Abstract
IMPORTANCE
Magnetic resonance imaging markers of incipient cognitive decline among healthy elderly individuals have become important for both clarifying the biological underpinnings of dementia and clinically identifying healthy individuals at high risk of cognitive decline. Even though the role of hippocampal atrophy is well known in the later stages of decline, the ability of fornix-hippocampal markers to predict the earliest clinical deterioration is less clear.
OBJECTIVES
To examine the involvement of the hippocampus-fornix circuit in the very earliest stages of cognitive impairment and to determine whether the volumes of fornix white matter and hippocampal gray matter would be useful markers for understanding the onset of dementia and for clinical intervention.
DESIGN
A longitudinal cohort of cognitively normal elderly participants received clinical evaluations with T1-weighted magnetic resonance imaging and diffusivity scans during repeated visits over an average of 4 years. Regression and Cox proportional hazards models were used to analyze the relationships between fornix and hippocampal measures and their predictive power for incidence and time of conversion from normal to impaired cognition.
SETTING
A cohort of community-recruited elderly individuals at the Alzheimer Disease Center of the University of California, Davis.
PARTICIPANTS
A total of 102 cognitively normal elderly participants, with an average age of 73 years, recruited through community outreach using methods designed to enhance ethnic diversity.
MAIN OUTCOMES AND MEASURES
Our preliminary hypothesis was that fornix white matter volume should be a significant predictor of cognitive decline among normal elderly individuals and that fornix measures would be associated with gray matter changes in the hippocampus.
RESULTS
Fornix body volume and axial diffusivity were highly significant predictors (P = .02 and .005, respectively) of cognitive decline from normal cognition. Hippocampal volume was not significant as a predictor of decline but was significantly associated with fornix volume and diffusivity (P = .004).
CONCLUSIONS AND RELEVANCE
This could be among the first studies establishing fornix degeneration as a predictor of incipient cognitive decline among healthy elderly individuals. Predictive fornix volume reductions might be explained at least in part by clinically silent hippocampus degeneration. The importance of this finding is that white matter tract measures may become promising candidate biomarkers for identifying incipient cognitive decline in a clinical setting, possibly more so than traditional gray matter measures.
An increasingly important focus of aging and dementia research is the earliest departure from normal cognition into cognitive decline among cognitively healthy elderly individuals. A variety of recent research1–5 has suggested that macroscopic structural changes in the brain, which are identifiable through magnetic resonance imaging (MRI), may be discernible years before measurable cognitive loss. These MRI markers of incipient cognitive decline among healthy elderly individuals have become especially important for both clarifying the biological underpinnings of dementia and clinically identifying healthy individuals at high risk of cognitive decline. Recognizing that neurodegenerative diseases such as Alzheimer disease (AD) will be most effectively prevented or slowed when treated early, identifying such cases of increased risk using widely available procedures such as brain MRI is becoming increasingly vital.
Although hippocampal atrophy is one of the earliest and most studied macroscopic changes associated with the AD process,2 changes to the fornix and other regions structurally connected to the hippocampus are still being delineated. The fornix consists of axons emerging from the cornu ammonis 1 (CA1) and subiculum subfields of the hippocampus and primarily innervating neurons in the mammillary bodies.6–10 This hippocampus-fornix circuitry is essential to episodic memory consolidation.11–14 Recent work in our laboratory has found significant associations between hippocampal atrophy and microstructural degeneration of the fornix among individuals with memory impairment and dementia.15
Compared with the hippocampus, however, the fornix has been far less studied as a predictor of cognitive change throughout the AD process. In particular, although the integrity of the fornix decreases with age in healthy individuals and can be used as a predictor of the conversion from mild cognitive impairment (MCI) to AD,12,16–18 few studies have assessed the fornix as a predictor of conversion from normal cognition to MCI.1 Moreover, the predictive powers of the hippocampus and the fornix have rarely been compared in the same study. Finally, it is unclear whether fornix volume19 or measures of microstructural integrity17,18 (ie, fractional anisotropy [FA] or axial and radial diffusivities), more clearly identify AD-related changes.
The present study aims to address these issues. It compares measures of fornix morphometry and microstructural integrity with measures of hippocampus subfield morphometry as predictors of conversion from normal cognition to MCI or AD. It also analyzes the strength of associations among hippocampus measures and fornix measures. We explored the extent to which each of these measures of the fornix-hippocampal circuit are associated with one another and the extent to which they each predict conversion from normal to impaired cognition.
Methods
Participants
The participants for our study consisted of 102 cognitively normal individuals recruited into the Longitudinal Cohort of the Alzheimer Disease Center of the University of California, Davis. Participants were recruited through community outreach using methods designed to enhance ethnic diversity.20 All participants provided informed consent before participating in our study.
Clinical Evaluation
Each participant received multidisciplinary clinical evaluations at the Alzheimer Disease Center of the University of California, Davis, at baseline and at approximately annual follow-up examinations. Evaluations included a detailed medical history and physical and neurological examinations. Diagnosis of cognitive syndromes (MCI or AD) were made according to standardized criteria21 by a consensus conference of clinicians. The Clinical Dementia Rating Scale Sum of Boxes score22 was assessed as a measure of clinically relevant functional impairment.
Neuropsychological Testing
The psychometrically matched cognitive measures collected at each evaluation were from the Spanish and English Neuropsychological Assessment Scales.23–26 The present study used the Spanish and English Neuropsychological Assessment Scales to measure episodic memory and executive function. These measures do not have appreciable floor or ceiling effects for participants in this sample and have linear measurement properties across a broad ability range.23,24
Magnetic Resonance Imaging
Each participant received a structural T1-weighted MRI scan and a diffusion-tensor imaging (DTI) sequence at a date close to the baseline clinical evaluation. All images were acquired at the University of California, Davis, Imaging Research Center. The T1-weighted spoiled gradient recalled echo acquisition had the following parameters: repetition time, 9.1 milliseconds; flip angle, 15°; field of view, 24 cm; and slice thickness, 1.5 mm. The 2-dimensional axial-oblique single-shot spin-echo planar DTI sequence had the following parameters: echo time, 9.4 milliseconds; repetition time, 8000 milliseconds; flip angle, 90°; field of view, 24 cm; and slice thickness, 5 mm. The B value was 1000 s/mm2 with 6 gradient directions collected 4 times each, plus 2 B0 images. Diffusivity summary parameters (FA and axial and radial diffusivities) were calculated using the FSL toolbox.27
Fornix Volume and Diffusivity Analysis
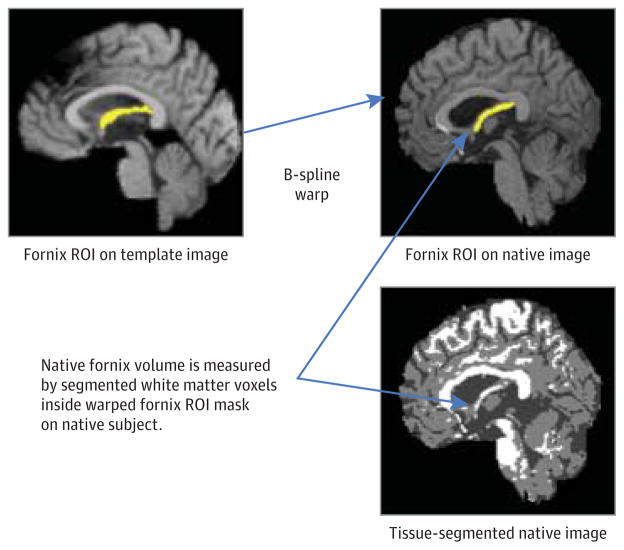
Native fornix volume and diffusivity measurements were calculated using previously described automated methods.15 The crux of the technique is the automatic deformation of a template fornix body region of interest (ROI) onto each participant’s image (Figure 1) to delineate the native fornix. We focused on the fornix body, but we excluded the crura because they are difficult to match or segment precisely and have also been excluded in other recent studies (eg, see Oishi et al18). To minimize partial volume artifacts in each participant’s native fornix ROI, each T1-weighted image was segmented into gray matter, white matter, and cerebrospinal fluid compartments,28 and only voxels labeled white matter voxels were used to calculate fornix properties, including volume, FA, radial diffusivity, and axial diffusivity.15 With this method, previous investigations by our group15 found biologically plausible correlations among fornix white matter integrity, hippocampal subregions, and episodic memory,15 and this method was further validated in the present study by visual confirmation that the ROI was an accurate fit onto each native fornix body.
Figure 1. Template-Based Automatic Fornix Volume Determination.
The diagram illustrates the combined use of automatic region-of-interest (ROI) fitting and tissue segmentation for designating the white matter voxels of the fornix body.
To obtain robust region-level summaries of diffusion properties from DTI, we implemented a variation of the tract-based spatial statistics procedure.29 Specifically, we computed a fornix crest line of maximal FA intensity on each participant’s FA image warped to template space, and this crest line consisted of voxels having the highest FA value within each coronal slice of the fornix ROI. The coronal slicing plane was approximately orthogonal to the fornix body orientation, thus approximating the orthogonal sampling prescribed by tract-based spatial statistics. Mean values of FA, axial diffusivity, and radial diffusivity among all crest-line voxel slices were used as the primary outcome measures in the following analysis.
Hippocampus Volume and Local Radial Thickness
The hippocampi were hand traced on the native T1-weighted images according to a previously described protocol that covered the anterior two-thirds of the hippocampus, including most of the CA1 and subiculum whose axons reside in the fornix.7,15 The reliability level of this method is high.15,30 To obtain sensitive measurements of local hippocampal shape variation, we implemented the radial distance technique described by Thompson et al,31 thus allowing the lengths of the radial lines to serve as localized hippocampal thickness measures in subsequent statistical analysis. The CA1 and subiculum ROIs were created by a neurologist expert in neuroanatomy, as described previously.15 We computed the mean thickness over each of these ROIs to summarize hippocampal size at a subfield level.
Statistical Analysis
Prediction of Cognitive Decline
We used the Cox proportional hazards model32 to estimate the influence of hippocampus and fornix markers on the risk of conversion from normal cognition to MCI or AD. For participants who developed cognitive impairment (ie, from normal cognition to MCI or dementia), we estimated the time to conversion as the elapsed interval between baseline evaluation and the date at which the impairment was diagnosed. Participants who remained without cognitive impairment by their last visit were censored at that date.
Each of several predictors was tested in a sequential model-building approach, as in previous analyses by our group.30 First, we constructed a reference survival model containing only the fixed effects of baseline age, years of education, and sex. Next, we constructed a series of “single-factor” models, each containing the effects of the reference model along with 1 additional factor. The following factors, measured at baseline, were evaluated in single-factor models: episodic memory; executive function; Mini-Mental State Examination score; fornix body white matter volume; fornix body FA, radial diffusivity, and axial diffusivity; CA1 right and CA1 left hippocampal radial thicknesses; total hippocampal volume; and log-transformed white matter hyperintensity volume and vascular risk. The vascular risk score was measured as the sum of 6 factors (stroke, transient ischemic attach, diabetes mellitus, hyperlipidemia, hypertension, and heart disease) and is reported as a percentage. Volumetric measures (fornix volume, white matter hyperintensity volume, and hippocampal volume) were normalized by whole-head size. To standardize effect size across all measures, all measurement variables were converted to z scores based on their distribution of values in the patient population.
For each single-factor model, we used a likelihood ratio to determine whether the model produced a significant increase of predictive power over the reference model. All factors whose likelihood ratio test passed a P value threshold of .10 were included in a final multiple-effects model. Thus, the multiple-factor model contained all the effects in the reference model and all single effects of cognitive or MRI variables passing this test of improved predictive power.
Relations Among Fornix and Hippocampus Measures
Subsequent analysis explored whether a reduced fornix volume reflected an isolated anatomical lesion or, as suggested by our earlier analyses, broader injury to a more extended circuit including both the hippocampus and the fornix.15 Pairwise correlations among fornix and hippocampus variables were assessed. In addition, a linear multiregression model was estimated with age; sex; fornix FA, axial diffusivity, and radial diffusivity; vascular risk factors; and hippocampal measures as predictors and with fornix body volume as the outcome. We then used principal components analysis to identify patterns of associations among fornix volume, fornix diffusivity measures, and hippocampus volume and thickness.
Results
Participants
Of the 102 participants who were cognitively normal at baseline, 20 subsequently progressed (“converted”) to MCI (18 participants) or AD (2 participants), and 82 remained normal throughout the time frame of our study. These so-called converters were followed up for an average of 4.4 years, and the so-called nonconverters were followed up for an average of 3.8 years. The demographic characteristics of the cohort are summarized in Table 1.
Table 1.
Demographic Characteristics of Converters and Nonconvertersa
| Characteristic | Mean (SD) | |
|---|---|---|
| Converters (n = 20) | Nonconverters (n = 82) | |
| Age,b y | 78.1 (6.9) | 71.4 (5.9) |
| Sex, No. | ||
| Male | 4 | 29 |
| Female | 16 | 53 |
| Education, y | 12.1 (3.4) | 12.2 (4.7) |
| MMSE score | 27.5 (2.5) | 28.2 (1.7) |
| z Score | ||
| Episodic | −0.16 (0.8) | 0.11 (0.8) |
| Executive | −0.32 (0.5) | −0.02 (0.5) |
| APOE4, % | 26 | 26 |
| Observation interval, y | 4.4 (2.1) | 3.8 (2.3) |
| Time to convert, y | 3.0 (1.9) | |
Abbreviation: MMSE, Mini-Mental State Examination.
Of 102 participants who were cognitively normal at baseline, 20 subsequently progressed (“converted”) to mild cognitive impairment (18 participants) or Alzheimer disease (2 participants), and 82 remained normal throughout the time frame of our study.
Significant difference.
Predictors of Conversion From Normal to Impaired Cognition
The only single-factor models passing the P < .10 threshold were those for fornix volume, fornix axial diffusivity, and mean radial thicknesses of the CA1 right and CA1 left hippocampal ROIs. Single-factor models for episodic memory, executive function, Mini-Mental State Examination score, fornix FA, for-nix radial diffusivity, total hippocampal volume, white matter hyperintensity volume, and vascular risk factors did not pass the significance threshold.
The multiple-factor model, which included age, education, sex, fornix volume, fornix axial diffusivity, and mean radial distances of the CA1 right and CA1 left hippocampal ROIs, had significantly greater explanatory power than the reference model under a likelihood ratio test (P < .001). Table 2 summarizes the significance and hazard ratios for all effects in the multiple-effects model. Greater age, greater fornix axial diffusivity, and lesser fornix volume were independently associated with greater risk of conversion. Increases of 1 SD in fornix body volume, fornix axial diffusivity, and age were associated, respectively, with a 53% reduction in risk, a 25% increase in risk, and a 15% increase in risk.
Table 2.
Significances and HRs for All Factors in Proportional Hazards Modela
| Factor | P Valueb | Mean HR (95% CI) |
|---|---|---|
| Age | <.001c | 1.15 (1.06–1.24) |
| Education | .14 | 0.90 (0.78–1.04) |
| Sex (male vs female) | .15 | 2.28 (0.76–8.57) |
| Hippocampus radial thickness | ||
| CA1 right | .08 | 1.22 (0.62–2.60) |
| CA1 left | .57 | 0.50 (0.22–1.09) |
| Fornix body white volume | .02c | 0.47 (0.24–0.89) |
| Fornix axial diffusivity | .005c | 1.25 (1.02–1.46) |
Abbreviations: CA1, cornu ammonis 1; HR, hazard ratio.
Combining nonbrain factors with fornix body white matter volume and fornix axial diffusivity.
Determined by use of the χ2 test.
Significant factor.
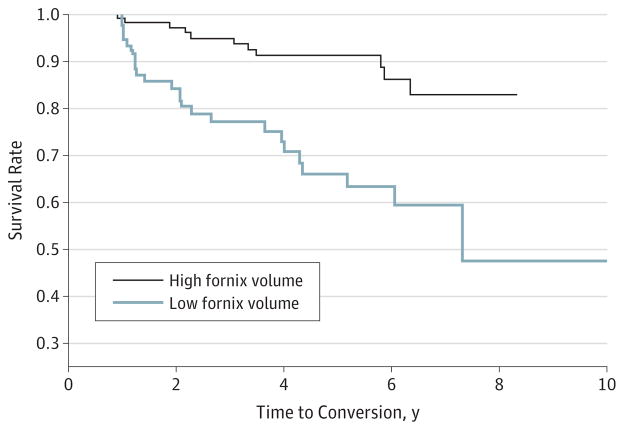
For a graphical depiction of the effect of fornix volume on conversion risk, we split participants into 2 groups based on a fornix volume threshold midpoint between the mean value among converters and the mean value among nonconverters (normalized volume ×1000 = 0.19). Survival curves for these 2 groups suggest greater survivorship among those with a greater fornix volume (Figure 2).
Figure 2. Group Conversion Trajectories.
The percentage of participants remaining cognitively normal are compared for high fornix volume (>0.19; normalized volume ×1000 per intracranial volume) vs low fornix volume (<0.19), graphed as a function of conversion time.
Relations Among Fornix and Other Measures
Single Regression Models
As expected, the volumes of the hippocampus and CA1 radial distance measures were highly correlated (mean r > 0.6, P < .001). Similarly, there was a significant association between fornix white matter volume and fornix FA (r = 0.31, P = .002) but not between fornix volume and radial diffusivity. Total hippocampal volume was also significantly associated with both fornix FA (r = 0.30, P = .002) and fornix white matter volume (r = 0.41 P < .001), whereas hippocampal CA1 area radial thickness also had strong associations with fornix volume (CA1 left: r = 0.23, P = .02; CA1 right: r = 0.29, P = .002). There was no significant association between fornix and whole-brain white matter hyperintensity or between fornix and vascular risk score.
Multiregression Model
Table 3 summarizes the multiple regression model with age, sex, fornix diffusivities, vascular risk score, and the hippocampal measures as predictors and with fornix white matter volume as the outcome. Total hippocampal volume (P = .02) was the only significant predictor, with fornix body FA suggesting a trend-level association (P = .11). Radial diffusivity and axial diffusivity were not significant. The model with all covariates explained 27% of the variance in fornix body volume.
Table 3.
Parameter Estimates From Multiregression Modela
| Factor | P Valueb |
|---|---|
| Age | .64 |
| Sex | .12 |
| Hippocampus | |
| CA1 right | .81 |
| CA1 left | .76 |
| Total hippocampal volume (right and left) | .02c |
| Brain white matter hyperintensity | .16 |
| Vascular risk score | .42 |
| Fornix body | |
| Axial diffusivity | .86 |
| Radial diffusivity | .62 |
| Fractional anisotropy | .11 |
Abbreviation: CA1, cornu ammonis 1.
Having fornix body white matter volume as outcome with joint inputs combining nonbrain factors with fornix fractional anisotropy, normalized hippocampal volume, and left and right CA1 mean radius. The R2 for the model was 0.27.
Determined by use of the F test.
Significant factor.
Principal Component Analysis
Principal component analysis identified 3 components that together explained 87% of the variance. Component 1 loaded heavily (0.5 in magnitude) on each of the hippocampal measures, whereas component 2 loaded heavily (0.6 in magnitude) on fornix FA and on fornix volume. Component 3 loaded extremely heavily on fornix FA and on fornix volume (magnitudes of 0.7 and 0.63, respectively), with much lighter loadings (magnitudes no greater than 0.2) on the hippocampal measures. All hippocampal measures thus were loaded most heavily on component 1, whereas the fornix measures had heavy loadings (all >0.5 in magnitude) on each of the components 2 and 3.
Discussion
Fornix Body Volume as Predictor of Cognitive Decline From Normal
Our results suggest that fornix variables (volume and axial diffusivity) are measurable brain factors that precede the earliest clinically relevant deterioration of cognitive function among cognitively normal elderly individuals. Although hippocampus measures have been studied much more deeply in relation to age-associated cognitive decline, our direct comparison between fornix and hippocampus measures suggests that fornix properties have a superior ability to identify incipient cognitive decline among healthy elderly individuals. The key importance of this finding is that it suggests that white matter tract measures may prove to be promising candidate biomarkers for predicting incipient cognitive decline among cognitively normal individuals in a clinical setting, possibly more so than traditional gray matter measures.
The association between fornix degeneration and eventual cognitive decline is plausible owing to the important role the hippocampus-fornix circuitry plays in memory consolidation. But this finding does not clarify what biological events may cause fornix degeneration early in the course of clinically significant cognitive decline. Although further work is necessary to address causality, our analysis of associations between fornix and hippocampus measures supports a model in which fornix degeneration reflects early-stage degeneration of a broader hippocampus-fornix circuit. Specifically, regression analysis suggested that there are correlations between hippocampus and fornix measures. These hippocampus-fornix associations echo similar findings from prior studies15,17 of individuals in more advanced stages of cognitive decline. The possible biological substrates for joint hippocampus and fornix degeneration could include Wallerian-like degeneration of fornix axons secondary to early AD-related neuronal soma injury in the hippocampus, and primary axonal injury due to the toxic effects of β-amyloid on oligodendrocytes, which can lead to soma injury as a secondary consequence.33,34 Meanwhile, vascular risk scores and brain white matter hyperintensity burden were not significantly associated with fornix measures, thus reducing the prospect that cerebrovascular disease burden is a significant driver for fornix degeneration. Longitudinal cognitive and disease biomarker data are required to better distinguish between these hypotheses.
Greater water diffusivity along the direction of travel of the axon (axial diffusivity) is often presented as an indicator of white matter tract integrity, but, in our analysis, it was associated with increased risk of cognitive conversion. A plausible scenario for this finding is that, in the course of axonal damage, axial diffusivity at first decreases owing to axonal breakdown35 but subsequently increases again as microglia clear the axonal fragments that have been impeding the parallel diffusion of water molecules.36,37 Two recent studies38,39 suggesting that increases in axial diffusivity are associated with reductions in FA support this view. Experiments with axonal resections in mice have also suggested that axial diffusivity is the most sensitive diffusivity measure of axonal injury.40 However, the lack of a significant association of either radial diffusivity or axial diffusivity with fornix body volume (but a trending significant association of FA with fornix body volume) indicates that we cannot attribute early fornix changes solely to demyelination or axonal loss separately. Longitudinal DTI studies charting individual diffusivity changes over time will be required to clarify this situation.
Comparison With Other Recent Studies
To our knowledge, ours is the first study to find that fornix body volume is a significant predictor of cognitive decline in cognitively normal individuals. This finding is consistent with the recent study by Zhuang et al,1 who found that FA values within the fornix and several other brain regions critical to memory (the precuneus, parahippocampal cingulum, and parahippocampal gyrus white matter) predicted conversion to amnestic MCI, with the precuneus appearing to be the strongest such predictor, and also with a follow-up study from the same group41 that compared 2 amnestic MCI cohorts to find a temporal order of disintegration. After cognitive impairment, microstructural damage appeared earlier in the fornix than in the uncinate and parahippocampal gyri, suggesting a primary role for the fornix in early cognitive decline. This supports our findings about the fornix’s predictive power regarding the conversion of cognitively normal individuals. The key difference between our study and these studies,1,41 as well as earlier studies showing the association between the fornix and impaired cognition in MCI and AD, is that our study shows the fornix predicting future decline in cognitively normal participants.
We also note that our findings appear to be inconsistent with the findings of the study by Copenhaver et al,19 who concluded that cross-sectional fornix differences between cognitively normal individuals and patients with MCI were not significant. Our contrasting conclusions may be due in part to differing methods of ROI creation. Their ROI measures were hand drawn and based on a detailed protocol. Other ROI approaches have used 1 or several slices17,18 or even individual voxels.42 Such approaches may possibly introduce greater spurious variability in fornix measures, reducing the statistical power, in contrast to our automated ROI delineation.
Limitations
Our main limitation was the small number of cognitively normal converters for whom MRI, DTI, and hippocampal ROI data were available, thus limiting the stability and statistical power of survival and regression models. A second limitation was that our hippocampal ROIs were hand drawn, potentially weakening the statistical power of association between the shape of the fornix and the shape of the hippocampus.
Conclusions
Our study finds that fornix volume is a predictor of conversion from normal cognition to clinically relevant cognitive impairment. This volume loss may be a consequence of cell body loss in the hippocampus leading to axon deterioration or possibly amyloidosis; future research is required to clarify the biological substrates. These results extend previous findings of the association between the fornix and the conversion from MCI to AD by suggesting that fornix degeneration may also predict the departure from normal brain aging.
Footnotes
Conflict of Interest Disclosures: None reported.
Author Contributions: Study concept and design: Fletcher, Raman, Mungas, Carmichael.
Acquisition of data: Fletcher, Huebner, Liu, Mungas, DeCarli.
Analysis and interpretation of data: All authors.
Drafting of the manuscript: Fletcher, Raman, Huebner, Liu, Carmichael.
Critical revision of the manuscript for important intellectual content: Fletcher, Mungas, Carmichael, DeCarli.
Statistical analysis: All authors.
Obtained funding: Mungas, DeCarli.
Administrative, technical, or material support: Mungas, DeCarli.
Study supervision: Fletcher, DeCarli.
References
- 1.Zhuang L, Sachdev PS, Trollor JN, et al. Microstructural white matter changes in cognitively normal individuals at risk of amnestic MCI. Neurology. 2012;79(8):748–754. doi: 10.1212/WNL.0b013e3182661f4d. [DOI] [PubMed] [Google Scholar]
- 2.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack CR, Jr, Vemuri P, Wiste HJ, et al. Alzheimer’s Disease Neuroimaging Initiative. Evidence for ordering of Alzheimer disease biomarkers. Arch Neurol. 2011;68(12):1526–1535. doi: 10.1001/archneurol.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silbert LC, Dodge HH, Perkins LG, et al. Trajectory of white matter hyperintensity burden preceding mild cognitive impairment. Neurology. 2012;79(8):741–747. doi: 10.1212/WNL.0b013e3182661f2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggleton JP, Vann SD, Saunders RC. Projections from the hippocampal region to the mammillary bodies in macaque monkeys. Eur J Neurosci. 2005;22(10):2519–2530. doi: 10.1111/j.1460-9568.2005.04450.x. [DOI] [PubMed] [Google Scholar]
- 7.Duvernoy HM. The Human Hippocampus. Berlin, Germany: Springer-Verlag; 1998. [Google Scholar]
- 8.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 9.Saunders RC, Aggleton JP. Origin and topography of fibers contributing to the fornix in macaque monkeys. Hippocampus. 2007;17(5):396–411. doi: 10.1002/hipo.20276. [DOI] [PubMed] [Google Scholar]
- 10.Thomas AG, Koumellis P, Dineen RA. The fornix in health and disease: an imaging review. Radiographics. 2011;31(4):1107–1121. doi: 10.1148/rg.314105729. [DOI] [PubMed] [Google Scholar]
- 11.Aggleton JP, Brown MW. Interleaving brain systems for episodic and recognition memory. Trends Cogn Sci. 2006;10(10):455–463. doi: 10.1016/j.tics.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Metzler-Baddeley C, Jones DK, Belaroussi B, Aggleton JP, O’Sullivan MJ. Frontotemporal connections in episodic memory and aging: a diffusion MRI tractography study. J Neurosci. 2011;31(37):13236–13245. doi: 10.1523/JNEUROSCI.2317-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudebeck SR, Scholz J, Millington R, Rohenkohl G, Johansen-Berg H, Lee ACH. Fornix microstructure correlates with recollection but not familiarity memory. J Neurosci. 2009;29(47):14987–14992. doi: 10.1523/JNEUROSCI.4707-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsivilis D, Vann SD, Denby C, et al. A disproportionate role for the fornix and mammillary bodies in recall versus recognition memory. Nat Neurosci. 2008;11(7):834–842. doi: 10.1038/nn.2149. [DOI] [PubMed] [Google Scholar]
- 15.Lee DY, Fletcher E, Carmichael OT, et al. Sub-regional hippocampal injury is associated with fornix degeneration in Alzheimer’s disease. Front Aging Neurosci. 2012;4:1–10. doi: 10.3389/fnagi.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang SH, Cho SH, Chang MC. Age-related degeneration of the fornix in the human brain: a diffusion tensor imaging study. Int J Neurosci. 2011;121(2):94–100. doi: 10.3109/00207454.2010.531894. [DOI] [PubMed] [Google Scholar]
- 17.Mielke MM, Okonkwo OC, Oishi K, et al. Fornix integrity and hippocampal volume predict memory decline and progression to Alzheimer’s disease. Alzheimers Dement. 2012;8(2):105–113. doi: 10.1016/j.jalz.2011.05.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oishi K, Mielke MM, Albert M, Lyketsos CG, Mori S. The fornix sign: a potential sign for Alzheimer’s disease based on diffusion tensor imaging. J Neuroimaging. 2012;22(4):365–374. doi: 10.1111/j.1552-6569.2011.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Copenhaver BR, Rabin LA, Saykin AJ, et al. The fornix and mammillary bodies in older adults with Alzheimer’s disease, mild cognitive impairment, and cognitive complaints: a volumetric MRI study. Psychiatry Res. 2006;147(2–3):93–103. doi: 10.1016/j.pscychresns.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Hinton L, Carter K, Reed BR, et al. Recruitment of a community-based cohort for research on diversity and risk of dementia. Alzheimer Dis Assoc Disord. 2010;24(3):234–241. doi: 10.1097/WAD.0b013e3181c1ee01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mungas D, Beckett L, Harvey D, et al. Heterogeneity of cognitive trajectories in diverse older persons. Psychol Aging. 2010;25(3):606–619. doi: 10.1037/a0019502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9(suppl 1):173–176. doi: 10.1017/s1041610297004870. discussion 177–178. [DOI] [PubMed] [Google Scholar]
- 23.Crane PK, Narasimhalu K, Gibbons LE, et al. Item response theory facilitated co-calibrating cognitive tests and reduced bias in estimated rates of decline. J Clin Epidemiol. 2008;61(10):1018.e9–1027.e9. doi: 10.1016/j.jclinepi.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mungas D, Reed BR, Crane PK, Haan MN, González H. Spanish and English Neuropsychological Assessment Scales (SENAS): further development and psychometric characteristics. Psychol Assess. 2004;16(4):347–359. doi: 10.1037/1040-3590.16.4.347. [DOI] [PubMed] [Google Scholar]
- 25.Mungas D, Reed BR, Haan MN, González H. Spanish and English neuropsychological assessment scales: relationship to demographics, language, cognition, and independent function. Neuropsychology. 2005;19(4):466–475. doi: 10.1037/0894-4105.19.4.466. [DOI] [PubMed] [Google Scholar]
- 26.Mungas D, Reed BR, Tomaszewski Farias S, DeCarli C. Criterion-referenced validity of a neuropsychological test battery: equivalent performance in elderly Hispanics and non-Hispanic Whites. J Int Neuropsychol Soc. 2005;11(5):620–630. doi: 10.1017/S1355617705050745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Fletcher E, Singh B, Harvey D, Carmichael O, DeCarli C. Adaptive image segmentation for robust measurement of longitudinal brain tissue change. Conf Proc IEEE Eng Med Biol Soc. 2012:5319–5322. doi: 10.1109/EMBC.2012.6347195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 30.Carmichael O, Mungas D, Beckett L, et al. MRI predictors of cognitive change in a diverse and carefully characterized elderly population. Neurobiol Aging. 2012;33(1):83–95. doi: 10.1016/j.neurobiolaging.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson PM, Hayashi KM, De Zubicaray GI, et al. Mapping hippocampal and ventricular change in Alzheimer disease. Neuroimage. 2004;22(4):1754–1766. doi: 10.1016/j.neuroimage.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 32.Cox DR. Regression models and life-tables. [Accessed August 12, 2013];J R Stat Soc, B. 1972 34(2):187–220. http://www.jstor.org/discover/10.2307/2985181?uid=3739656&uid=2&uid=4&uid=3739256&sid=21102546342337. [Google Scholar]
- 33.Desai MK, Mastrangelo MA, Ryan DA, Sudol KL, Narrow WC, Bowers WJ. Early oligodendrocyte/myelin pathology in Alzheimer’s disease mice constitutes a novel therapeutic target. Am J Pathol. 2010;177(3):1422–1435. doi: 10.2353/ajpath.2010.100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desai MK, Sudol KL, Janelsins MC, Mastrangelo MA, Frazer ME, Bowers WJ. Triple-transgenic Alzheimer’s disease mice exhibit region-specific abnormalities in brain myelination patterns prior to appearance of amyloid and tau pathology. Glia. 2009;57(1):54–65. doi: 10.1002/glia.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vernooij MW, de Groot M, van der Lugt A, et al. White matter atrophy and lesion formation explain the loss of structural integrity of white matter in aging. Neuroimage. 2008;43(3):470–477. doi: 10.1016/j.neuroimage.2008.07.052. [DOI] [PubMed] [Google Scholar]
- 36.O’Dwyer L, Lamberton F, Bokde ALW, et al. Multiple indices of diffusion identifies white matter damage in mild cognitive impairment and Alzheimer’s disease. PLoS One. 2011;6(6):e21745. doi: 10.1371/journal.pone.0021745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomalla G, Glauche V, Weiller C, Röther J. Time course of wallerian degeneration after ischaemic stroke revealed by diffusion tensor imaging. J Neurol Neurosurg Psychiatry. 2005;76(2):266–268. doi: 10.1136/jnnp.2004.046375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Acosta-Cabronero J, Williams GB, Pengas G, Nestor PJ. Absolute diffusivities define the landscape of white matter degeneration in Alzheimer’s disease. Brain. 2010;133(pt 2):529–539. doi: 10.1093/brain/awp257. [DOI] [PubMed] [Google Scholar]
- 39.Bosch B, Arenaza-Urquijo EM, Rami L, et al. Multiple DTI index analysis in normal aging, amnestic MCI and AD: relationship with neuropsychological performance. Neurobiol Aging. 2012;33(1):61–74. doi: 10.1016/j.neurobiolaging.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Budde MD, Xie M, Cross AH, Song SK. Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. J Neurosci. 2009;29(9):2805–2813. doi: 10.1523/JNEUROSCI.4605-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhuang L, Sachdev PS, Trollor JN, et al. Microstructural white matter changes, not hippocampal atrophy, detect early amnestic mild cognitive impairment. PLoS One. 2013;8(3):e58887. doi: 10.1371/journal.pone.0058887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ringman JM, O’Neill J, Geschwind D, et al. Diffusion tensor imaging in preclinical and presymptomatic carriers of familial Alzheimer’s disease mutations. Brain. 2007;130(pt 7):1767–1776. doi: 10.1093/brain/awm102. [DOI] [PubMed] [Google Scholar]