Abstract
Background:
Several clinical trials have demonstrated the safety and effectiveness of oral tenofovir disoproxil fumarate (TDF), with or without emtricitabine (FTC), as pre-exposure prophylaxis (PrEP) for reducing the risk of HIV acquisition. Adherence to the study product was insufficient to demonstrate the effectiveness of FTC/TDF in 2 PrEP clinical trials conducted among women (FEM-PrEP and the Vaginal and Oral Interventions to Control the Epidemic study), but further analyses of adherence in these studies may inform PrEP demonstration projects and future HIV prevention clinical trials.
Methods:
We randomly selected a subcohort of 150 participants randomized to FTC/TDF in 3 FEM-PrEP sites (Bondo, Kenya; Bloemfontein, South Africa; and Pretoria, South Africa) to examine adherence levels over time and to assess factors associated with adherence, based on plasma tenofovir and intracellular tenofovir diphosphate drug concentrations in specimens collected at 4-week visit intervals.
Results:
We observed drug concentrations consistent with good adherence in 28.5% of all visit intervals when drug was available to use, but only 12% of participants achieved good adherence throughout their study participation. In multivariate analysis, the Bloemfontein site [odds ratio (OR): 2.43; 95% confidence interval (CI): 1.32 to 4.48] and liking the pill color (OR: 2.93; 95% CI: 1.18 to 7.27) were positively associated with good adherence, whereas using oral contraceptive pills at enrollment was negatively associated with good adherence (OR: 0.37; 95% CI: 0.18 to 0.74).
Conclusions:
Most participants did not regularly adhere to the study product throughout their trial participation, although a small minority did. Few factors associated with good adherence to the study product were identified in FEM-PrEP.
Key Words: pre-exposure prophylaxis, FEM-PrEP, adherence, women, Africa
INTRODUCTION
Numerous randomized clinical trials have assessed the safety and effectiveness of oral tenofovir disoproxil fumarate (TDF), with or without emtricitabine (FTC), as pre-exposure prophylaxis (PrEP) for reducing the risk of HIV acquisition. Recently, oral TDF was found effective for preventing HIV among people who inject drugs.1 Oral FTC/TDF had already been shown to reduce HIV acquisition among HIV-uninfected partners in serodiscordant couples,2 heterosexual men and women,3 and transgender women and men who have sex with men.4 However, 2 trials conducted among women, FEM-PrEP and the Vaginal and Oral Interventions to Control the Epidemic (VOICE) study, were unable to demonstrate the effectiveness of oral FTC/TDF (FEM-PrEP and VOICE) or oral TDF alone (VOICE) because of low adherence to the study product.5,6
In HIV prevention clinical trials, healthy volunteers were asked to adhere to an investigational product that may or may not prevent HIV acquisition and, moreover, may be a placebo. Consequently, the adherence patterns and factors associated with adherence to the study product within a placebo-controlled PrEP trial may or may not be similar to the patterns and factors influencing adherence to a marketed efficacious product outside the clinical trial context. Nonetheless, a better understanding of adherence and nonadherence within a trial may help in developing approaches for supporting participant adherence in PrEP demonstration projects and in future trials of new HIV prevention technologies.
Several factors have previously been shown to be associated with low adherence to the study product in PrEP clinical trials. In Partners PrEP, depending on the measure used, these factors were younger age, heavy alcohol use, sexual behavior, and months on study product.7 In VOICE, young single participants had lower adherence than older married participants.6 Adherence to the study product was measured using pill counts, drug concentrations, and electronic pill bottle monitoring in Partners PrEP2,7 and by drug concentrations in VOICE.6 In FEM-PrEP, a preliminary case–control analysis among 124 participants randomized to FTC/TDF (33 who seroconverted and 91 matched HIV-negative controls) found that adherence was low in the majority of participants. Among the HIV-uninfected controls, 24% had evidence of recent pill use (≥10 ng/mL tenofovir in plasma) at 2 consecutive visits matched to the infection window of participants who seroconverted.5 For this article, we measured adherence in a larger prospective subcohort of participants selected for analysis of drug concentrations to assess the association between baseline, time-dependent, and poststudy factors and drug concentrations consistent with good levels of adherence, and to describe patterns of adherence over time.
METHODS
Overview of the FEM-PrEP Clinical Trial
Initiated in 2009, FEM-PrEP was a phase 3, randomized, double-blind, placebo-controlled trial to assess the safety and effectiveness of once-daily oral FTC/TDF for the prevention of HIV in women at higher risk of HIV in Bondo, Kenya; Bloemfontein and Pretoria, South Africa; and Arusha, Tanzania. At screening, women were considered to be at “higher risk” if they had had sex at least once within the past 2 weeks or had had sex with more than 1 sexual partner in the past month. The primary effectiveness and safety results, including a description of the overall study population, have been previously published.5 In brief, participants were randomized to either once-daily FTC/TDF or placebo. They were asked to use the study product for 52 weeks and attend study visits every 4 weeks. The study visits included HIV testing, adherence counseling,8 safety assessments, free contraceptive counseling and method provision (contraceptive use at enrollment was an eligibility requirement), provision of study product, and HIV risk reduction services including counseling, free condoms, and treatment of curable sexually transmitted infections (STIs). A total of 2120 participants were enrolled, and 82% completed the study.5 All associated ethics and regulatory committees approved the trial, and all participants provided written informed consent. The trial closed early in April 2011 because of a lack of effect.9
Data Collection
We used data collected from face-to-face quantitative questionnaires about (1) demographics at screening, (2) HIV risk perceptions at enrollment, (3) sexual partners and sexual behaviors at enrollment and at quarterly visits, and (4) study product acceptability and perception of study product assignment at the time of permanent product withdrawal. We also used data on adverse events, on STI test results at screening, and from blood samples collected at each study visit through 52 weeks. We chose to use drug concentrations as the measure of adherence rather than self-reported adherence and pill counts because we have previously documented that adherence was strongly overestimated when those measures were used.10
Sample Selection and Analysis of Drug Concentrations
After the trial, we randomly selected 50 participants who were assigned FTC/TDF and who attended at least 1 follow-up visit from each of the sites where HIV infections occurred (Bloemfontein, Bondo, and Pretoria). No participants in the Arusha site contributed more than 16 weeks of follow-up data, and none seroconverted; therefore, we did not include participants from Arusha in this analysis. The resulting subcohort of 150 participants was scheduled to make 1364 visits between weeks 4 and 52 of follow-up (accounting for the early closure of the trial), of which, 55 visits (4.0%) were missed. We further excluded 122 visits (8.9%) when the participant did not have study product available to use (eg, because of protocol-required product interruption), 24 visits (1.8%) where no or insufficient specimen was available for the analysis of drug concentrations, and 2 visits (0.1%) when the visit interval was less than 10 days long. Specimens from the remaining 1161 visit intervals (7.8 per participant) were included in our analysis; the mean length of these visit intervals was 27.9 days with a range of 15–39 days. The final sample size provided at least 80% power to detect a doubling in the odds of good adherence based on reasonable assumptions about the overall proportion of visit intervals with good adherence, the proportions of participants with a given characteristic, and the correlation among repeated measures.
Concentrations of tenofovir (TFV) in plasma were used to characterize recent pill use (ie, within the previous 10 days). Because of its longer half-life, intracellular tenofovir diphosphate (TFV-DP) can be used to assess adherence over longer periods.11 Although FEM-PrEP did not collect peripheral blood mononuclear cells for this purpose, we were able to use a novel and validated assay to assess TFV-DP in upper layer packed cells (ULPCs; consisting primarily of platelets, lymphoctyes, monocytes, and granulocytes) from specimens that were collected at each visit for a different purpose (retrospective detection of HIV PCR-DNA if the participant seroconverted).12
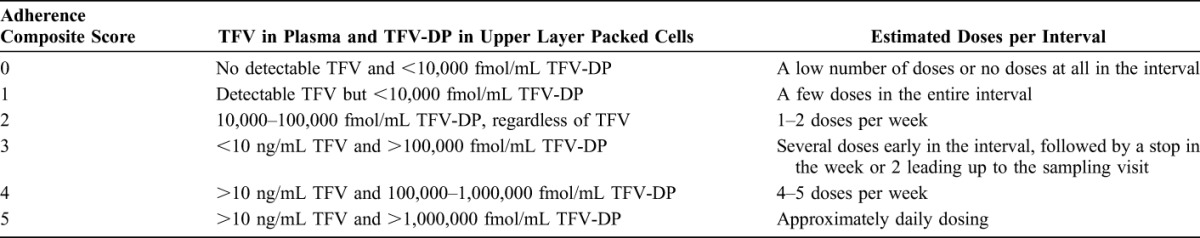
In consultation with the study pharmacologist, the TFV in plasma and intracellular TFV-DP concentration data were used to develop a semi-ordinal composite adherence score for each 4-week visit interval. Scores ranged from 0 (no detectable TFV and <10,000 fmol/mL TFV-DP, consistent with low or no doses of drug in the interval) to 5 (>10 ng/mL TFV in plasma and >1,000,000 fmol/mL TFV-DP, consistent with taking the product nearly every day) (Table 1). Good adherence (scores 4 or 5) was defined as having TFV in plasma exceeding 10 ng/mL and intracellular TFV-DP in ULPCs exceeding 100,000 fmol/mL; a participant taking study drug 4 or more times each week over the preceding 28 days was expected to exhibit drug concentrations in these ranges.
TABLE 1.
Qualitative Adherence Composite Scores, Corresponding TFV and TFV-DP Concentrations, and Estimated Doses per Interval
Statistical Methods
We performed logistic regression to assess associations between baseline, time-dependent, and poststudy factors and good adherence in each 4-week interval using robust variance estimation to account for repeated measures on participants. Bivariate analyses were conducted, followed by multivariate analysis that included factors significantly associated (P < 0.05) with good adherence in the bivariate analyses. The final model was simplified using stepwise variable selection.
The prespecified factors that we hypothesized might be associated with adherence were included in the analysis. Baseline variables included site, age, education, marital status, living with partner, perception of HIV risk in the next 4 weeks, using effective contraception at screening, using oral contraceptive pills (OCPs) at enrollment, and having an STI or bacterial vaginosis on or before enrollment. Time-dependent variables included having missed a previous visit, type of partner(s) in the previous 4 weeks, having had sex without using a condom in the previous 4 weeks, knowledge of partner having HIV, having had a gastrointestinal event, and time in study. The poststudy variables we assessed were participants' beliefs about their randomization arm and pill attributes.
RESULTS
Demographics and Baseline Characteristics
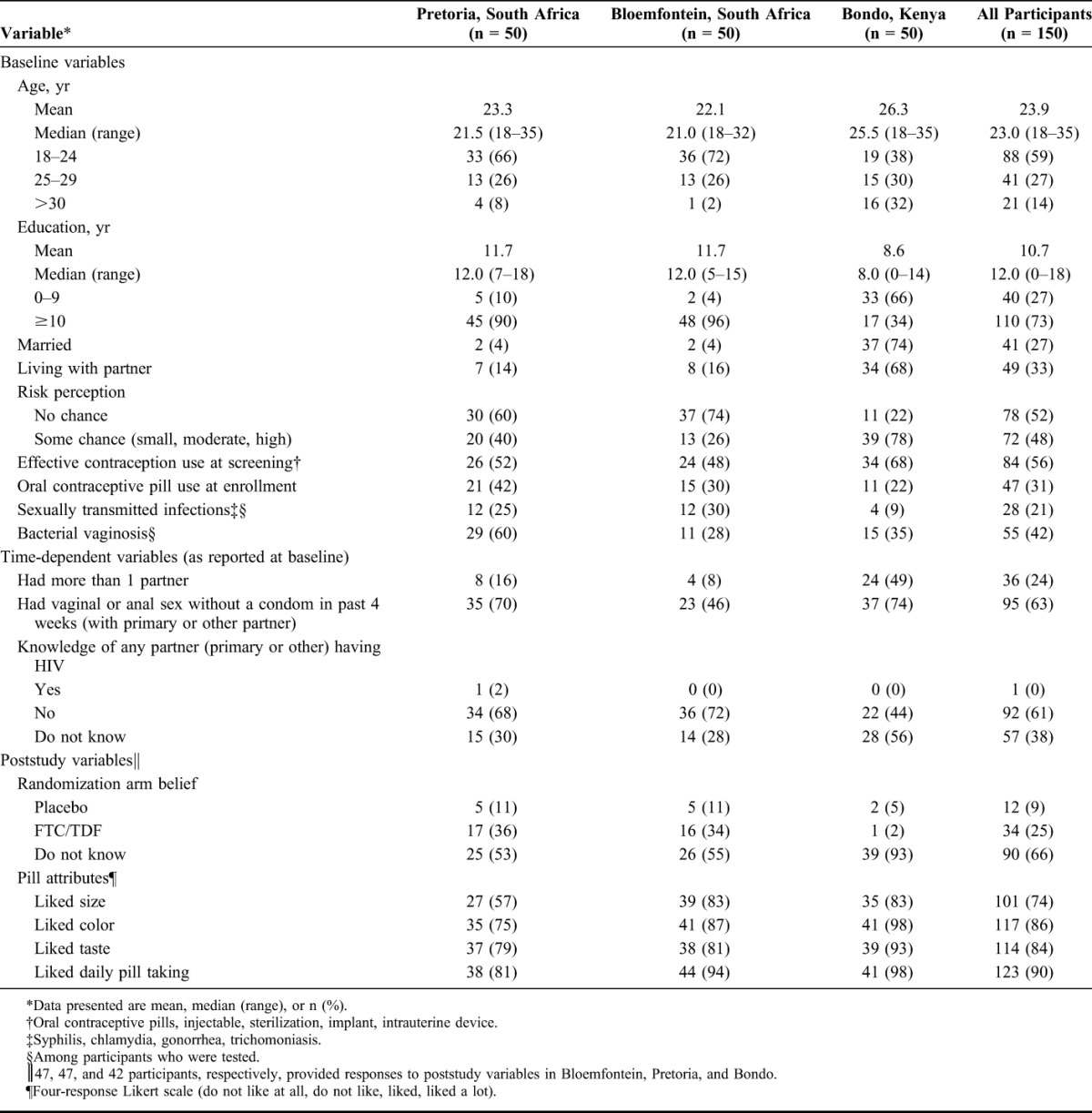
Within our subcohort, participants in the 2 South African sites (Bloemfontein and Pretoria) had similar demographic and other baseline characteristics, although these characteristics differed from those of participants in Kenya (Bondo) (Table 2). Participants from Bloemfontein and Pretoria were younger (72% and 66%, respectively, ages: 18–24 years) than participants from Bondo (38%, ages: 18–24) and had more years of education (mean, 11.7 years in both Bloemfontein and Pretoria versus 8.6 years in Bondo). Marital status and number of sexual partners also differed: 74% of participants in Bondo and 4% of participants in both South African sites were married, and 49% of participants in Bondo versus 8% in Bloemfontein and 16% in Pretoria reported having more than 1 sexual partner at baseline. In addition, more participants in Bondo (56%) than in Bloemfontein (28%) and Pretoria (30%) were unsure whether any of their sexual partners had HIV. Similar proportions of participants in Pretoria (70%) and Bondo (74%) reported that they had had sex without using a condom at least once in the past 4 weeks with any sexual partner compared with a smaller proportion of participants in Bloemfontein (46%). Fewer participants in Bondo (22%) than in Bloemfontein (30%) and Pretoria (42%) reported using OCPs at enrollment to fulfill their contraceptive use requirement for participating in the study.
TABLE 2.
Demographic Characteristics of Subcohort and Other Baseline, Time-Dependent, and Poststudy Variables
Poststudy Variables
At the end of the trial, the majority of participants from all the 3 sites (55% in Bloemfontein, 53% in Pretoria, and 93% in Bondo) said that they did not know whether they were randomized to FTC/TDF or placebo. Among those participants in Bloemfontein (n = 21) and Pretoria (n = 22) who did postulate their randomization assignment, 77% correctly guessed that that they had been assigned FTC/TDF (Table 2). Almost all participants in Bondo (98%) and most participants in Bloemfontein and Pretoria (87% and 75%, respectively) reported liking the color of the study product, and fewer participants in Pretoria (57%) than in Bondo and Bloemfontein (both 83%) reported liking the size of the study product.
Overall Visit Interval Adherence
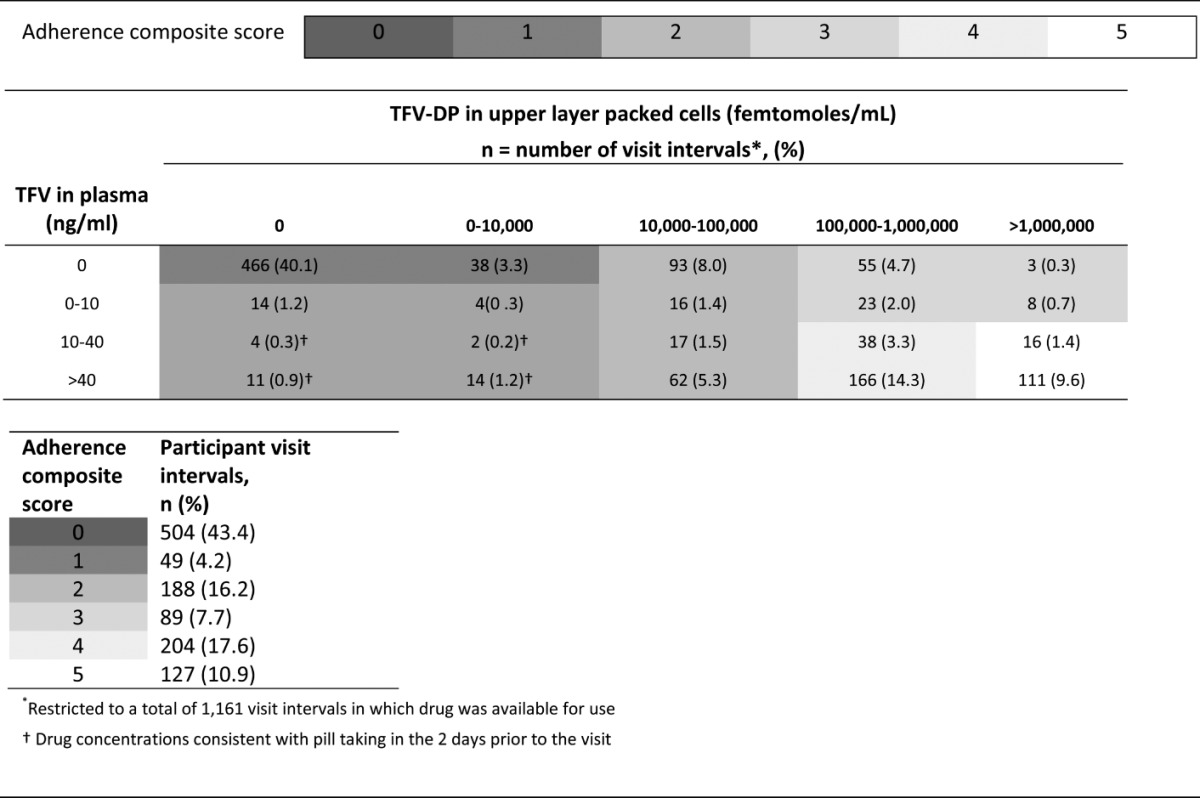
Fewer than 50 participants in the subcohort contributed 52 weeks of data because of early closure of the trial. Despite the availability of drug, 43.4% of all observed visit intervals had a composite adherence score of 0 (Table 3), and we observed only 28.5% of all visit intervals in the 2 highest composite adherence categories. There was also little evidence of participants exclusively taking drug in the day or 2 before clinic visits to give the appearance of adhering, also referred to as “white coat adherence.” Of the 553 visit intervals with nonquantifiable concentrations of TFV-DP (<10,000 fmol/mL) and thus little or no long-term use, only 31 (5.6%) had TFV plasma concentrations consistent with pill taking in the 2 days before the visit (≥10 ng/mL). Similarly, in the majority of visit intervals demonstrating recent pill use (TFV concentrations of ≥10 ng/mL), the intracellular TFV-DP concentrations (≥100,000 fmol/mL) were consistent with using the study product fairly regularly throughout the interval (Table 3).
TABLE 3.
Adherence Composite Scores, Listed by Plasma TFV and Intracellular TFV-DP Concentrations and by Participant Visit Intervals
Patterns of Adherence Over Time
Figure 1 displays the frequencies of adherence scores by scheduled follow-up visit among participants in the subcohort who had the study product available for use during the visit interval. The use of study product seemed to decrease over time, with 35% of participants having good adherence (scores 4 and 5) at week 4 compared with 23% having good adherence at week 52. Conversely, nonuse of the study product (a score of 0) generally increased over time.
FIGURE 1.
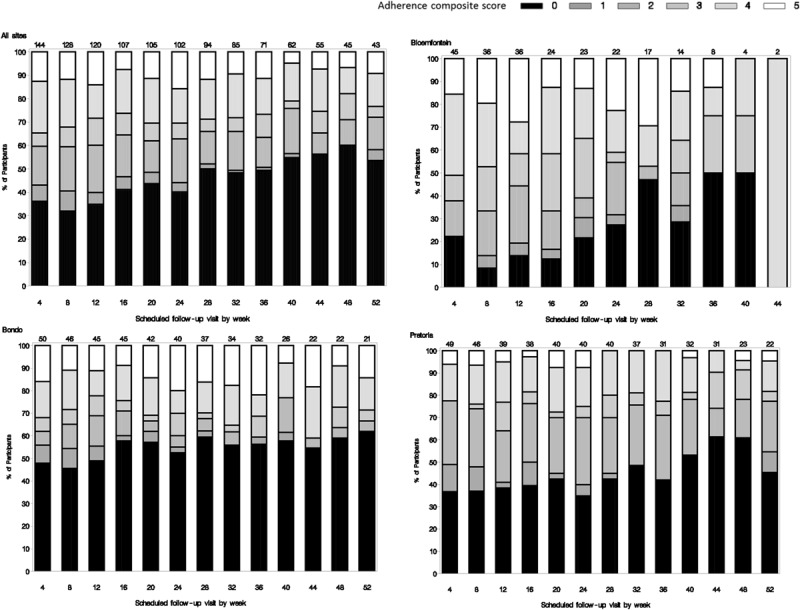
Adherence score frequencies by scheduled follow-up visit.
Figure 1 also shows the frequencies of adherence scores by site. Participants from the 2 South Africa sites seemed to have the greatest variability in study product adherence, although participants in Bloemfontein contributed the least amount of follow-up data. Bondo had the highest percentage of participants with no evidence of study product use over time.
At the individual level, 83 participants (55%) had at least 1 study interval with drug concentrations consistent with good adherence. However, throughout their trial participation, only 18 participants (12%) had drug concentrations consistent with good adherence (scores 4 and 5; none of whom contributed a full 52 weeks of follow-up before study closure), 34 (23%) consistently had no or low drug concentrations (scores 0 and 1), 8 (5%) had intermittent but non-zero adherence over time (scores 2 or 3), and the majority (n = 90; 60%) had drug concentrations that generally fluctuated across all adherence scores.
Factors Associated With Adherence
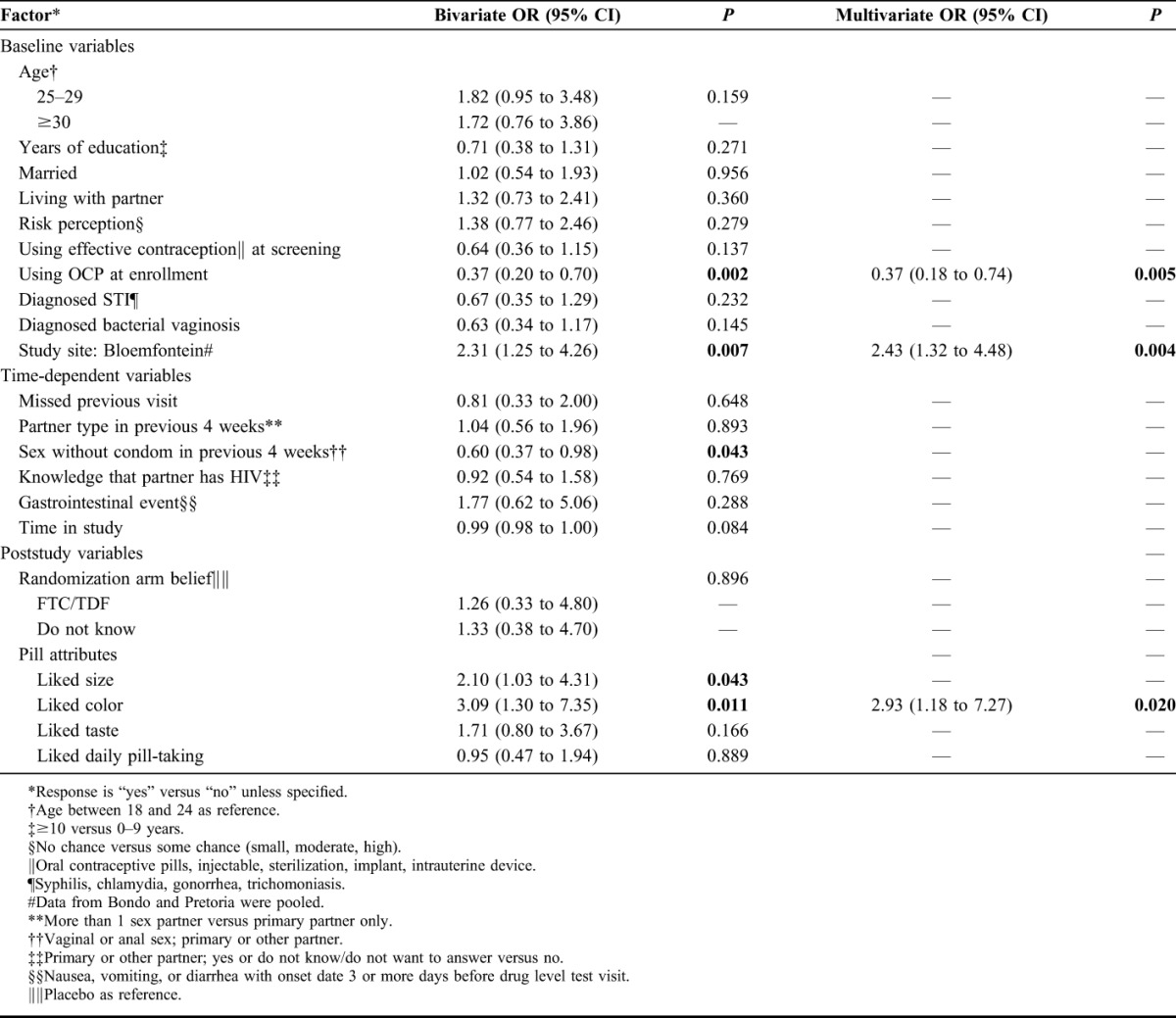
In bivariate analyses, we found that being from the Bloemfontein site [odds ratio (OR): 2.31; 95% confidence interval (CI): 1.25 to 4.26 when pooling data from the other sites], liking the color of the pill (OR: 3.09; 95% CI: 1.30 to 7.35) and liking the size of the pill (OR: 2.10; 95% CI: 1.03 to 4.31) were significantly associated with good adherence (Table 4). We also found that using OCPs at enrollment (OR: 0.37; 95% CI: 0.20 to 0.70) and reporting having had sex without using a condom in the 4-week interval before drug measurement (OR: 0.60; 95% CI: 0.37 to 0.98) were negatively associated with good adherence. We saw a trend toward decreased adherence over time, but this was not significant in bivariate analysis (OR: 0.99 per week in study; 95% CI: 0.98 to 1.00). In multivariate analysis, being from the Bloemfontein site (OR: 2.43; 95% CI: 1.32 to 4.48) and liking the color of the pill (OR: 2.93; 95% CI: 1.18 to 7.27) remained positively associated with good adherence and using OCPs at enrollment remained negatively associated with good adherence (OR: 0.37; 95% CI: 0.18 to 0.74) (Table 4).
TABLE 4.
Bivariate and Multivariate Regression of Factors Associated With Good Adherence
DISCUSSION
Overall adherence among the prospective subcohort described here was similar to the adherence levels we previously reported from a preliminary case–control analysis5; the majority of participants did not regularly take their study product, if they took it at all. Our findings suggest that a considerable minority of participants (23% of our subcohort) may have joined FEM-PrEP without ever intending to take the study product because their drug concentrations starting at week 4 showed that they rarely, if ever, took drug. Although 55% of participants had at least 1 visit interval consistent with good adherence when drug was available to use, only a small minority of the subcohort participants—12% across all the 3 sites—seemed to regularly take the study product for the length of trial participation. It is plausible that these participants joined FEM-PrEP with the intent to adhere as instructed and maintained their interest and motivation throughout their time in the trial. Adherence among the majority of participants (60%), however, fluctuated substantially over time, suggesting that there could be factors that influenced adherence at different time points during the trial. Our findings also suggest that “white coat adherence,” where participants take their study pills in advance of their study visits to seem adherent, was not common.
We found few factors associated with good study product adherence. Before the drug concentration data were available, we had hypothesized that adherence among participants who were already using effective contraception at screening would be somewhat higher (compared with participants initiating contraception because of trial eligibility criteria) because they had already shown the willingness and ability to adopt preventive health behaviors.13 However, we did not confirm such an effect. We did find that participants who chose OCPs as their contraceptive at enrollment (regardless of previous contraceptive method use), and hence were asked to take 2 pills daily as part of the study, were much less likely to adhere than participants who chose another contraceptive method. It is unknown whether participants who opted to use OCPs differed in other unmeasured factors that would have affected adherence (irrespective of OCP use), if the request to take 2 daily pills led to poor adherence, or a combination of both. Nonetheless, this observation may have programmatic implications and should be assessed in demonstration projects. Furthermore, we found no association between perceived HIV risk measured only at enrollment and good adherence throughout the trial, which may be partly due to how HIV risk perception was measured (ie, perception of risk in the subsequent 4 weeks). Additional secondary analyses of contraception and risk perceptions are underway and may shed further light on the relationship between these factors and adherence.
It is unclear why participants from Bloemfontein had better adherence. They contributed less time before study closure, but their adherence was also generally better in the first few months of follow-up than in the other sites. It is possible that unmeasured or as-yet unidentified individual factors, such as alcohol use or concerns about taking an investigational drug, or the broader social context, such as varying community support for clinical trials or the clinic environment, influenced adherence and differed across sites.
We excluded data from visit intervals where drug was not available to use (eg, because of missed product supply visits or protocol-defined product withdrawals) because including those results would have masked adherence relationships of interest. We also did not impute drug concentration data that were missing when participants missed the next visit after receiving the study product. Although the latter missing data could have introduced some bias, we did not find any evidence that missing study visits were associated with greater or lesser adherence in ensuing intervals when product was available. Another potential limitation of our analyses was that we were not able to develop a strictly ordinal score relating overall adherence in 4-week intervals to drug concentrations. TFV in plasma concentrations and TFV-DP in ULPCs reflect adherence during different portions of the visit interval, and different patterns of adherence could lead to similar drug concentrations when measured only once every 28 days. Hence, we chose to model factors associated with good adherence, which was reasonably well defined using available drug concentration data. Moreover, most visit intervals (76.1%) were consistent with either very poor or good adherence, suggesting that a dichotomized outcome based on the higher adherence concentrations targeted in the protocol was appropriate.
Our overall adherence results are similar to the adherence findings in VOICE, in which fewer than 33% of samples from a random sample of participants assigned to FTC/TDF showed evidence of TFV at quarterly visits.6 We did not, however, find any association between younger unmarried participants and lower adherence to the study product, as demonstrated in VOICE.6 This may be because the 2 studies used different methods to measure adherence (from results currently available, VOICE assessed factors associated with a detectable TFV plasma concentration of ≥0.3 ng/mL and also included samples when participants had missed their previous visit) or because of differences in the study populations.
Moving forward, it will be crucial to continue focusing on the development and evaluation of new methods to enhance adherence counseling and other participant support for achieving and maintaining good adherence in trials of HIV prevention technologies and PrEP demonstration projects. The low adherence demonstrated in FEM-PrEP and VOICE also provides evidence in support of investigator review of aggregated drug concentration data throughout trial implementation to ensure that participants assigned to the study drug are actually taking it, rather than relying on self-reported measures to assess adherence during the trial. This is being performed in some ongoing HIV prevention clinical trials and demonstration projects.
In summary, we found that only a small minority of FEM-PrEP participants regularly adhered to the study product. In interpreting these results, we must recognize that participants were asked to adhere to the daily use of either an investigational HIV prevention drug or a placebo. FEM-PrEP participants were reminded at each study visit that the purpose of the research was to determine whether FTC/TDF was effective for HIV prevention. They were also counseled at each study visit to use HIV risk reduction methods of known effectiveness, such as condoms and partner reduction, because it was not known whether FTC/TDF could prevent HIV acquisition and because they may have been randomized to the placebo, which could not protect against HIV.
Ultimately, taking a daily pill for HIV prevention may not be feasible or acceptable for some African women at high risk of HIV, but we cannot eliminate this option based solely on inconsistent evidence from clinical trials assessing the effectiveness of PrEP. Demonstration projects are planned or under way, in which all participants are provided FTC/TDF, now that it is a proven efficacious drug for reducing the risk of HIV acquisition through sex, and are counseled on the importance of adherence. The data from these projects will allow for a better assessment of the role of daily oral antiretroviral drugs for PrEP, particularly among African women at high risk of HIV.
ACKNOWLEDGMENTS
The authors are grateful to the women who participated in the FEM-PrEP trial, to the study staff, to the communities who partnered with us to conduct the trial, and to all of our collaborators in Africa, Belgium, and the United States. The views expressed in this publication do not necessarily reflect those of FHI 360, the funding agencies, or Gilead Sciences, Inc.
APPENDIX. 1. FEM-PrEP Study Group
Walter Agingu, Julie Ambia, Jesse Asewe, Irith De Baetselier, Savi Chetty-Tulsee, Sarah Chiduo, Tania Crucitti, Katrien Fransen, John Gardner Gaddiel, Haddie Kiernan, Stella Kirkendale, Tharnija Lalbahadur, Michele Lanham, Temu Lucky, Paul Mak'Oketch, Shumani Makatu, Mookho Malahleha, Justin Mandala, Makanda Mankalimeng, Martha Masaki, Timothy Mastro, Kevin McKenna, Modie Constance Monedi, Sarah Mullins, Kavita Nanda, Jacob Odhiambo, Immaculate Olango, Paul Omullo, Zablon Omungo, Fred Owino, Caleb Parker, Malebo Ratlhagana, Ilse Reblin, Lisa Saylor, Phumzile Siguntu, Joseph Short Skhosana, Amanda Troxler, Valentine Veena, Lalitha Venkatasubramanian, Gustav Venter, and Christina Wong.
Footnotes
FEM-PrEP was conducted under 2 grants funded by the United States Agency for International Development (USAID): the Contraceptive and Reproductive Health Technologies and Research Utilization Program and the Preventive Technologies (Agreement No. GHO-A-00-09-00016-00). Early support was also provided by the Bill & Melinda Gates Foundation. Gilead Sciences, Inc. donated FTC/TDF and placebo. The drug concentration analyses were performed using equipment provided by the University of North Carolina at Chapel Hill Center for AIDS Research (CFAR), an NIH funded program P30 AI50410.
The authors have no conflicts of interest to disclose.
For a complete listing of participating members of FEM-PrEP Study Group, see Appendix 1.
REFERENCES
- 1.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083–2090 [DOI] [PubMed] [Google Scholar]
- 2.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434 [DOI] [PubMed] [Google Scholar]
- 4.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marrazzo JM, Ramjee G, Nair G, et al. Pre-exposure prophylaxis for HIV in women: daily oral tenofovir, oral tenofovir-emtricitabine, or vaginal tenofovir gel in the VOICE Study (MTN 003) [26LB]. Paper presented at: The 20th Conference on Retroviruses and Opportunistic Infections; March 3–6, 2013; Atlanta, GA
- 7.Haberer JE, Baeten JM, Campbell J, et al. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med. 2013;10:e1001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amico KR, Mansoor LE, Corneli A, et al. Adherence support approaches in biomedical HIV prevention trials: experiences, insights and future directions from four multisite prevention trials. AIDS Behav. 2013;17:2143–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.FHI360. Press release: FHI to initiate orderly closure of FEM-PrEP. 2011. Available at: www.fhi360.org/fhi-statement-fem-prep-hiv-prevention-study, Accessed May 7, 2014 [Google Scholar]
- 10.Taylor D, Amico KR, Brown E. Asking about and measuring adherence. Paper presented at: The International Microbicides Conference; April 15–18, 2012; Sydney, Australia
- 11.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4:151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams JL, Sykes C, Menezes P, et al. Tenofovir diphosphate and emtricitabine triphosphate concentrations in blood cells compared with isolated peripheral blood mononuclear cells: a new measure of antiretroviral adherence? J Acquir Immune Defic Syndr. 2013;62:260–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor D.The contraceptive use requirement and related findings in the FEM-PrEP trial: were existing contraceptive users more adherent to protocol? Paper presented at: The International Microbicides Conference; April 15–18, 2012; Sydney, Australia.


