Abstract
All-carbon quaternary stereocenters have posed significant challenges in the synthesis of complex natural products. These important structural motifs have inspired the development of broadly applicable palladium-catalyzed asymmetric allylic alkylation reactions of unstabilized non-biased enolates for the synthesis of enantioenriched α-quaternary products. This microreview outlines key considerations in the application of palladium-catalyzed asymmetric allylic alkylation reactions and presents recent total syntheses of complex natural products that have employed these powerful transformations for the direct, catalytic, enantioselective construction of all-carbon quaternary stereocenters.
Keywords: Total synthesis, Asymmetric catalysis, Natural products, Palladium, Allylation, All-carbon quaternary stereocenters
1. Introduction and Background
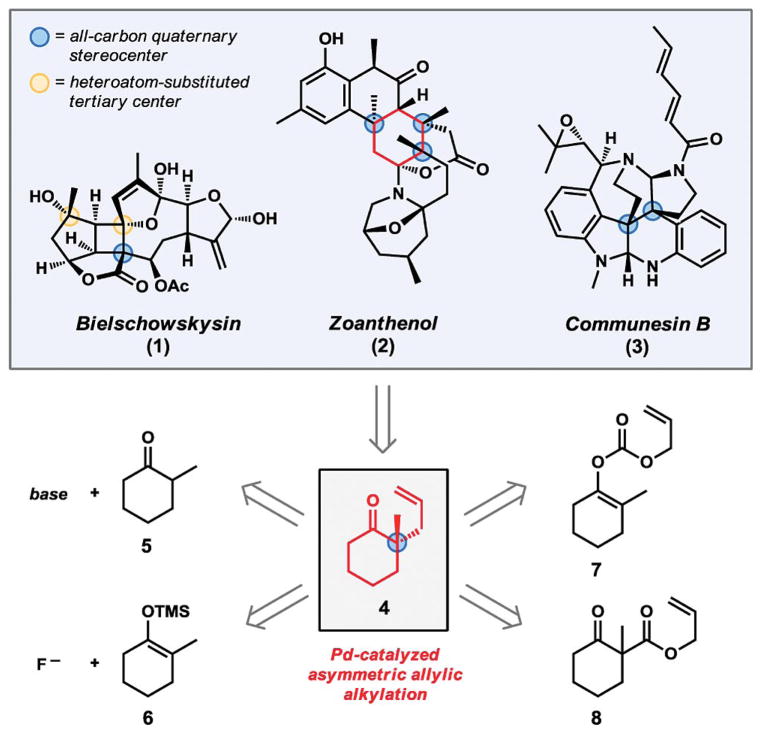
Complex natural products serve a vital role in chemistry as a driving force for the invention of new chemical transformations.[1] The fundamental synthetic challenges posed by all-carbon quaternary stereocenters[2] contained in many natural products have inspired the development of new synthetic methods for the enantioselective construction of these important motifs. In particular, the research area of Pd-catalyzed asymmetric allylic alkylation[3,4] has been advanced significantly in response to synthetic limitations identified during efforts directed towards complex molecules (Figure 1). Modern catalytic enantioselective methods of this type have led to the development of new strategies for the efficient and direct assembly of challenging cyclic core structures in many natural products and have additionally provided powerful and broadly applicable tools for the functionalization of highly substituted ketone-derived enolates. In this short review, the direct construction of all-carbon quaternary stereocenters at nucleophilic enolates by means of Pd-catalyzed allylic alkylation reactions is discussed within the broader context of challenges originating from total synthesis.
Figure 1.
Natural products as an inspiration for the development of asymmetric catalysis.
An all-carbon quaternary stereocenter – that is, one composed of a central carbon atom bound to four carbon substituents (Figure 1) – should be distinguished from a heteroatom-substituted tertiary center (with one heteroatom and three carbon substituents).
In the following discussion, particular attention is given to reported syntheses that have appeared since the most recent reviews.[5] Total syntheses featuring enantioselective tertiary stereocenter formation[6] or catalyst-controlled diastereoselective transformations[7] are not discussed in detail in this microreview.
2. Reaction Design and Mechanistic Considerations
Pd-catalyzed allylic alkylation reactions have found increasingly wide synthetic applications, due to the continued evolution of the methodology. The invention of highly enantioselective transformations for the assembly of all-carbon quaternary stereocenters has facilitated efficient asymmetric syntheses of numerous complex natural products. Although a number of factors have been instrumental in the success of these Pd-catalyzed reactions in complex settings, advances in substrate scope and identification of suitable chiral ligands for these substrate types have had a direct impact on the general utility of these reactions.
2.1. General Aspects of Pd-Catalyzed Asymmetric Allylic Alkylation
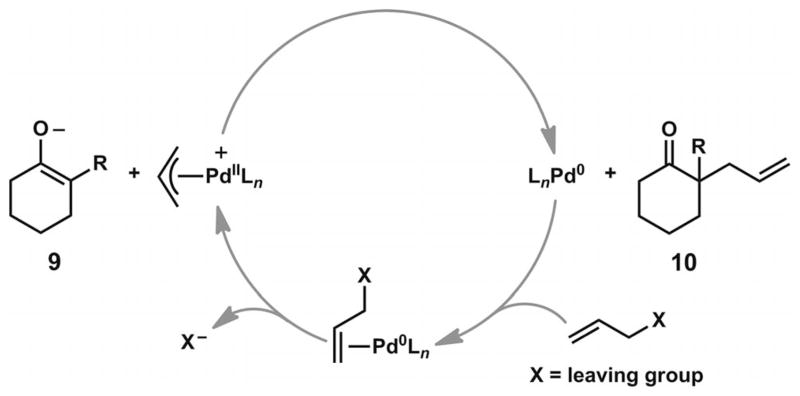
In a typical allylic alkylation reaction, a Pd0 complex undergoes initial olefin coordination and subsequent oxidative addition to an allyl electrophile (Scheme 1). Expulsion of the leaving group leads to a cationic PdII π-allyl complex. Upon combination with an enolate in the reaction mixture, subsequent C–C bond formation can lead to the α-quaternary ketone product and regenerate the initial Pd0 complex. The precise reaction mechanism can vary, depending on the choice of ligand, palladium precursor, substrate, allyl source, additive, and solvent.
Scheme 1.
General mechanism for Pd-catalyzed allylic alkylation reactions.
2.2. Development of Methods for Enolate Generation
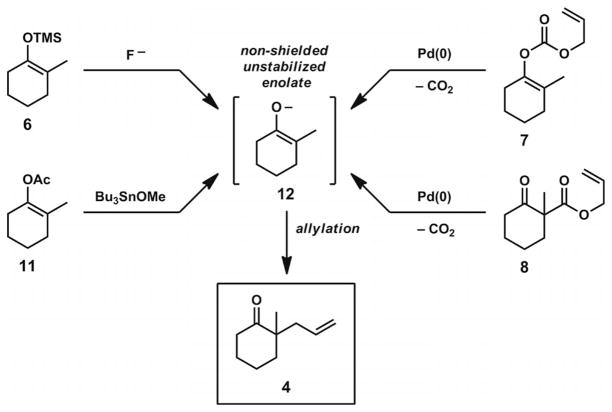
Various methods have been employed for the generation of ketone enolates for Pd-catalyzed asymmetric allylic alkylation reactions, but important advances in the past decade have rendered these transformations more practical and useful for the preparation of complex molecules. An unavoidable and general problem in the allylation of differentially substituted ketones with multiple acidic sites is the formation of isomeric enolates, which can proceed to afford different products in the presence of a palladium π-allyl complex (Scheme 2, A).
Scheme 2.
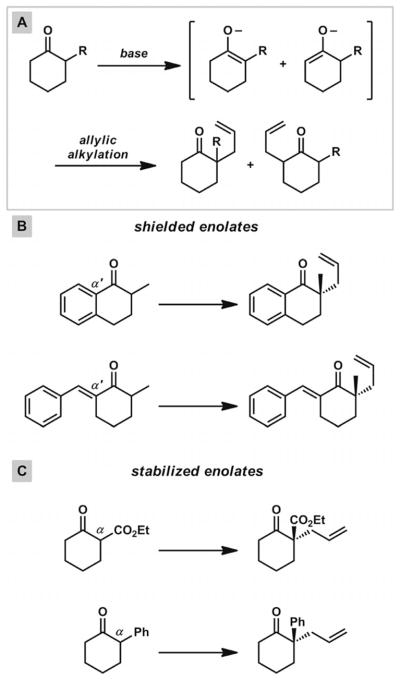
The enolate alkylation problem and approaches to selective enolate formation.
To circumvent this problem, many groups have employed substrates that contain either α′-blocking groups to shield undesired deprotonation sites (Scheme 2, B) or α-electron withdrawing groups to provide large reductions in pKa at the desired deprotonation sites (Scheme 2, C). Although both of these strategies have afforded control of regioselective deprotonations in asymmetric alkylation reactions, and enantioselective transformations on substrates of these types have been documented,[8,9,10,11] this approach can introduce unwanted functional groups into cyclic ketone scaffolds. The modification or removal of these vestigial functionalities from the α-quaternary ketone products can greatly diminish the general application of these compounds in total synthesis.
The general synthesis of chiral α-quaternary ketone building blocks by means of Pd-catalyzed asymmetric allylic alkylation reactions of non-biased unstabilized enolates constituted a major synthetic challenge until the past decade. The lack of established methods for the preparation of relatively simple compounds such as ketone 4 (Figure 1) in high ee presented a significant obstacle to the synthesis of complex natural products with all-carbon quaternary stereocenters.
Alternative methods for selective enolate generation can be found in the work of Tsuji and co-workers in the 1980s (Scheme 3). By use of either enol acetate[12] or silyl enol ether[13] substrates in the presence of appropriate additives, it was possible to unmask the latent enolates as single isomers. Subsequent allylic alkylation provided the α-quaternary ketone products without ancillary group incorporation. Additionally, Tsuji’s work showed that it was possible to incorporate allyl fragments into substrates by use of allyl enol carbonates[14] or allyl β-keto esters.[15,16] The in situ formation of both an allyl electrophile and an enolate nucleophile could be conveniently initiated by a Pd0 catalyst with both of these substrate types. With all of these methods explored by Tsuji, the enolates formed under the reaction conditions maintain high regiochemical fidelity and proceed smoothly to afford the corresponding allylation products. Although these methods provided promising strategies for generating enolates in a widely applicable manner, asymmetric variants of these transformations did not surface until over 20 years later.
Scheme 3.
Tsuji reactions for the allylation of non-shielded, non-stabilized enolates.
2.3. Development of Asymmetric Allylic Alkylation Reactions
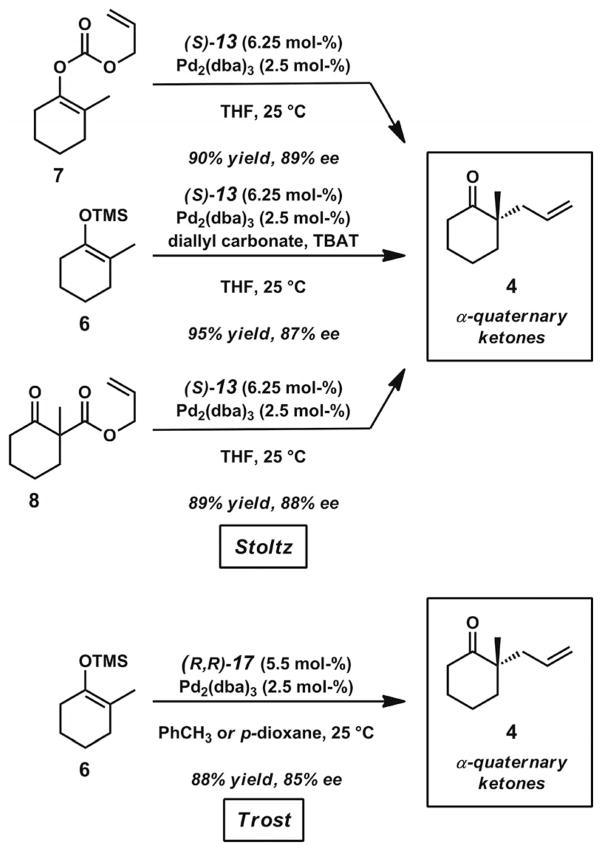
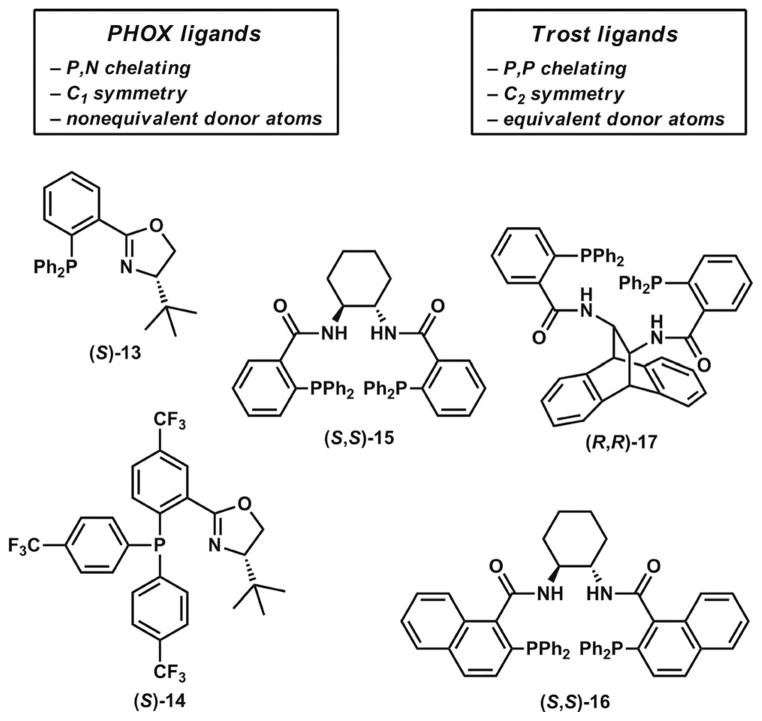
In the past decade, contributions predominantly from the Stoltz and Trost groups have helped address the difficulty of performing Pd-catalyzed asymmetric allylic alkylations on non-stabilized unbiased enolates to give α-quaternary ketones. In the earliest report by Stoltz in 2004, numerous chiral bidentate ligands were screened for their ability to promote high asymmetric induction in reactions with allyl enol carbonate and silyl enol ether substrates (Scheme 4).[17a] Ultimately, it was found that treatment of these substrates with the combination of (S)-tBu-PHOX (13, Figure 2) and [Pd2(dba)3] provided the highest degrees of enantioenrichment in the α-quaternary ketone products. Other P,N- and P,P-chelating ligands were investigated, but they proved to be less effective under the optimized conditions. Carbocyclic substrates with various ring sizes could undergo transformation to give product in high ees. Examples of benzannulated and non-benzannulated substrates were reported.
Scheme 4.
Pd-catalyzed asymmetric allylic alkylation reactions with allyl enol carbonate, silyl enol ether, and β-keto ester substrate classes.
Figure 2.
Selected chiral ligands for Pd-catalyzed asymmetric allylic alkylation reactions.
Shortly afterward, a study by the Trost group in 2005 involved the development of a different catalyst system for the decarboxylative allylic alkylation of allyl enol carbonates (Scheme 4).[18] A screening of various Trost bis-phosphane ligands with modified diamine backbones and aryl-phosphanes found that ligand (R,R)-17 (Figure 2) was most effective for this transformation. Complexation of this ligand with [Pd2(dba)3]·CHCl3 provided an effective catalyst for the synthesis of chiral α-quaternary ketone products with various ring sizes. Although most of the examples consist of benzannulated substrates, two examples of non-benzannulated ketones were also presented. Notably, several cyclic ketones with incorporated heterocycles could also be prepared. The catalyst system was additionally shown to be applicable for the formation of α-tertiary ketones.
Subsequent work by the Stoltz group extended the asymmetric alkylation methodology with the PHOX ligand system to β-keto ester substrates (Scheme 4).[17b,17c] The starting materials are racemates, so the destruction and reconstruction of stereochemical information through the intermediacy of a prochiral enolate must take place in order for these compounds to form asymmetric alkylation products in what has been termed a stereoablative process.[19] Despite this key mechanistic difference, these substrates demonstrated yields and levels of asymmetric induction similar to those obtained with the silyl enol ethers and enol carbonates. The development of reactions for this class of substrates had practical advantages because various α-substituents could be introduced under relatively mild conditions and because β-keto ester substrates typically have higher thermal and chemical stabilities than silyl enol ether and enol carbonate substrates.
The bis-phosphane and phosphanyloxazoline-type ligands described in the examples above (Figure 2) have enjoyed notable success in the construction of challenging all-carbon quaternary stereocenters to prepare key intermediates for natural product synthesis. Trost ligands[20] possess C2 symmetry and equivalent donor atoms as well as amide functionality capable of hydrogen bonding. To date, numerous ligands with modified backbone scaffolds and arylphosphane substitution have been reported. Structural modification of these ligands has led to changes in reactivity in cases with multiple possible asymmetric alkylation pathways. PHOX ligands,[21] pioneered by Pfaltz, Helmchen, and Williams, have also proven to be useful ligands for asymmetric allylic alkylation reactions. These ligands possess C1 symmetry and non-equivalent donor atoms. This lack of symmetry has important ligand design implications, because the oxazoline and arylphosphane regions of the ligand can be tuned somewhat independently. Overall, the ligand classes have unique and complementary steric and electronic features that make them particularly useful and adaptable for Pd-catalyzed asymmetric allylic alkylation reactions in total synthesis.
3. Catalytic Asymmetric Synthesis of Natural Products
The development of Pd-catalyzed asymmetric allylic alkylation reactions of non-biased unstabilized enolates for the synthesis of α-quaternary ketone products has provided a powerful tool for total synthesis. The following case studies not only illustrate important advances in synthetic chemistry, but also provide examples of how broader, longstanding problems in the field have been identified and overcome in the process of constructing complex molecules.
3.1. Total Syntheses of Hamigeran B and Allocyathin B2
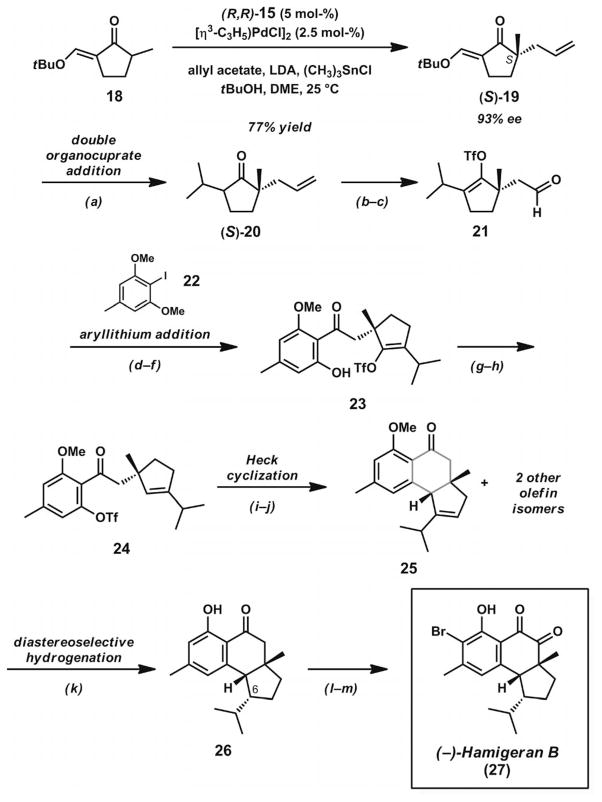
The synthetic potential of Pd-catalyzed allylic alkylation reactions for the assembly of all-carbon quaternary stereo-centers was illustrated by the early enantioselective syntheses of hamigeran B (27, Scheme 5)[22] and allocyathin B2 (37, Scheme 6, below)[23] by the Trost group. The common component for both of these syntheses was enantioenriched exocyclic vinylogous ester 19.
Scheme 5.
Total synthesis of hamigeran B. Reaction conditions: a) (CH3)2CuLi, Et2O, −20 °C (89% yield). b) LDA, THF, −78→0 °C; then PhN(Tf)2 (87% yield). c) OsO4 (3.77 mol-%), NMO, THF, H2O; then NaIO4. d) Iodoarene 22, DME, −55 °C; then aldehyde 21. e) Dess–Martin periodinane, NaHCO3, CH2Cl2, 75% yield (three steps). f) BCl3, CH2Cl2, −20 °C (85% yield). g) Pd(OAc)2 (10 mol-%), dppf (20 mol-%), HCO2H, Et3N, DMF, 70 °C (94% yield). h) Tf2O, CH2Cl2, pyridine, 0 °C (94 % yield). i) Pd(OAc)2, dppb, K2CO3, PhCH3. j) BBr3, CH2Cl2, −78 °C (51% yield, two steps). k) Ir black, H2 (1500 psi), EtOH (>99% yield). l) SeO2, cat. AcOH, p-dioxane (90% yield). m) NBS, iPr2NH (5 mol-%), CH2Cl2 (85% yield).
Scheme 6.
Total synthesis of allocyathin B2. Reaction conditions: a) (CH3)2CuLi, Et2O, −20 °C (89% yield). b) LDA, THF, −78→0 °C; then PhN(Tf)2 (96% yield). c) [Pd2(dba)3]·CHCl3 (2.5 mol-%), PPh3 (20 mol-%), CuI (5 mol-%), TMS-acetylene, nBuNH2, 50 °C (85% yield). d) OsO4 (1 mol-%), NMO; then NaIO4 (87% yield). e) NaBH4, MeOH (94% yield). f) PPh3, I2, imidazole (97% yield). g) tBuLi, ZnCl2, THF, −78 °C →r.t.; then [Pd(PPh3)4] (5 mol-%), vinyl iodide 30. h) K2CO3, MeOH (74% yield, two steps). i) nBuLi, THF, −78 °C; then Boc2O, −78 →r.t. (99% yield). j) TBAF, THF (52–55% yield). k) CpRu(CH3CN)3PF6 (20 mol-%), DMF (1 equiv.), butan-2-one, room temp. (48% yield of 35, or 55% combined yield, 6.7:1 E/Z ratio). l) PhS(O)CH2CN, piperidine, PhH (75% yield). m) Pd/C (10%), EtOAc, H2 (1 atm) (83% yield). n) DIBAL, CH2Cl2, −78 °C. o) KOH, MeOH, 60 °C (51% yield, two steps).
In order to form the requisite quaternary stereocenter, the development of an effective asymmetric allylic alkylation reaction for exocyclic vinylogous ester 18 was needed. The treatment of this compound with allyl acetate as the allyl source, LDA as base, trimethyltin chloride as Lewis acid, and [(η3-C3H5)PdCl]2 and chiral ligand (S,S)-15 (Figure 2) as catalyst precursors in DME at 0 °C provided α-quaternary ketone (R)-19 (Scheme 6, below) in 93% yield but only 12% ee. Extensive reaction optimization revealed that the use of tBuOH as an additive greatly improved asymmetric induction and provided optimized reaction conditions leading to the formation of product 19 in 87% yield and 91% ee. Additionally, it was found that reduced catalyst loadings could also be employed to obtain similar results. The exocyclic vinylogous ester functionality not only served an important purpose as an α′-blocking group to prevent the formation of isomeric enolates, but also enabled subsequent transformations later in the synthetic sequence.
The convergent synthetic approach to hamigeran B (27)[22] sought to unite an aryl fragment with a cyclopentenyl fragment containing an all-carbon quaternary stereo-center (Scheme 5). Subsequent formation of the central six-membered ring would provide the core of the target. In order to proceed toward hamigeran B (27), it was necessary to obtain the S enantiomer of 19 generated from the optimization studies. By application of Trost ligand (R,R)-15 under otherwise identical reaction conditions, vinylogous ester (S)-19 was obtained in 77% yield and 93% ee. Subsequent treatment with lithium dimethylcuprate in Et2O led to the formation of cyclopentanone 20, containing an all-carbon quaternary stereocenter. Triflate formation and oxidative cleavage of the allyl group provided aldehyde 21. Nucleophilic addition of the aryllithium of 22, followed by oxidation of the intermediate alcohol with Dess–Martin periodinane and selective monodemethylation with BCl3, gave ketone 23. Enol triflate reduction under palladium catalysis conditions, formation of the aryl triflate 24, and application of Heck cyclization conditions in the presence of Pd(OAc)2, dppb, and K2CO3 in toluene afforded a mixture of isomeric disubstituted, trisubstituted, and tetrasubstituted olefinic tricycles. After BBr3-induced demethylation, phenolic trisubstituted olefin 25 could be isolated from the mixture in 51% yield over two steps. The selection of appropriate hydrogenation conditions proved crucial for the formation of the remaining stereocenter. Treatment of alkene 25 with 1500 psi H2 in the presence of Pd/C in ethanol led to the undesired C(6) epimer, but hydrogenation with Ir black in ethanol directly provided the desired tricycle 26. Late-stage diketone formation with SeO2 and acetic acid in p-dioxane, followed by regioselective arene bromination, led to hamigeran B (27).
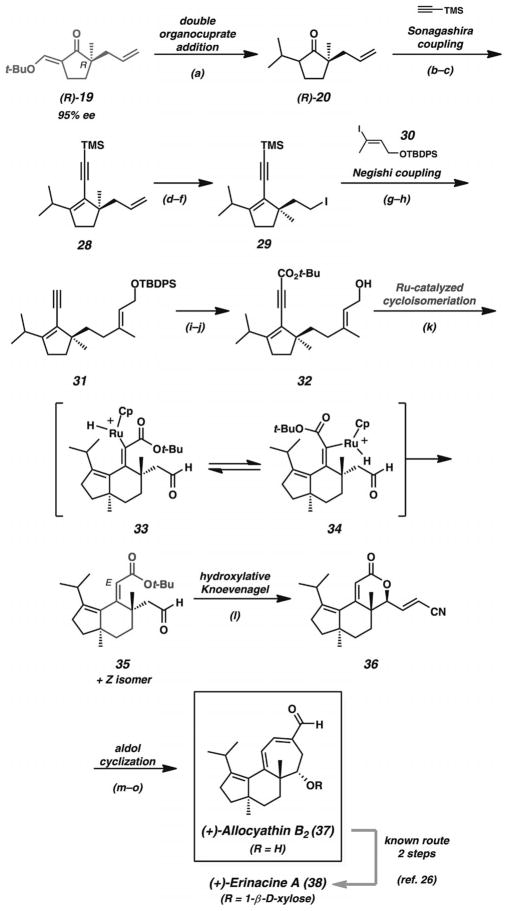
By employing the enantiomeric chiral intermediate (R)-19, the Trost group completed the synthesis of allocyathin B2 (37, Scheme 6) shortly after their investigations of hamigeran B (27). The challenging central cyclohexane ring bearing two all-carbon quaternary stereocenters presented an opportunity to test the group’s methods for Ru-catalyzed enyne cycloisomerizations. Exocyclic vinylogous ester 19 underwent a double organocuprate addition to give ketone 20 as in the earlier synthesis of hamigeran B (27). Triflation and Sonagashira coupling provided alkyne 28. Oxidative olefin cleavage, aldehyde reduction, and terminal alcohol substitution provided iodide 29. Lithium/halogen exchange and zincation enabled a Negishi coupling with iodide 30. Esterification of the alkyne and alcohol deprotection provided enyne 32. Treatment of this compound under the group’s previously developed conditions for Ru-catalyzed cycloisomerization[24] provided a mixture of E and Z olefin isomers. An investigation of various ester groups found that the tert-butyl ester provided the best ratio of E/Z olefin isomers. Ultimately, the E isomer could be isolated and advanced to unsaturated lactone 35 by a hydroxylative Knoevenagel reaction with phenylsulfinyl acetonitrile.[25] Hydrogenation of the less hindered disubstituted double bond, hydride reduction, and aldol cyclization completed the total synthesis of allocyathin B2 (37). The preparation of this compound also constituted a formal synthesis of erinacine A (38) based on prior work by Snider.[26] By applying their asymmetric alkylation methodology, the Trost group achieved the divergent total syntheses of hamigeran B (27) and allocyathin B2 (37) with exocyclic vinylogous ester 19 as the common precursor.
3.2. Total Syntheses of Dichroanone and Liphagal
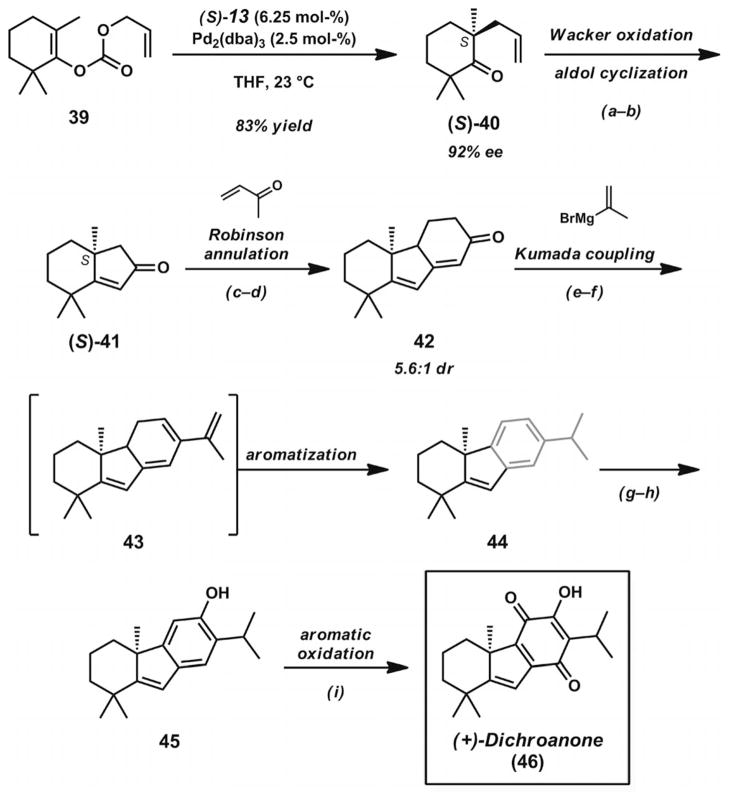
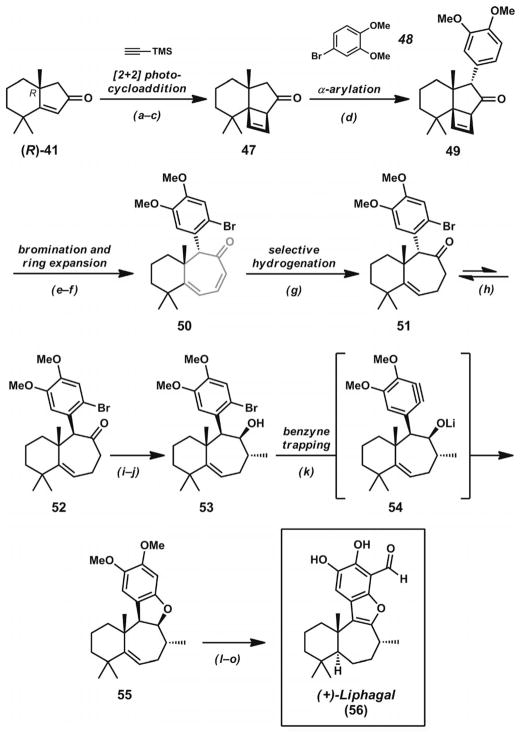
After the development of a Pd·PHOX catalyst system for the catalytic construction of α-quaternary cyclic ketones,[17a] the Stoltz group sought to prepare the unique and highly substituted carbocyclic structures of dichroanone (46, Scheme 7)[27] and liphagal (56, Scheme 8, below).[28] Central to their divergent synthetic approach to these natural products was the preparation of bicyclic enone 41 (Scheme 7), which contains two quaternary carbons in close proximity in the cyclohexane ring. The synthetic routes to both of these natural products began with the Pd-catalyzed asymmetric decarboxylative allylic alkylation of cyclic enol carbonate 39. The addition of this compound to a solution of [Pd2(dba)3] and (S)-tBu-PHOX (13, Figure 2) in THF (or TBME) led to enantioenriched ketone 40 in 83% yield and 92% ee. The presence of the α′-methyl groups in the substrate did not appear to impede catalysis. Through Wacker oxidation and intramolecular aldol cyclization by previously developed protocols,[17a] bicyclic ketone 41 could be obtained in 74% yield over two steps. The key enone possesses a prevalent substitution pattern found not only in dichroanone (46) and liphagal (56), but also in many other terpenoid natural products.
Scheme 7.
Total synthesis of dichroanone. Reaction conditions: a) PdCl2 (5 mol-%), Cu(OAc)2·H2O (25 mol-%), O2 (1 atm), DMA/H2O (7:1), 23 °C, Parr shaker (77% yield). b) KOH (0.45 equiv.), xylenes, 110 °C, Dean–Stark (96% yield). c) LiHMDS, THF, 0→23 °C; then methyl vinyl ketone, −78 °C; then aq. NH4Cl, −78→23 °C (72% yield). d) Powdered KOH (2 equiv.), xylenes, 110 °C, Dean–Stark (80% yield). e) LDA, THF, −78 °C; then PhN(Tf)2, −78→23 °C. f) Isopropenylmagnesium bromide (2 equiv.), [Pd(PPh3)4] (5 mol-%), THF, 23 °C; then 6 M aq. HCl, 23 °C (65% yield, two steps). g) Cl2HCOCH3. TiCl4, CH2Cl2, −78 → 23 °C (79% yield). h) Aq. H2O2, aq. H2SO4, THF/MeOH/H2O (2:5:1), 23 °C (74% yield). i) IBX (1.2 equiv.), CHCl3, 23 °C; then C6F5SH (4 equiv.), 23 °C; then O2 (1 atm), NaOH (10 equiv.), MeOH, 23→75 °C; then 6 M aq. HCl, 23 °C (35% yield).
Scheme 8.
Total synthesis of liphagal. Reaction conditions: a) TMS-acetylene, UV-B lamps, acetone. b) BF3·OEt2, CH2Cl2. c) TBAF, THF (68% yield, three steps). d) [Pd(P(tBu)3)2] (5 mol-%), 4-bro-moveratrole, NaOtBu, THF, microwave irradiation, 120 °C (67% yield). e) Br2 (1.8 equiv.), CHCl3 (65% yield). f) Microwave irradiation, o-dichlorobenzene, 250 °C (68% yield). g) PtO2 (20 mol-%), H2 (1 atm), EtOAc (69% yield). h) NaOMe, MeOH, 65 °C (78% yield, three cycles). i) LDA, THF, −78→0 °C; then CH3I, −78→0 °C (68% yield). j) DIBAL, PhCH3 (91% yield). k) LDA (3 equiv.), THF, −20 °C (83% yield). l) Pd/C (19 mol-%), H2 (1 atm), EtOH, 21 °C (97% yield). m) NO+BF4−, CH3CN, 0 °C (70% yield). n) nBuLi, TMEDA, THF, 0 °C; then DMF, 0→21 °C (70% yield). o) BI3, CH2Cl2 (45 % yield).
The S enantiomer of bicyclic enone 41 was advanced towards dichroanone (46) by the de novo construction of the fully substituted quinone nucleus. A Robinson annulation sequence followed by vinyl triflate formation and Pd-catalyzed Kumada coupling with isopropenylmagnesium bromide led to isopropyl arene 44. The reaction likely proceeds through coupling product 43, which can undergo facile aromatization to give the benzannulated ring system. Titanium-mediated formylation of the aromatic ring proceeded smoothly to give an intermediate aldehyde, which was converted into the corresponding phenol 45 by Baeyer–Villiger oxidation. Careful generation of a reactive intermediate o-quinone could be achieved by treatment of the tricycle with IBX. Subsequent trapping of the reactive intermediate with pentafluorothiophenol, reoxidation with NaOH/MeOH/O2, and hydrolysis with aqueous HCl completed the synthesis of dichroanone (46). Notably, the total synthesis proceeded without the use of protecting groups and provided an asymmetric route to this family of quinone norditerpenoids.
Whereas the route to dichroanone demonstrated the utility of bicyclic enone 41 in α-functionalization reactions, the synthesis of liphagal[28] (56, Scheme 8) demonstrated further synthetic applications for this bicyclic scaffold. With enone (R)-41 available by allylic alkylation of substrate 39 with (R)-tBu-PHOX (13, Figure 2), a photoinduced [2+2] cycloaddition with TMS-acetylene was performed (Scheme 8). Treatment of the crude cycloadduct with BF3·OEt2, followed by TBAF, led to cyclobutene 47. The strained ketone underwent α-arylation with 4-bromoveratrole (48) under microwave irradiation conditions to give highly functionalized tricycle 49. Arene bromination of this compound proved to be remarkably chemoselective, with the strained cyclobutene remaining intact during the transformation. Subsequent microwave-assisted thermal ring expansion provided conjugated cycloheptadienone 50. Selective hydrogenation of the less substituted double bond, followed by base-mediated epimerization, led to bicycle 52 after two equilibration cycles. LDA-mediated methylation of the nonconjugated cycloheptenone and DIBAL reduction provided alcohol 53. Treatment of this compound with LDA led to a reactive aryne intermediate, which could react intramolecularly with the adjacent lithium alkoxide to provide dihydrobenzofuran 55. Hydrogenation of the remaining olefin provided the necessary stereochemistry at the junction of the six- and seven-membered rings. Dihydrobenzofuran oxidation, followed by arene formylation and phenol demethylation, provided liphagal (56). Taken together, the two syntheses demonstrate the unique synthetic utility of bicyclic enone 41, which is formed from the decarboxylative asymmetric allylic alkylation of enol carbonate 39.
3.3. Total Syntheses of Elatol, α-Chamigrene, and Laurencenone C
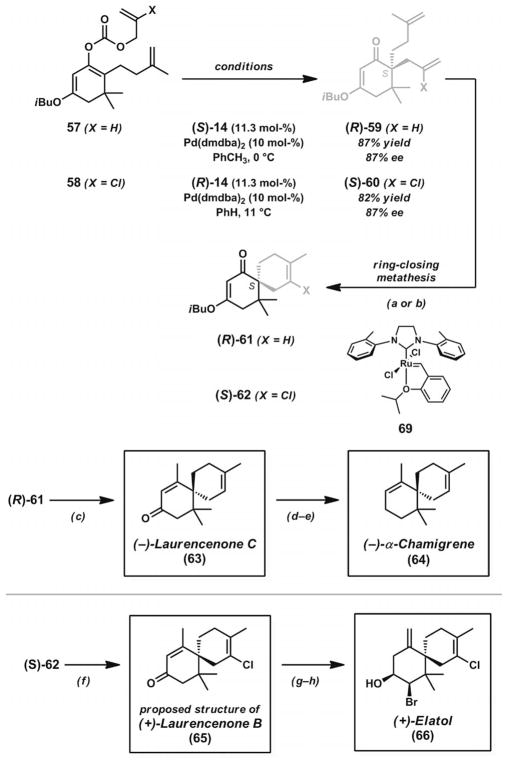
After successfully demonstrating the utility of the allyl enol carbonate approach[17a] for enolate generation in the asymmetric construction of quaternary stereocenters, the Stoltz group sought to extend the scope of the asymmetric decarboxylative allylic alkylation reactions in order to gain access to the chamigrene family of natural products[29] (Scheme 9). These compounds are each distinguished by a central all-carbon quaternary stereocenter connecting two highly substituted six-membered rings, as shown by the structures of laurencenone C (63) and α-chamigrene (64). The halogenation patterns for members such as elatol (66) presented additional synthetic challenges. In order to arrive at these natural products, a flexible synthetic route was needed.
Scheme 9.
Total synthesis of laurencenone C, α-chamigrene, the proposed structure of laurencenone B, and elatol. Reaction conditions: a) substrate 59, Grubbs–Hoveyda third-generation catalyst (69, 5 mol-%), PhH, 60 °C (97% yield). b) Substrate 60, Grubbs–Hoveyda third-generation catalyst (69, 5 mol-%), PhH, 60 °C (97% yield). c) MeLi, CeCl3, THF, −78→0 °C; then 10% aq. HCl, 0→23 °C (80% yield). d) BF3·OEt2, HSCH2CH2SH, MeOH, 23 °C (92 % yield). e) Na(0), Et2O/NH3(l), −60→reflux (44% yield). f) MeLi, CeCl3, THF, −78→0 °C; then 10% aq. HCl, 0→23 °C (89% yield). g) Br2, 48% aq. HBr, AcOH, 23 °C. h) DIBAL, THF, −78→60 °C (32% yield, two steps).
The gem-dimethyl group adjacent to the desired alkylation site in substrates 57 and 58 presented a formidable challenge for the decarboxylative allylic alkylation methodology with the Pd·PHOX catalyst system. Investigation of enol carbonate and β-keto ester substrates with (S)-tBu-PHOX (13, Figure 2) led to the product in 81% ee, but levels of conversion were consistently poor in both cases. Faced with these synthetic difficulties, ligand modifications were explored to promote the desired C–C bond-forming step in the catalytic cycle. It was reasoned that through employment of the electron-deficient PHOX derivative 14 (Figure 2), the increased electrophilicity of the derived Pd π-allyl complex should override the steric constraints imposed by the substrate. The combination of electron-deficient PHOX ligand 14 and [Pd(dmdba)2] in toluene or benzene ultimately provided the most effective conditions. Vinylogous ester 59 could be obtained in 87% yield and 87% ee, whereas the analogous chlorinated vinylogous ester 60 could be obtained in 82% yield and 87% ee. In the presence of catalyst, these substrates could be converted into products below ambient temperature. In addition to these two substrates, the reaction conditions could also be applied to numerous α-substituted analogues.
From enantioenriched vinylogous esters 59 and 60, it was envisioned that ring-closing metathesis to form a trisubstituted or tetrasubstituted olefin should provide the spirobicylic core of the chamigrene natural products (Scheme 9). Unfortunately, the application of commonly used ruthenium metathesis catalysts 67 and 68 (Figure 3) gave unsatisfactory results. Use of the metathesis catalyst 69,[30] concurrently developed by the Grubbs laboratory, provided an effective solution. Trisubstituted olefin 61 and chlorinated tetrasubstituted olefin 62 were both formed in excellent yields. Notably, the advances enabled by ruthenium complex 69 provided one of the first examples of effective ring-closing metathesis to form highly substituted halogenated olefins.
Figure 3.
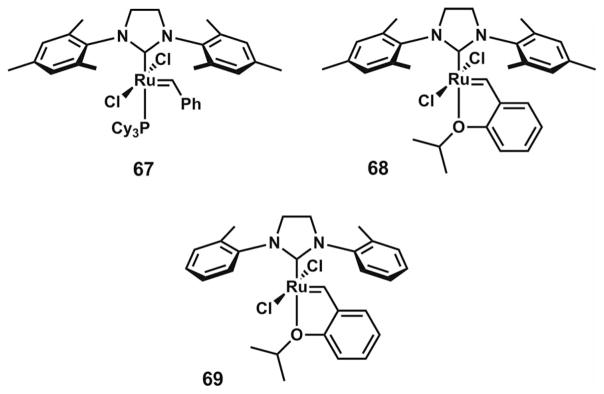
Ruthenium olefin metathesis catalysts.
With the spirocyclic core of the target natural products secured, short sequences enabled the synthesis of various chamigrene natural products. The addition of methyllithium to spirocycle 61 in the presence of CeCl3 activator, followed by acid workup, provided laurencenone C (63). Formation of the corresponding thioketal, followed by dissolving metal reduction, provided α-chamigrene (64) in only two additional steps. Application of the same methyllithium addition and acidic workup conditions to vinylogous ester 62 enabled the formation of enone 65, a chlorinated analogue of 63. Although this structure had been reported as laurencenone B, the spectra did not match those of the published compound. Nevertheless, the compound was brominated and reduced with DIBAL to afford elatol (66). Overall, three spirocyclic natural products in the family were prepared by this unified asymmetric alkylation/ring-closing metathesis strategy.
3.4. Formal Synthesis of Platencin
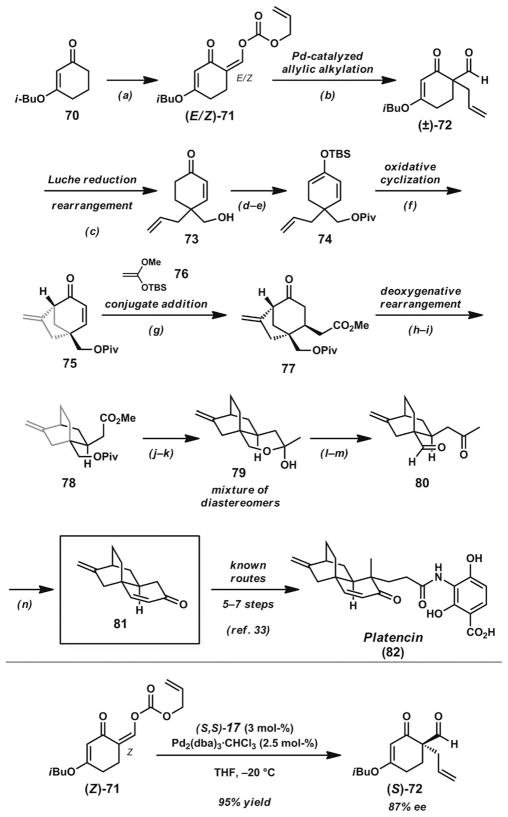
Maier completed a formal synthesis of platencin (82, Scheme 10)[31] by employing a decarboxylative allylic alkylation reaction to establish the substitution around a central quaternary carbon atom joining three different rings. Beginning from vinylogous ester 70, formylation and allyl enol carbonate construction provided rapid access to allylic alkylation substrate 71 as a mixture of E and Z isomers. Treatment with catalytic Pd(OAc)2 and PPh3 provided allylation product (±)-72. Subsequent Luche reduction and rearrangement under acidic conditions provided enone 73, esterification of the primary alcohol and silylation gave silyl dienol ether 74, and aerobic oxidative cyclization under Toyota’s conditions[32] afforded [3.2.1] bicycle 75. Addition of silyl ketene acetal 76 provided conjugate adduct 77, subsequent tosylhydrazone formation and skeletal rearrangement led to [2.2.2] bicycle 78, and Weinreb amide formation and treatment with methyllithium gave hemiacetal 79 as an inconsequential mixture of diastereomers. Reduction and oxidation of this compound provided the ring-opened keto-aldehyde 80. A straightforward aldol cyclization completed the advanced intermediate 81 employed in numerous syntheses of (±)-platencin (82).[33]
Scheme 10.
Formal synthesis of (±)-platencin. Reaction conditions: a) NaH, HCO2iBu, 0 °C; then ClCO2allyl, cat. KH, THF, 0 °C. b) Pd(OAc)2 (1.3 mol-%), PPh3, THF, 20 °C (92% yield, two steps). c) NaBH4, CeCl3·7H2O, MeOH, 0 °C; then pTsOH, H2O/Et2O, room temp. d) PivCl (2 equiv.), pyridine (4 equiv.), DMAP (0.05 equiv.), CH2Cl2, room temp. (94% yield, two steps). e) LDA (1.5 equiv.), TBSCl (2 equiv.), HMPA (1 equiv.), THF, −80 °C→r.t. (88% yield). f) O2, Pd(OAc)2 (5.8 mol-%), DMSO (85 % yield). g) H2C=C(OMe)OTBS (76, 1.5 equiv.), TiCl4 (1.2 equiv.), CH2Cl2, −80 °C (88% yield). h) TsNHNH2 (1.3 equiv.), MeOH, 60 °C (95% yield). i) NaCNBH3, ZnCl2, MeOH, 60 °C (60% yield). j) HCl·NH(OMe)Me (6 equiv.), Me3Al (5 equiv.), CH2Cl2, 0 °C. k) MeLi (6 equiv.), Et2O, −80→−30 °C. l) LiAlH4 (1 equiv.), Et2O, −80→0 °C (85% yield, two steps). m) (COCl)2 (5.8 equiv.), DMSO (9 equiv.), −80 °C, Et3N (73% yield). n) NaOH (6.5 equiv.), EtOH, 20 °C, 20 h (87% yield).
To provide an asymmetric entry into this route, Maier returned to the allylic alkylation step with a focus on the development of an enantioselective variant. Treatment of (Z)-71 with catalytic [Pd2(dba)3]·CHCl3 and ligand (S,S)-17 (Figure 2) in THF at 0 °C provided access to α-quaternary product 72 in 78% ee. Further optimization revealed that decreasing the reaction temperature to −20 °C provided improved results, with the desired compound isolable in 95% yield and 87% ee. As observed in Trost’s initial studies on decarboxylative alkylations of allyl enol carbonates,[18] the chiral diamine scaffold of ligand 17 proved effective for quaternary stereocenter formation. As a demonstration of the essentially neutral conditions of the decarboxylative alkylation reaction, the potentially sensitive aldehyde functionality underwent minimal side reactions after quaternary stereocenter formation.
3.5. Formal Synthesis of Hamigeran B
A report from the Stoltz group outlined an intramolecular aldol strategy for the construction of the tricyclic framework of hamigeran B (27, Scheme 11).[34] The synthetic plan targeted the early construction of an α-quaternary tetralone fragment through a Pd-catalyzed decarboxylative allylic alkylation reaction.[17a] Facile access to enol carbonate 83 enabled rapid evaluation of reaction conditions for the construction of the key quaternary stereocenter. The initial evaluation of (S)-tBu-PHOX (13, Figure 2) and [Pd2(dba)3] as catalyst precursors in THF provided functionalized tetralone 84 in 71% yield and 88% ee. The application of the electron-deficient ligand 14 (Figure 2) provided greater levels of asymmetric induction, with the product isolable in 83% yield and 94% ee. Subsequent olefin cross-metathesis with methyl vinyl ketone provided enone 85 for the evaluation of the planned tandem conjugate reduction and aldol cyclization. Treatment of the compound with Stryker’s reagent ([Ph3PCuH]6) gave desired β-hydroxyketone 86 along with the uncyclized reduction product. Dehydration of compound 86 led to a tricyclic enone 87, previously reported by Miesch,[35] and completed an asymmetric formal synthesis of hamigeran B (27). The synthesis provides another example of the beneficial effect of electron-deficient PHOX ligand 14 in enantioselective allylic alkylation reactions. When taken together with Trost’s earlier total synthesis,[22] the value of asymmetric allylic alkylation reactions can be seen in the different chiral ketone synthons available for the construction of a common natural product target.
Scheme 11.
Formal synthesis of hamigeran B. Reaction conditions: a) methyl vinyl ketone (10 equiv.), Grubbs–Hoveyda second-generation catalyst (68, 10 mol-%), PhH, 35 °C (66% yield). b) [Ph3PCuH]6 (0.5 equiv.), PhCH3, −40 °C. c) SOCl2 (15 equiv.), DMAP (30 mol-%), pyridine, 0 °C (62 % yield).
3.6. Total Synthesis of Carissone and Cassiol
The dual syntheses of carissone (99, Scheme 13, below)[36] and cassiol (105, Scheme 14, below)[37] by the Stoltz group were enabled by the unique synthetic potential of vinylogous thioester-derived β-keto ester substrates. Whereas Stoltz developed the first asymmetric allylic alkylation reactions for cycloalkanone-derived β-keto ester substrates[17b] (Scheme 4), Trost succeeded in extending the substrate scope to vinylogous thioester-derived β-keto ester substrates (Scheme 12).[38] In the presence of chiral ligand 17 (Figure 2), α-quaternary vinylogous thioesters such as 89 were prepared in high ees. These products could readily be converted into γ-quaternary enone derivatives through Stork–Danheiser-type transformations. Vinylogous ester-derived β-keto ester substrates were also evaluated, but conversion was typically sluggish, due to the greater orbital overlap of oxygen relative to sulfur.
Scheme 13.
Total synthesis of carissone and formal synthesis of α-eudesmol. Reaction conditions: a) allylic bromide 93, Mg(0), I2, 0→23 °C (94% yield). b) Grubbs second-generation catalyst (67, 3 mol-%), PhH, 40 °C (99% yield). c) Rh/Al2O3 (5 mol-%), H2 (1 atm), MeOH. d) HCl, THF (56% yield, two steps). e) Dess–Martin periodinane, CH2Cl2, 0→23 °C. f) NaClO2, NaH2PO4, 2-meth-ylbut-2-ene, tBuOH/H2O; then CH2N2 (87% yield, two steps). g) CeCl3·7H2O, NaBH4, MeOH, −45 °C. h) MeMgBr, THF, 0→26 °C (73% yield, two steps). i) MnO2, 4 Å MS, CH2Cl2 (100% yield).
Scheme 14.
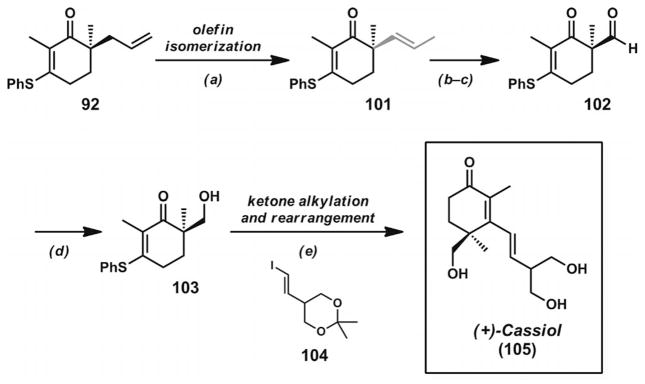
Total synthesis of cassiol. Reaction conditions:a) [PdCl2(CH3CN)2] (10 mol-%), PhH, 60 °C (99% yield, 13:1 ratio 101/92). b) OsO4 (10 mol-%), DABCO, K3Fe(CN)6, K2CO3, tBuOH/H2O (1:1), 35 °C. c) Pb(OAc)4, PhH, 30 °C (70% yield). d) Li(OtBu)3AlH, THF, 0 °C (85% yield). e) Vinyl iodide 104, tBuLi, Et2O, −78 °C; then vinylogous thioester 103; then aq. HCl, TBME (36% yield).
Scheme 12.
Extension of β-keto ester substrate scope to vinylogous ester scaffolds.
Concurrent work in the Stoltz group sought to develop a PHOX-based catalyst system for the preparation of these useful compounds and their further application in the total synthesis of natural products. Investigation of β-keto ester and enol carbonate derivatives of α′-substituted isobutyl vinylogous esters provided low levels of conversion and unsatisfactory ee values, but the asymmetric allylic alkylation of vinylogous thioester-derived β-keto ester substrate 91 (Scheme 13) with [Pd2(pmdba)3] and (S)-tBu-PHOX (13, Figure 2) led to enantioenriched product 92 in 85% yield and 92% ee.
Further manipulations provided an asymmetric synthetic route to eudesmane sesquiterpenoids such as carissone and α-eudesmol[36] (Scheme 13). The addition of the Grignard reagent of bromide 93, followed by acid-promoted ketone transposition, led to enone 94. Ring-closing metathesis with Grubbs second-generation catalyst (67) led to fused bicycle 95. Selective hydrogenation of the less substituted double bond and desilylation provided hydroxy enone 96. Oxidation of the alcohol, followed by carboxylate methylation, led to enone 97. Subsequent Luche reduction of the enone and dimethylation of the ester led to diol 98. Allylic alcohol oxidation provided carissone (99). Notably, the assembly of diol 98 also completed a formal synthesis of (−)-α-eudesmol (100) by interception of Aoyama’s route.[39]
The concise synthesis of cassiol (105, Scheme 14)[37] also illustrated the utility of α-quaternary vinylogous thioesters formed through decarboxylative asymmetric allylic alkylation reactions. The key enantioenriched vinylogous thioester 92 was subjected to palladium-catalyzed olefin isomerization, oxidative olefin cleavage, and aldehyde reduction steps to provide alcohol 103. Treatment of this compound with the vinyllithium derivative of 104, followed by aqueous acid workup, led to cassiol (105) in a concise sequence. Both of the syntheses completed by the Stoltz group illustrate the conversion of α-quaternary vinylogous thioesters into γ-quaternary cyclohexenones in a unified catalytic asymmetric strategy directed towards natural products.
3.7. Total Syntheses of Flustramides A and B and Flustramines A and B
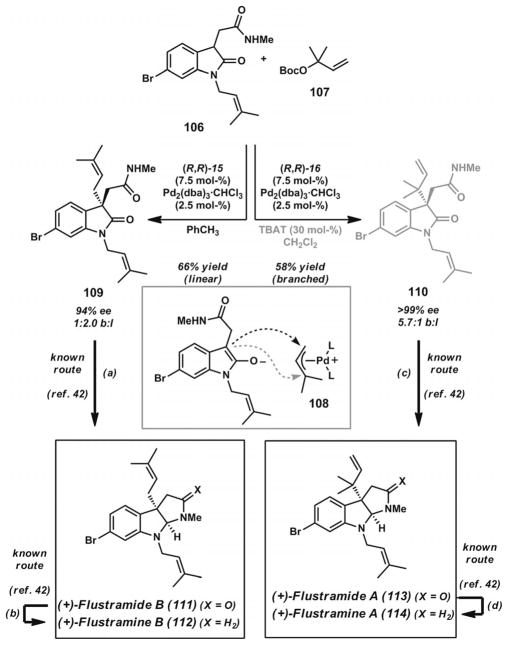
Trost devised a novel and concise strategy for the synthesis of highly substituted pyrroloindoline and pyrroloindolone-type alkaloids such as flustramines A and B and flustramides A and B (111–114, Scheme 15).[40] His group sought to develop reactions based on chiral Pd π-prenyl complex 108, which can undergo regioselective asymmetric alkylation by an oxindole enolate to produce either the linear prenylated product or the branched reverse prenylated product. Past work has shown that C–C bond formation predominantly occurs at the less substituted allyl terminus,[41] favoring linear product 109, but the successful modification of reaction conditions would provide branched isomer 110, which would be difficult to form by alternative means given the proximity of two quaternary carbons.
Scheme 15.
Total syntheses of flustramides A and B and flustramines A and B. Reaction conditions: a) AlH3·NMe2Et, THF, −15 °C (95% yield). b) AlH3·NMe2Et, THF, room temp. (97% yield). c) AlH3·NMe2Et, THF, −15 °C (92% yield). d) AlH3·NMe2Et, THF, room temp. (90% yield).
With this overall problem in mind, a variety of reaction conditions were evaluated to provide potential access to the linear or branched prenylated products. Carbonate 107 was identified as an excellent prenyl source and masked alkoxide base, generating one equivalent of each after oxidative addition and decarboxylation of the substrate by Pd0. With carbonate 107, chiral phenyl bisphosphane ligand 15 (Figure 2), and [Pd2(dba)3]·CHCl3 in toluene, a 1:2 ratio of branched/linear products was observed, and the linear product 109 could be isolated in 66% yield and 94% ee. Conversely, combination of the naphthyl bis-phosphane ligand 16 (Figure 2), [Pd2(dba)3]·CHCl3, and catalytic TBAT (tetrabutylammonium triphenylsilyldifluorosilicate) in CH2Cl2 led to a 5.7:1 ratio of branched/linear products with the branched product 110 isolable in 58% yield and greater than 99% ee. These results show that the careful choice and modification of the chiral ligand can greatly influence the regioselectivity of the asymmetric alkylation event leading to an all-carbon quaternary stereocenter. Additionally, the general method could be extended to geranylation reactions,[40] providing impressive access to vicinal all-carbon quaternary stereocenters. Trost’s studies show that it is possible to exert control over the regioselectivity, enantio-selectivity, and diastereoselectivity of asymmetric alkylation reactions with these chiral ligand systems.
Adoption of Kawasaki’s reductive amide cyclization route[42] provided access to the highly substituted flustramine and flustramide alkaloids. Oxindole reduction of linear alkylation product 109 and branched alkylation product 110 with alane-dimethylethylamine complex at low temperature provided (+)-flustramide A (111) and (+)-flustramide B (113). Treatment of these compounds with additional reductant at ambient temperature effected lactam reduction to give (+)-flustramine A (112) and (+)-flustramine B (114). The synthetic route powerfully enables divergent access to C(3)-quaternary prenylated and reverse prenylated oxindole scaffolds through appropriate choice of chiral ligand. In general, examples of the catalytic, asymmetric synthesis of vicinal quaternary stereocenters are quite rare.
3.8. Total Syntheses of Cyanthiwigins B, F, and G
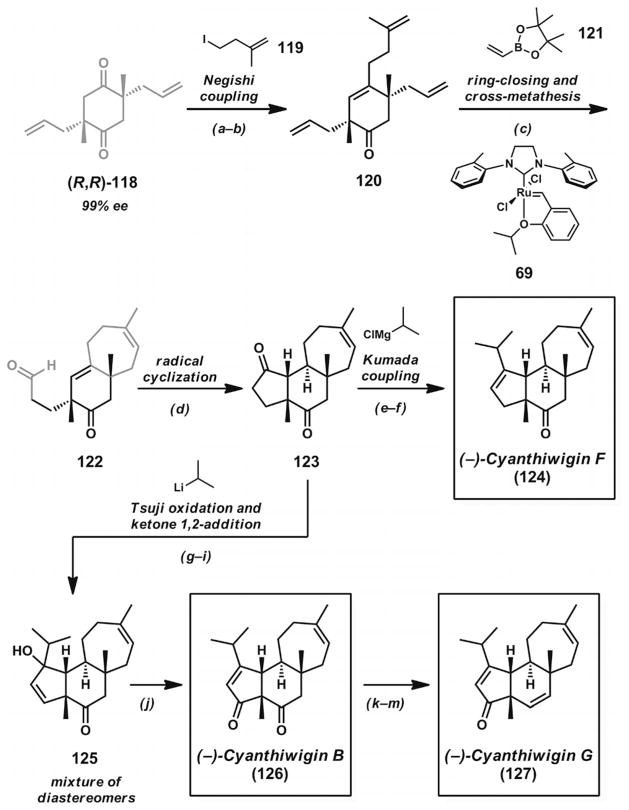
The investigations into Pd-catalyzed allylic alkylations in total synthesis described above sought to establish one quaternary stereocenter at a time. The highly substituted cyclohexane core of the cyathane diterpenoids motivated the Stoltz group to develop new double asymmetric decarboxylative allylic alkylation reactions for efficient construction of multiple all-carbon quaternary stereocenters on a single molecule of substrate. These efforts culminated in efficient total syntheses of cyanthiwigins B, F, and G (124, 126, and 127, Scheme 17, below).[43]
Scheme 17.
Total syntheses of cyanthiwigins B, F, and G. Reaction conditions: a) KHMDS, PhN(Tf)2, THF, −78 °C (73 % yield). b) Zn(0), TMSCl, 1,2-dibromoethane, alkyl iodide 119, THF, 65 °C; then [Pd(PPh3)4] (5 mol-%) (78% yield). c) Vinylboronic ester 121, Grubbs–Hoveyda third-generation catalyst (69, 10 mol-%), PhH, 60 °C; then NaBO3, THF/H2O (51% yield). d) tBuSH, AIBN, PhH, 80 °C. e) KHMDS, PhN(Tf)2, THF, −78 °C (60% yield). f) iPrMgCl, CuCN, THF; [Pd(dppf)Cl2] (10 mol-%) (41% yield). g) KHMDS, THF −78 °C; then allyl chloroformate. h) [Pd2(pmdba)3] (5 mol-%), CH3CN, 80 °C (57% yield, two steps). i) CeCl3, iPrLi, THF, −78 °C (76% yield, mixture of diastereomers). j) PCC, CH2Cl2 (86% yield). k) NaBH4, MeOH/CH2Cl2, 25 °C. l) MnO2, CH2Cl2 (15% yield, two steps). m) Martin’s sulfurane, CDCl3 (48% yield).
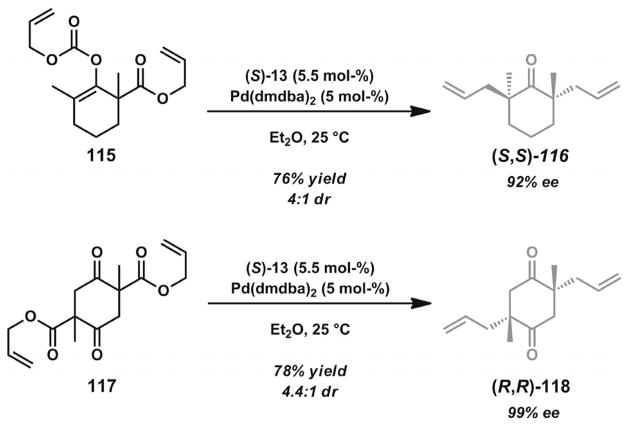
The Stoltz group investigated reactions of substrate 115 (Scheme 16) in a mixture of catalytic [Pd(dmdba)2] and (S)-tBu-PHOX (13, Figure 2) in Et2O.[17b] After two sequential asymmetric alkylation events, diketone 116 could be obtained in 76% yield, 92% ee, and 4:1 dr. Studies on the related bis-β-keto ester double alkylation substrate 117 provided product 118 in 78% yield, 99% ee, and 4.4:1 dr.[43] These impressive levels of asymmetric induction can be explained by statistical amplification resulting from multiple asymmetric transformations occurring in sequence.[44,45] The efficiency of these transformations for the assembly of two all-carbon quaternary stereocenters in the same ring system provided an excellent foundation for further synthetic efforts directed towards the cyathane diterpenoids.
Scheme 16.
Double enantioselective decarboxylative allylic alkylation reactions.
With double alkylation product 118 to hand, the synthesis of numerous members of the cyanthiwigin family could be completed (Scheme 17). Desymmetrization of the diketone was achieved by triflation and Negishi coupling to give enone 120. Subsequent ring-closing metathesis and cross-metathesis with Grubbs–Hoveyda third-generation catalyst (69) in the presence of vinylboronate 121 provided aldehyde 122. Radical cyclization completed the remaining cyclo-pentane ring. Subsequent triflation and Kumada coupling afforded cyanthiwigin F (124).
Diketone 123 was further functionalized to related natural products cyanthiwigin B (126) and cyanthiwigin G (127) in a short, concise sequence (Scheme 17). By means of a Tsuji oxidation[46] and treatment of the intermediate enone with isopropyllithium in the presence of a CeCl3 activator, adduct 125 could be formed as an inconsequential mixture of diastereomers. Oxidative transposition of the tertiary allylic alcohol enabled the completion of cyanthiwigin B (126). Reduction of the ketone and enone moieties with NaBH4, followed by reoxidation of the allylic alcohol, afforded an intermediate alcohol, which could be eliminated by use of Martin’s sulfurane to give cyanthiwigin G (127). The unified approach to these natural products was enabled by the development of a doubly enantioselective and diastereoselective allylic alkylation of a single substrate compound. The efficient synthetic route provided unique access to the highly substituted cyclohexanoid core of the cyathane diterpenoids.
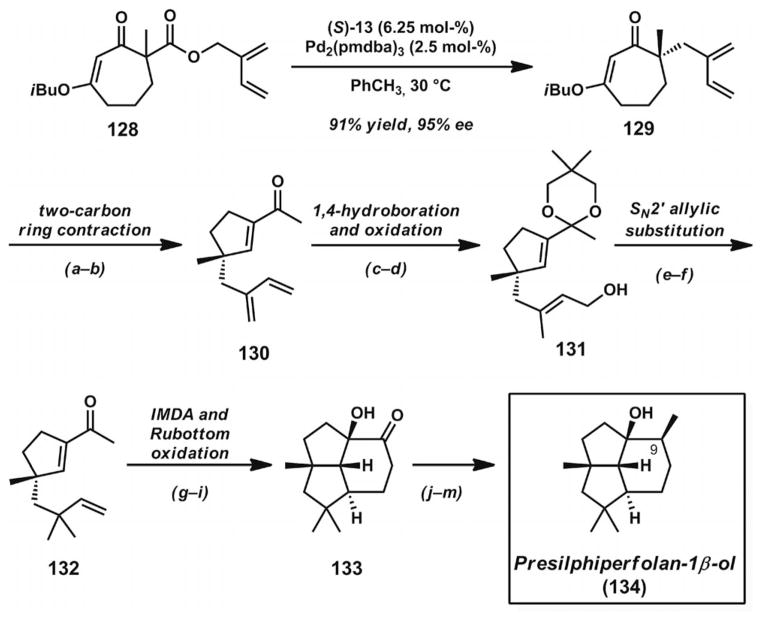
3.9. Total Synthesis of Presilphiperfolan-1β-ol
Recent work in the Stoltz group has demonstrated the utility of asymmetric allylic alkylation reactions in the construction of functionality-rich γ-quaternary acylcyclopentenes,[47] which proved to be key intermediates for the total synthesis of presilphiperfolan-1β-ol (134, Scheme 18).[48] Treatment of β-keto ester substrate 128 with [Pd2(pmdba)3] and (S)-tBu-PHOX [(S)-13, Figure 2] in toluene at 30 °C[17b,47] led to the efficient formation of isoprenyl vinylogous ester 129 in 91% yield and 95% ee. An unusual two-carbon ring contraction was achieved through an initial LiAlH4 reduction with acid workup followed by base-promoted rearrangement, to give acylcyclopentene 130 in excellent yield. Although these conditions resemble the Stork–Danheiser-type transformations illustrated in the syntheses of chamigrene natural products (Scheme 9), platencin (Scheme 10), carissone (Scheme 13), and cassiol (Scheme 14), the unusual stability of the intermediate β-hydroxycycloheptanone enabled the formation of acylcyclopentene 130 rather than the more conventional isomeric cycloheptenone elimination product.[47] Subsequent ketalization with neopentyl glycol and 1,4-hydroboration/oxidation with catalytic Ni(cod)2 and PCy3 by Morken’s procedure[49] led to allylic alcohol 131. Activation of the alcohol as an allylic phosphate provided the necessary substrate for a CuCN-catalyzed SN2′ allylic substitution with dimethylzinc. The more functionalized acylcyclopentene 132 underwent silylation, intramolecular Diels–Alder cyclization, and Rubottom oxidation to provide a 90% combined yield of oxidized cycloadducts over three steps, with a 28% yield of the desired diastereomer 133. Additional Wittig methylenation, silylation, diastereoselective hydrogenation, and desilylation steps completed the total synthesis of presilphiperfolan-1β-ol (134).[48]
Scheme 18.
Total synthesis of presilphiperfolan-1β-ol. Reaction conditions: a) LiAlH4, Et2O, 0 °C (96% yield). b) LiOH, TFE, THF, 60 °C (96% yield). c) Neopentyl glycol, PPTS (5 mol-%), PhH, reflux (94% yield). d) HBPin, Ni(cod)2 (5 mol-%), PCy3 (5 mol-%), PhCH3, 23 °C; then 3 M aq. NaOH, H2O2, THF, 0→23 °C (81% combined yield, 63% yield of desired linear isomer 131). e) Diethyl chlorophosphate, Et3N, DMAP (20 mol-%), CH2Cl2, 0→23 °C (84% yield). f) Zn(CH3)2, CuCN (10 mol-%), THF, −78→23 °C; then 10% aq. HCl (94% yield). g) TBSOTf, Et3N, CH2Cl2, 0→23 °C (98% yield). h) p-Dioxane, 170 °C, sealed tube. i) DMDO, acetone, −78 °C; then TBAF, −78→23 °C (90 % combined yield, two steps, 1:2 dr, 28% yield of desired diastereomer). j) KOtBu, Ph3PCH3Br, THF, 0→23 °C (90% yield). k) HMDS, imidazole, TMSCl, CH2Cl2 (96% yield). l) PtO2 (5 mol-%), EtOAc, H2 (1 atm). m) TBAF, THF, reflux (92% yield, two steps).
The diastereoselective preparation of the 9-epimer of pre-silphiperfolan-1β-ol (9α-Me) was also achieved,[48] but the spectrum of this compound did not match that reported for the natural product. After extensive spectroscopic analysis and evaluation of the likely biosynthetic pathway, it was concluded that the epimeric presilphiperfolanol was most likely not a natural product.
The synthetic studies leading to presilphiperfolan-1β-ol (134) provided an important opportunity to expand the scope of π-allyl electrophiles for α-quaternary stereocenter construction. The examination of a 2-(vinyl)allyl electrophile in the synthesis complements the Stoltz group’s earlier investigation of internal π-allyl substitution in the synthesis of chamigrene natural products[29] (Scheme 9). These studies, together with the Trost group’s exploration of terminal π-allyl substitution in the synthesis of flustramine and flustramide indole alkaloids[40] (Scheme 15), illustrate the wide range of π-allyl electrophiles that can be employed in asymmetric allylic alkylation reactions.
4. Conclusion and Outlook
The pursuit of complex targets has provided inspiration for the development of Pd-catalyzed asymmetric allylic alkylation reactions for the construction of challenging all-carbon quaternary stereocenters. The synthetic methods developed in the past ten years have provided chemists with useful tools for the assembly of densely substituted ring systems, and consequently, the total synthesis of complex natural products. Although major advances have been made, broader examination of reaction scope, development of new catalyst systems, and coupling of palladium enolate chemistry to other powerful bond-forming processes has the potential to reshape the field in future.
Note Added in Proof
Several research groups have recently reported methods for asymmetric allylic alkylation of enolates derived from N-heterocyclic frameworks and applied them to the synthesis of polycyclic alkaloid natural products. The Stoltz laboratory employed the PHOX ligands (S)-13 or (S)-14 (Figure 2) with [Pd2(dba)2] or [Pd2(pmdba)3] to achieve the syntheses of α-quaternary lactams and related heterocycles (Scheme 19).[50] In their report, they also demonstrated the concise formal synthesis of rhazinilam (138).[51] The important lactam building blocks can be readily applied to the synthesis of quebrachamine and other related alkaloids (139).[52] Recent findings from the same laboratory have generalized the substrate scope to include additional lactam and vinylogous amide-type substrates.[53] Concurrent investigation of the asymmetric alkylation of carbazolone substrates by the Lupton group[54] enabled the successful formal synthesis of kopsihainanine A[55] (Scheme 20). Very recently, the Shao group also documented a similar approach in their total synthesis of (−)-aspidospermidine and (+)-kopsihainanine A.[56]
Scheme 19.
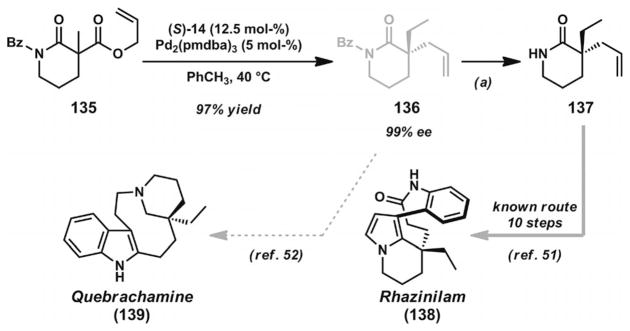
Formal synthesis of rhazinilam and synthetic approach to quebrachamine. Reaction conditions: a) LiOH·H2O, MeOH, 23 °C (96% yield).
Scheme 20.
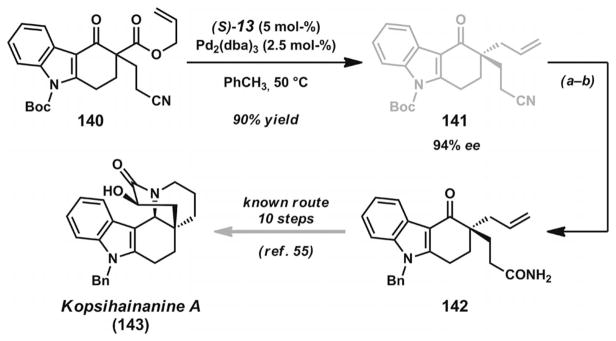
Formal synthesis of kopsihainanine A. Reaction conditions: a) HCO2H, b) K2CO3, BnBr, acetone, 55 °C (94% yield, two steps).
Acknowledgments
The authors thank National Institutes of Health – National Institute of General Medical Sciences (grant number R01GM080269-01), Roche, Abbott Laboratories, Amgen, Boehringer Ingelheim, the Gordon and Betty Moore Foundation, and Caltech for awards and financial support. Nick O’Connor, Kelly Kim, and Christopher Haley are acknowledged for editorial assistance.
Biographies

Allen Y. Hong was born in San Francisco, California in 1984. He began studying chemistry in 2004 at UC Berkeley. In the autumn of 2005 he joined Prof. Richmond Sarpong’s laboratory and studied rhodium-catalyzed reactions for the synthesis of highly functionalized furans. Allen graduated from UC Berkeley in 2006 with a B.S. in chemical biology. After completing his undergraduate education, he moved south to Pasadena, California in 2007 to begin doctoral studies in synthetic organic chemistry at the California Institute of Technology. In January 2008 he joined the research group of Prof. Brian M. Stoltz to study asymmetric catalysis and total synthesis, focusing on the synthesis of presilphiperfolanol natural products with the aid of Pd-catalyzed asymmetric allylic alkylation reactions. Allen earned his Ph.D. in chemistry in 2012. In January 2013 he continued the southward trend and began NIH-sponsored postdoctoral research under the guidance of Prof. Chris D. Vanderwal at UC Irvine.

Brian M. Stoltz was born in Philadelphia, PA, in 1970 and obtained his B.S. degree from the Indiana University of Pennsylvania in Indiana, PA. After graduate work at Yale University in the laboratories of John L. Wood and an NIH postdoctoral fellowship at Harvard in the Corey laboratories, he took a position at the California Institute of Technology. A member of the Caltech faculty since 2000, he is currently Professor of Chemistry. His research interests lie in the development of new methodology for general applications in synthetic chemistry.
References
- 1.For a review discussing our strategy of using natural product structures to drive the development of enantioselective catalysis, see: Mohr JT, Krout MR, Stoltz BM. Nature. 2008;455:323–332. doi: 10.1038/nature07370.
- 2.For excellent general reviews on the catalytic enantioselective generation of quaternary stereocenters, see: Martin SF. Tetrahedron. 1980;36:419–460.Fuji K. Chem Rev. 1993;93:2037–2066.Corey EJ, Guzman-Perez A. Angew Chem. 1998;110:402–415. doi: 10.1002/(SICI)1521-3773(19980302)37:4<388::AID-ANIE388>3.0.CO;2-V.Angew Chem Int Ed. 1998;37:388–401. doi: 10.1002/(SICI)1521-3773(19980302)37:4<388::AID-ANIE388>3.0.CO;2-V.Christoffers J, Mann A. Angew Chem. 2001;113:4725–4732.Angew Chem Int Ed. 2001;40:4591–4597. doi: 10.1002/1521-3773(20011217)40:24<4591::aid-anie4591>3.0.co;2-v.Denissova I, Barriault L. Tetrahedron. 2003;59:10105–10146.Douglas CJ, Overman LE. Proc Natl Acad Sci USA. 2004;101:5363–5367. doi: 10.1073/pnas.0307113101.Christoffers J, Baro A. Adv Synth Catal. 2005;347:1473–1482.Quaternary Stereocenters: Christoffers J, Baro A, editors. Challenges and Solutions for Organic Synthesis. Wiley; Weinheim, Germany: 2005. Trost BM, Jiang C. Synthesis. 2006:369–396.Cozzi PG, Hilgraf R, Zimmermann N. Eur J Org Chem. 2007:5969–5994.Das JP, Marek I. Chem Commun. 2011;47:4593–4623. doi: 10.1039/c0cc05222a.
- 3.For general reviews discussing palladium-catalyzed asymmetric allylic alkylation, see: Trost BM. Acc Chem Res. 1996;29:355–364.Trost BM, Van Vranken DL. Chem Rev. 1996;96:395–422. doi: 10.1021/cr9409804.Helmchen G. J Organomet Chem. 1999;576:203–214.Pfaltz A, Lautens M. In: Comprehensive Asymmetric Catalysis. Jacobsen EN, Pfaltz A, Yamamoto H, editors. Vol. 2. Springer; New York: 1999. pp. 833–884.Trost BM, Lee C. In: Catalytic Asymmetric Synthesis. 2. Ojima I, editor. Wiley-VCH; New York: 2000. pp. 593–649.Trost BM. Chem Pharm Bull. 2002;50:1–14. doi: 10.1248/cpb.50.1.Trost BM. J Org Chem. 2004;69:5813–5837. doi: 10.1021/jo0491004.Lu Z, Ma S. Angew Chem. 2008;120:264–303.Angew Chem Int Ed. 2008;47:258–297. doi: 10.1002/anie.200605113.Trost BM. Org Process Res Dev. 2012;16:185–194. doi: 10.1021/op200294r.
- 4.For reviews discussing Pd-catalyzed decarboxylative allylic alkylation reactions, see: Tunge JA, Burger EC. Eur J Org Chem. 2005:1715–1726.Mohr JT, Stoltz BM. Chem Asian J. 2007;2:1476–1491. doi: 10.1002/asia.200700183.Weaver JD, Recio A, III, Grenning AJ, Tunge JA. Chem Rev. 2011;111:1846–1913. doi: 10.1021/cr1002744.
- 5.For an earlier review discussing the application of Pd-catalyzed allylic alkylation reactions in total synthesis, see: Trost BM, Crawley ML. Chem Rev. 2003;103:2921–2944. doi: 10.1021/cr020027w.
- 6.For some recent examples of Pd-catalyzed enantioselective allylic alkylation reactions of ketone enolates leading to the formation of chiral tertiary centers at the nucleophile in the context of total synthesis, see: nigellamine A2: Bian J, Van Wingerden M, Ready JM. J Am Chem Soc. 2006;128:7428–7429. doi: 10.1021/ja061559n.hyperolactone C and biyouyanagin A: Du C, Li L, Li Y, Xie Z. Angew Chem. 2009;121:7993–7996.Angew Chem Int Ed. 2009;48:7853–7856. doi: 10.1002/anie.200902908.Tekturna (aliskiren): Hanessian S, Chénard E. Org Lett. 2012;14:3222–3225. doi: 10.1021/ol301332f.
- 7.For some recent examples of catalyst-controlled Pd-catalyzed diastereoselective allylic alkylation reactions in the context of total synthesis, see: spirotryprostatin B: Trost BM, Stiles DT. Org Lett. 2007;9:2763–2766. doi: 10.1021/ol070971k.epothilone D: Prantz K, Mulzer J. Chem Eur J. 2010;16:485–506. doi: 10.1002/chem.200901567.drechslerines A and B: Hagiwara H, Fukushima M, Kinugawa K, Matsui T, Hoshi T, Suzuki T. Tetrahedron. 2011;67:4061–4068.
- 8.Hayashi T, Kanehira K, Hagihara T, Kumada M. J Org Chem. 1988;53:113–120. [Google Scholar]
- 9.a) Sawamura M, Nagata H, Sakamoto H, Ito Y. J Am Chem Soc. 1992;114:2586–2592. [Google Scholar]; b) Sawamura M, Sudoh M, Ito Y. J Am Chem Soc. 1996;118:3309–3310. [Google Scholar]; c) Kuwano R, Ito Y. J Am Chem Soc. 1999;121:3236–3237. [Google Scholar]; d) Kuwano R, Uchida K, Ito Y. Org Lett. 2003;5:2177–2179. doi: 10.1021/ol034665s. [DOI] [PubMed] [Google Scholar]
- 10.a) Trost BM, Ariza X. Angew Chem. 1997;109:2749–2751. [Google Scholar]; Angew Chem Int Ed Engl. 1997;36:2635–2637. [Google Scholar]; b) Trost BM, Radinov R, Grenzer EM. J Am Chem Soc. 1997;119:7879–7880. [Google Scholar]; c) Trost BM, Schroeder GM. J Am Chem Soc. 1999;121:6759–6760. [Google Scholar]; d) Trost BM, Schroeder GM, Kristensen J. Angew Chem. 2002;114:3642–3645. doi: 10.1002/1521-3773(20020916)41:18<3492::AID-ANIE3492>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2002;41:3492–3495. doi: 10.1002/1521-3773(20020916)41:18<3492::AID-ANIE3492>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]; e) Trost BM, Schroeder GM. Chem Eur J. 2005;11:174–184. doi: 10.1002/chem.200400666. [DOI] [PubMed] [Google Scholar]
- 11.a) You SL, Hou XL, Dai LX, Cao BX, Sun J. Chem Commun. 2000:1933–1934. [Google Scholar]; b) You SL, Hou XL, Dai LX, Zhu XZ. Org Lett. 2001;3:149–151. doi: 10.1021/ol0067033. [DOI] [PubMed] [Google Scholar]
- 12.Tsuji J, Minami I, Shimizu I. Tetrahedron Lett. 1983;24:4713–4714. [Google Scholar]
- 13.Tsuji J, Minami I, Shimizu I. Chem Lett. 1983:1325–1326. [Google Scholar]
- 14.Tsuji J, Minami I, Shimizu I. Tetrahedron Lett. 1983;24:1793–1796. [Google Scholar]
- 15.Shimizu I, Yamada T, Tsuji J. Tetrahedron Lett. 1980;21:3199–3202. [Google Scholar]
- 16.Saegusa published very similar work with β-keto esters simultaneously with Tsuji’s work, see: Tsuda T, Chujo Y, Nishi S, Tawara K, Saegusa T. J Am Chem Soc. 1980;102:6381–6384.
- 17.a) Behenna DC, Stoltz BM. J Am Chem Soc. 2004;126:15044–15045. doi: 10.1021/ja044812x. [DOI] [PubMed] [Google Scholar]; b) Mohr JT, Behenna DC, Harned AM, Stoltz BM. Angew Chem. 2005;117:7084–7087. doi: 10.1002/anie.200502018. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2005;44:6924–6927. doi: 10.1002/anie.200502018. [DOI] [PubMed] [Google Scholar]; c) Behenna DC, Mohr JT, Sherden NH, Marinescu SC, Harned AM, Tani K, Seto M, Ma S, Novák Z, Krout MR, McFadden RM, Roizen JL, Enquist JA, Jr, White DE, Levine SR, Petrova KV, Iwashita A, Virgil SC, Stoltz BM. Chem Eur J. 2011;17:14199–14223. doi: 10.1002/chem.201003383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.a) Trost BM, Xu J. J Am Chem Soc. 2005;127:2846–2847. doi: 10.1021/ja043472c. [DOI] [PubMed] [Google Scholar]; b) Trost BM, Xu J, Schmidt T. J Am Chem Soc. 2009;131:18343–18357. doi: 10.1021/ja9053948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.For a review discussing catalytic enantioselective stereoablative processes, see: Mohr JT, Ebner DC, Stoltz BM. Org Biomol Chem. 2007;5:3571–3576. doi: 10.1039/b711159m.
- 20.For an early report of Trost ligands in Pd-catalyzed asymmetric alkylation reactions, see: Trost BM, Van Vranken DL, Bingel C. J Am Chem Soc. 1992;114:9327–9343.
- 21.For early reports of PHOX ligands, see: von Matt P, Pfaltz A. Angew Chem. 1993;105:614–615.Angew Chem Int Ed Engl. 1993;32:566–568.Sprinz J, Helmchen G. Tetrahedron Lett. 1993;34:1769–1772.Dawson GJ, Frost CG, Williams JMJ, Coote SJ. Tetrahedron Lett. 1993;34:3149–3150.
- 22.a) Trost BM, Pissot-Soldermann C, Chen I, Schroeder GM. J Am Chem Soc. 2004;126:4480–4481. doi: 10.1021/ja0497025. [DOI] [PubMed] [Google Scholar]; b) Trost BM, Pissot-Soldermann C, Chen I. Chem Eur J. 2005;11:951–959. doi: 10.1002/chem.200400558. [DOI] [PubMed] [Google Scholar]
- 23.a) Trost BM, Dong L, Schroeder GM. J Am Chem Soc. 2005;127:2844–2845. doi: 10.1021/ja0435586. [DOI] [PubMed] [Google Scholar]; b) Trost BM, Dong L, Schroeder GM. J Am Chem Soc. 2005;127:10259–10268. doi: 10.1021/ja051547m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.a) Trost BM, Toste FD. J Am Chem Soc. 1999;121:9728–9729. [Google Scholar]; b) Trost BM, Toste FD. J Am Chem Soc. 2000;122:714–715. [Google Scholar]; c) Trost BM, Toste FD. J Am Chem Soc. 2002;124:5025–5036. doi: 10.1021/ja012450c. [DOI] [PubMed] [Google Scholar]
- 25.a) Nokami J, Mandai T, Imakura Y, Nishiuchi K, Kawada M, Wakabayashi S. Tetrahedron Lett. 1981;22:4489–4490. [Google Scholar]; b) Ono T, Tamaoka T, Yuasa Y, Matsuda T, Nokami J, Wakabayashi S. J Am Chem Soc. 1984;106:7890–7893. [Google Scholar]; c) Trost BM, Mallart S. Tetrahedron Lett. 1993;34:8025–8028. [Google Scholar]
- 26.Snider BB, Vo NH, O’Neil SV, Foxman BM. J Am Chem Soc. 1996;118:7644–7645. [Google Scholar]
- 27.McFadden RM, Stoltz BM. J Am Chem Soc. 2006;128:7738–7739. doi: 10.1021/ja061853f. [DOI] [PubMed] [Google Scholar]
- 28.Day JJ, McFadden RM, Virgil SC, Kolding H, Alleva JL, Stoltz BM. Angew Chem. 2011;123:6946–6950. doi: 10.1002/anie.201101842. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:6814–6818. doi: 10.1002/anie.201101842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.a) White DE, Stewart IC, Grubbs RH, Stoltz BM. J Am Chem Soc. 2008;130:810–811. doi: 10.1021/ja710294k. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) White DE, Stewart IC, Seashore-Ludlow BA, Grubbs RH, Stoltz BM. Tetrahedron. 2010;66:4668–4686. doi: 10.1016/j.tet.2010.04.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart IC, Ung T, Pletnev AA, Berlin JM, Grubbs RH, Schrodi Y. Org Lett. 2007;9:1589–1592. doi: 10.1021/ol0705144. [DOI] [PubMed] [Google Scholar]
- 31.Varseev GN, Maier ME. Angew Chem. 2009;121:3739–3742. [Google Scholar]; Angew Chem Int Ed. 2009;48:3685–3688. doi: 10.1002/anie.200900447. [DOI] [PubMed] [Google Scholar]
- 32.Toyota M, Wada T, Fukumoto K, Ihara M. J Am Chem Soc. 1998;120:4916–4925. [Google Scholar]
- 33.a) Nicolaou KC, Tria GS, Edmonds DJ. Angew Chem. 2008;120:1804–1807. doi: 10.1002/anie.200800066. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:1780–1783. doi: 10.1002/anie.200800066. [DOI] [PubMed] [Google Scholar]; b) Hayashida J, Rawal VH. Angew Chem. 2008;120:4445–4448. [Google Scholar]; Angew Chem Int Ed. 2008;47:4373–4376. doi: 10.1002/anie.200800756. [DOI] [PubMed] [Google Scholar]; c) Yun SY, Zheng JC, Lee D. Angew Chem. 2008;120:6297–6299. [Google Scholar]; Angew Chem Int Ed. 2008;47:6201–6203. doi: 10.1002/anie.200801587. [DOI] [PubMed] [Google Scholar]; d) Tiefenbacher K, Mulzer J. Angew Chem. 2008;120:6294–6295. [Google Scholar]; Angew Chem Int Ed. 2008;47:6199–6200. doi: 10.1002/anie.200801441. [DOI] [PubMed] [Google Scholar]; e) Waalboer DCJ, Schaapman MC, van Delft FL, Rutjes FPJT. Angew Chem. 2008;120:6678–6680. [Google Scholar]; Angew Chem Int Ed. 2008;47:6576–6578. doi: 10.1002/anie.200802912. [DOI] [PubMed] [Google Scholar]; f) Nicolaou KC, Toh QY, Chen DYK. J Am Chem Soc. 2008;130:11292–11293. doi: 10.1021/ja804588r. [DOI] [PubMed] [Google Scholar]; Nicolaou KC, Toh QY, Chen DYK. J Am Chem Soc. 2008;130:14016. doi: 10.1021/ja804588r. [DOI] [PubMed] [Google Scholar]; g) Austin KAB, Banwell MG, Willis AC. Org Lett. 2008;10:4465–4468. doi: 10.1021/ol801647h. [DOI] [PubMed] [Google Scholar]
- 34.Mukherjee H, McDougal NT, Virgil SC, Stoltz BM. Org Lett. 2011;13:825–827. doi: 10.1021/ol102669z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miesch L, Welsch T, Rietsch V, Miesch M. Chem Eur J. 2009;15:4394–4401. doi: 10.1002/chem.200802309. [DOI] [PubMed] [Google Scholar]
- 36.Levine SR, Krout MR, Stoltz BM. Org Lett. 2009;11:289–292. doi: 10.1021/ol802409h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrova KV, Mohr JT, Stoltz BM. Org Lett. 2009;11:293–295. doi: 10.1021/ol802410t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trost BM, Bream RN, Xu J. Angew Chem. 2006;118:3181–3184. doi: 10.1002/anie.200504421. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2006;45:3109–3112. doi: 10.1002/anie.200504421. [DOI] [PubMed] [Google Scholar]
- 39.Aoyama Y, Araki Y, Konoike T. Synlett. 2001;9:1452–1454. [Google Scholar]
- 40.Trost BM, Malhotra S, Chan WH. J Am Chem Soc. 2011;133:7328–7331. doi: 10.1021/ja2020873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kazmaier U, Stolz D, Krämer K, Zumpe FL. Chem Eur J. 2008;14:1322–1329. doi: 10.1002/chem.200701332. [DOI] [PubMed] [Google Scholar]
- 42.Kawasaki T, Shinada M, Kamimura D, Ohzono M, Ogawa A. Chem Commun. 2006:420–422. doi: 10.1039/b512485a. [DOI] [PubMed] [Google Scholar]
- 43.a) Enquist JA, Jr, Stoltz BM. Nature. 2008;453:1228–1231. doi: 10.1038/nature07046. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Enquist JA, Jr, Virgil SC, Stoltz BM. Chem Eur J. 2011;17:9957–9969. doi: 10.1002/chem.201100425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langenbeck W, Triem G. Z Phys Chem Abt A. 1936;117:401–409. [Google Scholar]
- 45.a) Vigneron JP, Dhaenens M, Horeau A. Tetrahedron. 1973;29:1055–1059. [Google Scholar]; b) Rautentrauch V. Bull Soc Chim Fr. 1994;131:515–524. [Google Scholar]; c) Baba SE, Sartor K, Poulin J, Kagan H. Bull Soc Chim Fr. 1994;131:525–533. [Google Scholar]
- 46.a) Shimizu I, Tsuji J. J Am Chem Soc. 1982;104:5844–5846. [Google Scholar]; b) Shimizu I, Minami I, Tsuji J. Tetrahedron Lett. 1983;24:1797–1800. [Google Scholar]; c) Minami I, Takahashi K, Shimizu I, Kimura T, Tsuji J. Tetrahedron. 1986;42:2971–2977. [Google Scholar]
- 47.a) Hong AY, Krout MR, Jensen T, Bennett NB, Harned AM, Stoltz BM. Angew Chem. 2011;123:2808–2812. doi: 10.1002/anie.201007814. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:2756–2760. doi: 10.1002/anie.201007814. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hong AY, Bennett NB, Krout MR, Jensen T, Harned AM, Stoltz BM. Tetrahedron. 2011;67:10234–10248. doi: 10.1016/j.tet.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Bennett NB, Hong AY, Harned AM, Stoltz BM. Org Biomol Chem. 2012;10:56–59. doi: 10.1039/c1ob06189e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hong AY, Stoltz BM. Angew Chem. 2012;124:9812–9816.Angew Chem Int Ed. 2012;51:9674–9678. doi: 10.1002/anie.201205276.this communication is corrected by: Hong AY, Stoltz BM. Angew Chem. 2013;125:2201.Angew Chem Int Ed. 2013;52:2147.
- 49.a) Ely RJ, Morken JP. J Am Chem Soc. 2010;132:2534–2535. doi: 10.1021/ja910750b. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ely RJ, Morken JP. Org Synth. 2011;88:342–352. [Google Scholar]
- 50.Behenna DC, Liu Y, Yurino T, Kim J, White DE, Virgil SC, Stoltz BM. Nature Chem. 2012;4:130–133. doi: 10.1038/nchem.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magnus P, Rainey T. Tetrahedron. 2001;57:8647–8651. [Google Scholar]
- 52.Amat M, Lozano O, Escolano C, Molins E, Bosch J. J Org Chem. 2007;72:4431–4439. doi: 10.1021/jo070397q. [DOI] [PubMed] [Google Scholar]
- 53.Bennett NB, Duquette DC, Kim J, Liu W-B, Marziale AN, Behenna DC, Virgil SC, Stoltz BM. Chem Eur J. doi: 10.1002/chem.201300030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gartshore CJ, Lupton DW. Angew Chem. 2013;125 doi: 10.1002/ange.201209069;. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2013;52 doi: 10.1002/anie.201209069. Early View. [DOI] [Google Scholar]
- 55.Jing P, Yang Z, Zhao C, Zheng H, Fang B, Xie X, She X. Chem Eur J. 2012;18:6729–6732. doi: 10.1002/chem.201200867. [DOI] [PubMed] [Google Scholar]
- 56.Li Z, Zhang S, Wu S, Shen X, Zou L, Wang F, Li X, Peng F, Zhang H, Shao Z. Angew Chem. 2013;125 doi: 10.1002/ange.201209878;. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2013;52 doi: 10.1002/anie.201209878. [DOI] [Google Scholar]