Abstract
Objectives
In classifying Crohn’s disease (CD) location, proximal (L4) disease includes esophagogastroduodenal (EGD) and jejunal disease. Our aim was to determine the influence of proximal disease on outcomes of behavior and need for surgery and to determine if there was significant clinical heterogeneity between EGD and jejunal disease.
Methods
We performed a cross-sectional query of the NIDDK IBD Genetics Consortium (IBDGC) database of patients with a confirmed diagnosis of CD and phenotyped per the IBDGC manual. Presence of any L4, L4-EGD, L4-jejunal and non-L4 disease (L1-ileal, L2-colonic, and L3-ileocolonic) was compared with demographic features including age, race, ethnicity, smoking and IBD family history, diagnosis age, disease duration, clinical outcomes of inflammatory, stricturing or penetrating behavior, and CD abdominal surgeries. Univariate and multivariable analyses were performed with R.
Results
Among 2105 patients with complete disease location data, 346 had L4 disease (175 L4-EGD, 115 L4-jejunal, and 56 EGD and jejunal) with 321 having concurrent L1-L3 disease. 1759 had only L1-L3 disease. L4 vs. non-L4 patients were more likely (p<0.001) to be younger at diagnosis, non-smokers, have co-existing ileal involvement and have stricturing disease. L4- jejunal vs. L4-EGD patients were at least twice as likely (p<0.001) to have had ileal disease, stricturing behavior, and any or multiple abdominal surgeries. Remarkably, L4-jejunal patients had more (p<0.001) stricturing behavior and multiple abdominal surgeries than non-L4 ileal disease patients. Logistic regression showed stricturing risks were ileal (without proximal) site (OR 3.18; 95% CI 2.23-4.64), longer disease duration (OR 1.33/decade; 1.19-1.49), jejunal site (OR 2.90; 1.89-4.45), and older age at diagnosis (OR 1.21/decade; 1.10-1.34). Multiple surgeries risks were disease duration (OR 3.74/decade; 3.05-4.64), penetrating disease (OR 2.60; 1.64-4.21), and jejunal site (OR 2.39; 1.36-4.20), with short duration from diagnosis to first surgery protective (OR 0.87/decade to 1st surgery; 0.84-0.90).
Conclusion
Jejunal disease is a significantly greater risk factor for stricturing disease and multiple abdominal surgeries than either EGD or ileal (without proximal) disease. The Montreal site classification should be revised to include separate designations for jejunal and EGD disease.
Introduction
Crohn’s disease (CD) results in transmural inflammation involving the colon, the small intestine, and occasionally the stomach and esophagus. Systems to classify disease location include the Vienna and Montreal classifications. In 1998, The Vienna classification was proposed.1 The Vienna classification classified disease into four categories by location: L1, terminal ileum (TI) without colonic involvement; L2, colonic involvement without TI involvement; L3, ileocolonic disease involving both the TI and colon; and L4, disease proximal to the TI without TI or colonic involvement. Because many patients have both L1-L3 and L4 disease, a new classification system was proposed. In 2005, the Montreal working party reclassified L4 disease as proximal disease that can coexist with L1- L3 disease.2
Disease site is associated with complicated disease and need for surgery. lleal disease is associated with stricturing and fistulizing CD phenotypes.3 Ileal disease is also associated with the need for CD-related surgery including small bowel resection, strictureplasty and abscess drainage.3 Most patients with proximal disease also have concurrent ileal disease. Proximal disease has also been found to be a risk factor for complicated disease and need for surgery.4,5 However, the role of proximal disease, independent of ileal disease has not been examined. Additionally, among those with proximal disease, the contribution of esophagogastroduodenal (EGD) versus jejunal involvement has not been evaluated.
We sought to examine the role of proximal CD on disease behavior and the need for abdominal surgery. We further aimed to examine the role of proximal CD with EGD involvement compared with jejunal disease. Finally, we aimed to examine the effect of EGD or jejunal disease independent of ileal involvement. We used a cross-sectional study design including information on disease location, behavior and surgery from the National Institute of Digestive and Kidney Disease Inflammatory Bowel Disease Genetics Consortium (NIDDK-IBDGC) to achieve these aims.
Methods
Population
We queried the phenotype database of the National Institute of Diabetes and Digestive and Kidney Diseases Inflammatory Bowel Disease Genetics Consortium (IBDGC) repository.6,7 This is a large database of patients with diagnosis of inflammatory bowel disease (IBD) recruited from the United States, Puerto Rico and Canada for genetic research studies. Requirements for case recruitment are any patients with a confirmed diagnosis of CD, ulcerative colitis (UC) or IBD undetermined type (IBDU) according to standardized diagnostic criteria.6 Phenotyping was performed by clinicians with experience in managing patients with inflammatory bowel disease and was conducted according to an established Phenotype Operating Manual. The validity of the phenotype classification using the definitions and procedures outlined in the manual was previously described.6 We included CD patients recruited between January 2003 and December 2011 who had complete data on the presence or absence of all proximal disease locations (esophagus, stomach, duodenum, and jejunum) and complete data to determine presence and absence of disease in the ileum and colon (i.e., L1, L2, or L3 locations).
Phenotyping – disease location and behavior
Please refer to the phenotyping manual in Supplement 1. For CD, disease location is confined to the maximal macroscopic involvement at any time during the disease course up to the time of phenotyping. Most patients were last phenotyped at the time of enrollment; very few patients (in our sample, only 2%) were rephenotyped after initial enrollment. Location is based on endoscopy (excluding wireless capsule endoscopy, which was not routinely available), radiographic (barium studies, CT and MRI), and operative reports. Patients are phenotyped as to whether macroscopic disease is present, absent or unknown for any or all of the following sites: EGD, jejunal, ileal, colorectal, and perianal locations. In contrast to the Montreal and Vienna classifications, ileal disease site defines any area of ileal involvement, and is not limited to the terminal ileum. Absence of a particular disease site requires that the patients have manifested no evidence of macroscopic disease at one or more endoscopic and/or radiographic examinations of the site categorized. Otherwise location is coded as unknown. Notably, phenotyping is based on available data; testing is performed according to clinical indication (e.g. a patient without upper gastrointestinal symptoms would not necessarily undergo an upper endoscopy). Additionally, the details regarding the modalities used for phenotyping were not recorded on a patient-per-patient basis.
Disease behavior is classified as B1 (inflammatory - disease without evidence of stricture or penetrating disease), B2 (stricturing – constant luminal narrowing with prestenotic dilation and/or obstructive symptoms, but without penetrating disease), and B3 (penetrating – bowel perforation, intra-abdominal fistula, inflammatory mass or abscess not related to a postoperative complication) as described previously.2,6 For surgeries, data is collected on the number of abdominal surgeries (bowel resection, strictureplasty or abscess drainage) and the number of years between diagnosis and the first surgery.
Demographics
Demographic information was entered for each study subject on sex, year of birth, race, tobacco use and ethnicity. Data on cases includes date of diagnosis, duration of disease (time from diagnosis until most recent available medical records at the time of phenotyping), family history of IBD (1st degree relative with IBD), smoking status at the time of diagnosis, disease location, disease behavior, and surgical history.
Statistical analyses
Five main disease location comparisons were analyzed with the outcomes of stricturing disease and surgery. These groups were 1) proximal (L4) disease versus non-L4 (L1-L3 without proximal) disease; 2) L4-EGD (EGD without jejunal) disease versus non-L4 disease; 3) L4-jejunal (jejunal without EGD) disease versus non-L4 disease; 4) L4-EGD versus L4-jejunal disease; 5) the role of proximal disease independent of ileal disease. Differences in mean age and disease duration by proximal disease status were compared with two-sided t-tests. Years from diagnosis to first surgery was analyzed by log rank test. Statistical significance was designated as p<0.01. Two-by-two and two-by-three comparison results for categorical variables were obtained by using Chi-squared tests. Finally, we created multivariable models of stricturing disease and surgery by proximal disease status and EGD versus jejunal involvement (with or without distal disease) accounting for all potential confounders (age, sex, race, smoking status at diagnosis, IBD family history in parents, siblings or children, and disease duration). All analyses were performed in R.8
Results
Characteristics of included patients by disease location
There were 1759 patients with non-L4 disease and 346 patients with L4 disease. Of these, patients, 175 (51%) had L4-EGD disease, while 115 (33%) had L4-jejunal disease. The complete flowchart of patients is presented in Figure 1.
Figure 1.

Flowchart of patient characteristics by disease location. CD, Crohn’s disease; L1, ileal location; L2, colonic location; L3, ileocolonic location; L4, proximal disease; EGD, esophagogastroduodenal
Proximal disease (any L4 disease) versus non-L4 disease
Patients with L4 disease had an earlier age at diagnosis (21.2 vs. 25.4 years; p<0.001) and were less likely to be smokers at the time of diagnosis (15% vs. 22%; p=0.003) (Table 1). L4 patients were less likely to have concurrent L2 disease (12% vs. 21%; p<0.001) and were more likely to have stricturing (B2) behavior (32% vs. 23%; p<0.001). Additionally, L4 patients were more likely to have had 2 or more abdominal surgeries than non-L4 patients (23% vs. 17%; p=0.007). Because there were so few patients with isolated L4 disease (without concurrent L1-L3 disease, see Figure 1), this group was not analyzed separately.
Table 1.
Disease site comparisons. Percentage in parentheses.
| Total (n=2105) | Non-L4 (n=1,759) | L4 (n=346) | L4 vs. Non-L4 | L4-EGD (n=175) | L4-EGD vs. Non-L4 | L4-Jej (n=115) | L4-Jej vs. Non-L4 |
|---|---|---|---|---|---|---|---|
| Females (m=0) | 941(53) | 161 (47) | p=0.02 | 91 (52) | p=0.76 | 51 (44) | p=0.07 |
| Race (m=5): | p=0.89 | p=0.33 | p=0.17 | ||||
| Caucasians | 1245 (71) | 250 (72) | 132 (75) | 79 (69) | |||
| African Americans | 260 (15) | 49 (14) | 25 (14) | 13 (11) | |||
| Other | 249 (14) | 47 (14) | 18 (10) | 23 (20) | |||
| Hispanics (m=14)1 | 255 (14) | 32 (9) | p=0.01 | 11 (6) | p=0.004 | 18 (16) | p=0.83 |
| Smoking at Diagnosis (m=45): | 382 (22) | 51 (15) | p=0.003 | 31 (18) | p=0.26 | 14 (12) | p=0.02 |
| 1st Degree Relative with IBD (m=176) | 294 (17) | 54 (16) | p=0.65 | 23 (13) | p=0.27 | 16 14) | p=0.52 |
| Age at Diagnosis – mean ± SD (m=0) | 25.4±12.3 | 21.2±11.4 | p<0.001 | 19.7±11.0 | p<0.001 | 23.0±10.7 | p=0.04 |
| Disease Duration (yr) – mean ± SD (m=0) | 10.1±9.9 | 9.2±9.4 | p=0.12 | 7.0±8.1 | p<0.001 | 11.5±9.4 | p=0.15 |
| Years from Diagnosis to First Surgery – mean ± SD (m=996) | 4.7±6.1 | 4.7±6.0 | p=0.96 | 4.9±5.8 | p=0.88 | 4.5±6.1 | p=0.76 |
| Distal Disease Location (m=81): | p=0.001* | p=0.06* | p<0.001* | ||||
| L1 | 520 (30) | 103 (30) | p=0.63 | 37 (21) | p=0.04 | 47 (41) | p=0.008 |
| L2 | 374 (21) | 41 (12) | p<0.001 | 35 (20) | p=0.93 | 3 (3) | p<0.001 |
| L3 | 809 (46) | 177 (51) | p=0.01 | 92 (53) | p=0.04 | 59 (51) | p=0.21 |
| Disease Behavior (m=25): | p=0.001* | p=0.03* | p<0.001* | ||||
| B1 | 829 (47) | 143 (41) | p=0.07 | 100 (57) | p=0.009 | 24 (21) | p<0.001 |
| B2 | 398 (23) | 111 (32) | p<0.001 | 32 (18) | p=0.25 | 52 (45) | p<0.001 |
| B3 | 513 (29) | 86 (25) | p=0.13 | 39 (22) | p=0.08 | 38 (33) | p=0.44 |
| No. of Abdominal Surgeries (m=15) – mean ± SD: | 0.8±1.0 | 1.1±1.3 | p=0.006 | 0.5±0.9 | p=0.01 | 1.6±1.6 | p<0.001 |
| 0 | 927 (53) | 167 (48) | p=0.17 | 113 (65) | p=0.003 | 29 (25) | p<0.001 |
| ≥1 | 821 (47) | 175 (51) | p=0.17 | 61 (35) | p=0.003 | 85 (74) | p<0.001 |
| ≥2 | 298 (17) | 80 (23) | p=0.007 | 24 (14) | p=0.32 | 44 (38) | p<0.001 |
Level of significance – p<0.01.
Missing patients included in denominator for all analyses.
EGD – esophageal, gastric or duodenal; Jej – jejunal; m – missing; SD – standard deviation.
Chi-squared test for 3×2 table
Hispanic – marked as separate field from race
L4-EGD disease versus non-L4 disease
Patients with L4-EGD disease were younger at diagnosis (19.7 vs. 25.4 years; p<0.001), had a shorter disease duration at phenotyping (7.0 vs. 10.1; p<0.001), were less likely to be of Hispanic ethnicity (6% vs. 14%; p=0.004), and had fewer abdominal surgeries (35% vs. 47%; p=0.003) than patients with non-L4 disease (Table 1). The distribution of concurrent disease location (L1, L2 or L3) as well as disease behavior (B1, B2 or B3) showed no differences between cases with L4-EGD disease and those with non-L4 disease.
L4-jejunal disease versus non-L4 disease
L4 jejunal patients were significantly less likely to have concurrent colonic (L2) disease (3% vs. 21%; p<0.001) than patients with non-L4 disease (Table 1). They were also more likely to have stricturing (B2) behavior (45% vs. 23%; p<0.001) and less likely to have inflammatory (B1) behavior (21% vs. 47%; p<0.001); rates of penetrating disease were not different. Finally, L4-jejunal patients were more likely to have had one or more abdominal surgeries (74% vs. 47%; p<0.001) as well as multiple abdominal surgeries (38% vs. 17%; p<0.001) than non-L4 patients. There were 56 patients with both jejunal and EGD disease. This group had disease behavior more consistent with L4-jejunal patients than L4-EGD patients (available upon request).
L4-jejunal versus L4-EGD disease
Patients with L4-jejunal disease were more likely to have concurrent ileal (L1) disease (41% vs. 21%; p=0.001) and less likely to have concurrent colonic (L2) disease (3% vs. 21%; p<0.001) (Fig. 2A), more likely to have stricturing disease (45% vs. 18%; p<0.001) less likely to have inflammatory disease (21% vs. 57%; p<0.001) (Fig. 2B), and more likely to have had one or more (74% vs. 35%; p<0.001) as well as multiple abdominal surgeries (38% vs. 14%; p<0.001) (Fig. 2C).
Figure 2.


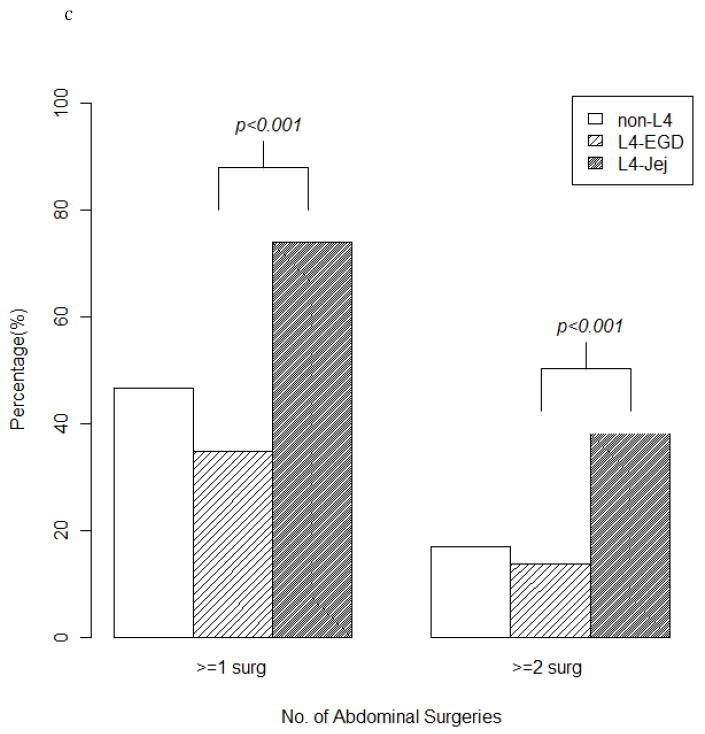
(a) Three groups (L4-EGD, L4-jejujnal, and non-L4 disease) comparing concurrent disease location. (b) Three groups (L4-EGD, L4-jejunal, and non-L4 disease) comparing disease behavior. (c) Three groups (L4-EGD, L4-jejujnal, and non- L4 disease) comparing abdominal surgery rates. EGD, esophagogastroduodenal; Jej, jejunal; L4, proximal disease.
Role of L4-jejunal disease independent of ileal disease
92% of patients with L4-jejunal disease had concurrent ileal disease (either L1 or L3). We compared L4-jejunal disease patients who had concurrent L1 or L3 disease with non-L4 patients with L1 or L3 disease. In the chi-squared model, patients with the L4-jejunal and ileal disease were still more likely to have stricturing behavior (P<0.001) in addition to multiple abdominal surgeries (P<0.001) than patients with ileal disease only.
Independent predictors of stricturing behavior and multiple abdominal surgeries
Two logistic regression models were created to calculate risks of stricturing (B2) behavior (Table 2) and 2 or more abdominal surgeries (Table 3). Statistically significant independent risk factors for stricturing behavior were ileal involvement (OR 3.18; 95% CI 2.23-4.64), longer duration of disease (OR 1.33 per decade; 1.19-1.49), L4-jejunal disease (OR 2.90; 1.89-4.45), and older age at diagnosis (OR 1.21 per decade; 1.10-1.34). Significant independent risk factors for 2 or more abdominal surgeries were increased duration of disease (OR 3.74 per decade; 3.05-4.64), presence of penetrating (B3) behavior (OR 2.60; 1.64-4.21), and L4-jejunal disease (OR 2.39; 1.36-4.20). Longer duration of time from diagnosis to the first surgery was protective (OR 0.87 per decade; 0.84-0.90). Results were similar for independent predictors of one or more abdominal surgeries (available upon request). L4-EGD disease was neither predictive of stricturing disease or multiple abdominal surgeries (p = 0.39 and p = 0.30, respectively).
Table 2.
Multivariable logistic analysis: predictors of stricturing (B2) behavior. Rows sorted by significance of estimation.
| (n=1810) | OR | 95% CI | P-value |
|---|---|---|---|
| Ileal involvement (L1+L3) | 3.18 | 2.23 -4.64 | <0.001 |
| Disease duration (decade) | 1.33 | 1.19-1.49 | <0.001 |
| L4-jejunal (vs. non-L4) | 2.90 | 1.89-4.45 | <0.001 |
| Age at diagnosis (decade) | 1.21 | 1.10-1.34 | <0.001 |
| Hispanic Ethnicity | 1.57 | 0.87-2.84 | 0.13 |
| L4-EGD (vs. non-L4) | 0.82 | 0.51-1.28 | 0.39 |
| African American race | 1.34 | 0.68-2.63 | 0.40 |
| Caucasian race | 1.19 | 0.66-2.16 | 0.57 |
| Female | 1.03 | 0.82-1.29 | 0.82 |
| Family history of IBD | 1.01 | 0.73-1.33 | 0.90 |
| Smoking at diagnosis | 1.01 | 0.77-1.33 | 0.93 |
Rows sorted by significance of estimation.
Level of significance – p<0.01.
OR – odds ratio; CI – confidence interval; IBD - inflammatory bowel disease; EGD - esophagogastroduodenal
Table 3.
Multivariable logistic analysis: predictors of multiple (≥2) abdominal surgeries. Rows sorted by significance of estimation.
| (n=954) | OR | OR 95% CI | P-value |
|---|---|---|---|
| Disease duration (decade) | 3.74 | 3.05-4.64 | <0.001 |
| Time from diagnosis to first surgery (decade) | 0.87 | 0.84-0.90 | <0.001 |
| Penetrating (B3) disease (vs. B1) | 2.60 | 1.64-4.21 | <0.001 |
| L4-jejunal (vs. non-L4) | 2.39 | 1.36-4.20 | 0.002 |
| Hispanics | 2.78 | 1.17-6.70 | 0.02 |
| African Americans | 2.85 | 1.04-8.06 | 0.04 |
| Caucasians | 2.53 | 1.05-6.37 | 0.04 |
| Ileal involvement (L1+L3) | 1.87 | 1.04-3.51 | 0.04 |
| L4-EGD (vs. non-L4) | 1.42 | 0.73-2.69 | 0.30 |
| Family history of IBD | 0.82 | 0.54-1.23 | 0.33 |
| Stricturing (B2) disease (vs. B1) | 1.24 | 0.74-2.08 | 0.42 |
| Smoking at diagnosis | 1.08 | 0.75-1.56 | 0.68 |
| Females | 1.05 | 0.76-1.46 | 0.76 |
| Age at diagnosis (decade) | 0.99 | 0.84-1.15 | 0.85 |
Level of significance – p<0.01.
OR- odds ratio; CI – confidence interval; IBD – inflammatory bowel disease; EGD - esophagogastroduodenal
Discussion
Patients with L4 disease were more likely to have stricturing behavior and multiple abdominal surgeries than patients with non-L4 disease. This association was only observed among patients with jejunal involvement (L4-jejunal) and not in patients with L4-EGD involvement. Furthermore, patients with L4-jejunal disease had higher rates of stricturing behavior and multiple abdominal surgeries than those with non-L4 ileal disease. In a logistic regression model, longer disease duration as well as L4-jejunal disease were independent risk factors for both stricturing disease and multiple abdominal surgeries. By contrast, L4-EGD disease was not predictive for these outcomes.
Although currently grouped together by the Montreal classification system as L4 disease, the natural history of L4-EGD and L4-jejunal disease are very different from one another. Only the latter is predictive of stricturing complications and need for multiple surgeries. For this reason, we propose that the Montreal Classification should be amended to reflect this important difference. In part based upon a preliminary report of these findings,9 the recent Paris classification of pediatric CD has incorporated this separation whereby L4a represents disease is proximal to the ligament of Treitz while L4b represents disease that is distal to the ligament of Treitz and proximal to the distal one-third of the ileum.10
The overall rates of L4 disease observed in the IBDGC cohort (16%) is similar to that found in other studies. Rates of macroscopic gastroduodenal disease seen in a large pediatric cohort11 ranged between 5% to 13%, while in a population cohort study of children and adults in Denmark, disease proximal to the ileum was found in 8% to 19% of CD patients.12 Likewise, other studies have found evidence that L4 disease may carry a predisposition for more aggressive disease. Wolters et al. prospectively studied 358 patients with CD beginning from diagnosis and examined the relationship between phenotype at diagnosis (based on the Vienna classification) and disease recurrence.4 Although only 20 (6%) patients had disease proximal to the TI, proximal disease was the only significant positive predictor for combined surgical and non-surgical disease recurrence in a Cox proportional hazard model (p<0.01). These findings were echoed in a cohort of 132 Chinese patients with CD, 30 (23%) of who had L4 disease at diagnosis as based on the Montreal classification.5 L4 phenotype was found to be associated with more stricturing (47%) and penetrating disease (30%) behavior than patients with non-L4 disease (19% and 4%, respectively; p<0.001), and patients with L4 disease were more likely to undergo major surgery (67%) than those without L4 disease (37%) (p<0.001). In a Cox proportional hazard model, L4 disease independently predicted hospitalization but not the need for major surgery. Finally, other studies have observed that patients with jejunoileal disease are more likely to require aggressive medical and nutritional therapy.13,14 Our study, with more than 10-fold the number of L4 patients than other reports, was the only study to divide proximal (L4) disease into esophagogastroduodenal versus jejunal involvement. By making this separation, we were able to show that overall predisposition of proximal disease towards greater risk of stricturing behavior and more surgery was solely due to the approximately two-fifths of L4 patients with jejunal involvement.
As previously noted, in contrast to the Vienna and Montreal classifications, the IBDGC phenotyping manual did not include ileal disease proximal to the TI as L4 disease. This was a matter of practicality: phenotyping for ileal site was primarily by small bowel series radiographic reports and in many cases, these reports do not distinguish the portion of ileum where disease involvement was found. By contrast, it is usually clear when disease involves the jejunum radiographically, and this site specificity is usually detailed in radiographic reports. By identifying jejunal disease as predictive of stricturing disease and multiple surgeries, a unique at-risk group has been identified. It is unknown whether proximal ileal disease would also have an independent risk of stricturing and multiple surgeries as we found for jejunal disease. Making this determination would likely require primary review of radiographs, ideally in a prospectively phenotyped cohort.
Our findings are important for two reasons. First, it may impact design and interpretation of future studies with regard to proximal disease site correlations with genotypes, serology and other biomarkers. Second, patients with L4-jejunal disease may be an attractive at-risk target population for future studies of “top-down” approaches to medical management of CD because of their high rates of requiring multiple abdominal surgeries.
Although we found a strong association between jejunal involvement and stricturing disease multiple abdominal surgeries, causality remains unclear. Patients with jejunal CD could be uniquely prone to the development of strictures in the jejunum; however, there is no obvious mechanical or pathophysiologic reason why jejunal disease should be any different from ileal disease. A more likely explanation is that jejunal disease is a marker for more extensive small bowel involvement. The high rate of concurrent ileal disease among L4-jejunal patients further supports this theory but this needs to be confirmed by independent studies. Note that the effect of L4-jejunal disease on multiple surgeries was not a result of requiring earlier surgical intervention: time from diagnosis to first surgery for L4-jejunal patients was no shorter than that for L4-EGD or non-L4 patients.
Notably, smoking at the time of diagnosis was not found to be an independent risk factor for either stricturing behavior or multiple abdominal surgeries. Prior studies have shown smoking strongly associated with both outcomes.15,16 Univariate analysis did find that smoking at diagnosis is strongly associated with both stricturing behavior (OR 1.59 (1.22-2.09), P=0.001) and multiple abdominal surgeries (OR 1.97 (1.53-2.53), P<0.001). However, because smoking was also highly associated in univariate analyses with disease duration, greater age at diagnosis, and penetrating disease, it did not show up as an independent predictor in multivariable analyses.
Limitations
The modalities used for phenotyping were not recorded on a patient-by-patient basis. Instead, tests were performed based on clinical indication. Because not all patients underwent the most sensitive test available for picking up disease in a given location, rates of L4-jejunal disease were probably underestimated. However, the disease which was detected was probably more clinically relevant. Furthermore, rates of detecting L4-jejunal Crohn’s did not increase over time as one might predict with the adoption of newer more sensitive imaging techniques such as CT or MR enterography (data available upon request). Other limitations include 1) Inter-rater reliability was found to be only fair for EGD disease (Kappa statistic 0.36).6 By contrast, inter-rater reliability for jejunal disease was rated as good with an overall Kappa statistic of 0.66, similar to ileal and colonic disease and 2) Because most patients were only phenotyped on one occasion, it is possible that L4 disease developed after requiring abdominal surgery; however, we think this scenario would be atypical.
In conclusion, the disease course of patients with L4 disease differs significantly depending on whether or not the disease involves the jejunum. L4-jejunal disease, but not L4-EGD was independently predictive of stricturing phenotype and multiple abdominal surgeries. We therefore recommend that the Montreal classification for adults be modified to separate L4 disease into different subcategories.
Supplementary Material
Study Highlights.
What is current knowledge:
Small studies show proximal Crohn’s disease location having increased history of surgery for Crohn’s disease and complicated disease behavior.
Most proximal disease occurs with concurrent ileal, ileocolonic or colonic disease involvement.
What is new here:
A large study confirms proximal disease as having significant risk for stricturing behavior and multiple abdominal surgeries.
Proximal disease limited to the jejunum has a different clinical course than proximal disease limited to the esophagus, stomach or duodenum (EGD disease): jejunal but not EGD disease is associated with ileal involvement, stricturing behavior and any or multiple surgeries for Crohn’s disease.
In patients with ileal (L1 or L3) disease site, the additional presence of jejunal disease significantly increases the risk for stricturing behavior or multiple abdominal surgeries than does ileal site without any proximal disease.
Acknowledgments
We are indebted to the patients and their families with IBD who gave of themselves to participate in this study and make this study possible. We gratefully acknowledge assistance with the following individuals for assistance with recruitment and patient phenotyping for Cedars Sinai Medical Center Esther Torres (University of Puerto Rico); Assistance with Johns Hopkins Satellite Recruitment from Ann Silverman, MD (Henry Ford Health System); Jason Hou, MD (Baylor University); Kim L. Isaacs MD (University of North Carolina); John F. Valentine MD (University of Florida); John J. Kuemmerle MD (Virginia Commonwealth University); James D. Lewis, MD (University of Pennsylvania); Michael S. Gold MD, (Washington Hospital Center); Duane T. Smoot MD, (Howard University); Ioannis Oikonomou, MD, (Yale University); Theodore M. Bayless MD, (Johns Hopkins University), Lisa Datta, MS (Johns Hopkins University), Patricia Ushry (Johns Hopkins University) and Ming-Hsi Wang, MD. We thank the Quebec Inflammatory Bowel Disease Genetic Consortium and the following individuals for their participation in this study: Edmond Jean Bernard, M.D., F.R.C.P. (Hôpital Hôtel Dieu), Albert Cohen, M.D., F.R.C.P.(C.) (Jewish General Hospital), Guy Aumais, M.D., C.S.P.Q., F.R.C.P. (Hôpital Maisonneuve Rosemont), Gilles Jobin, M.D., F.R.C.P., M.Sc., Diane Langelier, M.D. (Centre Hospitalier Universitaire de Sherbrooke), Colette Deslandres, M.D. (Hôpital Sainte-Justine), Daniel Gaudet, M.D., Ph.D. (Centre Hospitalier de la Sagamie-Chicoutimie), and Sylviane Forget, M.D., F.R.C.P.(C.) (Montreal Children’s Hospital). We would like to express our gratitude to the following physicians from the University of Toronto: Zane Cohen, M.D., Robin McLeod, M.D., and Gordon R. Greenberg, M.D.
Financial support: This study was supported by Harvey M. and Lynn P. Meyerhoff Inflammatory Bowel Disease Center and the Morton Hyatt Family (ML, SH, SRB); NIH Grants DK62422 and DK62429), DK62420 (RHD); DK62432 (JDR); DK62423 (MSS); and DK62413 (KDT and DPM).
Footnotes
Specific author contributions: Study conception, design, supervision, data analysis and interpretation, and writing of the first draft: Mark Lazarev; Statistical analysis, Data Interpretation and writing first draft: Chengrui Huang; Patient recruitment, phenotyping and administrative support: Alain Bitton, Judy H. Cho, Richard H. Duerr, Dermot P. McGovern, Deborah D. Proctor, Miguel Regueiro, John D. Rioux, Kent D. Taylor, Mark S. Silverberg, A. Hillary Steinhart; Database design: Phillip P. Schumm; Study design, statistical analysis, data interpretation and writing first draft: Susan Hutfless; Study design, supervision, data interpretation, administrative support and writing first draft: Steven R. Brant. All authors were involved in critical revision of the paper for important intellectual content.
CONFLICT OF INTEREST: none
Guarantor of the article: Mark Lazarev
References
- 1.Gasche C, Scholmerich J, Brynskov J, et al. A simple classification of Crohn’s disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis. 2000;6(1):8–15. doi: 10.1097/00054725-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(Suppl A):5–36. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 3.Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann Surg. 2000;23(1):38–45. doi: 10.1097/00000658-200001000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolters FL, Russel MG, Sijbrandij J, et al. Phenotype at diagnosis predicts recurrence rates in Crohn’s disease. Gut. 2006;55:1124–30. doi: 10.1136/gut.2005.084061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow DK, Sung J, Yu JC, et al. Upper gastrointestinal tract phenotype of Crohn’s disease is associated with early surgery and further hospitalization. Inflamm Bowel Dis. 2009;15:551–7. doi: 10.1002/ibd.20804. [DOI] [PubMed] [Google Scholar]
- 6.Dassopoulos T, Nguyen GC, Bitton, et al. Assessment of reliability and validity of IBD phenotyping within the National Institues of Diabetes and Digestive and Kidney Diseases (NIDDK) IBD Genetics Consortium (IBDGC) Inflamm Bowel Dis. 2007;13:975–83. doi: 10.1002/ibd.20144. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen GC, Torres EA, Reguiero M, et al. Inflammatory bowel disease characteristics among African Americans, Hispanics, and non-Hispanic whites: Characterization of a large North American Cohort. Am J Gastroenterol. 2006;101:1012–23. doi: 10.1111/j.1572-0241.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- 8.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2008. URL http://www.R-project.org. [Google Scholar]
- 9.Lazarev M, Hutfless S, Bitton A, et al. Divergence in L4 Crohn’s disease: jejunal, not esophagogastroduodenal involvement, is protective of L2 disease location, and a risk for stricturing behavior and multiple abdominal surgeries. Report from the NIDDK-IBDGC Registry; Presented at Digestive Disease Week; New Orleans. May 2010; abstract 760. [Google Scholar]
- 10.Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011;17(6):1314–21. doi: 10.1002/ibd.21493. [DOI] [PubMed] [Google Scholar]
- 11.Heyman MB, Kirschner B, Gold B, et al. Children with early-onset inflammatory bowel disease (IBD): Analysis of a pediatric IBD consortium registry. J Pediatr. 2005;146:35–40. doi: 10.1016/j.jpeds.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 12.Jess T, Riis L, Vind I, et al. Changes in clinical characteristics, course, and prognosis of inflammatory bowel disease during the last 5 decades: A population-based study from Copenhagen, Denmark. Inflamm Bowel Dis. 2007;13(4):481–9. doi: 10.1002/ibd.20036. [DOI] [PubMed] [Google Scholar]
- 13.Higuero T, Merle C, Thiefin G, et al. Jejunoileal Crohn’s disease: a case-control study. Gastroenterol Clin Biol. 2004;28:160–66. doi: 10.1016/s0399-8320(04)94871-3. [DOI] [PubMed] [Google Scholar]
- 14.Attard TM, Horton KM, DeVito K, et al. Pediatric Jejunoileitis: A severe Crohn’s disease phenotype that requires intensive nutritional management. Inflamm Bowel Dis. 2004;10(4):357–60. doi: 10.1097/00054725-200407000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Cosnes J, Carbonnel F, Beaugerie L, et al. Effects of cigarette smoking on the long-term course of Crohn’s disease. Gastroenterology. 1996;110(2):424–31. doi: 10.1053/gast.1996.v110.pm8566589. [DOI] [PubMed] [Google Scholar]
- 16.Timmer A, Sutherland LR, Martin F. Oral contraceptive use and smoking are risk factors for relapse in Crohn’s disease. The Canadian Mesalamine for Remission of Crohn’s Disease Study Group. Gastroenterology. 1998;114(6):1143–50. doi: 10.1016/s0016-5085(98)70419-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


