Abstract
Pancreatic cancer is a highly lethal disease with a poor prognosis characterized by local and systemic disease progression. Both radiation and chemotherapy play important roles in the management of this disease. However, in order to improve standard therapy many molecularly targeted agents are being developed. Glycogen synthase kinase 3β (GSK3β) participates in a multitude of cellular processes and is a newly proposed therapeutic target in pancreatic cancer. This review will discuss both the oncogenic and tumor suppressor functions of GSK3β in pancreatic cancer with an emphasis on the roles of GSK3β in tumor cell survival and sensitivity to radiation and chemotherapy.
Keywords: GSK3β, Pancreatic cancer, c-Met, Radiation
Introduction
Pancreatic cancer, the fourth leading cause of cancer-related death in the United States is characterized by both local and systemic disease progression [1,2]. Newer treatment strategies for metastatic disease include FOLFIRINOX and Abraxane in combination with the standard chemotherapy, gemcitabine [3-5]. In the locally-advanced disease setting chemoradiation is superior to chemotherapy (gemcitabine) alone [6,7]. Since surgical resection offers the only potential cure for this disease but is possible in a minority of patients, efforts are under way to improve therapy for both locally advanced and metastatic disease. Therefore, identification of novel therapeutic targets and development of mechanism-based targeted therapy in combination with existing treatment regimens is of great interest and has the potential to improve the efficacy of current therapy against this deadly disease.
GSK3β is being investigated as a therapeutic target for the treatment of a variety of human diseases including cancer. GSK3β regulates many important processes in cancer cells such as the cell cycle, proliferation, differentiation and apoptosis [8-10]. On the other hand, the mechanisms underlying GSK3β regulation of tumorigenesis and cancer progression are still obscure and to date, whether GSK3β is an oncogene or a tumor suppressor remains controversial. Given the multiple functions of GSK3β, it may act as both an oncogene and a tumor suppressor, depending upon cell properties and contexts as well as the stage of tumorigenesis. In this review, we will discuss both the oncogenic and tumor suppressor functions of GSK3β in the context of pancreatic cancer.
GSK3β
GSK3α and GSK3β are encoded by two distinct genes and share 85% identity, especially in their kinase domains [8,9,11,12]. Interestingly, while these two isoforms do show redundancy in biological function, many studies have defined unique roles of GSK3β in physiological and pathological processes. GSK3β was first identified more than 30 years ago as one of the key kinases involved in glycogen metabolism [13,14]. GSK3β mediates insulin signaling by phosphorylating and inactivating glycogen synthase. Interestingly, GSK3β possesses its activity in unstimulated and resting cells, and extracellular signals diminish its activity by phosphorylation at Serine 9 (Ser9) mediated by AKT and other kinases as well as by a lack of phosphorylation at Tyrosine 216 (Tyr216) [8,15]. Insulin therefore promotes glycogen formation through inhibition of GSK3β.
Other functions independent of glycogen regulation occur as GSK3β phosphorylates many S/TPXXpS/T motif-containing proteins, targeting them for subsequent ubiquitin-mediated proteasomal degradation [11,16]. In most cases, GSK3β substrates require prior phosphorylation in the -4 position for subsequent GSK3β-mediated phosphorylation [16]. Phosphorylation of substrates by GSK3β and other kinases such as Casein Kinase (CK) and dual-specificity tyrosinephosphorylated and regulated kinase (DYRK) generates the optimal substrate conformation for binding of SCF (SKP1, Cullin and F-box protein complex)-type E3 ligases [11,17,18]. Several F-box proteins including SKP2, FBXW7, and β-TrCP, are able to recognize these GSK3β-phosphorylated motifs (degron) on the substrates for targeted degradation [19]. Through this cascade, GSK3β influences gene transcription by degradation of cytosolic transcription factors before nuclear translocation.
GSK3β as a Tumor Suppressor
GSK3β substrate proteins play crucial roles in a wide spectrum of cellular processes, including the cell cycle, transcription, and signal transduction. Most of them are functionally inhibited by GSK3β- mediated phosphorylation (Figure 1) and some key oncoproteins are well known substrates of GSK3β, such as β-catenin, c-Myc, Cyclin D, Cyclin E, and c-Jun [20-22]. β-catenin is the prototypical example of an oncoprotein that is negatively regulated by GSK3β. It is well-known that GSK3β is a negative regulator of Wnt/β-catenin signaling pathway such that in the absence of Wnt ligand, the scaffold protein Axin facilitates GSK3β-mediated phosphorylation of β-catenin resulting in β-catenin degradation by β-TrCP [23]. Activation of Wnt signaling causes disruption of the GSK3β-Axin-APC complex and inhibits β-catenin degradation, which in turn binds the T-cell factor (TCF)/Lymphoid Enhancer Factor (LEF) family of transcription factors and positively regulates transcription of oncogenes such as Cyclin D and c-Myc. Thus, GSK3β suppresses β-catenin activation through proteosome targeting while inhibition of GSK3β allows accumulation of β-catenin followed by nuclear translocation and β-catenin-dependent gene transcription.
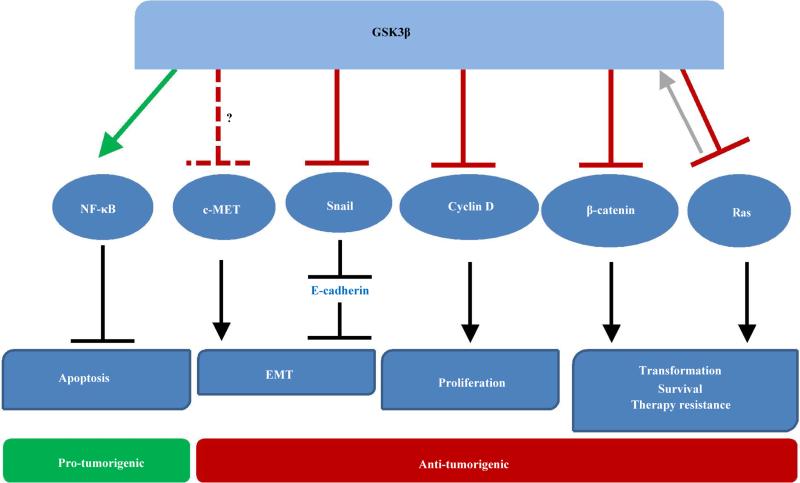
Figure 1.
The functions of GSK3β in pancreatic cancer. Active GSK3β has both tumor promoting and tumor inhibiting activities shown in green and red, respectively. GSK3β positively regulates NF-κB activity, resulting in inhibition of apoptosis and survival. On the other hand, GSK3β directly targets multiple proteins with oncogenic properties (RAS, β-catenin, Cyclin D, and Snail) for ubiquitin-mediated degradation. Data presented in this review also suggest a putative role for GSK3β in negatively regulating c-MET.
Connections between GSK3β and other oncoproteins, such as K-RAS are emerging. In the context of Wnt pathway regulation, GSK3β was found to phosphorylate RAS, ultimately leading to ubiquitination by β-TrCP-E3 ligase and subsequent degradation of RAS in colon cancer models [24]. Since K-RAS mutations occur in more than 90% of pancreatic carcinomas, it is important for future studies to determine whether GSK3β negatively regulates K-RAS stability in pancreatic cancer. The action of GSK3β to degrade oncogenic proteins such as K-RAS, c-Myc, and Cyclin D suggests that GSK3β possesses some tumor suppressor properties.
GSK3β as an Oncoprotein
In contrast to these tumor suppressor properties, GSK3β oncogenic properties are largely thought to be mediated by inhibition of apoptosis. GSK3β is required for cell survival during development, as GSK3β-/- mice die at embryonic day 13.5 due to hepatocyte apoptosis [25]. This phenotype is attributable to the function of GSK3β in activation of NF-κB to inhibit tumor necrosis factor (TNF)-induced apoptosis [25]. GSK3β was also found to prevent apoptosis in a number of human cancer cell lines, further supporting an oncogenic role of GSK3β in cancer [26-28]. Moreover, in cancers such as those of the colon [27], pancreas [29], and kidney [30]. GSK3β is offen overexpressed, suggesting its oncogenic association. Finally, it has been suggested that the effects of GSK3β on glycogen production may elicit positive effects on cancer cell proliferation and survival, further suggesting an oncogenic function [31].
Role of GSK3β In Pancreatic Cancer
The functions of GSK3β in the normal pancreas are seemingly distinct from the functions of GSK3β in pancreatic cancers which, in the vast majority of cases arise from epithelial cells within the exocrine system. In contrast, in the normal pancreas GSK3β plays an important role in the endocrine system, specifically in the beta cells of the islets. Here, GSK3β is known to negatively regulate beta cell DNA synthesis, cell cycle progression, and cell mass, as conditional knockout or pharmacological inhibition of GSK3β promotes these activities [32,33]. In pancreatic cancers GSK3β protein expression is increased relative to non-neoplastic pancreatic tissues, which varies throughout disease progression. Both nuclear and cytoplasmic GSK3β are overexpressed in pancreatic cancer cells as compared to normal pancreatic ductal and acinar cells. Specifically, increased expression of cytoplasmic GSK3β occurs in early PanIN lesions and in well/moderately -differentiated pancreatic cancer tissues [29]. Intriguingly, with disease progression to less differentiated phenotypes, the GSK3β expression pattern shifts from cytoplasmic to nuclear with GSK3β kinase activity contributing to its nuclear localization [29]. This dynamic expression pattern of GSK3β in pancreatic cancer development implicates different functions of GSK3β during disease progression, with GSK3β acting in the cytoplasm during the early stage of tumorigenesis (initiation, proliferation and transformation) and in the nucleus during later stages of disease (proliferation, survival and metastasis) [29]. Furthermore, overexpression of GSK3β was found to be partially attributable to K-RAS-mediated transcriptional upregulation of GSK3β [34]. Subsequent studies have found that pancreatic tumor tissues have higher levels of active GSK3β (pGSK3β Y216), but lower levels of inactive GSK3β (pGSK3β S9) as compared to non-neoplastic tissues [35,36]. However, whether GSK3β expression correlates with patient survival remains to be determined and will be an important aspect for further defining the role of GSK3β in pancreatic cancer. Despite these seemingly oncogenic properties of GSK3β in pancreatic cancer, established tumor suppressor functions in other cancers [12] as well as recent data regarding the role of GSK3β in radiation survival (described below) [37] suggest that GSK3β may have some tumor suppressor functions in pancreatic cancer as well.
Promotion of Chemotherapy Sensitivity by GSK3β Inhibition
The aberrant expression and disease-promoting activity of GSK3β indicates that it may serve as a therapeutic target in pancreatic cancer. Indeed, several studies have found that GSK3β inhibition reduces cell proliferation, increases cell apoptosis and sensitizes cells to death by different stimuli (Table 1) [26,38,39]. These phenotypes resulting from GSK3β inhibition are attributable to a variety of mechanisms including disruption of NF-κB-mediated survival [26,40] activation of tumor suppressors (p53 and Rb) [41], increased expression of the proapoptotic factor Bim [42], and inhibition of human telomerase reverse transcriptase and telomerase [35]. It has been reported that GSK3β is involved in NF-κB mediated cell survival and acts as a tumor promoter [26,40]. Consistent with this observation, a decrease in cellular GSK3β or pharmacologic inhibition of GSK3β activity reduced tumor cell survival and proliferation. GSK3β inhibition by the small molecule AR-A014418 sensitized pancreatic cancer cells to apoptosis induced by TRAIL (tumor necrosis factor-related apoptosis inducing ligand) both in vitro and in tumor xenografts [38]. In addition, AR-A014418 was shown to sensitize the Panc-1 pancreatic cell line model to gemcitabine both in vitro and in vivo [36,39]. The role of GSK3β in chemotherapy resistance, however, is controversial, as Mamaghani et al. [43] reported an overall lack of synergy and in some cases even antagonism between GSK3β inhibition (by AR-A014418) and gemcitabine in a panel of 6 pancreatic cancer cell lines. Further investigation is needed to determine the nuances of GSK3β inhibition as a means of sensitizing pancreatic cancer cells to chemotherapy.
Table 1.
Consequences of GSK3β inhibition in pancreatic cancer cells.
| Inhibitor | Cell type | Consequences | Reference |
|---|---|---|---|
| AR-A014418 | Panc-1 | Decreased proliferation and tumor growth, gemcitabine sensitization | 39 |
| AR-A014418 | Panc-1, MiaPaCa-2, BxPC-3 | Decreased proliferation, gemcitabine sensitization (in vitro & in vivo), radiosensitization, decreased migration & invasion | 36 |
| siGSK3β & AR-A014418 | MiaPaCa-2, Panc-1 | Decreased NFκB activity, caspase-3 cleavage, decreased proliferation | 40 |
| AR-A014418 | BxPC-3, Panc-1, HPAC, PK-1, PK-8 | Decreased proliferation, no gemcitabine sensitization (except Panc-1) | 43 |
| AR-A014418 | Panc-1, BxPC-3 | Increased TRAIL-induced apoptosis (in vitro & in vivo) | 38 |
| AR-A014418, shGSK3β, SB216763 | MiaPaCa-2, BxPC-3 | Decreased proliferation, increased apoptosis | 26 |
| shGSK3β, LiCI | BxPC-3 & Panc-1 | Resistance to radiation (in vitro & in vivo) | 37 |
| siGSK3β | Panc-1 | Resistance to radiation, increased HGF, c-Met activation | [Figure 4] |
Promotion of Radiation Therapy Resistance by GSK3β Inhibition
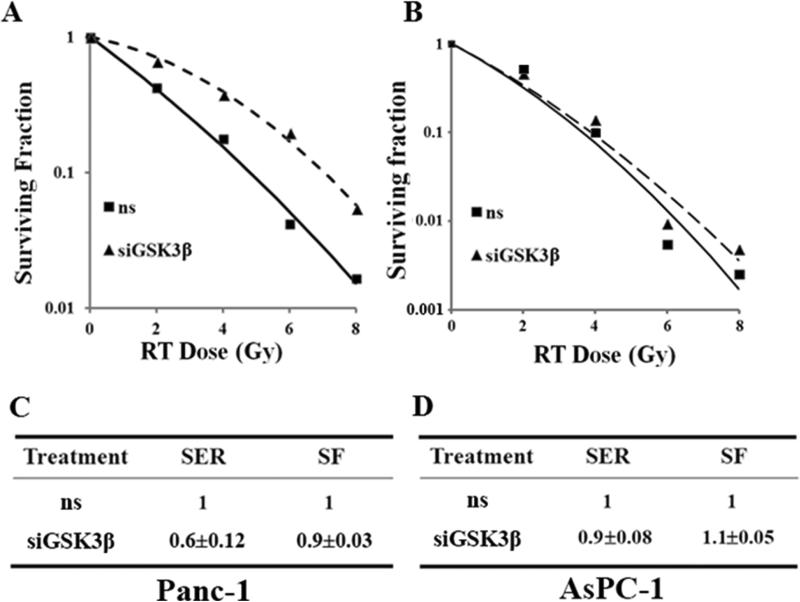
Given the importance of radiation therapy in the management of locally advanced pancreatic cancer, the role of GSK3β in radiation survival has been investigated [37]. Our previous work has shown that radiation inhibits GSK3β activity by inducing Ser9 phosphorylation, while protein kinase Cβ (PKCβ) inhibition by enzastaurin prevents radiation-induced Ser9 phosphorylation and causes radiosensitization of pancreatic cancers both in vitro and in vivo [44]. Furthermore, inhibition of GSK3β by genetic or pharmacological inhibitors (shRNA or Lithium Chloride, respectively) causes radioresistance of pancreatic cancer cells and pancreatic tumor xenografts (BxPC-3, Panc-1) [37]. This observation has been confirmed in additional studies which demonstrate that siRNA-mediated inhibition of GSK3β causes radiation resistance in Panc-1 and to a lesser extent in AsPC-1 pancreatic cancer cells (Figure 2). In addition, introduction of a non-degradable point mutant of β-catenin (S33Y) mimicked the effects of GSK3β inhibition on radiation survival in pancreatic cancer cells [37]. Mechanistic studies revealed that radioresistance modulated by GSK3β inhibition is partly attributable to activation of the Wnt/β-catenin pathway as radioresistance is associated with stabilization of β-catenin and upregulation of β-catenin target genes [37]. These results suggest that radiosensitization of pancreatic cancer would be achieved by the activation of the Wnt/GSK3β axis to trigger β-catenin degradation, rather than by the inhibition of GSK3β activity. In contrast, another study found that inhibition of GSK3β by AR-A014418 produced modest radiosensitization in pancreatic cancer cells (Panc-1 and MiaPaCa-2) although this effect was not confirmed by an independent method of GSK3β inhibition or in animal models [36]. Taken together, it appears that radiation resistance in response to pharmacological inhibition of GSK3β is variable and may be dependent on the specific GSK3β inhibitor used. However, there is general agreement that genetic inhibition of GSK3β produces radiation resistance warranting detailed future investigations, including the use of genetically modified mouse models (e.g. KrasG12D pancreatic ductal adenocarcinoma model [45]) with targeted manipulation of GSK3β.
Figure 2. GSK3β silencing causes radiation resistance in pancreatic cancer cells.
Panc-1 (A, C) and AsPC-1 (B, D) cells were transfected using Oligofectamine (Invitrogen) with ns (non-specific) or GSK3β siRNA (60 nM; Dharmacon) and 48 hours post-transfection were treated with ionizing radiation (RT; 0-8 Gy). Twenty-four hours later, cells were seeded in 60-mm dishes in triplicate for clonogenic survival. After 10 to 12 days of growth, colonies with more than 50 cells were scored. C, D, The Sensitization Enhancement Ratio (SER) was calculated from the radiation survival curves (A,B) as the ratio of the mean inactivation dose (according to the methods of Fertil et al. [59]) for ns siRNA-treated cells to siGSK3β-treated cells and are the mean ± SD of n=2 independent experiments. SER values<1 indicate radiation resistance. Cytotoxicity from siGSK3β treatment alone (in the absence of radiation) is expressed as the Surviving Fraction (SF).
Mechanisms Contributing to Radiation Survival Following GSK3β Inhibition
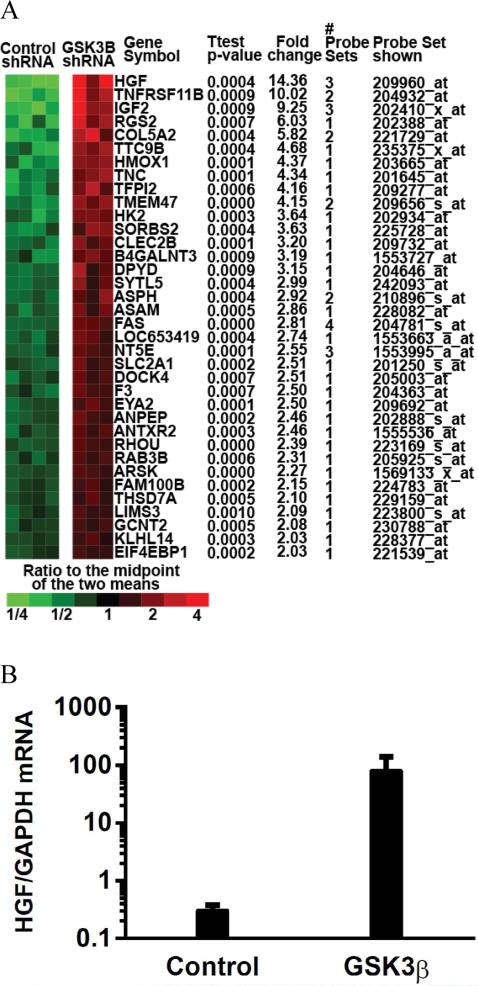
To further elucidate the mechanism by which GSK3β inhibition confers radiation resistance, we conducted a DNA microarray screen and found that the hepatocyte growth factor/scatter factor (HGF/SF), the ligand for the c-MET receptor was significantly upregulated after GSK3β silencing (Figure 3). While others have shown that c-MET is transcriptionally regulated by β-catenin [46], there are no prior reports of GSK3β/β-catenin modulation of HGF. Upon HGF binding, c-MET forms a homodimer and undergoes auto-phosphorylation at tyrosine residues 1234 and 1235 leading to activation of c-MET and downstream effectors such as RAS, focal adhesion kinase (FAK), and AKT, ultimately stimulating proliferation, survival, motility and invasion [47,48]. Deregulation of the HGF/c-MET axis occurs frequently in cancers, including those of the pancreas as a result of activating mutations, overexpression, as well as aberrant secretion (both autocrine and paracrine) of the HFG ligand [47,49]. While c-MET is not frequently mutated in pancreatic cancer, it is overexpressed at the protein level in a subset of pancreatic cancers and its activity is further promoted by HGF secreted from tumor-associated fibroblasts [50]. In addition, we found that pancreatic cancer cells are capable of autocrine-mediated c-Met activation which is increased upon GSK3β silencing (Figure 4A).
Figure 3.
Changes in gene expression in response to GSK3β silencing. A, Gene expression changes were determined in response to control or GSK3β shRNA in 3-4 Panc-1 tumors per treatment condition using an Affymetrix DNA microarray. Shown are distinct genes increased by an average of at least 2-fold relative to control shRNA with 2-sample t-test p-values of <0.001. The #probe sets indicate the number of probe sets used for each qualified gene. HGF was found to be the most up-regulated gene in response to GSK3β silencing. Microarray data, methods, and analysis have been deposited in the NCBI Gene Expression Omnibus [60] and are accessible through GEO Series accession number GSE35351 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE35351). B, Independent analyses using quantitative PCR confirmed increased HGF expression in response to GSK3β shRNA in Panc-1 cells. Data are the mean of n=2 ± SE independent experiments performed at least in duplicate..
Figure 4.
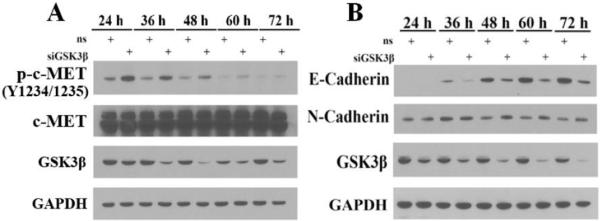
Inhibition of GSK3β induces c-MET activation and decreases E-Cadherin expression. AsPC-1 (A) and Panc-1 (B) cells transiently transfected with non-specific (ns) or GSK3β siRNA were collected at different time points following transfection and analyzed by immunoblotting. Membranes were probed with antibodies recognizing p-c-MET, GSK3β, c-MET, GAPDH (Cell Signaling), E-Cadherin, and N-Cadherin (BD Bioscience). Data are from a single representative experiment.
While c-MET has been shown to regulate radiation survival in other types of cancer [51,52], its role in pancreatic cancer has not previously been identified although irradiated fibroblasts were shown to induce c-Met activity in pancreatic cancer cells [53]. Consistent with HGF upregulation, we found phosphorylation of c-MET to be increased in response to GSK3β depletion in AsPC-1 pancreatic cancer cells (Figure 4A). Taken together, these findings warrant further investigation of the interactions between GSK3β and HGF/c-MET as well as their effects on radiation survival in pancreatic cancer.
Other potential mechanisms which may contribute to radioresistance in response to GSK3β inhibition include accumulation of GSK3β substrates such as RAS, β-catenin, and Snail which undergo ubiquitin-mediated degradation following GSK3β-mediated phosphorylation. Oncogenic activation or overexpression of RAS confers intrinsic radiation resistance in many human cancer cells [54]. Recently, a study indicated oncogenic K-RAS induces expression of an oncogenic kinase, Pim-1 and thus modulates radioresistance in pancreatic cancer [55]. Snail is a key transcription factor involved in Epithelial-Mesenchymal Transition (EMT) [56] which promotes EMT by transcriptional repression of E-Cadherin [57]. Through a mechanism similar to β-catenin destruction, GSK3β phosphorylates the degron of Snail, promoting subsequent β-TrCP-mediated degradation [56]. Therefore, inhibition of GSK3β blocks the phosphorylation and degradation of Snail, which in turn suppresses E-Cadherin expression thus promoting EMT [56]. Indeed, we found that depletion of GSK3β in Panc-1 cells results in the decreased expression of E-Cadherin and also consistent with EMT, a modest increase in N-cadherin (Figure 4B). Since EMT is associated with cancer stem cell-like properties and resistance to chemo- and radiation therapy [58], downregulation of E-cadherin in response to GSK3β depletion may represent yet another potential mechanism for radiation resistance.
Conclusions
The aberrant expression and elevated kinase activity of GSK3β in pancreatic cancer implicate its role as an oncogene. However, GSK3β also possesses many well-established tumor suppressor properties. While GSK3β is emerging as a therapeutic target in pancreatic cancer, some issues remain unresolved regarding the use of GSK3β inhibitors as a therapeutic strategy for the treatment of pancreatic cancer. First, activation of oncogenic signaling pathways (e.g. β-catenin, K-RAS) upon GSK3β inhibition may have detrimental effects in both tumor and normal cells, the latter of which could potentially result in transformation of normal cells. Second, our studies strongly suggest that targeting GSK3β renders pancreatic cancer cells resistant to radiation. This raises concerns in the context of combining GSK3β inhibitors with chemoradiation in pancreatic cancer. As GSK3β participates in a complex signaling network in which our current understanding is still incomplete, the development of GSK3β targeted agents for pancreatic cancer should progress with careful evaluation of both the potential oncogenic and tumor suppressor roles of GSK3β.
Acknowledgements
This work was supported by a Developmental Research Grant through P50CA130810 and R01CA163895 to MAM and CA170995 to YS.
Footnotes
Citation: Zhang Q, Bhojani MS, Josef EB, Spalding AC, Kuick R, et al. (2013) Glycogen Synthase Kinase 3β in Pancreatic Cancer and its Implications in Chemotherapy and Radiation Therapy. J Carcinogene Mutagene 4: 147. doi:10.4172/2157-2518.1000147
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Iacobuzio-Donahue CA, Fu B, Yachida S, Luo M, Abe H, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 4.Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29:4548–4554. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 6.Loehrer PJ, Feng Y, Cardenes H, Wagner L, Brell JM, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an eastern cooperative oncology group trial. J Clin Oncol. 2011;29:4105–4112. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Josef E, Schipper M, Francis IR, Hadley S, Ten-Haken R, et al. A phase I/II trial of intensity modulated radiation (IMRT) dose escalation with concurrent fixed-dose rate gemcitabine (FDR-G) in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2012;84:1166–1171. doi: 10.1016/j.ijrobp.2012.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs KM, Bhave SR, Ferraro DJ, Jaboin JJ, Hallahan DE, et al. GSK-3b: A Bifunctional Role in Cell Death Pathways. Int J Cell Biol. 2012;2012:930710. doi: 10.1155/2012/930710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harwood AJ. Regulation of GSK-3: a cellular multiprocessor. Cell. 2001;105:821–824. doi: 10.1016/s0092-8674(01)00412-3. [DOI] [PubMed] [Google Scholar]
- 11.Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 12.Luo J. Glycogen synthase kinase 3beta (GSK3beta) in tumorigenesis and cancer chemotherapy. Cancer Lett. 2009;273:194–200. doi: 10.1016/j.canlet.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Embi N, Rylatt DB, Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980;107:519–527. [PubMed] [Google Scholar]
- 14.Plyte SE, Hughes K, Nikolakaki E, Pulverer BJ, Woodgett JR. Glycogen synthase kinase-3: functions in oncogenesis and development. Biochim Biophys Acta. 1992;1114:147–162. doi: 10.1016/0304-419x(92)90012-n. [DOI] [PubMed] [Google Scholar]
- 15.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA, et al. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 16.Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8:83–93. doi: 10.1038/nrc2290. [DOI] [PubMed] [Google Scholar]
- 17.Lau AW, Fukushima H, Wei W. The Fbw7 and betaTRCP E3 ubiquitin ligases and their roles in tumorigenesis. Front Biosci (Landmark Ed) 2012;17:2197–2212. doi: 10.2741/4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y, Sun Y. CUL4B ubiquitin ligase in mouse development: a model for human X-linked mental retardation syndrome? Cell Res. 2012;22:1224–1226. doi: 10.1038/cr.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia L, Sun Y. SCF E3 ubiquitin ligases as anticancer targets. Curr Cancer Drug Targets. 2011;11:347–356. doi: 10.2174/156800911794519734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gregory MA, Qi Y, Hann SR. Phosphorylation by glycogen synthase kinase-3 controls c-myc proteolysis and subnuclear localization. J Biol Chem. 2003;278:51606–51612. doi: 10.1074/jbc.M310722200. [DOI] [PubMed] [Google Scholar]
- 21.Welcker M, Singer J, Loeb KR, Grim J, Bloecher A, et al. Multisite phosphorylation by Cdk2 and GSK3 controls cyclin E degradation. Mol Cell. 2003;12:381–392. doi: 10.1016/s1097-2765(03)00287-9. [DOI] [PubMed] [Google Scholar]
- 22.Wei W, Jin J, Schlisio S, Harper JW, Kaelin WG. The v-Jun point mutation allows c-Jun to escape GSK3-dependent recognition and destruction by the Fbw7 ubiquitin ligase. Cancer Cell. 2005;8:25–33. doi: 10.1016/j.ccr.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, et al. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 24.Jeong WJ, Yoon J, Park JC, Lee SH, Lee SH, et al. Ras stabilization through aberrant activation of Wnt/ß-catenin signaling promotes intestinal tumorigenesis. Sci Signal. 2012;5:ra30. doi: 10.1126/scisignal.2002242. [DOI] [PubMed] [Google Scholar]
- 25.Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, et al. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 26.Ougolkov AV, Fernandez-Zapico ME, Savoy DN, Urrutia RA, Billadeau DD. Glycogen synthase kinase-3beta participates in nuclear factor kappaB-mediated gene transcription and cell survival in pancreatic cancer cells. Cancer Res. 2005;65:2076–2081. doi: 10.1158/0008-5472.CAN-04-3642. [DOI] [PubMed] [Google Scholar]
- 27.Shakoori A, Ougolkov A, Yu ZW, Zhang B, Modarressi MH, et al. Deregulated GSK3beta activity in colorectal cancer: its association with tumor cell survival and proliferation. Biochem Biophys Res Commun. 2005;334:1365–1373. doi: 10.1016/j.bbrc.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 28.Liao X, Zhang L, Thrasher JB, Du J, Li B. Glycogen synthase kinase-3beta suppression eliminates tumor necrosis factor-related apoptosis-inducing ligand resistance in prostate cancer. Mol Cancer Ther. 2003;2:1215–1222. [PubMed] [Google Scholar]
- 29.Ougolkov AV, Fernandez-Zapico ME, Bilim VN, Smyrk TC, Chari ST, et al. Aberrant nuclear accumulation of glycogen synthase kinase-3beta in human pancreatic cancer: association with kinase activity and tumor dedifferentiation. Clin Cancer Res. 2006;12:5074–5081. doi: 10.1158/1078-0432.CCR-06-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bilim V, Ougolkov A, Yuuki K, Naito S, Kawazoe H, et al. Glycogen synthase kinase-3: a new therapeutic target in renal cell carcinoma. Br J Cancer. 2009;101:2005–2014. doi: 10.1038/sj.bjc.6605437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ougolkov AV, Billadeau DD. Targeting GSK-3: a promising approach for cancer therapy? Future Oncol. 2006;2:91–100. doi: 10.2217/14796694.2.1.91. [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Remedi MS, Pappan KL, Kwon G, Rohatgi N, et al. Glycogen synthase kinase-3 and mammalian target of rapamycin pathways contribute to DNA synthesis, cell cycle progression, and proliferation in human islets. Diabetes. 2009;58:663–672. doi: 10.2337/db07-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Tanabe K, Baronnier D, Patel S, Woodgett J, et al. Conditional ablation of Gsk-3β in islet beta cells results in expanded mass and resistance to fat feeding-induced diabetes in mice. Diabetologia. 2010;53:2600–2610. doi: 10.1007/s00125-010-1882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang JS, Koenig A, Harrison A, Ugolkov AV, Fernandez-Zapico ME, et al. Mutant K-Ras increases GSK-3β gene expression via an ETS-p300 transcriptional complex in pancreatic cancer. Oncogene. 2011;30:3705–3715. doi: 10.1038/onc.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mai W, Kawakami K, Shakoori A, Kyo S, Miyashita K, et al. Deregulated GSK3{beta} sustains gastrointestinal cancer cells survival by modulating human telomerase reverse transcriptase and telomerase. Clin Cancer Res. 2009;15:6810–6819. doi: 10.1158/1078-0432.CCR-09-0973. [DOI] [PubMed] [Google Scholar]
- 36.Kitano A, Shimasaki T, Chikano Y, Nakada M, Hirose M, et al. Aberrant glycogen synthase kinase 3β is involved in pancreatic cancer cell invasion and resistance to therapy. PLoS One. 2013;8:e55289. doi: 10.1371/journal.pone.0055289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson RL, Spalding AC, Zielske SP, Morgan M, Kim AC, et al. GSK3beta and beta-catenin modulate radiation cytotoxicity in pancreatic cancer. Neoplasia. 2010;12:357–365. doi: 10.1593/neo.92112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mamaghani S, Simpson CD, Cao PM, Cheung M, Chow S, et al. Glycogen synthase kinase-3 inhibition sensitizes pancreatic cancer cells to TRAIL-induced apoptosis. PLoS One. 2012;7:e41102. doi: 10.1371/journal.pone.0041102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimasaki T, Ishigaki Y, Nakamura Y, Takata T, Nakaya N, et al. Glycogen synthase kinase 3β inhibition sensitizes pancreatic cancer cells to gemcitabine. J Gastroenterol. 2012;47:321–333. doi: 10.1007/s00535-011-0484-9. [DOI] [PubMed] [Google Scholar]
- 40.Wilson W, Baldwin AS. Maintenance of constitutive IkappaB kinase activity by glycogen synthase kinase-3alpha/beta in pancreatic cancer. Cancer Res. 2008;68:8156–8163. doi: 10.1158/0008-5472.CAN-08-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyashita K, Kawakami K, Nakada M, Mai W, Shakoori A, et al. Potential therapeutic effect of glycogen synthase kinase 3beta inhibition against human glioblastoma. Clin Cancer Res. 2009;15:887–897. doi: 10.1158/1078-0432.CCR-08-0760. [DOI] [PubMed] [Google Scholar]
- 42.Marchand B, Tremblay I, Cagnol S, Boucher MJ. Inhibition of glycogen synthase kinase-3 activity triggers an apoptotic response in pancreatic cancer cells through JNK-dependent mechanisms. Carcinogenesis. 2012;33:529–537. doi: 10.1093/carcin/bgr309. [DOI] [PubMed] [Google Scholar]
- 43.Mamaghani S, Patel S, Hedley DW. Glycogen synthase kinase-3 inhibition disrupts nuclear factor-kappaB activity in pancreatic cancer, but fails to sensitize to gemcitabine chemotherapy. BMC Cancer. 2009;9:132. doi: 10.1186/1471-2407-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spalding AC, Watson R, Davis ME, Kim AC, Lawrence TS, et al. Inhibition of protein kinase Cbeta by enzastaurin enhances radiation cytotoxicity in pancreatic cancer. Clin Cancer Res. 2007;13:6827–6833. doi: 10.1158/1078-0432.CCR-07-0454. [DOI] [PubMed] [Google Scholar]
- 45.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 46.Boon EM, van der Neut R, van de Wetering M, Clevers H, Pals ST. Wnt signaling regulates expression of the receptor tyrosine kinase met in colorectal cancer. Cancer Res. 2002;62:5126–5128. [PubMed] [Google Scholar]
- 47.Blumenschein GR, Mills GB, Gonzalez-Angulo AM. Targeting the hepatocyte growth factor-cMET axis in cancer therapy. J Clin Oncol. 2012;30:3287–3296. doi: 10.1200/JCO.2011.40.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eder JP, Vande Woude GF, Boerner SA, LoRusso PM. Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin Cancer Res. 2009;15:2207–2214. doi: 10.1158/1078-0432.CCR-08-1306. [DOI] [PubMed] [Google Scholar]
- 49.Smolen GA, Sordella R, Muir B, Mohapatra G, Barmettler A, et al. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc Natl Acad Sci U S A. 2006;103:2316–2321. doi: 10.1073/pnas.0508776103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qian LW, Mizumoto K, Maehara N, Ohuchida K, Inadome N, et al. Co-cultivation of pancreatic cancer cells with orthotopic tumor-derived fibroblasts: fibroblasts stimulate tumor cell invasion via HGF secretion whereas cancer cells exert a minor regulative effect on fibroblasts HGF production. Cancer Lett. 2003;190:105–112. doi: 10.1016/s0304-3835(02)00517-7. [DOI] [PubMed] [Google Scholar]
- 51.De Bacco F, Luraghi P, Medico E, Reato G, Girolami F, et al. Induction of MET by ionizing radiation and its role in radioresistance and invasive growth of cancer. J Natl Cancer Inst. 2011;103:645–661. doi: 10.1093/jnci/djr093. [DOI] [PubMed] [Google Scholar]
- 52.Welsh JW, Mahadevan D, Ellsworth R, Cooke L, Bearss D, et al. The c-Met receptor tyrosine kinase inhibitor MP470 radiosensitizes glioblastoma cells. Radiat Oncol. 2009;4:69. doi: 10.1186/1748-717X-4-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohuchida K, Mizumoto K, Murakami M, Qian LW, Sato N, et al. Radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal interactions. Cancer Res. 2004;64:3215–3222. doi: 10.1158/0008-5472.can-03-2464. [DOI] [PubMed] [Google Scholar]
- 54.Bernhard EJ, Stanbridge EJ, Gupta S, Gupta AK, Soto D, et al. Direct evidence for the contribution of activated N-ras and K-ras oncogenes to increased intrinsic radiation resistance in human tumor cell lines. Cancer Res. 2000;60:6597–6600. [PubMed] [Google Scholar]
- 55.Xu D, Allsop SA, Witherspoon SM, Snider JL, Yeh JJ, et al. The oncogenic kinase Pim-1 is modulated by K-Ras signaling and mediates transformed growth and radioresistance in human pancreatic ductal adenocarcinoma cells. Carcinogenesis. 2011;32:488–495. doi: 10.1093/carcin/bgr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou BP, Deng J, Xia W, Xu J, Li YM, et al. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 57.Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol. 2004;24:306–319. doi: 10.1128/MCB.24.1.306-319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, et al. The epithelialmesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fertil B, Dertinger H, Courdi A, Malaise EP. Mean inactivation dose: a useful concept for intercomparison of human cell survival curves. Radiat Res. 1984;99:73–84. [PubMed] [Google Scholar]
- 60.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]