Abstract
Anti-mullerian hormone (AMH) is thought to reflect the growth of follicles and the ovarian function. However, the role of AMH in culture medium during in vitro maturation (IVM) on oocyte quality and subsequent development potential is unclear. The objective of this study is to investigate the effect of recombinant human AMH (rh-AMH) supplemented into IVM medium on oocyte quality. Cumulus-oocyte complexes (COCs) were obtained from ICR mice and cultured in vitro with the different concentrations (0–1,000 ng/ml) of rh-AMH. Following 16–18 h of culture, quantitative PCR and ELISA were performed to analyze GDF9 and BMP15 mRNA expression and protein production from the oocytes. Subsequently, in vitro fertilization (IVF) and early embryonic development were employed to further evaluate the quality of in vitro matured oocytes. The results showed that AMH was only expressed in cumulus cells but not in the oocytes. However, AMH most specific receptor, AMHR-II, was expressed in both oocytes and cumulus cells. The levels of GDF9 and BMP15 expression and blastocyst formation rate were significantly increased (p<0.05) when the IVM medium was supplemented with 100 ng/ml of rh-AMH. With AdH1-SiRNA/AMH for knocking down of AMH expression during IVM significantly reduced (p<0.05) the levels of GDF9 and BMP15 expression and blastocysts formation rate. These results suggest that AHM improves oocytes quality by up-regulating GDF9 and BMP15 expressions during IVM.
Introduction
Anti-mullerian hormone (AMH, also known as Mullerian inhibiting substance [MIS]), belonging to the transforming growth factor β (TGF-β) superfamily [1], is well known for its role in male sexual differentiation [2]. AMH signals through a receptor complex consisting of type I (AMHR-I) and type II (AMHR-II) receptors. AMH and AMHR-II are mutually specific [3], [4]. The formation of the AMH signaling complex is initiated by the binding of AMH to AMHRII, which activates type I receptor (AMHR-I) then phosphorylates the cytoplasmic Sma- and Mad-related proteins (Smads) in concert with other transcription factors to regulate responsive genes [5].
In female, AMH is only secreted by granulosa cells and cumulus cells. As studies have found that AMH functions inhibitory effects on follicle sensitivity to FSH and initial follicle recruitment [4]–[7], AMH has been thought to be an important player in two checkpoints that regulate the efficiency of primordial follicle pool usage and the choice of the dominant follicle: recruitment and selection, respectively. Earlier studies using anti-AMH-deficient mice suggested that AMH is involved in the regulation of primordial follicle recruitment [5]. It also has been demonstrated that AMH inhibits initiation of primordial follicle growth [6]. Therefore, AMH acts as an inhibitory growth factor in the ovary during the early stages of folliculogenesis. Since AMH is mainly produced in the growing ovarian follicles, it has been fully confirmed that serum AMH is positively related to the number of antral follicles, and the value of serum AMH has been widely used in predicting ovarian reverse for infertility treatment [8]–[13].
In addition, some studies found a positive correlation between the levels of AMH in follicular fluid (FF) and rates of fertilization/implantation/clinical pregnancy/high quality embryo in human in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) treatments [14]–[17]. Recently, Mehta et al. [18] reported that there is an inverse correlation of the AMH levels in FF with clinical pregnancy outcomes. However, Anckaert et al. [19] indicated that there is no correlation between the embryo quality and AMH in FF. Therefore, the correlation between the levels of AMH in FF and clinical outcomes is controversial. The role of AMH in affecting oocyte quality is still largely unknown
Immature oocyte retrieval followed by in vitro maturation (IVM) of those immature oocytes is a potentially useful treatment in assisted reproductive technology (ART) and female fertility preservation [20]. However, the clinical pregnancy and live birth rates have been shown lower in IVM oocytes comparing to the conventional gonadotropin stimulated in vivo matured oocytes [21]. It has been also shown that the there is higher early pregnancy losses with IVM oocytes [22]. Although a number of studies attempted to improve oocyte quality by modifying IVM medium, the results met limited success [23]. There is no information available for the effect of AMH supplemented into IVM medium on oocyte quality improvement.
The objective of the present study was to determine the effect of AMH on oocyte quality during IVM evaluated by both molecular evidence and subsequent embryonic development potential following IVF.
Materials and Methods
Animals
ICR mice were fed with a standard diet and maintained in a temperature-controlled room (20–22°C), on a 12/12 h light/dark cycle. All experiments were approved by Animal and Human Ethics Board of Nanjing Medical University, and were conducted in accordance with the Animal Research Committee Guidelines of Nanjing Medical University.
Isolation of Immature Cumulus-Oocyte Complexes (COCs) and In Vitro Maturation (IVM)
Immature COCs were obtained from female mice (6–8 weeks of age) stimulated with 10 IU equine chorionic gonadotropin (eCG) (Folligon; Intervet, Castle Hill, Australia). At 44 h post eCG injection, mice were euthanized by cervical dislocation. The ovaries were removed and placed in IVM medium, alpha modification of minimum essential medium (αMEM) (M4526, Sigma, USA) supplemented with 10% fetal bovine serum (FBS) (12003C, Sigma, Australia), 0.25 mM sodium pyruvate (11639, GIBCO, Australia), 50 mIU/ml recombinant human follicle-stimulating hormone (FSH) (F4021, Sigma, USA) and 3 ng/ml epidermal growth factor (EGF) (96-AF-100-15-1000, Peprotech, USA). COCs were isolated by puncturing of ovarian follicles with a needle, collected after washing twice and then subjected immediately to maturation in culture. Ten immature COCs were culture in each 30 µl droplet of IVM medium covered by mineral oil (MKBG7544V, Sigma, USA) at 37°C with 5% CO2 in air for 16–18 h [24].
Immunofluorescence Staining for AMH and AMHR-II
COC samples were fixed in 3–4% paraformaldehyde in phosphate buffered saline (PBS) for 25 minutes at room temperature, and then incubated for 30 minutes with PBS containing 0.01% triton X-100. The fixed and permeabilized COCs were further incubated with 1% bovine serum albumin (BSA)(A2058, Sigma, USA) in PBS for 30 minutes at room temperature, followed by incubating with anti-AMH (1∶50 dilution in PBS, SC- 6886, Santa Cruz, USA), anti-AMHR-II (1∶50 dilution in PBS, SC- 67287, Santa Cruz, USA) antibody in a humidified chamber at 4°C for overnight. COCs were washed with PBS and subsequently incubated with goat anti-rabbit IgG-TRITC (1∶50 dilution, BA1090, Boster, China) or rabbit anti-goat IgG-TRITC (1∶50 dilution, BA1091, Boster, China) for 1 hour at room temperature in dark. As the final step, COCs were incubated with 0.1 g/ml DAPI for 1 minute, and the images were captured with a fluorescence microscope (NIKON TE2000).
Extraction of RNA from Cumulus Cells and Oocytes/PCR and Real-Time Reverse-Transcription PCR
Total RNA isolation was performed using the RNeasy Micro Kit (145020967, Qiagen, USA) from oocytes and cumulus cells respectively. In-vitro RT-PCR was performed using Sensiscript Reverse Transcription Kit (A2058, Qiagen, USA) and oligo-dT (S0296, Takara, Japan) primer at 37°C for 1 hour. For real-time PCR reaction, cDNA was used as template for amplification to quantify the mRNA concentrations of the tested genes using Quanti Test SYBR Green PCR kits (204145, Takara, Japan). Quantification of gene expression was estimated by the standard curve method and each experiment was repeated at least three times. The PCR products (15 µl) were run on a 1.2% (wt/vol) agarose gel, stained with ethidiumbromide (25 mg/ml). The specific primer sequences were summarized in Table 1.
Table 1. Real-time PCR primer sequences.
| Gene | GenBank accession no. | Forward primer | Reverse primer | PCR size (bp) |
| AMH | NM_007445.2 | TGCTAGTCCTACATCTGGCTGA | GTCCAGGGTATAGCACTAACAGG | 120 |
| AMHRII | NM_144547.2 | GCAGCACAAGTATCCCCAAAC | GTCTCGGCATCCTTGCATCTC | 204 |
| GDF9 | NM_008110.2 | TGGAACACTTGCTCAAATCGG | GACATGGCCTCCTTTACCACA | 106 |
| BMP15 | NM_009757.4 | GAAAATGGTGAGGCTGGTAAAG | AGATGAAGTTGATGGCGGTAAA | 153 |
| GAPDH | NM_008084.2 | GGGTGGTCCAGGGTTTCTTACT | AGGTTGTCTCCTGCGACTTCA | 187 |
ELISA for GDF9 and BMP15 Proteins
COCs cultured supernatants were collected by centrifugation (20,00 g for 10 minutes) and stored at −80°C. The procedure was performed with a GDF9 ELISA Kit (SC-12244, Santa Cruz, USA) and a BMP15 ELISA Kit (E03B0394, Blue Gene, China) following the manufacturer's instructions. OD values were measured at 450 nm using a spectrophotometer (M680, Bio-Rad, USA). The concentrations of GDF9 and BMP15 in the samples were determined by comparing the OD values of the samples to the standard curve.
Construction of Recombinant Adenovirus
In order to further confirm the role of rh-AMH in regulating oocyte maturation, AdH1-SiRNA/AMH adenoviruses were generated by Sunbio Company (Shanghai, China). For AMH knockdown, recombinant adenoviruses AdH1-SiRNA/AMH expressing siRNA targeting AMH and AdH1-SiRNA/NS (non-silencing) were constructed. The potential target sequences for RNA interference (RNAi) were scanned with the siRNA Target Finder and Design Tool available from the Ambion Website. The selected target sequence (SiRNA/AMH), 5′-CCGGGCAGTTGCTAGTCCTACATTTCAAGAGAATGTAGGACTAGCAACTGCTTTTTTG-3′, corresponded to region 375–393 bp after the AMH start codon. The negative control (SiRNA/NS) sequence was 5′-CCGGTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTTG-3′. These sequences were sub-cloned into adenoviral shuttle vector pShuttle-H1 according to the method used by Shen et al [25]. The pShuttle-H1-siRNA/AMH and pShuttle-H1-siRNA/NS were then recombined with backbone plasmid pAdEasy-1 in BJ5183 bacteria. Adenovirus generation, amplification and titer examinations were performed according to the simplified system described by He et al [26]. Viral titer, determined by plaque assay in 293 cells, was 5.2×1010 ifu (infectious units)/ml in AdH1-SiRNA/AMH and 2.4×1011 ifu/ml in AdH1-SiRNA/NS.
Verification was performed in mouse granulosa cells before use in this study. After infecting the mouse granulosa cells with AdH1-SiRNA/AMH or the control AdH1-SiRNA/NS adenoviruses for 48 h, more than 80 percent of granulose cells were infected by recombinant adenoviruses andAMH expression was decreased over 80% after infection with AdH1-SiRNA/AMH compared to AdH1-SiRNA/NS (Figure S1). COCs were infected with AdH1-SiRNA with different multiplicity of infection (MOI) (3×106 ifu/COC, 3×105 ifu/COC, 3×104 ifu/COC, 3×103 ifu/COC, 3×102 ifu/COC) for 18 h, and showed that there were more than 90% cumulus infected by recombinant adenoviruses without obvious cell-damaging when cumulus cells were infected by AdH1-SiRNA/AMH with 3×104 ifu/COC. Therefore, COCs were cultured in vitro with AdH1-SiRNA/AMH or AdH1-SiRNA/NS at 3×104 ifu/COC respectively for the further experiments.
In Vitro Fertilization (IVF) and Embryonic Development Culture
Following 16–18 h of IVM, COCs were washed twice in IVF medium (HTF medium, In Vitro Care, USA), and then 10–15 COCs were placed into 50 µl of droplet IVF medium under mineral oil that were prepared at least 2 h in advance and equilibrated at 37°C in 5% CO2 incubator. Epididymal sperm suspensions are prepared from the male ICR mice, and pre-incubated for 60 minutes in IVM medium containing 9.0 mg/ml BSA to ensure sperm capacitation. The final concentration of 2×106 sperm/ml was introduced into 50 µl of droplet contained the maximum of 15 COCs. Sperm and oocytes were incubated together for 6 h, and then the oocytes were washed to place into 20 µl of KSOM+AA medium (MR-121-D, Millipore, USA) droplet under mineral oil for further developmental culture at 37°C a high humidified and 5% CO2 incubator. The cleavage rate was confirmed 24 h after insemination, and then the cleaved embryos continued culture until day 5 (120 h post-insemination) without changing the medium. At the end of culture, the percentages of blastocyst formation were assessed, and then provided for differential staining.
Differential Staining of Blastocysts
Blastocysts were fixed in 4% formaldehyde solution in PBS for 15 minutes at room temperature. They were washed in PBT (with 0.1% Tween 20) and permeabilized with 0.1% Triton in PBS for 15 minutes, followed by treating with primary antibodies overnight at 4°C. Primary antibodies used in this study were OCT4 (1∶200 dilution, 19857, Abcam, USA). Embryos were incubated with goat anti-rabbit IgG-TRITC (1∶50 dilution, BA1090, Boster, China) for 1 h at room temperature in dark. Finally, blastocysts were incubated with 0.1/ml DAPI for 1 min. The nuclei of blastocyst were observed immediately under fluorescence microscope (NIKON TE2000) equipped with a UV filter (with excitation set at 355–530 nm and emission at 465–615 nm with a long pass filter). The nuclei of the ICM were labeled with OCT4 appeared red and the nuclei of the total cell numbers (TCN) were stained with DAPI fluoresced blue.
Experimental Design
Localization of AMH and AMHR II in COCs
AMH works by interacting with specific receptors on the surfaces of the target cells. The best-known and most specific effects of AMH mediate through AMHR II [27]. To understand how AMH influences the quality of oocytes, the expression of AMH and AMHR II in COCs with mRNA levels were examined firstly, and then for the localization of AMH and AMHR-II in COCs with protein level were examined only for the cultured (16–18 hours) COCs by immunofluorescence staining.
Effects of different concentrations of rh-AMH on oocyte maturation in vitro
The IVM medium was supplemented with 0, 1, 10, 100 and 1,000 ng/ml of recombinant human (rh)-AMH respectively for IVM. At the end of IVM culture, COCs were denuded with 80 unit/ml hyaluronidase in IVM medium for assessment of oocyte maturity. The oocytes with extrusion of first polar body (1PB) were considered mature at metaphase-II (M-II) stage. Oocyte maturation rates in each group were compared in order to determine the optimal concentration of rh-AMH during IVM. The optimal concentration of rh-AMH during IVM was also further determined by both GDF9 and BMP 15, the key factors in oocyte maturation, in mRNA and protein levels.
Changes of GDF9 and BMP15 during IVM with or without rh-AMH
Since both mRNA expression and protein level of GDF9 and BMP15 were significantly higher in IVM medium supplemented with 100 ng/ml of rh-AMH, the changes of time course of GDF9 and BMP15 profiles were further examined during IVM (6, 12 and 18 hours respectively) in order to determine the effect of 100 ng/ml rh-AMH on oocyte quality during IVM.
Supplementation of rh-AMH into IVM medium on subsequent embryonic development
To determine the effect of rh-AMH on oocyte quality during IVM, COCs were cultured in IVM medium with or without 100 n/ml of rh-AMH, respectively. Following IVM and IVF, 2-cell stage embryos were recorded on the second day post-insemination, and blastocyst formation rates were observed on day 5 after embryo culture. To further confirm the role of rh-AMH in regulating oocyte quality, COCs were infected with AdH1-SiRNA/AMH or AdH1-SiRNA/NS adenoviruses during IVM. Then the embryonic developments (2-cell and blastocyst stages) in each group were compared following IVF. The quality of blastocysts was evaluated by the ratio of inner cell mass (ICM)/total cell number (TCN) following the differential staining.
Statistical Analysis
The data were processed for statistical analysis by SPSS 16.0 and are presented as the mean±SD. Statistical comparisons between each group were calculated by independent-sample t-test and p<0.05 were considered statistically significant.
Results
Localization of AMH and AMHR II in COCs
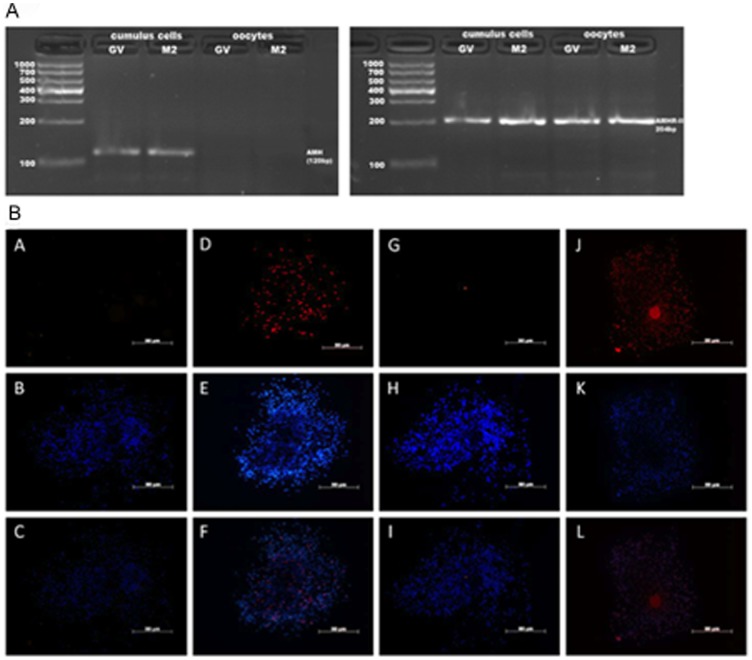
As shown in Figure 1, AMH was only expressed in cumulus and granulose cells before and after IVM, but not in the oocytes. However, AMHR II was expressed in both oocytes and cumulus cells before and after IVM.
Figure 1. Localization of AMH and AMHR-II by PCR and immunofluorescence staining.
(A) RNA was isolated from oocytes and cumulus cells from 30 COCs that were at GV stage or at MII stage after IVM, respectively. One-microliter amounts of cDNA were used as templates for PCR. (B) COCs after 16–18 h of culture in vitro were stained with mouse isotype IgGs as negative controls of AMH (A) and AMHR-II (G), AMH (D), AMHR-II (J) respectively, followed by TRITC conjugated secondary antibodies and DAPI (B,E,H,K), and merged images (C,F,I,L). Bars = 50 µm.
Effects of Different Concentrations of rh-AMH on Oocyte Maturation In Vitro
The oocyte maturation rates were not significantly different among groups when IVM medium was supplemented with 0, 1, 10, 100 and 1,000 ng/ml of rh-AMH for IVM (Table 2). Although there were no differences among groups for oocyte maturation, the expressions of GDF9 and BMP15 in oocytes and the levels of protein in IVM medium were a dose-dependent manner when rh-AMH at concentrations of 1–100 ng/ml. However, the expressions of GDF9 and BMP15 in the oocytes and the protein levels in IVM medium were significantly down-regulated at concentration of 1,000 ng/ml of rh-AMH during IVM compared to 100 ng/ml of rh-AMH group (Figure 2).
Table 2. The effect of different concentrations of rh-AMH in IVM-medium on mouse oocyte maturation following IVM.
| Concentration of rh-AMH (ng/ml) | No. of COCs examined | No. of oocytes matured (%, mean±SD)* |
| 0 | 115 | 111 (96.6±4.1) |
| 1 | 114 | 108 (94.8±4.5) |
| 10 | 112 | 105 (93.8±1.9) |
| 100 | 111 | 107 (96.1±6.1) |
| 1,000 | 118 | 110 (93.2±2.6) |
Data showed with 6 replicates.
*There are no significant differences among groups.
Figure 2. Effect of different concentrations of rh-AMH supplemented into IVM medium on GDF9 and BMP15 mRNA expression and protein production from the oocytes.
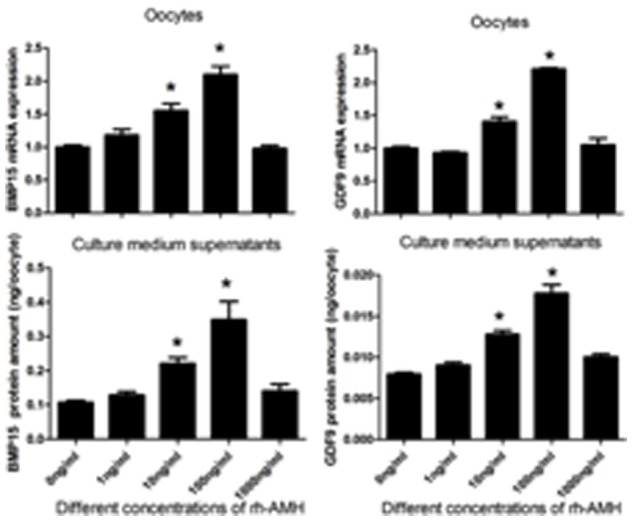
GDF9 and BMP15 mRNA in the oocytes were measured by RT-PCR, and GDF9 and BMP15 proteins in IVM-medium were measured by ELISA, respectively. Oocytes were harvested at 16–18 h after IVM. *Indicates significant differences (p<0.05) compared to the control group. Data were from 3 replicates.
Changes of GDF9 and BMP15 during IVM with or without rh-AMH
As shown in Figure 3, although there were no differences in both gene expressions and protein levels of GDF9 and BMP15 in the oocytes and IVM medium until COCs cultured to 6 h, the expressions of GDF9 and BMP15 were significantly increased (p<0.05) following 12 h of culture with rh-AMH compared to without rh-AMH in IVM medium. The levels of protein of GDF9 and BMP15 in IVM medium were significantly higher (p<0.05) when COCs were cultured with rh-AMH than without rh-AMH following culture at 18 h point.
Figure 3. Effect of IVM medium supplemented with or without 100/ml rh-AMH on GDF9 and BMP15 mRNA expression and protein production from the oocytes following IVM at different time points.
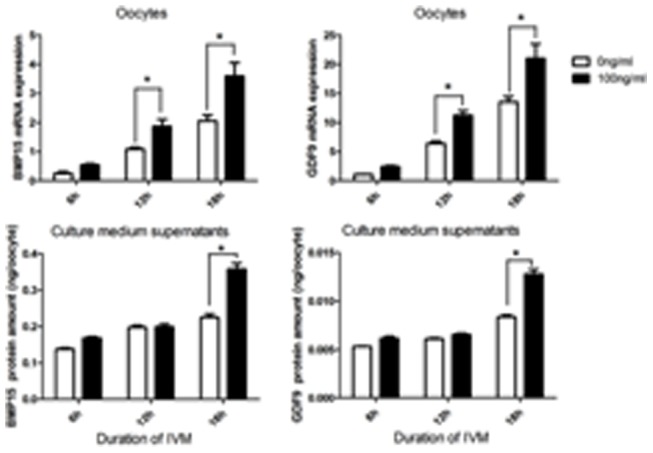
GDF9 and BMP15 mRNA expression in the oocytes were measured by RT-PCR, and GDF9, and BMP15 proteins in IVM-medium were measured by ELISA, respectively. Oocytes and IVM-medium supernatants were collected at 6, 12 and 18 h respectively during IVM. *Indicates significant differences (p<0.05) compared to the control group. Data were from 3 replicates.
Supplementation of rh-AMH into IVM Medium on Subsequent Embryonic Development
As shown in Figure 4A, the expression of AMH mRNA was significantly reduced (p<0.05) by AdH1-SiRNA/AMH adenovirus in cumulus cells. With AMH knocking down by AdH1-SiRNA/AMH, both BMP15 and GDF9 mRNA expressions were down-regulated significantly (p<0.05) (Figure 4B).
Figure 4. Effect of AMH knockdown on GDF9 and BMP15 mRNA expression from mouse cumulus cells and oocytes.
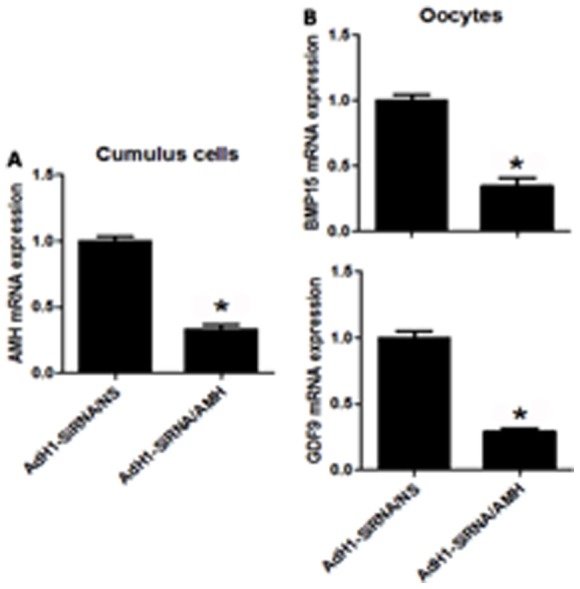
Cumulus cells and oocytes were harvested from COCs infected by AdH1-SiRNA/NS or AdH1-SiRNA/AMH during IVM, respectively. (A) Shows AMH mRNA expression in cumulus cells infected by AdH1-SiRNA/NS or AdH1-SiRNA/AMH; (B) Shows GDF9 and BMP15 mRNA expressions in oocytes from COCs infected by AdH1-SiRNA/NS or AdH1-SiRNA/AMH, respectively. *Indicates significant differences (p<0.05) compared to the control group. Data were from 3 replicates.
Table 3 shown, although there were no significant differences in the cleavage rates among groups, the blastocyst formation rate was significantly higher (p<0.05) in IVM medium supplemented with 100 ng/ml of rh-AMH group compared to the control group. Evidently the blastocyst formation rate was significantly decreased (p<0.05) in IVM medium treated AdH1-SiRNA/AMH group compared to AdH1-SiRNA/NS group.
Table 3. Subsequent embryonic development of immature COCs matured in IVM-medium without rh-AMH (Control) or with 100 ng/ml rh-AMH, AdH1-SiRNA/NS, AdH1-SiRNA/AMH respectively and followed by in vitro fertilization (IVF).
| Group of treatment | No. of COCs examined | No. of 2-cell embryos developed (%) | No. of blastocysts developed (%) |
| Control | 157 | 120 (76.4±5.4) | 38 (31.7±2.7) |
| rh-AMH | 150 | 120 (80.0±4.5) | 48 (40.0±3.3)* |
| AdH1-SiRNA/NS | 142 | 103 (73.1±8.9) | 35 (33.9±5.9) |
| AdH1-SiRNA/AMH | 135 | 95 (70.3±5.9) | 20 (20.6±2.9)** |
Data showed 3 replicates.
*Indicates significant difference compared to control group (P<0.05);
**indicates significant difference compared to AdH1-SiRNA/NS group (P<0.05).
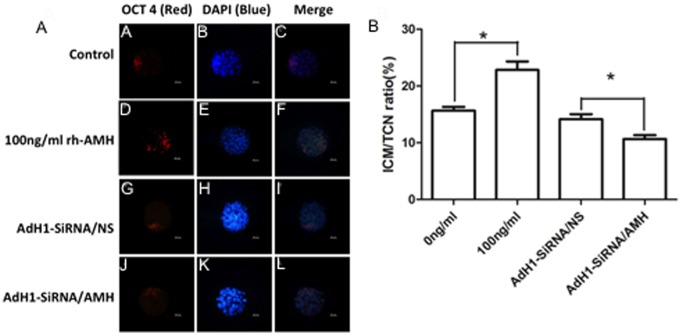
The quality of embryos was evaluated by ICM/TCN (Figure 5A). The ratio of ICM/TCN was significantly higher (p<0.05) in IVM medium with rh-AMH compared to without rh-AMH (Figure 5B). This was further confirmed by knocking down AMH with AdH1-SiRNA/AMH treated IVM medium group which significantly reduced (p<0.05) the ratio of ICM/TCN compared to AdH1-SiRNA/NS treated IVM medium group (Figure 5B).
Figure 5. Blastocyst quality was evaluated by the ratio of ICM/TCN.
(A) The blastocysts derived from the oocytes matured in vitro without rh-AMH (A–C) or with rh-AMH (D–F), and treated with AdH1-SiRNA/NS (G–I) and AdH1-SiRNA/AMH (J–K) respectively were stained with OCT4 for ICM (A, D, G, J), DAPI for TCN (B, E, H, K) and merged images (C, F, I, L). Bars = 20 µm. (B) The ratio of ICM/TCN represents by mean±SD in each group with 6 replicates. * Indicates significant differences (p<0.05) between the groups.
Discussion
The results of the present study demonstrated that AMH is only expressed in cumulus and granulose cells, but not in the oocytes and that AMHR II is expressed in both oocytes and cumulus cells. The results of the present study also demonstrated that the addition of 100 ng/ml of rh-AMH to IVM medium improves oocyte quality and that oocyte GDF9 and BMP15 expressions are down-regulated when COCs were treated by AdH1-SiRNA/AMH during IVM, resulting in reduced blastocyst formation rate. Therefore, these results suggest that AMH improves oocytes quality by up-regulating GDF9 and BMP15 mRNA expressions during IVM.
Studies have shown that AMH expression is strongest in pre-antral and small antral follicles, and then decreases continually [28]. AMHR-II is co-expressed with AMH in granulosa cells of growing follicles [29], [30]. In rat and sheep, AMH and AMHR-II are specifically expressed in granulosa cells, and there is no expression in the oocytes, theca and interstitial cells or expressed very little amount of AMH and AMHR-II in those cells [30]–[35]. Interestingly, our results indicated that AMH mRNA expression and protein only localized in cumulus cells (Figure 1). However, AMHR-II mRNA expression and protein were localized at both oocyte and cumulus cells.
AMH may have effects on both cumulus cells and oocytes through autocrine and paracrine when COCs were cultured in vitro. In rat [34] and sheep [35], the expression of AMHR-II was not observed in the oocytes from antral follicles. Therefore, the different observations could be due to technical variations between the studies or due to different species. In addition, as AMHR-II is a membrane receptor, using immunocytochemical method may not detect AMHR-II expression, because the cell membranes, especially large cells like oocytes, it may not be integrated properly in these studies. It has been reported that another member of TGF-β superfamily, BMP receptor IB (BMPR-IB) is located at both oocyte and granulosa cells in sheep ovary [36]. Same as sheep ovary, AMHR-II may be similar to this TGF-β superfamily receptor in mouse COCs. However, Sedes L et al. [37] found that AMH could recruit BMPR-IA in immature granulosa cells. Therefore, further study is required to confirm the linkage of those two receptors.
Belonging to the TGF-β superfamily, GDF9 and BMP15 play crucial roles in the follicular development, ovulation, oocyte maturation, and embryo development [38]–[41], and they are essential factors for folliculogenesis and female fertility [42]. It has been reported that GDF9 and BMP15 are closely associated with oocyte quality and embryo developmental potential [43]. In GDF9-deficient female mice, the ultrastructure of oocytes is abnormal; ovulation and oocyte fertilization rate are decreased in BMP15 knock-out model [44]. Also it has been reported that addition of exogenous GDF9 and BMP15 to IVM medium during IVM could increase blastocyst yield following IVF [45]. Since GDF9 and BMP15 are key factors that regulate oocyte maturation for subsequent embryo developmental potential, we have employed those two factors as molecule marker to evaluate oocyte quality in our study.
The concentration of AMH in mouse follicular fluid is still unclear, because mouse follicles are too small to aspirate its fluid. Therefore, we chose the range of AMH concentrations of human follicular fluid for our experiments. The concentrations of AMH in human small antral follicles (mean 790 ng/ml) were almost three orders of magnitude higher than in pre-ovulatory follicles (mean 1.17 ng/ml) [46]. AMH levels in follicular fluid, selected from follicles 4–8 mm in diameter, were significantly higher in women with anovulatory polycystic ovary syndrome (PCOS) (median 466.2 ng/ml) compared with normal-ovulatory controls (median 78.0 ng/ml) [47]. In addition, we chose these concentrations according to some other studies adding AMH into culture medium when cultured follicles or ovaries in vitro [48]–[50].
In rodents, AMH plays a decelerating role in the process of primordial follicle recruitment and follicular maturation [13]. Concerning possible effects of AMH on oocyte maturation, conflicting data have been reported. One study has shown that AMH inhibits oocyte meiosis in rat [51], but another study indicated that there is no effect of AMH on oocyte meiosis [52]. The results of our study confirmed that AMH has no inhibitive effect on oocyte meiosis with concentrations of 0–1,000 ng/ml (Table 2). In contrast, the concentration of 100 ng/ml of rh-AMH in IVM medium improves the oocyte quality in terms of GDF9 and BMP15 mRNA expression and protein secretion (Figure 2) as well as blastocyst formation rate and blastocyst quality (Table 3 and Figure 5). Interestingly, we found that levels of these key transcripts in the oocytes up-regulated with AMH following time course pattern during IVM (Figure 3), and down-regulated by AdH1-SiRNA/AMH when cumulus cells were infected by adenovirus during IVM (Figure 4). This result indicated that supplementation of rh-AMH into IVM medium improves oocyte quality during IVM.
In reproductive medicine, a serum AMH level has been recognized to be a useful diagnostic and prognostic tool. Since serum AMH is positively correlated to the number of antral follicles, the levels of serum AMH have been used as a reliable marker for the ovarian reserve [8] and a predictor of the ovarian response to controlled ovarian hyper-stimulation [53]. However, it still is unclear how AMH and FSH correlated during follicular development and oocyte maturation [28], [54], [55]. Currently, the mechanism behind this is largely unclear, so it needs further study to find out how AMH exerts its function associated with FSH and growth factors, such as EGF, during these processes.
In conclusion, the results of this study indicate that supplementation of 100 ng/ml of rh-AMH into IVM medium together with FSH and EGF improves oocyte quality. These results also indicate that rh-AMH improves oocytes quality by up-regulating GDF9 and BMP15 expression during IVM. These results suggest that IVM medium may be required rh-AMH supplementation during IVM in order to increase oocyte development competence.
Supporting Information
AMH knockdown experiments with recombinant adenoviruses AdH1-SiRNA/AMH targeting to AMH and with recombinant adenoviruses AdH-SiRNA/NS as control. Since the recombinant adenoviruses contain a green fluorescent protein (GFP) gene, GFP expression was visualized by fluorescence microscopy after transfected to granulosa cells with AdH1-SiRNA/AMH and AdH1-SiRNA/NS for 48 h. As shown in Figure S1A, more than 80 percent of granulose cells expressed green fluorescent light, which means these cells were infected by recombinant adenoviruses. These cells also were used for Western blot and the results were shown in Figure S1B. GAPDH was used as the standardized reference. The expressions of AMH were different in the granulosa cells which were infected by AdH1-SiRNA/AMH and AdH1-SiRNA/NS respectively. As shown in Figure S1 C, the expression of AMH was decreased over 80% after infection with AdH1-SiRNA/AMH for 48 h compared to AdH1-SiRNA/NS. The experiments were repeated three times. *Indicates significant differences (p<0.05).
(TIFF)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All data are included within the paper.
Funding Statement
This study was supported by a grant from Natural Sciences Foundation of China, No. NSFC81270746 to Ri-Cheng Chian. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. MacLaughlin DT, Donahoe PK (2002) Mullerian inhibiting substance: an update. Adv Exp Med Biol 511: 25–38; discussion 38–40. [DOI] [PubMed] [Google Scholar]
- 2. Josso N, Picard JY, Rey R, di Clemente N (2006) Testicular anti-Mullerian hormone: history, genetics, regulation and clinical applications. Pediatr Endocrinol Rev 3: 347–358. [PubMed] [Google Scholar]
- 3. di Clemente N, Jamin SP, Lugovskoy A, Carmillo P, Ehrenfels C, et al. (2010) Processing of anti-mullerian hormone regulates receptor activation by a mechanism distinct from TGF-beta. Mol Endocrinol 24: 2193–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Durlinger AL, Visser JA, Themmen AP (2002) Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction 124: 601–609. [DOI] [PubMed] [Google Scholar]
- 5. Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, et al. (1999) Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology 140: 5789–5796. [DOI] [PubMed] [Google Scholar]
- 6. Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, et al. (2002) Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology 143: 1076–1084. [DOI] [PubMed] [Google Scholar]
- 7. Fortune JE (2003) The early stages of follicular development: activation of primordial follicles and growth of preantral follicles. Anim Reprod Sci 78: 135–163. [DOI] [PubMed] [Google Scholar]
- 8. Ficicioglu C, Kutlu T, Baglam E, Bakacak Z (2006) Early follicular antimullerian hormone as an indicator of ovarian reserve. Fertil Steril 85: 592–596. [DOI] [PubMed] [Google Scholar]
- 9. Kevenaar ME, Meerasahib MF, Kramer P, van de Lang-Born BM, de Jong FH, et al. (2006) Serum anti-mullerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology 147: 3228–3234. [DOI] [PubMed] [Google Scholar]
- 10. Silberstein T, MacLaughlin DT, Shai I, Trimarchi JR, Lambert-Messerlian G, et al. (2006) Mullerian inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod 21: 159–163. [DOI] [PubMed] [Google Scholar]
- 11. Patrelli TS, Gizzo S, Sianesi N, Levati L, Pezzuto A, et al. (2012) Anti-Mullerian hormone serum values and ovarian reserve: can it predict a decrease in fertility after ovarian stimulation by ART cycles? PLoS One 7: e44571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li HW, Lee VC, Lau EY, Yeung WS, Ho PC, et al. (2013) Role of baseline antral follicle count and anti-Mullerian hormone in prediction of cumulative live birth in the first in vitro fertilisation cycle: a retrospective cohort analysis. PLoS One 8: e61095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Visser JA, de Jong FH, Laven JS, Themmen AP (2006) Anti-Mullerian hormone: a new marker for ovarian function. Reproduction 131: 1–9. [DOI] [PubMed] [Google Scholar]
- 14. Takahashi C, Fujito A, Kazuka M, Sugiyama R, Ito H, et al. (2008) Anti-Mullerian hormone substance from follicular fluid is positively associated with success in oocyte fertilization during in vitro fertilization. Fertil Steril 89: 586–591. [DOI] [PubMed] [Google Scholar]
- 15. Wunder DM, Guibourdenche J, Birkhauser MH, Bersinger NA (2008) Anti-Mullerian hormone and inhibin B as predictors of pregnancy after treatment by in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril 90: 2203–2210. [DOI] [PubMed] [Google Scholar]
- 16. Fanchin R, Mendez Lozano DH, Frydman N, Gougeon A, di Clemente N, et al. (2007) Anti-Mullerian hormone concentrations in the follicular fluid of the preovulatory follicle are predictive of the implantation potential of the ensuing embryo obtained by in vitro fertilization. J Clin Endocrinol Metab 92: 1796–1802. [DOI] [PubMed] [Google Scholar]
- 17. Hattori Y, Sato T, Okada H, Saito C, Sugiura-Ogasawara M (2013) Comparison of follicular fluid and serum anti-Mullerian hormone levels as predictors of the outcome of assisted reproductive treatment. Eur J Obstet Gynecol Reprod Biol 169: 252–256. [DOI] [PubMed] [Google Scholar]
- 18. Mehta BN, Chimote MN, Chimote NN, Nath NM, Chimote NM (2013) Follicular-fluid anti-Mullerian hormone (FF AMH) is a plausible biochemical indicator of functional viability of oocyte in conventional in vitro fertilization (IVF) cycles. J Hum Reprod Sci 6: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anckaert E, De Rycke M, Smitz J (2013) Culture of oocytes and risk of imprinting defects. Hum Reprod Update 19: 52–66. [DOI] [PubMed] [Google Scholar]
- 20. Chian RC, Uzelac PS, Nargund G (2013) In vitro maturation of human immature oocytes for fertility preservation. Fertil Steril 99: 1173–1181. [DOI] [PubMed] [Google Scholar]
- 21. Trounson A, Anderiesz C, Jones G (2001) Maturation of human oocytes in vitro and their developmental competence. Reproduction 121: 51–75. [DOI] [PubMed] [Google Scholar]
- 22. Smitz JE, Thompson JG, Gilchrist RB (2011) The promise of in vitro maturation in assisted reproduction and fertility preservation. Semin Reprod Med 29: 24–37. [DOI] [PubMed] [Google Scholar]
- 23. Gilchrist RB (2011) Recent insights into oocyte-follicle cell interactions provide opportunities for the development of new approaches to in vitro maturation. Reprod Fertil Dev 23: 23–31. [DOI] [PubMed] [Google Scholar]
- 24. Dunning KR, Watson LN, Sharkey DJ, Brown HM, Norman RJ, et al. (2012) Molecular filtration properties of the mouse expanded cumulus matrix: controlled supply of metabolites and extracellular signals to cumulus cells and the oocyte. Biol Reprod 87: 89. [DOI] [PubMed] [Google Scholar]
- 25. Shen C, Buck AK, Liu X, Winkler M, Reske SN (2003) Gene silencing by adenovirus-delivered siRNA. FEBS Lett 539: 111–114. [DOI] [PubMed] [Google Scholar]
- 26. He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, et al. (1998) A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A 95: 2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Visser JA, Themmen AP (2005) Anti-Mullerian hormone and folliculogenesis. Mol Cell Endocrinol 234: 81–86. [DOI] [PubMed] [Google Scholar]
- 28. Nilsson E, Rogers N, Skinner MK (2007) Actions of anti-Mullerian hormone on the ovarian transcriptome to inhibit primordial to primary follicle transition. Reproduction 134: 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. di Clemente N, Wilson C, Faure E, Boussin L, Carmillo P, et al. (1994) Cloning, expression, and alternative splicing of the receptor for anti-Mullerian hormone. Mol Endocrinol 8: 1006–1020. [DOI] [PubMed] [Google Scholar]
- 30. Baarends WM, Uilenbroek JT, Kramer P, Hoogerbrugge JW, van Leeuwen EC, et al. (1995) Anti-mullerian hormone and anti-mullerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology 136: 4951–4962. [DOI] [PubMed] [Google Scholar]
- 31. Ueno S, Takahashi M, Manganaro TF, Ragin RC, Donahoe PK (1989) Cellular localization of mullerian inhibiting substance in the developing rat ovary. Endocrinology 124: 1000–1006. [DOI] [PubMed] [Google Scholar]
- 32. Ueno S, Kuroda T, Maclaughlin DT, Ragin RC, Manganaro TF, et al. (1989) Mullerian inhibiting substance in the adult rat ovary during various stages of the estrous cycle. Endocrinology 125: 1060–1066. [DOI] [PubMed] [Google Scholar]
- 33. Hirobe S, He WW, Lee MM, Donahoe PK (1992) Mullerian inhibiting substance messenger ribonucleic acid expression in granulosa and Sertoli cells coincides with their mitotic activity. Endocrinology 131: 854–862. [DOI] [PubMed] [Google Scholar]
- 34. Hirobe S, He WW, Gustafson ML, MacLaughlin DT, Donahoe PK (1994) Mullerian inhibiting substance gene expression in the cycling rat ovary correlates with recruited or graafian follicle selection. Biol Reprod 50: 1238–1243. [DOI] [PubMed] [Google Scholar]
- 35. Bezard J, Vigier B, Tran D, Mauleon P, Josso N (1987) Immunocytochemical study of anti-Mullerian hormone in sheep ovarian follicles during fetal and post-natal development. J Reprod Fertil 80: 509–516. [DOI] [PubMed] [Google Scholar]
- 36. Wilson T, Wu XY, Juengel JL, Ross IK, Lumsden JM, et al. (2001) Highly prolific Booroola sheep have a mutation in the intracellular kinase domain of bone morphogenetic protein IB receptor (ALK-6) that is expressed in both oocytes and granulosa cells. Biol Reprod 64: 1225–1235. [DOI] [PubMed] [Google Scholar]
- 37. Sedes L, Leclerc A, Moindjie H, Cate RL, Picard JY, et al. (2013) Anti-Mullerian hormone recruits BMPR-IA in immature granulosa cells. PLoS One 8: e81551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Juengel JL, McNatty KP (2005) The role of proteins of the transforming growth factor-beta superfamily in the intraovarian regulation of follicular development. Hum Reprod Update 11: 143–160. [DOI] [PubMed] [Google Scholar]
- 39. Knight PG, Glister C (2006) TGF-beta superfamily members and ovarian follicle development. Reproduction 132: 191–206. [DOI] [PubMed] [Google Scholar]
- 40. Gilchrist RB, Lane M, Thompson JG (2008) Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update 14: 159–177. [DOI] [PubMed] [Google Scholar]
- 41. Hutt KJ, Albertini DF (2007) An oocentric view of folliculogenesis and embryogenesis. Reprod Biomed Online 14: 758–764. [DOI] [PubMed] [Google Scholar]
- 42. Juengel JL, Bodensteiner KJ, Heath DA, Hudson NL, Moeller CL, et al. (2004) Physiology of GDF9 and BMP15 signalling molecules. Anim Reprod Sci 82–83: 447–460. [DOI] [PubMed] [Google Scholar]
- 43. Qiao J, Feng HL (2011) Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum Reprod Update 17: 17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, et al. (2001) Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol 15: 854–866. [DOI] [PubMed] [Google Scholar]
- 45. Hussein TS, Thompson JG, Gilchrist RB (2006) Oocyte-secreted factors enhance oocyte developmental competence. Dev Biol 296: 514–521. [DOI] [PubMed] [Google Scholar]
- 46. Andersen CY, Byskov AG (2006) Estradiol and regulation of anti-Mullerian hormone, inhibin-A, and inhibin-B secretion: analysis of small antral and preovulatory human follicles' fluid. J Clin Endocrinol Metab 91: 4064–4069. [DOI] [PubMed] [Google Scholar]
- 47. Das M, Gillott DJ, Saridogan E, Djahanbakhch O (2008) Anti-Mullerian hormone is increased in follicular fluid from unstimulated ovaries in women with polycystic ovary syndrome. Hum Reprod 23: 2122–2126. [DOI] [PubMed] [Google Scholar]
- 48. Park JH, Maclaughlin DT, Teixeira JM (2011) The rate of in vitro maturation of primary follicles from adult mice and the quality of oocytes is improved in the absence of anti-mullerian hormone. Reprod Sci 18: 334–341. [DOI] [PubMed] [Google Scholar]
- 49. Carlsson IB, Scott JE, Visser JA, Ritvos O, Themmen AP, et al. (2006) Anti-Mullerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum Reprod 21: 2223–2227. [DOI] [PubMed] [Google Scholar]
- 50. Nilsson EE, Schindler R, Savenkova MI, Skinner MK (2011) Inhibitory actions of Anti-Mullerian Hormone (AMH) on ovarian primordial follicle assembly. PLoS One 6: e20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Takahashi M, Koide SS, Donahoe PK (1986) Mullerian inhibiting substance as oocyte meiosis inhibitor. Mol Cell Endocrinol 47: 225–234. [DOI] [PubMed] [Google Scholar]
- 52. Tsafriri A, Picard JY, Josso N (1988) Immunopurified anti-mullerian hormone does not inhibit spontaneous resumption of meiosis in vitro of rat oocytes. Biol Reprod 38: 481–485. [DOI] [PubMed] [Google Scholar]
- 53. La Marca A, Sighinolfi G, Radi D, Argento C, Baraldi E, et al. (2010) Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum Reprod Update 16: 113–130. [DOI] [PubMed] [Google Scholar]
- 54. Nelson SM, Yates RW, Fleming R (2007) Serum anti-Mullerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles–implications for individualization of therapy. Hum Reprod 22: 2414–2421. [DOI] [PubMed] [Google Scholar]
- 55. Veiga-Lopez A, Ye W, Padmanabhan V (2012) Developmental programming: prenatal testosterone excess disrupts anti-Mullerian hormone expression in preantral and antral follicles. Fertil Steril 97: 748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AMH knockdown experiments with recombinant adenoviruses AdH1-SiRNA/AMH targeting to AMH and with recombinant adenoviruses AdH-SiRNA/NS as control. Since the recombinant adenoviruses contain a green fluorescent protein (GFP) gene, GFP expression was visualized by fluorescence microscopy after transfected to granulosa cells with AdH1-SiRNA/AMH and AdH1-SiRNA/NS for 48 h. As shown in Figure S1A, more than 80 percent of granulose cells expressed green fluorescent light, which means these cells were infected by recombinant adenoviruses. These cells also were used for Western blot and the results were shown in Figure S1B. GAPDH was used as the standardized reference. The expressions of AMH were different in the granulosa cells which were infected by AdH1-SiRNA/AMH and AdH1-SiRNA/NS respectively. As shown in Figure S1 C, the expression of AMH was decreased over 80% after infection with AdH1-SiRNA/AMH for 48 h compared to AdH1-SiRNA/NS. The experiments were repeated three times. *Indicates significant differences (p<0.05).
(TIFF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All data are included within the paper.