Abstract
Chemotherapy-related cognitive deficits are a major neurological problem, but the underlying mechanisms are unclear. The death of neural stem/precursor cell (NSC) by cisplatin has been reported as a potential cause, but this requires high doses of chemotherapeutic agents. Cisplatin is frequently used in modern oncology, and it achieves high concentrations in the patient’s brain. Here we report that exposure to low concentrations of cisplatin (0.1µM) causes the loss of dendritic spines and synapses within 30 minutes. Longer exposures injured dendritic branches and reduced dendritic complexity. At this low concentration, cisplatin did not affect NSC viability nor provoke apoptosis. However, higher cisplatin levels (1µM) led to the rapid loss of synapses and dendritic disintegration, and neuronal--but not NSC—apoptosis. In-vivo treatment with cisplatin at clinically relevant doses also caused a reduction of dendritic branches and decreased spine density in CA1 and CA3 hippocampal neurons. An acute increase in cell death was measured in the CA1 and CA3 neurons, as well as in the NSC population located in the subgranular zone of the dentate gyrus in the cisplatin treated animals. The density of dendritic spines is related to the degree of neuronal connectivity and function, and pathological changes in spine number or structure have significant consequences for brain function. Therefore, this synapse and dendritic damage might contribute to the cognitive impairment observed after cisplatin treatment.
Keywords: cisplatin, chemotherapy, hippocampal dendritic spines, postsynaptic density-95 protein (PSD95), neural stem/precursor cells (NSCs), neuronal apoptosis, annexin V
INTRODUCTION
The cognitive deficits observed after treatment with chemotherapeutic drugs are a significant clinical problem, with a rapidly increasing impact on the quality of life of millions of Americans. One and a half million people are diagnosed with cancer every year in the US, and more than 60% survive for 20 years or more. 17–75% of the cancer survivors have persistent memory problems years after completion of chemotherapy (Hodgson et al., 2013; Myers, 2012). Cisplatin is frequently used in modern oncology, and it achieves high concentrations in the patient’s brain (Nakagawa et al., 1996). As such, cisplatin comes in contact with the normal neuronal populations of the brain -- including the neural stem cells (NSCs) and differentiated hippocampal neurons. Published studies report cognitive impairments in testicular (Schagen et al., 2008) and head and neck (Gan et al., 2011) cancer patients receiving chemotherapy regimens containing cisplatin. Our previous work showed that in-vitro, low-doses of cisplatin (2 µM) kills 50% of the human NSC, while a dose five times higher (10 µM) is required in order to kill 50% of patient-derived cancer (malignant glioma) stem-like cells (Gong et al., 2011).
In the hippocampus, excitatory synapses are located on tiny protrusions called dendritic spines. These dendritic spines are important because the molecular and cellular correlates of learning and memory are generally considered to take place at excitatory synapses (Kasai et al., 2010; Martin et al., 2000; Neves et al., 2008). Dendritic spines serve multiple roles; in addition to connectivity, they compartmentalize calcium and other signaling molecules, conferring specificity to changes in synaptic efficacy (Harris and Kater, 1994; Shepherd, 1996; Yuste, 2013). Hippocampal learning depends on these long-lasting changes in synaptic activity, and is influenced by the integrity of dendritic spines. Dendritic spines are dynamic and constantly changing in shape, size, and number in response to the environment. They are very sensitive to stress (Magarinos and McEwen, 1995) and the loss of dendritic integrity is reported in a number of neuro-degenerative conditions (Dumanis et al., 2009; Penzes et al., 2011). Only one study measured synaptic function in the presence of a chemotherapeutic agent and found an impairment in long-term potentiation (LTP) after a single-dose of cyclophosphamide in hippocampal slices (Lee et al., 2006), but no studies of dendritic spine integrity were conducted. Clinically, reduced hippocampal volume and loss of neurogenesis have been found in chemotherapy treated colon cancer patients (Schneiderman, 2004) and brain tumor patients (Monje et al., 2007), respectively. Since the disruption of dendritic spines is associated with cognitive impairments, it was reasonable to examine if low-doses of cisplatin altered spine integrity and potentially contributed to “chemo-brain.”
MATERIALS AND METHODS
Animals
All the data were generated using Sprague-Dawley rats (Harlan, Placentia, CA, USA). All experiments were approved by University Animal Care Committees and conformed to the NIH guidelines.
Dissociated hippocampal neuron and NSC cultures
Timed-pregnant Sprague Dawley rat dams gave birth in the UCI vivarium. Hippocampal neuron cultures were prepared on the day of pup birth (P0) from pups of either sex as previously described. Briefly, hippocampi were dissected and incubated in dissection solution (137 mM NaCl, 5.4 mM KCl, 0.17 mM Na2PO4, 0.22 mM KH2PO4, 33.3 mM D-glucose, and 43.8 mM sucrose in 9.9 mM HEPES pH=7.4) with 10 U/mL papain (Worthington). After removal of papain, cells were triturated and plated at a density of 400–600 cell/mm2 on 12 mm coverslips (Thermo Fisher) or (Sigma). Cultures were initially maintained in Neurobasal Medium (NBM) with B-27 (Invitrogen) at 36°C and 5% CO2. After 3–4 hours, half the culture medium was replaced with NBM pre-conditioned for 24 hours over 1–3 week old glia cell cultures (conditioned medium). Cultures were treated with 1µM arabinoside-cytosine (Sigma) on day in vitro 3 (DIV3) to inhibit glial proliferation and refreshed twice a week with conditioned medium.
Neurons were used for experiments on DIV17-21. The NSCs were isolated from the hippocampus of embryonic day 19 (E19) rats. The hippocampus was dissected from the fetal brain, and the cells were dispersed by incubation in 0.04% trypsin (Shetty, 2004). The cell suspension was plated onto poly-L-lysine coated dishes and maintained in DMEM+F12 medium with GlutaMAX, 20ng/mL of bFGF, 10ng/mL of EGF and StemPro neuronal supplement (Gibco). After 7 days in vitro the NSC were treated with different doses of cisplatin. RNSC-1 and RNSC-2 are two separate primary cultures, generated from individual pups from two distinctive litters. For both RNSC-1 and RNSC-2, every experimental point was repeated three times and the results presented are the means and SEM from three different experiments.
Immunocytochemistry (ICC)
Neurons were fixed with ice-cold 4% paraformaldehyde (PFA) in PBS pH=7.4 for 12 minutes. The following antibodies were used: mouse anti-PSD95 1:4000 (Thermo Fisher MA1-046), mouse anti-GFP 1:1000 (Sigma G6539) and mouse anti-Annexin V 1:400 in PBS (Abcam AB54775). The next day, coverslips were washed and incubated in the appropriate secondary antibodies conjugated to AlexaFluor488 or AlexaFluor594 at a concentration of 1:400 (Invitrogen). To calculate dendritic branching, mature hippocampal neurons (DIV 21) were fixed and coverslips incubated with the antibody against PSD95. 10–14 neurons per group were drawn using a Zeiss LSM Image Browser and Adobe Photoshop. Dendritic branching was evaluated using Sholl analysis, measuring total dendritic length and number of intersections at concentric circles at increasing distance from the soma (Chen et al., 2008). Spine density was quantified as the number of PSD-95 positive elements on dendritic branches. Spine density was expressed as the number of spines per 20 µm of dendrite length, comparing dendrites of the same order. More severe dendritic injury such as beading was also identified.
Green fluorescent protein (GFP) lentiviral infection
Recombinant lentiviruses expressing green fluorescent protein under the H1 promoter were produced by transient transfection in HEK293T cells. Supernatant was collected from transfected HEK293T cells and virus particles were titered to 2.5×105 particles per µL. Lentiviral infections were carried out on DIV13 and neurons were used on DIV17-21. All GFP expressing neurons were processed for anti-GFP ICC.
Quantitative RT-PCR (qRT-PCR) analysis
Total RNA was extracted using RNeasy Mini Kit (Qiagen, Germantown, MD, USA), and cDNA was generated using the iScript™ cDNA Synthesis Kit (Bio-rad, Hercules, CA, USA). Quantitative PCR reactions (iQ™ SYBR Green Supermix, Bio-rad) were conducted using a Bio-Rad CFX96 Real-time System, and the gene expression levels were normalized to those of GAPDH. The sequences for rat BDNF CDS and GAPDH primer sets were: BDNF CDS forward 5’-AAACGTCCACGGACAAGGCA-3’ reverse 5’-TTCTGGTCCTCATCCAGCAGC-3’ (Baj et al., 2013); GAPDH forward 5’-CCTTCATTGACCTCAACTACAT-3’, reverse 5’-CCAAAGTTGTCATGGATGACC-3’ (Suliman et al., 2003). All the primers were ordered from IDT, Integrated Device Technology, Inc., Coralville, Iowa, USA.
Chemotherapy application in-vivo and in-vitro
Young adult male Sprague-Dawley rats (250 gm) were treated with cisplatin dissolved in 0.9% NaCl (10 mg/kg, intraperitoneally) daily for two days. The control group was injected with an equal volume of 0.9% NaCl at the same time points. Three days after the last treatment, the animals were deeply anesthetized with sodium pentobarbital and then transcardially perfused with heparinized saline flush. In-vitro, cisplatin was made into a stock solution by dissolving in sterile water and diluting to a final concentration of 0.1µM or 1µM in neurobasal media (NBM+B27) or minimum essential media (DMEM+F12). After various time points the cells were fixed and the immunocytochemistry protocol was followed.
Dendritic arborization and spine visualization and quantification by Golgi staining
The rat brains were removed and immersed in Golgi-Cox solution for 9 days and in 30% sucrose for two days. Coronal sections through the hippocampus (200 µm) were processed in 10% NH4OH for 30 minutes and then fixed (Kodak Fix, Kodak). Individual Golgi-impregnated cells were reconstructed, and the dendritic branching as well as spines were quantified in the same way as for the in-vitro experiments described above.
Terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL Assay)
The TUNEL assay was performed using the NeuroTACS™ In Situ Apoptosis Detection Kit (Trevigen, Inc Gaithersburg, MD).
Systematic analyses and statistical considerations
Each experiment included 2–3 sister coverslips per treatment group, and neurons were sampled equally from each coverslip for imaging. At least 4 dendrites from a minimum of 2 separate neurons were analyzed per treatment group. Images were scaled for distance per pixel length, and the distance from the soma was measured and divided into 20 µm segments using ImageJ. For PSD95 quantification, each individual puncta was considered a separate spine and counts were not adjusted for puncta size. For GFP-expressing neurons, spines were defined by a clear neck and head protruding from the dendrite. Images for analysis were generated using confocal microscopy, Zeiss LSM 510 (Oberkochen, Germany). 3 µm z-series (0.5 µm steps) images were captured from dendrites that were distinct from other dendrites and dendritic crossings and extended at least 100 µm from the soma at 63× (NA 1.4) using an oil-immersion objective. Analysis of all treatment groups was accomplished using two-way repeated-measures (RM)-ANOVA. All RM-ANOVAs were followed by Bonferroni’s post-hoc multiple comparisons test. Significance levels were set at 0.05 and data are presented as the mean ± SEM. Data were analyzed using GraphPad Prism 5.0 Software.
RESULTS
Cisplatin reduces PSD95 puncta and dendritic spine density in a time- and dosedependent manner
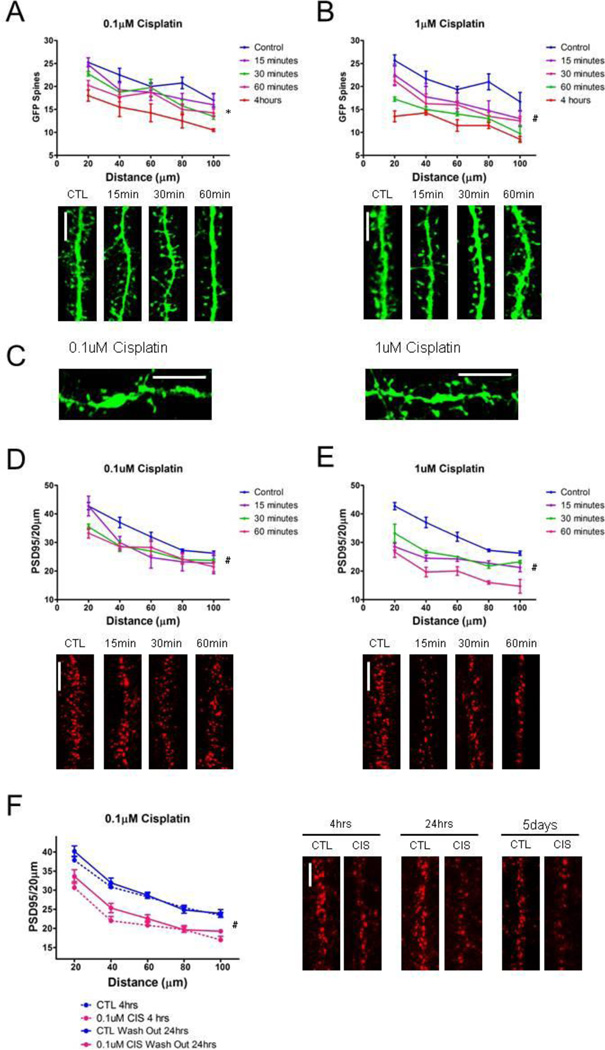
We treated cultured hippocampal neurons with cisplatin doses (0.1 µM and 1 µM) which were previously shown to be too low to reduce cancer (glioma stem) cell survival and/or proliferation (Gong et al., 2011). Hippocampal neurons are very sensitive to cisplatin, and even a very-low dose (0.1 µM) has early (30 minutes) and very pronounced effects on dendritic spine density. Cisplatin induces loss of excitatory synapses -- as quantified by using GFP expressing neurons to directly visualize dendritic spines (Figure 1A) and by a reduction in post-synaptic density-95 (PSD95) puncta (Figure 1D). A higher cisplatin dose (1 µM) causes a more rapid (as early as 15 minutes) and pronounced loss of GFP labeled spines and PSD95 puncta (Figure 1B and 1E). Very severe neuronal damage along with dendritic blebbing/beading is detected after four hours of treatment with either dose of cisplatin (Figure 1C).
Figure 1. Cisplatin reduces PSD95 puncta and dendritic spine density in a time- and dose-dependent manner.
(A) Graph and representative images of GFP filled spines depicting time-dependent (15–60 minutes) reduction in spine density after exposure to 0.1µM cisplatin (F(4,60) = 6.91, p = 0.002). (B) Dendritic spine loss is more prominent with a greater dose of cisplatin (1µM). The graph quantifying exposure to 1µM cisplatin at same time points shows a greater reduction in dendritic spines at all time points (F(4,56) = 7.66, p = 0.001). (C) Dendritic beading is evident at both doses at the 4 hour time point. (D) The loss of PSD95 occurs simultaneously to the reduction of dendritic spines. Graph quantifying the time-dependent reduction in PSD95 puncta after exposure to 0.1µM cisplatin (F(4,52) = 8.89, p = 0.001). Images of dendrites exposed to 0.1µM cisplatin and immunolabeled for PSD95. (E) Graph and representative images of dendrites immunolabeled for PSD95 depicting time-dependent reduction in PSD95 puncta after exposure to 1µM cisplatin (F(3,44) = 50.55, p < 0.001). Dendritic beading impeded quantification out to 100um for both doses at 4 hours, and therefore analysis was omitted. (F) Damage to dendritic spines by cisplatin is not reversible, as dendritic spine densities are significantly reduced after a 4 hour exposure to 0.1uM cisplain (F(1,88) = 32.43, p < 0.001) even after a 24 hour wash out period (F(1,88) = 22.78, p < 0.001). PSD95 density did not recover 5 days after washing out cisplatin, and low neuronal survival prohibited quantification at this time point. Scale bars approximately 5µm. (*p = 0.002, #p ≤ 0.001)
Cisplatin-induced loss of PSD95-labeled excitatory synapses is not reversible
In order to study chemotherapy administration in human patients, a model of limited drug exposure time was designed. As such, cultured hippocampal neurons were treated with cisplatin-containing medium (0.1 µM) for four hours, after which the medium was changed with fresh, drug free medium and the neurons were allowed to recover for twenty-four hours and for five days. Dendritic spines are often the first neuronal structure to be damaged in a toxic environment, but recover rapidly (on the order of minutes to an hour) in response to other insults such as stress and ischemia (Chen et al., 2008; Zhang et al., 2005). Damage to dendritic spines by cisplatin is not reversible, as dendritic spine densities are significantly reduced after a 4 hour exposure to 0.1µM cisplatin and no recovery is seen at either 24 hours or at five-days after the wash out (Figure 1F). The irreversibility of this dendritic spine damage might explain why the cognitive deficits persist in patients even years after they finish chemotherapy.
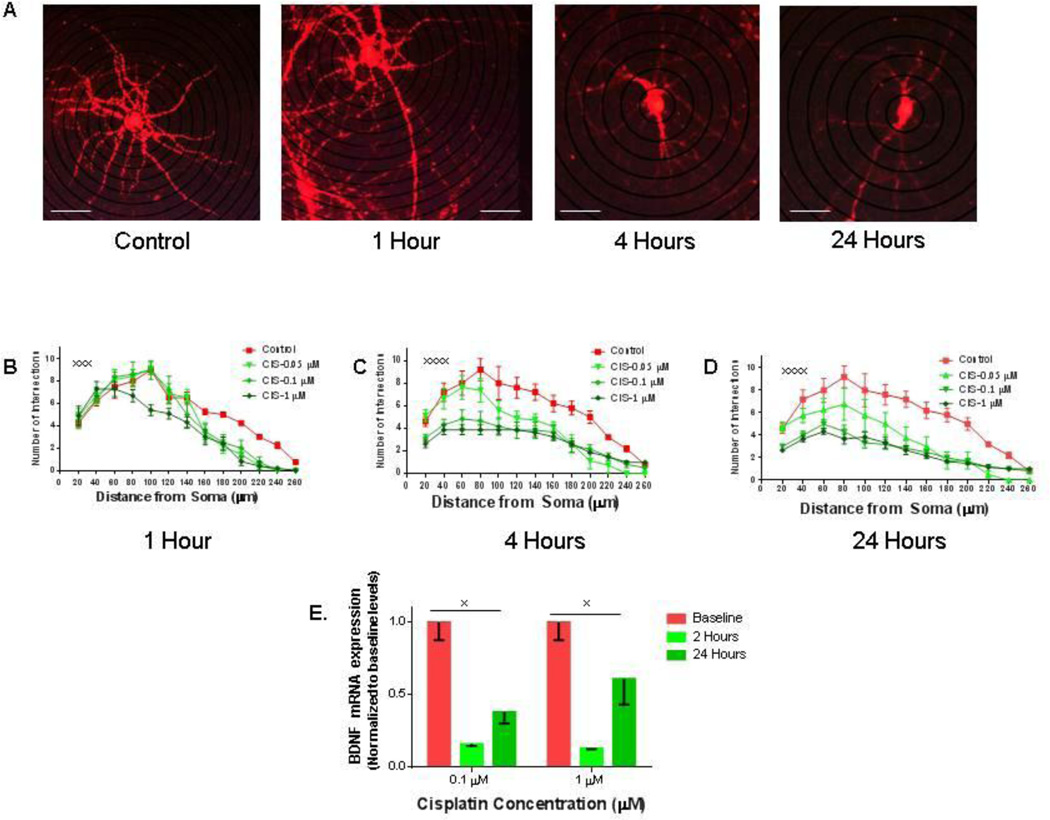
In-vitro treatment with cisplatin causes persistent reduction in dendritic branching
The dendrites of hippocampal neurons, like the synapses they carry, are highly vulnerable to toxin-induced dendritic regression (Avila-Costa et al., 2006). This is important because the excitatory synapses that are important to the processes of learning and memory are located on dendrites (Sheng and Hoogenraad, 2007). We therefore measured the number of dendritic branch points from soma in hippocampal neurons treated with cisplatin. The neurons treated with very-low dose cisplatin (0.1 µM) had had significantly fewer branching points starting at 4 hours after treatment, with a severe reduction of dendritic arborizations after 24 hours of treatment (Figure 2A, B, C and D). Higher cisplatin doses (1 µM) reduced dendritic complexity as early as one hour after the treatment, an effect that was more pronounced after 4 hours of treatment (Figure 2 B, C and D). The loss of dendritic arborization follows a dose-dependent and time-dependent pattern, with a more pronounced loss of branching proximal to the soma at higher doses of cisplatin and longer periods of exposure. The decrease in dendritic arborization could account for the reduced hippocampal volume seen in chemotherapy patients (Schneiderman, 2004).
Figure 2. In-vitro treatment with graded doses of cisplatin causes reduction in dendritic branching.
(A) Representative pictures of hippocampal neurons treated with 0.1 µM cisplatin (CIS) at various time points. (B–D) Quantification of dendritic branch points using Sholl analysis after one (B), four (C) and 24 (D) hours of treatment indicates that cisplatin reduces dendritic complexity all time points. Scale bars approximately 40 µm. Every point represents the average of 10 separate neurons. The experiments were repeated three times with similar results (statistical analysis for one hour: F (36, 468) = 2.162, xxxp = 0.0002, four hours F (48, 585) = 2.624, xxxxp < 0.0001 and 24 hours: F (48, 585) = 2.303, xxxxp < 0.0001). (E) Cisplatin treatment causes a significant decrease in brain-derived neurotrophic factor (BDNF) mRNA after exposure to 0.1µM or 1µM cisplatin at two and 24 hours (xp = 0.002).
Cisplatin treatment causes reduced brain-derived neurotrophic factor (BDNF) expression in cultured hippocampal neurons
Brain-derived neurotrophic factor (BDNF) is widely expressed in the developing and adult mammalian brain (Wetmore et al., 1990), and BDNF- stimulated intracellular signaling is critical for neuronal survival, morphogenesis, and plasticity (Lipsky and Marini, 2007). In hippocampus, BDNF stimulates actin signaling in dendritic spines and regulates dendritic spine integrity (An et al., 2008). Recent evidence has indicated that BDNF induced TrkB signaling could be a method of preventing cisplatin-induced cytotoxicity in neuroblastomas (Jaboin et al., 2003) and an upregulation in both BDNF and the TrKB receptor has been associated with cisplatin resistance in carcinoma cells (Lee et al., 2012). We now show that cisplatin treatment induces a severe decrease in the BDNF mRNA levels in primary hippocampal pyramidal neurons as early as two hours after treatment initiation. Furthermore, the BDNF levels only partially recover after 24 hours (Figure 2E). The reduction in BDNF likely contributes to the inability of the dendritic spines to recover from the in-vitro cisplatin exposure.
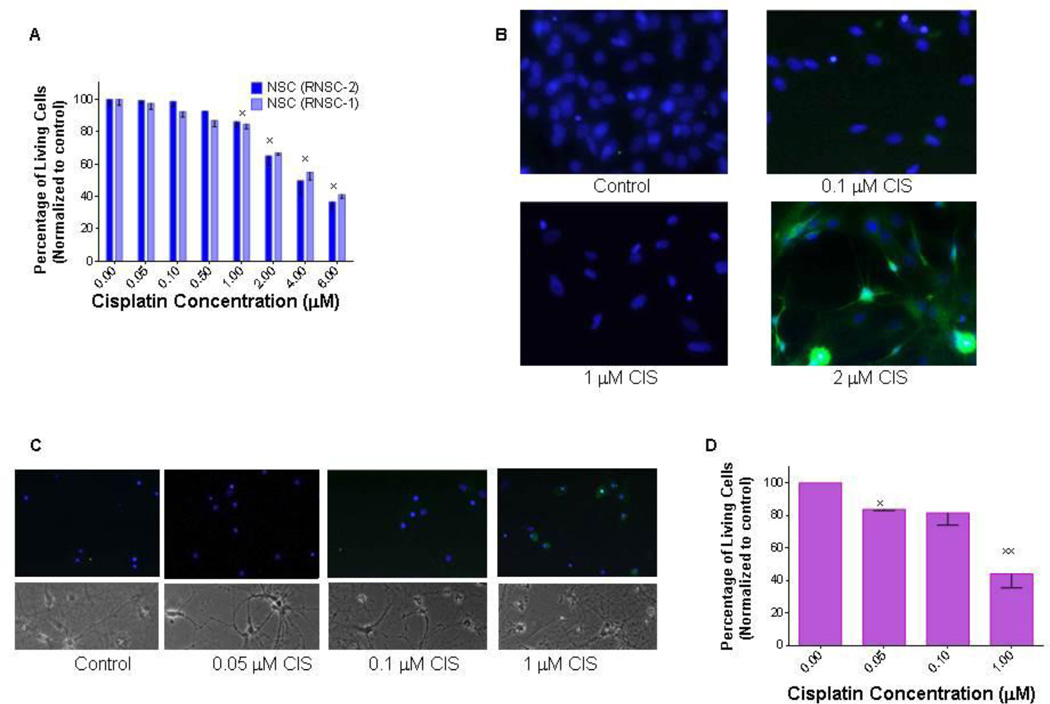
A very-low dose of cisplatin induces apoptosis in differentiated hippocampal neurons in-vitro
Identifying the population(s) of neurons that are more vulnerable to chemotherapy-induced damage is one of the most important questions in neuro-oncology. Therefore, we treated both neural stem cells (NSCs) and hippocampal neurons with graded doses of cisplatin. We report that NSCs are more resilient, and hippocampal neurons are more sensitive to cisplatin treatment. Higher doses of chemotherapy are needed to decrease NSC survival (Figure 3A) and induce apoptotic cell death (Figure 3B). Three days after treatment with 1 µM cisplatin, more than 85% of the cells survived (as compared with to control), while 2 µM cisplatin kills 34% of the cells, and an even higher dose (4 µM) is reduces cell survival by about half (Figure 3A). The minimum dose required in order to induce measureable apoptosis in NSC was 2 µM cisplatin. In contrast, in hippocampal neurons, a lower dose (1 µM) is needed to induce severely altered morphology on light microscopy (Figure 3C) and widespread cell damage death as seen by positive Annexin V staining (quantified in Figure 3D).
Figure 3. In-vitro treatment with graded doses of cisplatin leads to decreased survival of rat neural stem/precursor cells.
(A). In vitro treatment with graded doses of cisplatin (CIS) leads to a decreased number of NSCs (RNSC-1 and RNSC-2). The effect is significant at cisplatin doses of at least 1 µM (xp < 0.01). (B). Cisplatin treatment induces apoptosis in NSCsas visualized by the large number of Annexin V positive cells. (C). Even lower doses of cisplatin induce apoptosis (Annexin V positive cells) and loss of normal cellular architecture in differentiated hippocampal neurons. The experiments were repeated three times with similar results. (D) Quantification of Annexin V positive neurons as a percentage of total living cells (*xp < 0.001).
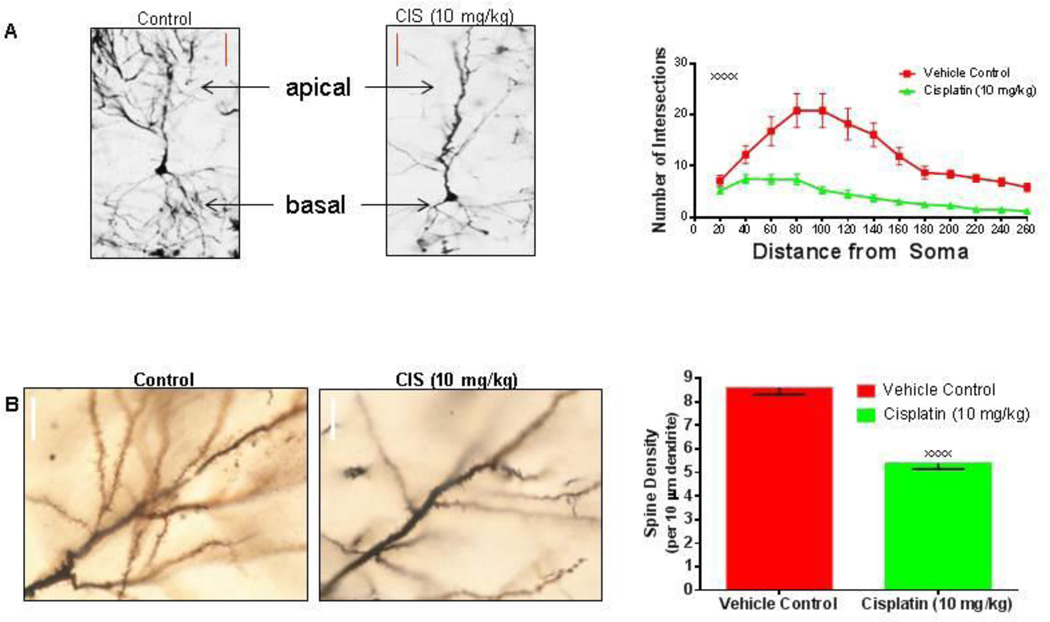
Cisplatin treatment severely affects the dendritic morphology of pyramidal neurons
A very important question was whether the dendritic changes seen in the in-vitro systems were also present in the brains of chemotherapy-treated animals, and therefore potentially in human patients. As such, Sprague Dawley rats were given a 10mg/kg regimen that was administered over two consecutive days. This type of treatment would directly translate to 60 mg/m2/day for two consecutive days (Reagan-Shaw et al., 2008) – which is a regimen administered to cancer patients every four weeks (Goorin et al., 2003). We report that cisplatin treatment can affect dendritic morphology of the pyramidal neurons as early as three days after treatment by severely reducing both the dendritic branching of both the apical and the basal dendrites (Figure 4A) and the number of dendritic spines (Figure 4B). The correlation of the dendrite and dendritic spine damage in-vivo provides evidence that similar events might occur in chemotherapy patients and could contribute to the impairments in learning and memory after cisplatin treatment.
Figure 4. Effects of in-vivo cisplatin treatment on dendritic morphology of pyramidal neurons.
(A) Representative Golgi-impregnated CA3 pyramidal neurons images and graph illustrate the acute reduction in dendritic branching three days after cisplatin treatment. Compared to controls, cisplatin treated rats (10 mg/kg) have significantly less dendrite branch points from the soma of pyramidal neurons (F (12, 234) = 3.828, xxxxp < 0.0001, n=6 rats per group). Scale bars approximately 40 µm. (B) Representative images and graph of CA3 dendritic spines depicting significant reduction in spine density after exposure to cisplatin (xxxxp < 0.0001). Scale bars approximately 10 µm.
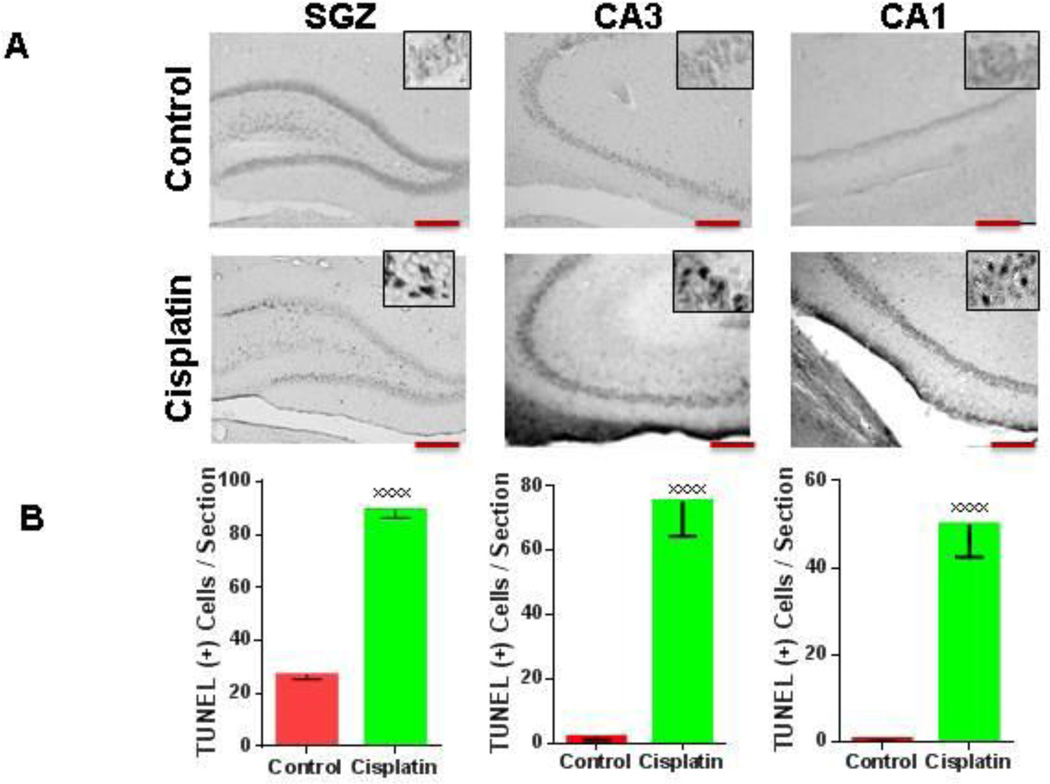
In-vivo administration of Cisplatin causes dose-dependent apoptotic cell death of the hippocampal CA1 and CA3 neurons as well as of the NSC situated in the subgranular zone (SGZ) of the dentate gyrus
As previously stated, decreased hippocampal volume is often observed in chemotherapy patients. This decrease in volume could be attributed to the reported reduction in dendritic complexity, apoptosis of the primary neurons of the hippocampus, or both. To answer this question we treated adult Sprague Dawley rats with cisplatin or the vehicle control (10mg/kg) for three days. The animals were sacrificed three days after the last cisplatin treatment and terminal-transferase-mediated dUTP nick-end-labeling (TUNEL) was performed to detect cells undergoing apoptosis. A very large increase in the number of TUNEL+ (apoptotic) nuclei was seen in the hippocampus of cisplatin treated animals as compared with the control group (Figure 5A and 5B), suggesting that therapeutic cisplatin doses can induce both neuronal as well as NSC apoptotic death.
Figure 5. Acute treatment with cisplatin leads to increased numbers of TUNEL(+) cells the CA1, CA3 areas as well as in the subgranular zone (SGZ)of the dentate gyrus.
(A) Coronal sections through the dorsal dentate gyrus from control and cisplatin treated adult male rats show an increase TUNEL positive cells in the SGZ, CA3, and CA1. Magnification used is 10× for the main panels and 40× for the inserts. Scale bars approximately 200 µm. (B) Quantification of TUNEL positive cells in control and CIS (10mg/kg) treated rats in the SGZ, CA3, and CA1 (from left to right, xxxxp < 0.0001).
DISCUSSION
The purpose of our current research is to delineate the effects of realistic and clinically relevant chemotherapy doses on neuronal integrity. Previously published data showed that neural stem/precursor cells (NSCs) are very sensitive to cisplatin, and are killed by cisplatin doses that do not effectively destroy cancer cells (Dietrich et al., 2006; Gong et al., 2011). We now report that even lower chemotherapy doses severely affect a different subpopulation of cells- the hippocampal neurons. This detrimental effect is specific to dendritic spines and branching and is both time- and dose-dependent. The synaptic damage is not reversible even when the drug is eliminated and the cells are allowed to recover for an extensive period of time (up to five days).
Before the initiation of our study, we performed an extensive literature review of clinically relevant cisplatin concentrations in in-vitro systems. The most accepted way to measure tissue penetrance is to determine the platinum concentration in the normal brain at the time of surgery or at autopsy (Stewart et al., 1982) in patients that received cisplatin chemotherapy. These patients received cisplatin for hours to more than 180 days preceding analysis. The published results of the above mentioned study showed that the platinum concentration in the brain varied between 0.33 and 2.9 µg/g. This platinum concentration is in similar range with the reported intracellular platinum accumulation in cell cultures treated with 5 µM of cisplatin (1.3–5.5 µg/g, Hermann, G et al., 2013) – and this concentration is significantly lower than the one we are now showing induces dendritic damage in cultured neurons.
Furthermore, we are finding both dendritic damage and neuronal and neural stem cell death occurs in-vivo, and can be seen in the brain of adult rats treated with chemotherapy doses directly translated (based on the latest U.S. Department of Health and Human Services Food and Drug Administration recommendations (Reagan-Shaw et al., 2008)) from clinically used chemotherapy regimens. Together, these data support the hypothesis that chemotherapy treatments can potentially induce both severe synaptic damage and neuronal cell loss.
CONCLUSION
We now propose that chemotherapy might have a dual effect on the brain. The initial memory deficits are caused by the loss of dendritic synaptic structures, while the neuro-degenerative conditions that often develop later are caused by the eventual death of hippocampal neurons. The neural stem/precursor cells, which are supposed to differentiate and replenish the damaged neurons, are also damaged by chemotherapy, and are not available in the necessary number to repair the loss -- which contributes to the persistent memory damage. Hence, the memory impairments becomes chronic, which is consistent with the data that patients experience cognitive difficulties for very long periods of time – even more than 20 years for breast cancer survivors (Koppelmans et al., 2012). However, the data presented by us in this manuscript are more reflective of the acute cognitive changes reported in cancer patients receiving active chemotherapy treatment (Brezden et al., 2000) and more research is needed into the long-term effects of chemotherapy on the brain of cancer survivors.
Highlights.
-
-
Low dose of cisplatin (0.1µM) reduces dendritic spine density in cultured hippocampal neurons.
-
-
1µM cisplatin causes apoptosis in hippocampal neurons but neural stem cells require higher doses.
-
-
In-vivo, cisplatin reduces dendritic complexity and spine density in hippocampal neurons.
-
-
Neuronal cell death is present in the CA1 and CA3 areas of cisplatin treated rats.
-
-
Cisplatin-induced reduction in spine density and neuronal death could lead to cognitive deficits
ACKNOWLEDGEMENTS
This study was supported by the National Institute for Neurological Diseases and Stroke Award number NS072234, by the UCI Cancer Center Award Number P30CA062203 from the National Cancer Institute and by research funds donated by Ralph and Suzanne Stern. We thank Dr. Tallie Z. Baram for her scientific mentoring and her constructive advice on experimental design and manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- An JJ, Gharami K, Liao GY, Woo NH, Lau AG, Vanevski F, Torre ER, Jones KR, Feng Y, Lu B, Xu B. Distinct role of long 3' UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell. 2008;134:175–187. doi: 10.1016/j.cell.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila-Costa MR, Fortoul TI, Nino-Cabrera G, Colin-Barenque L, Bizarro-Nevares P, Gutierrez-Valdez AL, Ordonez-Librado JL, Rodriguez-Lara V, Mussali-Galante P, Diaz-Bech P, Anaya-Martinez V. Hippocampal cell alterations induced by the inhalation of vanadium pentoxide (V(2)O(5)) promote memory deterioration. Neurotoxicology. 2006;27:1007–1012. doi: 10.1016/j.neuro.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Baj G, Del Turco D, Schlaudraff J, Torelli L, Deller T, Tongiorgi E. Regulation of the spatial code for BDNF mRNA isoforms in the rat hippocampus following pilocarpine-treatment: a systematic analysis using laser microdissection and quantitative real-time PCR. Hippocampus. 2013;23:413–423. doi: 10.1002/hipo.22100. [DOI] [PubMed] [Google Scholar]
- Brezden CB, Phillips K-A, Abdolell M, Bunston T, Tannock IF. Cognitive Function in Breast Cancer Patients Receiving Adjuvant Chemotherapy. Journal of Clinical Oncology. 2000;18:2695–2701. doi: 10.1200/JCO.2000.18.14.2695. [DOI] [PubMed] [Google Scholar]
- Chen Y, Dube CM, Rice CJ, Baram TZ. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J Neurosci. 2008;28:2903–2911. doi: 10.1523/JNEUROSCI.0225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J, Han R, Yang Y, Mayer-Proschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. Journal of biology. 2006;5:22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumanis SB, Tesoriero JA, Babus LW, Nguyen MT, Trotter JH, Ladu MJ, Weeber EJ, Turner RS, Xu B, Rebeck GW, Hoe HS. ApoE4 decreases spine density and dendritic complexity in cortical neurons in vivo. J Neurosci. 2009;29:15317–15322. doi: 10.1523/JNEUROSCI.4026-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan HK, Bernstein LJ, Brown J, Ringash J, Vakilha M, Wang L, Goldstein D, Kim J, Hope A, O'Sullivan B, Waldron J, Abdul Razak AR, Chen EX, Siu LL. Cognitive Functioning After Radiotherapy or Chemoradiotherapy for Head-and-Neck Cancer. International Journal of Radiation Oncology*Biology*Physics. 2011;81:126–134. doi: 10.1016/j.ijrobp.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Gong X, Schwartz PH, Linskey ME, Bota DA. Neural stem/progenitors and glioma stem-like cells have differential sensitivity to chemotherapy. Neurology. 2011;76:1126–1134. doi: 10.1212/WNL.0b013e318212a89f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorin AM, Schwartzentruber DJ, Devidas M, Gebhardt MC, Ayala AG, Harris MB, Helman LJ, Grier HE, Link MP. Presurgical Chemotherapy Compared With Immediate Surgery and Adjuvant Chemotherapy for Nonmetastatic Osteosarcoma: Pediatric Oncology Group Study POG-8651. Journal of Clinical Oncology. 2003;21:1574–1580. doi: 10.1200/JCO.2003.08.165. [DOI] [PubMed] [Google Scholar]
- Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- Hodgson KD, Hutchinson AD, Wilson CJ, Nettelbeck T. A meta-analysis of the effects of chemotherapy on cognition in patients with cancer. Cancer Treat Rev. 2013;39:297–304. doi: 10.1016/j.ctrv.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Jaboin J, Hong A, Kim CJ, Thiele CJ. Cisplatin-induced cytotoxicity is blocked by brain-derived neurotrophic factor activation of TrkB signal transduction path in neuroblastoma. Cancer Lett. 2003;193:109–114. doi: 10.1016/s0304-3835(02)00723-1. [DOI] [PubMed] [Google Scholar]
- Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 2010;33:121–129. doi: 10.1016/j.tins.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Koppelmans V, Breteler MM, Boogerd W, Seynaeve C, Gundy C, Schagen SB. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:1080–1086. doi: 10.1200/JCO.2011.37.0189. [DOI] [PubMed] [Google Scholar]
- Lee GD, Longo DL, Wang Y, Rifkind JM, Abdul-Raman L, Mamczarz JA, Duffy KB, Spangler EL, Taub DD, Mattson MP, Ingram DK. Transient improvement in cognitive function and synaptic plasticity in rats following cancer chemotherapy. Clin Cancer Res. 2006;12:198–205. doi: 10.1158/1078-0432.CCR-05-1286. [DOI] [PubMed] [Google Scholar]
- Lee J, Jiffar T, Kupferman ME. A novel role for BDNF-TrkB in the regulation of chemotherapy resistance in head and neck squamous cell carcinoma. PLoS One. 2012;7:e30246. doi: 10.1371/journal.pone.0030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky RH, Marini AM. Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticity. Ann N Y Acad Sci. 2007;1122:130–143. doi: 10.1196/annals.1403.009. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Monje ML, Vogel H, Masek M, Ligon KL, Fisher PG, Palmer TD. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol. 2007;62:515–520. doi: 10.1002/ana.21214. [DOI] [PubMed] [Google Scholar]
- Myers JS. Chemotherapy-related cognitive impairment: the breast cancer experience. Oncology nursing forum. 2012;39:E31–E40. doi: 10.1188/12.ONF.E31-E40. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Fujita T, Kubo S, Tokiyoshi K, Yamada M, Kanayama T, Hagiwara Y, Nakanomyo H, Hiraoka M. Difference in CDDP penetration into CSF between selective intraarterial chemotherapy in patients with malignant glioma and intravenous or intracarotid administration in patients with metastatic brain tumor. Cancer Chemother Pharmacol. 1996;37:317–326. doi: 10.1007/s002800050391. [DOI] [PubMed] [Google Scholar]
- Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. 2008;9:65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Schagen SB, Boogerd W, Muller MJ, Huinink ET, Moonen L, Meinhardt W, Van Dam FS. Cognitive complaints and cognitive impairment following BEP chemotherapy in patients with testicular cancer. Acta Oncologica. 2008;47:63–70. doi: 10.1080/02841860701518058. [DOI] [PubMed] [Google Scholar]
- Schneiderman B. Hippocampal volumes smaller in chemotherapy patients. Lancet Oncol. 2004;5:202. doi: 10.1016/s1470-2045(04)01443-3. [DOI] [PubMed] [Google Scholar]
- Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- Shepherd GM. The dendritic spine: a multifunctional integrative unit. J Neurophysiol. 1996;75:2197–2210. doi: 10.1152/jn.1996.75.6.2197. [DOI] [PubMed] [Google Scholar]
- Shetty AK. Progenitor cells from the CA3 region of the embryonic day 19 rat hippocampus generate region-specific neuronal phenotypes in vitro. Hippocampus. 2004;14:595–614. doi: 10.1002/hipo.10206. [DOI] [PubMed] [Google Scholar]
- Suliman HB, Carraway MS, Piantadosi CA. Postlipopolysaccharide oxidative damage of mitochondrial DNA. American journal of respiratory and critical care medicine. 2003;167:570–579. doi: 10.1164/rccm.200206-518OC. [DOI] [PubMed] [Google Scholar]
- Wetmore C, Ernfors P, Persson H, Olson L. Localization of brain-derived neurotrophic factor mRNA to neurons in the brain by in situ hybridization. Exp Neurol. 1990;109:141–152. doi: 10.1016/0014-4886(90)90068-4. [DOI] [PubMed] [Google Scholar]
- Yuste R. Electrical compartmentalization in dendritic spines. Annu Rev Neurosci. 2013;36:429–449. doi: 10.1146/annurev-neuro-062111-150455. [DOI] [PubMed] [Google Scholar]
- Zhang S, Boyd J, Delaney K, Murphy TH. Rapid reversible changes in dendritic spine structure in vivo gated by the degree of ischemia. J Neurosci. 2005;25:5333–5338. doi: 10.1523/JNEUROSCI.1085-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]