INTRODUCTION
As famously described by Charles Darwin and other scientists, there are organs and muscles in the human body that are “rudimentary” in nature, namely they have uncertain or no known current function(1). Additionally, natural selection also influences human development and morphology by encouraging more economic and precise function resulting in either agenesis or genesis of muscle. Accordingly, multiple reports of variations in current human anatomy resulting from such morphology have been described. Studies to date have focused on the physical description of individual muscle variations and possible functional significance. However, four muscles in the upper extremity not only show such variations, but, due to their demonstrated change in function, we hypothesize that the entire distal upper extremity is currently in the process of evolutionary change. These four muscles are: flexor digitorum superficialis to the fifth finger (FDS-V), anconeus, palmaris longus, and anconeus epitrochlearis (AE). Two of these muscles already appear to be in a rudimentary stage, one appears to be undergoing adaptive evolution, and one is stabilized and acting as a transient stability augmenter. The present study synthesizes, advances and extends previously described work about these muscles and extend the hypotheses that: (a) the FDS-V is currently under adaptive evolution, (b) the anconeus has currently stabilized its evolution and is serving as a transient stability augmenter during a short portion of the human lifespan, and (c) the entire distal upper extremity is currently in the process of undergoing evolutionary change.
HYPOTHESES
Quadrupedal or arboreal locomotion to habitual upright bipedal locomotion has caused changes to the necessary musculature of the upper extremity. Muscles that, in the past, may have been useful for quadrupedal or arboreal locomotion and fewer fine motor tasks may be no longer as useful in the modern environment. We hypothesize that evolution is currently underway in the human forearm, as demonstrated by four muscles that are in the midst of evolutionary change or have recently evolved to their current presentation. These four muscles are: (a) FDS-V, (b) anconeus, (c) palmaris longus, and (d) AE. From our review of the FDS-V, we hypothesize that the incidence of FDS-V in humans will continue to increase in order to perform fine tasks with precise range of motion. Regarding the anconeus muscle, which is always present in humans and is currently thought to be an extension of the triceps muscle with unknown current function, we hypothesize that this muscle has a primary function in infants as a stabilizer during the relatively brief period of human development where infants crawl and that it has currently stabilized evolutionarily. As humans grow, learn to walk and the elbow joint finishes developing in later childhood, we postulate that the anconeus takes on a more accessory-type role, which explains why the literature to date does not elucidate function of this muscle. Finally, we hypothesize that the entire distal upper extremity is currently in the process of undergoing evolutionary changes, as evidenced by the adaptive changes of FDS-V, the stabilization of the anconeus, and other changes occurring in the palmaris longus and the AE.
DISCUSSION
Flexor Digitorum Superficialis-V
The FDS-V is another muscle that has variable absence in humans. This muscle starts from the forearm and it inserts onto the radial and ulnar aspects of the proximal half of the middle phalanx of the fifth finger when it is present and is enervated by the median nerve(2). This muscle has been shown to be absent in 2% of the Japanese population(3), and others have reported its absence to be 6% (bilateral) and 6.8% (unilateral)(4). Contradictory hypotheses for the development of the FDS muscle belly(3) have been proposed, single origin (either antebrachial or palmar) and dual origin (both antebrachial and palmar). Kobayashi et al. referred to Yamada's supposition that the FDS muscle originates first in the palm and then migrates to the forearm(3) due to the results of their study as well as another reporting “brevis-type” variations of the FDS to the finger in question. It does not seem that “brevis-type” variations would be possible with a single origin of the FDS-V. However, the FDS muscle motor nerve is distributed in the forearm level and there is no recurrent branch that may have originated in the palm and goes back to the forearm for FDS innervation described yet. It is possible that the muscle portion of FDS comes from the forearm and the tendon portions originate from the hand because of previously described anomalies and the motor branch of the muscle. Furthermore, Shrewsbury and Kuczynski(5) noted that the FDS-V was absent in about 20% of the studied population. Interestingly, even if the tendon of FDS-V is absent, the distal components of the tendon are present(5). We have similarly noted an instance of a patient without an FDS but possessing intact distal components. Such instances are important for clinicians to be aware of in the event they encounter distal tendinous portions but not the FDS-V tendon itself, and may also support the theory that this muscle may have a dual origin and is formed in the hand first(5). It seems that, from research done so far, the dual-origin hypothesis may make more sense.
Comparing humans to other species, limbed amphibians and reptiles are similar to mammals in that they have flexor digitorum longus muscles. However, mammals have developed two layers in order to flex the digits: the flexor digitorum profundus and the FDS(6). The short hand muscles seen in reptiles and amphibians may have evolved proximally in mammals since there is no relation between palmaris longus and the FDS in reptiles and amphibians since the FDS location in the hand is replaced by a set of short, superficial finger flexors(7). In this case, Kobayashi et al. may be correct in restating Yamada's assertion that the FDS muscle originates first in the palm and then migrates to the forearm, however the nerves still emanate proximally(3). Intuitively, it makes sense for more primitive species to retain muscles in the hands because they do not use their hands for precise tool handling. Having less bulky muscles in the hand would be an advantageous evolutionary development for improved tool handling capabilities. In support of this theory, one may note that the fully oppositional motion of the human thumb is made possible by the distinct nature of the double saddle-shaped design of the first carpometacarpal joint. The first carpometacarpal joint is comprised of two articulating saddle-shaped bones facing one another, with the saddles meeting to form an “X”. Because of this unique configuration, the thenar area has two fewer muscles than would be present if the joint was otherwise shaped and thus keeps the hand less bulky(8). Some might argue that since the FDS-V is likely a less frequently used FDS muscle (when compared to FDS to the index finger, for example), it is less economically important to humans and we may continue to see more agenesis of this muscle; however, for the reasons outlined below, we hypothesize that, conversely, we will see increasing genesis of the FDS-V.
Other research has also confirmed the variability of the FDS-V in humans. In a study on 70 cadaveric hands, it was found that 13% of the hands had anatomical variations for the FDSV(9). Additionally, the variations noted were mostly irregular themselves, such as unusual variants of the FDS decussation and even complete absence of FDS muscles(9). Regarding the impact of cases in which the FDS-V is completely absent, research has indicated that the relatively common absence of this muscle can impact grip strength(10). Out of 171 subjects, it was found that the FDS-V was absent in 18.6% of females and 15.3% of males and in those subjects, grip strength was significantly lower than in subject groups with independent or common (attached to FDS of fourth finger) function of FDS-V(10). Another study, in contrast, found that there was no significant difference seen in grip strength between subjects who had an FDS-V and those who did not(11). Despite the wide variability of the presence of and the conflicting information as to the functional impact of the FDS-V, current clinical examination techniques are inadequate to discriminate among the possible variations or absence of FDS(12). There are also several muscle tendon variations that have been described in the flexor compartment of the forearm and, interestingly, most variations were related to the fifth finger(12). It is possible that the hand is evolving to either have decreased incidence of FDS-V or, more likely, it is possible that the incidence of FDS-V in humans will continue to increase in order to perform fine tasks (e.g., playing the piano or typewriting) or with precise range of motion, as humans often do in the modern environment. In this case, muscle tendon variations may be considered atavistic, in that they appear to represent a more primitive evolutionary presentation.
Anconeus
The anconeus is a muscle that originates from the lateral epicondyle of humerus and makes an insertion at the posterior olecranon process of the ulna. The radial nerve acts as the motor branch for this muscle(2). The anconeus is a muscle common to many species, is present in all mammals(6), and presents with similar anatomical location and possible proposed action in chimpanzees and Rhesus macaques. In other primates, the anconeus sometimes appears as a distinct muscle and in others appears as ill defined or a continuation of the triceps. Although the function of anconeus is unclear, it is linked with the extension of the forearm at the elbow in all non-human primates.
There is not clear consensus about function of the anconeus in humans and multiple studies have been conducted with varying conclusions. Gleason et al.'s EMG study supports Duchenne's original proposal for anconeus function, namely, that the anconeus abducts the ulna during pronation of the forearm. They observed electric silence in the muscle in flexion and extension of the elbow however they were able to show electric activity while the forearm pronates the axis of the second digit(13). Another EMG study performed on ten volunteers showed some muscle activity during both pronation and supination, but the researchers could not conclude the function of the anconeus(14). They postulated, however, that the muscle most likely acts as lateral stabilizer of the elbow joint(14). Another study suggested that the anconeus muscle is one of the elbow extensors along with the triceps and flexor carpi ulnaris muscles, however the role of the anconeus in elbow stabilization was not mentioned(15). The anconeus was also thought to be an important dynamic elbow stabilizer among triceps and brachialis according to O’Driscoll et al(16), and Molinier et al.’s(17) anatomy study shows close relation of the anconeus to the lateral head of the triceps muscle and they considered the two muscles to act synergistically. Additionally, they mentioned that the anconeus provides lateral stability for the extended elbow joint due to the close relationships between (i) the triceps and the anconeus and (ii) the joint capsule and the anconeus(17).
This proposed theory for the function of the anconeus muscle as a stabilizer for the elbow may be explained with mammalian humeroulnar joint evolutional theory explained by Jenkins(18). According to Jenkins, the pelycosaur humeroulnar joint, which is a less constrained joint than the human humeroulnar joint, has a torsional stress due to humeral rotation and the joint may disarticulate under torque. In order to prevent dislocation, the ulna rotates in conjunction with the humerus. As mammals evolved in the Jurassic period, the joint becomes more constrained and the forearm remains parallel to the humerus, which does a complex motion of rotation and adduction. Evolution continued so the joint becomes even more constrained and deep with a well-defined trochlea in nonhuman primates(18).
If the humeroulnar joint follows a common frame evolutionarily, it may be that in the earlier stages of tetrapod evolution, the joint was shallower requiring more active dynamic stabilization and, therefore, the anconeus muscle might have played a more primary role in earlier stages of humeroulnar joint evolution. In this way, the anconeus may have kept the forearm tracking properly while the humerus underwent the complex rotational motion with the tetrapodal gait. If this theory is correct, humans may not need this muscle anymore since the joint itself is anatomically stable. We postulate that it could be that the anconeus plays a more important role as a stabilizer in infancy during the relatively brief period of human development where infants crawl. As humans grow, learn to walk and the elbow joint finishes developing in later childhood, perhaps the anconeus takes on this more accessory-type role. The current literature does not have a comprehensive discussion of humeroulnar joint biomechanics during crawling and this may be an area meriting further research. It may be that the humeroulnar joint may be a relatively young joint evolutionarily. Due to the relative recent development of this joint and the transition to bipedalism, perhaps the anconeus will continue to adapt into a more rudimentary stage, unless there are stresses placed on it that stabilize the joint during a crucial period of human growth. In order to demonstrate whether or not the anconeus actually has any current function in modern humans, it may be informative to research and measure function of this muscle in crawling infants.
Evolution of the Distal Upper Extremity
Given the adaptive changes of the FDS-V and the current stabilization of the anconeus as hypothesized above, we also postulate that, due to these and additional changes occurring in the palmaris longus and the AE, the forearm as a whole is currently undergoing adaptive evolutionary change. The palmaris longus is a muscle that mostly appears in the human populations, varying among ethnic groups. This muscle is reported as one of the most variable muscles in the human body(19); its absence is reported between 3% and 63.9%(20). This muscle originates at the medial epicondyle of the humerus and inserts to the palmar aponeurosis. The median nerve acts as the motor branch for the palmaris longus(2). The function of the palmaris longus is uncertain in humans, but it is generally considered to be a contributor for flexing the hand at the wrist and tensing the palmar aponeurosis. Additionally, it is a well-known common option for use in tendon grafts.
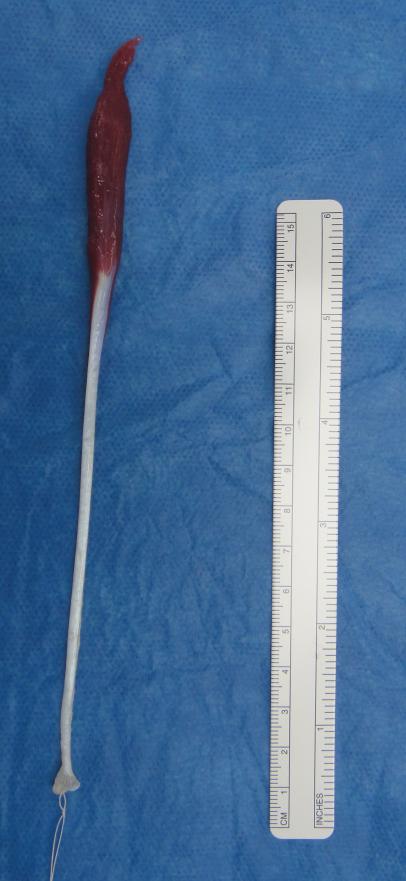
Most forearm muscles have a large and long muscle belly filling most of the length of the forearm and then, distally, the muscle becomes tendon 3-4 cm proximal of the wrist joint. The palmaris longus when present, however, has a long tendon and a short muscle belly [Figure 1], although variations have been found in humans wherein the muscle belly is more or less formed, attachment sites are varied, or accessory tendinous slips are present. Additionally, absence or presence of palmaris longus does not influence flexion of the wrist(21). These anatomical properties make the palmaris longus a popular choice for reconstructive purposes.
Figure 1.
Palmaris longus tendon harvested during reconstructive surgery of an 8-year-old female patient. Please note the short muscle belly and long tendinous portion of the graft.
So if the palmaris longus does not have any significant function since humans can perform the same tasks regardless of whether they have palmaris longus, why do most humans have this muscle? To explore the answer this question we looked at human evolution and function of this muscle in different species. In orangutans, the only strictly arboreal ape, the palmaris longus is always present(22). Having a strong palmaris longus muscle might therefore be more important in species such as orangutans that perform most daily activities in trees or use the forelimbs for ambulation, providing improved ability to grip and move in trees or with the forelimbs, and may be evolutionarily less important in other primates or in humans who are mostly terrestrial. The lower incidence of palmaris longus in other primates, who are more or completely terrestrial, may support this.
In other species, the palmaris longus is also variably found. Abdala and Diogo(6) performed a comprehensive comparison of anatomy among several species. They showed that salamanders, crocodiles, chickens and frogs do not have palmaris longus, however, semiaquatic turtles and rats have palmaris longus and some lizards also have it(6). According to the framework described by Abdala and Diogo, it makes sense for mammals have the muscle, but why? We postulate that since evolution wants to economize energetic output, it is possible that in the past, it may have been advantageous to have palmaris longus, but this may no longer be the case. However, according to current anatomical studies and the frequency of occurrence of the palmaris longus in humans and other species, it is likely that the palmaris longus is under regression and becoming a rudimentary muscle. This might be evidenced by the increase in size of the muscle bellies of the other muscles of the forearm and the relatively much smaller size of the palmaris longus. Additionally, although palmaris longus may vary among humans as described above, in general the smaller muscle belly and incidence of agenesis in humans is in contrast to the higher incidence and larger muscle belly in lower primates, mammals and other species. One can make the assumption that, if human evolution continues along similar lines wherein the muscle belly continues to phylogenetically reduce, it is expected that this muscle will eventually not be found in humans(23).
The AE, also known as epitrochleo-anconeus or anconeus sextus, is a muscle found in a variety of species. In humans it originates from the inferior surface of the medial epicondyle, crosses the ulnar nerve, and inserts on the olecranon. It is innervated from the ulnar nerve(24). The muscle function varies in different species, and the function in humans is unclear. In humans the AE is sometimes referred to as an independent muscle and sometimes as an accessory muscle or factor of the triceps brachii, whereas in other mammals it is always an independent structure. It is suggested that the muscle (i) serves to keep the ulnar nerve in position and guard the vessels that accompany it from pressure(24), and (ii) acts to assist the triceps brachii and the ligamentum cubiti mediale to support the median aspect of the elbow joint(25). This muscle presents with similar undifferentiated anatomic structure in gorillas, orangutans, and most other primates(26).
According to Dellon, the AE exists in only 11% of the human population, and the Osborne ligament is taking its place due to the increasing amount of work humans perform with a flexed elbow position. The Osborne ligament would provide more stability for the elbow when performing tasks in flexion(24). Husarik et al. performed an MRI study on sixty patients with asymptomatic elbows and found that 23% had an AE muscle(27). In Galton's study published in 1874(25), he performed dissections in Edentata and stated that this muscle was always present and well developed. He also declared that this muscle is not always seen in bats and occurs rarely in hoofed animals, but mentioned that the AE is seen very often in other mammals. In addition, he stated that it seen less frequently among the lower-order primates, is not seen in anthropoid apes, and seen only occasionally in humans as an anomaly(25). When a comparison of the AE's presence among the different species with an emphasis on the evolutionary track of the muscle is taken, there appears to be a reduction in the frequency of the occurrence of the muscle. There is a higher presence in the lower-order monkeys and lemurs, although it is not universally present among these(25). In any case, it seems to become lost among the anthropoid apes, and occurs again infrequently in humans, though called a variation(27) or anomaly(25).
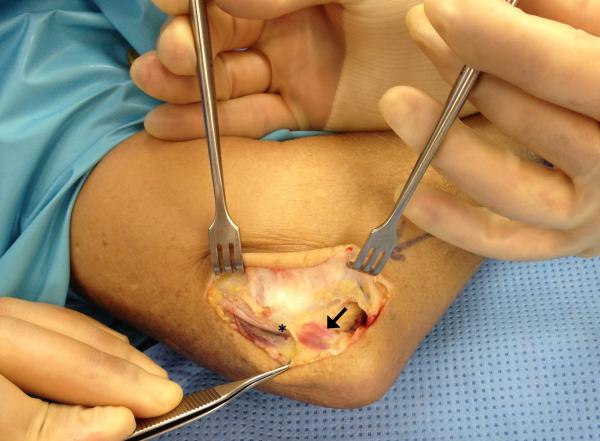
There is evidence that the AE is becoming or may be already considered rudimentary as its structure is retained from an earlier and more primitive condition of existence while in other mammals it is a necessary and functional mechanical appendage of the elbow joint(24). As over 70% of the human population does not even have this muscle(27), one can see that the muscle must not be necessary in humans and may be indicative of a late evolutionary process. Indeed, presence of an AE may not only be unnecessary, but may cause problems such as ulnar neuropathy, cubital tunnel syndrome and elbow pain(28, 29). It is therefore important for clinicians to be aware of the potential presence of the AE [Figure 2], as it may potentially be a contributing factor to patients presenting with elbow pain or neuropathy.
Figure 2.
Intraoperative image of cubital tunnel release showing the anconeus epitrochlearis. Please note a small anconeus epitrochlearis (black arrow) muscle and its relation to the ulnar nerve (asterisk).
In thinking about evolution, we tend to think of large branching moments in the evolutionary tree that occurred hundrends of thounsands or millions of years ago - Homo heidelbergensis Homo sapiens or Australopithecus anamensis and Australopithecus afarensis. In between those large branching “moments,” however, evolution also occurs on a more individual rather than a species level. Over time, these individual changes take hold if they are beneficial to the endurance of the species, playing a part in adaptation, natural selection and overall survival of the species. In this study we have examined four muscles of the distal upper extremity that appear to be currently undergoing evolutionary processes. It will be illuminative for researchers to continue to monitor the changes that these muscles are undergoing over time to see whether they continue to change or completely disappear in humans. Regarding current presentations of the FDS-V, it will also be of interest, especially to the fields of medicine and anatomy, to determine the origin (single origin vs. dual origin) and evolution of this muscle. Finally, with regard to the anconeus, the role of the anconeus as a potential stabilizer of the humeroulnar joint during infancy may be examined. Darwin wrote at length about various end-effects of natural selection on evolution, and we believe as discussed herein using the examples of these four muscles, that evolution is an ongoing process across a long-term continuum.
Acknowledgments
No support was received for this study.
Footnotes
All authors declare that there is no conflict of interest.
References
- 1.Darwin C. The descent of man, and selection in relation to sex. Princeton University Press; Princeton, N.J.: 1981. 1871. [Google Scholar]
- 2.Standring S, Ellis H, Healy JC, et al. Gray's anatomy: the anatomical basis of clinical practice. American Journal of Neuroradiology. 2005;26:2703. [Google Scholar]
- 3.Kobayashi N, Saito S, Wakisaka H, Matsuda S. Anomalous flexor of the little finger. Clin Anat. 2003;16:40–43. doi: 10.1002/ca.10057. [DOI] [PubMed] [Google Scholar]
- 4.Townley WA, Swan MC, Dunn RL. Congenital absence of flexor digitorum superficialis: implications for assessment of little finger lacerations. The Journal of hand surgery, European. 2010;35:417–418. doi: 10.1177/1753193409358523. [DOI] [PubMed] [Google Scholar]
- 5.Shrewsbury MM, Kuczynski K. Flexor digitorum superficialis tendon in the fingers of the human hand. The Hand. 1974;6:121–133. doi: 10.1016/0072-968x(74)90076-x. [DOI] [PubMed] [Google Scholar]
- 6.Abdala V, Diogo R. Comparative anatomy, homologies and evolution of the pectoral and forelimb musculature of tetrapods with special attention to extant limbed amphibians and reptiles. J Anat. 2010;217:536–573. doi: 10.1111/j.1469-7580.2010.01278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haines RW. The flexor muscles of the forearm and hand in lizards and mammals. J Anat. 1950;84:13–29. [PMC free article] [PubMed] [Google Scholar]
- 8.Brand PW, Hollister A. Clinical mechanics of the hand. Mosby Year Book; Burlington, MA: 1993. [Google Scholar]
- 9.Gonzalez MH, Whittum J, Kogan M, Weinzweig N. Variations of the flexor digitorum superficialis tendon of the little finger. Journal of hand surgery (Edinburgh, Scotland) 1997;22:277–280. doi: 10.1016/s0266-7681(97)80082-4. [DOI] [PubMed] [Google Scholar]
- 10.Bowman P, Johnson L, Chiapetta A, Mitchell A, Belusko E. The clinical impact of the presence or absence of the fifth finger flexor digitorum superficialis on grip strength. J Hand Ther. 2003;16:245–248. doi: 10.1016/s0894-1130(03)00040-1. [DOI] [PubMed] [Google Scholar]
- 11.Puhaindran ME, Sebastin SJ, Lim AYT, Xu WX, Chen YM. Absence of flexor digitorum superficialis tendon in the little finger is not associated with decreased grip strength. Journal of Hand Surgery (European Volume) 2008;33:205–207. doi: 10.1177/1753193408087229. [DOI] [PubMed] [Google Scholar]
- 12.Tan JS, Oh L, Louis DS. Variations of the flexor digitorum superficialis as determined by an expanded clinical examination. The Journal of hand surgery. 2009;34:900–906. doi: 10.1016/j.jhsa.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Gleason TF, Goldstein WM, Ray RD. The function of the anconeus muscle. Clinical orthopaedics and related research. 1985;192:147–148. [PubMed] [Google Scholar]
- 14.Basmajian JV, Griffin WR., Jr. Function of anconeus muscle. An electromyographic study. The Journal of bone and joint surgery. American. 1972;54:1712–1714. [PubMed] [Google Scholar]
- 15.An KN, Hui FC, Morrey BF, Linscheid RL, Chao EY. Muscles across the elbow joint: a biomechanical analysis. Journal of biomechanics. 1981;14:659–669. doi: 10.1016/0021-9290(81)90048-8. [DOI] [PubMed] [Google Scholar]
- 16.O Driscoll SW, Jupiter JB, King GJ, Hotchkiss RN, Morrey BF. The unstable elbow. Instructional course lectures-american academy of orthopaedic surgeons. 2001;50:89–104. [PubMed] [Google Scholar]
- 17.Molinier F, Laffosse J-M, Bouali O, Tricoire J-L, Moscovici J. The anconeus, an active lateral ligament of the elbow: new anatomical arguments. Surgical and radiologic anatomy. 2011;33:617–621. doi: 10.1007/s00276-010-0767-5. [DOI] [PubMed] [Google Scholar]
- 18.Jenkins FA. The functional anatomy and evolution of the mammalian humero-ulnar articulation. American Journal of Anatomy. 1973;137:281–297. doi: 10.1002/aja.1001370304. [DOI] [PubMed] [Google Scholar]
- 19.Cassell MD, Bergman RA. Palmaris longus muscle substituting for the ring finger slip of flexor digitorum superficialis. Anatomischer Anzeiger. 1990;171:201–204. [PubMed] [Google Scholar]
- 20.Sebastin SJ, Puhaindran ME, Lim AY, Lim IJ, Bee WH. The prevalence of absence of the palmaris longus--a study in a Chinese population and a review of the literature. Journal of hand surgery (Edinburgh, Scotland) 2005;30:525–527. doi: 10.1016/j.jhsb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Sebastin SJ, Lim AY, Bee WH, Wong TC, Methil BV. Does the absence of the palmaris longus affect grip and pinch strength? Journal of hand surgery (Edinburgh, Scotland) 2005;30:406–408. doi: 10.1016/j.jhsb.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Wehbé MA. Tendon graft donor sites. The Journal of hand surgery. 1992;17:1130–1132. doi: 10.1016/s0363-5023(09)91079-6. [DOI] [PubMed] [Google Scholar]
- 23.Erić M, Krivokuća D, Savović S, Lekšan I, Vučinić N. Prevalence of the palmaris longus through clinical evaluation. Surgical and radiologic anatomy. 2010;32:357–361. doi: 10.1007/s00276-009-0573-0. [DOI] [PubMed] [Google Scholar]
- 24.Dellon AL. Musculotendinous variations about the medial humeral epicondyle. Journal of hand surgery (Edinburgh, Scotland) 1986;11:175–181. doi: 10.1016/0266-7681(86)90254-8. [DOI] [PubMed] [Google Scholar]
- 25.Galton JC. On the Epitrochleo-Anconeus or Anconeus Sextus (Gruber) Journal of anatomy and physiology. 1874;9:168, 162–175. [PMC free article] [PubMed] [Google Scholar]
- 26.Diogo R, Wood B. Soft-tissue anatomy of the primates: phylogenetic analyses based on the muscles of the head, neck, pectoral region and upper limb, with notes on the evolution of these muscles. J Anat. 2011;219:273–359. doi: 10.1111/j.1469-7580.2011.01403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Husarik DB, Saupe N, Pfirrmann CWA, Jost B, Hodler J, Zanetti M. Elbow Nerves: MR Findings in 60 Asymptomatic Subjects—Normal Anatomy, Variants, and Pitfalls1. Radiology. 2009;252:148–156. doi: 10.1148/radiol.2521081614. [DOI] [PubMed] [Google Scholar]
- 28.Dekelver I, Van Glabbeek F, Dijs H, Stassijns G. Bilateral ulnar nerve entrapment by the M. anconeus epitrochlearis. A case report and literature review. Clinical rheumatology. 2012;31:1139–1142. doi: 10.1007/s10067-012-1991-7. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Dines JS, Gorman M, Limpisvasti O, Gambardella R, Yocum L. Anconeus Epitrochlearis as a Source of Medial Elbow Pain in Baseball Pitchers. Orthopedics. 2012;35:631. doi: 10.3928/01477447-20120621-39. [DOI] [PubMed] [Google Scholar]