Supplemental Digital Content is Available in the Text.
Key Words: Africa, HIV prevention, pre-exposure prophylaxis, serodiscordant couples, adherence, drug concentrations
Abstract
Background:
Antiretroviral pre-exposure prophylaxis (PrEP) is a novel HIV prevention strategy for which adherence is a known determinant of efficacy. Blood concentrations of PrEP medications are one objective marker of adherence.
Methods:
In a placebo-controlled PrEP efficacy trial of tenofovir disoproxil fumarate (TDF) and TDF with emtricitabine (FTC/TDF) among 4747 African women and men with an HIV-infected partner, we measured plasma tenofovir concentrations from participants in the active PrEP arms: 29 HIV seroconverters (cases) and 196 randomly selected controls who remained uninfected.
Results:
Among controls, 71% of visits had tenofovir concentrations >40 ng/mL, consistent with steady-state daily dosing, compared with 21% of cases at the visit HIV was first detected. Pill count data indicated that 96% of controls and 66% of cases had >80% adherence for these same visits. The estimated protective effect of PrEP against HIV, based on concentrations >40 ng/mL, was 88% (95% confidence interval: 60 to 96, P < 0.001) for individuals receiving TDF and 91% (95% confidence interval: 47 to 98, P = 0.008) for individuals receiving FTC/TDF. Controls had consistent patterns of PrEP concentrations during follow-up; among the 81% with concentrations >40 ng/mL at month 1, 75% maintained this concentration at month 12. Only 5 of 29 seroconverters seemed to be consistently adherent to PrEP. Tenofovir concentrations >40 ng/mL were associated with older age and shorter time on study; concentrations ≤40 ng/mL occurred more commonly when participants reported no sex with their HIV-infected partner.
Conclusions:
Plasma concentrations of tenofovir consistent with daily dosing were highly predictive of protection from HIV acquisition. Most of those who took PrEP seemed to have high and consistent adherence.
INTRODUCTION
Antiretroviral pre-exposure prophylaxis (PrEP) with once-daily tenofovir disoproxil fumarate (TDF) or combination emtricitabine (FTC/TDF) is an effective HIV prevention strategy for HIV-uninfected persons at high risk of infection. Four randomized placebo-controlled efficacy trials have demonstrated the efficacy of daily oral PrEP for HIV prevention among diverse populations, including men who have sex with men (MSM) from multiple countries, young African heterosexuals, African heterosexual HIV serodiscordant couples, and injection drug users in Thailand.1–4 PrEP clinical trials have also pointed to the critical need for high adherence to achieve HIV protection, with the degree of HIV protection across clinical trials (from 44% to 75%) strongly related to PrEP adherence in the trial population. Two efficacy trials of oral PrEP in young African women did not demonstrate HIV protection, as a result of very low adherence.5,6
In 1 PrEP clinical trial, the Partners PrEP Study among African HIV serodiscordant couples, the degree of HIV protection in the randomized comparison was 67% for TDF and 75% for FTC/TDF.4 By several indicators, including high visit-to-visit clinic attendance, clinic- and home-based pill counts of unused study product, medication electronic monitoring, and plasma drug levels, adherence to PrEP was high in the study population.7 In this study, we used the detection of tenofovir in plasma to estimate HIV protection efficacy by quantitative drug concentrations in plasma and to explore adherence sampled over time and its association with participant characteristics. To assess accuracy of pill count data to monitor adherence, detectable tenofovir in plasma was compared with clinic-based pill count data.
METHODS
Population
The Partners PrEP Study was a phase 3, randomized, double-blind, placebo-controlled, 3-arm clinical trial of daily oral TDF and FTC/TDF PrEP among HIV-uninfected members of HIV serodiscordant couples. Beginning in 2008, 4747 couples were randomized and followed at 9 clinical research sites in Kenya and Uganda. The design, procedures, and outcomes of the trial are described elsewhere.4 Briefly, HIV-uninfected partners were randomly assigned to once-daily TDF, FTC/TDF, or matching placebo and followed monthly for safety assessments and HIV seroconversion for up to 36 months. Plasma was stored at months 1, 3, and quarterly thereafter, plus at any visit where a participant tested positive for HIV. HIV-infected partners were not eligible for antiretroviral therapy under national guidelines at the time of enrollment but were monitored and actively referred for antiretroviral treatment initiation if they became eligible during follow-up. All couples received a package of HIV prevention services, including risk-reduction counseling, couples counseling, and condoms. In July 2011, the trial's independent Data and Safety Monitoring Board recommended public report of the results and discontinuation of the trial's placebo arm because of the demonstration of 67% efficacy for HIV protection with TDF and 75% efficacy with FTC/TDF. The trial was subsequently continued with all participants receiving active TDF or FTC/TDF PrEP. The present analysis includes data through July 2011.
Laboratory Procedures
HIV seroconversions were detected by rapid HIV tests conducted in duplicate at the study sites, with confirmatory testing by HIV Western blot. In addition, after seroconversion was confirmed, archived plasma samples from visits before seroconversion were tested for HIV RNA by polymerase chain reaction to more precisely determine the timing of HIV acquisition.
Plasma tenofovir concentrations were determined in selected archived plasma samples by previously described ultra-performance liquid chromatography-mass spectrometry assay methods.8,9 Tenofovir assays meet the FDA bioanalysis guidance values of within ±15% for precision and accuracy. Thawed aliquots of plasma with 13C5-TFV internal standard were protein precipitated with methanol. Samples underwent chromatographic separation using gradient elution with a Zorbax Eclipse XDB-C18 column, with positive electrospray ionization, and detection through multiple reaction monitoring using an LC-MS/MS system (Waters Acquity UPLC and Agilent 1100 HPLC, API4000 mass spectrometer; Applied Biosystem). Calibration standards for assay ranged from 0.31 to 1280 ng/mL.
Case–Cohort Study of Tenofovir Plasma Drug Concentrations
A case–cohort design in the active PrEP arms of the trial (ie, TDF and FTC/TDF arms) was used to assess the association between HIV infection and plasma concentrations of tenofovir. A cohort of 200 participants was randomly selected (100 from the TDF arm and 100 from the FTC/TDF arm), with all 30 HIV seroconverters from the PrEP arms comprising the cases (17 from the TDF arm and 13 from the FTC/TDF arm). Two participants from the cohort were lost to follow-up and 2 seroconverted to HIV, leaving 196 HIV-uninfected participants as the control population (100 TDF and 96 FTC/TDF). For these controls, 945 plasma samples were tested from available study visits 1, 3, 6, 12, 18, 24, 30, and 36 months after enrollment. Of the 30 seroconverters, 1 did not have stored samples after enrollment; for the remaining 29, 85 samples were tested from the same visit schedule as controls before HIV infection plus the visit at which HIV infection was first detected (either by HIV seroconversion or in a preseroconversion sample by HIV RNA polymerase chain reaction; for 14 of the 29 seroconverters, HIV RNA was detected in an archived plasma sample before seroconversion). In results previously reported from this clinical trial, plasma tenofovir concentrations from the time of seroconversion were analyzed, without consideration of HIV RNA detection before seroconversion.10
Statistical Analysis
Three threshold concentrations of plasma tenofovir levels were used to categorize participant's adherence: (1) >0.31 ng/mL (the assay limit of quantitation referred to hereafter as “detectable”), which is consistent with dosing within the last week; (2) >10 ng/mL, consistent with dosing in the last 2–3 days; and (3) >40 ng/mL, the lower 95% confidence interval (CI) 24 hours after dose for directly observed daily dosing at steady state.11–14 The temporal dosing references of these concentration thresholds are based on population-based values and will vary for a given individual because of pharmacokinetic (PK) differences within a population. Concentrations >40 ng/mL are consistently achieved with daily dosing but are also likely in persons who took a single dose in the last 24 hours. In PrEP trials reported to date, either the 0.31 ng/mL or the 10 ng/mL threshold has been used to define detectable tenofovir in plasma.1,6
The case–cohort analysis was performed using tenofovir concentrations in all participants irrespective of drug holds in the previous interval (eg, for pregnancy or evaluation of adverse events). Design calibration methods were used to compute hazard ratios from a Cox proportional hazards model for the 2-stage sampling of the case–cohort design. Information from covariates in all active arm participants was used to predict plasma drug concentrations from pill count coverage, age, and occupation. These models, through influence functions, were used to adjust the sampling for imbalances in the cohort selected for plasma testing.15,16 Estimates of HIV protective effect could be affected by potential confounding with factors associated with adherence and HIV risk, therefore the analysis was conducted both with drug levels as the only covariate and also adjusted for covariates found to correlate with adherence (gender, age, and any sex with study partner7). Analysis was conducted in the R survey package Version 3.0.1.17
Drug dispensing records and counts of the returned pills were used to calculate pill count “coverage,” defined as the percentage of days in the previous month pills were available and taken i.e. (pills dispensed–pills returned)/(elapsed days since last visit). Pill count coverage was zero where drug was not dispensed (eg, for protocol-specified holds such as pregnancy or adverse events) and incorporated imperfect coverage when there were insufficient pills for the elapsed days as a result of missed or late visits. When contrasting nonadherence measured by plasma drug concentrations with pill count coverage, we compared undetectable tenofovir concentrations and pill count coverage >80%, noting that pill count >80% corresponds to taking pills at least 22 of the previous 28 days and a pill taken in the last 6 days would result in detectable drug since tenofovir typically remains detectable for 14 days.13 Analysis of correlates of steady-state daily adherence (>40 ng/mL) in controls included time on study, sociodemographic characteristics (gender, age, education, occupation, and study arm), visit-level risk behaviors (any sex and any unprotected sex with study partner in the previous month, any outside partner, or polygamous marriage), and clinical characteristics of the HIV-infected partner (baseline HIV plasma viral load, baseline and follow-up CD4 counts, and initiation of antiretroviral therapy). Correlates of adherence were assessed using logistic regression with generalized estimating equation methods to account for repeated measures across visits, assuming an exchangeable covariance structure. Visits with participants on study drug hold were excluded.
Ethics
The study protocol was approved by the Ethics Review Committees at the University of Washington and each study site. The trial is registered with ClinicalTrials.gov (NCT00557245). All participants provided written informed consent.
RESULTS
Participant Characteristics
Demographic, clinical, and behavioral characteristics of cases and controls were similar to the entire active arm cohort in the trial (see Table, Supplemental Digital Content 1, http://links.lww.com/QAI/A519, which demonstrates characteristics of the study population), with the exception that CD4 counts in HIV-infected partners of seroconverters were lower by 114 cells per cubic millimeter compared with nonseroconverters (P = 0.002). The median number of samples tested in controls was 5 [interquartile range (IQR): 4–6] and in cases was 4 (IQR: 3–5), representing 96% and 90% of all possible visits, respectively.
Detection of Tenofovir in Plasma
Drug concentrations >40 ng/mL, consistent with steady-state dosing, were detected in 71% of samples from controls (72% TDF and 70% FTC/TDF; Table 1); for the 19 cases, concentrations >40 ng/mL were detected in 48% of samples, in visits before HIV infection (49% TDF and 47% FTC/TDF). At the first visit when HIV infection was detected, only 6 of 31 (21%) seroconverters had concentrations >40 ng/mL, although 9 had detectable tenofovir (7 on TDF, with 3 cases ≤40 ng/mL and 4 cases >40 ng/mL; 2 on FTC/TDF, both >40 ng/mL). Samples from controls and cases had similar, and low, rates of study drug interruptions because of pregnancy or clinical safety holds (20/945 control visits = 2.1% and 3/85 case visits = 3.5%, P = 0.11). Three of the 29 seroconverters, all on the TDF arm, who were on a drug hold at the time HIV infection was first detected (2 for pregnancy and 1 for a safety-related drug hold). Pill count data show a high proportion of visits with high adherence in controls (96%) and in cases, prior to HIV infection (91%), but substantially lower in cases' visits immediately before HIV acquisition (66%). In all groups, tenofovir concentrations indicate substantially lower adherence than pill count data.
TABLE 1.
Plasma Tenofovir Concentrations, Missed Visits, and Pill Count Coverage by Case–Control Status
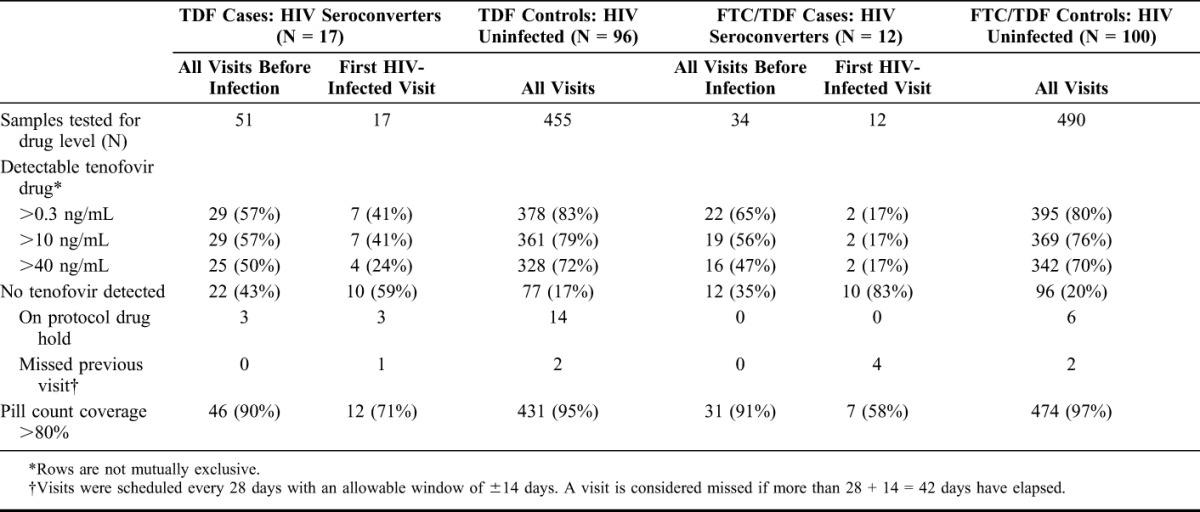
Quantitative concentrations of tenofovir, when detected, did not differ between controls and cases before infection [median: 81 ng/mL (IQR: 57–116) for controls versus median: 78 ng/mL (IQR: 52–118) for cases; (P = 0.6)]. In 9 cases with detectable tenofovir at the visit when HIV infection was detected, median tenofovir concentration was 49 ng/mL (IQR: 26–82), somewhat lower than controls (P = 0.08). Among controls, median tenofovir concentrations were similar in men and women (men: 83, IQR: 57–120; women: 78, IQR: 57–111; P = 0.5).
Participants who did not have detectable drug (<0.31 ng/mL) at their month 1 visit represented 11% of controls and consistently had low concentrations of drug (Fig. 1A), demonstrating a pattern of early nonadherence or noninitiation. Participants with detectable but low plasma concentrations (≤40 ng/mL) at month 1 (8%) subsequently show fluctuating tenofovir concentrations (Fig. 1B). Those with plasma concentrations >40 ng/mL at month 1 (81%) were largely consistent adherers; 100 of 133 (75%) of these month 1 adherers had concentrations >40 ng/mL at month 12 and 66 of 92 (72%) at month 18 (Fig. 1C). When tenofovir concentrations were undetectable in this group [eg, 20/133 (15%) at month 12 and 16/92 (17%) at month 18], only a minority (6 of 36, or 17%) were attributable to drug holds because of pregnancy or clinical safety events. In the seroconverters, where infection was detected at the last visit shown in Figure 1D, 5 of the 29 (17%) seroconverters had concentrations consistently >40 ng/mL throughout study follow-up; of these 5, 3 were women on the TDF arm, 1 a man, and 1 a woman on the FTC/TDF arm.
FIGURE 1.
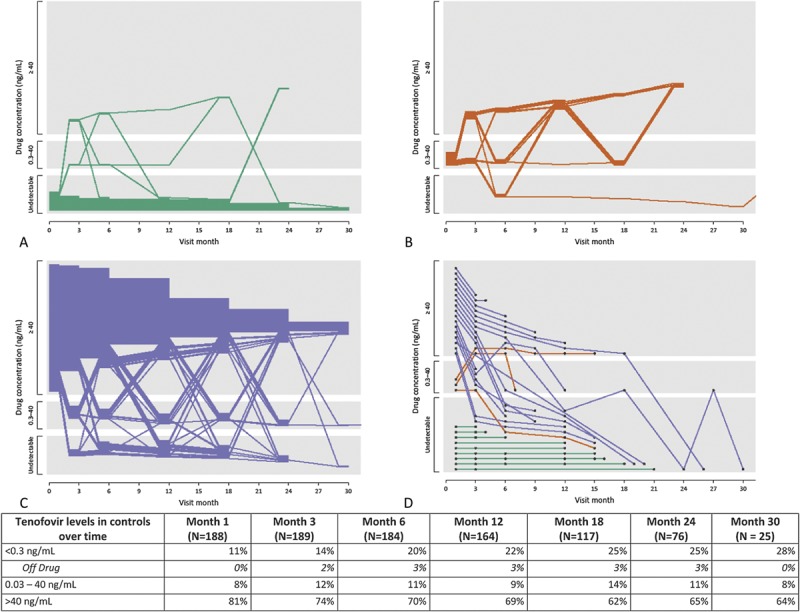
Change in plasma level detected by visit in control and cases. Levels of tenofovir detected are grouped by undetectable (<0.31), detectable but less than daily dosing (0.31, 40.0) and consistent with steady-state dosing (>40) ng/mL in plasma. Figures 1a, 1b and 1c show individual patterns in control participants grouped by the levels of tenofovir quantified at the month 1 visit (green ≤0.31, orange = 0.31, 40.0, blue ≥40) ; each participant is represented by one line and lines terminate at each participant's last visit. Figure 1d shows the plasma levels of the 29 seroconverters; the final value shown corresponds to the tenofovir plasma level at first visit at which HIV infection was detected. Off drug means on clinical drug hold as a result of pregnancy, AE or other reason.
Plasma Tenofovir Concentrations and HIV Protection
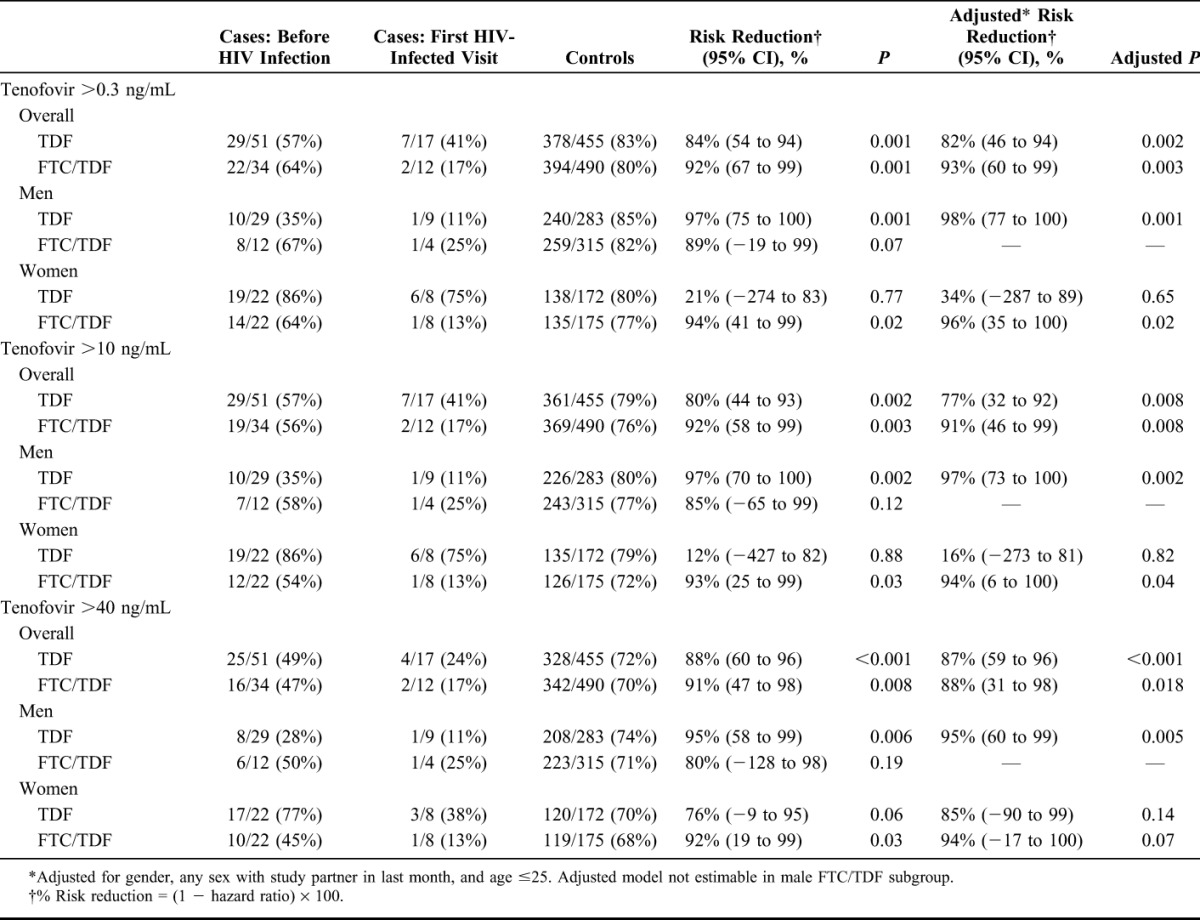
Plasma tenofovir concentrations at all 3 thresholds (>0.31, >10, and >40 ng/mL) were highly predictive of protection from HIV infection (Table 2). Plasma concentration of tenofovir >40 ng/mL was associated with HIV protection of 88% in the TDF arm (95% CI: 60% to 96%, P < 0.001) and 91% in the FTC/TDF arm (95% CI: 47% to 98%, P = 0.008), concentration of tenofovir >10 ng/mL with 80% HIV protection in the TDF arm (95% CI: 44% to 93%, P = 0.002) and 92% in the FTC/TDF arm (95% CI: 58% to 99%, P = 0.003), and tenofovir >0.3 ng/mL with 84% protection in the TDF arm (95% CI: 54% to 94%, P = 0.001) and 92% in the FTC/TDF arm (95% CI: 67% to 99%, P = 0.001). Separate gender subgroup analysis of TDF and FTC/TDF shows similar protection for men and women, with the exception of women in the TDF arm at the lower tenofovir thresholds, where association with protection at both the >0.3 and >10 ng/mL concentration were low, although with wide confidence limits (Table 2).
TABLE 2.
HIV Risk Reduction for 3 Threshold Concentrations of Tenofovir in Plasma
Predictors of High Plasma Tenofovir Concentrations
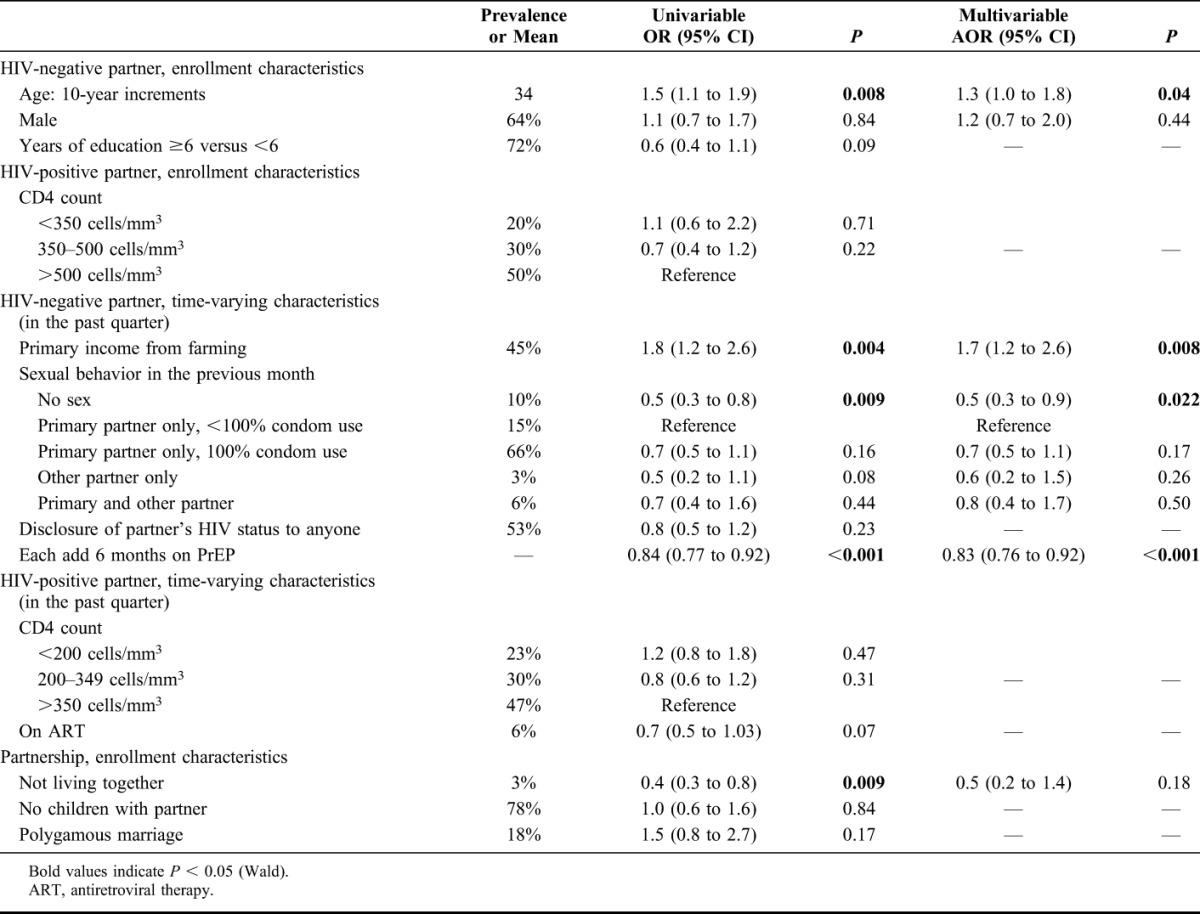
In multivariate analysis, correlates of tenofovir >40 ng/mL, consistent with daily adherence, were older age [adjusted odds ratio (aOR): 1.3, 95% CI: 1.0 to 1.8, for each increase of 10 years, P = 0.04] and couples whose primary income was from farming (aOR: 1.7, 95% CI: 1.2 to 2.6, P = 0.008). Not having sex compared with reporting unprotected sex with the HIV-infected study partner, aOR: 0.5, 95% CI: 0.3 to 0.9, P = 0.02) and longer time on study (aOR: 0.83, 95% CI: 0.76 to 0.92, for each additional 6 months on study drug, P < 0.001) were associated with lower likelihood of having tenofovir detected at >40 ng/mL (Table 3).
TABLE 3.
Univariable and Multivariable Regressions of Factors Correlating With Tenofovir >40 ng/mL in Active Arms of Study
PrEP Adherence: Pill Count Coverage Compared With Plasma Concentrations
The majority of control samples had tenofovir plasma concentrations consistent with pill count coverage in the previous month (Fig. 2); in visits with high pill count coverage (80%–103%), 592 of 779 (76%) had tenofovir plasma concentrations >40 ng/mL; with low pill count coverage (<80%), only 7 of 42 (17%) had tenofovir concentrations >40 ng/mL. For pill count coverage above 103% (coverage >100% occurs when fewer pills are returned than expected), tenofovir concentrations >40 ng/mL occurred in only 65 of 115 (57%). Notably, 141 of 936 (15%) had high pill count coverage (>80%) but undetectable tenofovir in plasma, indicating over-estimation of adherence by pill counts.
FIGURE 2.
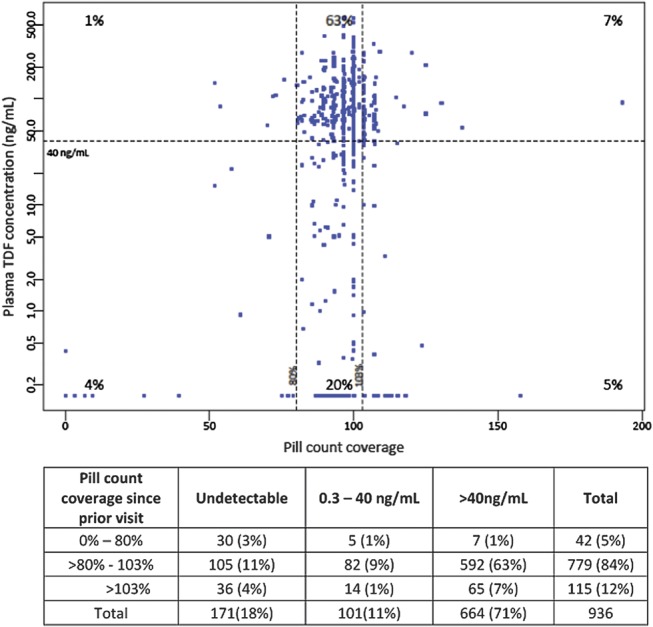
Pill count coverage and quantified tenofovir levels in plasma. Pill count coverage combines pill count and dispensing data to estimate the percentage of days since the previous visit that pills were taken.
DISCUSSION
Clinical trials in 4 different populations have provided strong evidence that daily oral PrEP is efficacious in preventing HIV infection.1–4 In each of these trials, analysis of blood tenofovir concentrations provided strong supporting objective evidence that PrEP taking behaviors, indicated by detectable tenofovir, conferred high levels of HIV protection. Our analysis of tenofovir concentrations at the first HIV-infected visit in the Partners PrEP Study showed HIV protection of 88% for TDF and 91% for FTC/TDF for participants with high adherence at steady-state levels (plasma tenofovir >40 ng/mL). This high level of protection was attributed to consistently high-drug concentrations from the majority of participants throughout follow-up. High concentrations were associated with older age and shorter time on study; plasma tenofovir concentrations ≤40 ng/mL occurred more commonly during study intervals when participants reported no sex with their HIV-infected partner, suggesting that participants' adherence to PrEP reflected their exposure and perceived risk.
A modeling study in MSM combined data on intracellular drug levels (concentration of tenofovir diphosphate, TFV-DP, in peripheral blood mononuclear cells) from a study with directly observed dosing of FTC/TDF to intracellular TFV-DP concentrations associated with HIV protection from a randomized clinical trial of PrEP. HIV transmission risk reduction for MSM was estimated to be 76% for 2 doses per week, 96% for 4 doses per week, and 99% for 7 doses per week.18 Our estimated efficacy for concentrations >40 and >10 ng/mL are comparably high, although we did not observe a trend with drug concentration, largely because risk reduction associated with any detectable tenofovir was very high in our context of >80% controls with detectable drug. Results from drug concentrations studies in PrEP trials consistently find very high levels of protection from HIV acquisition associated with the presence of drug, supporting high biologic efficacy of these drugs to prevent HIV.
Adherence behavior was largely consistent over time within individuals in our study: early nonadherers who were not using PrEP after 1 month continued as nonusers, and those who had high tenofovir concentrations at month 1 continued to be adherent to PrEP. Similar patterns of persistent adherers and early nonadherers have been observed for daily medication in other prevention fields.19,20 For implementation, our results might imply that persons who decide to initiate PrEP for HIV prevention will likely return for their PrEP drugs and use consistently (with counseling and support), whereas those not currently interested in PrEP may be unlikely to initiate or return for refills. These patterns of consistent use support the potential for cost effectiveness of targeted PrEP to those who are motivated to use PrEP.21–24 The high consistent adherence may be due in part to the context of a mutually disclosed serodiscordant relationship in the Partners PrEP Study, where partner support and known risk of HIV infection facilitate consistent adherence to a daily pill regimen.25,26 The success of a daily prevention medication among HIV serodiscordant couples motivates exploring where support for adherence behaviors from a friend or family member as an adherence “buddy” may have a similar effect for persons receiving PrEP outside an explicit couples setting.
High adherence, defined by plasma tenofovir concentrations, was related to older age, farming, and shorter length of time on study. Older age and shorter duration of use are similarly predictors of adherence in cardiovascular prevention27–31; participants whose primary income was from farming may have had less travel and time otherwise away from home that could disrupt daily pill taking. Notably, persons reporting no sex with their HIV-infected partner in the previous month had decreased adherence. A previous analysis from this same clinical trial found a similar association of lower adherence to PrEP during times with no sex, with adherence measured using electronic pill bottle openings, and unannounced home visit pills count.7 Together, these results suggest that periods of lower adherence to PrEP corresponded to periods of no sexual risk, a rational response to reduced risk of HIV exposure, raising the possibility that in implementation, PrEP users may pause use when not sexually active. Future work on adherence counseling, together with PK studies to inform drug timing, will be needed to determine whether to urge continued PrEP use even while not sexually active or to support discontinuation but develop approaches that ensure PrEP is restarted before re-initiation of sex.
Importantly, given the lack of efficacy of FTC/TDF and/or oral TDF in the FemPrEP and VOICE trials,5,6 high efficacy was observed with oral TDF in women in the Partners PrEP Study, which was comparable with FTC/TDF and did not differ from that of men. The lower protective effect for HIV associated with lower plasma tenofovir concentrations in women assigned to the TDF arm could be a chance imbalance. However, it could also suggest that protection against HIV acquisition for women using an oral TDF-only regimen is more significantly impacted than with oral FTC/TDF PrEP, if doses are missed. A PK study of a single dose of FTC/TDF in women showed similar plasma concentrations of tenofovir and FTC but 10-fold lower concentrations of tenofovir than FTC in cervicovaginal fluids and vaginal tissues at all time points.13 Our finding, in which efficacy is strongly associated with tenofovir >40 ng/mL but not with tenofovir >10 ng/mL, suggests that HIV protection in women with daily oral TDF may be less forgiving of missed dosing than FTC/TDF because plasma concentrations ≤40 ng/mL likely indicate a last dose more than 2 days ago.13
Importantly for clinical trials, participant-dependent measures of adherence, for example, self-reported and pill counts based on returned medication, can be substantially higher than demonstrated by biologic adherence measures.5,6,32 Specifically, in VOICE, adherence was 84%–88% by pill counts but 29%–30% by detectable tenofovir (≥0.31 ng/mL); in FEMPrEP, 88% by pill counts and 35% by detectable tenofovir (≥10 ng/mL); and in iPrEx, 89% by pill count and 51% by detectable tenofovir (≥10 ng/mL).1,5,6 Partners PrEP had 15% of visits with high adherence by pill count, yet undetectable tenofovir. We also found, as previously reported from an HIV prevention trial with twice daily dosing,32 that >100% adherence by pill count may indicate lower adherence or dumped study drug, as indicated by lower detectable drug concentrations. Understanding the reasons why pill counts can be influenced by participant's desire to seem adherent—thus over-estimating adherence—potentially include social desirability and/or fear of consequences in clinical trial participation; eliciting motivations for these behaviors remains a critical first step in developing and implementing effective interventions to enhance adherence and accuracy of self-reported adherence in clinical trials. Importantly, effectiveness trials of self-administered medication still rely on monitoring adherence using participant-dependent measures.33 The over-estimation of near-perfect adherence by pill count may indicate that interim evaluations of objective adherence measures, such as drug levels, are important adjunct measures to monitor whether there is sufficient uptake of the study drug to evaluate efficacy. Objective behavioral measures, such as electronic monitoring, in a subset of participants may be needed to study patterns of adherence.
A limitation of plasma concentrations as a biomarker of adherence is that extrapolation to degree of adherence over time relies on the assumption that a single point in time is a reliable indicator of behavior over the preceding time interval—in particular, “white-coat” dosing would result in high plasma levels that could, for example, explain tenofovir concentrations observed in seroconverters. In addition, concentrations in plasma do not measure cellular uptake and phosphorylation to the active metabolites, thus our data cannot be used to model risk reduction for different dosing intensities.
In summary, tenofovir concentrations in plasma, which were consistent with daily dosing, were highly predictive of protection from HIV. In a population at known risk for infection from an HIV-infected partner who was aware of their study participation and could provide adherence support, participants had consistent patterns of adherence over multiple time points, with the majority able to achieve and sustain PrEP adherence. These data strongly support the use of PrEP for the prevention of HIV infection in heterosexual men and women.
ACKNOWLEDGMENTS
We thank the study participants and the study team.
APPENDIX 1. Partners PrEP Study Team
University of Washington Coordinating Center and Central Laboratories, Seattle, WA: Connie Celum (principal investigator, protocol co-chair), Jared M. Baeten (medical director, protocol co-chair), Deborah Donnell (protocol statistician), Robert W. Coombs, Jairam R. Lingappa, M. Juliana McElrath. Study sites and site principal investigators: Eldoret, Kenya (Moi University, IN University): Kenneth H. Fife, Edwin Were; Kabwohe, Uganda (Kabwohe Clinical Research Center): Elioda Tumwesigye; Jinja, Uganda (Makerere University, University of Washington): Patrick Ndase, Elly Katabira; Kampala, Uganda (Makerere University): Elly Katabira, Allan Ronald; Kisumu, Kenya (Kenya Medical Research Institute, University of California San Francisco): Elizabeth Bukusi, Craig R. Cohen; Mbale, Uganda (The AIDS Support Organization, CDC-Uganda): Jonathan Wangisi, James D. Campbell, Jordan W. Tappero; Nairobi, Kenya (University of Nairobi, University of Washington): James Kiarie, Carey Farquhar, Grace John-Stewart; Thika, Kenya (University of Nairobi, University of Washington): Nelly R. Mugo; Tororo, Uganda (CDC-Uganda, The AIDS Support Organization): James D. Campbell, Jordan W. Tappero, Jonathan Wangisi. Data management was provided by DF/Net Research, Inc. (Seattle, WA) and site laboratory oversight was provided by Contract Laboratory Services (CLS) of the Wits Health Consortium (University of the Witwatersrand, Johannesburg, South Africa).
Footnotes
The Bill and Melinda Gates Foundation (grants 47,674 and OOPS52516) provided financial support for this study.
The authors have no conflicts of interest to disclose.
Presented in part as Paper #30 at the 19th Conference on Retroviruses and Opportunistic Infections, March 5–8, 2012, Seattle, WA.
D.D. and J.B. conducted the analysis and wrote the article. C.C. and J.M.B. were the lead investigators of this study and involved in study design, analysis, interpretation of results, and critically reviewing the article. N.N.B. and C.H. conducted the plasma testing. A.M., D.R.B., and J.E.H. were involved in implementation of the study and were involved in interpretation of the results. All authors reviewed and provided comments on the study protocol and article and approved the final article version.
For a complete listing of participating members of Partners PrEP Study Team, see Appendix 1.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
REFERENCES
- 1.Grant RM, Lama JR, Anderson PL, et al. Preexposure Chemoprophylaxis for HIV prevention in men who have sex with men. New Engl J Med. 2010;363:2587–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434 [DOI] [PubMed] [Google Scholar]
- 3.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083–2090 [DOI] [PubMed] [Google Scholar]
- 4.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marrazzo J, Ramjee G, Nair G, et al. Pre-Exposure Prophylaxis for HIV in Women: Daily Oral Tenofovir, Oral Tenofovir/Emtricitabine, or Vaginal Tenofovir Gel in the VOICE Study (MTN 003). 20th Conference on Retroviruses and Opportunistics Infections, 3–6 March 2013. Atlanta, GA
- 6.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haberer JE, Baeten JM, Campbell J, et al. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med. 2013;10:e1001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keller MJ, Madan RP, Torres NM, et al. A randomized trial to assess anti-HIV activity in female genital tract secretions and soluble mucosal immunity following application of 1% tenofovir gel. PLoS One. 2011;6:e16475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karim SS, Kashuba AD, Werner L, et al. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: implications for HIV prevention in women. Lancet. 2011;378:279–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women [Supplemental Material]. N Engl J Med. 2012;367:399–410 Available at: http://www.nejm.org/doi/full/10.1056/NEJMoa1108524. Accessed September 9, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blum MR, Chittick GE, Begley JA, et al. Steady-state pharmacokinetics of emtricitabine and tenofovir disoproxil fumarate administered alone and in combination in healthy volunteers. J Clin Pharmacol. 2007;47:751–759 [DOI] [PubMed] [Google Scholar]
- 12.Kiser JJ, Fletcher CV, Flynn PM, et al. Pharmacokinetics of antiretroviral regimens containing tenofovir disoproxil fumarate and atazanavir-ritonavir in adolescents and young adults with human immunodeficiency virus infection. Antimicrob Agents Chemother. 2008;52:631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patterson KB, Prince HA, Kraft E, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3:112re114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kakuda TN, Scholler-Gyure M, De Smedt G, et al. Assessment of the steady-state pharmacokinetic interaction between etravirine administered as two different formulations and tenofovir disoproxil fumarate in healthy volunteers. HIV Med. 2009;10:173–181 [DOI] [PubMed] [Google Scholar]
- 15.Breslow NE, Lumley T, Ballantyne CM, et al. Improved Horvitz-Thompson estimation of model Parameters from two-phase Stratified samples: Applications in Epidemiology. Stat Biosci. 2009;1:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lumley T. Complex Surveys: A Guide to Analysis Using R. Hoboken, NJ: John Wiley; 2010 [Google Scholar]
- 17. Survey: analysis of complex survey samples[computer program]. R package version 3.28. Lumley T, 2012.
- 18.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4:151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gueorguieva R, Wu R, Krystal JH, et al. Temporal patterns of adherence to medications and behavioral treatment and their relationship to patient characteristics and treatment response. Addict Behav. 2013;38:2119–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riegel B, Lee CS, Ratcliffe SJ, et al. Predictors of objectively measured medication nonadherence in adults with heart failure. Circ Heart Fail. 2012;5:430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schackman BR, Eggman AA. Cost-effectiveness of pre-exposure prophylaxis for HIV: a review. Curr Opin HIV AIDS. 2012;7:587–592 [DOI] [PubMed] [Google Scholar]
- 22.Gomez GB, Borquez A, Case KK, et al. The cost and impact of scaling up pre-exposure prophylaxis for HIV prevention: a systematic review of cost-effectiveness modelling studies. PLoS Med. 2013;10:e1001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walensky RP, Park JE, Wood R, et al. The cost-effectiveness of pre-exposure prophylaxis for HIV infection in South African women. Clin Infect Dis. 2012;54:1504–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hallett TB, Baeten JM, Heffron R, et al. Optimal uses of antiretrovirals for prevention in HIV-1 serodiscordant heterosexual couples in South Africa: a modelling study. PLoS Med. 2011;8:e1001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ware NC, Wyatt MA, Haberer JE, et al. What's love got to do with it? Explaining adherence to oral antiretroviral pre-exposure prophylaxis for HIV-serodiscordant couples. J Acquir Immune Defic Syndr. 2012;59:463–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Laughlin KN, Wyatt MA, Kaaya S, et al. How treatment partners help: social analysis of an African adherence support intervention. AIDS Behav. 2012;16:1308–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naderi SH, Bestwick JP, Wald DS. Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376,162 patients. Am J Med. 2012;125:882 e881–887 e881 [DOI] [PubMed] [Google Scholar]
- 28.Lewey J, Shrank WH, Bowry AD, et al. Gender and racial disparities in adherence to statin therapy: a meta-analysis. Am Heart J. 2013;165:665–678, 678 e661 [DOI] [PubMed] [Google Scholar]
- 29.Cheetham TC, Niu F, Green K, et al. Primary nonadherence to statin medications in a managed care organization. J Manag Care Pharm. 2013;19:367–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bardel A, Wallander MA, Svardsudd K. Factors associated with adherence to drug therapy: a population-based study. Eur J Clin Pharmacol. 2007;63:307–314 [DOI] [PubMed] [Google Scholar]
- 31.Ho PM, Bryson CL, Rumsfeld JS. Medication Adherence: Its Importance in cardiovascular outcomes. Circulation. 2009;119:3028–3035 [DOI] [PubMed] [Google Scholar]
- 32.Donnell DJ, Baeten JM, Hong T, et al. Correlation between pill counts and biologic effects in an HIV-1 prevention clinical trial: implications for measuring adherence. AIDS Behav. 2013;17:632–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baisley K, Baeten JM, Hughes JP, et al. Summary measures of adherence using pill counts in two HIV prevention trials: the need for Standardisation in reporting. AIDS Behav. 2013;17:3108–3119 [DOI] [PMC free article] [PubMed] [Google Scholar]


