Abstract
Background
Neurocysticercosis (NCC) is a frequent cause of epilepsy worldwide. Compared with the more common parenchymal brain cysts, extraparenchymal infections are difficult to manage and have a poor prognosis. Serological assays are used to detect circulating Taenia solium antigens or anti–T. solium antibodies in serum or cerebrospinal fluid (CSF) samples. There are no guidelines on whether to use serum or CSF specimens for a particular assay.
Methods
We obtained paired serum and CSF samples from 91 patients with NCC (48 had intraparenchymal NCC, and 43 had extraparenchymal NCC) for detection of antibodies, using an enzyme-linked immunotransfer blot (EITB) assay, and antigens, using a monoclonal antibody–based enzyme-linked immunosorbent assay (ELISA).
Results
For the intraparenchymal NCC group, the EITB assay yielded more true-positive results for serum samples, and the ELISA yielded slightly more true-positive results for CSF samples than for serum samples, but none of these differences were statistically significant. Most patients with calcified NCC were antibody positive but antigen negative. For extraparenchymal disease, all samples were antibody positive, and all but 2 were antigen positive, with most samples containing high antigen levels.
Conclusions
The sensitivity of antibody-detecting EITB assays is not increased through the use of CSF samples rather than serum samples. The antigen-detecting ELISA performed better for CSF samples than for serum samples, but for both specimen types it was less sensitive than the EITB assay. Active and inactive NCC are better differentiated from each other by the antigen-detecting ELISA, for both serum and CSF samples. High antigen levels suggest the presence of subarachnoid NCC.
Brain invasion by the larvae of the pork tapeworm Taenia solium, the agent of neurocysticercosis (NCC), is a major cause of seizures in most of the world [1–3]. The diagnosis of NCC has greatly improved in the past 25 years, after the introduction of sophisticated imaging techniques and with the improved validity of serological assays. Computed tomography (CT) [4] and, more recently, magnetic resonance imaging (MRI) [5] have demonstrated that intraparenchymal brain parasitic lesions are the most common presentation for NCC [6, 7]. Extraparenchymal lesions occur less frequently but are more difficult to manage. Intraparenchymal disease has a benign course, whereas extraparenchymal disease frequently causes hydrocephalus and is associated with a progressive evolution and significant mortality [8, 9].
The combined use of brain imaging and immunodiagnosis allows a precise diagnosis of NCC in most cases. Serological assays for confirmation of NCC can detect circulating anti–T. solium antibodies or T. solium antigens [10, 11]. Antibody detection is mostly a confirmatory tool, particularly for patients with compatible brain imaging findings or for neurologically symptomatic patients from regions of endemicity. Antigen detection supplements antibody detection by demonstrating the presence of live parasites. Antibody- and antigen-detecting immunodiagnostic tests have been used to examine serum and cerebrospinal fluid (CSF), with variable results [9, 12–16]. There are reasons to believe that the use of CSF could be advantageous for the immunodiagnosis of NCC. Antigens should be directly released to the CSF from neighboring parasites, and antibodies are present in the CSF because of filtration from the blood as well as local antibody production in the central nervous system (as previously demonstrated in persons with NCC) [17]). Lower nonspecific background reactions are also to be expected, owing to the lower protein content of the CSF. On the other hand, CSF is usually obtained through lumbar puncture, a painful and invasive procedure that is performed only in institutional settings and can be particularly risky for patients with intracranial hypertension [18, 19]. Serum samples are obtained by venipuncture, which involves minimal risks and is more acceptable to patients.
There are no clear guidelines on the use CSF for immunodiagnostic purposes in patients with NCC. We evaluated paired serum and CSF samples from patients with intraparenchymal NCC and patients with extraparenchymal NCC to assess whether, for each group, analysis of CSF specimens is more sensitive than analysis of serum specimens for detection of anti–T. solium antibodies and T. solium antigens.
MATERIALS AND METHODS
Samples
Archived records at the Cysticercosis Unit of the Instituto Nacional de Ciencias Neurologicas in Lima, Peru, were reviewed to identify patients from whom paired serum and CSF samples were collected between October 1991 and December 2006. Inclusion criteria specified that paired samples were collected within 30 days of each other, that at least 1 sample (serum or CSF) per pair had T. solium antibodies detected by an enzyme-linked immunotransfer blot (EITB) assay, and that the patient underwent at least 1 brain examination (by CT or MRI) within 90 days of sample collection. Samples were collected under different research studies duly approved by a registered institutional review board, with written records of informed consent that specified permission for future use of remaining biological samples. CSF samples had been obtained by lumbar puncture (spinal CSF) or during placement of ventriculoperitoneal shunts (ventricular CSF).
Demographic and Radiologic Information
Age, sex, and radiologic information (CT and/or MRI findings on the number, type, location, and stage of NCC lesions and the presence or absence of hydrocephalus) were collected from the records of patients who met the selection criteria described. The resulting database was anonymized by deleting any patient identifier code and then used for this study.
Patients were categorized into 2 groups. Group 1 comprised patients with intraparenchymal NCC and ≥1 lesion in the brain parenchyma, without extraparenchymal lesions or hydrocephalus. This group included patients with live, viable parasites (with or without calcifications), and patients with calcified lesions only. Group 2 comprised patients with extraparenchymal NCC. Patients in group 2 could also have intraparenchymal lesions; this group included patients with subarachnoid NCC (with or without ventricular cysts) and patients with intraventricular NCC only.
Study Design
In paired samples from each patient, an EITB assay was used to detect antibodies, as described by Tsang et al. [15], and a monoclonal antibody (MAb)–based capture ELISA (Ag-ELISA) was used to detect antigens, as described by Brandt et al. [11] and adapted by others [20, 21]. In each assay format, serum and CSF samples from the same patient were processed simultaneously, under the same conditions as described below, with observers blinded to the results for the other sample in each pair.
Assays
EITB assay
The EITB assay involves the separation of 7 glycoprotein antigens (GP50, GP42–39, GP24, GP21, GP18, GP14, and GP13) by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. The glycoproteins resolving in the gels are transferred to nitrocellulose membranes and immobilized. Antibody detection is performed by exposing antigen-loaded nitrocellulose strips to the problem sample. Sample incubation was performed according to the original description: serum and CSF were diluted in PBS (pH 7.2) with Tween 20 (PBS-T; 0.3%) and milk (5%) (a 1:50 dilution for serum and a 1:10 dilution for CSF). Samples were incubated overnight at 4°C with gentle shaking. On the next day, the membranes were washed 5 times with 0.3% PBS-T at 56°C. A 1:12,000 dilution of goat anti–human IgG in 0.3% PBS-T was added as a conjugate, and samples were incubated for 2 h at room temperature. After 5 more washings, 3.3′-diaminobenzidine tetrahydrochloride dihydrate diluted in PBS at 0.5 mg/mL was added as a substrate, and the reaction was developed with 30% hydrogen peroxide diluted 1:104. EITB results were expressed as the number of reactive bands (i.e., 0–7). A sample was considered positive if it showed ≥1 reactive band of antibody to the glycoprotein antigens.
Ag-ELISA
Nunc MaxiSorp plates were sensitized with 100 μL of trapping MAb (B158C11A10) in bicarbonate buffer at 5 μg/mL, shaken for 30 min at 37°C, and washed with 0.05% PBS-T. After that, 150 μL of blocking solution (PBS-T with 1% newborn calf serum) was added to all the wells, and incubation for 15 min was performed. Next, 100 μL of serum or CSF samples, pretreated with 5% trichloroacetic acid to break existing immune complexes [21], was added to each well, and plates were incubated for 15 min at 37°C with gentle shaking. Then, after the plates were washed 5 times, 100 μL of the second MAb (B60H8A4-BIOT, diluted 1:712 in blocking solution) was added to each well, and plates were incubated for 15 min at 37°C on a shaker. After another washing, 100 μL of streptavidin diluted 1:104 in blocking solution was added, and plates were incubated for 15 min at 37°C with gentle shaking. Plates were washed again 5 times, and 100 μL of o-phenylenediamine (OPD/H2O2) diluted in citrate buffer was added as substrate/chromogen, after which plates were incubated in the dark for 15 min. The reaction was stopped with 50μL of 2N H2SO4. Plates were read at 492/650 nm. A sample was considered positive if the optical density (OD) was greater than a cutoff defined by analyzing the values of 8 known negative samples by the Student t test. Results were also analyzed as a continuous variable, expressed as the percentage of positivity, obtained from dividing the sample optical density (×100) by that of a positive standard pool from known patients with NCC. The positive control is selected from the linear slope of a dilution curve of antigen, where the kinetics of reaction is higher. Any variation in processing conditions is reflected in changes in the OD reading for this known positive sample, thus providing a control for interplate variation.
Data Analysis
The proportions of serum and CSF samples with positive results were compared using the McNemar test for matched pairs of dichotomic variables. This comparison was conducted independently for EITB and ELISA results and was repeated for each of the 4 types of NCC. Similarly, the number of reactive bands found in serum and CSF samples by the EITB assay were compared using the paired Student t test, and the antigen levels measured by ELISA in serum and CSF specimens were compared using the Wilcoxon matched-pairs signed rank test. The correlation between antigen levels in serum and CSF was also assessed with the Spearman ρ correlation coefficient. Additionally, to evaluate whether spinal and ventricular CSF samples yielded similar results, 2 indicators were compared. The difference in the number of reactive bands detected by the EITB assay (calculated by subtracting the number of bands for serum specimens from the number for CSF specimens) and the ratio of antigen levels in CSF to those in serum were compared between spinal and ventricular CSF samples, using Mann-Whitney 2-sample U tests. Finally, antibody and antigen levels were compared between patients with intraparenchymal NCC and those with extraparenchymal NCC for both serum and CSF samples; patients with only calcific NCC were excluded from comparisons of antigen levels. All data were entered in Excel XP worksheets (Microsoft) and analyzed using Stata software (version 9.0; Stata). All confidence intervals (CIs) were calculated at the 95% confidence level, and results were considered statistically significant at P values of <.05.
RESULTS
Study Population
The final group included samples from 91 patients. Forty-eight (53%) were male, and 43 (47%) were female, and the ages ranged from 17 to 73 years (median age, 33 years [interquartile range, 24.5–46.5 years]). Forty-eight patients had intraparenchymal NCC only (41 had viable parasitic cysts, and 7 had calcified lesions only), and 43 had extraparenchymal disease (31 had subarachnoid cysticercosis, and 12 had intraventricular cysts only). The median age of patients with viable cysts was less than that of those with calcified lesions only although the difference was not statistically significant (29 vs. 38 years; P = .148, by the Mann-Whitney U test), and the median age for these 48 patients was less than that for patients with subarachnoid cysticercosis (30 vs. 45.5 years; P < .001, by the Mann-Whitney U test).
Intraparenchymal NCC
Antibody detection by the EITB assay
There were 48 paired serum and CSF samples from patients with intraparenchymal NCC. All serum samples, independent of the parasite stage, were antibody positive by the EITB assay. CSF samples from 5 patients (4 of 41 patients with viable intraparenchymal cysts and 1 of 7 patients with calcified cysts only) were negative for antibodies by the EITB assay (table 1). Thus, use of the EITB assay for detection of viable intraparenchymal NCC identified a slightly lower percentage of true-positive cases via analysis of CSF than via analysis of serum (90.2% [95% CI, 76.9%–97.3%] vs. 100% [95% CI, 91.4%–100%]; P = .125, by the McNemar test). The mean number (±SD) of reactive antibody bands detected by the EITB assay was greater for serum samples than for CSF samples (5.00 ± 1.91 bands vs. 4.17 ± 2.35 bands; P = .002, by the paired Student t test). Antibody seroprevalence among patients with calcified lesions only was similar to that among patients with viable cysts in this series.
Table 1. Positive results of an enzyme-linked immunotransfer blot (EITB) assay and a monoclonal antibody–based enzyme-linked immunosorbent assay (Ag-ELISA) for serum and cerebrospinal fluid (CSF) specimens from patients with intraparenchymal neurocysticercosis.
| Positive results, proportion (%) | ||||
|---|---|---|---|---|
| Assay, specimen | Patients with viable cysts |
P a | Patients with calcified cysts only |
P a |
| EITB | .125 | NS | ||
| Serum | 41/41 (100) | 7/7 (100) | ||
| CSF | 37/41 (90.2) | 6/7 (85.7) | ||
| Ag-ELISA | .219 | NS | ||
| Serum | 18/30 (60) | 1/7 (14.3) | ||
| CSF | 22/30 (73.3) | 2/7 (28.6) | ||
By the McNemar test.
Antigen detection by the Ag-ELISA
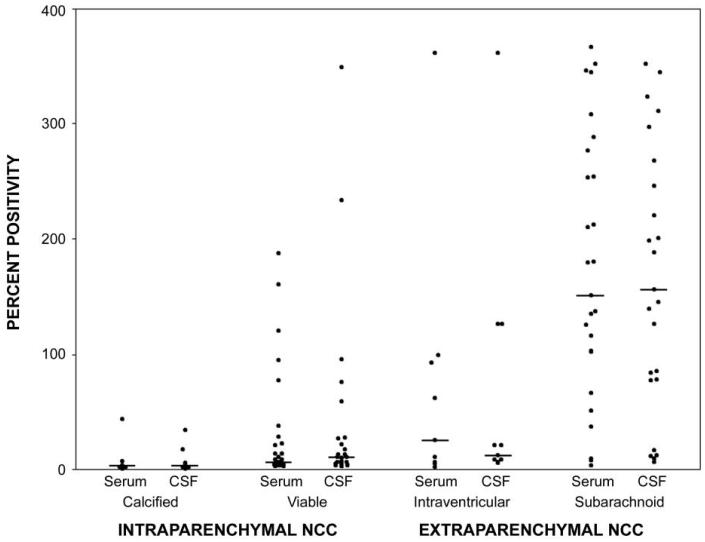
Paired serum and CSF samples from 37 patients with intraparenchymal NCC were available for Ag-ELISA. Viable intraparenchymal NCC was detected by Ag-ELISA for a lower proportion of serum specimens (18 of 30) than CSF specimens (22 of 30), although this difference was not statistically significant (P = .219, by the McNemar test). The prevalence of positive Ag-ELISA findings among patients with calcified lesions only was less than that among patients with viable cysts; this difference was significant for serum samples (1 [14.3%] of 7 vs. 18 [60%] of 30; P = .042, by the Fisher exact test) and marginal for CSF samples (2 [28.6%] of 7 vs. 22 [73.3%] of 30; P = .072, by the Fisher exact test). Antigen levels, expressed as the percentage of positivity, followed the same trend and were also significantly lower among patients with calcified lesions than among those with viable cysts for both serum (8.91 ± 15.67 vs. 29.30 ± 48.91; P = .020, by the Mann-Whitney U test) and CSF (7.62 ± 12.10 vs. 35.48 ± 75.63; P = .013, by the Mann-Whitney U test) (figure 1).
Figure 1.
Levels of circulating Taenia solium antigens in serum and cerebrospinal fluid (CSF), by neurocysticercosis (NCC) type. The percentage of positivity was obtained by dividing the sample optical density (×100) by that of a positive standard pool from known patients with NCC.
Extraparenchymal NCC
Comparison of spinal and ventricular CSF samples
Ventricular CSF samples were available only from the extraparenchymal NCC group and were obtained from 18 of 31 patients with subarachnoid NCC and 7 of 12 patients with ventricular NCC. CSF specimens obtained from shunts were similar to those obtained from lumbar puncture in terms of the numbers of reactive antibody band (P = .578) and antigen levels (P = .861) for patients with either subarachnoid or ventricular cysticercosis.
When CSF samples were compared with serum samples from the same patients, however, some differences appeared. Relative to findings for serum samples, CSF samples obtained from shunts had fewer reactive antibody bands detected by the EITB assay than did CSF samples obtained by lumbar puncture (1.92 ± 1.85 vs. 0.83 ± 1.50 fewer bands in CSF than in serum; P = .047, by the Mann-Whitney U test), reflecting proportionally fewer antibodies in CSF samples obtained from shunts. Similarly, when CSF antigen levels were assessed in relation to levels in serum for the same patient, the ratio of CSF levels to serum levels was significantly lower among CSF samples obtained from shunts than among those obtained by lumbar puncture (1.28 ± 1.67 vs. 3.17 ± 3.47; P = .049, by the Mann-Whitney U test), reflecting proportionally fewer antigens in the former.
Antibody detection by EITB assay
Paired serum and CSF samples were available for 43 patients with extraparenchymal NCC. All serum and CSF samples were EITB positive; patients with extraparenchymal NCC had more reactive antibody bands in serum specimens than in CSF specimens (6.28 ± 1.26 bands vs. 4.81 ± 1.76 bands; P < .001, by the paired Student t test). The differences in the number of positive antibody bands between serum and CSF samples were similar for patients with intraventricular NCC and those with subarachnoid NCC (table 2).
Table 2. Positive results of an enzyme-linked immunotransfer blot (EITB) assay and a monoclonal antibody–based enzyme-linked immunosorbent assay (Ag-ELISA) for serum and cerebrospinal fluid (CSF) specimens from patients with extraparenchymal neurocysticercosis (NCC).
| Positive results, proportion (%) | ||
|---|---|---|
| Assay, specimen | Patients with subarachnoid NCC |
Patients with intraventricular NCC |
| EITB | ||
| Serum | 31/31 (100) | 12/12 (100) |
| CSF | 31/31 (100) | 12/12 (100) |
| Ag-ELISA | ||
| Serum | 25/25 (100) | 7/9 (77.8) |
| CSF | 25/25 (100) | 9/9 (100) |
Antigen detection on ELISA
Paired serum and CSF samples from 34 patients with extraparenchymal NCC were available for Ag-ELISA. All samples, both serum and CSF, were positive by Ag-ELISA, with the exception of 2 serum samples from patients with ventricular NCC. Samples from patients with subarachnoid disease had higher antigen levels than those from patients with ventricular NCC (P = .016 for CSF and P = .013 for serum, by the Mann-Whitney U test) (table 2).
Relation between antigen levels in serum and CSF
In the whole study population, antigen levels in paired serum and CSF specimens were strongly correlated (Spearman ρ = 0.791; P < .001). This correlation was less marked but still highly significant for patients with subarachnoid NCC (r = 0.479, P = .015). Serum and CSF antigen levels were not significantly correlated for patients with intraventricular NCC (r = 0.226; P = .559).
Serological Differences between Intraparenchymal and Extraparenchymal NCC
Antibody detection by EITB
Samples from patients with extraparenchymal NCC had more reactive antibody bands than those from patients with intraparenchymal NCC. The difference was significant for serum specimens (6.28 ± 1.26 bands vs. 4.98 ± 1.97 bands; P < .001) but only marginal for CSF specimens (4.81 ± 1.76 bands vs. 4.08 ± 2.39 bands; P = .104).
Antigen detection on ELISA
Serum and CSF antigen levels were significantly higher among patients with extraparenchymal NCC than among those with viable intraparenchymal disease (P < .001 for both comparisons, by the Mann-Whitney U test); this analysis was performed after exclusion of patients with only calcified intraparenchymal lesions.
DISCUSSION
Brain imaging methods frequently yield nonspecific findings in NCC. Immunodiagnosis of NCC, typically performed using serum or CSF samples, is a helpful tool for diagnostic confirmation and follow-up. Despite the difficulties associated with CSF sample collection, there are no clear indications as to which type of samples should be used. This series demonstrated that there is basically no advantage to using CSF rather than serum for antibody detection with EITB assays. Among patients with intraparenchymal brain NCC, the use of CSF samples for antigen detection was associated with a 13% increase in case identification (P = not significant). Most patients with extraparenchymal NCC were strongly seropositive, so there were no apparent differences between serum and CSF findings in this group.
Immunodiagnosis can be done by detecting specific antibodies or antigens. In 1989, the introduction of the EITB assay (based on immunoblotting or Western blotting) with purified glycoprotein antigen provided a sensitive and specific marker of infection by T. solium and rapidly became the assay of choice for immunodiagnosis [15, 22, 23]. Also in 1989, an ELISA for antigen detection (Ag-ELISA) was reported to have reasonable performance [10]. A similar Ag-ELISA was described by a different group in 1992 and was improved in 1998 by changing the immunoglobulin isotype of the monoclonal antibodies from IgM to IgG [11, 20]. Although this assay does not have the sensitivity and specificity of the EITB assay, it may differentiate infections that involve live parasites and will probably become a very useful tool in monitoring the effect of antiparasitic treatment [24, 25].
NCC can locate anywhere in the nervous system and is associated with inflammation and subsequent disruption in the blood-brain barrier. CSF antibody levels increase according to the duration and severity of inflammation, as reported for multiple sclerosis [26], and also after the disruption of the blood-brain barrier [27, 28]. “De novo” immunoglobulin synthesis inside the central nervous system has been shown to occur in NCC [17]. In general, however, antibody levels are lower in CSF, because they mostly come from passive diffusion from the blood system [29, 30], although there is a gradient of increased protein concentration as the CSF descends along the neuroaxis [30, 31]. Consequently, the use of CSF yields weaker reactions and thus lower sensitivity but also a significant reduction in nonspecific reactions. Thus, for assay formats of lower specificity, such as indirect hemagglutination, complement fixation, or ELISA using nonpurified antigens, test performance is improved by using CSF instead of serum [31–35]. Highly specific assays can take advantage of the stronger reactivity of serum without the risk of disturbing increases in false-positive results [9, 15, 36–39].
The multiplicity of antigens and assay formats has thus made it difficult to compare antibody- and antigen-detecting assays in terms of their accuracy or their performance in different biological fluids. In this study, paired serum and CSF samples from patients with well-defined NCC types were tested for antibodies by using an EITB assay [15] and for antigens by using a MAb-based ELISA [11, 20, 21]. All serum samples and most CSF samples (those from ~10% of patients with intraparenchymal NCC were EITB negative) were positive by the EITB assay. Most patients with calcified lesions were antibody positive, confirming that antibody responses in persons with established NCC persist for years after successful cure [40]. In the original description of the EITB assay in NCC [15], the sensitivity with serum samples was estimated at 98%, similar to our findings. The original study, however, did not find differences in sensitivity with CSF samples, but only a small number of CSF samples were tested. In our series, antibody detection by EITB assay in CSF samples was slightly inferior to that in serum samples. Our findings are concordant with those of other, larger studies of NCC antibody detection in CSF samples [16, 36]. Because almost all patients were positive for antibodies at EITB assay (as usual in clinical cases), we could not compare EITB sensitivity in serum versus CSF in terms of positive or negative diagnoses. EITB results are usually expressed as positive or negative or as numbers of reactive bands (0–7). Patients with extraparenchymal NCC had more bands in both serum and CSF samples. Previous reports had described higher antibody concentration in extraparenchymal or malignant NCC [9, 12, 13, 41].
Unlike antibody detection, measurement of circulating antigen levels allows differentiation of NCC cases with viable parasites [42–44], with antigen levels correlating to the numbers and size of lesions [24, 25, 45]. For antigen detection, CSF samples were superior to serum samples in all types of NCC except subarachnoid NCC, for which both serum and CSF levels were extremely high. In most patients with viable intraparenchymal cysts, antigen levels were detectable, but at low levels. Antigen levels in patients with calcified intraparenchymal NCC were almost undetectable. In contrast, serum and CSF antigen levels in extraparenchymal disease were 10 times higher [43, 46]. High antigen levels should thus lead one to suspect the presence of extraparenchymal NCC. Serum and CSF antigen levels were strongly correlated for all types of NCC except intraventricular cysts.
In this series, there were clear differences in age by stage and type of NCC. The mean age for patients with calcified NCC was 9 years older than that for patients with viable intraparenchymal NCC. This finding is concordant with the classic work by Dixon and Lipscomb [47] that involved English soldiers, which described a median of 3–5 years between exposure and the onset of symptoms. Additionally, calcification of viable cysts after their degeneration has been estimated to take ~2 years [48]. Patients with extraparenchymal NCC were, on average, 13 years older than patients with viable intraparenchymal lesions. This time may represent prolonged latency due to slow growth of extraparenchymal larvae (symptoms probably appear or greatly worsen only after the size of the parasites causes mass effect or blocks CSF circulation and leads to hydrocephalus) [49]. Less likely, this lag time could arise from slow migration of cysts from neighboring parenchyma or from the lateral ventricles downward [50].
Our findings suggest that serum antibody detection by an EITB assay, using purified antigen, remains the assay of choice for diagnosis of NCC [15], with no improvement in performance (or even a slight decrease in sensitivity) with use of CSF rather than serum samples. EITB antibody detection is associated with a high rate of positive antibody reactions among patients with calcified NCC. Mab-based detection of circulating antigen by ELISA shows lower sensitivity, although its performance was somewhat better for CSF than for serum samples. Active and inactive infections are better differentiated by antigen detection in serum or CSF samples, and high antigen levels suggests the presence of extraparenchymal NCC, particularly subarachnoid disease, which is associated with a worse prognosis.
Acknowledgments
We are grateful to the personnel of the Cysticercosis Unit, Instituto de Ciencias Neurológicas, Lima, Peru, for their hard work in collecting and processing samples.
Financial support: National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants D43 TW01140 and TW06581); Bill and Melinda Gates Foundation (grant 23981 to V.C.W.T., A.E.G., R.H.G., H.H.G.).
STUDY GROUP MEMBERS
Members of the Cysticercosis Working Group in Peru who collaborated with the work described in this article include Juan Jimenez, Mary Luz Rodriguez, Yesenia Castillo, Patricia Arias, Milagritos Portocarrero, S. Manuel Martinez, and Herbert Saavedra (Instituto de Ciencias Neurológicas, Lima); and Manuela Verastegui (Universidad Peruana Cayetano Heredia, Lima).
Footnotes
Potential conflicts of interest: none reported.
Presented in part: 54th Annual Meeting of the American Society of Tropical Medicine and Hygiene, Washington, DC, 11–15 December 2005.
References
- 1.White AC., Jr. Neurocysticercosis: a major cause of neurological disease worldwide. Clin Infect Dis. 1997;24:101–13. doi: 10.1093/clinids/24.2.101. [DOI] [PubMed] [Google Scholar]
- 2.Del Brutto OH, Sotelo J. Neurocysticercosis: an update. Rev Infect Dis. 1988;10:1075–87. doi: 10.1093/clinids/10.6.1075. [DOI] [PubMed] [Google Scholar]
- 3.Commission on Tropical Diseases of the International League against Epilepsy Relationship between epilepsy and tropical diseases. Epilepsia. 1994;35:89–93. [PubMed] [Google Scholar]
- 4.Mervis B, Lotz JW. Computed tomography (CT) in parenchymatous cerebral cysticercosis. Clin Radiol. 1980;31:521–8. doi: 10.1016/s0009-9260(80)80037-7. [DOI] [PubMed] [Google Scholar]
- 5.Suss RA, Maravilla KR, Thompson J. MR imaging of intracranial cysticercosis: comparison with CT and anatomopathologic features. AJNR Am J Neuroradiol. 1986;7:235–42. [PMC free article] [PubMed] [Google Scholar]
- 6.Cuetter AC, Garcia-Bobadilla J, Guerra LG, Martinez FM, Kaim B. Neurocysticercosis: focus on intraventricular disease. Clin Infect Dis. 1997;24:157–64. doi: 10.1093/clinids/24.2.157. [DOI] [PubMed] [Google Scholar]
- 7.Agapejev S. Epidemiology of neurocysticercosis in Brazil. Rev Inst Med Trop Sao Paulo. 1996;38:207–16. doi: 10.1590/s0036-46651996000300008. [DOI] [PubMed] [Google Scholar]
- 8.Estanol B, Corona T, Abad P. A prognostic classification of cerebral cysticercosis: therapeutic implications. J Neurol Neurosurg Psychiatry. 1986;49:1131–4. doi: 10.1136/jnnp.49.10.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corona T, Pascoe D, Gonzalez-Barranco D, Abad P, Landa L, Estanol B. Anticysticercous antibodies in serum and cerebrospinal fluid in patients with cerebral cysticercosis. J Neurol Neurosurg Psychiatry. 1986;49:1044–9. doi: 10.1136/jnnp.49.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison LJ, Joshua GW, Wright SH, Parkhouse RM. Specific detection of circulating surface/secreted glycoproteins of viable cysticerci in Taenia saginata cysticercosis. Parasite Immunol. 1989;11:351–70. doi: 10.1111/j.1365-3024.1989.tb00673.x. [DOI] [PubMed] [Google Scholar]
- 11.Brandt JR, Geerts S, De Deken R, et al. A monoclonal antibody-based ELISA for the detection of circulating excretory-secretory antigens in Taenia saginata cysticercosis. Int J Parasitol. 1992;22:471–7. doi: 10.1016/0020-7519(92)90148-e. [DOI] [PubMed] [Google Scholar]
- 12.Mohammad IN, Heiner DC, Miller BL, Goldberg MA, Kagan IG. Enzyme-linked immunosorbent assay for the diagnosis of cerebral cysticercosis. J Clin Microbiol. 1984;20:775–9. doi: 10.1128/jcm.20.4.775-779.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosas N, Sotelo J, Nieto D. ELISA in the diagnosis of neurocysticercosis. Arch Neurol. 1986;43:353–6. doi: 10.1001/archneur.1986.00520040039016. [DOI] [PubMed] [Google Scholar]
- 14.Estrada JJ, Kuhn RE. Immunochemical detection of antigens of larval Taenia solium and anti-larval antibodies in the cerebrospinal fluid of patients with neurocysticercosis. J Neurol Sci. 1985;71:39–48. doi: 10.1016/0022-510x(85)90035-8. [DOI] [PubMed] [Google Scholar]
- 15.Tsang VC, Brand JA, Boyer AE. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium) J Infect Dis. 1989;159:50–9. doi: 10.1093/infdis/159.1.50. [DOI] [PubMed] [Google Scholar]
- 16.Proano-Narvaez JV, Meza-Lucas A, Mata-Ruiz O, Garcia-Jeronimo RC, Correa D. Laboratory diagnosis of human neurocysticercosis: double-blind comparison of enzyme-linked immunosorbent assay and electro-immunotransfer blot assay. J Clin Microbiol. 2002;40:2115–8. doi: 10.1128/JCM.40.6.2115-2118.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller BL, Staugaitis SM, Tourtellotte WW, et al. Intra-blood-brain barrier IgG synthesis in cerebral cysticercosis. Arch Neurol. 1985;42:782–4. doi: 10.1001/archneur.1985.04210090046013. [DOI] [PubMed] [Google Scholar]
- 18.Dufrense J. Citología práctica del líquido cefalorraquídeo. Ciba-Geigy; Basel, Switzerland: 1972. [Google Scholar]
- 19.van Crevel H, Hijdra A, de Gans J. Lumbar puncture and the risk of herniation: when should we first perform CT? J Neurol. 2002;249:129–37. doi: 10.1007/pl00007855. [DOI] [PubMed] [Google Scholar]
- 20.Van Kerckhoven I, Vansteenkiste W, Claes M, Geerts S, Brandt J. Improved detection of circulating antigen in cattle infected with Taenia saginata metacestodes. Vet Parasitol. 1998;76:269–74. doi: 10.1016/s0304-4017(97)00226-4. [DOI] [PubMed] [Google Scholar]
- 21.Dorny P, Vercammen F, Brandt J, Vansteenkiste W, Berkvens D, Geerts S. Sero-epidemiological study of Taenia saginata cysticercosis in Belgian cattle. Vet Parasitol. 2000;88:43–9. doi: 10.1016/s0304-4017(99)00196-x. [DOI] [PubMed] [Google Scholar]
- 22.Garcia HH, Gonzalez AE, Gilman RH. Diagnosis, treatment and control of Taenia solium cysticercosis. Curr Opin Infect Dis. 2003;16:411–9. doi: 10.1097/00001432-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Del Brutto OH, Rajshekhar V, White AC, Jr, et al. Proposed diagnostic criteria for neurocysticercosis. Neurology. 2001;57:177–83. doi: 10.1212/wnl.57.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zamora H, Castillo Y, Garcia HH, et al. Drop in antigen levels following successful treatment of subarachnoid neurocysticercosis. Am J Trop Med Hyg. 2005;73(Suppl):S41. [Google Scholar]
- 25.Nguekam Zoli AP, Ongolo-Zogo P, Dorny P, Brandt J, Geerts S. Follow-up of neurocysticercosis patients after treatment using an antigen detection ELISA. Parasite. 2003;10:65–8. doi: 10.1051/parasite/2003101p65. [DOI] [PubMed] [Google Scholar]
- 26.Forghani B, Cremer NE, Johnson KP, Fein G, Likosky WH. Comprehensive viral immunology of multiple sclerosis. III. Analysis of CSF antibodies by radioimmunoassay. Arch Neurol. 1980;37:616–9. doi: 10.1001/archneur.1980.00500590040004. [DOI] [PubMed] [Google Scholar]
- 27.Madrazo NI, Olhagaray B, Becerra M, Sandoval MA, Soto LR. Acute cysticercosis encephalitis: description of a histologically confirmed case. Neurosurgery. 1983;13:593–5. doi: 10.1227/00006123-198311000-00020. [DOI] [PubMed] [Google Scholar]
- 28.Estanol B, Juarez H, Irigoyen Mdel C, Gonzalez-Barranco D, Corona T. Humoral immune response in patients with cerebral parenchymal cysticercosis treated with praziquantel. J Neurol Neurosurg Psychiatry. 1989;52:254–7. doi: 10.1136/jnnp.52.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collis SC, Kimberlin RH. Further studies on changes in immunoglobulin G in the sera and CSF of Herdwick sheep with natural and experimental scrapie. J Comp Pathol. 1983;93:331–8. doi: 10.1016/0021-9975(83)90019-1. [DOI] [PubMed] [Google Scholar]
- 30.Braga FM, dos Reis-Filho JB, de Camargo-Lima JG. Ventriculo-lumbar gradient of concentration of total cerebrospinal fluid proteins. 1. Mechanisms of origin [in Portuguese] Arq Neuropsiquiatr. 1983;41:254–65. doi: 10.1590/s0004-282x1983000300006. [DOI] [PubMed] [Google Scholar]
- 31.Cho SY, Kim SI, Kang SY, et al. Evaluation of enzyme-linked immunosorbent assay in serological diagnosis of human neurocysticercosis using paired samples of serum and cerebrospinal fluid. Kisaengchunghak Chapchi. 1986;24:25–41. doi: 10.3347/kjp.1986.24.1.25. [DOI] [PubMed] [Google Scholar]
- 32.Bueno EC, Vaz AJ, Machado LD, Livramento JA. Neurocysticercosis: detection of IgG, IgA and IgE antibodies in cerebrospinal fluid, serum and saliva samples by ELISA with Taenia solium and Taenia crassiceps antigens. Arq Neuropsiquiatr. 2000;58:18–24. doi: 10.1590/s0004-282x2000000100003. [DOI] [PubMed] [Google Scholar]
- 33.da Silva MR, Maia AA, Espindola NM, Machado Ldos R, Vaz AJ, Henrique-Silva F. Recombinant expression of Taenia solium TS14 antigen and its utilization for immunodiagnosis of neurocysticercosis. Acta Trop. 2006;100:192–8. doi: 10.1016/j.actatropica.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Espinoza B, Ruiz-Palacios G, Tovar A, Sandoval MA, Plancarte A, Flisser A. Characterization by enzyme-linked immunosorbent assay of the humoral immune response in patients with neurocysticercosis and its application in immunodiagnosis. J Clin Microbiol. 1986;24:536–41. doi: 10.1128/jcm.24.4.536-541.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Correa D, Sandoval MA, Harrison LJ, et al. Human neurocysticercosis: comparison of enzyme immunoassay capture techniques based on monoclonal and polyclonal antibodies for the detection of parasite products in cerebrospinal fluid. Trans R Soc Trop Med Hyg. 1989;83:814–6. doi: 10.1016/0035-9203(89)90340-4. [DOI] [PubMed] [Google Scholar]
- 36.Diaz JF, Verastegui M, Gilman RH, et al. Immunodiagnosis of human cysticercosis (Taenia solium): a field comparison of an antibody-enzyme-linked immunosorbent assay (ELISA), an antigen-ELISA, and an enzyme-linked immunoelectrotransfer blot (EITB) assay in Peru. The Cysticercosis Working Group in Peru (CWG) Am J Trop Med Hyg. 1992;46:610–5. doi: 10.4269/ajtmh.1992.46.610. [DOI] [PubMed] [Google Scholar]
- 37.Ferrer E, Bonay P, Foster-Cuevas M, et al. Molecular cloning and characterisation of Ts8B1, Ts8B2 and Ts8B3, 3 new members of the Taenia solium metacestode 8 kDa diagnostic antigen family. Mol Biochem Parasitol. 2007;152:90–100. doi: 10.1016/j.molbiopara.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Bueno EC, Scheel CM, Vaz AJ, et al. Application of synthetic 8-kD and recombinant GP50 antigens in the diagnosis of neurocysticercosis by enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 2005;72:278–83. [PubMed] [Google Scholar]
- 39.Flisser A, Plancarte A, Correa D, et al. New approaches in the diagnosis of Taenia solium cysticercosis and taeniasis. Ann Parasitol Hum Comp. 1990;65(Suppl 1):95–8. doi: 10.1051/parasite/1990651095. [DOI] [PubMed] [Google Scholar]
- 40.Garcia HH, Gilman RH, Catacora M, et al. Serological evolution of neurocysticercosis patients after antiparasitic therapy. J Infect Dis. 1997;175:486–9. doi: 10.1093/infdis/175.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zini D, Farrell VJ, Wadee AA. The relationship of antibody levels to the clinical spectrum of human neurocysticercosis. J Neurol Neurosurg Psychiatry. 1990;53:656–61. doi: 10.1136/jnnp.53.8.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia HH, Harrison LJ, Parkhouse RM, et al. A specific antigen-detection ELISA for the diagnosis of human neurocysticercosis. The Cysticercosis Working Group in Peru. Trans R Soc Trop Med Hyg. 1998;92:411–4. doi: 10.1016/s0035-9203(98)91069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia HH, Gonzalez AE, Gilman RH, et al. Circulating parasite antigen in patients with hydrocephalus secondary to neurocysticercosis. Am J Trop Med Hyg. 2002;66:427–30. doi: 10.4269/ajtmh.2002.66.427. [DOI] [PubMed] [Google Scholar]
- 44.Nguekam A, Zoli AP, Vondou L, et al. Kinetics of circulating antigens in pigs experimentally infected with Taenia solium eggs. Vet Parasitol. 2003;111:323–32. doi: 10.1016/s0304-4017(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 45.Garcia HH, Parkhouse RM, Gilman RH, et al. Serum antigen detection in the diagnosis, treatment, and follow-up of neurocysticercosis patients. Trans R Soc Trop Med Hyg. 2000;94:673–6. doi: 10.1016/s0035-9203(00)90228-1. [DOI] [PubMed] [Google Scholar]
- 46.Fleury A, Hernandez M, Avila M, et al. Detection of HP10 antigen in serum for diagnosis and follow-up of subarachnoidal and intraventricular human neurocysticercosis. J Neurol Neurosurg Psychiatry. 2007;78:970–4. doi: 10.1136/jnnp.2006.107243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dixon H, Lipscomb F. Cysticercosis: an analysis and follow-up of 450 cases. Med Res Council Spec Rep Ser. 1961;299:1–57. [Google Scholar]
- 48.Handler LC, Mervis B. Cerebral cysticercosis with reference to the natural history of parenchymal lesions. AJNR Am J Neuroradiol. 1983;4:709–12. [PMC free article] [PubMed] [Google Scholar]
- 49.Cuetter AC, Andrews RJ. Intraventricular neurocysticercosis: 18 consecutive patients and review of the literature. Neurosurg Focus. 2002;12:e5. doi: 10.3171/foc.2002.12.6.6. [DOI] [PubMed] [Google Scholar]
- 50.Thomas B, Krishnamoorthy T. Migrating intraventricular cysticercus during MRI. Neurology. 2005;65:1321. doi: 10.1212/01.wnl.0000182296.59409.38. [DOI] [PubMed] [Google Scholar]