Abstract
Introduction
Exposure to endocrine disrupting chemicals (EDCs) has recently been linked to declining fertility in men in both developed and developing countries. Since many EDCs possess intrinsic estrogenic or androgenic activities, thus, the gonad is one of the major targets of EDCs.
Areas covered
For the past 2 decades, studies found in the literature regarding the disruptive effects of these EDCs on reproductive function in human males and also rodents were mostly focused on oxidative stress-induced germ cell apoptosis, disruption of steroidogenesis, abnormal sperm production and disruption of spermatogenesis in particular cell adhesion function and the blood–testis-barrier (BTB) function. Herein, we highlight recent findings in the field illustrating testis-specific proteins are also targets of EDCs.
Expert opinion
This information should be helpful in developing better therapeutic approach to manage ECD-induced reproductive toxicity. This information is also helpful to identify potential targets for male contraceptive development.
Keywords: blood–testis-barrier, endocrine disrupting chemicals, spermatogenesis, steroidogenesis, testis, testis-specific proteins
1. Introduction
Infertility affects about 15% of the married couples, among these, ~ 50% of the cases are contributed by reproductive dysfunction in men, and infertility is an emerging health issue after cancer and cardiovascular diseases across the world [1-3]. Generally, male factors that lead to infertility amongst married couples are either congenital, acquired or idiopathic, some of them are reversible and treatable [3,4]. Recent studies have shown that exposure to environmental toxicants is one of the major causes of idiopathic male infertility [5,6]. Some of the environmental toxicants are capable of disrupting endocrine systems in both humans and rodents and designed endocrine disrupting chemicals (EDCs). According to the US Environmental Protection Agency (EPA) at www.epa.gov/endocrine/, an EDC is defined as ‘an exogenous agent that interferes with the synthesis, secretion, transport, metabolism, binding, action or elimination of natural hormones in the body that are responsible for the maintenance of homeostasis, reproduction, development and/or behavior’. EDCs exert their effects primarily through nuclear hormone receptors such as estrogen receptor, androgen receptor and thyroid receptor, and/or through non-nuclear hormone receptor pathways such as orphan receptor and enzymatic pathways involving in steroidogenesis and metabolism [7]. As the result of industrial activities, many of these chemicals are now part of the food chain. For instance, phthalates and bisphenol A (BPA) used as plasticizers and plastic monomers that give polyvinyl chloride (PVC) its fluidity to improve flexibility and durability are found to leach out gradually over time from bottles and containers used by consumers. Organochlorine insecticide dichlorodiphenyltrichloroethane (DDT) synthesized in 1874 to control malaria and typhus has also contaminated our environment since then. A significant reduction of sperm quality in US veterans after exposure to dioxin was first reported in 1990 [8]. In short, EDCs are now detected in body fluids in humans, drinking water, food supplies, cosmetics and household products and textiles. Unfortunately, some of these toxicants (e.g., cadmium) have exceedingly long half-life, posing potential harmful events due to their gradual build-up in organs over time (Table 1). Most of the EDCs target the liver, kidneys, thyroid and testes. Males appear to be more susceptible to the adverse effect of EDCs versus females since most of the EDCs are anti-androgenic. For instance, BPA and cadmium are estrogenic while phthalates are anti-androgenic compounds [9-11].
Table 1. The acceptable daily intake/tolerable daily intake (ADI/TDI) dose and the half-life (t1/2) of common EDCs in humans.
| Types of chemicals |
Mechanism of actions/Refs. |
EDCs | Human elimination half-life |
Main route (source) of exposure |
Estimated daily intake (per kg body weight) |
ADI or TDI (adult) |
Refs. |
|---|---|---|---|---|---|---|---|
| Heavy metals |
Estrogenic [127-129] | Cadmium | > 20 years | Oral (seafood, rice) |
1.06 μg | 55 μg/kg/day | ATSDR, 1999 [130,131] |
| Lead | 28 – 36 days | Inhalation (old lead- based paints) |
0.043 μg | 50 μg/kg/day (25 μg/kg/day for children) |
ATSDR, 2005 [130] |
||
| Methyl mercury |
60 – 90 days | Oral (Fish, seafood) |
0 – 0.33 μg | 0.1 μg/kg/day | WHO, 2006 [130,132] |
||
| Plasticizers | Estrogenic [148] | Bisphenol A | < 2 hours | Oral | 34 ng | 50 μg/kg/day | EFSA, 2006 [133,134] |
| Anti-androgenic [135] | Phthalates (e.g., DEHP) |
10 hours | Oral, medical devices |
1.6 | 22 μg/kg/day | USEPA, 1998 [136,137] |
|
| Surfactants | Thyroid toxicity [138] | PFOS PFOA |
5.4 years 3.8 years |
Oral Oral |
1.6 ng 2.9 ng |
150 ng/kg/day 1.5 μg/kg/day |
EFSA, 2008 [139,140] |
ATSDR: Agency for Toxic Substances and Disease Registry; DEHP: Di-2-ethylhexyl phthalate; EFSA: European Food Safety Authority; PFOA: Perfluorooctanoic acid also known as C8 and perfluorooctanoate; PFOS: Perfluorooctanesulfonic acid or perfluorooctane sulfonate; USEPA: US Environmental Protection Agency;
WHO: World Health Organization; Oral, includes dairy products, meats, vegetables, fruits, seafood, beverage, and water.
ADI: acceptable daily intake; TDI: tolerable daily intake
In humans, development of the male reproductive system begins at ~ 7-week of pregnancy which continues until puberty at ~ 12 – 13 years of age, primarily mediated by the testicular hormones and hormones released at the hypothalamic-pituitary-testicular axis (HPT) [12], which in turn regulate Leydig and Sertoli cell function. It is noted that Leydig cells produce testosterone through steroidogenesis, whereas Sertoli cells release paracrine/autocrine factors (e.g., cytokines) and hormones (e.g., estrogen, inhibin, activin, follistatin) in the testis that support germ cell maturation. Although spermatogenesis begins at puberty, a series of events that take place during the prenatal, early postnatal and prepubertal stages are essential to prepare for the initiation of spermatogenesis (Figure 1). Spermatogenesis is tightly regulated by the HPT axis in mammals, which is sensitive to internal hormone fluctuations and external stimuli mediated by drugs, radiation, toxicants and development [13-16]. Human epidemiological studies also suggest a link between toxicant exposure and reduced fertility as manifested by reduced sperm count and sperm motility among men following toxicant exposure [17,18], implying these toxicants may disrupt germ cell maturation and/or adhesion. Low sperm count may also be related to an increase in exposure of men to estrogenic, anti-estrogenic or anti-androgenic chemicals, which exert their effects by mimicking naturally occurring steroids, affecting the expression of various receptors and/or activation/inhibition of sex hormone biosynthesis along the HPT axis as well as in the testis [19]. For instance, molecular signaling cascades were shown to be activated or inhibited following exposure of male rodents to EDCs, perturbing gene transcription and/or cell apoptosis. Herein, we summarize recent findings on the testis-specific targets commonly disrupted by EDCs. In this review, we limit our discussion on the impact of EDCs on spermatogenesis from the onset of pubertal to adult animals, including humans except for a few examples illustrated in Tables 2 and 3 regarding the effects of EDCs on Leydig cell function in immature and mature rodents, since cellular targets in fetal testes are different from adult animals. We divide our discussion into four topics in the sections below.
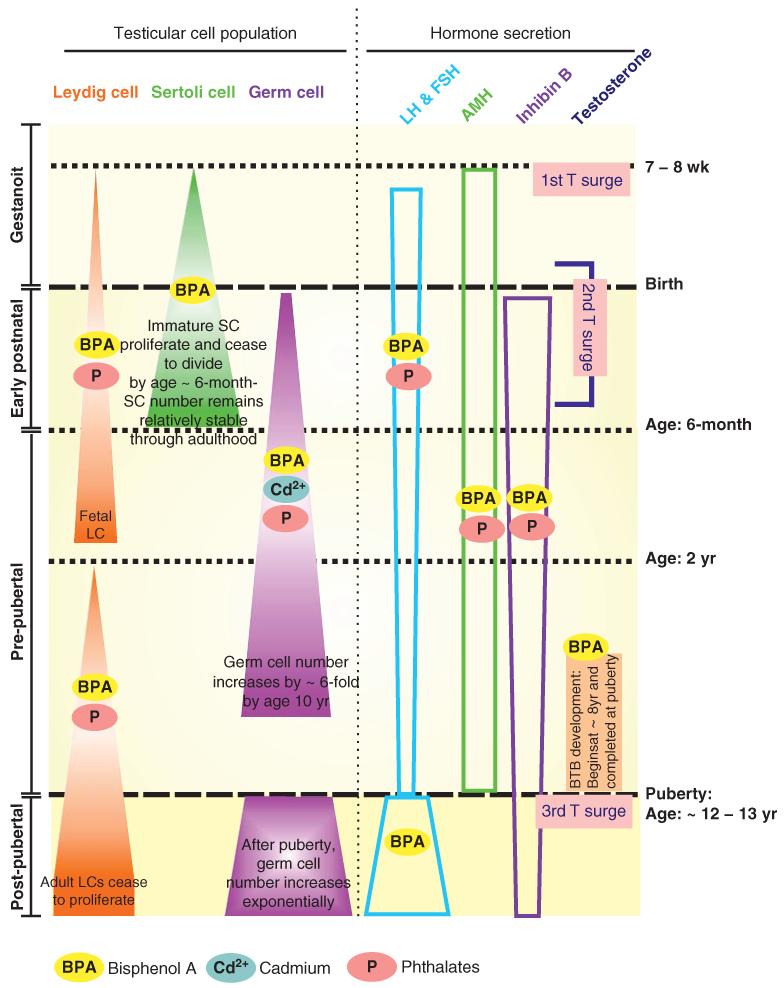
Figure 1. Different stages of testicular development in human males that are sensitive to EDCs.
Fetal Leydig cells (LC) proliferate beginning at gestational week 7 – 8, through the gestation and early postnatal life until 1.5-year of age. Perinatal exposure BPA increases LC proliferation via ERα activations, while phthalates decrease total LC numbers but increase aggregations of fetal LC. At the age 2-year, adult LC starts to proliferate until early adulthood, when then cease to divide. Prepubertal BPA exposure stimulates adult LC proliferation; while phthalates produce the opposite effect. Immature Sertoli cells (SC) proliferate until the SC population reaches its maximum number by 6-month of age which then cease to divide, and maturation of SC begins at the onset of puberty at ~ 12 – 13 years of age. Perinatal BPA exposure increases Sertoli cell numbers and this thus increases the testicular weight as shown in rat pups. Germ cells proliferation can be impaired by cadmium and phthalate exposure, while stimulated by BPA exposure [125,126]. Hormonal regulation during early development is mediated via pituitary gonadotropins (LH & FSH), anti-mullerian hormone (AMH) and inhibin B. Both BPA and phthalates increase the perinatal AMH and impair gonadotropins and inhibin B secretion. During the onset of puberty, the amplitude and frequency of the pulsative release of gonadotropins are up-regulated to accommodate the needs of gonadotropins. BPA exposure impairs their secretion which affects the biosynthesis of testicular steroids. In the seminiferous tubules, BTB development begins by ~ 8 years of age which is completed at puberty by ~ 12 – 13 years of age. Thereafter, preleptotene spermatocytes have to be transported across the BTB to enter the adluminal compartment to prepare for meiosis I/II. BPA exposure was found to specifically disrupting BTB integrity in premature rats suggesting BPA may be harmful to BTB development in humans. Throughout development, there are three testosterone (T) surges which stimulate specific events in the testis. The first testosterone surge occurs at gestational week 7 – 8, which stimulates the proliferation of testicular cells. The second testosterone surge increases the fetal LC populations. The last testosterone surge at the onset of puberty simulates Sertoli cell maturation which is essential to maintain spermatogenesis.
Table 2. Effects of EDCs on Leydig cell function in adult rodents*.
| EDCs | Exposure period | Dosage | Effects on Leydig cells | Refs. |
|---|---|---|---|---|
| PFOS | Adult mouse exposure for 21 days | 5 or 10 mg/kg/day | ↓ Pituitary LH mRNA level | [27] |
| ↓ Testicular IGF-1, IGF-1r and LHr gene expression |
||||
| ↓ Steroidogenic enzyme expression | ||||
| ↓ Serum testosterone | ||||
| Cadmium | Single injection and sacrifice after 0.48 – 144 h in adult rats |
10 μm/kg | ↓ Testicular LHr mRNA level | [29] |
| Methylmercury | Adult rat exposure for 14 days | 3 mg/kg/day | ↓ Serum testosterone level | [141] |
Table 3. Effects of EDCs on Leydig cell function in immature rodents*.
| EDCs | Exposure period |
Dosage | Effects | Refs. |
|---|---|---|---|---|
| Lead | Neonatal | 200 or 2000 ppm from PND 0 – 21 | ↓ LC populations in adult life | [144] |
| ↓ Steroidogenic enzymes, serum and testicular testosterone |
||||
| ↓ Weights of sex organ | ||||
| DEHP | Prepubertal | 10 mg/kg/day from PND 21 – 35 | ↓ Testosterone production | [145] |
| ↓ Steroidogenic enzyme productions | ||||
| BPA | Perinatal | 2.4 μg/kg/day from GD 12 – PND 21 | ↓ Serum testosterone and LH levels | [146] |
| ↓ Steriodogenic enzymes gene expression | ||||
| Mixture of BPA and DEHP |
Perinatal | 10 mg/kg/day from GD 0 – PND 21 | ↓ Gene expression levels of AMH, AR, StAR | [61] |
| ↓ Serum FSH, testosterone and progesterone levels and these persist in mature offsprings |
||||
| Cadmium chloride |
Prenatal | 0.5 mg/kg GD 13 – 17 | ↓ Fetal and adult serum testosterone levels | [147] |
| ↓ Steroidogenic enzyme expression |
2. Impact of EDCs on hormone dialogue: from HPG axis to testosterone action in the testis
2.1 Introduction
Puberty in humans and rodents is a neurologically initiated event that begins with the secretion of GnRH (gonadotropin-releasing hormone) from the hypothalamus under the influence of kisspeptin on GnRH neurons, which in turn stimulates the pituitary gonadotropins luteinizing hormone (LH) and follicle stimulating hormone (FSH) synthesis and secretion [20,21]. LH receptors are restricted to Leydig cells whereas FSH receptors are limited to Sertoli cells in the testis. Binding of LH onto its receptor in Leydig cells stimulate testosterone biosynthesis through steroidogenesis. Testosterone in turn initiates the onset of spermatogenesis at puberty and also regulates germ cell development through spermatogenesis. Gonadal steroidogenesis begins with signal transduction on stimulation by binding LH onto its G-protein-coupled LH receptor at the cell surface, which interacts with adenylate cyclase (AC) to increase intracellular cyclic AMP. Cyclic AMP then stimulates the protein kinase A to initiate cholesterol biosynthesis and transport. This activation of cAMP response element binding (CREB) protein and the subsequence transcription of downstream steroidogenic enzymes is the hallmark in steroid biogenesis [22]. The first and rate-limiting step of steroidogenesis is the transport of cholesterol from outer to inner mitochondrial membrane by the steroidogenic acute regulatory protein (StAR) (Figure 2). In the inner mitochondrial membrane, cytochrome P450 side-chain cleavage (P450scc) converts cholesterol to pregnenolone and it was further catalyzed into progesterone, and rostenedione and testosterone by 3β-hydroxysteroid dehydrogenase (3β-HSD), cytochrome P450 17A (CYP17A) and 17β-hydroxysteroid dehydrogenase (17β-HSD), respectively (Figure 2). Testosterone can be further converted to estrogen by the enzyme cytochrome P450 aromatase (AROM) (Figure 2). Expression of these enzymes and their intrinsic activities thus determine the bioavailability of steroid hormones and thereby the physiological outcomes in sperm production and fertility. In fact, these testis-specific enzymes are known to be the targets of multiple EDCs (Figure 2) [23,24].
Figure 2. A schematic drawing illustrating the effects of EDCs on steroidogenesis in mammalian Leydig cells.
Following the binding of LH to G-protein coupled LH receptor, it stimulates adenylate cyclase (AC), leading to a surge in intracellular cyclic AMP (cAMP). Activated cAMP and ligand binding of peroxisome proliferator activated receptor gamma (PPARγ) stimulate the phosphorylation of protein kinase A (PKA), which in turn initiate gene transcription of StAR protein. StAR gene expression can be further up-regulated by thyroid hormones and Insulin-like growth factor-1 (IGF-1) stimulation. StAR protein in the outer mitochondrial membrane is responsible for the transportation of precursor cholesterol to the inner membrane where P450scc (cholesterol side-chain cleavage enzyme, labeled as P450sc) was found. Cholesterol is converted to pregnenolone where it goes to the smooth endoplasmic reticulum (ER) for further enzymatic conversion to testosterone and estrogen. Different EDCs impose adverse effects which ultimately lead to the disrupted biosynthesis of male sex hormone testosterone that perturbs spermatogenesis.
2.2 Multiple steroidogenesis regulatory pathways in Leydig cells are the target of EDCs
Leydig cell steroidogenesis is one of the primary targets of EDCs. Perfluorinated compounds (PFCs), such as perfluorooctanoic acid (PFOA), perfluorooctane sulfonate (PFOS), perflorododecanoic acid (PFDOA), perfluorodecanoic acid (PFDA) and perfluoronanoic acid (PFNA) were shown to reduce serum testosterone level in rodents and also in spent medium of Leydig cells cultured in vitro (Figure 2) [25-27]. PFOS was also shown to down-regulate LH receptor gene expression without affecting pituitary LH mRNA levels in adult mice, suggesting disruptive effects of PFOS on LH receptor expression but not LH per se [27]. Similar effects were also reported in cadmium and lead treated rodents [28,29]. Tables 2 and 3 summarize effects of different EDCs on Leydig cell function in adult and immature rodents, respectively. In this context, it is of interest to note that any person is likely exposed to a variety of toxicants at any given time, exposure to a mixture of anti-androgens can also lead to a cumulative and dose-additive effect because of the relatively long half-life despite they mediate their effects through diverse mechanism of actions versus exposure to just a single toxicant at a time [30,31]. Thus, it is likely that we may underestimate the risks by exposing ourselves to multiple environmental toxicants even if they are present at relevant acceptable doses versus a single compound. Furthermore, recent studies have shown that ECD-induced endocrine disruptive effects, such as phthalates, in the fetal testis are species-specific [32] as shown in selected examples summarized in Tables 2 and 3. Besides aromatic EDCs, which are structurally similar to naturally occurring steroid hormones, heavy metals (e.g., cadmium, mercury and lead) also disrupt testicular steroid metabolism by inhibiting steroidogenic enzymatic activities (Figure 2) [33,34].
Besides testosterone, thyroid hormone released from the thyroid gland is also important for growth and development of the testis. In the testis, triiodothyronine (T3) transport into testicular cells (e.g., Leydig cells) through its transporter, once inside the cell, they bind to the retinoid X receptor/thyroid receptor (RXR/TR) to form heterodimer which induces transcription of steroidogenic factor-1 (SF-1) and subsequent up-regulates StAR gene (Figure 2) [35]. Recent human epidemiological study revealed that phthalates (and their metabolites), BPA and cadmium displayed an inverse relationship with thyroid hormone status in men [36]. Furthermore, thyroid hormone also modulates Leydig cell differentiation and steroidogenesis [37], implying that exposure to EDCs may affect steroidogenesis through their effects on thyroid hormone homeostasis. Other than thyroid hormone, a systematic and localized hormone, insulin-like growth factor-1 (IGF-1), which acts on IGF-1 receptor (IGF-1r) can also stimulate steroid hormone synthesis and StAR expression in Leydig cells. Studies in rodents have shown that PFOS inhibited IGF-1 and down-regulated its receptor expression in both the liver and the testis [27]. Other than the disruptive effects on the expression of steroidogenic enzymes, IGF-1 is also an anti-apoptotic factor for Leydig and Sertoli cells [38,39]. Furthermore, many EDCs, such as BPA, phthalates and PFCs, are known activators of peroxisome proliferator activated receptors (PPARs), which induced down-regulation of translocator protein (TSPO, former called peripheral-type benzodiazepine receptor) expression [7,40]. TSPO is a cholesterol-binding protein located in the outer mitochondrial membrane for steroid hormone biosynthesis. It interacts with StAR, and it requires for the binding, uptake and release of cholesterol from cytosol to the mitochondrion upon ligand stimulation [41]. These effects thus affect the rate-limiting step (i.e., transportation of precursor cholesterol into the inner mitochondrial membrane) of steroidogenesis, regulating testosterone production. Testosterone is the dominant male androgen. It is essential for male sexual development, initiation and maintenance of spermatogenesis. In addition, testosterone is not only an endocrine hormone that regulates male sexual organ development and to maintain testicular function in adulthood, it also regulates paracrine signaling in Sertoli cells to initiate and to maintain spermatogenesis. Figure 2 summarizes some of the likely target sites of EDCs in Leydig cells. In short, Leydig cell steroidogenesis is one of the most important targets of EDCs in mammals.
3. Impact of EDCs on sperm production in the testis through their effects on Sertoli and germ cells
The ultimate goal of spermatogenesis is the production of sperm for fertilization. Spermatogenesis is a highly regulated process and takes place in the seminiferous tubule where germ cells are nourished and supported by Sertoli cells, creating the seminiferous epithelium. Sertoli cells are essential to germ cell development in several ways. Sertoli cells provide the structural and nourishment support to developing germ cells, many of which are metabolically quiescence cells, in particular after meiosis when genetic materials are condensed and packed in the head of spermatids. Sertoli cells also secrete hormones (e.g., estrogen, inhibin), cytokines (e.g., TGF-βs), paracrine/autocrine factors (e.g., IGF-1) and proteins (e.g., transferrin, testin) necessary for germ cell development. Many of the signaling functions that coordinate the events of germ cell development take place at the Sertoli-germ cell interface, such as mediated by gap junctions [42]. Sertoli cells also confer immune privilege status to the testis by secreting immunosuppressive biomolecules, and also create the blood–testis-barrier (BTB) that anatomically divides the seminiferous epithelium into the basal and the adluminal compartment. Additionally, Sertoli cells serve as the scavenger to remove apoptotic germ cells and other cellular debris as they arise during spermatogenesis through phagocytosis. Similar to the Leydig cell, EDCs also target the Sertoli cell, disturbing hormone or protein secretions, inducing oxidative stress, blocking GJ communications and damaging the BTB integrity, contributing to infertility [5,42-45].
3.1 EDC-induced disruption of the local hormonal/autocrine/paracrine microenvironment in the testis
3.1.1 The somatotropic GH/IGF-1 axis
While testicular function is primarily regulated by the HPT axis, however, the somatotropic GH (growth hormone)/IGF-1 (insulin-like growth factor 1) axis is also an important functional axis that regulates reproductive function through its effects on Leydig cells for the onset of spermatogenesis [46]. IGF-1 is known to play a role in Sertoli cell proliferation and maturation, enhanced testicular steroidogenesis and initiation of spermatogenesis [46]. Both Sertoli and Leydig cells produce IGF-1 locally in the testis upon FSH and LH stimulation, respectively. Both systemic and localized IGF-1 production can stimulate androgen production by Leydig cells [46]. In addition, reduced IGF-1 and IGF-1 receptor (IGF-1r) expression promotes Sertoli cell death [39], highlighting the important role of IGF-1. A recent study demonstrated the inhibitory effect of PFOS exposure to male CD-1 mice on the expression of GH receptor, IGF-1 and IGF-1r in the liver and the testis [27]. In addition, exposure of rats to dioxin significantly reduced serum IGF-1 levels [47], illustrating EDCs induce systemic effects and injury to the testis. In short, the somatotropic GH/IGF-1 axis is an emerging target of EDCs.
3.1.2 Sertoli cell peptide hormones
Hormonal peptides such as anti-Müllerian hormone (AMH) and inhibin B are two Sertoli cell peptide hormones which are crucial to testis development and are targets of EDCs. Studies in humans have reported a tight relationship between various hormones and semen quality. For instance, semen quality negatively correlated with gonadotropins, but positively correlated with thyroid hormone and inhibin B levels [48]. Inhibin B is one of the peptide hormones secreted by Sertoli cells to serve as a feedback loop to reduce FSH biosynthesis and secretion by the pituitary gland. FSH promotes Sertoli cell function, increasing sperm count, sperm motility and spermatogenic status. Exposure to PFOS reduced inhibin B expression in adult mice, partially affected spermatogenesis that led to low sperm count. Exposure of male rats to bisphenol AF (an analog of BPA) also increased serum levels of gonadotropins and reduced the mRNA levels of testicular inhibin B [48]. Animal studies suggested that EDCs affected normal spermatogenesis by inhibiting inhibin B synthesis at the transcriptional level. Recent human studies demonstrated that the serum BPA level of infertile males was inversely associated with inhibin B, androgen and TSH; while positively association with FSH and FSH:inhibin B ratio [48,49]. Another human study also demonstrated that perinatal dioxin exposure led to lowered inhibin B and elevated FSH level and reduced semen quality, consistent with findings using BPA [50].
During the onset of puberty, testosterone plays a major role in initiating Sertoli cell maturation, testosterone is also necessary to support spermatogenesis during adulthood. Thus, proliferation and maturation of Sertoli cells is a downstream regulatory target of Leydig cell steroidogenic function. Testosterone acts on androgen receptors (ARs), which expressed exclusively in Sertoli cells in the testis since the onset of puberty [51]. Deletion of Sertoli AR led to the meiotic arrest and failure in spermiogenesis [52,53]. Androgens, AR and GnRH form a feedback regulatory loop that controls their normal physiological levels [54]. Circulating androgens act on AR in GnRH neurons in the hypothalamus to suppress GnRH secretion [55]. Any disruption of this axis produces both systemic and testicular effects that perturb sperm production. In fact, it is known that human semen quality is associated with the level of testicular androgen receptor and androgen sensitivity [56,57]. Under physiological conditions, testosterone produced by Leydig cells is bound and maintained at high level in the lumen of seminiferous tubules by Sertoli cell-specific androgen binding protein (ABP). High concentration of testosterone in the luminal fluid is essential to spermatogenesis and maturation of sperm in the epididymis [58] since testicular testosterone level is at least 100-fold higher than its level in the systemic circulation [59,60]. ABP production was stimulated by FSH and testosterone, which are the targets of many EDCs since EDCs lower ABP synthesis and/or expression, thereby affecting sperm maturation [61]. On the other hand, estrogen produced locally in the testis by Sertoli and germ cells is equally important to normal sperm production process [62-64]. Estrogen receptors are found in Sertoli and germ cells and they are crucial to spermatogenesis, in particular spermiogenesis [63,65]. Exposure of rodents to estrogenic compounds, such as BPA, dioxin and cadmium, that disrupts the homeostasis of estrogen in the microenvironment of the testis can impede spermatogenesis. Biosynthesis of estrogen is carried out by cytochrome P450 aromatase, which is responsible for the irreversibly aromatization of androgens to estrogens. Other than Leydig cells, aromatization can also take place in both Sertoli and germ cells, since both cell types express high level of cytochrome P450 aromatase [63,66]. Overexpression of aromatase was found to induce infertility and an increase in estrogen exposure also led to germ cell apoptosis [67,68], suggesting the importance of estrogen in maintaining the relative ratio of Sertoli:germ cell at ~ 1:30 – 1:50 in the seminiferous epithelium [69]. Estrogen in the testis, similar to androgen, may exert its effects through both genomic and non-genomic pathways. They can bind to estrogen receptors (ERα or ERβ) which are then translocated to the nucleus to interact with the estrogen responsive element (ERE) in multiple estrogen-dependent genes to activate transcription (Figure 3). Other than genomic pathway, G-protein coupled receptor 30 (GPR30) agonist or estrogens may also bind to GPR30 to stimulate the activation of secondary messengers or signaling cascades involving tyrosine kinase receptors and protein kinases, such as IGF-1R, P13 kinase/Akt, MEK/ERK, PKA and PKC, and subsequent stimulate gene transcription [70]. Figure 3 summaries some of events that take place in the seminiferous epithelium mediated by steroid hormones, which are also the targets of EDCs. Recent studies have shown that one of the functions of testosterone in the epithelium is to maintain cell adhesion, such as Sertoli cell adhesion at the BTB and Sertoli-spermatid adhesion in the apical ectoplasmic specialization (ES) [5,71-75]. Other studies have also demonstrated the importance of testosterone and estrogen in maintaining spermiogenesis [21,64]. This, thus, explains reduced sperm counts in rodents and men following exposure to EDCs since a reduced testosterone in the intratesticular environment impedes germ cell development and adhesion in the seminiferous epithelium.
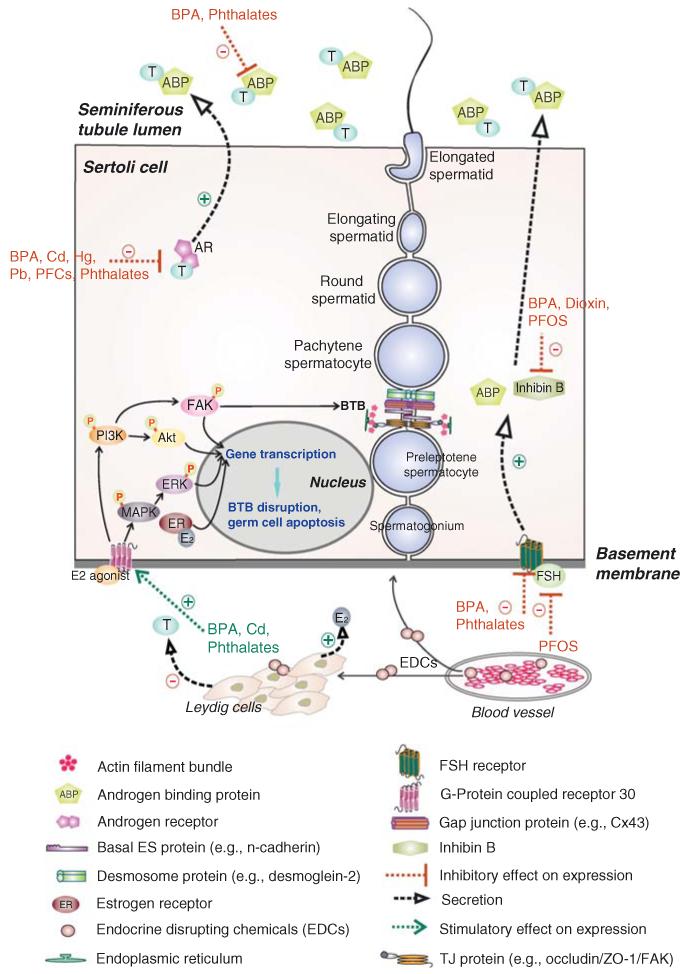
Figure 3. Effects of EDCs-induced changes in the mammalian seminiferous epithelium that disrupts spermatogenesis.
In the mammalian seminiferous epithelium, Sertoli cells are the nurse cells that provide structural and nourishment supports to developing germ cells. The BTB is formed by two adjacent Sertoli cells near the basement membrane. Co-existing tight junction (TJ), gap junction (GJ), and basal ES, which together with desmosome create the BTB. The BTB physically divides the seminiferous epithelium into the basal and the adluminal compartments. Preleptotene spermatocytes differentiate from type B spermatogonia must traverse the BTB to enter the adluminal compartment to differentiate into zygotene and pachytene spermatocytes to undergo meiosis I/II. Ligand binding of FSH onto its receptor as well as androgen onto its receptor stimulate Sertoli cells to secrete androgen binding protein (ABP), which is being used to maintain a high level of testosterone in the tubule lumen to assist sperm maturation. In addition to ABP, Sertoli cells also secrete inhibin B which maintain Sertoli cell normal functioning and increase sperm count/motility. Sertoli cells also secrete a wide range of autocrine, paracrine, and biological factors (such as anti-viral cytokines interferon γ). Sertoli and germ cells also secrete estrogen, which is crucial to maintain male reproductive function via the PI3K/FAK or PI3K/Akt and MAPK/ERK signaling pathways, regulating gene transcription, germ cell apoptosis and BTB function. EDCs have been shown to exert their effects at multiple levels in the seminiferous epithelium depicted herein to affect male reproductive function.
In this context, it is noted that FSH receptors are integral membrane proteins restricted to the Sertoli cell in the seminiferous epithelium [76,77], which is crucial to FSH function in the testis. However, studies from the knockout mouse models and human data have shown that suppression of FSH levels and signaling did not lead to male infertility even though FSH-deficient males are having smaller testes versus the control mice [78-80]. Likewise, the absence of inhibin beta B (also known as INHBB) does not alter male fertility and spermatogenesis [81-83]. In fact, FSH and inhibin are now known to be the hormones that fine-tune, but not essential to, spermatogenesis. Thus, it must be cautious about mechanistic relationships of the EDCs to the true target that results in a phenotype in males in rodents and humans, such as reduced sperm count and sperm quality. Since EDCs that affect the serum levels of FSH and/or inhibin B may not necessarily impede testicular function, instead, the noted phenotypes may be attributed entirely to a disruption of the Leydig cell, rather than the Sertoli cell, and perhaps through other yet-to-be defined biomolecules or cellular structures and/or signaling pathway(s).
4. Impact of EDCs on induction of reaction oxygen species
During spermatogenesis, apoptosis is crucial to maintain the number of germ cells in the seminiferous tubule through both intrinsic and extrinsic pathways [84]. This is physiologically necessary to maintain spermatogenesis since the number of Sertoli cells per testis in adult animals and humans after puberty remains stable, as Sertoli cells in adult males cease to divide [85]. Thus, each Sertoli cell in the rat testis was found to support ~ 30 – 50 developing germ cells [69,86]. Interestingly, an increase in testicular oxidative stress, however, was found to increase cell apoptosis and subsequent hypospermatogenesis [87]. Oxidative stress induced by environmental toxicants has long been link to male infertility [88]. In fact, many of the conditions which are known to have detrimental effects to male reproductive system are due to an induction of oxidative stress [89]. Besides altering hormonal changes, EDCs are also known to induce oxidative stress through a reduction of antioxidant enzyme (e.g., superoxide dismutase, dismutase, catalase and glutathione peroxidase) activities and/or an increase in lipid peroxidation [33,90-92]. In addition, EDC-induced oxidative stress was found to disrupt cell adhesion protein function between testicular cells through the phosphorylation of phosphoinositide 3-kinase/c-Src/focal adhesion kinase (PI3K/c-Src/FAK) signaling cascade [88]. An activation and phosphorylation of FAK and polarity protein Par6 have recently shown to induce internalization of tight junction and apical ES proteins, resulting in cell junction disruption and a loss of cell–cell communications between Sertoli cells as well as between Sertoli cells and spermatids [93,94]. Antioxidants can partially block some of these changes, illustrating oxidative stress may contribute, at least in part, to testicular injury following EDC exposure [95-98]. In fact, subfertile men caused by sperm DNA fragmentation can be therapeutically corrected to have successful pregnancy after antioxidant therapy for at least 3 months [99], suggesting oxidative stress plays a crucial role in male infertility, such as induced by EDCs, and antioxidant therapy may be one of the possible treatments to male infertility.
5. Impact of EDCs on the BTB
During spermatogenesis, type B spermatogonia transform into preleptotene spermatocytes residing in the basal compartment outside the BTB (Figure 4). However, preleptotene spermatocytes connected in ‘clones’ through intercellular bridges must traverse the BTB to enter the adluminal compartment while differentiate into zygotene and pachytene spermatocytes to prepare for meiosis I/II. If this event is disrupted, this leads to a disruption in spermatogenesis. For instance, it was shown that administration of non-steroidal estrogenic diethylstilbestrol (DES), also an environmental toxicant, a synthetic non-steroidal estrogen, and an EDC, to neonatal rats delayed the establishment of BTB by 4-week also delayed the first wave of spermiation by the same time due to failure of spermatocytes to enter meiosis [100]. Furthermore, treatment of neonatal rats with adjudin also delayed the assembly of a functional BTB and the onset of meiosis [101]. Additionally, treatment of adult rats with adjudin at approximately fivefold the normal dose which induced reversible infertility was found to permanently damage the BTB, 250 versus 50 mg/kg b.w., and this also led to irreversible infertility since these rats failed to reinitiate spermatogenesis, even though the population of spermatogonia in this group of rats was relatively unaffected versus normal rats or rats in the low-dose adjudin treated group [102]. In fact, cadmium is known to induce irreversible BTB disruption [103-105], and fertility in these rats also failed to rebound. Collectively, these findings illustrate that the BTB is a likely target of toxicants, when it is exposed to toxicants in particular through an acute dose that leads to its permanent damage, infertility will follow [106].
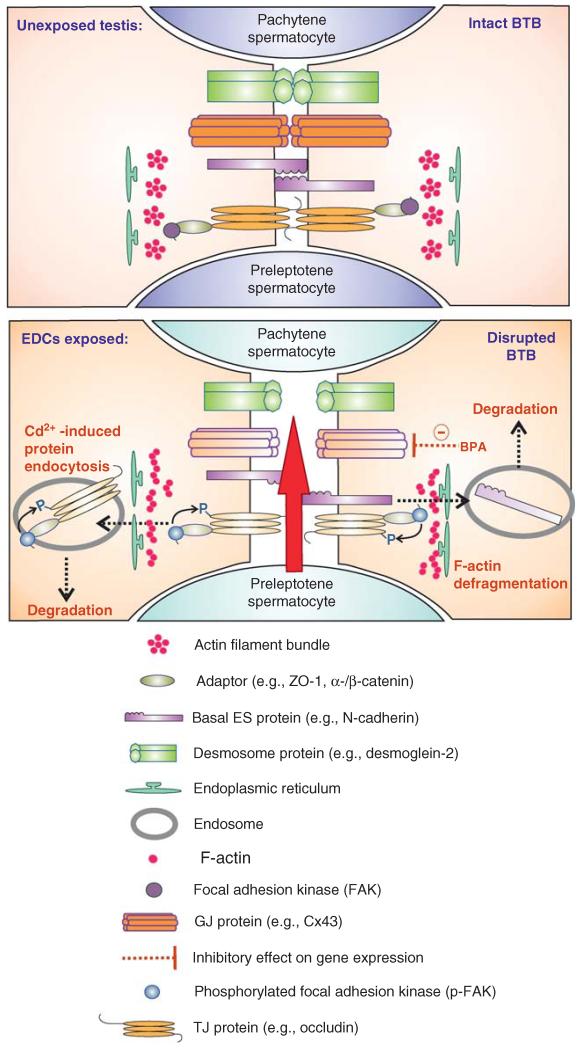
Figure 4. EDCs-induced BTB disruption.
The BTB is an important structure in the testis to maintain spermatogenesis. One of the hallmark ultrastructures of the BTB is the actin filament bundles that lie perpendicular to the apposing Sertoli cell plasma membranes which are sandwiched in-between endoplasmic reticulum and the plasma membrane. Exposure of rodents to heavy metal cadmium and BPA, both are EDCs, disrupted the BTB integrity by inducing defragmentation of actin filament bundles at the BTB, thereby enhancing endocytosis of tight junction- and basal ES-integral membrane proteins, destabilizing the BTB integrity. In addition, BPA down-regulates the expression of GJ protein connexin-43 (Cx43), further perturbing the BTB integrity. On EDC exposure, the BTB is “open”, disrupting spermatogenesis, leading to aspermatogenesis.
At present, the precise mechanism(s) by which cadmium disrupts BTB function is not known, however, recent studies have shed new information regarding cadmium-induced tissue barrier injury. It was shown that cadmium mediated its effects at cell junction through E-cadherin [107,108]. Since E-cadherin is a Ca2+-dependent cell adhesion molecule, it is likely that Cd2+ competes with Ca2+ to binding onto the E-cadherin [109], thereby altering the function of E-cadherin and its cellular distribution at the cell–cell interface [110], disrupting cell adhesion, thereby leading to tissue-barrier injury in the kidney and blood vessels [111,112]. The reason that the BTB is even more susceptible to the endothelial tight junction-barrier to cadmium toxicity is plausibly due to the unusual molecular layout of cell adhesion molecules at the BTB. In other blood–tissue barriers, such as in the kidney and blood vessels, tight junctions ‘seal’ off the access of toxicants to crucial signal molecules behind the blood–tissue barrier. In the testis, the BTB is composed of intermingled cell junctions, namely tight junction, basal ES, gap junction and desmosome. Furthermore, signaling molecules that are usually being seal-off from the toxicants, such as focal adhesion kinase (FAK) is an integrated component of the occludin-ZO-1 complex [94,113], and they are readily accessible by toxicants. Thus, it was shown that the disruption of the BTB occurred prior to the disruption of the microvessels located in the interstitial space by as much as ~ 14-h following a single dose of cadmium administration through i.p. [104,105]. Furthermore, FAK appears to be an important target of signal transduction during cadmium-induced testis injury. For instance, it was shown that a knockdown of FAK by RNAi would insensitize the Sertoli cells to cadmium-induced tight junction-permeability barrier disruption [114], illustrating FAK can also be a therapeutic target to manage cadmium-induced male infertility. A recent study has shown that the FAK isoform that confers BTB integrity is p-FAK-Tyr407, however, overexpression of p-FAK-Tyr397 was found to perturb the Sertoli cell tight junction function [115]. These data suggest that cadmium may exert its effects by altering the homeostasis of these two isoforms of FAK at the BTB, thereby it can rapidly disrupt the BTB function. More important, cadmium was recently shown to down-regulate the expression of drug efflux transporters, such as P-glycoprotein at the Sertoli cell BTB [116]. Taken collectively, these data illustrate that a short exposure of the testis to cadmium can rapidly dismantle the entire molecular defense system in the testis in which this toxicant can initially downregulate the expression of efflux drug transporters, making it easily for the toxicant to enter the testis, reaching its molecular targets FAK and cadherins, impeding cell adhesion function at the site, thereby disrupting spermatogenesis (Figure 4).
Besides cadmium, BPA was also shown to disrupt BTB function in rodents. However, the BTB in adult rats was found to be very resistant to the disruptive effects of BPA, however, exposure of immature rats to BPA at age 20 – 25 day postpartum (dpp) was found to delay the assembly of the BTB [13], illustrating there is a narrow ‘window’ in the testis during BTB development that is sensitive to BPA exposure. This disruptive effect may be related, at least in part, to the half-life of BPA versus cadmium, at ~ 2-h versus ~ 20-year (Table 1). The observation on the disruptive effects of BPA on the Sertoli cell BTB function in immature rats reported from our laboratory [13] was consistent with another study published in the same year in which neonatal rats exposed to BPA was shown to induce down-regulation on the expression of connexin-43 at the Sertoli cell BTB in these rats, coupled with an impairment on their fertility [117]. These finding, thus, support the notion that developing BTB in rats is sensitive to BPA toxicity, and supporting the concept that the BTB is crucial to spermatogenesis. Additionally, exposure of immature rodents to plasticizers BPA and phthalates simultaneously was also found to down-regulate CX43 expression in Sertoli and Leydig cells [117,118]. Furthermore, using an in vitro model to study BTB function in which Sertoli cells cultured in vitro established a functional tight junction-permeability barrier which mimics the BTB in vivo, treatment of this Sertoli cell epithelium by BPA was found to induce a transient disruption of the barrier function since the removal of BPA could ‘reseal’ the disrupted barrier [13]. This disruptive effects of BPA on the Sertoli cell tight junction function appeared to be mediated by a mis-localization of BTB proteins connexin 43, ZO-1, N-cadherin and occludin, in which these proteins redistributed from the Sertoli cell–cell interface, and moved into the cell cytosol, thereby destabilizing the tight junction-permeability barrier [13]. In short, these findings illustrate that immature animals and perhaps children and infants are sensitive to BPA, and repeated exposure to this toxicant, possibly including phthalates, can impair BTB function, affecting fertility in adulthood as shown in animal studies.
6. Conclusion
Declining fertility or sperm quality in men has recently been linked to the exposure of multiple environmental toxicants, which are found to interfere with the mammalian endocrine system [119,120]. Human epidemiological studies have illustrated the possible association between declining male sperm quality and exposure of men to environmental pollutants [17,18,121,122]. Earlier studies revealed that nearly all the components of the male reproductive tract are potential targets of EDCs. Some of them affect the HPT axis, which governs reproductive development and function, thereby disturbing normal physiological function of the testis. The main function of mammalian testes is the production of male sex hormones and sperm throughout adulthood. For instance, enzymatic pathway in steroidogenesis is targeted by nearly all commonly found EDCs, like BPA, phthalates, heavy metals and PFCs, disrupting androgen production. This inhibition may be receptor-mediated or through direct effects on gene transcription. Recent studies also suggest that the BTB is an emerging target of EDCs in the testis in particular in immature animals, such as infants and children. Long-term and low-dose exposures of developing animals to EDCs are needed to assess the disruptive effects of EDCs in future studies.
7. Expert opinion
EDCs have now become an integrated component of our environment since they are widely used in virtually every household item. However, studies in the last ~ 2 – 3 decades have shown that they can cause various metabolic and reproductive disorders in both rodents and humans. Thousands of reports based on animal studies can now be found in the literature, illustrating many of these EDCs can perturb reproductive functions, such as changes in testis weight, other accessory sex organs, hormone levels, sperm count and fertility, plus epigenetic effects. However, many of these studies were criticized regarding their physiological relevance since many animal studies were based on results of utilizing acute doses in order to display observable phenotypes, the levels of which may never be achieved among individuals in the general public. However, some EDCs have exceedingly long half-life and high level of these EDCs can be accumulated in the mammalian body over time, perhaps years and decades. Furthermore, administration of a single EDC may mask the physiological effects and its health risk since each person is being exposed to multiple types of EDCs simultaneously. For instance, when animals were exposed to a mixture of phthalates (called dose addition model) and compared to effects that obtained individually, it was found that a mixture of phthalates produced additive effects [123], which are worse than the sum of the combined effects. Thus, changes that were reported following low-dose exposure to a single EDC may not necessarily illustrate the ‘real’ health risk. Of course, it is also important to perform long-term low-dose studies using a combination of common EDCs to assess their health risks. There are also studies in the field that illustrate the use of active ingredients in ginseng-ginsenosides that can prevent BPA-induced apoptosis, vimentin filament collapse and phosphorylation of ERK1/2 in 15P-1 Sertoli cell lines [124], illustrating some of the risk can possibly be managed. The major weakness in the field is that the underlying molecular mechanism(s) by which EDCs affect male reproductive function remain largely unknown. For instance, even though some of the molecular targets of EDCs are highlighted herein, how EDCs reduce semen quality and sperm count in men remains largely unknown? These mechanistic studies are crucial to provide better therapeutic treatments to men with idiopathic infertility. Unfortunately, most reports found in the literature for the last decade or two are correlation studies in rodents and humans, reporting phenotypes regarding changes in reproductive function and/or andrological parameters following exposure to EDCs. This lack of mechanistic and functional studies may be due to the declining funding in the field. However, a better coordinated effort by investigators may alleviate the issue of budget constraint to some extends. However, recent studies that ECDs, such as cadmium and BPA, exert their effects at the cell junction in the seminiferous epithelium in particular the blood–testis barrier may be an important area of research that deserves attention. Furthermore, the role of ECDs in transcriptional regulation of proteins crucial to spermatogenesis also requires much needed research. We are hopeful that advances in technology, such as the use of gene profiling and genomics/proteomics approaches, can bring new initiatives to the field.
Article highlights.
Exposure to environmental toxicants is one of the major causes of idiopathic male infertility, and male-factors accounts for 50% of the infertility among infertile couples.
Multiple testis-specific targets of EDCs have been identified, ranging from steroidogenesis to different cellular events of spermatogenesis.
The BTB is an emerging target of EDCs.
Specific windows during development in laboratory animals, such as in perinatal and neonatal rats, are more sensitive to exposure of EDCs, illustrating children and infants may be more susceptible to exposure of EDCs.
Strategies for treatment of toxicant-induced testicular injury may be developed based on recent reports in the field, such as the use of ginseng.
This box summarizes key points contained in the article.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (NICHD U54 HD029990, Project 5 to C.Y.C.; R01 HD056034 to C.Y.C.; and The Hong Kong General Research Fund (GRF) (HKBU261812) to C.K.C.W.
Footnotes
Declaration of interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Bibliography
Papers of special note have been highlighted as either of interest
(•) or of considerable interest
(••) to readers.
- 1.Jungwirth A, Giwercman A, Tournaye H, et al. European Association of Urology guidelines on male infertility: the 2012 update. Eur Urol. 2012;62:324–32. doi: 10.1016/j.eururo.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 2.Ray A, Shah A, Gudi A, et al. Unexplained infertility: an update and review of practice. Reprod Biomed Online. 2012;24:591–602. doi: 10.1016/j.rbmo.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 3••.Kilchevsky A, Honig S. Male factor infertility in 2011: semen quality, sperm selection and hematospermia. Nat Rev Urol. 2012;9:68–70. doi: 10.1038/nrurol.2011.234. This review provides an important recent update on male factor that contributes to infertility in married couples.
- 4.Harnisch B, Oates R. Genetic disorders related to male factor infertility and their adverse consequences. Semin Reprod Med. 2012;30:105–15. doi: 10.1055/s-0032-1307418. [DOI] [PubMed] [Google Scholar]
- 5.Cheng CY, Wong EWP, Lie PPY, et al. Environmental toxicants and male reproductive function. Spermatogenesis. 2011;1:2–13. doi: 10.4161/spmg.1.1.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6•.Nordkap L, Joensen UN, Blomberg JM, et al. Regional differences and temporal trends in male reproductive health disorders: semen quality may be a sensitive marker of environmental exposures. Mol Cell Endocrinol. 2012;355:221–30. doi: 10.1016/j.mce.2011.05.048. This study summarizes latest findings that declining semen quality is a senstive index of environmental toxicant exposures.
- 7.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayes HM, Tarone RE, Casey HW, et al. Excess of seminomas observed in Vietnam service U.S. military working dogs. J Natl Cancer Inst. 1990;82:1042–6. doi: 10.1093/jnci/82.12.1042. [DOI] [PubMed] [Google Scholar]
- 9.Taylor JA, Richter CA, Ruhlen RL, et al. Estrogenic environmental chemicals and drugs: mechanisms for effects on the developing male urogenital system. J Steroid Biochem Mol Biol. 2011;127:83–95. doi: 10.1016/j.jsbmb.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Safe S. Cadmium’s disguise dupes the estrogen receptor. Nat Med. 2003;9:1000–1. doi: 10.1038/nm0803-1000. [DOI] [PubMed] [Google Scholar]
- 11.Kortenkamp A, Faust M. Combined exposures to anti-androgenic chemicals: steps towards cumulative risk assessment. Int J Androl. 2010;33:463–74. doi: 10.1111/j.1365-2605.2009.01047.x. [DOI] [PubMed] [Google Scholar]
- 12.Marty MS, Chapin RE, Parks LG, et al. Development and maturation of the male reproductive system. Birth Defects Res B Dev Reprod Toxicol. 2003;68:125–36. doi: 10.1002/bdrb.10015. [DOI] [PubMed] [Google Scholar]
- 13•.Li MW, Mruk DD, Lee WM, et al. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: is this a suitable model for studying blood-testis barrier dynamics? Int J Biochem Cell Biol. 2009;41:2302–14. doi: 10.1016/j.biocel.2009.05.016. This is the first report demonstrating BPA has a disruptive effect on BTB integrity in immature rats.
- 14.Amory JK. Drug effects on spermatogenesis. Drugs Today (Barc) 2007;43:717–24. doi: 10.1358/dot.2007.43.10.1131829. [DOI] [PubMed] [Google Scholar]
- 15.Hou WG, Zhao J, Li Z, et al. Effects of electromagnetic pulse irradiation on the mouse blood-testicle barrier. Urology. 2012;80:225 e1–6. doi: 10.1016/j.urology.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Campion S, Catlin N, Heger N, et al. Male reprotoxicity and endocrine disruption. EXS. 2012;101:315–60. doi: 10.1007/978-3-7643-8340-4_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jurewicz J, Hanke W, Radwan M, et al. Environmental factors and semen quality. Int J Occup Med Environ Health. 2009;22:305–29. doi: 10.2478/v10001-009-0036-1. [DOI] [PubMed] [Google Scholar]
- 18.Hauser R. The environment and male fertility: recent research on emerging chemicals and semen quality. Semin Reprod Med. 2006;24:156–67. doi: 10.1055/s-2006-944422. [DOI] [PubMed] [Google Scholar]
- 19.Mocarelli P, Gerthoux PM, Patterson DG, Jr, et al. Dioxin exposure, from infancy through puberty, produces endocrine disruption and affects human semen quality. Environ Health Perspect. 2008;116:70–7. doi: 10.1289/ehp.10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharpe RM. Regulation of spermatogenesis. In: Knobil E, Neill JD, editors. The physiology of reproduction. Raven Press; New York: 1994. pp. 1363–434. [Google Scholar]
- 21.O’Donnell L, Meachem SJ, Stanton PG, et al. Endocrine regulation of spermatogenesis. In: Neill JD, editor. Physiology of reproduction. 3rd edition Elsevier; Amsterdam: 2006. pp. 1017–69. [Google Scholar]
- 22•.Manna PR, Chandrala SP, Jo Y, et al. cAMP-independent signaling regulates steroidogenesis in mouse Leydig cells in the absence of StAR phosphorylation. J Mol Endocrinol. 2006;37:81–95. doi: 10.1677/jme.1.02065. This paper provides the data on the critical timing of puberty and EDC exposure that leads to phenotypic changes.
- 23.Scott HM, Mason JI, Sharpe RM. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev. 2009;30:883–925. doi: 10.1210/er.2009-0016. [DOI] [PubMed] [Google Scholar]
- 24.Gray LE, Ostby J, Furr J, et al. Effects of environmental antiandrogens on reproductive development in experimental animals. Human Reprod Update. 2001;7:248–64. doi: 10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- 25.Shi Z, Feng Y, Wang J, et al. Perfluorododecanoic acid-induced steroidogenic inhibition is associated with steroidogenic acute regulatory protein and reactive oxygen species in cAMP-stimulated Leydig cells. Toxicol Sci. 2010;114:285–94. doi: 10.1093/toxsci/kfq014. [DOI] [PubMed] [Google Scholar]
- 26.Shi Z, Ding L, Zhang H, et al. Chronic exposure to perfluorododecanoic acid disrupts testicular steroidogenesis and the expression of related genes in male rats. Toxicol Lett. 2009;188:192–200. doi: 10.1016/j.toxlet.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Wan HT, Zhao YG, Wong MH, et al. Testicular signaling is the potential target of perfluorooctanesulfonate-mediated subfertility in male mice. Biol Reprod. 2011;84:1016–23. doi: 10.1095/biolreprod.110.089219. [DOI] [PubMed] [Google Scholar]
- 28.Doumouchtsis KK, Doumouchtsis SK, Doumouchtsis EK, et al. The effect of lead intoxication on endocrine functions. J Endocrinol Invest. 2009;32:175–83. doi: 10.1007/BF03345710. [DOI] [PubMed] [Google Scholar]
- 29.Gunnarsson D, Nordberg G, Selstam G. Differential effects of cadmium on the gene expression of seven-transmembrane-spanning receptors and GAPDH in the rat testis. Toxicol Lett. 2007;168:51–7. doi: 10.1016/j.toxlet.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 30.Rider CV, Furr J, Wilson VS, et al. A mixture of seven antiandrogens induces reproductive malformations in rats. Int J Androl. 2008;31:249–62. doi: 10.1111/j.1365-2605.2007.00859.x. [DOI] [PubMed] [Google Scholar]
- 31.Rider CV, Furr JR, Wilson VS, et al. Cumulative effects of in utero administration of mixtures of reproductive toxicants that disrupt common target tissues via diverse mechanisms of toxicity. Int J Androl. 2010;33:443–62. doi: 10.1111/j.1365-2605.2009.01049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32••.Johnson KJ, Heger NE, Boekelheide K. Of mice and men (and rats): phthalate-induced fetal testis endocrine disruption is species-dependent. Toxicol Sci. 2012;129:235–48. doi: 10.1093/toxsci/kfs206. This important review summarizes findings that phthalate-induced defects in endocrine function in immature animals and humans are species-dependent.
- 33.Pandya C, Pillai P, Nampoothiri LP, et al. Effect of lead and cadmium co-exposure on testicular steroid metabolism and antioxidant system of adult male rats. Andrologia. 2012;44(Suppl 1):813–22. doi: 10.1111/j.1439-0272.2010.01137.x. [DOI] [PubMed] [Google Scholar]
- 34.Tan SW, Meiller JC, Mahaffey KR. The endocrine effects of mercury in humans and wildlife. Crit Rev Toxicol. 2009;39:228–69. doi: 10.1080/10408440802233259. [DOI] [PubMed] [Google Scholar]
- 35.Manna PR, Roy P, Clark BJ, et al. Interaction of thyroid hormone and steroidogenic acute regulatory (StAR) protein in the regulation of murine Leydig cell steroidogenesis. J Steroid Biochem Mol Biol. 2001;76:167–77. doi: 10.1016/s0960-0760(00)00156-4. [DOI] [PubMed] [Google Scholar]
- 36.Meeker JD, Ferguson KK. Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in U.S. adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007-2008. Environ Health Perspect. 2011;119:1396–402. doi: 10.1289/ehp.1103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner MS, Wajner SM, Maia AL. Is there a role for thyroid hormone on spermatogenesis? Microsc Res Tech. 2009;72:796–808. doi: 10.1002/jemt.20759. [DOI] [PubMed] [Google Scholar]
- 38.Colon E, Zaman F, Axelson M, et al. Insulin-like growth factor-I is an important antiapoptotic factor for rat leydig cells during postnatal development. Endocrinology. 2007;148:128–39. doi: 10.1210/en.2006-0835. [DOI] [PubMed] [Google Scholar]
- 39.Froment P, Vigier M, Negre D, et al. Inactivation of the IGF-I receptor gene in primary Sertoli cells highlights the autocrine effects of IGF-I. J Endocrinol. 2007;194:557–68. doi: 10.1677/JOE-07-0258. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Ramdhan DH, Naito H, et al. Ammonium perfluorooctanoate may cause testosterone reduction by adversely affecting testis in relation to PPARalpha. Toxicol Lett. 2011;205:265–72. doi: 10.1016/j.toxlet.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan J, Papadopoulos V. Transcriptional regulation of translocator protein (Tspo) via a SINE B2-mediated natural antisense transcript in MA-10 Leydig cells. Biol Reprod. 2012;86:147, 1–15. doi: 10.1095/biolreprod.111.097535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li MWM, Mruk DD, Cheng CY. Gap junctions and blood-tissue barriers. Adv Exp Med Biol. 2012;763:260–80. doi: 10.1007/978-1-4614-4711-5_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siu ER, Mruk DD, Porto CS, et al. Cadmium-induced testicular injury. Toxicol Appl Pharmacol. 2009;238:240–9. doi: 10.1016/j.taap.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mruk DD, Cheng CY. Environmental contaminants. Is male reproductive helath at risk? Spermatogenesis. 2011;1:283–90. doi: 10.4161/spmg.1.4.18328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pointis G, Gilleron J, Carette D, et al. Testicular connexin 43, a precocious molecular target for the effect of environmental toxicants on male fertility. Spermatogenesis. 2011;1:303–17. doi: 10.4161/spmg.1.4.18392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chandrashekar V, Zaczek D, Bartke A. The consequences of altered somatotropic system on reproduction. Biol Reprod. 2004;71:17–27. doi: 10.1095/biolreprod.103.027060. [DOI] [PubMed] [Google Scholar]
- 47.Croutch CR, Lebofsky M, Schramm KW, et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) and 1,2,3,4,7,8-hexachlorodibenzo-p-dioxin (HxCDD) alter body weight by decreasing insulin-like growth factor I (IGF-I) signaling. Toxicol Sci. 2005;85:560–71. doi: 10.1093/toxsci/kfi106. [DOI] [PubMed] [Google Scholar]
- 48.Meeker JD, Godfrey-Bailey L, Hauser R. Relationships between serum hormone levels and semen quality among men from an infertility clinic. J Androl. 2007;28:397–406. doi: 10.2164/jandrol.106.001545. [DOI] [PubMed] [Google Scholar]
- 49.Meeker JD, Calafat AM, Hauser R. Urinary bisphenol A concentrations in relation to serum thyroid and reproductive hormone levels in men from an infertility clinic. Environ Sci Technol. 2010;44:1458–63. doi: 10.1021/es9028292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mocarelli P, Gerthoux PM, Needham LL, et al. Perinatal exposure to low doses of dioxin can permanently impair human semen quality. Environ Health Perspect. 2011;119:713–18. doi: 10.1289/ehp.1002134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rey RA, Musse M, Venara M, et al. Ontogeny of the androgen receptor expression in the fetal and postnatal testis: its relevance on Sertoli cell maturation and the onset of adult spermatogenesis. Microsc Res Tech. 2009;72:787–95. doi: 10.1002/jemt.20754. [DOI] [PubMed] [Google Scholar]
- 52••.De Gendt K, Swinnen JV, Saunders PT, et al. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA. 2004;101:1327–32. doi: 10.1073/pnas.0308114100. This paper reports the Sertoli cell-specific androgen receptor knockout in testes leads to an arrest in meiosis that impairs spermatogenesis.
- 53.Chang C, Chen Y, Yeh S, et al. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci USA. 2004;101:6876–81. doi: 10.1073/pnas.0307306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boukari K, Meduri G, Brailly-Tabard S, et al. Lack of androgen receptor expression in Sertoli cells accounts for the absence of anti-Mullerian hormone repression during early human testis development. J Clin Endocrinol Metab. 2009;94:1818–25. doi: 10.1210/jc.2008-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shakil T, Hoque AN, Husain M, et al. Differential regulation of gonadotropin-releasing hormone secretion and gene expression by androgen: membrane versus nuclear receptor activation. Mol Endocrinol. 2002;16:2592–602. doi: 10.1210/me.2002-0011. [DOI] [PubMed] [Google Scholar]
- 56.Massin N, Bry H, Vija L, et al. Healthy birth after testicular extraction of sperm and ICSI from an azoospermic man with mild androgen insensitivity syndrome caused by an androgen receptor partial loss-of-function mutation. Clin Endocrinol (Oxf) 2012;77:593–8. doi: 10.1111/j.1365-2265.2012.04402.x. [DOI] [PubMed] [Google Scholar]
- 57.Lazaros L, Xita N, Takenaka A, et al. Semen quality is influenced by androgen receptor and aromatase gene synergism. Hum Reprod. 2012;27(12):3385–92. doi: 10.1093/humrep/des334. [DOI] [PubMed] [Google Scholar]
- 58.Grover A, Smith CE, Gregory M, et al. Effects of FSH receptor deletion on epididymal tubules and sperm morphology, numbers, and motility. Mol Reprod Dev. 2005;72:135–44. doi: 10.1002/mrd.20303. [DOI] [PubMed] [Google Scholar]
- 59.Turner TT, Jones CC, Howards SS, et al. On the androgen microenvironment of maturing spermatozoa. Endocrinology. 1984;115:1925–32. doi: 10.1210/endo-115-5-1925. [DOI] [PubMed] [Google Scholar]
- 60.Zirkin BR. Spermatogenesis: its regulation by testosterone and FSH. Semin Cell Dev Biol. 1998;9:417–21. doi: 10.1006/scdb.1998.0253. [DOI] [PubMed] [Google Scholar]
- 61.Xi W, Wan HT, Zhao YG, et al. Effects of perinatal exposure to bisphenol A and di(2-ethylhexyl)-phthalate on gonadal development of male mice. Environ Sci Pollut Res Int. 2011;19:2515–27. doi: 10.1007/s11356-012-0827-y. [DOI] [PubMed] [Google Scholar]
- 62.Robertson KM, O’Donnell L, Jones ME, et al. Impairment of spermatogenesis in mice lacking a functional aromatase (cyp 19) gene. Proc Natl Acad Sci USA. 1999;96:7986–91. doi: 10.1073/pnas.96.14.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63••.Carreau S, Hess RA. Oestrogens and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1517–35. doi: 10.1098/rstb.2009.0235. This review summarizes findings on the crucial significance of estrogens in spermatogenesis, in particular the impact of germ cell aromatase on spermatogenesis.
- 64••.O’Donnell L, Robertson KM, Jones ME, et al. Estrogen and spermatogenesis. Endocr Rev. 2001;22:289–318. doi: 10.1210/edrv.22.3.0431. This elegant review summarizes the role of estrogen in spermatogenesis.
- 65.Lucas TFG, Pimenta MT, Pisolato R, et al. 17β-estradiol signaling and regulation of Sertoli cell function. Spermatogenesis. 2011;1:318–24. doi: 10.4161/spmg.1.4.18903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carreau S, Delalande C, Galeraud-Denis I. Mammalian sperm quality and aromatase expression. Microsc Res Tech. 2009;72:552–7. doi: 10.1002/jemt.20703. [DOI] [PubMed] [Google Scholar]
- 67.Assinder S, Davis R, Fenwick M, et al. Adult-only exposure of male rats to a diet of high phytoestrogen content increases apoptosis of meiotic and post-meiotic germ cells. Reproduction. 2007;133:11–19. doi: 10.1530/rep.1.01211. [DOI] [PubMed] [Google Scholar]
- 68.Li X, Rahman N. Impact of androgen/estrogen ratio: lessons learned from the aromatase over-expression mice. Gen Comp Endocrinol. 2008;159:1–9. doi: 10.1016/j.ygcen.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 69.Weber JE, Russell LD, Wong V, et al. Three dimensional reconstruction of a rat stage V Sertoli cell: II. Morphometry of Sertoli-Sertoli and Sertoli-germ cell relationships. Am J Anat. 1983;167:163–79. doi: 10.1002/aja.1001670203. [DOI] [PubMed] [Google Scholar]
- 70.Carreau S, Bouraima-Lelong H, Delalande C. Estrogens in male germ cells. Spermatogenesis. 2011;1:90–4. doi: 10.4161/spmg.1.2.16766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng J, Watkins SC, Walker WH. Testosterone activates mitogen-activated protein kinase via Src kinase and the epidermal growth factor receptor in Sertoli cells. Endocrinology. 2007;148:2066–74. doi: 10.1210/en.2006-1465. [DOI] [PubMed] [Google Scholar]
- 72.Wang RS, Yeh S, Tzeng CR, et al. Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice. Endocr Rev. 2009;30:119–32. doi: 10.1210/er.2008-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shupe J, Cheng J, Puri P, et al. Regulation of Sertoli-germ cell adhesion and sperm release by FSH and nonclassical testosterone signaling. Mol Endocrinol. 2011;25:238–52. doi: 10.1210/me.2010-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Siu ER, Mruk DD, Porto CS, et al. Cadmium-induced testicular injury. Toxicol Appl Pharmacol. 2009;238:240–9. doi: 10.1016/j.taap.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O’Donnell L, Stanton P, Bartles J, et al. Sertoli cell ectoplasmic specializations in the seminiferous epithelium of the testosterone-suppressed adult rat. Biol Reprod. 2000;63:99–108. doi: 10.1095/biolreprod63.1.99. [DOI] [PubMed] [Google Scholar]
- 76.Griswold MD, Heckert L, Linder C. The molecular biology of the FSH receptor. J Steroid Biochem Mol Biol. 1995;53:215–18. doi: 10.1016/0960-0760(95)00049-6. [DOI] [PubMed] [Google Scholar]
- 77.Menon KM, Menon B. Structure, function and regulation of gonadotropin receptors - a perspective. Mol Cell Endocrinol. 2012;356:88–97. doi: 10.1016/j.mce.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumar TR, Wang Y, Lu N, et al. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–4. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- 79.Siegel ET, Kim HG, Nishimoto HK, et al. The molecular basis of impaired follicle-stimulating hormone action: evidence from human mutations and mouse models. Reprod Sci. 2013;20:211–33. doi: 10.1177/1933719112461184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tapanainen JS, Aittomaki K, Min J, et al. Men homozygous for an inactivating mutation of the follicle-stimulating hormone (FSH) receptor gene present variable suppression of spermatogenesis and fertility. Nat Genet. 1997;15:205–6. doi: 10.1038/ng0297-205. [DOI] [PubMed] [Google Scholar]
- 81.McNeilly AS. Diagnostic applications for inhibin and activins. Mol Cell Endocrinol. 2012;359:121–5. doi: 10.1016/j.mce.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 82.Jorgensen N, Liu F, Andersson AM, et al. Serum inhibin-b in fertile men is strongly correlated with low but not high sperm counts: a coordinated study of 1,797 European and US men. Fertil Steril. 2010;94:2128–34. doi: 10.1016/j.fertnstert.2009.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chang H, Brown CW, Matzuk MM. Genetic analysis of the mammalian transforming growth factor-beta superfamily. Endocr Rev. 2002;23:787–823. doi: 10.1210/er.2002-0003. [DOI] [PubMed] [Google Scholar]
- 84.Shaha C, Tripathi R, Mishra DP. Male germ cell apoptosis: regulation and biology. Philos Trans R Soc Lond B Biol Sci. 2010;365:1501–15. doi: 10.1098/rstb.2009.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Orth JM. Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anat Rec. 1982;203:485–92. doi: 10.1002/ar.1092030408. [DOI] [PubMed] [Google Scholar]
- 86.Wong V, Russell LD. Three-dimensional reconstruction of a rat stage V Sertoli cell: I. Methods, basic configuration, and dimensions. Am J Anat. 1983;167:143–61. doi: 10.1002/aja.1001670202. [DOI] [PubMed] [Google Scholar]
- 87.Turner TT, Lysiak JJ. Oxidative stress: a common factor in testicular dysfunction. J Androl. 2008;29:488–98. doi: 10.2164/jandrol.108.005132. [DOI] [PubMed] [Google Scholar]
- 88.Wong EW, Cheng CY. Impacts of environmental toxicants on male reproductive dysfunction. Trends Pharmacol Sci. 2011;32:290–9. doi: 10.1016/j.tips.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Makker K, Agarwal A, Sharma R. Oxidative stress & male infertility. Indian J Med Res. 2009;129:357–67. [PubMed] [Google Scholar]
- 90.Boujbiha MA, Hamden K, Guermazi F, et al. Impairment of spermatogenesis in rats by mercuric chloride: involvement of low 17beta-estradiol level in induction of acute oxidative stress. Biol Trace Elem Res. 2011;142:598–610. doi: 10.1007/s12011-010-8774-2. [DOI] [PubMed] [Google Scholar]
- 91.D’Cruz SC, Jubendradass R, Jayakanthan M, et al. Bisphenol A impairs insulin signaling and glucose homeostasis and decreases steroidogenesis in rat testis: an in vivo and in silico study. Food Chem Toxicol. 2012;50:1124–33. doi: 10.1016/j.fct.2011.11.041. [DOI] [PubMed] [Google Scholar]
- 92.Priya PH, Reddy PS. Effect of restraint stress on lead-induced male reproductive toxicity in rats. J Exp Zool A Ecol Genet Physiol. 2012;317:455–65. doi: 10.1002/jez.1738. [DOI] [PubMed] [Google Scholar]
- 93.Wong EWP, Mruk DD, Lee WM, et al. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:9657–62. doi: 10.1073/pnas.0801527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Siu ER, Wong EW, Mruk DD, et al. An occludin-focal adhesion kinase protein complex at the blood-testis barrier: a study using the cadmium model. Endocrinology. 2009;150:3336–44. doi: 10.1210/en.2008-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Acharya UR, Mishra M, Patro J, et al. Effect of vitamins C and E on spermatogenesis in mice exposed to cadmium. Reprod Toxicol. 2008;25:84–8. doi: 10.1016/j.reprotox.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 96.Menezo YJ, Hazout A, Panteix G, et al. Antioxidants to reduce sperm DNA fragmentation: an unexpected adverse effect. Reprod Biomed Online. 2007;14:418–21. doi: 10.1016/s1472-6483(10)60887-5. [DOI] [PubMed] [Google Scholar]
- 97.Ognjanovic BI, Markovic SD, Ethordevic NZ, et al. Cadmium-induced lipid peroxidation and changes in antioxidant defense system in the rat testes: protective role of coenzyme Q(10) and vitamin E. Reprod Toxicol. 2010;29:191–7. doi: 10.1016/j.reprotox.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 98.Vani K, Kurakula M, Syed R, et al. Clinical relevance of vitamin C among lead-exposed infertile men. Genet Test Mol Biomarkers. 2012;16:1001–6. doi: 10.1089/gtmb.2012.0027. [DOI] [PubMed] [Google Scholar]
- 99.Gil-Villa AM, Cardona-Maya W, Agarwal A, et al. Role of male factor in early recurrent embryo loss: do antioxidants have any effect? Fertil Steril. 2009;92:565–71. doi: 10.1016/j.fertnstert.2008.07.1715. [DOI] [PubMed] [Google Scholar]
- 100.Toyama Y, Ohkawa M, Oku R, et al. Neonatally administered diethylstilbestrol retards the development of the blood-testis barrier in the rat. J Androl. 2001;22:413–23. [PubMed] [Google Scholar]
- 101.Kopera IA, Su L, Bilinska B, et al. An in vivo study on adjudin and blood-testis barrier dynamics. Endocrinology. 2009;150:4724–33. doi: 10.1210/en.2008-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mok KW, Mruk DD, Lee WM, et al. Spermatogonial stem cells alone are not sufficient to re-initiate spermatogenesis in the rat testis following adjudin-induced infertility. Int J Androl. 2012;35:86–101. doi: 10.1111/j.1365-2605.2011.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hew KW, Heath GL, Jiwa AH, et al. Cadmium in vivo causes disruption of tight junction-associated microfilaments in rat Sertoli cells. Biol Reprod. 1993;49:840–9. doi: 10.1095/biolreprod49.4.840. [DOI] [PubMed] [Google Scholar]
- 104•.Setchell BP, Waites GMH. Changes in the permeability of the testicular capillaries and of the “blood-testis barrier” after injection of cadmium chloride in the rat. J Endocrinol. 1970;47:81–6. doi: 10.1677/joe.0.0470081. This is the study that first reports the blood-testis barrier is an early target of cadmium toxicity in the testis.
- 105.Wong CH, Mruk DD, Lui WY, et al. Regulation of blood-testis barrier dynamics: an in vivo study. J Cell Sci. 2004;117:783–98. doi: 10.1242/jcs.00900. [DOI] [PubMed] [Google Scholar]
- 106.Cheng CY, Mruk DD. The blood-testis barrier and its implications for male contraception. Pharmacol Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107•.Prozialeck W. Evidence that E-cadherin may be a target for cadmium toxicity in epithelial cells. Toxicol Appl Pharmacol. 2000;164:231–49. doi: 10.1006/taap.2000.8905. This is the first report that demonstrated E-cadherin is an initial target of cadmium toxicity.
- 108.Prozialeck WC, Grunwald GB, Dey PM, et al. Cadherins and NCAM as potential targets in metal toxicity. Toxicol Appl Pharmacol. 2002;182:255–65. doi: 10.1006/taap.2002.9422. [DOI] [PubMed] [Google Scholar]
- 109.Prozialeck WC, Lamar PC. Interaction of cadmium (Cd2+) with a 13-residue polypeptide analog of a putative calcium-binding motif of E-cadherin. Biochem Biophys Acta. 1999;1451:93–100. doi: 10.1016/s0167-4889(99)00077-4. [DOI] [PubMed] [Google Scholar]
- 110.Prozialeck WC, Lamar PC, Lynch SM. Cadmium alters the localization of N-cadherin, E-cadherin, and beta-catenin in the proximal tubule epithelium. Toxicol Appl Pharmacol. 2003;189:180–95. doi: 10.1016/s0041-008x(03)00130-3. [DOI] [PubMed] [Google Scholar]
- 111.Prozialeck WC, Edwards JR, Nebert DW, et al. The vascular system as a target of metal toxicity. Toxicol Sci. 2008;102:207–18. doi: 10.1093/toxsci/kfm263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Prozialeck WC, Edward JR. Cell adhesion molecules in chemical-induced renal injury. Pharmacol Ther. 2007;114:74–93. doi: 10.1016/j.pharmthera.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Siu MKY, Mruk DD, Lee WM, et al. Adhering junction dynamics in the testis are regulated by an interplay of beta1-integrin and focal adhesion complex (FAC)-associated proteins. Endocrinology. 2003;144:2141–63. doi: 10.1210/en.2002-221035. [DOI] [PubMed] [Google Scholar]
- 114.Siu ER, Wong EW, Mruk DD, et al. Focal adhesion kinase is a blood-testis barrier regulator. Proc Natl Acad Sci USA. 2009;106:9298–303. doi: 10.1073/pnas.0813113106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lie PPY, Mruk DD, Mok KW, et al. Focal adhesion kinase-Tyr407 and - Tyr397 exhibit antagonistic effects on blood-testis barrier dynamics in the rat. Proc Natl Acad Sci USA. 2012;109:12562–7. doi: 10.1073/pnas.1202316109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116•.Su L, Mruk DD, Cheng CY. Regulation of drug transporters in the testis by environmental toxicant cadmium, steroids and cytokines. Spermatogenesis. 2012;2:285–93. doi: 10.4161/spmg.22536. This report demonstrates toxicants regulate the expression of drug transporters in the testis.
- 117.Salian S, Doshi T, Vanage G. Neonatal exposure of male rats to Bisphenol A impairs fertility and expression of Sertoli cell junctional proteins in the testis. Toxicology. 2009;265:56–67. doi: 10.1016/j.tox.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 118.Liu K, Lehmann KP, Sar M, et al. Gene expression profiling following in utero exposure to phthalate esters reveals new gene targets in the etiology of testicular dysgenesis. Biol Reprod. 2005;73:180–92. doi: 10.1095/biolreprod.104.039404. [DOI] [PubMed] [Google Scholar]
- 119.Vandenberg LN, Colborn T, Hayes TB, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lagos-Cabre R, Moreno RD. Contribution of environmental pollutants to male infertility: a working model of germ cell apoptosis induced by plasticizers. Biol Res. 2012;45:5–14. doi: 10.4067/S0716-97602012000100001. [DOI] [PubMed] [Google Scholar]
- 121.Xu W, Bao H, Liu F, et al. Environmental exposure to arsenic may reduce human semen quality: associations derived from a Chinese cross-sectional study. Environ Health. 2012;11:46. doi: 10.1186/1476-069X-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Toft G, Jonsson BA, Lindh CH, et al. Exposure to perfluorinated compounds and human semen quality in Arctic and European populations. Human Reprod. 2012;27:2532–40. doi: 10.1093/humrep/des185. [DOI] [PubMed] [Google Scholar]
- 123.Howdeshell KL, Wilson VS, Furr J, et al. A mixture of five phthalate esters inhibits fetal testicular testosterone production in the Sprague-Dawley rat in a cumulative, dose-additive manner. Toxicol Sci. 2008;105:153–65. doi: 10.1093/toxsci/kfn077. [DOI] [PubMed] [Google Scholar]
- 124.Wang L, Hao J, Hu J, et al. Protective effects of ginsenosides against Bisphenol A-induced cytotoxicity in 15P-1 Sertoli cells via extracellular signal-regulated kinase 1/2 signalling and antioxidant mechanisms. Basic Clin Pharmacol Toxicol. 2012;111:42–9. doi: 10.1111/j.1742-7843.2012.00857.x. [DOI] [PubMed] [Google Scholar]
- 125.Geoffroy-Siraudin C, Perrard MH, Ghalamoun-Slaimi R, et al. Ex-vivo assessment of chronic toxicity of low levels of cadmium on testicular meiotic cells. Toxicol Appl Pharmacol. 2012;262:238–46. doi: 10.1016/j.taap.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 126.Lehraiki A, Racine C, Krust A, et al. Phthalates impair germ cell number in the mouse fetal testis by an androgen- and estrogen-independent mechanism. Toxicol Sci. 2009;111:372–82. doi: 10.1093/toxsci/kfp153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shanle EK, Xu W. Endocrine disrupting chemicals targeting estrogen receptor signaling: identification and mechanisms of action. Chem Res Toxicol. 2011;24:6–19. doi: 10.1021/tx100231n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Choe SY, Kim SJ, Kim HG, et al. Evaluation of estrogenicity of major heavy metals. Sci Total Environ. 2003;312:15–21. doi: 10.1016/S0048-9697(03)00190-6. [DOI] [PubMed] [Google Scholar]
- 129.Wang YD, Chen XY, Wu YM, et al. Experiment study on the estrogen-like effect of compounds of mercury, chromium and manganese. Wei Sheng Yan Jiu. 2005;34:49–51. [PubMed] [Google Scholar]
- 130.Yang Y, Li F, Bi X, et al. Lead, zinc, and cadmium in vegetable/crops in a zinc smelting region and its potential human toxicity. Bull Environ Contam Toxicol. 2011;87:586–90. doi: 10.1007/s00128-011-0388-7. [DOI] [PubMed] [Google Scholar]
- 131.Barrett JR. Rice is a significant source of methylmercury: research in China assesses exposures. Environ Health Perspect. 2010;118:a398. doi: 10.1289/ehp.118-a398a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Karjalainen AK, Hallikainen A, Hirvonen T, et al. Estimated intake levels for Finnish children of methylmercury from fish. Food Chem Toxicol. 2013;54:70–7. doi: 10.1016/j.fct.2012.02.074. [DOI] [PubMed] [Google Scholar]
- 133.Hengstler JG, Foth H, Gebel T, et al. Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A. Crit Rev Toxicol. 2011;41:263–91. doi: 10.3109/10408444.2011.558487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lakind JS, Naiman DQ. Daily intake of bisphenol A and potential sources of exposure: 2005-2006 National Health and Nutrition Examination Survey. J Expo Sci Environ Epidemiol. 2011;21:272–9. doi: 10.1038/jes.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Howdeshell KL, Rider CV, Wilson VS, et al. Mechanisms of action of phthalate esters, individually and in combination, to induce abnormal reproductive development in male laboratory rats. Environ Res. 2008;108:168–76. doi: 10.1016/j.envres.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 136.Ge RS, Chen GR, Tanrikut C, et al. Phthalate ester toxicity in Leydig cells: developmental timing and dosage considerations. Reprod Toxicol. 2007;23:366–73. doi: 10.1016/j.reprotox.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 137.Guo Y, Zhang Z, Liu L, et al. Occurrence and profiles of phthalates in foodstuffs from China and their implications for human exposure. J Agric Food Chem. 2012 doi: 10.1021/jf3021128. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 138.Melzer D, Rice N, Depledge MH, et al. Association between serum perfluorooctanoic acid (PFOA) and thyroid disease in the U.S. National Health and Nutrition Examination Survey. Environ Health Perspect. 2010;118:686–92. doi: 10.1289/ehp.0901584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Olsen GW, Burris JM, Ehresman DJ, et al. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115:1298–305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fromme H, Tittlemier SA, Volkel W, et al. Perfluorinated compounds–exposure assessment for the general population in Western countries. Int J Hyg Environ Health. 2009;212:239–70. doi: 10.1016/j.ijheh.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 141.Fossato da Silva DA, Teixeira CT, Scarano WR, et al. Effects of methylmercury on male reproductive functions in Wistar rats. Reprod Toxicol. 2011;31:431–9. doi: 10.1016/j.reprotox.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 142.Ye L, Su ZJ, Ge RS. Inhibitors of testosterone biosynthetic and metabolic activation enzymes. Molecules. 2011;16:9983–10001. doi: 10.3390/molecules16129983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Svechnikov KV, Izzo G, Landreh L, et al. Endocrine disruptors and Leydig cell function. J Biomed Biotechnol. 2010 doi: 10.1155/2010/684504. pii:684504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang H, Ji YL, Wang Q, et al. Maternal lead exposure during lactation persistently impairs testicular development and steroidogenesis in male offspring. J Appl Toxicol. 2012 doi: 10.1002/jat.2795. doi: 10.1002/jat.2795; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 145.Hannas BR, Lambright CS, Furr J, et al. Dose-response assessment of fetal testosterone production and gene expression levels in rat testes following in utero exposure to diethylhexyl phthalate, diisobutyl phthalate, diisoheptyl phthalate, and diisononyl phthalate. Toxicol Sci. 2011;123:206–16. doi: 10.1093/toxsci/kfr146. [DOI] [PubMed] [Google Scholar]
- 146.Akingbemi BT, Sottas CM, Koulova AI, et al. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology. 2004;145:592–603. doi: 10.1210/en.2003-1174. [DOI] [PubMed] [Google Scholar]
- 147.Ji YL, Wang H, Liu P, et al. Effects of maternal cadmium exposure during late pregnant period on testicular steroidogenesis in male offspring. Toxicol Lett. 2011;205:69–78. doi: 10.1016/j.toxlet.2011.05.233. [DOI] [PubMed] [Google Scholar]
- 148.Matsushima A, Kakuta Y, Teramoto T, et al. Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERR gamma. J Biochem. 2007;142:517–24. doi: 10.1093/jb/mvm158. [DOI] [PubMed] [Google Scholar]