Abstract
Clinical observations have suggested that antagonism of 5-HT2A receptors may benefit patients with parkinsonian symptomatology. The mechanism of the antiparkinsonian effects of 5-HT2A receptor antagonists has not been fully elucidated. We have shown that the selective 5-HT2A receptor antagonist M100907 [R-(+)-alpha-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenethyl)]-4-piperidinemethanol] improved motor impairments in mice treated with the parkinsonian neurotoxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). In Parkinson's disease (PD) patients and animal models of parkinsonism dopamine denervation is associated with increased cortico-striatal glutamatergic transmission. We hypothesized that 5-HT2A receptor antagonists may exert their antiparkinsonian effects by decreasing striatal glutamate. Here, using in vivo microdialysis, we have shown an increased basal level of extracellular striatal glutamate when measured three weeks after MPTP administration. The local administration of M100907 to the striatum significantly decreased striatal extracellular glutamate levels in MPTP-treated and saline treated mice. Basal extracellular serotonin (5-HT) levels were also elevated, whereas dopamine (DA) levels were significantly reduced in the striatum of MPTP-treated mice. Infusion of M100907 into the striatum produced no effect on dopamine or 5-HT levels. Local application of tetrodotoxin suppressed glutamate, 5-HT and DA concentrations in striatal dialysates in the presence or absence of M100907. The striatal expression of the glutamate transporter GLT1 was unchanged. However, there was an upregulation of the expression of 5-HT2A receptors in the striatum in MPTP-treated animals. Our data provide further evidence of enhanced glutamatergic neurotransmission in parkinsonism and demonstrate that blocking 5-HT2A receptors in the striatum will normalize glutamatergic neurotransmission.
Keywords: glutamate, M100907, microdialysis, parkinsonism, MPTP, serotonin
1. Introduction
Parkinson's disease (PD) is a neurological disease associated with a decrease in dopamine (DA) in the striatum which is the result of the degeneration of dopamine producing neurons in the substantia nigra pars compacta. DA replacement, with L-3,4-dihydroxyphenylalanine (L-DOPA), is the predominant treatment of PD. Unfortunately, most patients develop dyskinesia (abnormal involuntary movements) and motor fluctuations within a few years of L-DOPA therapy (Nutt, 1990; Hurtig, 1997; Obeso et al., 2000; Ahlskog and Muenter, 2001). Consequently, there is a clear need to identify non-dopaminergic drug targets to provide fewer side effects while maintaining therapeutic efficacy.
In PD patients and animal models of parkinsonism, dopamine denervation induces an increase in corticostriatal glutamatergic transmission (Anglade et al., 1996; Ingham et al., 1998; Meshul et al., 1999). Accordingly, in vivo microdialysis and proton magnetic resonance spectroscopy have revealed increased glutamate concentrations in the striatum of MPTP-treated mice (Robinson et al., 2003; Chassain et al., 2008). Because hyper-glutamatergic drive is associated with parkinsonism, treatment strategies that counteract glutamatergic activity may provide alternatives to conventional dopaminergic- focused therapies.
It is well known that the atypical antipsychotic drugs e.g. clozapine cause fewer extrapyramidal motor deficits in schizophrenic patients (Kane, 2001). The favorable side effect profile has been attributed to their potent 5-HT2 receptor antagonism in relation to weak dopamine D2 receptor antagonism (Meltzer, 1991). Clozapine has been shown to be effective at alleviating catalepsy induced by haloperidol (Murphy and Feldon, 2000), or the selective dopamine D1 antagonist SCH 23390, and the dopamine D2 antagonist raclopride (Ahlqvist et al., 2003). It has been reported that the non-selective 5-HT2A receptor antagonist ritanserin reduced haloperidol-induced catalepsy in rats (Lucas et al., 1997; Young et al., 1999). Recently, we have shown that the selective 5-HT2A receptor antagonist M100907 but not the selective 5-HT2C receptor antagonist SB206553 improved motor impairments in mice treated with the dopaminergic neurotoxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP; Ferguson et al., 2010). The data suggest that antagonism of 5-HT2A receptors could exert an anti-parkinsonian activity.
A number of studies have demonstrated a widespread distribution of 5-HT2A receptors in the striatum (Pompeiano et al., 1994; Ward and Dorsa, 1996; Mijnster et al., 1997; Bubser et al., 2001) and may suggest that 5-HT2A receptors may play a role in regulating striatal glutamate transmission. For example, microdialysis in the cortex has revealed that the 5-HT2A receptor antagonist M100907 blocks increases in extracellular glutamate levels elicited by the 5-HT2A/2C receptor agonist, 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI; Scruggs et al., 2003).
In the current studies, we determined whether there is increase in basal extracellular glutamate levels in the striatum of mice treated with MPTP and whether infusion of M100907 into the striatum will attenuate the elevation in extracellular glutamate. In view of the well documented interaction between 5-HT and DA systems (Di Matteo et al., 2008), we also assessed the effect of M100907 on striatal extracellular DA.
2. Materials and methods
2.1. Animals
Male C57BL/6J mice, 70-77 days of age at the start of experiments, were obtained from Jackson Labs (Bar Harbor, ME). Animals were group housed, with food and water available ad libitum. All studies were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and under the oversight of the Meharry Medical College Animal Care and Use Committee. All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize alternatives to in vivo techniques, if available.
2.2. MPTP treatment
Mice were injected (i.p.) with 20 mg/kg MPTP (Sigma–Aldrich, St Louis, MO, USA) or saline (10 mL/kg) every 2 hours for a total of four injections, resulting in a cumulative dose of 80 mg/kg (Ferguson et al., 2010). The mortality rate in our cohort of animals was five percent. All experiments were carried out three weeks after MPTP administration.
2.3. Surgical and microdialysis procedures
Two weeks after MPTP or saline treatment, mice were implanted with a chronic indwelling guide cannula; 5-7 days later the mice were used in dialysis sessions examining the ability of the 5-HT2A antagonist M100907 to modulate glutamate release in the striatum. One day prior to use, the efficiency of transmitter recovery by the probe was determined by collecting three 10-minute samples (perfusion flow rate of 2 μL/min) after placing the probe in a solution of glutamate (200 pg/μL) in artificial cerebrospinal fluid (aCSF; 140 mM NaCl, 3.4 mM KCl, 1.5 mM CaCl2, 1.0 mM MgCl2, 1.4 mM NaH2PO4, and 4.85 mM NaHPO4, pH 7.4). Two groups of mice (control or MPTP-treated) were used in the experiment to assess the effects of M100907. Mice were anesthesized under isoflurane for stereotaxic surgery to place guide cannula (Plastics One; Roanoke, VA) into the right striatum (anterior-posterior, +0.6 mm; dorso-ventral, −4.2 mm; and lateral, 2.0 mm relative to bregma). A dual dental adhesive (Plastics One; Roanoke, VA) was applied to the skull surface and base of the cannula, and then built up with a small amount of dental acrylic compound. Five to seven days post-operatively, the dialysis probe (1.5 mm active exchange surface) was inserted and the animal was placed in a Plexiglas dialysis chamber. The swivel assembly and attached tubing was carefully counterbalanced to allow free movement of the mouse. The dialysis probe was perfused at 0.2 μL/min with aCSF for one hour, after which the flow rate was increased to 2 μL/min. Five 20min baseline samples were collected, after which the selective 5-HT2A antagonist M100907 (100 nM) was administered through the dialysis probe and an additional five fractions were collected. Afterwards a mixture of M100907 (100 nM) and the sodium channel blocker, tetrodotoxin (TTX; 1nM) was administered through the dialysis probe and a final five fractions were collected. At the end of the experiment mice were deeply anesthetized with sodium pentobarbital (60 mg/kg, i.p.), perfused intracardially with 4% paraformaldehyde and serial coronal sections (40 μm) were cut through the striatum and stained with cresyl violet. If the placement was not correct (i.e., outside the striatum), the data from that animal were discarded. The levels of amino acids in the dialysate were determined using reverse phase HPLC-EC and fluorescent detection. Aminobutyric acid was added to dialysis samples as an internal standard. Samples were derivatized using o-phthalaldehyde and loaded into an autosampler for injection onto a 1.5 micron C18 column (Alltech Associates; Deerfield, IL). The mobile phase was 100 mM sodium phosphate buffer containing 10% methanol (pH 3.70) and flow rate was set at 1.2 ml/min with the column temperature maintained at 40°C. The glutamate and GABA derivatization products were detected with a RF-10Axl fluorescence detector (Shimadzu Corp; Kyoto, Japan) and an electrochemical detector (ESA; Chelmford, MA) placed in series. Mean baseline levels of glutamate and GABA were calculated by averaging the concentrations of the five basal dialysate samples. If any baseline sample from an animal varied by more than 30% of the mean, it was eliminated; data from animals with less than three basal samples were not included in the analysis.
2.4. Immunohistochemistry
Animals were deeply anesthetized with isoflurane and then transcardially perfused with 4% paraformaldehyde in 0.1M phosphate buffer after a brief perfusion with phosphate buffer. The brains were removed from the cranium, postfixed in 4% paraformaldehyde overnight, and then cryoprotected in 30% sucrose in phosphate buffer for 1-2 days. The brains were sectioned on a freezing microtome at a thickness of 40 μm in the coronal plane (Bubser et al., 2001). Localization of tyrosine hydroxylase (TH)-positive neurons was performed by using the Chemicon rabbit anti-TH with donkey anti rabbit biotinylated secondary antibody (Chemicon, Temecular, CA). In brief, sections were incubated for 48 h at 4°C in primary antibody for TH, a rabbit polyclonal antibody raised against amino acids 32-47 of the N-terminus of the rat TH protein (Chemicon # P07101, Millipore, Temecular, CA). The primary antibody was diluted 1:1000 in 0.1 M PBS containing 1% normal horse serum and 0.2% Triton X-100. The sections were incubated in secondary antibody for 90 min at room temperature followed by incubation in ABC reagent (Vector, according to the manufacturer's directions) for 90 min at room temperature. The reaction product was visualized using nickel-enhanced diaminobenzidine (DAB kit, Vector, 12- min exposure). The slices were then washed in buffer, mounted on gelatin-coated slides, air- dried, and coverslipped. For a negative control, elimination of the primary antibody resulted in a complete lack of tissue immunolabeling.
Stereological assessment of the number of TH-immunoreactive neurons in 40 μm thick coronal sections cut through the substantia nigra was performed using the Stereologer software package (Stereology Resource Center; Chester, MD) at the Morphology Core Laboratory of Meharry Medical College (Nayyar et al., 2009). The two-stage (Nv × Vref) approach using the optical dissector and Cavalieri method (West and Gundersen, 1990) was used to calculate the total number of TH-immunoreactive cells in animals subjected to saline or MPTP-treatment regimen. All immunohistochemical analyses were done by persons unaware of the treatment condition of the animals.
2.5. Preparation of striatal synaptosomes
All procedures were carried out at 4°C. After decapitating the mice, the dorsolateral striatum was dissected from 1.0 mm thick coronal slices and immediately homogenized in 4 ml of a cold 0.32 M sucrose solution using 10 up-and-down strokes of a pre-chilled Teflon/glass homogenizer. The homogenate was centrifuged at 1000 × g for 10 min and the supernatant was carefully collected and stored at 4 °C. The pellet was resuspended in 5 ml of cold 0.32 M sucrose solution and centrifuged again at 1000 × g for 10 min. The two supernatants were pooled and centrifuged for 30 min at 17,500 × g. After discarding the supernatant, the final pellet containing the synaptosomes was resuspended in 1 mL of ice-cold Krebs–Ringer buffer (120 mM NaCl, 4.8 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, 6 mM glucose, 1.3 mM CaCl2, pH 7.6). Protein concentration was determined and samples diluted in Krebs–Ringer to a protein concentration of 50 μg/ml.
2.6. Immunoblot analyses
Striatal proteins were extracted using T-PER extraction reagent (Pierce BioTechnology; Rockville, IL); the protein concentration of the supernatant was determined by the BCA protein assay (Pierce BioTechnology). Protein was loaded and separated on a 10% SDS-PAGE gel under reducing conditions, and transferred onto PVDF membranes. Nonspecific binding was blocked by incubation with phosphate-buffered saline containing 0.05% Tween-20 and 5% nonfat dry milk for 1 hour. The membranes were incubated in blocking solution containing anti-5-HT2AR or GLT1 (1:1000; Sigma Chemical Co., St Louis, MO) and β-actin as a loading control (1:5000; Chemicon), and the proteins revealed by an immunoperoxidase method with ECL detection (Amersham Biosciences Inc., Piscataway, NJ). The resultant signals were analyzed using an Alpha Imager™ 2000 Digital Imaging System (Alpha Innotech Corp; San Leandro, CA).
2.7. Statistical analysis
Microdialysis data are expressed as percentages of basal values, averaged from five pre-drug fractions. Microdialysis data presented as a histogram were analyzed by two-way ANOVA with lesion (MPTP treatment) and drug as independent factors, followed by Tukey's post-hoc tests when indicated by a significant main effect on the ANOVA. Student's t test was used to compare measures of 5-HT2A, TH and GLT1 immunoreactivity in saline and MPTP-treated animals.
3. Results
3.1. Effects of MPTP treatment on dopamine neurons in the substantia nigra
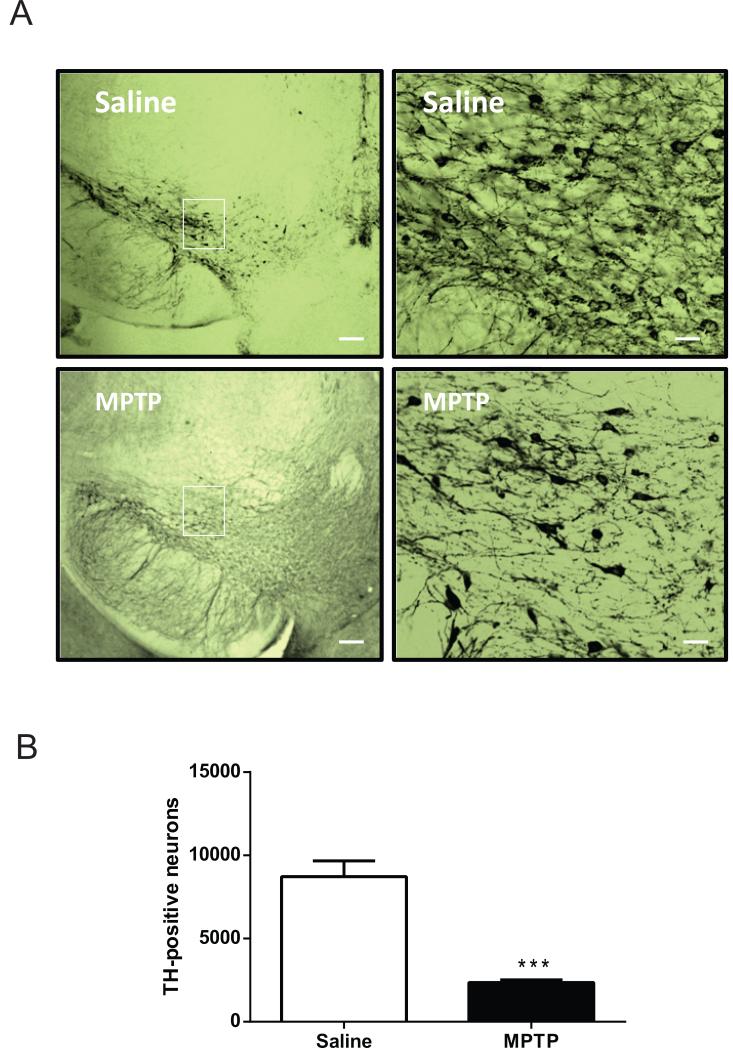
To quantify the extent of nigrostriatal damage caused by MPTP treatment, the number of TH-immunoreactive neurons within the substantia nigra pars compacta was determined using unbiased stereological methods. An example of TH immunolabeling within the substantia nigra pars compacta of a saline- and MPTP-treated animal is illustrated in Fig. 1. Three weeks after the last dose of the neurotoxin or saline, there was a significant decrease in the number of substantia nigra pars compacta TH-immunoreactive neurons in the MPTP-treated group compared to the saline-treated group. There was a 73% decrease in TH-immunoreactive neurons after MPTP-treatment compared to the saline group (Fig. 1; P < 0.001).
Figure 1.
Tyrosine hydroxylase expression in the substantia nigra pars compacta of saline and MPTP-treated mice. (A) Photomicrographs illustrating tyrosine hydroxylase expression in the substantia nigra pars compacta. Magnification of inset is shown on the right panels. Scale bar: 50 μm on left panels. Scale bar: 10 μm on right panels (B) Stereological counts of tyrosine hydroxylase immunopositive neurons in the substantia nigra pars compacta of saline and MPTP-treated mice. There was a significant difference between saline and MPTP-treated mice (n = 6/group). *** significantly different from saline-treated (P < 0.001), by Student's t-test.
3.2. Effects of M100907 and TTX infusion on glutamate Levels in the dorsal striatum
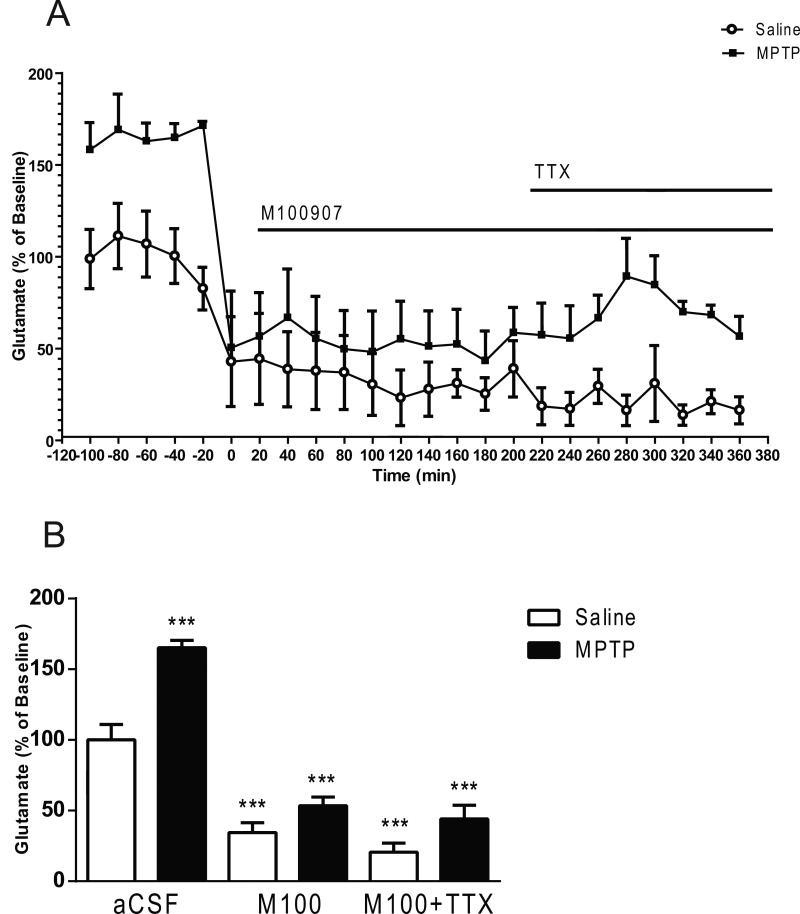
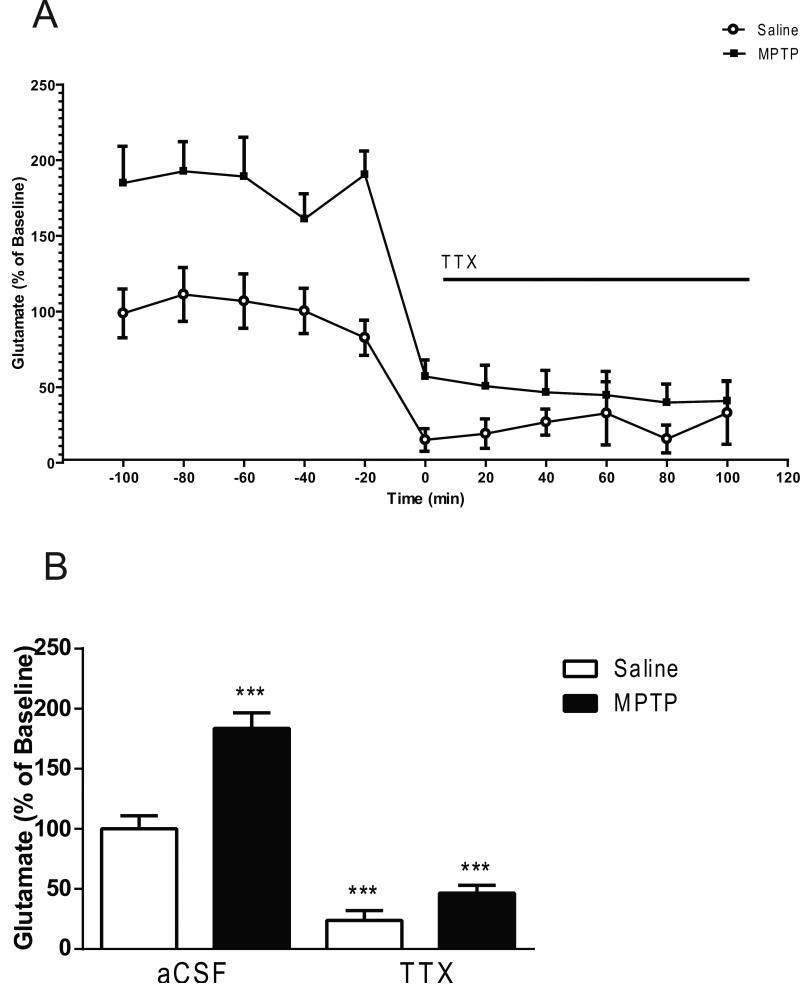
All in vivo microdialysis experiments were carried out 3 weeks after the last MPTP administration. The mean basal extracellular glutamate levels in striatal dialysates obtained from saline treated mice were 3.41 ± 0.24 pmol/μL, (mean ± S.E.M.; n= 30). In local application experiments, baseline samples were collected from the striatum after a 2 hour perfusion, and basal extracellular levels remained stable before drug perfusion. A two-ANOVA revealed main effects of lesion produced by MPTP treatment (F1,42 = 29.05, p < 0.0001), drug treatment (F2,42 = 90.18, p < 0.0001) and lesion × drug interaction (F2,42 = 4.856; p < 0.05) on extracellular glutamate (Fig. 2). MPTP-treated mice exhibited a greater than 60% increase in basal extracellular glutamate levels compared to the saline-treated mice (Fig. 2). Post hoc analysis using the Tukey's multiple comparison test showed that local perfusion of 100 nM M100907 into the dorsal striatum significantly decreased basal glutamate levels in saline (p<0.0001) and MPTP (p < 0.0001)-treated mice, compared with the baseline levels of the saline-treated mice. Extracellular glutamate was further decreased (p < 0.0001) subsequent to administration of M100907 and TTX (Fig 2). TTX perfusion is a powerful in vivo method for differentiating between action potential-dependent and action potential-independent drug-induced neurotransmitter release (Westerink et al., 1987). The addition of 1μL TTX to the perfusion fluid reduced extracellular glutamate in saline and MPTP-treated mice (lesion; F1,18 = 124.3, P < 0.0001; TTX; F1,18 = 31.01, p < 0.0001; lesion x TTX interaction; F1,18 = 10.11, p < 0.05) (Fig. 3). Extracellular glutamate was decreased by 73% (p<0.0001) in the saline-treated and 75% (p < 0.0001) in the MPTP-treated mice, in comparison to basal levels of each respective treatment group (Fig 3).
Figure 2.
Effect of M100907 on Glutamate Levels in the Dorsal Striatum. Data are expressed as percentages of values in vehicle-injected control mice. Mice were treated with four doses of MPTP (20 mg/kg, ip) or saline (10 ml/kg, ip) (n = 11/group) every 2 hours for a total of four doses. Dialysis was carried out 7 days after cannula implantation (3 weeks after MPTP treatment). (A) Four baseline samples were first collected and then a challenge dose of M100907 (100 nM) and an additional five samples were collected. Finally a challenge dose of M100907 (100 nM) and TTX (1 μM) and five samples were collected. (B) The time course data are represented as a histogram. *** Significantly different from saline-treated mice (P < 0.001); in two-way ANOVA with Tukey's post hoc comparison. The line segments on time course graphs indicate duration of drug administration.
Figure 3.
Effect of tetrodotoxin (TTX) on Glutamate Levels in the Dorsal Striatum. Data are expressed as percentages of values in vehicle-injected control mice. Mice were treated with four doses of MPTP (20 mg/kg, ip) or saline (10 ml/kg, ip) (n = 6/group) every 2 hours for a total of four doses. Dialysis was carried out 7 days after the cannula implantation. (A) Five baseline samples were first collected and then a challenge dose of TTX (1 μM) and six samples were collected. (B) The time course data are represented as a histogram. *** Significantly different from saline-treated mice (P < 0.001); in two-way ANOVA with Tukey's post hoc comparison. The line segments on time course graphs indicate duration of drug administration.
3.3. Effects of M100907 and TTX on 5-HT levels in the dorsal striatum
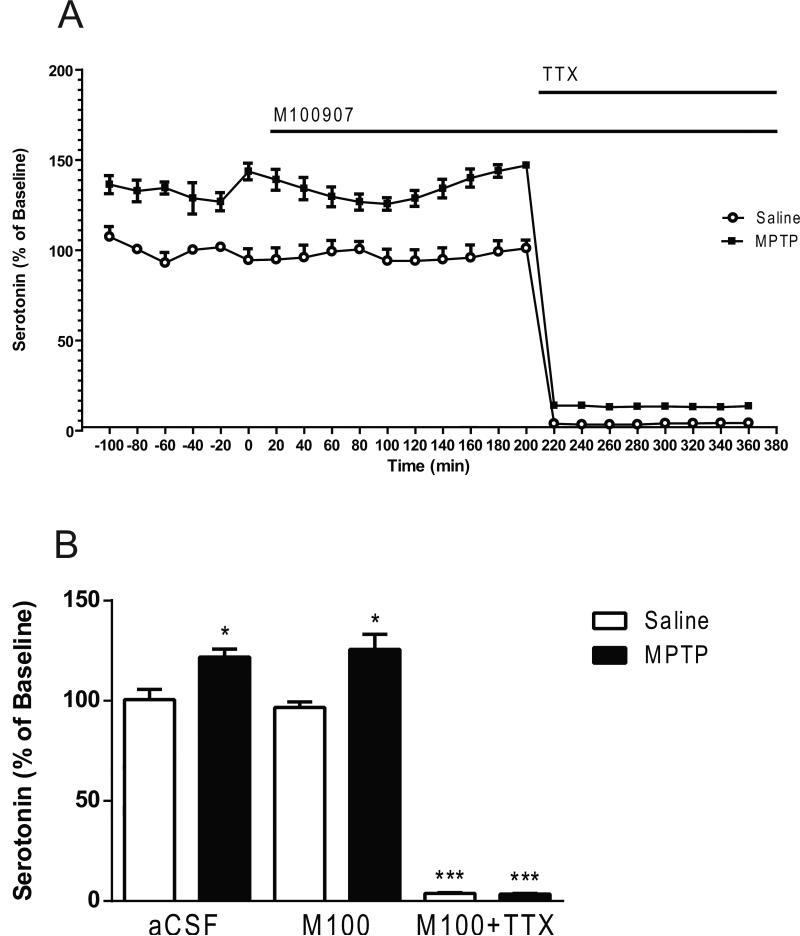
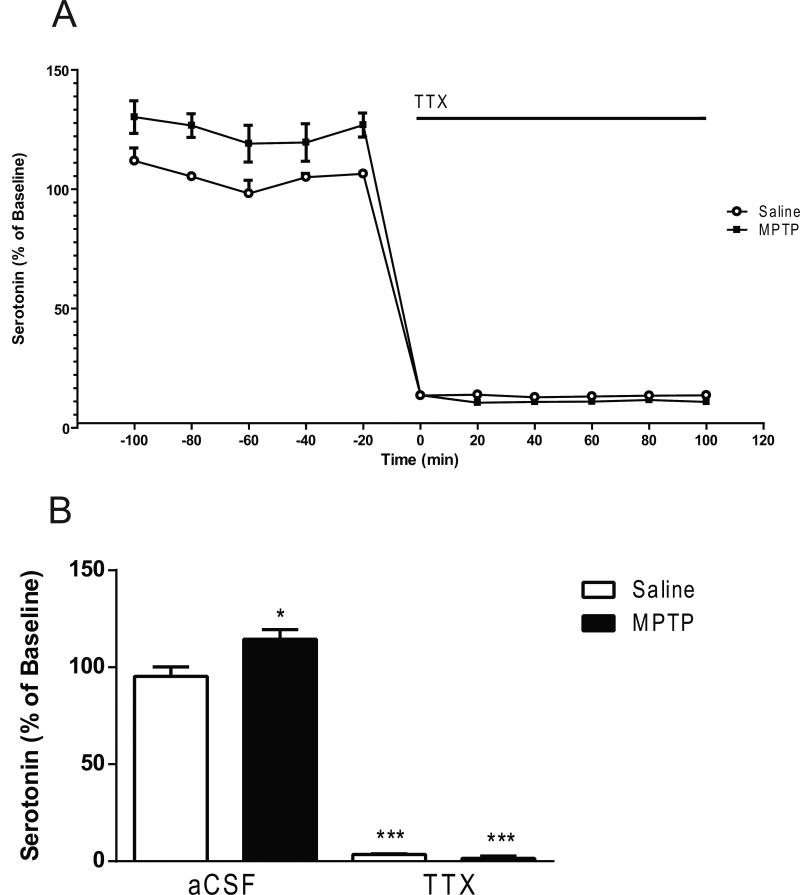
Two-way ANOVA revealed significant main effects (lesion; F1,42 = 16.03, p<0.001; drug; F2,42 = 298.1, p < 0.0001; lesion × drug interaction; F2,42 = 4.47, p < 0.05) (Fig. 4). Post hoc analysis using the Tukey's multiple comparison test revealed a significant increase (21%) of basal serotonin levels in the MPTP-treated mice (p < 0.05) compared to the saline-treated mice (0.664 ± 0.087 fmol/5 μL sample, mean ± S.E.M.; n= 30) (Fig. 4). Post hoc analysis using the Tukey's multiple comparison test revealed no significant decreases in 5-HT levels subsequent to M100907 application (Fig. 4). However, serotonin levels were significantly decreased in the saline-treated (p < 0.0001) and the MPTP-treated mice (p < 0.0001) with the co-administration of M100907 and TTX. In the absence of M100907 the addition of 1μL TTX to the perfusion fluid reduced serotonin by 96% in the saline-treated (p < 0.0001) and 99 % in the MPTP-treated mice (p<0.0001), in comparison to basal levels of each respective treatment group (lesion; F1,18 = 7.490, P < 0.05; TTX; F1,18 = 1068, p < 0.0001; lesion × TTX interaction; F1,18 = 11.33, p < 0.01) (Fig. 5).
Figure 4.
Effect of M100907 on Serotonin Levels in the Dorsal Striatum. Data are expressed as percentages of values in vehicle-injected control mice. Mice were treated with four doses of MPTP (20 mg/kg, ip) or saline (10ml/kg, ip) (n = 11/group) every 2 hours for a total of four doses. Dialysis was carried out 7 days after cannula implantation. (A) Four baseline samples were first collected and then a challenge dose of M100907 (100 nM) and an additional five samples were collected. Finally a challenge dose of M100907 (100 nM) and TTX (1 μM) and five samples were collected.(B) The time course data are represented as a histogram. * Significantly different from saline-treated mice (P < 0.05); *** Significantly different from saline-treated mice (P < 0.001); in two-way ANOVA with Tukey's post hoc comparison. The line segments on time course graphs indicate duration of drug administration.
Figure 5.
Effect of TTX on Serotonin Levels in the Dorsal Striatum. Data are expressed as percentages of values in vehicle-injected control mice. Mice were treated with four doses of MPTP (20 mg/kg, ip) or saline (10 ml/kg, ip) (n = 6/group) every 2 hours for a total of four doses. Dialysis was carried out 7 days after cannula implantation. (A) Five baseline samples were first collected and then a challenge dose of TTX (1 μM) and six samples were collected. (B) The time course data are represented as histogram. * Significantly different from saline-treated mice (P < 0.05); *** Significantly different from saline-treated mice (P < 0.001); in twoway ANOVA with Tukey's post hoc comparison. The line segments on time course graphs indicate duration of drug administration.
3.4. Effect of M100907 and TTX on DA Levels in the dorsal striatum
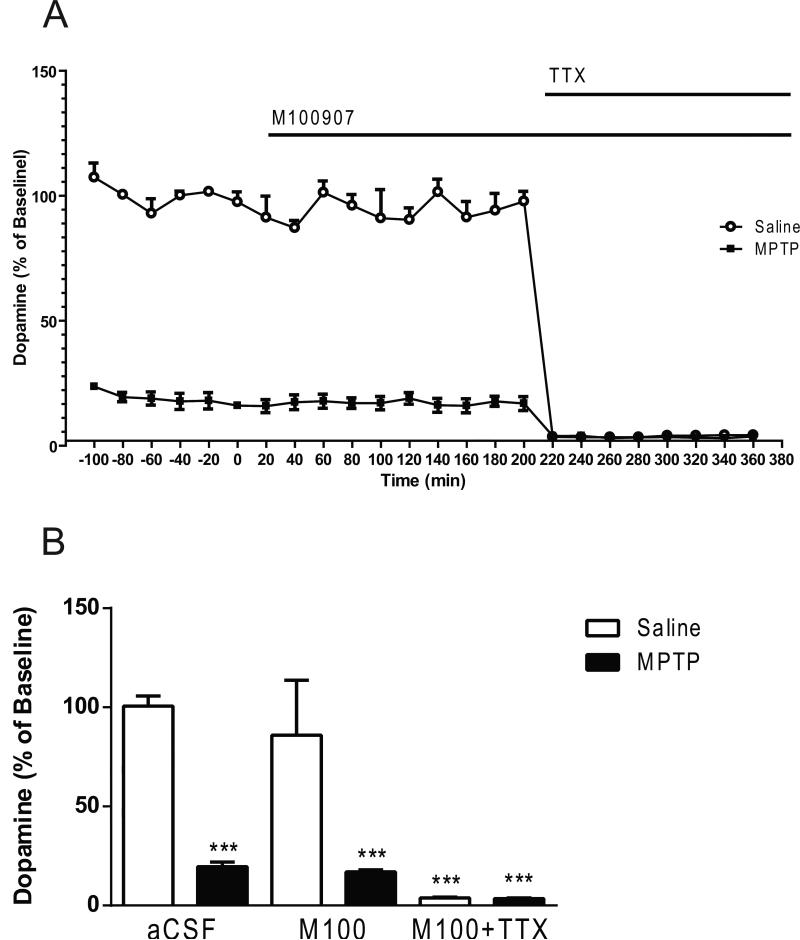
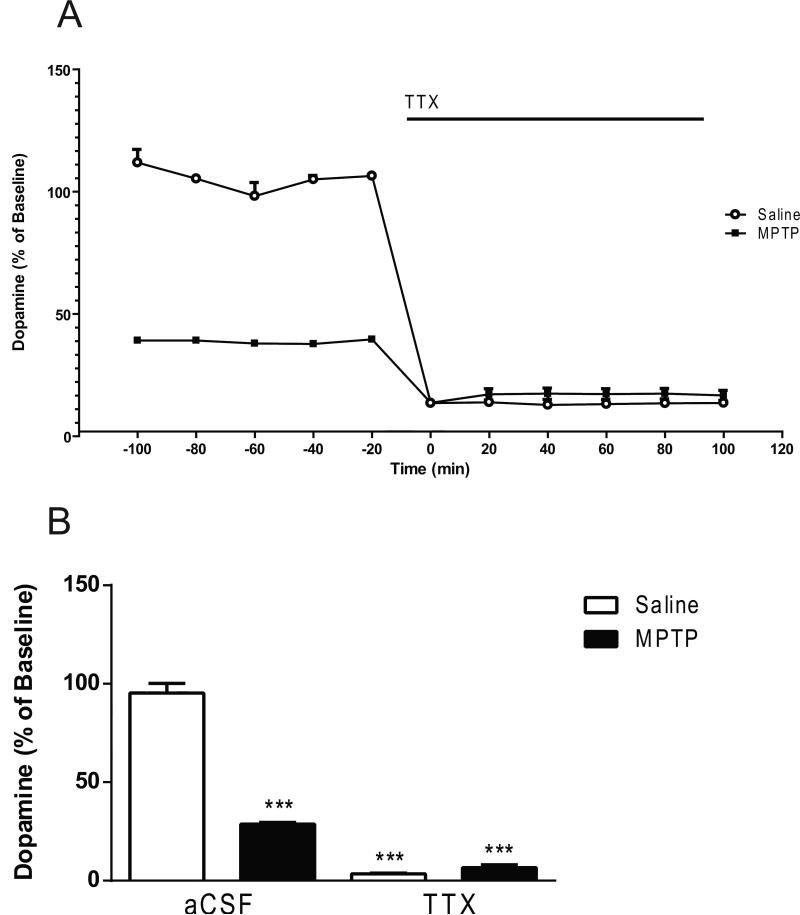
Two-way ANOVA indicated significant main effects of treatment (lesion; F1,42 = 155 , p < 0.0001; drug effect, F2,42 = 76.23, p < 0.0001 and lesion × drug interaction F2,42 = 38.93, p < 0.0001) (Fig. 6). The basal dopamine levels were 80% lower in the MPTP-treated mice (p < 0.0001) compared to the saline-treated mice (1.71 ± 0.05 fmol/ 5 μL sample, mean ± S.E.M.; n= 30) (Fig. 6). Post hoc analysis using the Tukey's multiple comparison test revealed no significant decreases in the dopamine levels subsequent to M100907 application. However, dopamine levels were significantly decreased in the saline-treated (p < 0.0001) and the MPTP-treated mice (p < 0.0001) with the administration of M100907 and TTX (Fig. 6). In the absence of M100907 the addition of 1μL TTX to the perfusion fluid reduced dopamine by 97% in the saline-treated (p < 0.0001) and 79% in the MPTP-treated mice (p<0.0001), in comparison to basal levels of each respective treatment group (lesion; F1,18 = 197.8, P < 0.0001; TTX; F1,18 = 638.5, p < 0.0001; lesion × TTX interaction; F1,18 = 239.4, p < 0.0001) (Fig. 7).
Figure 6.
Effect of M100907 on Dopamine Levels in the Dorsal Striatum.
Data are expressed as percentages of values in vehicle-injected control mice. Mice were treated with four doses of MPTP (20 mg/kg, ip) or saline (10 ml/kg, ip) (n = 11/group) every 2 hours for a total of four doses. Dialysis was carried out 7 days after cannula implantation. (A) Four baseline samples were first collected and then a challenge dose of M100907 (100 nM) and an additional five samples were collected. Finally a challenge dose of M100907 (100 nM) and TTX (1 μM) and five samples were collected. (B) The time course data is represented in histogram. *** Significantly different from saline-treated mice (P < 0.001); in two-way ANOVA with Tukey's post hoc comparison. The line segments on time course graphs indicate duration of drug administration.
Figure 7.
Effect of TTX on Dopamine Levels in the Dorsal Striatum. Data are expressed as percentages of values in vehicle-injected control mice. Mice were treated with four doses of MPTP (20 mg/kg, ip) or saline (10 ml/kg, ip) (n = 11/group) every 2 hours for a total of four doses. Dialysis was carried out 7 days after cannula implantation. (A) Five baseline samples were first collected and then a challenge dose of TTX (1 μM) and six samples were collected. (B) The time course data is represented in histogram. *** Significantly different from saline-treated mice (P < 0.001); in two-way ANOVA with Tukey's post hoc comparison. The line segments on time course graphs indicate duration of drug administration.
3.5. Glutamate transporter expression in the striatum
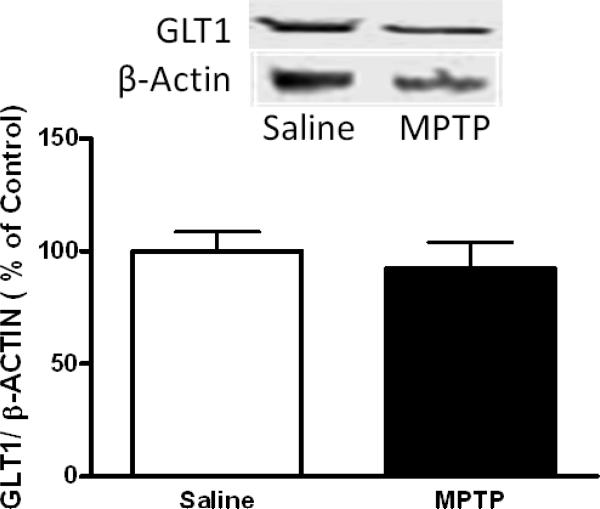
To determine if changes in the basal level of striatal glutamate could be the result of alterations in the density of glutamate transporter (GLT1), semi-quantitative western blot analysis was conducted on tissue from the dorsal striatum. An example of western blot for GLT1 in a saline-and MPTP -treated group is illustrated in Fig. 8. The GLT1 protein was not significantly affected (p > 0.05) by MPTP treatment.
Figure 8.
Expression of GLT1 in the Dorsal Striatum. Western blot analysis of GLT1 expression in homogenate prepared from the dorsal striatum. Exposure to MPTP did not significantly affect the expression of the GLT1 protein expression in the dorsal striatum. Mice were treated with four doses of MPTP (20 mg/kg, ip) or saline (10 ml/kg, ip) every 2 hours for a total of four doses. Optical density values are shown in relation to saline group after normalization to corresponding β-actin bands. Error bars represent mean ±SEM.
3.6. 5-HT2A Receptor Expression in the Striatum
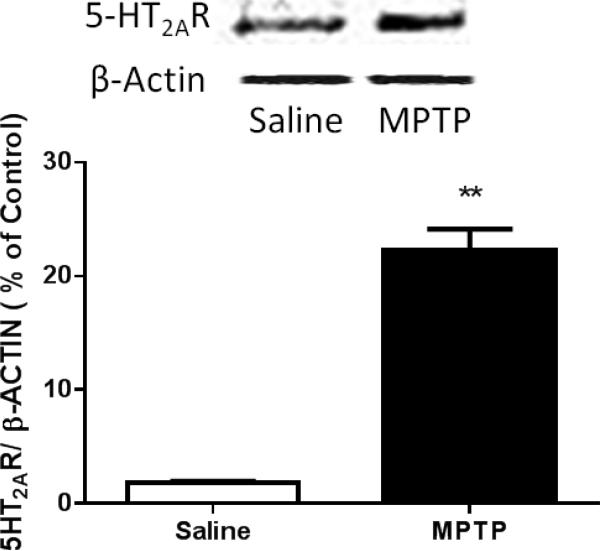
We analyzed the expression of 5-HT2A receptors in the dorsolateral striatum, a major area of input of cortico-striatal projections (McGeorge and Faull, 1989). Synaptosomes prepared from tissue dissected from the dorsal striatum were subjected to Western blot analysis and probed with antibodies for 5-HT2A receptors. The 5-HT2A receptor protein was highly expressed in both saline-treated and MPTP-treated mice (Fig. 9). The 5-HT2A receptor protein was robustly upregulated (p < 0.01) in the MPTP-treated mice in comparision to the saline-treated mice.
Figure 9.
Expression of 5-HT2A receptor (5-HT2AR) in the Dorsal Striatum. Western blot analysis of 5-HT2A receptor expression in synaptosomes prepared from the dorsal striatum. Exposure to MPTP resulted in up regulation of the 5-HT2A receptor. Mice were treated with four doses of MPTP (20 mg/kg, ip) or saline (10 ml/kg, ip) every 2 hours for a total of four doses. Optical density values are shown in relation to saline group after normalization to corresponding β-actin bands. ** significantly different from saline-treated mice (n = 5/group) (P < 0.01). Error bars represent mean ±SEM.
4. Discussion
The current study shows that the administration of the neurotoxin MPTP results in an increase in the basal level of extracellular striatal glutamate when measured 3 weeks after MPTP administration. In addition the MPTP regimen evokes increased serotonergic neurotransmission as reflected in elevated striatal levels of 5-HT. The local administration of the selective 5-HT2A receptor antagonist M100907 to the striatum significantly decreased striatal extracellular glutamate levels in the MPTP-treated mice and the saline-treated mice. It is important to note that M100907 administered into the striatum by reverse microdialysis, produced no effect on DA or 5-HT release in this region. Glutamate, DA, and 5-HT concentrations in striatal dialysates were suppressed by local application of TTX both in the presence and absence of M100907. Overall, these data illustrate that under the experimental conditions used, dialysate glutamate, 5-HT, and DA concentrations are largely derived from neuronal, impulse-dependent release in the striatum. These findings suggest that M100907 is acting directly on corticostriatal projections to inhibit the neuronal release of glutamate in the striatum. In addition we noted an increased expression of 5-HT2A receptors but no changes in GLT-1 in the striatum of MPTP-treated mice.
It has been well established that in PD (Anglade et al., 1996) and rodent models (Ingham et al., 1993; Meshul et al., 2000), nigrostriatal DA depletion leads to increased diameter of postsynaptic density in glutamatergic axo-spinous synapses, suggesting that corticostriatal activity may be increased. In line with these observations, there is evidence for an increase in the basal extracellular levels of striatal glutamate in MPTP-treated mice (Robinson et al., 2003; Holmer et al., 2005; Chassain et al., 2008) and 6-hydroxydopamine-lesioned rats (Lindefors and Ungerstedt, 1990; Meshul et al., 1999; Meshul and Allen 2000; Jonkers et al., 2002; Walker et al., 2009). These findings are in agreement with our studies, though some investigators did not detect any changes in extracellular striatal glutamate (Corsi et al., 2003; Galeffi et al., 2003; Robelet et al., 2004). The discrepancy may be attributable to differences in the PD model used or differences in survival times after lesioning.
The control of the levels of extracellular glutamate is the function of the sodium-dependent transporters (Sheldon et al., 2007). Of the five members of the family of reuptake transporters, GLT-1 is the primary transporter that regulates the extracellular levels of glutamate (Suchak et al., 2003; Maragakis and Rothstein, 2004). There is the possibility that the increased extracellular levels of glutamate associated with loss of DA could result from downregulation of striatal GLT-1. Whereas some groups have reported downregulation of GLT-1 following dopaminergic lesioning (Holmer et al., 2005; Chung et al., 2008), others have observed an upregulation of striatal GLT-1 (Massie et al., 2010). We and others did not detect changes in striatal GLT-1 expression (Lievens et al., 2001). It has been reported that alterations in GLT-1 expression following 6-hydroxydopamine injections is transient and could explain these contradictory findings (Massie et al., 2010). Another possible explanation is that other factors besides glutamate uptake may play a role in influencing the extracellular level of glutamate.
It has been well documented that activation of 5-HT2A receptors in the cortex evokes the release of glutamate (Aghajanian and Marek, 1999; Scruggs et al., 2000, 2003). We observed increased basal levels of 5-HT coupled with the upregulation of 5-HT2A receptor expression. Our data suggest that an enhanced 5-HT2A-mediated neurotransmission in the corticostriatal pathway may contribute to the increase in glutamatergic signaling associated with DA depletion in PD.
4.1. Striatal 5-HT2A neurotransmission and its implications in PD
L-DOPA is arguably the most effective treatment for PD, but patients invariably develop motor fluctuations and dyskinesias after chronic treatment (Lang and Lozano, 1998; Obeso et al., 2000; Dauer and Przedborski, 2003; Fahn, 2003; Nutt and Wooten, 2005). Therefore efforts towards the development of alternative non-dopaminergic treatments are warranted.
Modulation of striatal dopamine release by 5-HT2A compounds has been well investigated. Results have shown that while 5-HT2A receptor activation has no effect on basal dopamine release, stimulated dopamine release is facilitated (Ichikawa and Meltzer, 1995; Gobert and Milan, 1999; Lucas and Spampinato, 2000; Kuroki et al., 2003). Furthermore, it has been noted that 5-HT2A receptor antagonists do not alter striatal dopamine levels when administered under basal conditions (Sorensen et al., 1993; Schmidt and Fadayel, 1996; De Deuwaerdere and Spampinto, 1999; Gobert et al., 2000) but attenuate increases in dopamine release evoked by psychostimulant administration (Schmidt et al., 1994; Porras et al., 2002; Auclair et al., 2004). Under the conditions of our study, it is unlikely that the antiparkinsonian effects of the 5-HT2A antagonist M100907 could be attributed to its effects on dopamine homeostasis in the striatum. How 5-HT2A receptors may modulate motor function can be derived from our understanding of current models of basal ganglia anatomy and physiology (Fig 10). The striatum is the primary input nucleus of the basal ganglia. It receives excitatory glutamatergic input from the cerebral cortex. The major output nuclei of the basal ganglia, the internal globus pallidus (GPi) and the substantia nigra pars reticulata (SNr), receive information from the striatum via two major pathways. The direct pathway consists of monosynaptic inhibitory projections from the striatum to the output nucleus (Fig 10). The net excitatory polysynaptic projections which include the external globus pallidus (GPe) and the subthalamic nucleus (STN), terminating in the output nuclei constitutes the indirect pathway. At the striatal level, dopamine acting on dopamine D1 receptors, facilitates transmission along the direct pathway and inhibits transmission along the indirect pathway through dopamine D2 receptors. It is thought that the delicate balance between inhibition of the output nuclei by the direct pathway and excitation by the indirect pathway is critical for normal control of motor activity, and that modulation of striatal activity by dopamine plays a crucial role in maintaining this balance. In the parkinsonian state, dopamine deficiency leads to an overall increase in excitatory drive in the GPi-SNr, increasing the inhibitory output from GPi-SNr and thus decreased activity in the thalamocortical motor centers (Fig 10). Accordingly, it has been observed that in PD (Anglade et al., 1996) and rodent models (Ingham et al., 1993; Meshul et al., 2000), nigrostriatal DA depletion leads to increased diameter of postsynaptic density in glutamatergic axo-spinous synapses, suggesting that corticostriatal activity may be increased. In line with these observations, there is evidence for an increase in the basal extracellular levels of striatal glutamate in MPTP-treated mice (Robinson et al., 2003; Holmer et al., 2005; Chassain et al., 2008) and 6-hydroxydopamine-lesioned rats (Lindefors and Ungerstedt, 1990; Meshul et al., 1999; Meshul and Allen 2000; Jonkers et al., 2002; Walker et al., 2009). Counteracting the glutamatergic hyperactivity in the striatum may alleviate parkinsonian motor deficits.
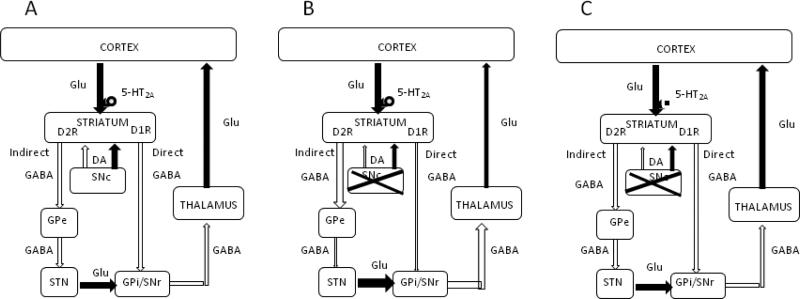
Figure 10.
Basal ganglia circuitry showing proposed antiparkinsonian activity of 5-HT2A receptor antagonists. Excitatory projections are depicted as closed arrows and inhibitory projections are shown as open arrows. (A) The striatum is the primary input nucleus of the basal ganglia. It receives excitatory glutamatergic input from the cerebral cortex. The major output nuclei of the basal ganglia, the internal globus pallidus (GPi) and the substantia nigra pars reticulata (SNr), receive information from the striatum via two major pathways. The direct pathway consists of monosynaptic inhibitory projections from the striatum to the output nucleus. The net excitatory polysynaptic projections which include the external globus pallidus (GPe) and the subthalamic nucleus (STN), terminating in the output nuclei constitutes the indirect pathway. At the striatal level, dopamine acting on dopamine D1 receptors, facilitates transmission along the direct pathway and inhibits transmission along the indirect pathway through dopamine D2 receptors. It is thought that the delicate balance between inhibition of the output nuclei by the direct pathway and excitation by the indirect pathway is critical for normal control of motor activity, and that modulation of striatal activity by dopamine plays a crucial role in maintaining this balance. (B) In the parkinsonian state, loss of dopamine in the substantia nigra pars compacta (SNc) leads to an overall increase in excitatory drive in the GPi-SNr, increasing the inhibitory output from GPi-SNr and thus decreased activity in the thalamocortical motor centers. Activation of 5-HT2A receptors on the terminals of corticostriatal neurons increase glutamatergic transmission. (C) Blocking 5-HT2A receptors will dampen the increased glutamatergic drive arising from dopamine deficiency and restore normal function of the basal ganglia and improve parkinsonian motor deficits.
In situ hybridization and immunohistochemical studies have revealed widespread distribution of 5-HT2A receptors in the striatum (Pompeiano et al., 1994; Ward and Dorsa, 1996; Mijnster et al., 1997; Bubser et al., 2001), but the major source of 5-HT2A receptors appears to be the heteroceptors located on the terminals of the cortico-striatal glutamatergic axons (Bubser et al., 2001). As such, the organization of 5-HT2A-containing afferents to the striatum offers an anatomical substrate for the ability of 5-HT2A antagonists to modulate the dysfunctional basal ganglia circuitry that may be responsible for parkinsonian symptoms. Activation of 5-HT2A heteroceptors in several brain areas has been shown to evoke glutamate release (Aghajanian and Marek, 1997; Scruggs et al., 2000, 2003). We hypothesize that 5-HT2A receptor antagonists may restore motor function by normalizing the overactive glutamatergic drive resulting from DA depletion (Fig 10).
Several studies have examined the 5-HT2A antagonists in PD for their potential effects on LDOPA-induced dyskinesia. The 5-HT2A receptor inverse agonist pimavanserin alleviated LDOPA-induced dyskinesia in the MPTP-lesioned parkinsonian monkey (Vanover et al., 2008) and PD patients (Roberts, 2006). At odds with this finding, the selective 5-HT2A receptor antagonist volinanserin (M100907) failed to reduce L-DOPA-induced dyskinesia in 6-OHDA-lesioned rat (Taylor et al., 2006). Despite the discrepancy it appears that increased serotonergic neurotransmission may play a role in L-DOPA-induced dyskinesia since chronic L-DOPA treatment led to increased 5-HT2A receptor expression in the striatum and cortex of MPTP-lesioned macaques (Riahi et al., 2011; Huot et al., 2012). Increased 5-HT2A receptor mediated neurotransmission will enhance glutamatergic neurotransmission by evoking glutamate release (Aghajanian and Marek, 1999; Scruggs et al., 2003). As we have shown, inhibition of glutamate release in the corticostriatal pathway may be a possible mechanism for the antidyskinetic actions of 5-HT2A receptor antagonists.
5. Conclusions
In conclusion, our studies reveal an increased glutamatergic and serotonergic neurotransmission in the striatum of the parkinsonian mouse model. 5-HT2A receptor antagonists attenuated striatal glutamate with no effect on striatal serotonin or dopamine. Considering that excessive glutamatergic tone is thought to be a pathophysiological feature of Parkinson's disease our findings demonstrate that further exploration of 5-HT2A receptor antagonists as potential therapeutic target for PD is warranted.
There was elevated basal striatal glutamate three weeks after MPTP administration.
Striatal serotonin was elevated, but dopamine was reduced in MPTP-treated mice.
Local infusion of M100907 to the striatum decreased extracellular glutamate levels.
Local Infusion of M100907 had no effect on striatal serotonin or dopamine levels.
Glutamatergic transmission in PD may be normalized by 5-HT2A receptor blockade.
Acknowledgements
We are indebted to Dr. Elaine Sanders-Bush, Vanderbilt University for the generous gift of M100907. This work was supported by National Institute of Neurological Diseases and Stroke of the National Institutes of Health under award number U01NS041071. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- L-DOPA
L-3,4-dihydroxyphenylalanine
- 5-HT
serotonin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajanian GK, Marek GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36:589–599. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin, via 5-HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Res. 1999;825:161–171. doi: 10.1016/s0006-8993(99)01224-x. [DOI] [PubMed] [Google Scholar]
- Ahlqvist J, Isacson R, Wahlestedt C, Salmi P. Anti-cataleptic effects of clozapine, but not olanzapine and quetiapine, on SCH 23390- or raclopride-induced catalepsy in rats. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2003;13:177–182. doi: 10.1016/s0924-977x(03)00004-x. [DOI] [PubMed] [Google Scholar]
- Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16:448–458. doi: 10.1002/mds.1090. [DOI] [PubMed] [Google Scholar]
- Anglade P, Mouatt-Prigent A, Agid Y, Hirsch E. Synaptic plasticity in the caudate nucleus of patients with Parkinson's disease. Neurodegeneration. 1996;5:121–128. doi: 10.1006/neur.1996.0018. [DOI] [PubMed] [Google Scholar]
- Auclair A, Blanc G, Glowinski J, Tassin JP. Role of serotonin 2A receptors in the D-amphetamine-induced release of dopamine: comparison with previous data on alpha1b-adrenergic receptors. J Neurochem. 2004;91:318–326. doi: 10.1111/j.1471-4159.2004.02714.x. [DOI] [PubMed] [Google Scholar]
- Bubser M, Backstrom JR, Sanders-Bush E, Roth BL, Deutch AY. Distribution of serotonin 5-HT(2A) receptors in afferents of the rat striatum. Synapse. 2001;39:297–304. doi: 10.1002/1098-2396(20010315)39:4<297::AID-SYN1012>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Chassain C, Bielicki G, Durand E, Lolignier S, Essafi F, Traore A, Durif F. Metabolic changes detected by proton magnetic resonance spectroscopy in vivo and in vitro in a murin model of Parkinson's disease, the MPTP-intoxicated mouse. J Neurochem. 2008;105:874–882. doi: 10.1111/j.1471-4159.2007.05185.x. [DOI] [PubMed] [Google Scholar]
- Chung EK, Chen LW, Chan YS, Yung KK. Downregulation of glial glutamate transporters after dopamine denervation in the striatum of 6-hydroxydopamine-lesioned rats. J Comp Neurol. 2008;511:421–437. doi: 10.1002/cne.21852. [DOI] [PubMed] [Google Scholar]
- Corsi C, Pinna A, Gianfriddo M, Melani A, Morelli M, Pedata F. Adenosine A2A receptor antagonism increases striatal glutamate outflow in dopamine-denervated rats. Eur J Pharmacol. 2003;464:33–38. doi: 10.1016/s0014-2999(03)01352-9. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- De Deurwaerdere P, Spampinato U. Role of serotonin(2A) and serotonin(2B/2C) receptor subtypes in the control of accumbal and striatal dopamine release elicited in vivo by dorsal raphe nucleus electrical stimulation. J Neurochem. 1999;73:1033–1042. doi: 10.1046/j.1471-4159.1999.0731033.x. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Pierucci M, Esposito E, Crescimanno G, Benigno A, Di Giovanni G. Serotonin modulation of the basal ganglia circuitry: therapeutic implication for Parkinson's disease and other motor disorders. Prog Brain Res. 2008;172:423–463. doi: 10.1016/S0079-6123(08)00921-7. [DOI] [PubMed] [Google Scholar]
- Fahn S. Description of Parkinson's disease as a clinical syndrome. Ann N Y Acad Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- Ferguson MC, Nayyar T, Deutch AY, Ansah TA. 5-HT2A receptor antagonists improve motor impairments in the MPTP mouse model of Parkinson's disease. Neuropharmacology. 2010;59:31–36. doi: 10.1016/j.neuropharm.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeffi F, Bianchi L, Bolam JP, Della Corte L. The effect of 6-hydroxydopamine lesions on the release of amino acids in the direct and indirect pathways of the basal ganglia: a dual microdialysis probe analysis. Eur J Neurosci. 2003;18:856–868. doi: 10.1046/j.1460-9568.2003.02795.x. [DOI] [PubMed] [Google Scholar]
- Gobert A, Millan MJ. Serotonin (5-HT)2A receptor activation enhances dialysate levels of dopamine and noradrenaline, but not 5-HT, in the frontal cortex of freely-moving rats. Neuropharmacology. 1999;38:315–317. doi: 10.1016/s0028-3908(98)00188-9. [DOI] [PubMed] [Google Scholar]
- Gobert A, Rivet JM, Lejeune F, Newman-Tancredi A, Adhumeau-Auclair A, Nicolas JP, Cistarelli L, Melon C, Millan MJ. Serotonin(2C) receptors tonically suppress the activity of mesocortical dopaminergic and adrenergic, but not serotonergic, pathways: a combined dialysis and electrophysiological analysis in the rat. Synapse. 2000;36:205–221. doi: 10.1002/(SICI)1098-2396(20000601)36:3<205::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Holmer HK, Keyghobadi M, Moore C, Meshul CK. l-dopa-induced reversal in striatal glutamate following partial depletion of nigrostriatal dopamine with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neuroscience. 2005;136:333–341. doi: 10.1016/j.neuroscience.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Huot P, Johnston TH, Winkelmolen L, Fox SH, Brotchie JM. 5-HT2A receptor levels increase in MPTP-lesioned macaques treated chronically with L-DOPA. Neurobiology of aging. 2012;33:194, e195–115. doi: 10.1016/j.neurobiolaging.2010.04.035. [DOI] [PubMed] [Google Scholar]
- Hurtig HI. Problems with current pharmacologic treatment of Parkinson's disease. Exp Neurol. 1997;144:10–16. doi: 10.1006/exnr.1996.6380. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Meltzer HY. DOI, a 5-HT2A/2C receptor agonist, potentiates amphetamine-induced dopamine release in rat striatum. Brain Res. 1995;698:204–208. doi: 10.1016/0006-8993(95)00865-n. [DOI] [PubMed] [Google Scholar]
- Ingham CA, Hood SH, Taggart P, Arbuthnott GW. Plasticity of synapses in the rat neostriatum after unilateral lesion of the nigrostriatal dopaminergic pathway. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:4732–4743. doi: 10.1523/JNEUROSCI.18-12-04732.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham CA, Hood SH, van Maldegem B, Weenink A, Arbuthnott GW. Morphological changes in the rat neostriatum after unilateral 6-hydroxydopamine injections into the nigrostriatal pathway. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1993;93:17–27. doi: 10.1007/BF00227776. [DOI] [PubMed] [Google Scholar]
- Jonkers N, Sarre S, Ebinger G, Michotte Y. MK801 suppresses the L-DOPA-induced increase of glutamate in striatum of hemi-Parkinson rats. Brain Res. 2002;926:149–155. doi: 10.1016/s0006-8993(01)03147-x. [DOI] [PubMed] [Google Scholar]
- Kane JM. Extrapyramidal side effects are unacceptable. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2001;11(Suppl 4):S397–403. doi: 10.1016/s0924-977x(01)00109-2. [DOI] [PubMed] [Google Scholar]
- Kuroki T, Meltzer HY, Ichikawa J. 5-HT 2A receptor stimulation by DOI, a 5-HT 2A/2C receptor agonist, potentiates amphetamine-induced dopamine release in rat medial prefrontal cortex and nucleus accumbens. Brain Res. 2003;972:216–221. doi: 10.1016/s0006-8993(03)02516-2. [DOI] [PubMed] [Google Scholar]
- Lang AE, Lozano AM. Parkinson's disease. First of two parts. N Engl J Med. 1998;339:1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- Lievens JC, Salin P, Nieoullon A, Kerkerian-Le Goff L. Nigrostriatal denervation does not affect glutamate transporter mRNA expression but subsequent levodopa treatment selectively increases GLT1 mRNA and protein expression in the rat striatum. J Neurochem. 2001;79:893–902. doi: 10.1046/j.1471-4159.2001.00644.x. [DOI] [PubMed] [Google Scholar]
- Lindefors N, Ungerstedt U. Bilateral regulation of glutamate tissue and extracellular levels in caudate-putamen by midbrain dopamine neurons. Neurosci Lett. 1990;115:248–252. doi: 10.1016/0304-3940(90)90463-j. [DOI] [PubMed] [Google Scholar]
- Lucas G, Bonhomme N, De Deurwaerdere P, Le Moal M, Spampinato U. 8-OH-DPAT, a 5-HT1A agonist and ritanserin, a 5-HT2A/C antagonist, reverse haloperidol-induced catalepsy in rats independently of striatal dopamine release. Psychopharmacology (Berl) 1997;131:57–63. doi: 10.1007/s002130050265. [DOI] [PubMed] [Google Scholar]
- Lucas G, Spampinato U. Role of striatal serotonin2A and serotonin2C receptor subtypes in the control of in vivo dopamine outflow in the rat striatum. J Neurochem. 2000;74:693–701. doi: 10.1046/j.1471-4159.2000.740693.x. [DOI] [PubMed] [Google Scholar]
- Maragakis NJ, Rothstein JD. Glutamate transporters: animal models to neurologic disease. Neurobiol Dis. 2004;15:461–473. doi: 10.1016/j.nbd.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Massie A, Goursaud S, Schallier A, Vermoesen K, Meshul CK, Hermans E, Michotte Y. Time-dependent changes in GLT-1 functioning in striatum of hemi-Parkinson rats. Neurochemistry international. 2010;57:572–578. doi: 10.1016/j.neuint.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. The mechanism of action of novel antipsychotic drugs. Schizophrenia bulletin. 1991;17:263–287. doi: 10.1093/schbul/17.2.263. [DOI] [PubMed] [Google Scholar]
- Meshul CK, Allen C. Haloperidol reverses the changes in striatal glutamatergic immunolabeling following a 6-OHDA lesion. Synapse. 2000;36:129–142. doi: 10.1002/(SICI)1098-2396(200005)36:2<129::AID-SYN6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Meshul CK, Cogen JP, Cheng HW, Moore C, Krentz L, McNeill TH. Alterations in rat striatal glutamate synapses following a lesion of the cortico- and/or nigrostriatal pathway. Exp Neurol. 2000;165:191–206. doi: 10.1006/exnr.2000.7467. [DOI] [PubMed] [Google Scholar]
- Meshul CK, Emre N, Nakamura CM, Allen C, Donohue MK, Buckman JF. Time-dependent changes in striatal glutamate synapses following a 6-hydroxydopamine lesion. Neuroscience. 1999;88:1–16. doi: 10.1016/s0306-4522(98)00189-4. [DOI] [PubMed] [Google Scholar]
- Mijnster MJ, Raimundo AG, Koskuba K, Klop H, Docter GJ, Groenewegen HJ, Voorn P. Regional and cellular distribution of serotonin 5-hydroxytryptamine2a receptor mRNA in the nucleus accumbens, olfactory tubercle, and caudate putamen of the rat. J Comp Neurol. 1997;389:1–11. [PubMed] [Google Scholar]
- Murphy CA, Feldon J. Low-dose clozapine pretreatment partially prevents haloperidol-induced deficits in conditioned active avoidance. Behavioural pharmacology. 2000;11:307–316. doi: 10.1097/00008877-200006000-00014. [DOI] [PubMed] [Google Scholar]
- Nayyar T, Bubser M, Ferguson MC, Diana Neely M, Shawn Goodwin J, Montine TJ, Deutch AY, Ansah TA. Cortical serotonin and norepinephrine denervation in parkinsonism: preferential loss of the beaded serotonin innervation. Eur J Neurosci. 2009;30:207–216. doi: 10.1111/j.1460-9568.2009.06806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt JG. Levodopa-induced dyskinesia: review, observations, and speculations. Neurology. 1990;40:340–345. doi: 10.1212/wnl.40.2.340. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Wooten GF. Clinical practice. Diagnosis and initial management of Parkinson's disease. N Engl J Med. 2005;353:1021–1027. doi: 10.1056/NEJMcp043908. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Olanow CW, Nutt JG. Levodopa motor complications in Parkinson's disease. Trends Neurosci. 2000;23:S2–7. doi: 10.1016/s1471-1931(00)00031-8. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Porras G, Di Matteo V, Fracasso C, Lucas G, De Deurwaerdere P, Caccia S, Esposito E, Spampinato U. 5-HT2A and 5-HT2C/2B receptor subtypes modulate dopamine release induced in vivo by amphetamine and morphine in both the rat nucleus accumbens and striatum. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;26:311–324. doi: 10.1016/S0893-133X(01)00333-5. [DOI] [PubMed] [Google Scholar]
- Riahi G, Morissette M, Parent M, Di Paolo T. Brain 5-HT(2A) receptors in MPTP monkeys and levodopa-induced dyskinesias. Eur J Neurosci. 2011;33:1823–1831. doi: 10.1111/j.1460-9568.2011.07675.x. [DOI] [PubMed] [Google Scholar]
- Robelet S, Melon C, Guillet B, Salin P, Kerkerian-Le Goff L. Chronic L-DOPA treatment increases extracellular glutamate levels and GLT1 expression in the basal ganglia in a rat model of Parkinson's disease. Eur J Neurosci. 2004;20:1255–1266. doi: 10.1111/j.1460-9568.2004.03591.x. [DOI] [PubMed] [Google Scholar]
- Roberts C. ACP-103, a 5-HT2A receptor inverse agonist. Current opinion in investigational drugs. 2006;7:653–660. [PubMed] [Google Scholar]
- Robinson S, Freeman P, Moore C, Touchon JC, Krentz L, Meshul CK. Acute and subchronic MPTP administration differentially affects striatal glutamate synaptic function. Exp Neurol. 2003;180:74–87. doi: 10.1016/s0014-4886(02)00050-x. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Fadayel GM. Regional effects of MK-801 on dopamine release: effects of competitive NMDA or 5-HT2A receptor blockade. J Pharmacol Exp Ther. 1996;277:1541–1549. [PubMed] [Google Scholar]
- Schmidt CJ, Sullivan CK, Fadayel GM. Blockade of striatal 5-hydroxytryptamine2 receptors reduces the increase in extracellular concentrations of dopamine produced by the amphetamine analogue 3,4-methylenedioxymethamphetamine. J Neurochem. 1994;62:1382–1389. doi: 10.1046/j.1471-4159.1994.62041382.x. [DOI] [PubMed] [Google Scholar]
- Scruggs JL, Patel S, Bubser M, Deutch AY. DOI-Induced activation of the cortex: dependence on 5-HT2A heteroceptors on thalamocortical glutamatergic neurons. J Neurosci. 2000;20:8846–8852. doi: 10.1523/JNEUROSCI.20-23-08846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scruggs JL, Schmidt D, Deutch AY. The hallucinogen 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) increases cortical extracellular glutamate levels in rats. Neurosci Lett. 2003;346:137–140. doi: 10.1016/s0304-3940(03)00547-0. [DOI] [PubMed] [Google Scholar]
- Sheldon AL, Robinson MB. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochemistry international. 2007;51:333–355. doi: 10.1016/j.neuint.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen SM, Kehne JH, Fadayel GM, Humphreys TM, Ketteler HJ, Sullivan CK, Taylor VL, Schmidt CJ. Characterization of the 5-HT2 receptor antagonist MDL 100907 as a putative atypical antipsychotic: behavioral, electrophysiological and neurochemical studies. J Pharmacol Exp Ther. 1993;266:684–691. [PubMed] [Google Scholar]
- Suchak SK, Baloyianni NV, Perkinton MS, Williams RJ, Meldrum BS, Rattray M. The ‘glial’ glutamate transporter, EAAT2 (Glt-1) accounts for high affinity glutamate uptake into adult rodent nerve endings. J Neurochem. 2003;84:522–532. doi: 10.1046/j.1471-4159.2003.01553.x. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Bishop C, Ullrich T, Rice KC, Walker PD. Serotonin 2A receptor antagonist treatment reduces dopamine D1 receptor-mediated rotational behavior but not L-DOPA-induced abnormal involuntary movements in the unilateral dopamine-depleted rat. Neuropharmacology. 2006;50:761–768. doi: 10.1016/j.neuropharm.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Vanover KE, Betz AJ, Weber SM, Bibbiani F, Kielaite A, Weiner DM, Davis RE, Chase TN, Salamone JD. A 5-HT2A receptor inverse agonist, ACP-103, reduces tremor in a rat model and levodopa-induced dyskinesias in a monkey model. Pharmacol Biochem Behav. 2008;90:540–544. doi: 10.1016/j.pbb.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RH, Koch RJ, Sweeney JE, Moore C, Meshul CK. Effects of subthalamic nucleus lesions and stimulation upon glutamate levels in the dopamine-depleted rat striatum. Neuroreport. 2009;20:770–775. doi: 10.1097/WNR.0b013e32832ad556. [DOI] [PubMed] [Google Scholar]
- Ward RP, Dorsa DM. Colocalization of serotonin receptor subtypes 5-HT2A, 5-HT2C, and 5-HT6 with neuropeptides in rat striatum. J Comp Neurol. 1996;370:405–414. doi: 10.1002/(SICI)1096-9861(19960701)370:3<405::AID-CNE10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- Westerink BH, Tuntler J, Damsma G, Rollema H, de Vries JB. The use of tetrodotoxin for the characterization of drug-enhanced dopamine release in conscious rats studied by brain dialysis. Naunyn-Schmiedeberg's archives of pharmacology. 1987;336:502–507. doi: 10.1007/BF00169306. [DOI] [PubMed] [Google Scholar]
- Young CD, Bubser M, Meltzer HY, Deutch AY. Clozapine pretreatment modifies haloperidol-elicited forebrain Fos induction: a regionally-specific double dissociation. Psychopharmacology (Berl) 1999;144:255–263. doi: 10.1007/s002130051001. [DOI] [PubMed] [Google Scholar]