β1-integrin, the binding partner of ILK, caused delayed behavioral aging and reduced mortality of Drosophila (Goddeeris et al., 2003). These observations suggest that integrin signaling may constitute a novel, conserved longevity pathway, yet the molecular underpinnings remain unknown.
Many characterized longevity mechanisms are known to confer increased resistance to cellular and environmental stressors, such as high temperature. Heat stress activates the conserved transcription factor heat-shock factor-1 (HSF-1) via a multistep process involving oligomerization, post-translational modification, and nuclear translocation. In the nucleus, HSF-1 upregulates the transcription of many genes encoding heat-shock proteins. Collectively, this universal systemic response to elevated temperatures is known as the heat-shock response (HSR). In C. elegans, the HSR involves a circuit of neurons, AFD and AIY, that sense temperature and communicate the systemic activation of HSF-1 (Prahlad et al., 2008). Notably, overexpression of HSF-1 is sufficient to extend the lifespan of C. elegans (Hsu et al., 2003), and overexpression of the HSF-1 target genes hsp-16 and Hsp70 promotes longevity in C. elegans and Drosophila, respectively (Tatar et al., 1997; Hsu et al., 2003; Walker & Lithgow, 2003). These findings underscore the pivotal role of HSF-1 and the HSR in organismal aging.
In this study, we investigated the relationship between ILK, the HSR, and longevity. We found that HSF-1 was required for the extended lifespan of C. elegans depleted of pat-4/ILK during adulthood. Moreover, inhibition of pat-4/ILK and several other components of the integrin-signaling complex in adult nematodes also increased their thermotolerance. We found that pat-4/ILK inhibition increased hsf-1 transcription and translation, resulting in the induction of a subset of HSF-1 target genes. Activation of HSF-1 required the function of AFD and AIY thermosensory neurons, known to be essential for establishing the HSR, but PAT-4/ILK was not expressed in these neurons. Instead, we found PAT-4/ILK to be expressed in neighboring neurons, in addition to its known expression in body-wall muscle and gonad. Of these tissues, we found neurons, and possibly the gonad, but not muscle to be important for the beneficial effects of pat-4/ILK reduction. These findings are the first to demonstrate a direct link between ILK and HSF-1, and suggest that PAT-4/ILK systemically modulates stress resistance and longevity in a cell nonautonomous manner via thermosensory neurons.
Results
Reduction of pat-4/ILK during adulthood increases lifespan without affecting muscle integrity
We previously identified a role for pat-4/ILK in lifespan determination during a genome-wide RNAi screen in C. elegans to identify novel longevity genes (Hansen et al., 2005). This novel role for ILK in lifespan determination appears to be conserved, because Drosophila heterozygous ilk mutants are also long-lived (Nishimura et al., 2014). To examine the novel longevity role of ILK in more detail, we reduced pat-4/ILK levels in C. elegans by feeding RNAi (i.e., using the pat-4/ILK bacterial RNAi clone previously identified in the screen mentioned above). PAT-4/ILK is essential for the development of C. elegans and plays an important role in muscle–cell integrity and cytoskeletal attachment in the embryo (Williams & Waterston, 1994; Mackinnon et al., 2002). Consistent with this, we found that animals fed pat-4/ILK dsRNA-expressing bacteria from hatching (i.e., whole-life RNAi treatment) showed adult-onset paralysis with minimal head movement (Fig. 1A), which was accompanied by irreversible aggregation and collapse of myosin filaments in the body-wall muscles (Fig. 1C,E) (Meissner et al., 2009). Because PAT-4/ILK is also expressed in the C. elegans pharynx (Mackinnon et al., 2002), we measured the pharyngeal pumping rate in animals subjected to pat-4/ILK RNAi from hatching. These animals showed markedly reduced pumping rates throughout their lifespan compared with animals fed bacteria containing empty vector (Fig. 1G). Such defects in pharyngeal pumping may reduce food intake and thus lead to dietary restriction, a longevity paradigm that extends lifespan in many species (Mair & Dillin, 2008). In principle, then, ‘mechanical’ dietary restriction could contribute to the longevity observed in C. elegans subjected to pat-4/ILK RNAi (Hansen et al., 2005; Table S1, Supporting information).
Figure 1.
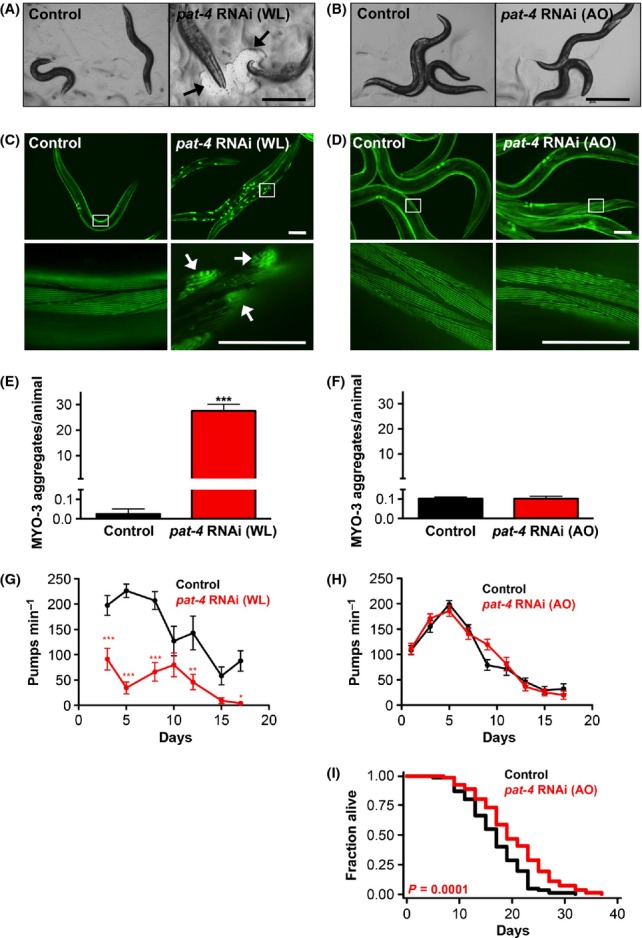
Inhibition of pat-4/ILK in adult C. elegans prolongs lifespan without inducing gross morphological defects. (A, B) Micrographs of N2 wild-type animals on day 2 (A) or day 5 (B) of adulthood. Animals were raised from hatching on bacteria containing empty vector or expressing pat-4/ILK dsRNA (whole-life, WL; A) or were raised on control bacteria and transferred to pat-4/ILK RNAi on day 1 of adulthood (adult-only, AO; B). Scale bars = 600 μm. Only WL pat-4/ILK RNAi caused paralysis, creating areas where the bacterial lawn was absent (A, arrows). (C, D) Fluorescence micrographs of animals expressing myo-3p::gfp::myo-3, taken on day 2 (C) or day 5 (D) of adulthood. Animals were raised as described for (A) and (B), respectively. Boxes indicate sections enlarged in the lower panels. Only WL pat-4/ILK RNAi resulted in MYO-3::GFP aggregates (C, arrows). Scale bars = 100 μm. (E, F) Quantification of MYO-3::GFP aggregates in animals raised as described for (C) and (D), respectively. Mean + SEM of N = 38–42 animals. ***P < 0.0001 (Student’s t-test). The experiment was repeated three times with similar results. (G, H) Pharyngeal pumping rates were measured in N2 wild-type animals raised as described for (A) and (B), respectively. Mean + SEM of N = 9–22. *P < 0.05, **P < 0.01, ***P < 0.001 of multiple comparison of RNAi treatment per day (Two-way anova: RNAi effect F(1,110) = 99.1, P < 0.0001, with an interaction of age and RNAi treatment F(6,110) = 3.61, P = 0.003). The experiment was repeated twice in N2 animals and twice in sterile CF512 animals with similar results. (I) Lifespan analysis of N2 animals transferred to bacteria expressing control (gfp) or pat-4/ILK dsRNA on day 1 of adulthood (AO). Similar lifespan extension was observed in a previous RNAi screen in which gene knockdown was initiated at the L4 larval stage (Curran & Ruvkun, 2007). Statistical analysis with log-rank (Mantel-Cox) test. The experiment was performed >10 times with similar results (see Table S1 for additional data). All experiments were performed at 20 °C.
We next analyzed the function of PAT-4/ILK in adult nematodes by initiating pat-4/ILK RNAi on day 1 of adulthood (i.e., adult-only RNAi treatment); this treatment reduced pat-4/ILK mRNA levels by ~40% by day 3 (Fig. S1, Supporting information). This reduction in pat-4/ILK during adulthood increased the mean lifespan of C. elegans by ~10–30% (Fig. 1I; Table S1, Supporting information), similar to whole-life RNAi treatment (Hansen et al., 2005; Table S1, Supporting information). Notably, these animals did not become paralyzed or form myosin aggregates at any point during their lifespan, and their appearance was indistinguishable from that of control animals fed bacteria containing empty vector (Fig. 1B,D,F). This indicates that reducing PAT-4/ILK during adulthood extends lifespan without majorly affecting body-wall muscle integrity. Moreover, we found no difference in pharyngeal pumping rates between control animals and animals subjected to pat-4/ILK RNAi from day 1 of adulthood (Fig. 1H). This is in contrast with the pharyngeal pumping defect we observed in animals subjected to whole-life pat-4/ILK RNAi and implies that the longevity induced by pat-4/ILK reduction during adulthood is unlikely due to ‘mechanical’ dietary restriction. Thus, these data indicate that decreasing the expression of PAT-4/ILK during adulthood can extend the lifespan of C. elegans without affecting body-wall or pharyngeal muscle integrity.
PAT-4/ILK reduction induces stress response genes
Many organisms, including C. elegans, exhibit increased resistance to stress when subjected to conserved lifespan-extending treatments or pertubations (Johnson et al., 2001). To determine whether inhibition of pat-4/ILK might evoke a stress response, we examined the induction of three commonly used stress reporters in C. elegans: the oxidative stress reporters sod-3p::gfp (Libina et al., 2003) and gst-4p::gfp (Link & Johnson, 2002), and heat-stress reporter hsp-16.2p::gfp (Mendenhall et al., 2012). We found that expression of all three reporters was increased in multiple tissues, most notably in the intestine, in 3-day-old animals subjected to pat-4/ILK RNAi from day 1 of adulthood (Fig. 2A–D). Consistently, we found the mRNA levels of sod-3 and hsp-16.2 significantly upregulated upon reduction in pat-4/ILK (Fig. 3C). Thus, inhibition of pat-4/ILK induced expression of genes typically upregulated in response to environmental stressors, consistent with the mobilization of a stress response.
Figure 2.

Inhibition of PAT-4/ILK in adult C. elegans leads to the induction of stress reporters, thermotolerance, and longevity via HSF-1. (A–C) Fluorescence micrographs (A–C) and quantification of relative GFP intensity (D) of wild-type animals expressing sod-3p::gfp (A), gst-4p::gfp (B), or hsp-16.2p::gfp (C). Animals were transferred to bacteria containing empty vector or expressing pat-4/ILK dsRNA on day 1, and images were captured on day 3. Exposure times were 1 s for sod-3p::gfp and hsp-16.2p::gfp, and 600 ms for gst-4p::gfp. Scale bars = 200 μm. For imaging of the hsp-16.2p::gfp reporter, animals were incubated for 1 h at 36 °C followed by 2 h at 20 °C. The experiments were repeated at least three times with similar results. N = 9–12. ***P < 0.001 (two-way anova). (E) Survival of N2 wild-type (WT) C. elegans after 8 h incubation at 36 °C. Animals were transferred to bacteria containing empty vector or expressing pat-4/ILK dsRNA on day 1, and survival was measured on day 3. Mean + SEM. **P < 0.01 (Student’s t-test). The experiment was performed a total of 13 times (see Table S2 for additional data). (F) Survival of 3-week-old ilk54-wCS/+ and wCS (control) Drosophila was measured after 85 min incubation at 36 °C. Mean + SEM of N = 40 *P < 0.05 (Student’s t-test). The experiment was repeated three times with similar results. (G) Survival of hsf-1(sy441) mutants after 7 h incubation at 36 °C. Animals were transferred to bacteria containing empty vector or expressing pat-4/ILK dsRNA on day 1, and survival was measured on day 3. Mean + SEM. *P < 0.05, **P < 0.01 (Student’s t-test). See Table S4 for additional data. (H) Lifespan analysis of WT animals and hsf-1(sy441) mutants transferred to bacteria expressing control (gfp) or pat-4/ILK dsRNA on day 1 of adulthood. Statistical analysis with log-rank (Mantel-Cox) test. The experiment was repeated three times with similar results (see Table S1 for additional data). All experiments were performed at 20 °C.
Figure 3.

HSF-1 levels and activity are increased in C. elegans in response to pat-4/ILK reduction. (A) Representative electrophoretic mobility shift assay performed on nuclear extracts from 2-day-old N2 wild-type animals raised from hatching on bacteria containing empty vector or expressing pat-4/ILK dsRNA. Animals were either unstressed or exposed to heat shock at 37 °C for 90 min before harvest. Arrows indicate HSF-1 bound to biotin-labeled oligonucleotides containing the heat-shock element (HSF-1::HSE) or unbound oligonucleotides (free probes). The experiment was performed three times with similar results. (B) Quantification of electrophoretic mobility shift assays. Results are the Mean + SD of three independent experiments (including that shown in (A), normalized to the control. ***P < 0.001 (two-way anova). (C–D) Quantitative RT–PCR of total RNA from 2-day-old N2 animals raised from hatching on bacteria containing empty vector or expressing pat-4/ILK dsRNA. mRNA levels of known HSF-1 target genes (C) or pat-4/ILK and hsf-1 (D) were normalized to the housekeeping genes ama-1 and nhr-23. We confirmed that pat-4/ILK RNAi did not affect expression of an hsp-70p(C12C8.1)::gfp construct (data not shown), corroborating these results. Mean + SEM of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 (one-way anova). (E) Western blot analysis of HSF-1 in total protein extracts from 2-day-old N2 animals raised from hatching on bacteria containing empty vector or expressing pat-4/ILK dsRNA. Animals were either unstressed or exposed to heat shock at 37 °C for 90 min before harvest. The experiment was performed five times. (F) Quantification of relative HSF-1 protein levels. Results are the Mean + SD of five independent experiments (including that shown in E), normalized to the loading control (β-actin), and compared to the unstressed animals expressing empty vector. *P < 0.05, **P < 0.01, ***P < 0.001 (two-way anova).
Inhibition of pat-4/ILK confers thermotolerance in both C. elegans and Drosophila
We next asked whether inhibition of pat-4/ILK could induce stress resistance in C. elegans by examining the response to increased temperature. We focused on this type of stress as heat stress causes disruption of the cytoskeleton in several species (Walter et al., 1990; Fisher et al., 1996), and PAT-4/ILK is localized at this site in C. elegans (Mackinnon et al., 2002). To test survival at elevated temperatures, we subjected animals to pat-4/ILK RNAi from day 1 of adulthood and on day 3 incubated them at 36 °C for 8 h. Survival was scored by the response to a gentle stimulus to the head region. We found that animals subjected to pat-4/ILK RNAi prior to the heat shock were indeed more resistant to elevated temperatures than animals fed control bacteria (Fig. 2E, Table S2, Supporting information).
Because ILK depletion also extends the lifespan of Drosophila (Nishimura et al., 2014), we next asked whether the thermotolerance phenotype might represent a conserved response to ILK inhibition. For this, vials containing 3-week-old Drosophila ilk outcrossed heterozygotes or wild-type flies were submerged in a 36 °C water bath for 85 min. Under these conditions, we observed that both male and female flies expressing reduced levels of ilk were significantly more thermotolerant than their wild-type counterparts (Fig. 2F), consistent with our findings in C. elegans. These observations indicate that the elevated thermotolerance exhibited by animals with reduced ILK levels may be a conserved protective mechanism and could contribute to the extended lifespan observed in both C. elegans (Hansen et al., 2005; Curran & Ruvkun, 2007) and Drosophila (Nishimura et al., 2014).
Stress resistance is influenced by many integrin-signaling complex components
The integrin-signaling complex acts as a physical link between the extracellular matrix and the cytoskeleton. To identify other integrin-signaling complex components that might affect thermotolerance in C. elegans, we used RNAi to systematically reduce the levels of such integrin-complex components in adult animals and then analyzed the effects on thermotolerance. We also tested the effects of RNAi-depleting other cytoskeletal components that, similar to whole-life pat-4/ILK RNAi treatment (Fig. 1A,C), can induce detachments in the cytoskeletal lattice, as visualized by MYO-3 aggregate accumulation (Meissner et al., 2009). In repeated experiments, we identified several RNAi clones that consistently induced thermotolerance, including pat-6/Parvin, deb-1/Vinculin, and unc-89/Obscurin (Table S2, Supporting information). We note that a pat-6/Parvin RNAi clone was identified in the previously mentioned RNAi-longevity screen (Hansen et al., 2005), and we found that pat-6/Parvin RNAi treatment in adult animals was capable of extending C. elegans lifespan to a similar extent as pat-4/ILK RNAi (data not shown). Other RNAi clones tested also induced thermotolerance but with more variable results; for example, kin-32/FAK (Focal Adhesion Kinase), unc-98, unc-95/paxillin, unc-52/perlecan, myo-3/myosin heavy chain, and unc-97/PINCH (Tables S2 and S3, Supporting information). Taken together, our data suggest that thermotolerance can be increased by reducing the expression of a number of integrin-complex components, in addition to pat-4/ILK. Interestingly, most of the RNAi clones we tested caused some degree of disruption of PAT-4/ILK expression (i.e., in M-lines and dense bodies in body-wall muscle cells) when fed to animals from hatching (Fig. S2, Table S3, Supporting information), suggesting that their thermotolerant phenotypes may be mediated, at least in part, via pat-4/ILK.
HSF-1 is required for thermotolerance and longevity induced by pat-4/ILK reduction
We next sought to determine how PAT-4/ILK may regulate thermotolerance in C. elegans. We focused on analyzing the role of three major stress-regulating transcription factors, DAF-16/FoxO, SKN-1/Nrf, and HSF-1, which regulate the genes we assayed earlier, that is, sod-3, gst-4, and hsp-16.2, respectively (see also Fig. 2A–C). For this, we examined the expression of the genes in the transcription factor mutants daf-16(mu86), skn-1(RNAi), and hsf-1(sy441). pat-4/ILK RNAi failed to increase in the expression of sod-3p::gfp, gst-4p::gfp, and hsp-16.2p::gfp in these mutants (Fig. S3B,D Supporting information), suggesting that depletion of pat-4/ILK may induce expression of heat- and oxidative stress response genes through the regulation of DAF-16/FoxO, SKN-1/Nrf, and HSF-1. To determine whether these transcription factors were required for the thermotolerant phenotype induced by pat-4/ILK RNAi, we measured the survival of daf-16/FoxO, skn-1/Nrf, and hsf-1 mutants exposed to elevated temperatures. Interestingly, pat-4/ILK RNAi during adulthood induced thermotolerance in daf-16(mu86) mutants and skn-1(zu135) mutants (Table S4, Supporting information), but had no effect on thermotolerance in hsf-1(sy441) mutants (Fig. 2G, Table S4, Supporting information), even though pat-4/ILK levels can be potently reduced in hsf-1 mutants (Fig. S4, Supporting information). We also eliminated the possibility that hsf-1 mutants were inherently incapable of elevated thermotolerance by showing that hsf-1(sy441) mutants subjected to RNAi targeting the insulin/IGF-1 receptor daf-2/InR indeed displayed increased resistance to heat stress (Table S4, Supporting information).
Although daf-16/FoxO and skn-1/Nrf1 were not required for thermotolerance caused by pat-4/ILK inhibition, we noted that both the DAF-16/FoxO target gene sod-3 and the SKN-1/Nrf1 target gene gst-4 were induced by pat-4/ILK RNAi. We therefore tested whether the sod-3p::gfp and gst-4p::gfp reporters could be induced in hsf-1(sy441) mutants upon inhibition of pat-4/ILK and found that hsf-1 was at least partially required for the induction of both sod-3p::gfp and gst-4p::gfp (Fig. S3C,D, Supporting information). These results demonstrated that sod-3 and gst-4 are regulated not only by DAF-16/FoxO and SKN-1/Nrf, but also by HSF-1, at least in response to pat-4/ILK reduction. We note that hsp-16.2 can also be regulated by DAF-16/FoxO to some extent (Hsu et al., 2003). In contrast to the stress-reporter induction, we found that daf-16/FoxO and skn-1/Nrf were not required for pat-4/ILK RNAi-induced thermotolerance (Table S4, Supporting information). Additionally, the lifespan extension of animals subjected to whole-life or adult-only pat-4/ILK RNAi is daf-16/FoxO-independent (Hansen et al., 2005; Curran & Ruvkun, 2007). It is possible that additional stress responses, besides thermotolerance are engaged in pat-4/ILK RNAi-treated animals via DAF-16/FoxO or other transcription factors. Collectively, these data demonstrate that hsf-1 is required for the induction of stress-responsive genes and thermotolerance in animals with reduced PAT-4/ILK levels.
To determine whether hsf-1 was similarly required for the longevity of animals subjected to pat-4/ILK RNAi, we performed epistasis experiments with hsf-1(sy441) mutant animals. Reduction in pat-4/ILK during adulthood did not extend the lifespan of hsf-1(sy441) mutants, whereas inhibition of daf-2/InR did (Fig. 2H, Table S1, Supporting information). The latter result further links stress responses to longevity and supports the possibility that hsf-1-dependent induction of stress-response genes could underlie the novel lifespan-determining function of PAT-4/ILK, at least in C. elegans.
PAT-4/ILK reduction increases HSF-1 activity
Having shown that HSF-1 was required for the extended longevity and thermotolerance of animals with reduced PAT-4/ILK levels, we next asked whether PAT-4/ILK could regulate HSF-1 activity. For this, we performed electrophoretic mobility shift assays to measure the binding of HSF-1 to a sequence encompassing the heat-shock element (HSE) of a target gene (Voellmy, 2004; Chiang et al., 2012). We found that HSF-1–HSE binding was not only increased in nuclear extracts from C. elegans raised on empty-vector bacteria and exposed to heat shock of 37 °C for 90 min, as previously reported (Hsu et al., 2003; Chiang et al., 2012), but also in nuclear extracts from C. elegans raised under normal temperature conditions (20 °C) on bacteria expressing pat-4/ILK dsRNA (Fig. 3A,B). There was no further increase in the binding of HSF-1 upon additional heat shock (Fig. 3A,B), which indicated that the pat-4/ILK RNAi treatment had saturated the binding of HSF-1 to the available HSE elements or that a similar process of HSF-1 activation occurs in animals upon heat shock and reduction in pat-4/ILK.
To verify that increased binding of HSF-1 to HSEs translated into increased transcriptional activity, we performed quantitative RT–PCR of HSF-1 target genes known to be regulated in response to heat shock from animals raised at 20 °C and subjected to pat-4/ILK RNAi. We found that pat-4/ILK inhibition upregulated the HSF-1 target genes unc-23 and aip-1 (Fig. 3C) (GuhaThakurta et al., 2002; Hsu et al., 2003) as well as sod-3 and hsp-16.2, corroborating our results with the stress reporter genes (Figs 3C, and 2A,C). In contrast, other HSF-1-regulated genes, such as sip-1 and hsp-70 (C12C8.1 and F44E5.4), and several small heat-shock proteins (hsp-16.1, hsp-16.49, and hsp-12.6) were unchanged, suggesting that only a subset of heat shock-inducible HSF-1 target genes are relevant for the increased longevity and thermotolerance observed in C. elegans raised at normal temperature with reduced pat-4/ILK levels (Fig. 3C).
We next investigated the mechanism by which pat-4/ILK RNAi upregulated HSF-1 target genes by analyzing hsf-1 gene expression. Interestingly, hsf-1 mRNA levels were significantly increased by pat-4/ILK RNAi (Fig. 3D). This was unexpected because we had previously found that neither heat-shock treatment nor reduction in daf-2/InR in C. elegans increase hsf-1 transcription (Hsu et al., 2003; Chiang et al., 2012). Consistent with the increase in hsf-1 transcription, we found that animals subjected to pat-4/ILK RNAi contained increased levels of HSF-1 protein, and the level was comparable with that observed in control animals following heat shock (Fig. 3E,F). HSF-1 protein expression in animals subjected to pat-4/ILK RNAi was not further increased by heat shock (Fig. 3E,F). Thus, reduction in pat-4/ILK induced transcription and translation of hsf-1, and upregulated the expression of a subset of known HSF-1 target genes in C. elegans without any temperature insults. Taken together, these observations suggest that a specialized HSF-1-mediated heat-stress response is activated in animals with reduced levels of pat-4/ILK.
Thermosensory neurons are required for pat-4/ILK-regulated thermotolerance and longevity
Upon exposure to high temperatures, C. elegans elicit a canonical heat-shock response (HSR) that is regulated systemically by signals from a neuronal circuit that senses temperature and regulates thermotaxis (Fig. 4A) (Jeong et al., 2012). Specifically, the AFD thermosensory neurons (i.e., the neuronal pair AFDR and AFDL) detect temperature through a nucleotide-gated channel TAX-2/TAX-4, which requires the upstream function of several guanylyl cyclases, one of which is GCY-8. The sensory input is signaled through the AIY interneurons, (i.e., the neuronal pair AIYR and AIYL) which require the LIM homeodomain protein TTX-3 for function (Mori & Ohshima, 1995). Upon perception of heat shock, the AFD and AIY neurons regulate a HSR in distal tissues of the animals (Prahlad et al., 2008; Prahlad & Morimoto, 2011). To determine whether the AFD and AIY neurons play a similar role in pat-4/ILK-modulated thermotolerance, we examined the loss-of-function mutants gcy-8 and ttx-3 after feeding with pat-4/ILK dsRNA-expressing or control bacteria. We found that pat-4/ILK RNAi treatment during adulthood failed to increase thermotolerance (Fig. 4B, Table S5, Supporting information) as well as longevity (Fig. 4C,D, Table S1, Supporting information) of the gcy-8(oy44) and ttx-3(ks5) mutants compared with wild-type animals. These observations confirm that, as with the canonical HSR, functional thermosensory neuron AFD and interneuron AIY are required for thermotolerance and longevity in animals with reduced pat-4/ILK levels.
Figure 4.

Inhibition of pat-4/ILK in adult C. elegans requires functional thermosensory neurons to promote thermotolerance, longevity, and induction of the stress reporter hsp-16.2p::gfp. (A) Schematic model of temperature sensing. The AFD neurons sense temperature via the nucleotide-gated channel TAX-2/TAX-4 (not shown), which engages the guanylyl cyclase GCY-8. This event activates the LIM homeodomain protein TTX-3 in the interneurons AIY to ultimately direct thermotactic movement (Mori & Ohshima, 1995). (B) Survival of N2 wild-type (WT) animals, and gcy-8(oy44) and ttx-3(ks5) mutants after 8 h incubation at 36 °C. Animals were transferred to bacteria containing empty vector or expressing pat-4/ILK dsRNA on day 1 and survival was measured on day 3. Mean + SEM. ***P < 0.001 (two-way anova). The experiment was repeated three times with similar results (see Table S5 for additional data). (C–D) Lifespan analysis of WT animals, gcy-8(oy44) mutants (C), and ttx-3(ks5) mutants (D) transferred to bacteria expressing control (gfp) or pat-4/ILK dsRNA on day 1 of adulthood. The same wild-type control is presented in (C) and (D) because the three strains were tested in the same experiment. Statistical analysis with log-rank (Mantel-Cox) test. The experiment was repeated three times with similar results (see Table S1 for additional data). All experiments were carried out at 20 °C. (E) Fluorescence micrographs of wild-type animals, gcy-8(oy44) mutants, and ttx-3(ks5) mutants expressing hsp-16.2p::gfp. Animals were transferred to bacteria containing empty vector or bacteria expressing pat-4/ILK dsRNA on day 1, and images were captured on day 3. Exposure time was 1 s. Prior to imaging, animals were incubated for 1 h at 36 °C followed by 2 h at 20 °C. Scale bars = 200 μm. (F) Quantification of relative GFP intensity of the animals shown in (E). N = 7–12. ***P < 0.001 (two-way anova). The experiment was repeated three times with similar results.
To further explore the role of the AFD thermosensory neurons and AIY interneurons, we introduced the pat-4/ILK-responsive stress reporter hsp-16.2p::gfp into the gcy-8(oy44) and ttx-3(ks5) mutants. In contrast to wild-type animals, gcy-8(oy44) and ttx-3(ks5) mutants failed to induce hsp-16.2::gfp expression, including in the intestine (Fig. 4E–F). Consistent with this observation, transcription of hsp-16.2 and of other tested HSF-1 target genes was unchanged in gcy-8(oy44) and ttx-3(ks5) mutants subjected to pat-4/ILK RNAi (Fig. S5, Supporting information). Moreover, hsf-1 transcript levels were not increased in these mutants (Fig. S5, Supporting information). Thus, functional AFD and AIY neurons are likely required for the effects of pat-4/ILK reduction not only for the expression of hsf-1 but also for the induction of hsf-1 target genes in the intestine, a distal tissue not reported to express PAT-4/ILK. Taken together with our thermotolerance- and lifespan analyses on gcy-8 and ttx-3 mutants, these results indicate that pat-4/ILK mediates effects via the AFD thermosensory neurons and the AIY interneurons.
pat-4/ILK functions in neurons, but not in muscle, to mediate longevity
To learn more about how PAT-4/ILK elicits organismal effects on lifespan and thermotolerance via the AFD thermosensory neurons and AIY interneurons, we next investigated where in the animal PAT-4/ILK functions. As a start, we asked whether pat-4/ILK acted cell autonomously in these thermosensory neurons. We examined the expression of pat-4/ILK specifically in AFD and AIY neurons using a transcriptional pat-4p::gfp reporter construct (Dupuy et al., 2007) expressed in combination with gcy-8p::mCherry or ttx-3p::rfp constructs. Surprisingly, we found that pat-4p::gfp was neither expressed in AFD (Fig. 5A) nor in AIY neurons (Fig. 5B), but was present in adjacent cells, which may be ADF and/or AIM neurons (chemosensory/oxygensensory neurons and interneurons, respectively). Importantly, we detected no difference in the thermotactic behavior of control animals and those subjected to pat-4/ILK RNAi in adulthood (Fig. S6, Supporting information). Thus, although the stress response mediated by pat-4/ILK reduction requires the presence of functional AFD and AIY neurons (Fig. 4E–F), these thermosensory neurons may not directly mediate the effects on thermotolerance and longevity, suggesting that PAT-4/ILK functions cell-nonautonomously outside the AFD and AIY neurons.
Figure 5.
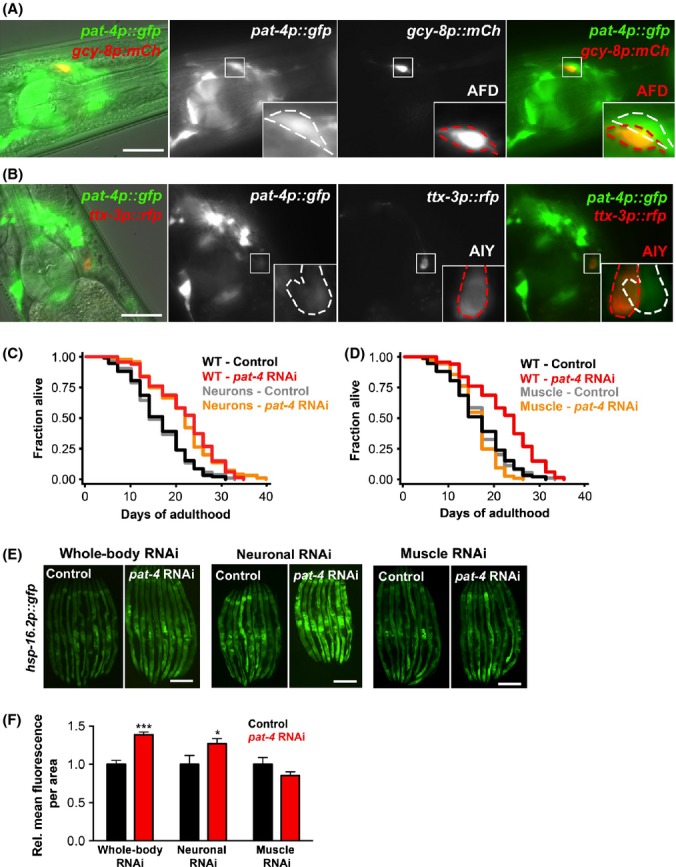
Inhibition of pat-4/ILK in neurons, but not in muscle, of adult C. elegans promotes longevity and induction of the stress reporter hsp-16.2p::gfp. (A–B) Micrographs showing the pharynx of animals co-expressing pat-4p::gfp and gcy-8p::mCherry (A) or ttx-3p::rfp (B) on day 1 of adulthood. Images of an overlay of the fluorescence channels with DIC (first column), of the fluorescence channels (second and third columns), and an overlay of the fluorescence channels (fourth column) are shown. The outline of the single cells expressing pat-4p::gfp (white) and the cell body of one of the AFD (A) or AIY (B) neurons (red) are highlighted in the magnified insets. Scale bars = 20 μm. (C–D) Lifespan analysis of N2 wild-type (WT) animals, neuronal-specific RNAi strain (sid-1; rab-3p::sid-1) (C), and muscle-specific RNAi strain (sid-1; myo-3p::sid-1) transferred to bacteria expressing empty vector or pat-4/ILK dsRNA on day 1 of adulthood. The same wild-type control is presented in (C) and (D) because the three strains were tested in the same experiment. Statistical analysis with log-rank (Mantel-Cox) test. The experiment was repeated three times with similar results (see Table S1 for additional data and Fig. S5 for information on RNAi effects in the sid-1 transgenic strains). All experiments were carried out at 20 °C. (E) Fluorescence micrographs of wild-type animals (‘whole-body’ RNAi), neuronal-specific RNAi strain (sid-1; rab-3p::sid-1) and muscle-specific RNAi strain (sid-1; myo-3p::sid-1) expressing hsp-16.2p::gfp. Animals were transferred to bacteria containing empty vector or bacteria expressing pat-4/ILK dsRNA on day 1 and images were captured on day 3. Exposure time was 1 s. Prior to imaging, animals were incubated for 1 h at 36 °C followed by 2 h at 20 °C. Scale bars = 200 μm. (F) Quantification of relative GFP intensity of the animals shown in (E). N = 9–14. *P < 0.05, ***P < 0.001 (two-way anova). The experiment was repeated three times with similar results.
PAT-4/ILK is expressed in several mechanosensory neurons different from the cells we identified above, as well as in body-wall muscle cells, the pharynx and in somatic gonad structures (Mackinnon et al., 2002). To specifically investigate the role of PAT-4/ILK in neurons and muscle, we used tissue-specific sid-1 RNAi strains. sid-1 mutants are deficient in transporting dsRNA molecules from one tissue to another (Winston et al., 2002) but have been engineered to express the SID-1 dsRNA transporter under a neuronal- (sid-1(qt9); rab-3p::sid-1) or body-wall-muscle-specific promoter (sid-1(qt9); myo-3p::sid-1) (see legend for Fig. S5, Supporting information for more information on RNAi effects in these animals). Using these strains, we found that adult-only pat-4/ILK RNAi treatment significantly increased the lifespan of animals when pat-4/ILK was reduced in neurons, indicating that PAT-4/ILK-expressing neurons are important in conferring the lifespan extension (Fig 5C, Table S1, Supporting information). Consistent with these observations, we found that pat-4/ILK RNAi in neurons induced hsp-16.2p::gfp expression, including in the intestine (Fig. 5E–F). In contrast, reduction in pat-4/ILK in body-wall muscles neither increased lifespan (Fig. 5D, Table S1, Supporting information) nor induced the hsp-16.2p::gfp reporter (Fig. 5E–F), even though pat-4/ILK levels were potently reduced in the muscle-specific RNAi strain in response to this RNAi treatment (Fig. S5C, Supporting information). In summary, our analyses using tissue-specific RNAi strains indicate that neurons, but not muscle, constitute a critical organ in which pat-4/ILK functions to cause systemic effects on the animal. As the AFD thermosensory neurons and the AIY interneurons are similarly required for these effects but do not express PAT-4/ILK, our results indicate that pat-4/ILK reduction causes thermotolerance and lifespan extension as well as stress responses in tissues not expressing PAT-4/ILK (such as the intestine) via cell nonautonomous signaling (see model in Fig. 6).
Figure 6.
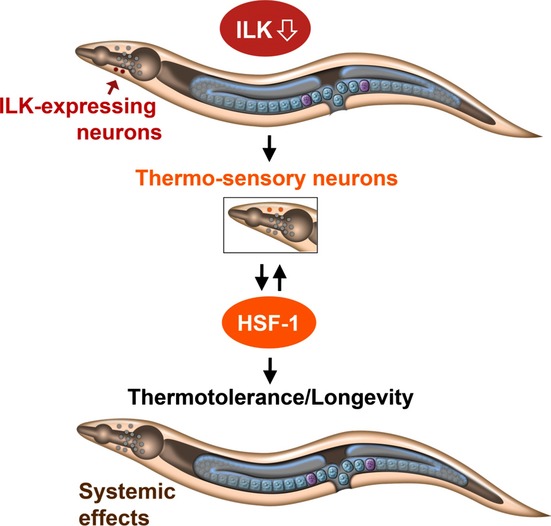
Model for how PAT-4/ILK modulates thermotolerance and longevity in C. elegans. In this organism, depletion of pat-4/ILK in PAT-4/ILK-expressing neurons, localized for example, in the head region of the animal as shown here, activates the transcription factor HSF-1 via specific thermosensory neurons also localized in the head (which do not express pat-4/ILK), thereby ensuring the induction of an HSF-1 meditated stress response in distal tissues (e.g., the intestine), as well as thermotolerance and longevity. It is possible that additional PAT-4/ILK-expressing tissues/organs, such as the somatic gonad, are also important for the longevity function of PAT-4/ILK in C. elegans. A notable exception may be the body-wall muscle, as reduction in pat-4/ILK in this tissue failed to induce longevity and stress responses; see text for details.
Discussion
Inhibition of components of the integrin-signaling complex extends lifespan in both Drosophila and C. elegans (Goddeeris et al., 2003; Hansen et al., 2005; Curran & Ruvkun, 2007; Nishimura et al., 2014) raising the possibility that integrin signaling constitutes a conserved longevity pathway. In this study, we characterized the molecular mechanisms by which one component of this complex, PAT-4/ILK, modulates lifespan in C. elegans. Reduced ILK levels rendered both C. elegans and Drosophila more resistant to heat stress, suggesting that increased thermotolerance may be one mechanism by which ILK modulates aging. We also identified HSF-1, the key regulator of the canonical HSR, as a critical effector of the thermotolerance induced by pat-4/ILK depletion, providing the first connection between HSF-1 and ILK. Specifically, we found that ILK regulates the expression of hsf-1 and of specific stress response genes via a neuronal circuit that includes neurons involved in thermosensation to establish a systemic stress response that may mediate, or accompany, pat-4/ILK RNAi-induced lifespan extension in C. elegans (Fig. 6).
The observation that a reduction in PAT-4/ILK levels increased hsf-1 transcription in C. elegans was particularly noteworthy, as neither heat stress nor reduced insulin/IGF-1 signaling increases hsf-1 mRNA levels (Hsu et al., 2003; Chiang et al., 2012), and increased hsf-1 transcript levels have so far only been demonstrated in breast cancer tumor cells (Santagata et al., 2011). To our knowledge, our study is the first to show that transcriptional regulation of hsf-1 is functionally relevant to an organismal stress response.
The HSF-1-mediated stress response in C. elegans requires the thermosensory neurons AFD and AIY for the systemic induction of multiple HSF-1 target genes, such as heat-shock proteins necessary for organismal proteostasis (Prahlad et al., 2008; Prahlad & Morimoto, 2011). We found that lifespan extension, thermotolerance, HSF-1 upregulation, and hsp-16.2 induction following pat-4/ILK inhibition were all dependent on the function of the AFD and AIY neurons, similar to events occurring during a canonical heat-shock response (HSR). However, pat-4/ILK RNAi upregulated only a subset of known heat-inducible HSF-1 targets and induced transcription of hsf-1 itself in animals raised at normal temperatures, implying that the loss of pat-4/ILK induced a noncanonical HSR. Interestingly, differential activation of HSF-1 targets in response to heat stress has been demonstrated in certain cell types; for example, human retinoblastoma cells fail to upregulate Hsp70 upon heat shock, even though HSF-1 is activated and Hsp90 is induced (Mathur et al., 1994). Thus, atypical or specialized HSRs can be mounted at least in some mammalian cells, similar to our demonstration of a pat-4/ILK RNAi-induced HSR in C. elegans.
While PAT-4/ILK was not expressed in the AFD and AIY thermosensory neurons, we observed that depletion of pat-4/ILK from other neurons was sufficient to increase C. elegans lifespan and induce the expression of the HSF-1-target gene hsp-16.2. In contrast, the main site of PAT-4/ILK expression, the body-wall muscles, did not seem to be important for the beneficial organismal effects of PAT-4/ILK. PAT-4/ILK-expressing neurons may therefore be a major tissue from which PAT-4/ILK reduction conveys organismal effects on thermotolerance and longevity. We note that it is possible that additional PAT-4/ILK-expressing tissues may contribute as well, as we found that gonad-less gon-2(q3888) mutants, which lack all gonad structures when raised at the nonpermissive temperature (i.e., somatic gonad and germline), failed to become thermotolerant upon reduction in pat-4/ILK (Table S6, Supporting information). Future experiments are needed to fully clarify how PAT-4/ILK-expressing tissues contribute to C. elegans lifespan determination. Likewise, it will be interesting to elucidate the signals that are required for the intertissue communication between PAT-4/ILK-expressing neurons (and possibly other sites), and AFD and AIY thermosensory neurons, that ultimately regulate the systemic HSF-1-mediated stress response observed in C. elegans with reduced PAT-4/ILK levels.
How could reduced levels of PAT-4/ILK increase thermotolerance and extend organismal lifespan in C. elegans in an hsf-1-dependent fashion? One possible mechanism could include alterations in the signaling pathways regulated by ILK downstream of the integrin-signaling complex (Qin & Wu, 2012). For example, HSF-1 might be negatively regulated by the ILK substrate glycogen synthase 3 (GSK3), as observed, for example, in mammalian cells (Xavier et al., 2000). Another hypothesis could relate to the scaffolding role of ILK in the integrin complex. We and others have shown that reducing PAT-4/ILK levels in animals from hatching leads to gaps in the cytoskeletal lattice, myofilament detachment, and collapse of MYO-3 in body-wall muscle cells (Meissner et al., 2009 and this study). We did not observe such prominent phenotypes in animals in which pat-4/ILK was inhibited only during adulthood; however, adult-only inhibition of pat-4/ILK could be speculated to cause subtle cytoskeletal disruptions in certain PAT-4/ILK-expressing tissues/cells. In turn, such cytoskeletal disruptions may resemble, and be perceived by HSF-1, as an aggregation-like state. While future experiments are needed to directly investigate these two nonmutually exclusive hypotheses, we have observed that whole-life reduction in pat-4/ILK can lead to an early onset of aggregation of polyglutamine-expansion repeats in C. elegans (Fig. S7, Supporting information). Moreover, aggregation of human Aβ protein is promoted in C. elegans following whole-life inhibition of other integrin-complex components (i.e., the integrins pat-2 and pat-3) (Jensen et al., 2012). It remains to be directly addressed whether inhibition of pat-4/ILK or other integrin-complex components in adult animals could similarly promote a cellular milieu permissive for protein aggregation or misfolding. In such a scenario, activation of HSF-1 could potentially take place via chaperone displacement. For example, Hsp90, which under normal conditions is constitutively bound to HSF-1 to keep HSF-1 in an inactive state, might act as the sensor for subtle cytoskeletal disruptions and dissociate from HSF-1. Interestingly, Hsp90 was recently shown to directly interact with ILK in mammalian cells to facilitate ILK’s interaction with alpha-parvin (Radovanac et al., 2013). It remains to be determined whether this interaction between Hsp90 and ILK could represent a functional connection to HSF-1.
In conclusion, we have shown that genetic reduction in the integrin-signaling complex component pat-4/ILK activates HSF-1 to induce a neuron-dependent, noncanonical HSR in C. elegans. Our study suggests that fine-tuning of such an HSR through neuroendocrine signals can regulate normal physiological processes such as stress responses and aging, and may thus be relevant to ILK-mediated, age-related pathologies such as cancer and cardiomyopathies.
Experimental procedures
Strains
Strains were maintained and cultured under standard conditions at 20 °C using E. coli OP50 as a food source (Brenner, 1974). For RNAi experiments, animals were grown on HT115 bacteria (see below). For experiments with the temperature-sensitive strains CF512, CF2201 and CF2253, animals were raised at 25 °C until adulthood. See Table S7 (Supporting information) for strains used and created for this study.
RNA interference (RNAi)
All RNAi bacterial clones (expressing dsRNA for gene of interest) used to feed C. elegans for gene inactivation were verified by sequencing and cultured as specified in Supporting Information.
Pharyngeal pumping
Pharyngeal pumping was measured by counting the number of contractions in the posterior bulb of the pharynx during a 30-s interval. If pharyngeal pumping of a particular animal was irregular, multiple measurements were performed and an average was calculated. Between 20 and 40 animals of each strain were assayed on each day of the experiment, and an average was calculated.
Lifespan analysis
Lifespan was measured at 20 °C as previously described (Hansen et al., 2005), with RNAi treatments initiated as indicated in the text and figure legends. Animals were scored as dead if they failed to respond to gentle prodding with a platinum wire pick. Censoring occurred if animals desiccated on the edge of the plate, escaped, ruptured, or suffered from internal hatching. Statistical analysis was performed using stata software (StataCorp, College Station, TX, USA). P values were calculated with the log-rank (Mantel-Cox) method.
Analysis and imaging of stress reporters
Induction of stress reporters was analyzed by imaging transgenic animals on media plates after anesthetizing the animals with M9 medium containing 0.1% NaN3. Images were acquired with a Leica DFC310 FX camera using the exposure times indicated in the figure legends. Image analysis was performed with imagej software (National Institutes of Health, Bethesda, MD, USA), by tracing the intestine and measuring the integrated density of GFP fluorescence.
Imaging of animals with reporter constructs in thermosensory neurons and body-wall muscles was performed at higher magnification using a Zeiss Imager Z1. Fluorescence and differential interference contrast (DIC) microscopy was performed after mounting animals on a 2% agarose pad in M9 medium, both containing 0.1% NaN3.
C. elegans and Drosophila thermotolerance assays
The survival of C. elegans at elevated temperatures was measured after an 8 h incubation at 36 °C (Hansen et al., 2007). For each strain, 4 plates with ~20 animals per plate were incubated in a single layer in a HERAtherm incubator (Thermo Fisher, Waltham, MA). Animals were scored as dead if they failed to respond to gentle prodding with a platinum wire pick.
The survival of Drosophila at elevated temperatures was measured after an 85 min incubation in a 36 °C waterbath. ilk54 (Zervas et al., 2001) was introgressed into the wCS wild-type background for six generations, and the backcrossed line is referred to as ilk54-wCS. Virgin female and male flies were collected and aged at 25 °C. For the thermotolerance assay, 3-week-old flies were briefly anesthetized with CO2, separated into groups of 10 flies per food vial, and allowed to recover overnight. The flies were then transferred to empty vials and placed in a waterbath at 36 °C for 85 min. Completely immobile flies were scored as dead.
Electrophoretic mobility shift assay and Western blot analysis
All biochemical procedures were performed as described in the Supporting Information (Chiang et al., 2012).
Quantitative RT–PCR
Quantitative RT–PCR was performed as previously described (Chiang et al., 2012; Lapierre et al., 2013). Preparation of C. elegans RNA and details of PCR conditions are provided in the Supporting Information. See Table S8 (Supporting information) for primer sequences.
Statistical analysis
Statistical significance was determined by Student’s t-test and one- or two-way anova using graphpad prism software (La Jolla, CA, USA), except where noted otherwise.
Acknowledgments
We would like to thank all members of the Hansen Laboratory and Dr. Sreekanth Chalasani for fruitful discussions, and Drs. Louis Lapierre, Deepti Wilkinson, and Anne O’Rourke for feedback on the manuscript. We thank Drs. Andrew Dillin and Roger Tsien for sharing reagents, and the Kenyon and Shen labs for providing strains. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center (CGC), which is funded by the Office of Research Infrastructure Programs (P40 OD010440). This project was supported by an Ellison Foundation New Scholar in Aging Award and a Glenn Award for Research in Biological Mechanisms of Aging to MH, and an American Association for Cancer Research/Astellas Pharma Global Development Inc. Fellowship in Basic Cancer Research Grant and an Ellison Medical Foundation/AFAR postdoctoral fellowship to CK. MH is supported by the National Institutes of Health (NIA).
Author contributions
CK and MH designed the study, analyzed and interpreted the data, and wrote the manuscript. TTC with the help of XY conducted the HSF-1 Western blot and EMSA assays in ALH’s laboratory. MN and HHC performed the Drosophila stress experiments in RB’s laboratory. AED performed repeats of some lifespan experiments, and AED, BO, CCC, and SG performed repeats of some thermotolerance experiments. SG constructed the kin-32 RNAi clone, outcrossed several strains, and provided comments on the manuscript. MH outcrossed several strains and performed repeats of some thermotolerance experiments. SP created the tissue-specific RNAi strains and NB outcrossed AGD855 in Dr. Andrew Dillin’s laboratory. CK performed all other experiments. RB and AHL contributed to the study design and data interpretation, and provided comments on the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
Fig. S1 pat-4/ILK mRNA levels are reduced in C. elegans by whole-life and adult-only RNAi treatments.
Fig. S2 Effects of reduction of integrin-complex components in C. elegans expressing GFP-tagged PAT-4/ILK.
Fig. S3 Effects on stress-inducible GFP reporters following inhibition of pat-4/ILK in adult C. elegans transcription factor mutants.
Fig. S4 pat-4/ILK mRNA levels are reduced in hsf-1 mutants.
Fig. S5 Reduction of pat-4/ILK in gcy-8 and ttx-3 mutants does not increase transcription of HSF-1 target genes.
Fig. S6 Thermotactic behavior is intact in C. elegans with reduced PAT-4/ILK levels.
Fig. S7 Whole-life inhibition of pat-4/ILK increases aggregation of polyglutamine-expansion repeats in C. elegans.
Table S1 Lifespan analysis of various strains subjected to pat-4/ILK RNAi.
Table S2 Survival analysis of wild-type animals subjected to RNAi against components of the integrin complex and cytoskeleton during adulthood and incubated at 36°C.
Table S3 Summary of phenotypes observed in animals subjected to RNAi of integrin complex and cytoskeletal components.
Table S4 Survival analysis of transcription factor mutants subjected to pat-4/ILK RNAi during adulthood and incubated at 36°C.
Table S5 Survival analysis of mutants with defects in neurons involved in thermosensation subjected to pat-4/ILK RNAi during adulthood and incubated at 36°C.
Table S6 Survival analysis of gon-2 mutants subjected to pat-4/ILK RNAi during adulthood and incubated at 36°C.
Table S7 C. elegans strains used in this study.
Table S8 Sequences of quantitative RT-PCR primers used in this study.
References
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang WC, Ching TT, Lee HC, Mousigian C, Hsu AL. HSF-1 regulators DDL-1/2 link insulin-like signaling to heat-shock responses and modulation of longevity. Cell. 2012;148:322–334. doi: 10.1016/j.cell.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran SP, Ruvkun G. Lifespan regulation by evolutionarily conserved genes essential for viability. PLoS Genet. 2007;3:e56. doi: 10.1371/journal.pgen.0030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy D, Bertin N, Hidalgo CA, Venkatesan K, Tu D, Lee D, Rosenberg J, Svrzikapa N, Blanc A, Carnec A, Carvunis AR, Pulak R, Shingles J, Reece-Hoyes J, Hunt-Newbury R, Viveiros R, Mohler WA, Tasan M, Roth FP, Le Peuch C, Hope IA, Johnsen R, Moerman DG, Barabasi AL, Baillie D, Vidal M. Genome-scale analysis of in vivo spatiotemporal promoter activity in Caenorhabditis elegans. Nat. Biotechnol. 2007;25:663–668. doi: 10.1038/nbt1305. [DOI] [PubMed] [Google Scholar]
- Fisher BR, Heredia DJ, Brown KM. Heat-induced alterations in embryonic cytoskeletal and stress proteins precede somite malformations in rat embryos. Teratog. Carcinog. Mutagen. 1996;16:49–64. doi: 10.1002/(SICI)1520-6866(1996)16:1<49::AID-TCM6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Goddeeris MM, Cook-Wiens E, Horton WJ, Wolf H, Stoltzfus JR, Borrusch M, Grotewiel MS. Delayed behavioural aging and altered mortality in Drosophila beta integrin mutants. Aging Cell. 2003;2:257–264. doi: 10.1046/j.1474-9728.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- GuhaThakurta D, Palomar L, Stormo GD, Tedesco P, Johnson TE, Walker DW, Lithgow G, Kim S, Link CD. Identification of a novel cis-regulatory element involved in the heat shock response in Caenorhabditis elegans using microarray gene expression and computational methods. Genome Res. 2002;12:701–712. doi: 10.1101/gr.228902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Hsu AL, Dillin A, Kenyon C. New genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS Genet. 2005;1:119–128. doi: 10.1371/journal.pgen.0010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 2007;6:95–110. doi: 10.1111/j.1474-9726.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- Jensen LT, Moller TH, Larsen SA, Jakobsen H, Olsen A. A new role for laminins as modulators of protein toxicity in Caenorhabditis elegans. Aging Cell. 2012;11:82–92. doi: 10.1111/j.1474-9726.2011.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong DE, Artan M, Seo K, Lee SJ. Regulation of lifespan by chemosensory and thermosensory systems: findings in invertebrates and their implications in mammalian aging. Front Genet. 2012;3:218. doi: 10.3389/fgene.2012.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TE, de Castro E, Hegi de Castro S, Cypser J, Henderson S, Tedesco P. Relationship between increased longevity and stress resistance as assessed through gerontogene mutations in Caenorhabditis elegans. Exp. Gerontol. 2001;36:1609–1617. doi: 10.1016/s0531-5565(01)00144-9. [DOI] [PubMed] [Google Scholar]
- Knoll R, Postel R, Wang J, Kratzner R, Hennecke G, Vacaru AM, Vakeel P, Schubert C, Murthy K, Rana BK, Kube D, Knoll G, Schafer K, Hayashi T, Holm T, Kimura A, Schork N, Toliat MR, Nurnberg P, Schultheiss HP, Schaper W, Schaper J, Bos E, Den Hertog J, van Eeden FJ, Peters PJ, Hasenfuss G, Chien KR, Bakkers J. Laminin-alpha4 and integrin-linked kinase mutations cause human cardiomyopathy via simultaneous defects in cardiomyocytes and endothelial cells. Circulation. 2007;116:515–525. doi: 10.1161/CIRCULATIONAHA.107.689984. [DOI] [PubMed] [Google Scholar]
- Lange A, Wickstrom SA, Jakobson M, Zent R, Sainio K, Fassler R. Integrin-linked kinase is an adaptor with essential functions during mouse development. Nature. 2009;461:1002–1006. doi: 10.1038/nature08468. [DOI] [PubMed] [Google Scholar]
- Lapierre LR, De Magalhaes Filho CD, McQuary PR, Chu CC, Visvikis O, Chang JT, Gelino S, Ong B, Davis AE, Irazoqui JE, Dillin A, Hansen M. The TFEB orthologue HLH-30 regulates autophagy and modulates longevity in Caenorhabditis elegans. Nat Commun. 2013;4:2267. doi: 10.1038/ncomms3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libina N, Berman JR, Kenyon C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell. 2003;115:489–502. doi: 10.1016/s0092-8674(03)00889-4. [DOI] [PubMed] [Google Scholar]
- Link CD, Johnson CJ. Reporter transgenes for study of oxidant stress in Caenorhabditis elegans. Methods Enzymol. 2002;353:497–505. doi: 10.1016/s0076-6879(02)53072-x. [DOI] [PubMed] [Google Scholar]
- Mackinnon AC, Qadota H, Norman KR, Moerman DG, Williams BD. C. elegans PAT-4/ILK functions as an adaptor protein within integrin adhesion complexes. Curr. Biol. 2002;12:787–797. doi: 10.1016/s0960-9822(02)00810-2. [DOI] [PubMed] [Google Scholar]
- Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu. Rev. Biochem. 2008;77:727–754. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- Mathur SK, Sistonen L, Brown IR, Murphy SP, Sarge KD, Morimoto RI. Deficient induction of human hsp70 heat shock gene transcription in Y79 retinoblastoma cells despite activation of heat shock factor 1. Proc. Natl Acad. Sci. USA. 1994;91:8695–8699. doi: 10.1073/pnas.91.18.8695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald PC, Fielding AB, Dedhar S. Integrin-linked kinase–essential roles in physiology and cancer biology. J. Cell Sci. 2008;121:3121–3132. doi: 10.1242/jcs.017996. [DOI] [PubMed] [Google Scholar]
- Meissner B, Warner A, Wong K, Dube N, Lorch A, McKay SJ, Khattra J, Rogalski T, Somasiri A, Chaudhry I, Fox RM, Miller DM, 3rd, Baillie DL, Holt RA, Jones SJ, Marra MA, Moerman DG. An integrated strategy to study muscle development and myofilament structure in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000537. doi: 10.1371/journal.pgen.1000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendenhall AR, Tedesco PM, Taylor LD, Lowe A, Cypser JR, Johnson TE. Expression of a single-copy hsp-16.2 reporter predicts life span. J. Gerontol. A Biol. Sci. Med. Sci. 2012;67:726–733. doi: 10.1093/gerona/glr225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I, Ohshima Y. Neural regulation of thermotaxis in Caenorhabditis elegans. Nature. 1995;376:344–348. doi: 10.1038/376344a0. [DOI] [PubMed] [Google Scholar]
- z M, Kumsta C, Kaushik G, Diop S, Ding Y, Bisharat-Kernizan J, Catan H, Ross R, Engler A, Bodmer R, Hansen M, Ocorr K. Dual role of β1-integrin and integrin-linked kinase in modulating cardiac aging. Aging Cell. 2014 doi: 10.1111/acel.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postel R, Vakeel P, Topczewski J, Knoll R, Bakkers J. Zebrafish integrin-linked kinase is required in skeletal muscles for strengthening the integrin-ECM adhesion complex. Dev. biol. 2008;318:92–101. doi: 10.1016/j.ydbio.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Prahlad V, Morimoto RI. Neuronal circuitry regulates the response of Caenorhabditis elegans to misfolded proteins. Proc. Natl Acad. Sci. USA. 2011;108:14204–14209. doi: 10.1073/pnas.1106557108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahlad V, Cornelius T, Morimoto RI. Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons. Science. 2008;320:811–814. doi: 10.1126/science.1156093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Wu C. ILK: a pseudokinase in the center stage of cell-matrix adhesion and signaling. Curr. Opin. Cell Biol. 2012;24:607–613. doi: 10.1016/j.ceb.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radovanac K, Morgner J, Schulz JN, Blumbach K, Patterson C, Geiger T, Mann M, Krieg T, Eckes B, Fassler R, Wickstrom SA. Stabilization of integrin-linked kinase by the Hsp90-CHIP axis impacts cellular force generation, migration and the fibrotic response. EMBO J. 2013;32:1409–1424. doi: 10.1038/emboj.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santagata S, Hu R, Lin NU, Mendillo ML, Collins LC, Hankinson SE, Schnitt SJ, Whitesell L, Tamimi RM, Lindquist S, Ince TA. High levels of nuclear heat-shock factor 1 (HSF1) are associated with poor prognosis in breast cancer. Proc. Natl Acad. Sci. USA. 2011;108:18378–18383. doi: 10.1073/pnas.1115031108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Khazaeli AA, Curtsinger JW. Chaperoning extended life. Nature. 1997;390:30. doi: 10.1038/36237. [DOI] [PubMed] [Google Scholar]
- Voellmy R. On mechanisms that control heat shock transcription factor activity in metazoan cells. Cell Stress Chaperones. 2004;9:122–133. doi: 10.1379/CSC-14R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker GA, Lithgow GJ. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell. 2003;2:131–139. doi: 10.1046/j.1474-9728.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- Walter MF, Petersen NS, Biessmann H. Heat shock causes the collapse of the intermediate filament cytoskeleton in Drosophila embryos. Dev. Genet. 1990;11:270–279. doi: 10.1002/dvg.1020110405. [DOI] [PubMed] [Google Scholar]
- Williams BD, Waterston RH. Genes critical for muscle development and function in Caenorhabditis elegans identified through lethal mutations. J. Cell Biol. 1994;124:475–490. doi: 10.1083/jcb.124.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston WM, Molodowitch C, Hunter CP. Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science. 2002;295:2456–2459. doi: 10.1126/science.1068836. [DOI] [PubMed] [Google Scholar]
- Wu C, Dedhar S. Integrin-linked kinase (ILK) and its interactors: a new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complexes. J. Cell Biol. 2001;155:505–510. doi: 10.1083/jcb.200108077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier IJ, Mercier PA, McLoughlin CM, Ali A, Woodgett JR, Ovsenek N. Glycogen synthase kinase 3beta negatively regulates both DNA-binding and transcriptional activities of heat shock factor 1. J. Biol. Chem. 2000;275:29147–29152. doi: 10.1074/jbc.M002169200. [DOI] [PubMed] [Google Scholar]
- Zervas CG, Gregory SL, Brown NH. Drosophila integrin-linked kinase is required at sites of integrin adhesion to link the cytoskeleton to the plasma membrane. J. Cell Biol. 2001;152:1007–1018. doi: 10.1083/jcb.152.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 pat-4/ILK mRNA levels are reduced in C. elegans by whole-life and adult-only RNAi treatments.
Fig. S2 Effects of reduction of integrin-complex components in C. elegans expressing GFP-tagged PAT-4/ILK.
Fig. S3 Effects on stress-inducible GFP reporters following inhibition of pat-4/ILK in adult C. elegans transcription factor mutants.
Fig. S4 pat-4/ILK mRNA levels are reduced in hsf-1 mutants.
Fig. S5 Reduction of pat-4/ILK in gcy-8 and ttx-3 mutants does not increase transcription of HSF-1 target genes.
Fig. S6 Thermotactic behavior is intact in C. elegans with reduced PAT-4/ILK levels.
Fig. S7 Whole-life inhibition of pat-4/ILK increases aggregation of polyglutamine-expansion repeats in C. elegans.
Table S1 Lifespan analysis of various strains subjected to pat-4/ILK RNAi.
Table S2 Survival analysis of wild-type animals subjected to RNAi against components of the integrin complex and cytoskeleton during adulthood and incubated at 36°C.
Table S3 Summary of phenotypes observed in animals subjected to RNAi of integrin complex and cytoskeletal components.
Table S4 Survival analysis of transcription factor mutants subjected to pat-4/ILK RNAi during adulthood and incubated at 36°C.
Table S5 Survival analysis of mutants with defects in neurons involved in thermosensation subjected to pat-4/ILK RNAi during adulthood and incubated at 36°C.
Table S6 Survival analysis of gon-2 mutants subjected to pat-4/ILK RNAi during adulthood and incubated at 36°C.
Table S7 C. elegans strains used in this study.
Table S8 Sequences of quantitative RT-PCR primers used in this study.


